Design, Synthesis, and Biological Evaluation of Novel Tomentosin Derivatives in NMDA-Induced Excitotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Studies
2.2.1. Cell Viability Significantly Increased following Treatment with Tomentosin Derivatives
2.2.2. Tomentosin Derivatives Prepared to Decrease Free-Radical Production
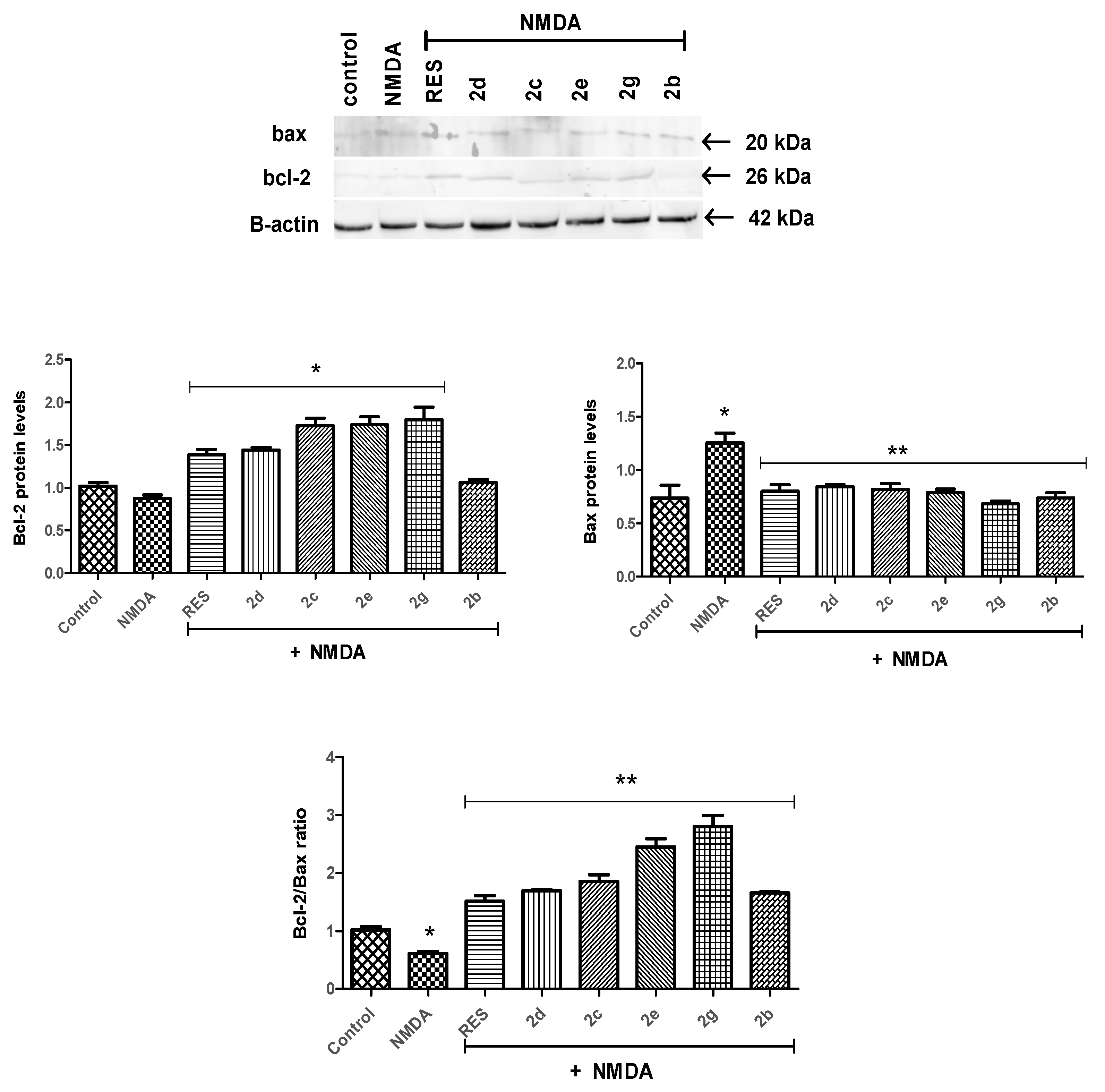
2.2.3. Bcl-2/Bax Ratio Was Altered by Tomentosin Derivatives
3. Materials and Methods
3.1. Chemistry Methods
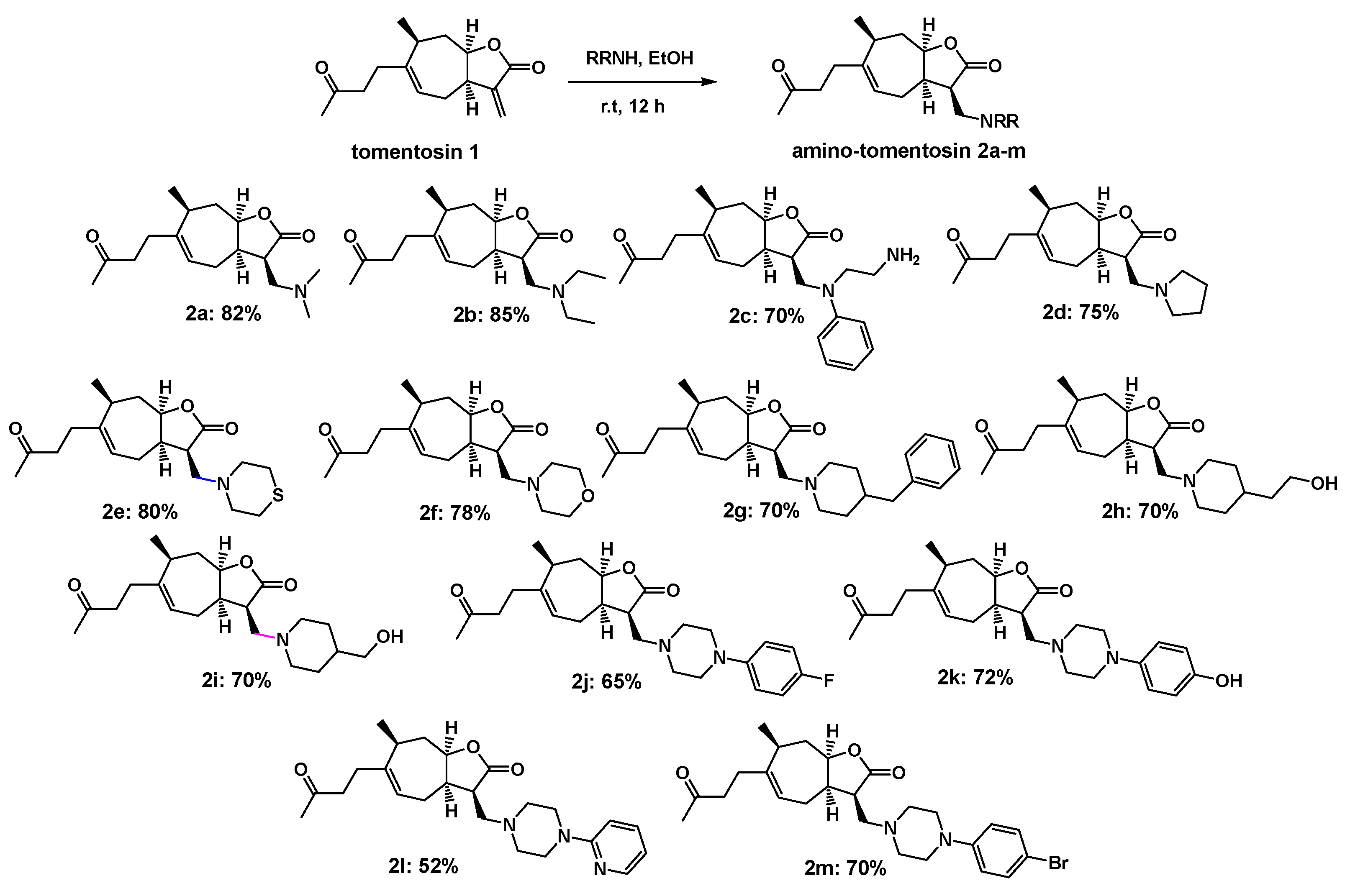
3.1.1. Synthesis of Amino-Tomentosin 2a–m
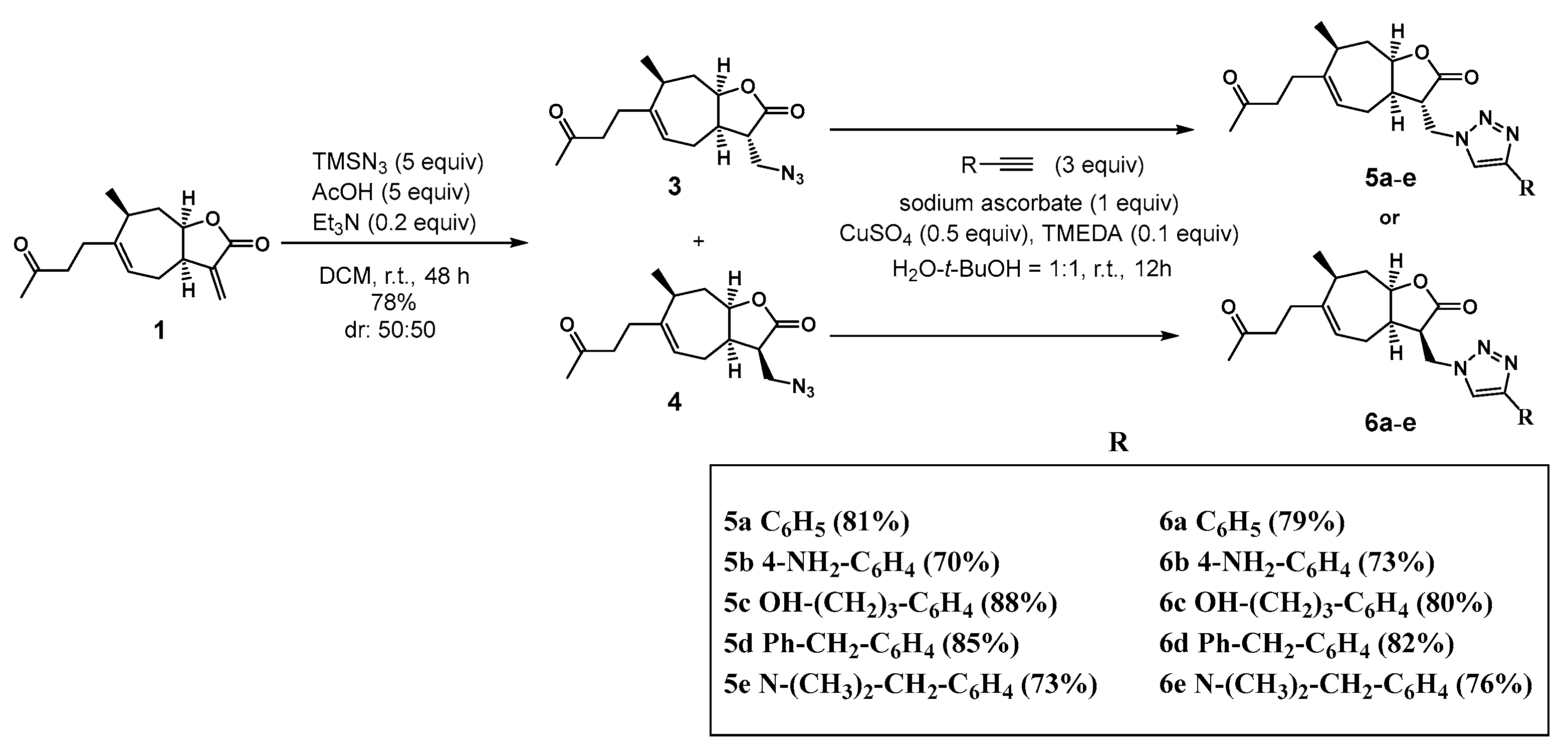
3.1.2. Synthesis of 13-Azido-11,13-Dihydrotomentosin 3 and 4
3.1.3. Synthesis of 1,2,3-Triazolo-Tomentosin 5a–e and 6a–e
4. Biological Methods and Materials
4.1. Materials
4.2. Cell Culture and Treatments
4.3. Cell Viability Assay
4.4. Free-Radical Generation (DCF-DA Assay)
4.5. Protein Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.W.; Zhang, X.I.A.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, M.F.; McMurtray, A.M. Neurodegenerative disorders. In Encyclopedia of Stress, 2nd ed.; Fink, G., McEwen, B., De Kloet, E.R., Rubin, R., Chrousos, G., Steptoe, A., Rose, N., Craig, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Barkus, C.; McHugh, S.B.; Sprengel, R.; Seeburg, P.H.; Rawlins, J.N.P.; Bannerman, D.M. Hippocampal NMDA receptors and anxiety: At the interface between cognition and emotion. Eur. J. Pharmacol. 2010, 626, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruman, I.I.; Mattson, M.P. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J. Neurochem. 1999, 72, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003, 26, 81–89. [Google Scholar] [CrossRef]
- Lindsay, J.; Esposti, M.D.; Gilmore, A.P. Bcl-2 proteins and mitochondria--specificity in membrane targeting for death. Biochim. Biophys. Acta. 2011, 1813, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.T.; Teijido, O.; Antonsson, B.; Dejean, L.M.; Manon, S. Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-xL: Keep your friends close but your enemies closer. Int. J. Biochem. Cell. Biol. 2013, 45, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Saghatelian, A. Emerging roles of lipids in BCL-2 family-regulated apoptosis. Biochim. Biophys. Acta 2013, 1831, 1542–1554. [Google Scholar] [CrossRef]
- Pollard, H.; Charriaut-Marlangue, C.; Cantagrel, S.; Represa, A.; Robain, O.; Moreau, J.; Ben-Ari, Y. Kainate-induced apoptotic cell death in hippocampal neurons. Neuroscience 1994, 63, 7–18. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Liu, N.; Huang, S.S.; Lin, M.M.; Qin, S.; Wu, J.C.; Liang, Z.Q.; Qin, Z.H.; Wang, Y. NADPH protects against kainic acid-induced excitotoxicity via autophagy-lysosome pathway in rat striatum and primary cortical neurons. Toxicology 2020, 435, 152408. [Google Scholar] [CrossRef] [PubMed]
- Gaidin, S.G.; Turovskaya, M.V.; Gavrish, M.S.; Babaev, A.A.; Mal’tseva, V.N.; Blinova, E.V.; Turovsky, E.A. The selective BDNF overexpression in neurons protects neuroglial networks against OGD and glutamate-induced excitotoxicity. Int. J. Neurosci. 2019, 26, 363–383. [Google Scholar] [CrossRef]
- Domin, H.; Zięba, B.; Gołembiowska, K.; Kowalska, M.; Dziubina, A.; Śmiałowska, M. Neuroprotective potential of mGluR5 antagonist MTEP: Effects on kainate-induced excitotoxicity in the rat hippocampus. Pharmacol Rep. 2010, 62, 1051–1061. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsten, T.; Ross, A.J.; King, A.; Zong, W.X.; Rathmell, J.C.; Shiels, H.A.; Ulrich, E.; Waymire, K.G.; Mahar, P.; Frauwirth, K.; et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000, 6, 1389–1399. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, N.J.; McGown, A.T.; Nduka, J.; Hadfield, J.A.; Pritchard, R.G. Cytotoxic Michael-type amine adducts of alpha-methylene lactones alantolactone and isoalantolactone. Bioorg. Med. Chem. Lett. 2001, 11, 429–431. [Google Scholar] [CrossRef]
- Woods, J.R.; Mo, H.; Bieberich, A.A.; Alavanja, T.; Colby, D.A. Fluorinated amino-derivatives of the sesquiterpene lactone, parthenolide, as (19)f NMR probes in deuterium-free environments. J. Med. Chem. 2001, 54, 7934–7941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasim, S.; Crooks, P.A. Antileukemic activity of aminoparthenolide analogs. Bioorg. Med. Chem. Lett. 2008, 18, 3870–3873. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, P.; Chiappini, I.; Fardella, G.; Menghini, A. A New eudesmane acid from Dittrichia (Inula) viscosa. Planta Med. 1985, 51, 471. [Google Scholar] [CrossRef]
- Zeggwagh, N.A.; Ouahidi, M.L.; Lemhadri, A.; Eddouks, M. Study of hypoglycaemic and hypolipidemic effects of Inula viscosa L. aqueous extract in normal and diabetic rats. J. Ethnopharmacol. 2006, 108, 223–227. [Google Scholar] [CrossRef]
- Willuhn, G.; Skibinski, A.; Schmidt, T.J. Structure revision of xanthalongin and further sesquiterpene lactones from flowers of Arnica longifolia. Planta Med. 1998, 64, 635–639. [Google Scholar] [CrossRef]
- Cohen, Y.; Wang, W.; Ben-Daniel, B.H.; Ben-Daniel, Y. Extracts of inula viscosa control downy mildew of grapes caused by plasmopara viticola. Phytopathology 2006, 96, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Fontana, G.; La Rocca, S.; Passannanti, S.; Paternostro, M.P. Sesquiterpene compounds from Inula viscosa. Nat. Prod. Res. 2007, 21, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Rozenblat, S.; Grossman, S.; Bergman, M.; Gottlieb, H.; Cohen, Y.; Dovrat, S. Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem. Pharmacol. 2008, 75, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Abrham, G.; Dovrat, S.; Bessler, H.; Grossman, S.; Nir, U.; Bergman, M. Inhibition of Inflammatory Cytokine Secretion by Plant-Derived Compounds Inuviscolide and Tomentosin: The Role of NFκB and STAT1. Open Pharmacol. J. 2010, 4, 36–44. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Xanthane sesquiterpenoids: Structure, synthesis and biological activity. Nat. Prod. Rep. 2011, 28, 824–842. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Kim, S.G.; Kim, M.J.; Lee, J.; Choi, B.K.; Jin, M.H.; Lee, E. Suppressive effect of tomentosin on the production of inflammatory mediators in RAW264.7 cells. Biol. Pharm. Bull. 2014, 37, 1177–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaki, M.; Oukhrib, A.; Hiebel, M.A.; Berteina-Raboin, S.; Akssira, M. Michael-Type amine adducts of α-Methylene-γ-Lactones tomentosin. Mor. J. Chem. 2019, 7, 580–586. [Google Scholar] [CrossRef]
- Zaki, M.; Oukhrib, A.; El Hakmaoui, A.; Hiebel, M.A.; Berteina-Raboin, S.; Akssira, M. Synthesis of novel 1,2,3-triazole-substituted tomentosins. Z. Nat. B 2019, 74, 273–281. [Google Scholar] [CrossRef]
- Popik, P.; Layer, R.T.; Fossom, L.H.; Benveniste, M.; Geter-Douglass, B.; Witkin, J.M.; Skolnick, P. NMDA antagonist properties of the putative anti addictive drug, ibogaine. J. Pharmacol. Exp. Ther. 1995, 275, 753–760. [Google Scholar]
- Zhang, J.M.; Hu, G.Y. Huperzine A, a nootropic alkaloid, inhibits N-methyl-D-aspartate-induced current in rat dissociated hippocampal neurons. Neuroscience 2001, 105, 663–669. [Google Scholar] [CrossRef]
- Yang, L.; Xie, J.; Almoallim, H.S.; Alharbi, S.A.; Chen, Y. Tomentosin inhibits cell proliferation and induces apoptosis in MOLT-4 leukemia cancer cells through the inhibition of mTOR/PI3K/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22719. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef]
- Klein, J.A.; Ackerman, S.L. Oxidative stress, cell cycle, and neurodegeneration. J. Clin. Investig. 2003, 111, 785–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armagan, G.; Kanit, L.; Yalcin, A. D-serine induces oxidative stress in rat brain. Drug Chem. Toxicol. 2011, 34, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Armagan, G.; Kanit, L.; Yalcin, A. Effects of non-steroidal anti-inflammatory drugs on D-serine-induced oxidative stress in vitro. Drug Chem. Toxicol. 2012, 35, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Armagan, G.; Turunc, E.; Kanit, L.; Yalcin, A. Neuroprotection by mefenamic acid against D-serine: Involvement of oxidative stress, inflammation and apoptosis. Free. Radic. Res. 2012, 46, 726–739. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [Green Version]
- Armagan, G.; Keser, A.; Atalayın, Ç.; Dağcı, T. Tideglusib protects neural stem cells against NMDA receptor overactivation. Pharmacol. Rep. 2015, 67, 823–831. [Google Scholar] [CrossRef]
- Merghoub, N.; El Btaouri, H.; Benbacer, L.; Gmouh, S.; Trentesaux, C.; Brassart, B.; Attaleb, M.; Madoulet, C.; Wenner, T.; Amzazi, S.; et al. Tomentosin induces telomere shortening and caspase-dependent apoptosis in cervical cancer cells. J. Cell Biochem. 2016, 118, 1689–1698. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, H.; Dong, X.; Yang, Z.; Chang, W. Tomentosin induces apoptotic pathway by blocking inflammatory mediators via modulation of cell proteins in AGS gastric cancer cell line. J. Biochem. Mol. Toxicol. 2020, 34, e22501. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, J.; Nam, M.J.; Choi, Y.S.; Park, S. Tomentosin Displays Anti-Carcinogenic Effect in Human Osteosarcoma MG-63 Cells via the Induction of Intracellular Reactive Oxygen Species. Int. J. Mol. Sci. 2019, 20, 1508. [Google Scholar] [CrossRef] [Green Version]


| % Cell Viability | ||||
|---|---|---|---|---|
| Compound | 10 µM | 1 µM | 0.1 µM | 0.01 µM |
| 2a | 57.95 ± 0.96 | 66.74 ± 2.06 | 71.78 ± 8.02 | 59.14 ± 4.78 |
| 2b | 70.58 ± 5.96 | 77.37 ± 9.29 # | 72.45 ± 7.83 | 67.16 ± 6.15 |
| 2c | 68.95 ± 3.17 | 70.42 ± 3.57 | 82.89 ± 12.01 # | 65.49 ± 1.63 |
| 2d | 72.11 ± 4.76 | 69.74 ± 4.46 | 105.8 ± 4.70 # | 101.15 ± 8.55 # |
| 2e | 67.32 ± 1.51 | 80.74 ± 4.07 # | 63.78 ± 2.35 | 67.16 ± 6.81 |
| 2f | 70.00 ± 2.63 | 63.74 ± 3.23 | 71.45 ± 3.25 | 64.42 ± 2.11 |
| 2g | 75.74 ± 10.84 | 83.63 ± 12.47 # | 70.62 ± 3.23 | 64.56 ± 4.23 |
| 2h | 61.32 ± 1.27 | 66.11 ± 4.36 | 71.51 ± 3.57 | 73.59 ± 9.11 |
| 2i | 67.21 ± 0.92 | 73.11 ± 4.37 | 70.90 ± 5.56 | 65.16 ± 3.22 |
| 2j | 68.63 ± 2.01 | 74.79 ± 10.73 | 74.69 ± 5.44 | 59.47 ± 4.23 |
| 2k | 71.21 ± 4.45 | 67.42 ± 6.72 | 67.37 ± 7.03 | 62.75 ± 1.94 |
| 2l | 64.21 ± 2.94 | 64.63 ± 2.69 | 63.17 ± 3.66 | 63.22 ± 3.28 |
| 2m | 69.37 ± 2.24 | 64.16 ± 2.39 | 68.18 ± 7.18 | 63.42 ± 6.82 |
| 2 mM NMDA | 52.14 ± 3.36 ** | |||
| % Cell Viability | ||||
|---|---|---|---|---|
| Compound | 10 µM | 1 µM | 0.1 µM | 0.01 µM |
| 5a | 61.11 ± 0.97 | 63.83 ± 0.56 | 79.44 ± 1.51 # | 65.02 ± 3.49 |
| 6a | 58.00 ± 1.35 | 60.20 ± 1.78 | 77.50 ± 3.42 # | 62.42 ± 3.23 |
| 5b | 58.82 ± 1.88 | 59.73 ± 3.16 | 65.56 ± 4.81 | 61.95 ± 2.61 |
| 6b | 58.78 ± 3.49 | 60.11 ± 0.66 | 66.83 ± 2.09 | 62.01 ± 3.12 |
| 5c | 56.40 ± 1.88 | 61.24 ± 4.63 | 65.76 ± 2.45 | 60.68 ± 2.65 |
| 6c | 55.67 ± 1.95 | 57.91 ± 3.56 | 66.23 ± 3.09 | 62.15 ± 0.92 |
| 5d | 57.96 ± 3.45 | 58.52 ± 0.81 | 77.53 ± 2.71 # | 60.34 ± 1.11 |
| 6d | 55.93 ± 1.68 | 56.01 ± 2.91 | 77.90 ± 3.88 # | 60.21 ± 1.93 |
| 5e | 57.14 ± 1.10 | 56.66 ± 2.91 | 63.95 ± 2.25 | 60.81 ± 0.87 |
| 6e | 60.37 ± 0.95 | 59.73 ± 1.17 | 67.37 ± 2.73 | 63.49 ± 1.22 |
| 2 mM NMDA | 52.14 ± 3.36 ** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaki, M.; Loubidi, M.; Bilgiç, T.; Birim, D.; Akssira, M.; Dagcı, T.; Berteina-Raboin, S.; Saso, L.; Khouili, M.; Armagan, G. Design, Synthesis, and Biological Evaluation of Novel Tomentosin Derivatives in NMDA-Induced Excitotoxicity. Pharmaceuticals 2022, 15, 421. https://doi.org/10.3390/ph15040421
Zaki M, Loubidi M, Bilgiç T, Birim D, Akssira M, Dagcı T, Berteina-Raboin S, Saso L, Khouili M, Armagan G. Design, Synthesis, and Biological Evaluation of Novel Tomentosin Derivatives in NMDA-Induced Excitotoxicity. Pharmaceuticals. 2022; 15(4):421. https://doi.org/10.3390/ph15040421
Chicago/Turabian StyleZaki, Mohamed, Mohammed Loubidi, Tuğçe Bilgiç, Derviş Birim, Mohamed Akssira, Taner Dagcı, Sabine Berteina-Raboin, Luciano Saso, Mostafa Khouili, and Güliz Armagan. 2022. "Design, Synthesis, and Biological Evaluation of Novel Tomentosin Derivatives in NMDA-Induced Excitotoxicity" Pharmaceuticals 15, no. 4: 421. https://doi.org/10.3390/ph15040421
APA StyleZaki, M., Loubidi, M., Bilgiç, T., Birim, D., Akssira, M., Dagcı, T., Berteina-Raboin, S., Saso, L., Khouili, M., & Armagan, G. (2022). Design, Synthesis, and Biological Evaluation of Novel Tomentosin Derivatives in NMDA-Induced Excitotoxicity. Pharmaceuticals, 15(4), 421. https://doi.org/10.3390/ph15040421









