Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Results
2.1. Patients
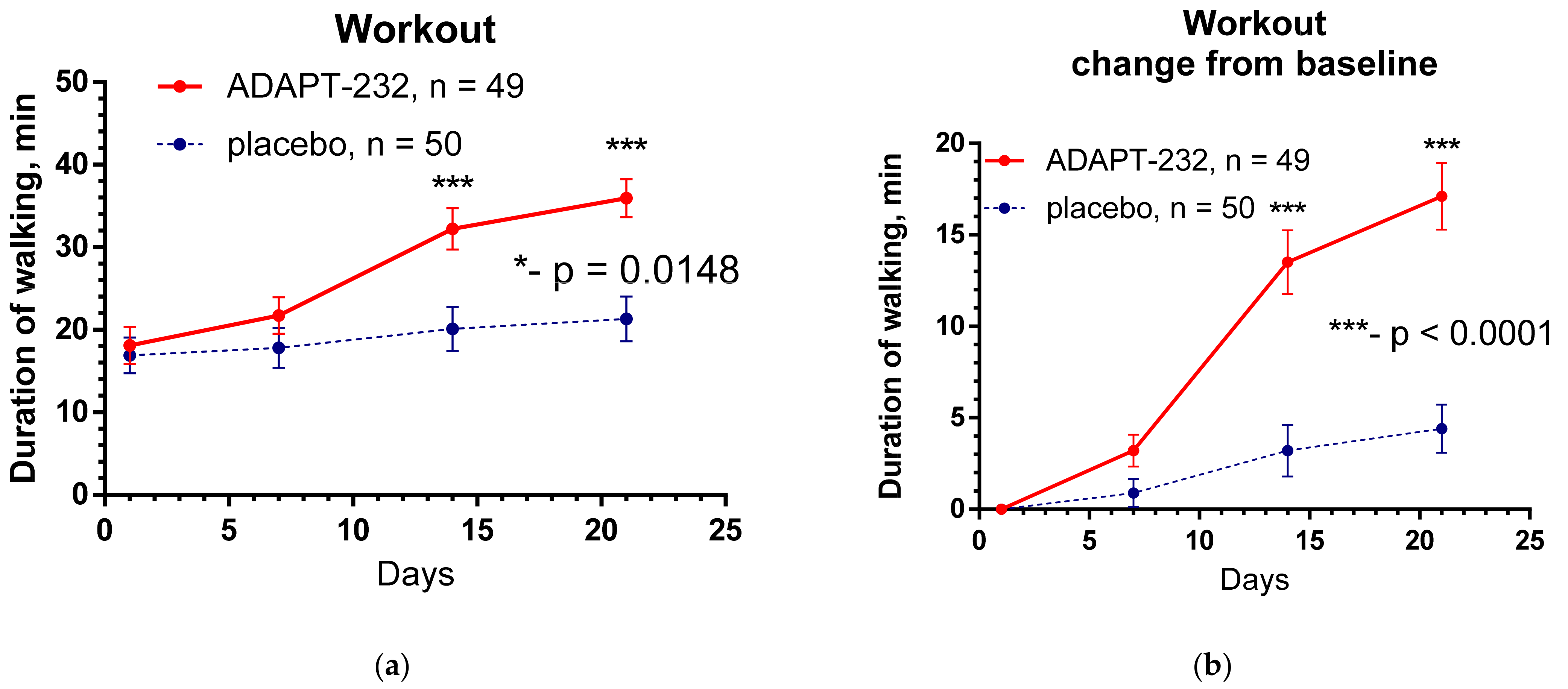
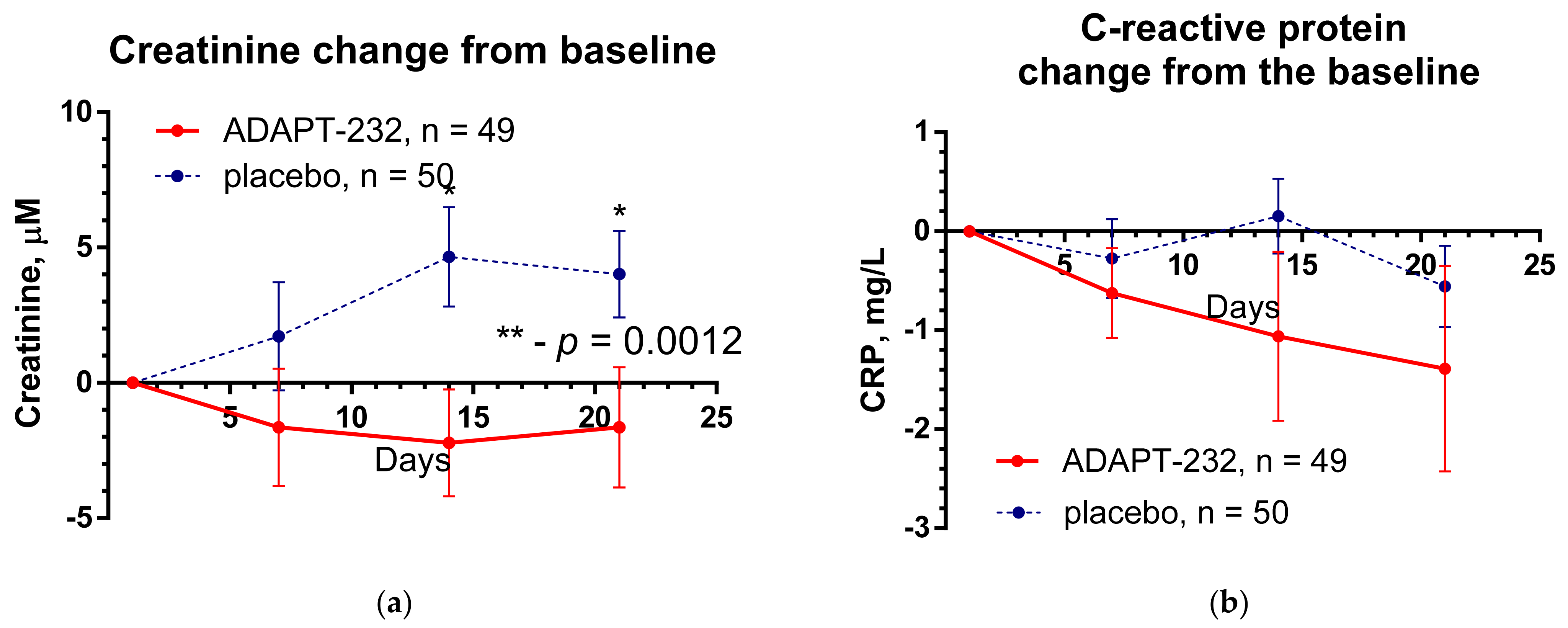
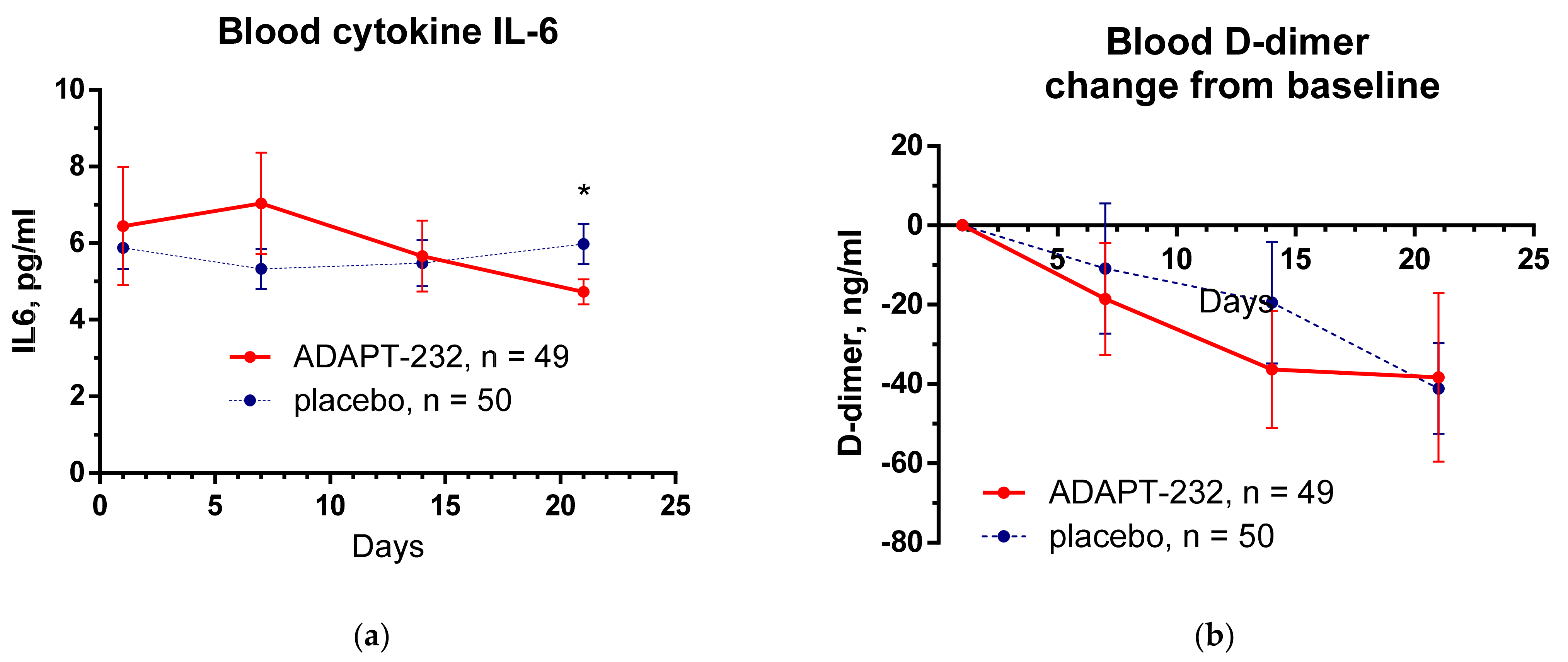
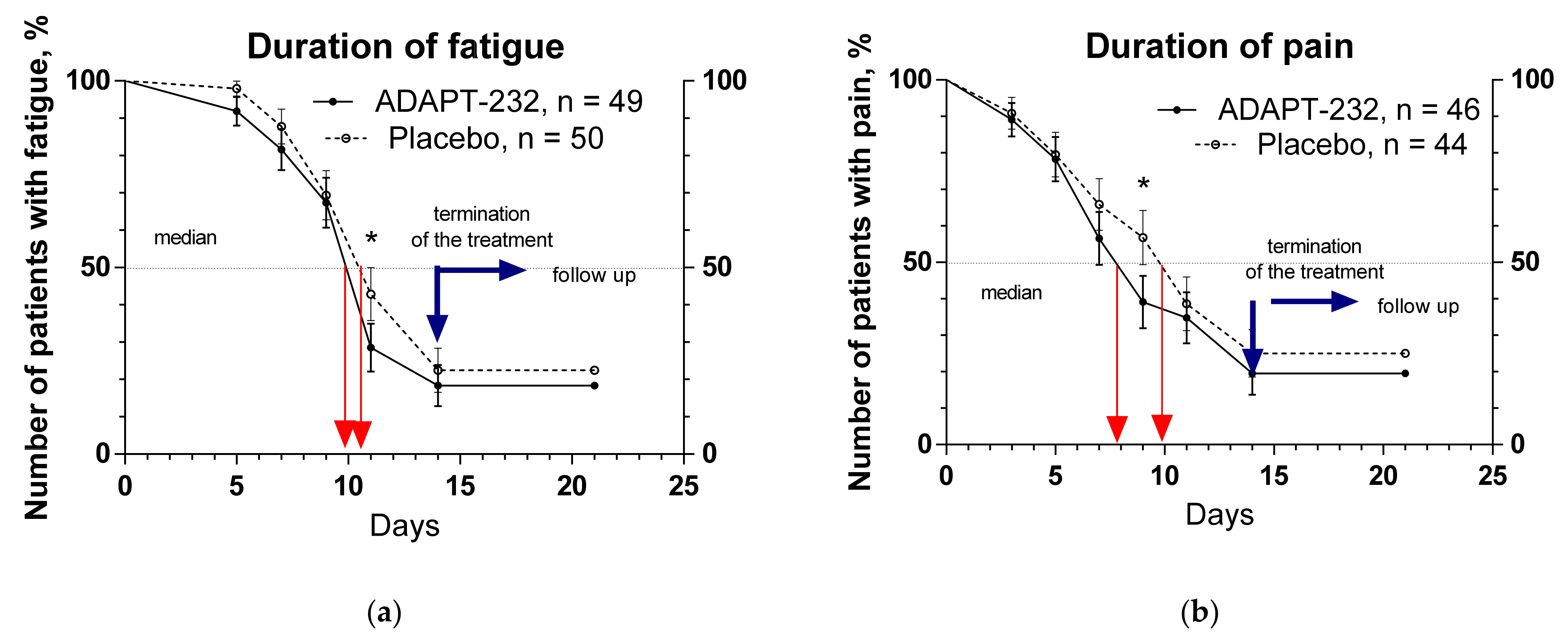
2.2. Efficacy
2.3. Safety
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Population
4.2.1. Recruitment and Screening
4.2.2. Inclusion and Exclusion Criteria
4.2.3. Participant Withdrawal
4.2.4. Data Sets Analyzed
4.3. Intervention and Comparator
4.3.1. Doses and Treatment Regimens
4.3.2. Randomization and Blinding
4.3.3. Allocation Concealment
4.3.4. Implementation and Blinding
4.3.5. Evaluation of Compliance
4.4. Efficacy and Safety Outcomes and Endpoints
- Duration of symptoms of Long COVID: time (days) from randomization to when symptoms disappear. Time frame: change from baseline during the treatment period and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
- Number of participants clinically recovered: number of participants without symptoms of Long COVID. Time frame: change from baseline during the treatment period and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
- Length of homestay/sick-listed: time (days) at home/sick-listed. Time frame: change from baseline during the treatment period and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
- The severity of the Long COVID symptoms: time from randomization to relief of total and individual Long COVID symptoms. Patients were assessed for changes in clinical signs: headache, fatigue, physical activity, depression and anxiety, anosmia, ageusia, hair loss, cough, fever, sweat, pain in muscles, chest, and joints. The medians and hazard ratio were measured and compared between groups. Time frame: change from baseline during the treatment period and follow-up (through 21 days after randomization).
- Physical activity and daily workout: assessed by Habitual Physical Activity Questionnaire Score and duration of walking (min). Time frame: change from baseline during the treatment period and follow-up (from Day 1 to Day 14 and day 21 after randomization).
- Cognitive performance score: d2 test of attention and memory. Time frame: change from baseline during the period of the treatment and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
- The severity of anxiety and depression was assessed by Hospital Anxiety and Depression Scale (HADS). Time frame: change from baseline during the treatment period and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
- The severity of anxiety assessed by Hamilton Anxiety Rating Scale. Time frame: change from baseline during the treatment period and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
- Hypercoagulation marker: D-dimer, concentration in the serum, pg/L. Time frame: change from baseline during the period of the treatment and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
- Immune response marker: IL-6 concentration in the serum, pg/mL. Time frame: change from baseline during the treatment period and follow-up (from Day 1 to Day 14 and day 21 after randomization).
- Inflammatory marker: C-reactive protein in serum, mg/L. Time Frame: change from baseline during the period of the treatment and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
- Inflammatory marker: creatinine in serum, μM. Time frame: change from baseline during the treatment period and follow-up (from Day 1 to Day 14 and Day 21 after randomization).
4.5. Statistical Analysis
Sample Size Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Graaf, M.A.; Antoni, M.L.; Ter Kuile, M.M.; Arbous, M.S.; Duinisveld, A.J.F.; Feltkamp, M.C.W.; Groeneveld, G.H.; Hinnen, S.C.H.; Janssen, V.R.; Lijfering, W.M.; et al. Short-term outpatient follow-up of COVID-19 patients: A multidisciplinary approach. eClinicalMedicine 2021, 32, 100731. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. medRxiv 2021. the preprint server for health sciences. Available online: https://www.nature.com/articles/s41598-021-95565-8.pdf (accessed on 14 February 2022). [CrossRef]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Billig Rose, E.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. CDC COVID-19 Response Team, IVY Network Investigators. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network—United States, March–June 2020. MMWR Morb. Mort. Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Callard, F.; Perego, E. How and why patients made Long COVID. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.; et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 00542-2020. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Long COVID. Available online: https://clinicaltrials.gov/ct2/results?term=Long+COVID&cond=COVID-19&age_v=&gndr=&type=Intr&rslt=&Search=Apply (accessed on 14 February 2022).
- ClinicalTrials.gov. COVID-19 Long-Haulers Study. Available online: https://clinicaltrials.gov/ct2/show/NCT04678830 (accessed on 14 February 2022).
- ClinicalTrials.gov. Zilucoplan® in Improving Oxygenation and Short- and Long-Term Outcome of COVID-19 Patients with Acute Hypoxic Respiratory Failure. Available online: https://clinicaltrials.gov/ct2/show/NCT04382755 (accessed on 14 February 2022).
- ClinicalTrials.gov. WHO COVID-19—Evaluation of the Efficacy of Probiotics to Reduce the Occurrence of Long COVID. Available online: https://clinicaltrials.gov/ct2/show/NCT05080244 (accessed on 14 February 2022).
- ClinicalTrials.gov. Phase 2 Study of RSLV-132 in Subjects with Long COVID. Available online: https://clinicaltrials.gov/ct2/show/NCT04944121 (accessed on 14 February 2022).
- ClinicalTrials.gov. Homeopathic Treatment of Post-Acute COVID-19 Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT05104749 (accessed on 14 February 2022).
- ClinicalTrials.gov. Feasibility of Cannabidiol for the Treatment of Long COVID. Available online: https://clinicaltrials.gov/ct2/show/NCT04997395 (accessed on 14 February 2022).
- ClinicalTrials.gov. Efficacy of Montelukast in Mild-Moderate Respiratory Symptoms in Patients with Long-COVID-19: (E-SPERANZA). Available online: https://clinicaltrials.gov/ct2/show/NCT04695704 (accessed on 14 February 2022).
- ClinicalTrials.gov. Pilot Study Into LDN and NAD+ for Treatment of Patients with Post-COVID-19 Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT04604704 (accessed on 14 February 2022).
- ClinicalTrials.gov. Feasibility Pilot Clinical Trial of Omega-3 Supplement vs. Placebo for Post COVID-19 Recovery Among Health Care Workers. Available online: https://clinicaltrials.gov/ct2/show/NCT05121766 (accessed on 14 February 2022).
- ClinicalTrials.gov. HElping Alleviate the Longer-Term Consequences of COVID-19 (HEAL-COVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04801940 (accessed on 14 February 2022).
- ClinicalTrials.gov. A Randomised-Controlled Trial of an Oral Microbiome Immunity Formula in Recovered COVID-19 Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT04950803 (accessed on 14 February 2022).
- ClinicalTrials.gov. COVID-OUT: Early Outpatient Treatment for SARS-CoV-2 Infection (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04510194 (accessed on 14 February 2022).
- ClinicalTrials.gov. LYT-100 in Post-Acute COVID-19 Respiratory Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT04652518 (accessed on 14 February 2022).
- ClinicalTrials.gov. Vortioxetine for Post-COVID-19 Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT05047952 (accessed on 14 February 2022).
- ClinicalTrials.gov. Coenzyme Q10 as Treatment for Long Term COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04960215 (accessed on 14 February 2022).
- ClinicalTrials.gov. Special Chinese Medicine Out-Patient Programme for Discharged COVID-19 Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT0444605 (accessed on 14 February 2022).
- ClinicalTrials.gov. Spironolactone and Dexamethasone in Patients Hospitalized With COVID-19 (SPIDEX-II). Available online: https://clinicaltrials.gov/ct2/show/NCT04826822 (accessed on 14 February 2022).
- ClinicalTrials.gov. The Effect of Micellized Food Supplements on Health-related Quality of Life in Patients With Post-Acute COVID-19 Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT05150782 (accessed on 14 February 2022).
- ClinicalTrials.gov. Efficacy of Hydroxychloroquine (HCQ) as Post Exposure Prophylaxis (PEP) for Prevention of COVID-19 (PEP-CQ). Available online: https://clinicaltrials.gov/ct2/show/NCT04408456 (accessed on 14 February 2022).
- ClinicalTrials.gov. CACOLAC: Citrulline Administration in the Hospital Patient in Intensive Care for COVID-19 Acute Respiratory Distress Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT04404426 (accessed on 14 February 2022).
- ClinicalTrials.gov. Recovering Damaged Cells for Sequelae Caused by COVID-19, SARS-CoV-2. Available online: https://clinicaltrials.gov/ct2/show/NCT04846010 (accessed on 14 February 2022).
- ClinicalTrials.gov. Efficacy, Safety, Tolerability of AXA1125 in Fatigue Predominant PASC. Available online: https://clinicaltrials.gov/ct2/show/NCT05152849 (accessed on 14 February 2022).
- ClinicalTrials.gov. Evaluation of the Effect of Long-Term Lipid-Lowering Therapy in STEMI Patients with Coronavirus Infection COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04900155 (accessed on 14 February 2022).
- Post COVID-19 Syndrome and the Gut-Lung Axis. Available online: https://clinicaltrials.gov/ct2/show/NCT04813718 (accessed on 14 February 2022).
- ClinicalTrials.gov. A Study of Immune System Proteins in Participants with Mild to Moderate COVID-19 Illness. Available online: https://clinicaltrials.gov/ct2/show/NCT04634409 (accessed on 14 February 2022).
- ClinicalTrials.gov. Efficacy Evaluation of Shen Cao Gan Jiang Tang on Mild and Moderate COVID-19 Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT05055427 (accessed on 14 February 2022).
- Li, L.; Gou, C.Y.; Li, X.M.; Song, W.Y.; Wang, X.J.; Li, H.Y.; Li, H.Y.; Li, X.H. Effects of Chinese Medicine on Symptoms, Syndrome Evolution, and Lung Inflammation Absorption in COVID-19 Convalescent Patients during 84-Day Follow-up after Hospital Discharge: A Prospective Cohort and Nested Case-Control Study. Chin. J. Integr. Med. 2021, 27, 245–251. [Google Scholar] [CrossRef]
- May, B.C.; Gallivan, K.H. Levocetirizine and montelukast in the COVID-19 treatment paradigm. Int. Immunopharmacol. 2022, 103, 108412. [Google Scholar] [CrossRef]
- Cordero, F.M.; Monne, S.B.; Ortega, J.A.; García-Sangenís, A.; Puèrtolas, O.C.; Contreras-Martos, S.; Muñoz, G.A.; Escolà, R.M.; Joué, M.B.; Pedrós, R.M.; et al. Double-blind placebo-controlled randomized clinical trial to assess the efficacy of montelukast in mild to moderate respiratory symptoms of patients with long COVID: E-SPERANZA COVID Project study protocol. Trials 2022, 23, 19. [Google Scholar] [CrossRef]
- Narimanian, M.; Badalyan, M.; Panosyan, V.; Gabrielyan, E.; Panossian, A.; Wikman, G.; Wagner, H. Impact of Chisan (ADAPT-232) on the quality-of-life and its efficacy as an adjuvant in the treatment of acute non-specific pneumonia. Phytomedicine 2005, 12, 723–729. [Google Scholar] [CrossRef]
- Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Panossian, A.; Wikman, G. Double-blind, placebo-controlled, randomized study of the single-dose effects of ADAPT-232 on cognitive functions. Phytomedicine 2010, 17, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Hovhannisyan, A.; Nylander, M.; Wikman, G.; Panossian, A.G. Efficacy of Adaptogenic Supplements on Adapting to Stress: A Randomized, Controlled Trial. J. Athl. Enhanc. 2015, 19, 2. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Current Clin. Pharmacol. 2009, 4, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Hamm, R.; Kadioglu, O.; Wikman, G.; Efferth, T. Synergy and Antagonism of Active Constituents of ADAPT-232 on Transcriptional Level of Metabolic Regulation of Isolated Neuroglial Cells. Front. Neurosci. 2013, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Y.; Ou, W.; Ming, F.; Liang, G.; Qian, Y.; Cai, Q.; Dong, S.; Hu, S.; Wang, W.; et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: A cohort study. J. Transl. Med. 2020, 18, 406. [Google Scholar] [CrossRef]
- Sabaka, P.; Koščálová, A.; Straka, I.; Hodosy, J.; Lipták, R.; Kmotorková, B.; Kachlíková, M.; Kušnírová, A. Role of interleukin 6 as a predictive factor for a severe course of COVID-19: Retrospective data analysis of patients from a long-term care facility during COVID-19 outbreak. BMC Infect. Dis. 2021, 21, 308. [Google Scholar] [CrossRef]
- Sonnweber, T.; Boehm, A.; Sahanic, S.; Pizzini, A.; Aichner, M.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: A prospective observational cohort study. Respir. Res. 2020, 21, 276. [Google Scholar] [CrossRef]
- Cione, E.; Siniscalchi, A.; Gangemi, P.; Cosco, L.; Colosimo, M.; Longhini, F.; Luciani, F.; De Sarro, G.; G&SPWorking Group Berrino, L.; D’Agostino, B.; et al. Neuron-specific enolase serum levels in COVID-19 are related to the severity of lung injury. PLoS ONE 2021, 16, e0251819. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney Outcomes in Long COVID. J. Am. Soc. Nephrol. 2021, 32, 2851–2862. Available online: https://jasn.asnjournals.org/content/early/2021/08/25/ASN.2021060734 (accessed on 14 February 2022). [CrossRef] [PubMed]
- Głowacka, M.; Lipka, S.; Młynarska, E.; Franczyk, B.; Rysz, J. Acute Kidney Injury in COVID-19. Int. J. Mol. Sci. 2021, 22, 8081. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.; Parikh, C.R. Long COVID and kidney disease. Nat. Rev. Nephrol. 2021, 17, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.H.; Cheng, Y.C.; Luo, R.; Zhang, C.X.; Ge, S.W.; Xu, G. Recovery of new-onset kidney disease in COVID-19 patients discharged from hospital. BMC Infect. Dis. 2021, 21, 397. [Google Scholar] [CrossRef]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef]

| Unit | Group A ADAPT-232, n = 50 | Group B Placebo n = 50 | Signif. of Difference | |||
|---|---|---|---|---|---|---|
| Parameters | Mean | S.D. | Mean | S.D. | p-Value | |
| Age | years | 49.52 | 12.57 | 48.18 | 15.13 | 0.6312 a |
| Gender | Male/Female | 7/42 | 7/43 | |||
| BMI | kg/m2 | 26.93 | 4.054 | 26.62 | 4.750 | 0.7196 a |
| Start of symptoms | weeks | 2.720 | 0.6402 | 2.760 | 0.6247 | 0.7525 b |
| Compliance | % | 101.0 | 1.998 | 100.8 | 2.437 | 0.1804 b |
| Fatigue | A.U. | 1.694 | 0.585 | 1.780 | 0.648 | 0.489 a |
| Headache | A.U. | 1.184 | 0.565 | 1.100 | 0.707 | 0.518 a |
| Respiration problems | A.U. | 0.449 | 0.6145 | 0.4600 | 0.7060 | 0.876 b |
| Organoleptic disorders | A.U. | 0.5600 | 0.7602 | 0.4400 | 0.5014 | 0.778 b |
| Hair loss | A.U. | 0.8660 | 0.1237 | 0.8953 | 0.1266 | 0.4996 a |
| Body temperature increase | C | <37 | <37 | >0.05 | ||
| Cough | AU | 0.7347 | 0.6047 | 0.6000 | 0.6061 | 0.2711 a |
| Pain in muscles, chest, and joints | A.U. | 1.245 | 0.5962 | 1.160 | 0.6503 | 0.5002 a |
| Sweatiness | A.U. | 1.204 | 0.7065 | 1.120 | 0.7730 | 0.6417 b |
| Stay at home/sick-listed | days | 0.3265 | 0.4738 | 0.8000 | 2.356 | 0.6866 b |
| Physical activity | A.U. | 16.43 | 2.850 | 15.56 | 4.305 | 0.3702 b |
| Physical activity (daily walk) | min | 5.534 | 0.0628 | 5.162 | 0.0757 | 0.7004 a |
| Decreased attention (d2 test) | %E (errors) | 25.51 | 16.70 | 26.43 | 18.93 | 0.9849 b |
| Anxiety (mild 14–17; moderate 18–24; severe > 25) | HAM-A score | 16.46 | 5.388 | 16.44 | 4.634 | 0.8674 b |
| Depression (mild 8–10, moderate 11–14, severe > 15) | HADS score | 15.30 | 4.595 | 15.78 | 4.473 | 0.5978 a |
| Blood serum cytokines IL-6 (normal level < 5.186) | pg/mL | 6.443 | 10.95 | 5.883 | 3.919 | 0.3763 b |
| D-dimer (normal level < 250) | pg/L | 133.3 | 195.8 | 105.2 | 99.46 | 0.9492 b |
| C-reactive protein (normal level < 350) | mg/L | 5.578 | 8.313 | 10.35 | 18.74 | 0.6630 b |
| Creatinine (female 52–92, male 65–120) | μM | 77.18 | 11.50 | 75.50 | 10.69 | 0.5557 b |
| Total Leukocyte count, WBC | 103 u/L | 8.96 | 12.11 | 7.55 | 4.42 | 0.44 |
| Erythrocytes, RBC | 106 u/L | 4.57 | 0.39 | 4.63 | 0.46 | 0.51 |
| Hemoglobin, Hb | g/L | 128.26 | 11.77 | 130.84 | 11.39 | 0.27 |
| Hematocrit, HCT | % | 38.53 | 3.30 | 39.12 | 3.46 | 0.39 |
| Platelet Count | 103 u/L | 240.16 | 57.65 | 251.86 | 59.64 | 0.32 |
| Absolute Neutrophil count | 103 u/L | 60.89 | 8.30 | 60.49 | 8.60 | 0.81 |
| Total Lymphocyte count | 103 u/L | 31.13 | 7.75 | 30.03 | 9.33 | 0.52 |
| Monocyte count | 103 u/L | 2.79 | 3.43 | 3.05 | 3.40 | 0.70 |
| Eosinophil count | 103 u/L | 4.36 | 1.61 | 4.22 | 1.57 | 0.65 |
| Basophil count | 103 u/L | 0.09 | 0.24 | 0.07 | 0.22 | 0.75 |
| Erythrocyte sedimentation rate, ESR | mm/h | 19.56 | 11.18 | 15.94 | 9.53 | 0.08 |
| Group A ADAPT | Group B Placebo | Significance of Difference | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Mean | SD | n | Mean | SD | n | p Value |
| Fatigue, days | 11.96 | 4.899 | 49 | 12.98 | 0.648 | 50 | 0.2662 b |
| Headache, days | 10.40 | 5.336 | 45 | 9.244 | 4.700 | 41 | 0.3582 b |
| Respiration problems, days | 6.579 | 3.485 | 19 | 6.895 | 5.259 | 19 | 0.7619 b |
| Organoleptic disfunctions, days | 11.58 | 6.543 | 26 | 10.64 | 5.728 | 22 | 0.8383 b |
| Hair loss, days | 16.70 | 5.823 | 33 | 15.41 | 5.231 | 37 | 0.3317 a |
| Cough, days | 8.094 | 4.855 | 32 | 6.259 | 4.809 | 27 | 0.0219 b |
| Pain in muscles, chest and joints, days | 10.67 | 6.056 | 46 | 11.77 | 6.243 | 44 | 0.3668 b |
| Sweatiness, days | 12.07 | 6.211 | 44 | 12.24 | 6.102 | 41 | 0.7605 b |
| Average duration of all symptoms, days | 7.640 | 2.724 | 50 | 7.463 | 2.469 | 50 | 0.5375 b |
| Stay at home/sick-listed, days | 5.918 | 9.089 | 49 | 5.940 | 9.224 | 50 | 0.9839 b |
| Treatment | Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 Screening | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 14 | Day 21 | |
| Doctor’s visits | 1 Baseline | 2 | 3 | 4 | ||||
| Eligibility check/Information | * | |||||||
| Informed consent | * | |||||||
| Clinical examination | * | * | * | * | ||||
| Enrollment and allocation to intervention | * | |||||||
| Treatment (Kan Jang and placebo) | * | * | * | * | * | * | * | |
| Biomarker assessments | ||||||||
| Body temperature (fever) | * | * | * | * | * | * | * | * |
| COVID-19 PCR test | * | * | ||||||
| Blood serum cytokine IL-6 (pg/mL) | * | * | * | * | ||||
| D-dimer (pg/L) | * | * | * | * | ||||
| C-reactive protein (mg/L) | * | * | * | * | ||||
| Creatinine μM | * | * | * | |||||
| Clinician and observer reported outcomes assessments | ||||||||
| Cognitive performance (tests forattention and memory): d2 test | * | * | * | * | ||||
Tests for anxiety/depression:
| * | * | * | * | ||||
| Drug intake accountability | * | |||||||
| Adverse events | * | * | * | |||||
| Patient-reported outcomes assessments | ||||||||
Long COVID symptoms:
| * | * | * | * | * | * | * | * |
| Workout, min | * | * | * | * | ||||
| Physical activity (questionnaire) | * | * | * | * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karosanidze, I.; Kiladze, U.; Kirtadze, N.; Giorgadze, M.; Amashukeli, N.; Parulava, N.; Iluridze, N.; Kikabidze, N.; Gudavadze, N.; Gelashvili, L.; et al. Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals 2022, 15, 345. https://doi.org/10.3390/ph15030345
Karosanidze I, Kiladze U, Kirtadze N, Giorgadze M, Amashukeli N, Parulava N, Iluridze N, Kikabidze N, Gudavadze N, Gelashvili L, et al. Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals. 2022; 15(3):345. https://doi.org/10.3390/ph15030345
Chicago/Turabian StyleKarosanidze, Irina, Ushangi Kiladze, Nino Kirtadze, Mikhail Giorgadze, Nana Amashukeli, Nino Parulava, Neli Iluridze, Nana Kikabidze, Nana Gudavadze, Lali Gelashvili, and et al. 2022. "Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial" Pharmaceuticals 15, no. 3: 345. https://doi.org/10.3390/ph15030345
APA StyleKarosanidze, I., Kiladze, U., Kirtadze, N., Giorgadze, M., Amashukeli, N., Parulava, N., Iluridze, N., Kikabidze, N., Gudavadze, N., Gelashvili, L., Koberidze, V., Gigashvili, E., Jajanidze, N., Latsabidze, N., Mamageishvili, N., Shengelia, R., Hovhannisyan, A., & Panossian, A. (2022). Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals, 15(3), 345. https://doi.org/10.3390/ph15030345







