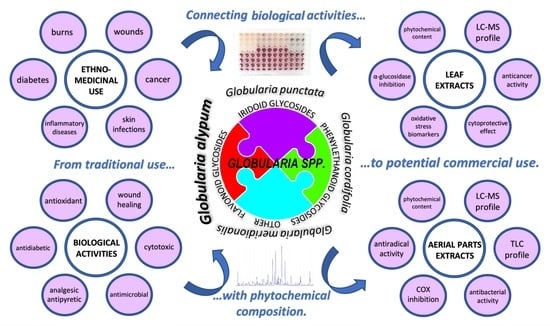
Globularia alypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Content
2.1.1. Phytochemical Content of Leaf Extracts
2.1.2. Phytochemical Content of Aerial Parts Extracts
2.2. LC-MS Profile
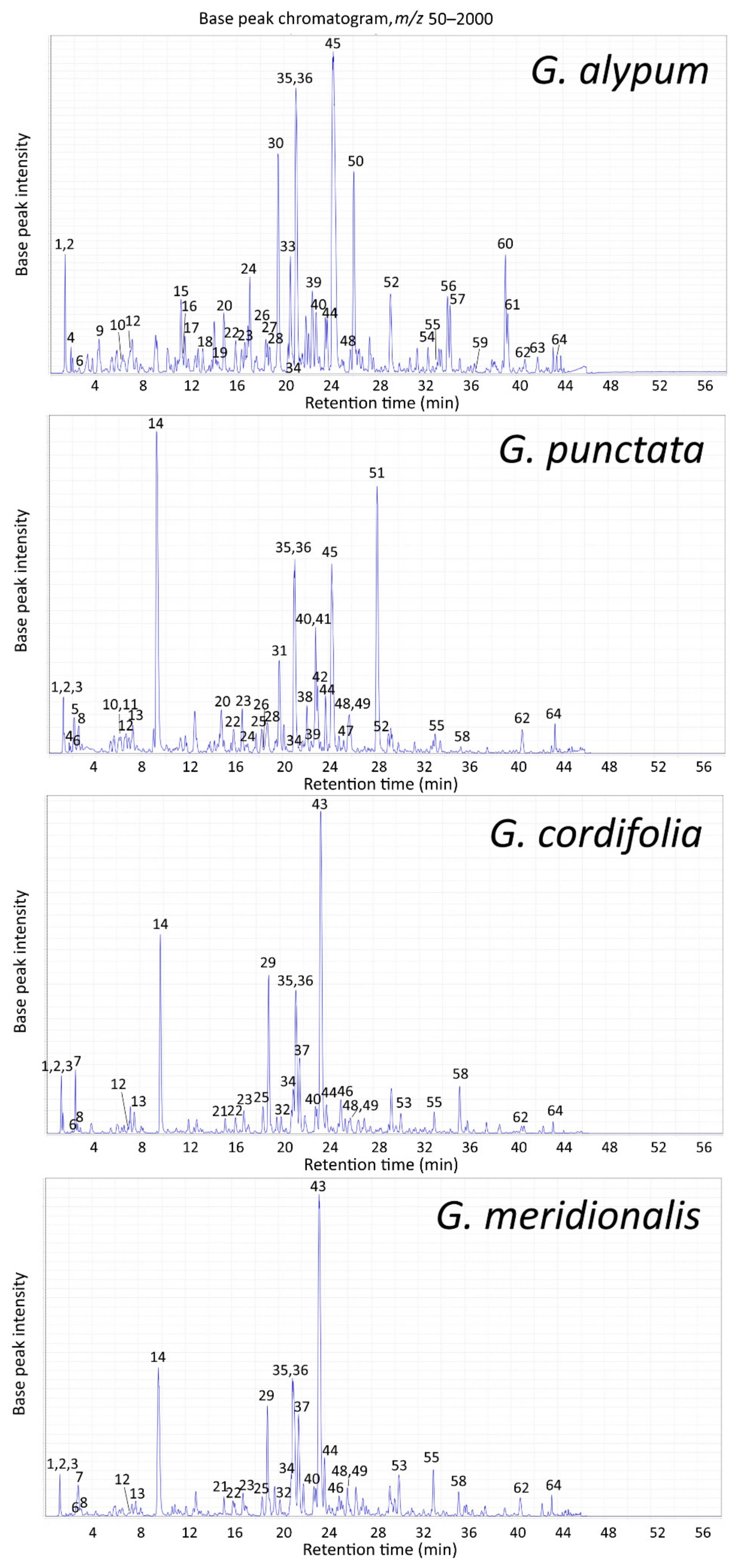
2.2.1. Compound Identification and LC-MS Profile of Leaf Extracts
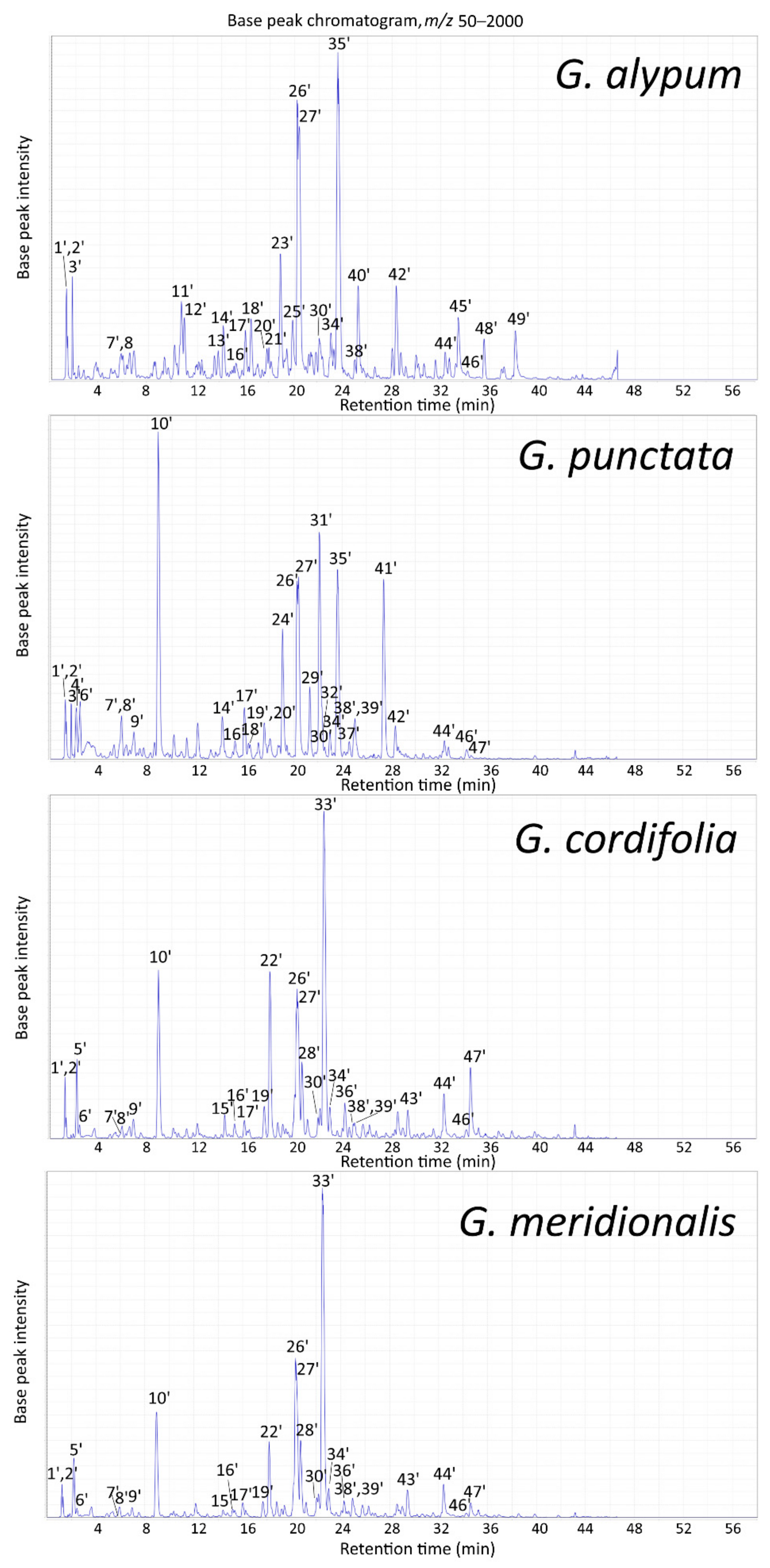
2.2.2. LC-MS Profile of Aerial Parts Extracts
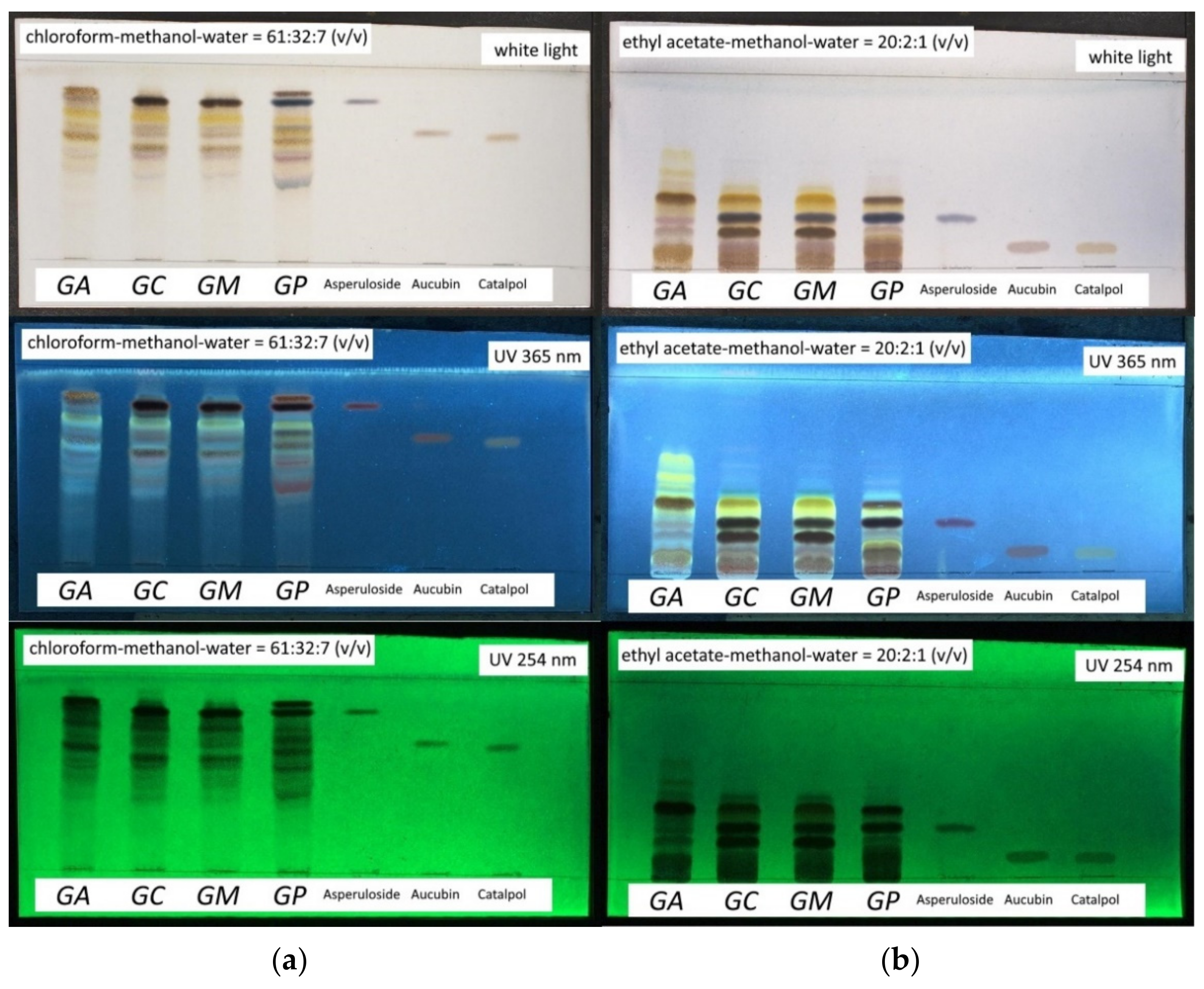
2.2.3. TLC Profile of Aerial Parts Extracts
2.3. Antidiabetic and Antioxidant Potential
2.3.1. α-Glucosidase Inhibition
2.3.2. Oxidative Stress Biomarkers
2.3.3. DPPH Radical Scavenging Activity
2.4. Anti-Inflammatory Potential
2.5. Antibacterial Potential
2.6. Anticancer Potential
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Sample Preparation
3.4. Phytochemical Content
3.5. HPLC-PDA-ESI-MSn Analysis
3.6. TLC Analysis
3.7. Assessment of Antidiabetic and Antioxidant Potential
3.7.1. α-Glucosidase Activity
3.7.2. Cell Culture and Treatment
3.7.3. Oxidative Stress Biomarkers and Cell Viability Assessment
3.7.4. DPPH Radical Scavenging Activity Assay
3.7.5. TLC Bioautography Assay
3.8. Assessment of Anti-Inflammatory Potential
3.8.1. PGE2 Assay
3.8.2. TMPD Assay
3.9. Assessment of Antibacterial Potential
3.9.1. Bacterial Strains and Inoculum and Sample Preparation
3.9.2. Well Diffusion Method
3.9.3. Serial Broth Microdilution Method with Agar Sub-Cultivation
3.10. Assessment of Anticancer Potential
3.10.1. Cell Culture and Treatment
3.10.2. Cell Viability Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2021.
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.E.B.F.; Moreira, N.C.S.; Sakamoto-Hojo, E.T. Mechanisms underlying the pathophysiology of type 2 diabetes: From risk factors to oxidative stress, metabolic dysfunction, and hyperglycemia. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 874–875, 503437. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Neves, J.M.; Duarte, B.; Pinto, M.; Formiga, A.; Neves, J. Diabetic foot infection: Causative pathogens and empiric antibiotherapy considerations—The experience of a tertiary center. Int. J. Low. Extrem. Wounds 2019, 18, 122–128. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Ansarry, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; Builders, P., Ed.; IntechOpen: London, UK, 2018; pp. 11–30. [Google Scholar] [CrossRef] [Green Version]
- Asraoui, F.; Kounnoun, A.; El Cadi, H.; Cacciola, F.; El Majdoub, Y.O.; Alibrando, F.; Mandolfino, F.; Dugo, P.; Mondello, L.; Louajri, A. Phytochemical investigation and antioxidant activity of Globularia alypum L. Molecules 2021, 26, 759. [Google Scholar] [CrossRef]
- Bahlil, Y.; Krouf, D.; Mellouk, Z.; Taleb-Dida, N.; Guenzet, A. Favorable effects of Globularia alypum on cardiometabolic markers in high fructose-fed rats. Nutr. Food Sci. 2020, 51, 605–620. [Google Scholar] [CrossRef]
- Mohamed, T.; Souiy, Z.; Achour, L.; Hamden, K. Anti-obesity, anti-hyperglycaemic, anti-antipyretic and analgesic activities of Globularia alypum extracts. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]
- Ghlissi, Z.; Kallel, R.; Sila, A.; Harrabi, B.; Atheymen, R.; Zeghal, K.; Bougatef, A.; Sahnoun, Z. Globularia alypum methanolic extract improves burn wound healing process and inflammation in rats and possesses antibacterial and antioxidant activities. Biomed. Pharmacother. 2016, 84, 1488–1495. [Google Scholar] [CrossRef]
- Katiri, A.; Barkaoui, M.; Msanda, F.; Boubaker, H. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in the Tizi n’ Test Region (Taroudant Province, Morocco). J. Pharmacogn. Nat. Prod. 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Telli, A.; Esnault, M.-A.; Khelil, A.O.E.H. An ethnopharmacological survey of plants used in traditional diabetes treatment in south-eastern Algeria (Ouargla province). J. Arid. Environ. 2016, 127, 82–92. [Google Scholar] [CrossRef]
- El-Ghazouani, F.; El-Ouahmani, N.; Teixidor-Toneu, I.; Yacoubi, B.; Zekhnini, A. A survey of medicinal plants used in traditional medicine by women and herbalists from the city of Agadir, southwest of Morocco. Eur. J. Integr. Med. 2021, 42, 101284. [Google Scholar] [CrossRef]
- El-Mokasabi, F.M.; Al-Sanousi, M.F.; El-Mabrouk, R.M. Taxonomy and ethnobotany of medicinal plants in Eastern Region of Libya. J. Environ. Sci. Toxicol. Food Technol. 2018, 12, 14–23. [Google Scholar] [CrossRef]
- Abouri, M.; El Mousadik, A.; Msanda, F.; Boubaker, H.; Saadi, B.; Cherifi, K. An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int. J. Med. Plants 2012, 1, 99–123. [Google Scholar]
- Afifi-Yazar, F.U.; Kasabri, V.; Abu-Dahab, R. Medicinal plants from Jordan in the treatment of cancer: Traditional uses vs. in vitro and in vivo evaluations—Part 1. Planta Med. 2011, 77, 1203–1209. [Google Scholar] [CrossRef] [Green Version]
- Leporatti, M.L.; Ghedira, K. Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobiol. Ethnomed. 2009, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Helmstädter, A. Ethnopharmacology in the work of Melville William Hilton-Simpson (1881–1938)—Historical analysis and current research opportunities. Pharmazie 2016, 71, 352–360. [Google Scholar] [CrossRef]
- Irki, S.; Mahmoudi, Y.; Hamidi, N. Histological study and cytotoxic effect of Globularia alypum leaves. Algerian J. Nat. Prod. 2019, 7, 714–719. [Google Scholar] [CrossRef]
- Friščić, M.; Bucar, F.; Hazler Pilepić, K. LC-PDA-ESI-MSn analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spectrom. 2016, 51, 1211–1236. [Google Scholar] [CrossRef]
- Friščić, M.; Maslo, S.; Garić, R.; Maleš, Ž.; Hazler Pilepić, K. Comparative analysis of specialized metabolites and antioxidant capacity in vitro of different natural populations of Globularia spp. Acta Bot. Croat. 2018, 77, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sertić, M.; Crkvenčić, M.; Mornar, A.; Hazler Pilepić, K.; Nigović, B.; Maleš, Ž. Analysis of aucubin and catalpol content in different plant parts of four Globularia species. J. Appl. Bot. Food Qual. 2015, 88, 209–214. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [Green Version]
- Sipahi, H.; Becker, K.; Gostner, J.M.; Charehsaz, M.; Kirmizibekmez, H.; Schennach, H.; Aydin, A.; Fuchs, D. Effects of globularifolin on cell survival, nuclear factor-κB activity, neopterin production, tryptophan breakdown and free radicals in vitro. Fitoterapia 2014, 92, 85–92. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, X.; Ran, Q.; Yang, K.; Wen, Y.; Li, H.; Wang, F. Globularifolin exerts anticancer effects on glioma U87 cells through inhibition of Akt/mTOR and MEK/ERK signaling pathways in vitro and inhibits tumor growth in vivo. Biochimie 2017, 142, 144–151. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Liu, M.; Wang, X.; Zhang, L.; Gong, C. Globularifolin inhibits CAMA-1 human breast cancer cell line via cell cycle arrest, apoptosis and inhibition of PI3K/AKT signalling pathway. Trop. J. Pharm. Res. 2018, 17, 1711–1716. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, M.R.; Conforti, F.; Statti, G.; Pirisi, F.M.; Menichini, F. Antioxidant and anti- cholinesterase activity of Globularia meridionalis extracts and isolated constituents. Nat. Prod. Commun. 2012, 7, 1015–1020. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pérez, C.; Zengin, G.; Segura-Carretero, A.; Lobine, D.; Mahomoodally, M.F. Chemical fingerprint and bioactivity evaluation of Globularia orientalis L. and Globularia trichosantha Fisch. & C. A. Mey. using non-targeted HPLC-ESI-QTOF-MS approach. Phytochem. Anal. 2018, 30, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Hazler Pilepić, K.; Friščić, M.; Duran, A.; Maslo, S.; Garić, R.; Čuljak, S.; Šutalo, K. Contribution to Globularia phylogeny based on nuclear ribosomal spacer and two chloroplast DNA regions. Period. Biol. 2016, 118, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Tutin, T.G. Globularia L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 282–283. [Google Scholar]
- Amessis-Ouchemoukh, N.; Abu-Reidah, I.M.; Quirantes-Piné, R.; Rodríguez-Pérez, C.; Madani, K.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Tentative characterisation of iridoids, phenylethanoid glycosides and flavonoid derivatives from Globularia alypum L. (Globulariaceae) leaves by LC-ESI-QTOF-MS. Phytochem. Anal. 2014, 25, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Bouriche, H.; Kada, S.; Senator, A.; Demirtas, I.; Ozen, T.; Toptanci, B.C.; Kizil, G.; Kizil, M. Phenolic content and biomolecule oxidation protective activity of Globularia alypum extracts. Braz. Arch. Biol. Technol. 2017, 60, e17160409. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Kollmann, A.; Khlifi, S.; Ducrot, P.-H. Antioxidative effect of compounds isolated from Globularia alypum L. structure-activity relationship. LWT Food Sci. Technol. 2007, 40, 1246–1252. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Bassarello, C.; Piacente, S.; Çaliş, İ. Phenylethyl glycosides from Globularia alypum growing in Turkey. Helv. Chim. Acta 2008, 91, 1525–1532. [Google Scholar] [CrossRef]
- Feriani, A.; del Mar Contreras, M.; Talhaoui, N.; Gómez-Caravaca, A.M.; Taamalli, A.; Segura-Carretero, A.; Ghazouani, L.; El Feki, A.; Allagui, M.S. Protective effect of Globularia alypum leaves against deltamethrin-induced nephrotoxicity in rats and determination of its bioactive compounds using high-performance liquid chromatography coupled with electrospray ionization tandem quadrupole-time-of-flight mass spectrometry. J. Funct. Foods 2017, 32, 139–148. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Akbay, P.; Sticher, O.; Çaliş, İ. Iridoids from Globularia dumulosa. Z. Naturforsch. C 2003, 58, 181–186. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Çaliş, İ.; Piacente, S.; Pizza, C. Phenolic compounds from Globularia cordifolia. Turk. J. Chem. 2004, 28, 455–460. [Google Scholar]
- Kirmizibekmez, H.; Bassarello, C.; Piacente, S.; Akaydin, G.; Çaliş, İ. Flavonoid, phenylethanoid and iridoid glycosides from Globularia aphyllanthes. Z. Naturforsch. B 2009, 64, 252–256. [Google Scholar] [CrossRef]
- Merghache, S.; Zerriouh, M.; Merghache, D.; Tabti, B.; Djaziri, R.; Ghalem, S. Evaluation of hypoglycaemic and hypolipidemic activities of globularin isolated from Globularia alypum L. in normal and streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Klimek, B. Acylated 6-hydroxyluteolin diglucosides from Globularia elongata. Phytochemistry 1988, 27, 255–258. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Çaliş, İ.; Akbay, P.; Sticher, O. Iridoid and bisiridoid glycosides from Globularia cordifolia. Z. Naturforsch. C 2003, 58, 337–341. [Google Scholar] [CrossRef]
- Ben Hassine, B.; Bui, A.; Mighri, Z. Contribution a l’etude des plantes medicinales Tunisiennes. Identification des acides phenols de Globularia alypum L. par C.C.M. bidimensionnelle et H.P.L.C. J. Soc. Chim. Tunisie 1982, 1, 3–10. [Google Scholar]
- Ben Hassine, B.; Bui, A.M.; Mighri, Z.; Cavé, A. Flavonoïdes et anthocyanes de Globularia alypum L. Plantes Méd. Phytothér. 1982, 16, 197–205. [Google Scholar]
- Liu, S.-K.; Hao, H.; Bian, Y.; Ge, Y.-X.; Lu, S.; Xie, H.-X.; Wang, K.-M.; Tao, H.; Yuan, C.; Zhang, J.; et al. Discovery of new α-glucosidase inhibitors: Structure-based virtual screening and biological evaluation. Front. Chem. 2021, 9, 639279. [Google Scholar] [CrossRef]
- Ouffai, K.; Azzi, R.; Abbou, F.; Mahdi, S.; El Haci, I.A.; Belyagoubi-Benhammou, N.; Bekkara, F.A.; Lahfa, F.B. Phenolics compounds, evaluation of Alpha-amylase, alpha-glucosidase inhibitory capacity and antioxidant effect from Globularia alypum L. Vegetos 2021, 34, 477–484. [Google Scholar] [CrossRef]
- Hajji, N.; Jabri, M.-A.; Tounsi, H.; Wanes, D.; Ben El Hadj Ali, I.; Boulila, A.; Marzouki, L.; Sebai, H. Phytochemical analysis by HPLC-PDA/ESI-MS of Globularia alypum aqueous extract and mechanism of its protective effect on experimental colitis induced by acetic acid in rat. J. Funct. Foods 2018, 47, 220–228. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, H.-J.; Li, P.-F.; Yang, Y.-B.; Wu, L.-H.; Chou, G.-X.; Wang, Z.-T. Diterpenoids and phenylethanoid glycosides from the roots of Clerodendrum bungei and their inhibitory effects against angiotensin converting enzyme and α-glucosidase. Phytochemistry 2014, 103, 196–202. [Google Scholar] [CrossRef]
- Hadrich, F.; Bouallagui, Z.; Junkyu, H.; Isoda, H.; Sayadi, S. The α-glucosidase and α-amylase enzyme inhibitory of hydroxytyrosol and oleuropein. J. Oleo Sci. 2015, 64, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inibitory activity on intestinal α-glucosidase. J. Enzyme Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef]
- Kawabata, J.; Mizuhata, K.; Sato, E.; Nishioka, T.; Aoyama, Y.; Kasai, T. 6-Hydroxyflavonoids as α-glucosidase inhibitors from marjoram (Origanum majorana) leaves. Biosci. Biotechnol. Biochem. 2003, 67, 445–447. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem. Toxicol. 2014, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of diabetes-induced liver damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zennaki, S.; Krouf, D.; Taleb-Senouci, D.; Bouchenak, M. Globularia alypum L. lyophilized methanolic extract decreases hyperglycemia and improves antioxidant status in various tissues of streptozotocin-induced diabetic rats. J. Compl. Integr. Med. 2009, 6, 34. [Google Scholar] [CrossRef]
- Khlifi, D.; Hamdi, M.; El Hayouni, A.; Cazaux, S.; Souchard, J.P.; Couderc, F.; Bouajila, J. Global chemical composition and antioxidant and anti-tuberculosis activities of various extracts of Globularia alypum L. (Globulariaceae) leaves. Molecules 2011, 16, 10592–10603. [Google Scholar] [CrossRef] [Green Version]
- Taleb-Dida, N.; Krouf, D.; Bouchenak, M. Globularia alypum aqueous extract decreases hypertriglyceridemia and ameliorates oxidative status of the muscle, kidney, and heart in rats fed a high-fructose diet. Nutr. Res. 2011, 31, 488–495. [Google Scholar] [CrossRef]
- Djellouli, F.; Kaddour, A.; Krouf, D. The antioxidant and anti-inflammatory effect of Globularia alypum aqueous extract in hypercholesterolemic rats. South Asian J. Exp. Biol. 2021, 11, 492–502. [Google Scholar] [CrossRef]
- Fournial, A.; Grizaud, C.-M.; Mondon, P.; Le Moigne, C. Extract of Plant Origin of Globularia and Method for Obtaining Said Extract by In Vitro Plant Culture. US Patent 10,758,473 B2, 1 September 2020. [Google Scholar]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed tannins as antioxidants in ruminants—Effectiveness and action mechanisms to improve animal antioxidant status and oxidative stability of products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef]
- Mahbob, E.N.M.; Ahmad, R.; Ahmad, S. Nitric oxide (NO) radical inhibitory of Hedyotis philippinensis and its marker compound, asperuloside. Mal. J. Fund. Appl. Sci. 2014, 10, 7–11. [Google Scholar] [CrossRef]
- Hajji, N.; Wannes, D.; Jabri, M.-A.; Rtibi, K.; Tounsi, H.; Abdellaoui, A.; Sebai, H. Purgative/laxative actions of Globularia alypum aqueous extract on gastrointestinal-physiological function and against loperamide-induced constipation coupled to oxidative stress and inflammation in rats. Neurogastroenterol. Motil. 2020, 32, e13858. [Google Scholar] [CrossRef] [Green Version]
- Çaliş, İ.; Kirmizibekmez, H.; Rüegger, H.; Sticher, O. Phenylethanoid glycosides from Globularia trichosantha. J. Nat. Prod. 1999, 62, 1165–1168. [Google Scholar] [CrossRef]
- Çaliş, İ.; Kirmizibekmez, H.; Taşdemir, D.; Sticher, O.; Ireland, C.M. Sugar esters from Globularia orientalis. Z. Naturforsch. C 2002, 57, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P.-F.; Yu, C.-C.; Chu, P.-M.; Hsieh, P.-L. Verbascoside protects gingival cells against high glucose-induced oxidative stress via PKC/HMGB1/RAGE/NFκB pathway. Antioxidants 2021, 10, 1445. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Khlifi, S.; Kollmann, A.; Kerhoas, L.; El Abbouyi, A.; Ducrot, P.H. Iridoid glucosides from the aerial parts of Globularia alypum L. (Globulariaceae). Chem. Pharm. Bull. 2006, 54, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Taghzouti, O.K.; Balouiri, M.; Ouedrhiri, W.; Ech chahad, A.; Romane, A. In vitro evaluation of the antioxidant and antimicrobial effects of Globularia alypum L. extracts. J. Mater. Environ. Sci. 2016, 7, 1988–1995. [Google Scholar]
- Mansour, R.B.; Wasli, H.; Serairi-Beji, R.; Bourgou, S.; Dakhlaoui, S.; Selmi, S.; Khamessi, S.; Hammami, M.; Ksouri, R.; Megdiche-Ksouri, W. In vivo gastroprotective effect and biological potentialities of six Tunisian medicinal plants using multivariate data treatment. Plant Biosyst. 2020, 1–12. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jo, Y.H.; Kim, S.B.; Turk, A.; Oh, K.-E.; Hwang, B.Y.; Lee, K.Y.; Lee, M.K. Identification of antioxidant constituents of the aerial part of Plantago asiatica using LC–MS/MS coupled DPPH assay. Phytochem. Lett. 2018, 26, 20–24. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Burgos, C.; Muñoz-Mingarro, D.; Navarro, I.; Martín-Cordero, C.; Acero, N. Neuroprotective potential of verbascoside isolated from Acanthus mollis L. leaves through its enzymatic inhibition and free radical scavenging ability. Antioxidants 2020, 9, 1207. [Google Scholar] [CrossRef]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 inhibitors in cancer: A review of oncology and medicinal chemistry literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Li, P.; Ding, X.; Sui, H.C.; Rao, S.; Hsu, C.-H.; Leung, W.-P.; Cheng, G.-J.; Wang, P.; Zhu, B.T. Mechanism for the reactivation of the peroxidase activity of human cyclooxygenases: Investigation using phenol as a reducing cosubstrate. Sci. Rep. 2020, 10, 15187. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant capacity and phenolic contents of some Mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind. Crop. Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Sahpaz, S.; Garbacki, N.; Tits, M.; Bailleul, F. Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J. Ethnopharmacol. 2002, 79, 389–392. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ali Reza, A.S.M.; Abdullah-Al-Mamun, M.; Kabir, M.S.H.; Nasrin, M.S.; Akhter, S.; Arman, M.S.I.; Rahman, M.A. Antinociceptive and anxiolytic and sedative effects of methanol extract of Anisomeles indica: An experimental assessment in mice and computer aided models. Front. Pharmacol. 2018, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, Q.; Shan, Z.; Zhao, Y.; Li, M.; Wang, B.; Zheng, X.; Feng, W. The protective effect and mechanism of catalpol on high glucose-induced podocyte injury. BMC Complement. Alter. Med. 2019, 19, 244. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Yap, K.H.; Rao, V.; Choudhury, H. Multiple biological effects of an iridoid glucoside, catalpol, and its underlying molecular mechanisms. Biomolecules 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Lu, X.; Wei, T.; Dong, Y.; Cai, Z.; Tang, L.; Liu, M. Asperuloside and asperulosidic acid exert an anti-inflammatory effect via suppression of the NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages. Int. J. Mol. Sci. 2018, 19, 2027. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, X.; Li, X.; Yang, J.; Liu, W.; Yang, L.; Liu, B. Study on the protective effect and mechanism of Liriodendrin on radiation enteritis in mice. J. Radiat. Res. 2022, 128, 1–8. [Google Scholar] [CrossRef]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside protects pancreatic β-cells against ER-stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef]
- Çaliş, İ.; Kirmizibekmez, H.; Sticher, O. Iridoid glycosides from Globularia trichosantha. J. Nat. Prod. 2001, 64, 60–64. [Google Scholar] [CrossRef]
- Kadioğlu, S.; Kadioğlu, B.; Karagöz Sezel, K. Ethnobotanical properties of natural plants in Kop Mountain Pass (Bayburt/ Turkey). BioDiCon 2021, 14, 264–276. [Google Scholar] [CrossRef]
- Pessoa e Costa, T.; Duarte, B.; João, A.L.; Coelho, M.; Formiga, A.; Pinto, M.; Neves, J. Multidrug-resistant bacteria in diabetic foot infections: Experience from a portuguese tertiary centre. Int. Wound J. 2020, 17, 1835–1839. [Google Scholar] [CrossRef]
- Macdonald, K.E.; Boeckh, S.; Stacey, H.J.; Jones, J.D. The microbiology of diabetic foot infections: A meta-analysis. BMC Infect. Dis. 2021, 21, 770. [Google Scholar] [CrossRef] [PubMed]
- Shanab, S.; Doro, B.; Auzi, A. Phytochemical screening and antibacterial activity of Libyan Globularia alypum. Khalij-Libya J. Dent. Med. Res. 2021, 5, 11–22. [Google Scholar] [CrossRef]
- Boussoualim, N.; Trabsa, H.; Krache, I.; Arrar, L.; Baghiani, A. Anti-bacterial and β-lactamase inhibitory effects of Anchusa azurea and Globularia alypum extracts. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 742–749. [Google Scholar]
- Bouabdelli, F.; Djelloul, A.; Kaid-Omar, Z.; Semmoud, A.; Addou, A. Antimicrobial activity of 22 plants used in urolithiasis medicine in Western Algeria. Asian Pac. J. Trop. Dis. 2012, 2, 530–535. [Google Scholar] [CrossRef]
- Kraza, L.; Mourad, S.M.; Halis, Y. In vitro investigation of the antioxidant and antimicrobial effects of hydro-alcoholic and aqueous extracts of Globularia alypum L. ASN 2020, 7, 46–58. [Google Scholar] [CrossRef]
- Bogdadi, H.A.A.; Kokoska, L.; Havlik, J.; Kloucek, P.; Rada, V.; Vorisek, K. In vitro antimicrobial activity of some Libyan medicinal plant extracts. Pharm. Biol. 2007, 45, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Volk, R.-B. A newly developed assay for the quantitative determination of antimicrobial (anticyanobacterial) activity of both hydrophilic and lipophilic test compounds without any restriction. Microbiol. Res. 2008, 163, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Akroum, S.; Bendjeddou, D.; Satta, D.; Lalaoui, K. Antibacterial activity and acute toxicity effect of flavonoids extracted from Mentha longifolia. Am.-Eur. J. Sci. Res. 2009, 4, 93–96. [Google Scholar]
- Wang, M.; Firman, J.; Liu, L.S.; Yam, K. A review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Nazemiyeh, H.; Rahman, M.M.; Gibbons, S.; Nahar, L.; Delazar, A.; Ghahramani, M.-A.; Talebpour, A.-H.; Sarker, S.D. Assessment of the antibacterial activity of phenylethanoid glycosides from Phlomis lanceolata against multiple-drug-resistant strains of Staphylococcus aureus. J. Nat. Med. 2008, 62, 91–95. [Google Scholar] [CrossRef]
- Shikanga, E.A.; Combrinck, S.; Regnier, T. South African Lippia herbal infusions: Total phenolic content, antioxidant and antibacterial activities. S. Afr. J. Bot. 2010, 76, 567–571. [Google Scholar] [CrossRef] [Green Version]
- Radev, R. Pharmacological effects of phenylethanoid glycosides. J. Clin. Med. 2010, 3, 20–23. [Google Scholar]
- Dafni, U.; Tsourti, Z.; Alatsathianos, I. Breast cancer statistics in the European Union: Incidence and survival across European countries. Breast Care 2019, 14, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [Green Version]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Sandhu, N.; Knox, S.J. A review of newly diagnosed glioblastoma. Front. Oncol. 2021, 10, 574012. [Google Scholar] [CrossRef]
- Yang, B.; Wang, N.; Wang, S.; Li, X.; Zheng, Y.; Li, M.; Song, J.; Zhang, F.; Mei, W.; Lin, Y.; et al. Network-pharmacology-based identification of caveolin-1 as a key target of Oldenlandia diffusa to suppress breast cancer metastasis. Biomed. Pharmacother. 2019, 112, 108607. [Google Scholar] [CrossRef]
- Artanti, N.; Hanafi, M.; Andriyani, R.; Saraswati, V.; Udin, Z.; Lotulung, P.D.; Fujita, K.I.; Usuki, Y. Isolation of an anti-cancer asperuloside from Hedyotis corymbosa L. J. Trop. Life Sci. 2015, 5, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Mahibalan, S.; Rao, P.C.; Khan, R.; Basha, A.; Siddareddy, R.; Masubuti, H.; Fujimoto, Y.; Begum, A.S. Cytotoxic constituents of Oldenlandia umbellata and isolation of a new symmetrical coumarin dimer. Med. Chem. Res. 2016, 25, 466–472. [Google Scholar] [CrossRef]
- Bawadi, H.A.; Bansode, R.R.; Trappey II, A.; Truax, R.E.; Losso, J.N. Inhibition of Caco-2 colon, MCF-7 and Hs578T breast, and DU 145 prostatic cancer cell proliferation by water-soluble black bean condensed tannins. Cancer Lett. 2005, 218, 153–162. [Google Scholar] [CrossRef]
- Şenol, H.; Tulay, P.; Ergören, M.Ç.; Hanoğlu, A.; Çaliş, İ.; Mocan, G. Cytotoxic effects of verbascoside on MCF-7 and MDA-MB-231. Turk. J. Pharm. Sci. 2021, 18, 637–644. [Google Scholar] [CrossRef]
- Khalaf, H.A.A.; Jasim, R.A.; Ibrahim, I.T. Verbascoside—A review of its antitumor activities. Pharmacol. Pharm. 2021, 12, 109–126. [Google Scholar] [CrossRef]
- Ma, Y.C.; Zhang, M.; Xu, W.T.; Feng, S.X.; Lei, M.; Yi, B. Chemical constituents from Callicarpa nudiflora and their cytotoxic activities. China J. Chin. Mater. Med. 2014, 39, 3094–3101. [Google Scholar]
- Mansour, R.B.; Gargouri, B.; Gargouri, B.; Elloumi, N.; Jilani, I.B.H.; Ghrabi-Grammar, Z.; Lassoued, S. Investigation of antioxidant activity of alcoholic extract of Globularia alypum L. J. Med. Plants Res. 2012, 6, 4193–4199. [Google Scholar] [CrossRef]
- Lee, K.-W.; Kim, H.J.; Lee, Y.S.; Park, H.-J.; Choi, J.-W.; Ha, J.; Lee, K.-T. Acteoside inhibits human promyelocytic HL-60 leukemia cell proliferation via inducing cell cycle arrest at G0/G1 phase and differentiation into monocyte. Carcinogenesis 2007, 28, 1928–1936. [Google Scholar] [CrossRef]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assay for differentiation of glutathione S-transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [CrossRef]
- Ellman, G.L. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 1958, 74, 443–450. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095497. [Google Scholar] [CrossRef]
- Yang, U.J.; Park, T.-S.; Shi, S.-M. Protective effect of chlorophyllin and lycopene from water spinach extract on cytotoxicity and oxidative stress induced by heavy metals in human hepatoma cells. J. Toxicol. Environ. Health A 2013, 76, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, Y.-D.; Tang, F.; Sun, J. TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa textilis McClure. Molecules 2012, 17, 12297–12311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiebich, B.L.; Grozdeva, M.; Hess, S.; Hüll, M.; Danesch, U.; Bodensieck, A.; Bauer, R. Petasites hybridus extracts in vitro inhibit COX-2 and PGE2 release by direct interaction with the enzyme and by preventing p42/44 MAP kinase activation in rat primary microglial cells. Planta Med. 2005, 71, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Reiniger, E.A.; Bauer, R. Prostaglandin-H-synthase (PGHS)-1 and -2 microtiter assays for the testing of herbal drugs and in vitro inhibition of PGHS-isoenzyms by polyunsaturated fatty acids from Platycodi radix. Phytomedicine 2006, 13, 164–169. [Google Scholar] [CrossRef]
- Copeland, R.A.; Williams, J.M.; Giannaras, J.; Nurnberg, S.; Covington, M.; Pinto, D.; Pick, S.; Trzaskos, J.M. Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proc. Natl. Acad. Sci. USA 1994, 91, 11202–11206. [Google Scholar] [CrossRef] [Green Version]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2006; pp. 188–191. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved standard-, 9th ed.; CLSI document M07-A9; CLSI: Wayne, NE, USA, 2012. [Google Scholar]
- Lee, D.D.; Lee, E.Y.; Jeong, S.H.; Chang, C.L. Evaluation of a colorimetric broth microdilution method for antimicrobial susceptibility testing using 2,3,5-triphenyltetrazolium chloride. Korean J. Clin. Microbiol. 2007, 10, 49–53. [Google Scholar]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Madunić, J.; Matulić, M.; Friščić, M.; Hazler Pilepić, K. Evaluation of the cytotoxic activity of Hypericum spp. on human glioblastoma A1235 and breast cancer MDA MB-231 cells. J. Environ. Sci. Health Part A 2016, 51, 1157–1163. [Google Scholar] [CrossRef]
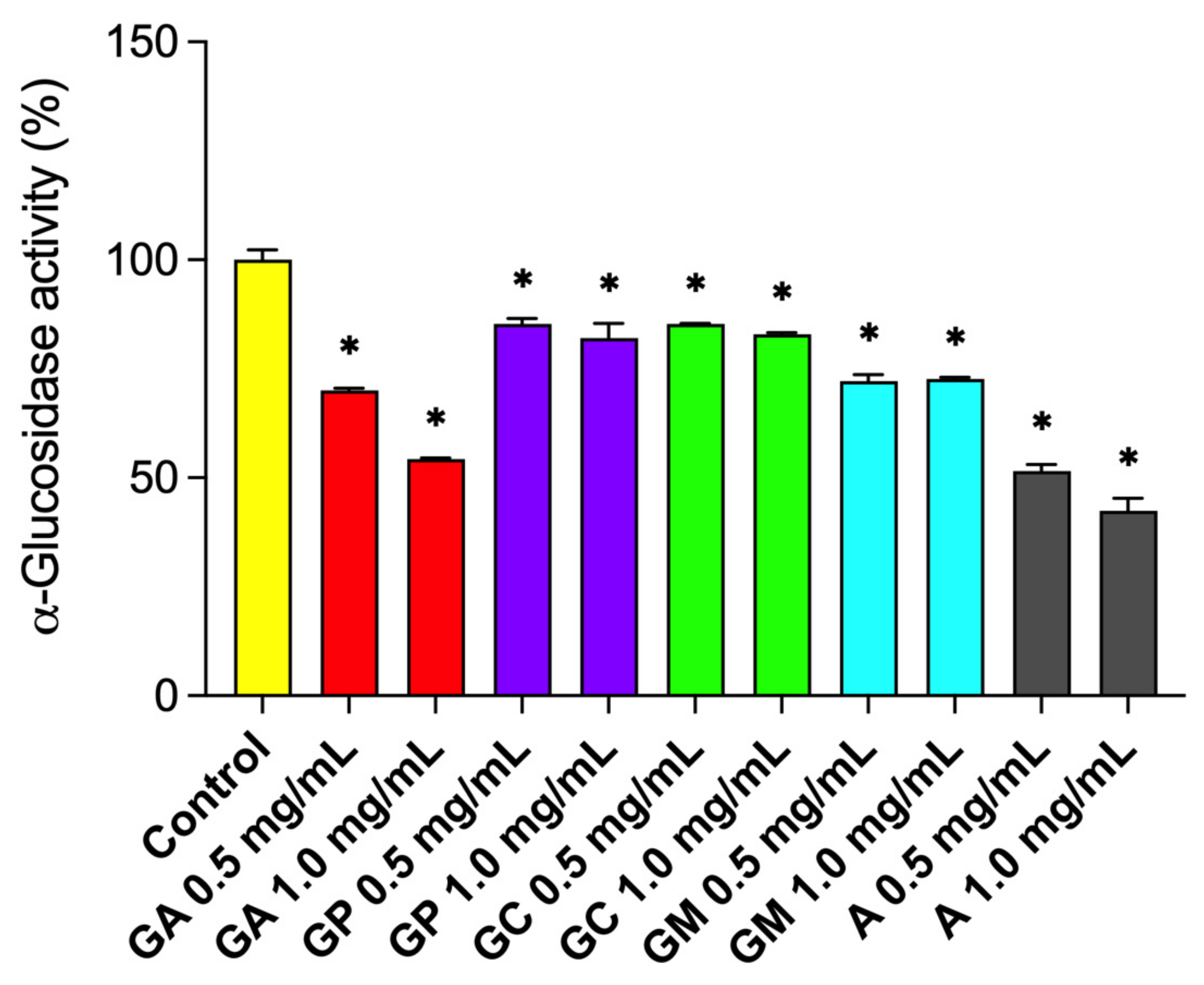

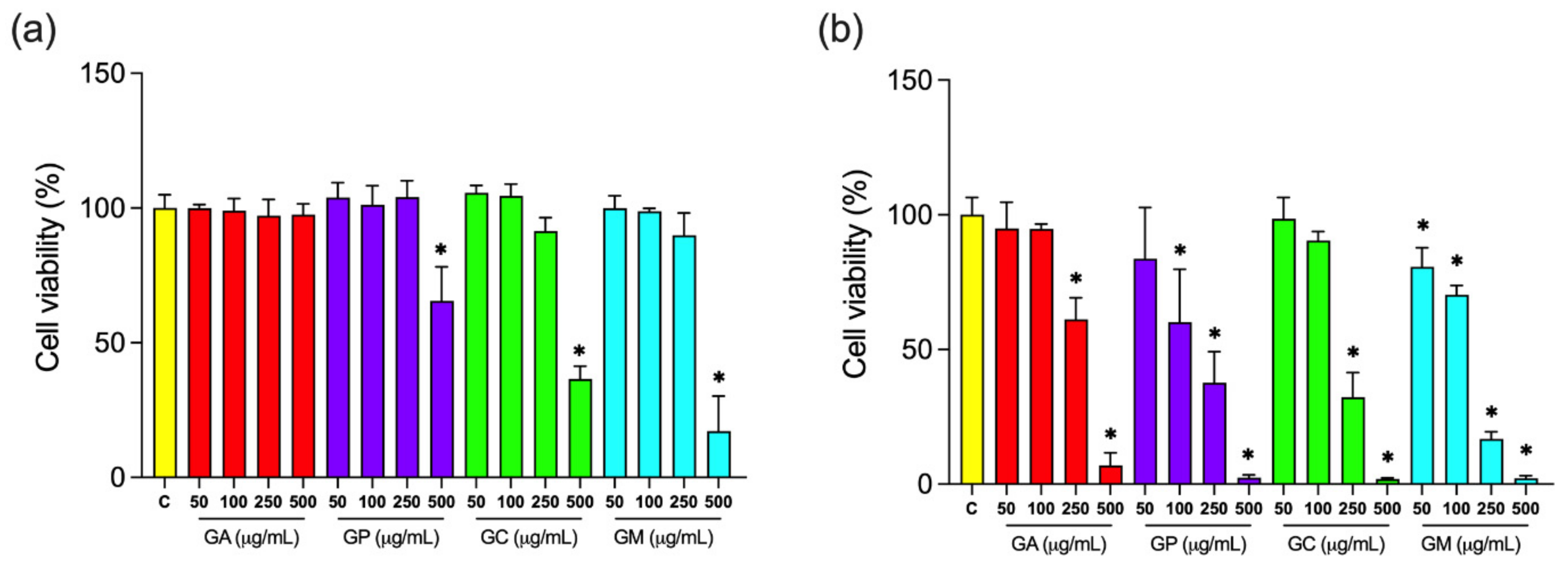
| Constituents * | G. alypum | G. punctata | G. cordifolia | G. meridionalis |
|---|---|---|---|---|
| Antidiabetic Potential | ||||
| Total phenolics a | 130.46 ± 5.99 A | 98.50 ± 1.23 C | 111.35 ± 3.30 B | 123.44 ± 0.77 A |
| Flavonoids b | 30.43 ± 0.29 C | 48.49 ± 2.37 A | 36.54 ± 1.45 B | 39.72 ± 0.60 B |
| Iridoids c | 27.49 ± 3.08 D | 343.33 ± 4.88 A | 311.23 ± 6.20 B | 247.37 ± 2.70 C |
| Condensed tannins d | 3.00 ± 0.06 D | 4.07 ± 0.11 C | 10.02 ± 0.28 A | 6.21 ± 0.11 B |
| Anticancer Potential | ||||
| Total phenolics a | 131.39 ± 2.89 B | 152.69 ± 4.87 A | 157.31 ± 4.29 A | 159.82 ± 2.34 A |
| Flavonoids b | 32.26 ± 1.37 C | 63.03 ± 0.72 A | 42.23 ± 0.67 B | 43.57 ± 0.50 B |
| Iridoids c | 12.07 ± 0.18 D | 440.04 ± 8.73 A | 290.82 ± 7.49 C | 310.25 ± 4.30 B |
| Condensed tannins d | 2.66 ± 0.09 C | 7.71 ± 0.25 B | 8.76 ± 0.08 A | 8.88 ± 0.27 A |
| Constituents * | G. alypum | G. punctata | G. cordifolia | G. meridionalis |
|---|---|---|---|---|
| Total phenolics a | 112.34 ± 2.17 A | 79.92 ± 2.18 C | 95.59 ± 1.62 B | 98.90 ± 3.93 B |
| Flavonoids b | 26.85 ± 0.46 B | 43.25 ± 0.31 A | 26.19 ± 0.40 BC | 25.42 ± 0.24 C |
| Peak | tR (min) | m/z (Major Ion in Full MS Indicated in Bold) | Compound Identification (Compound Class Abbreviation) | GA | GP | GC | GM |
|---|---|---|---|---|---|---|---|
| 1 | 1.2 | 217 [M + 35Cl]−, 219 [M + 37Cl]− | Mannitol (O) a | + | + | + | + |
| 2 | 1.2 | 377 [M + 35Cl]−, 379 [M + 37Cl]− | Sucrose (O) a | + | + | + | + |
| 3 | 1.2 | 191 [M–H]− | Quinic acid (O) a | – | + | + | + |
| 4 | 1.7 | 407 [M–H + HCOOH]− | Catalpol (I) a | + | + | – | – |
| 5 | 2.2 | 389 [M–H]−, 779 [2M–H]− | Scandoside (I) a | – | + | – | – |
| 6 | 2.5 | 391 [M–H + HCOOH]− | Aucubin (I) a | + | + | + | + |
| 7 | 2.5 | 407 [M–H + HCOOH]− | Monomelittoside (5-Hydroxyaucubin) (I) a | – | – | + | + |
| 8 | 2.6 | 371 [M–H]−, 417 [M–H + HCOOH]− | Deacetylasperuloside (I) a | – | + | + | + |
| 9 | 4.1 | 315 [M–H]− | 1′-O-Hydroxytyrosol glucoside (P) b | + * | – | – | – |
| 10 | 6.1 | 341 [M–H]− | Caffeoylglucoside isomer (O) a | + | + | – | – |
| 11 | 6.2 | 373 [M–H]−, 419 [M–H + HCOOH]− | Gardoside (I) a | – | + | – | – |
| 12 | 6.9 | 505 [M–H]− | Hebitol II (6′-O-Caffeoyl-β-d-glucopyranosyl-(1→6)-mannitol) (O) a | + | + | + | + |
| 13 | 7.4 | 431 [M–H]−, 863 [2M–H]− | Asperulosidic acid (I) a | – | + | + | + |
| 14 | 9.4 | 459 [M–H + HCOOH]− | Asperuloside (I) a | – | + | + | + |
| 15 | 11.2 | 519 [M–H]−, 565 [M–H + HCOOH]− | Globularitol (6′-O-Feruloyl-β-d-glucopyranosyl-(1→6)-mannitol) (O) a | + | – | – | – |
| 16 | 11.3 | 523 [M–H]− | Verminoside (6-O-Caffeoylcatalpol) (I) a | + | – | – | – |
| 17 | 11.6 | 433 [M–H + HCOOH]− | Geniposide (I) b | + * | – | – | – |
| 18 | 13.1 | 593 [M–H]− | Vicenin-2 (Apigenin-6,8-di-C-glucoside) (F) a | + | – | – | – |
| 19 | 14.4 | 507 [M–H]–, 553 [M–H + HCOOH]− | Specioside (6-O-(p-Coumaroyl)-catalpol) (I) a | + | – | – | – |
| 20 | 14.8 | 625 [M–H]− | 6-Hydroxyluteolin 7-O-sophoroside (F) a | + | + | – | – |
| 21 | 15.4 | 701 [M–H + HCOOH]− | 6′-O-Benzoyldeacetylasperulosidic acid glucoside (I) b | – | – | + * | + * |
| 22 | 16.1 | 463 [M–H]−, 927 [2M–H]− | 6-Hydroxyluteolin 7-O-glucoside (F) a | + | + | + | + |
| 23 | 16.7 | 415 [M–H]−, 461 [M–H + HCOOH]− | Alpinoside (I) a | + | + | + | + |
| 24 | 17.1 | 555 [M–H + HCOOH]− | Globularinin (I) a | + | + | – | – |
| 25 | 18.6 | 493 [M–H]−, 987 [2M–H]− | 6′-O-Benzoyldeacetylasperulosidic acid (I) b | – | + * | + * | + * |
| 26 | 18.6 | 555 [M–H + HCOOH]− | Globularimin (I) a | + | + | – | – |
| 27 | 18.6 | 787 [M–H + HCOOH]− | Liriodendrin ((+)-Syringaresinol di-O-β-glucopyranoside) (L) a | + | – | – | – |
| 28 | 18.7 | 463 [M–H]− | Isoquercitrin (Quercetin 3-O-glucoside) (F) a | + | + | – | – |
| 29 | 19.1 | 511 [M–H + HCOOH]− | 6′-O-Benzoylmonomelittoside (5-Hydroxydumuloside) (I) a | – | – | + | + |
| 30 | 19.5 | 477 [M–H]− | Calceolarioside A (Desrhamnosyl verbascoside) (P) a | + | – | – | – |
| 31 | 19.8 | 787 [M–H]−, 1575 [2M–H]− | 6-Hydroxyluteolin 7-O-(6′′′-O-caffeoyl)-sophoroside (F) a | – | + | – | – |
| 32 | 20.2 | 495 [M–H + HCOOH]− | 6-O-Benzoylaucubin (I) a | – | – | + | + |
| 33 | 20.6 | 477 [M–H]− | Calceolarioside B (Desrhamnosyl isoverbascoside) (P) a | + | – | – | – |
| 34 | 21.2 | 477 [M–H]− | Nepetin 7-O-glucoside (6-Methoxyluteolin 7-O-glucoside) (F) b | + | + * | + * | + * |
| 35 | 21.2 | 785 [M–H]− | Rossicaside A (P) a | + | + | + | + |
| 36 | 21.2 | 623 [M–H]−, 1247 [2M–H]− | Verbascoside (Acteoside) (P) a | + | + | + | + |
| 37 | 21.8 | 653 [M–H]−, 1307 [2M–H]− | Methoxyverbascoside isomer (P) b | – | – | + * | + * |
| 38 | 22.1 | 443 [M–2H]2–, 887 [M–H]− | Trichosanthoside B (P) a | – | + | – | – |
| 39 | 22.5 | 539 [M–H + HCOOH]− | Globularidin (I) a | + | + | – | – |
| 40 | 22.8 | 623 [M–H]− | Isoverbascoside (Isoacteoside) (P) a | + | + | + | + |
| 41 | 22.9 | 755 [M–H]−, 1511 [2M–H]− | Trichosanthoside A (P) a | – | + | – | – |
| 42 | 23.1 | 771 [M–H]− | 6-Hydroxyluteolin 7-O-(6′′′-O-(p-coumaroyl))-sophoroside (F) a | – | + | – | – |
| 43 | 23.5 | 511 [M–H + HCOOH]− | Globularifolin (10-O-Benzoylmonomelittoside) (I)a | – | – | + | + |
| 44 | 23.7 | 623 [M–H]− | Forsythoside A (P) a | + | + | + | + |
| 45 | 24.3 | 537 [M–H + HCOOH]− | Globularin (10-O-trans-Cinnamoylcatalpol) (I) a | + | + | – | – |
| 46 | 25.2 | 477 [M–H]−, 523 [M–H + HCOOH]− | 6′-O-Benzoyldeacetylalpinoside (I) a | – | – | + | + |
| 47 | 25.3 | 755 [M–H]− | Arenarioside (P) a | – | + | – | – |
| 48 | 25.7 | 637 [M–H]−, 683 [M–H + HCOOH]− | Leucosceptoside A (P) a | + | + | + | + |
| 49 | 26.1 | 517 [M–H]−, 563 [M–H + HCOOH]− | 10-O-(p-Coumaroyl)-deacetylasperuloside (I) b | – | + * | + * | + * |
| 50 | 26.1 | 491 [M–H]−, 983 [2M–H]− | 6′-O-Feruloyl-1′-O-hydroxytyrosol glucoside (P) b | + * | – | – | – |
| 51 | 28.2 | 521 [M–H + HCOOH]− | Besperuloside (10-O-Benzoyldeacetylasperuloside) (I) a | – | + | – | – |
| 52 | 29.2 | 573 [M–H + HCOOH]− | Globularioside (I) a | + | + | – | – |
| 53 | 30.4 | 653 [M–H]−, 835 [M–H + mannitol]− | Demethoxycentaureidin 6,4′-dimethyl ether (F) a | – | – | + | + |
| 54 | 32.4 | 813 [M–H]− | Alpinoside-alpinoside dimer (I) a | + | – | – | – |
| 55 | 33.2 | 961 [M–H]− | Globusintenoside isomer (P) a | + | + | + | + |
| 56 | 34.1 | 639 [M–H]− | Desrhamnosyl 6′-O-caffeoylverbascoside (P) b | + * | – | – | – |
| 57 | 34.3 | 785 [M–H]− | 6′-O-Caffeoylverbascoside (P) a | + | – | – | – |
| 58 | 35.5 | 889 [M–H]− | Benzoylrossicaside A isomer (P) b | – | + * | + * | + * |
| 59 | 36.5 | 935 [M–H + HCOOH]− | Globuloside A (Alpinoside-globularin dimer) (I) a | + | – | – | – |
| 60 | 39.1 | 799 [M–H]− | Galypumoside B (6′-O-Feruloylverbascoside) (P) a | + | – | – | – |
| 61 | 39.3 | 653 [M–H]−, 689 [M + 35Cl]−, 691 [M + 37Cl]− | Desrhamnosyl galypumoside B (P) b | + * | – | – | – |
| 62 | 40.7 | 327 [M–H]− | Oxo-dihydroxy-octadecenoic acid (O) a | + | + | + | + |
| 63 | 41.8 | 789 [M–H]− | Galypumoside C (6′-O-Menthiafoloylverbascoside) (P) b | + | – | – | – |
| 64 | 43.4 | 329 [M–H]− | Trihydroxy-octadecenoic acid (O) a | + | + | + | + |
| Peak | tR (min) | m/z (Major Ion in Full MS Indicated in Bold) | Compound Identification (Compound Class Abbreviation) | GA | GP | GC | GM |
|---|---|---|---|---|---|---|---|
| 1′ | 1.2 | 217 [M + 35Cl]–, 219 [M + 37Cl]– | Mannitol (O) a | + | + | + | + |
| 2′ | 1.2 | 377 [M + 35Cl]–, 379 [M + 37Cl]– | Sucrose (O) a | + | + | + | + |
| 3′ | 1.7 | 407 [M–H + HCOOH]– | Catalpol (I) a | + | + | – | – |
| 4′ | 2.1 | 389 [M–H]–, 779 [2M–H]– | Scandoside (I) a | – | + | – | – |
| 5′ | 2.2 | 407 [M–H + HCOOH]– | Monomelittoside (5-Hydroxyaucubin) (I)a | – | – | + | + |
| 6′ | 2.4 | 371 [M–H]–, 417 [M–H + HCOOH]– | Deacetylasperuloside (I) a | – | + | + | + |
| 7′ | 5.9 | 341 [M–H]– | Caffeoylglucoside isomer (O) a | + | + | + | + |
| 8′ | 5.9 | 373 [M–H]–, 419 [M–H + HCOOH]– | Gardoside (I) a | + | + | + | + |
| 9′ | 6.9 | 431 [M–H]–, 863 [2M–H]– | Asperulosidic acid (I) a | – | + | + | + |
| 10′ | 8.9 | 459 [M–H + HCOOH]– | Asperuloside (I) a | – | + | + | + |
| 11′ | 10.7 | 523 [M–H]–, 1047 [2M–H]– | Verminoside (6-O-Caffeoylcatalpol) (I) a | + | – | – | – |
| 12′ | 10.9 | 433 [M–H + HCOOH]– | Geniposide (I) b | + * | – | – | – |
| 13′ | 13.7 | 507 [M–H]–, 553 [M–H + HCOOH]– | Specioside (6-O-(p-Coumaroyl)-catalpol) (I) a | + | – | – | – |
| 14′ | 14.1 | 625 [M–H]–, 671 [M–H + HCOOH]–, 1251 [2M–H]– | 6-Hydroxyluteolin 7-O-sophoroside (F) a | + | + | – | – |
| 15′ | 14.4 | 701 [M–H + HCOOH]– | 6′-O-Benzoyldeacetylasperulosidic acid glucoside (I) b | – | – | + * | + * |
| 16′ | 15.2 | 463 [M–H]–, 927 [2M–H]– | 6-Hydroxyluteolin 7-O-glucoside (F) a | + | + | + | + |
| 17′ | 15.9 | 415 [M–H]–, 461 [M–H + HCOOH]– | Alpinoside (I) a | + | + | + | + |
| 18′ | 16.4 | 555 [M–H + HCOOH]– | Globularinin (I) a | + | + | – | – |
| 19′ | 17.6 | 493 [M–H]–, 987 [2M–H]– | 6′-O-Benzoyldeacetylasperulosidic acid (I) b | – | + * | + * | + * |
| 20′ | 17.8 | 555 [M–H + HCOOH]– | Globularimin (I) a | + | + | – | – |
| 21′ | 17.9 | 463 [M–H + HCOOH–324]–, 787 [M–H + HCOOH]– | Liriodendrin ((+)-Syringaresinol di-O-β-glucopyranoside) (L) a | + | – | – | – |
| 22′ | 18.1 | 511 [M–H + HCOOH]– | 6′-O-Benzoylmonomelittoside (5-Hydroxydumuloside) (I)a | – | – | + | + |
| 23′ | 18.8 | 477 [M–H]– | Calceolarioside A (Desrhamnosyl verbascoside) (P) a | + | – | – | – |
| 24′ | 19.1 | 787 [M–H]–, 1575 [2M–H]– | 6-Hydroxyluteolin 7-O-(6′′′-O-caffeoyl)-sophoroside (F) a | – | + | – | – |
| 25′ | 19.8 | 477 [M–H]– | Calceolarioside B (Desrhamnosyl isoverbascoside) (P) a | + | – | – | – |
| 26′ | 20.2 | 785 [M–H]– | Rossicaside A (P) a | + | + | + | + |
| 27′ | 20.4 | 623 [M–H]–, 1247 [2M–H]– | Verbascoside (Acteoside) (P) a | + | + | + | + |
| 28′ | 20.7 | 653 [M–H]–, 1307 [2M–H]– | Methoxyverbascoside isomer (P) b | – | – | + * | + * |
| 29′ | 21.3 | 443 [M–2H]2–, 887 [M–H]– | Trichosanthoside B (P) a | – | + | – | – |
| 30′ | 22.0 | 623 [M–H]– | Isoverbascoside (Isoacteoside) (P) a | + | + | + | + |
| 31′ | 22.1 | 755 [M–H]–, 1511 [2M–H]– | Trichosanthoside A (P) a | – | + | – | – |
| 32′ | 22.4 | 771 [M–H]– | 6-Hydroxyluteolin 7-O-(6′′′-O-(p-coumaroyl)-sophoroside (F)a | – | + | – | – |
| 33′ | 22.5 | 511 [M–H + HCOOH]– | Globularifolin (10-O-Benzoylmonomelittoside) (I) a | – | – | + | + |
| 34′ | 23.0 | 623 [M–H]– | Forsythoside A (P) a | + | + | + | + |
| 35′ | 23.6 | 537 [M–H + HCOOH]– | Globularin (10-O-trans-Cinnamoylcatalpol) (I) a | + | + | – | – |
| 36′ | 24.2 | 477 [M–H]–, 523 [M–H + HCOOH]– | 6′-O-Benzoyldeacetylalpinoside (I) a | – | – | + | + |
| 37′ | 24.6 | 755 [M–H]– | Arenarioside (P) a | – | + | – | – |
| 38′ | 24.9 | 637 [M–H]–, 683 [M–H + HCOOH]– | Leucosceptoside A (P) a | + | + | + | + |
| 39′ | 25.1 | 517 [M–H]–, 563 [M–H + HCOOH]– | 10-O-(p-Coumaroyl)-deacetylasperuloside (I) b | – | + * | + * | + * |
| 40′ | 25.2 | 491 [M–H]–, 983 [2M–H]– | 6′-O-Feruloyl-1′-O-hydroxytyrosol glucoside (P) b | + * | – | – | – |
| 41′ | 27.4 | 521 [M–H + HCOOH]– | Besperuloside (10-O-Benzoyldeacetylasperuloside) (I) a | – | + | – | – |
| 42′ | 28.3 | 573 [M–H + HCOOH]– | Globularioside (I) a | + | + | – | – |
| 43′ | 29.4 | 653 [M–H]–, 835 [M–H + mannitol]–, 1307 [2M–H]– | Demethoxycentaureidin 6,4′-dimethyl ether (F) a | – | – | + | + |
| 44′ | 32.4 | 961 [M–H]– | Globusintenoside isomer (P) a | + | + | + | + |
| 45′ | 33.5 | 785 [M–H]– | 6′-O-Caffeoylverbascoside (P) a | + | – | – | – |
| 46′ | 34.2 | 269 [M–H]– | Apigenin (F) a | + | + | + | + |
| 47′ | 34.6 | 889 [M–H]– | Benzoylrossicaside A isomer (P) b | – | + * | + * | + * |
| 48′ | 35.6 | 935 [M–H + HCOOH]– | Globuloside A (Alpinoside-globularin dimer) (I) a | + | – | – | – |
| 49′ | 38.2 | 799 [M–H]– | Galypumoside B (6′-O-Feruloylverbascoside) (P) a | + | – | – | – |
| G. alypum | G. punctata | G. cordifolia | G. meridionalis | Gallic Acid | Trolox |
|---|---|---|---|---|---|
| 17.25 | 24.19 | 22.68 | 20.41 | 0.64 | 2.71 |
| Assay | G. alypum | G. punctata | G. cordifolia | G. meridionalis | Indomethacin |
|---|---|---|---|---|---|
| TMPD | 51.3 ± 17.4 A | 39.9 ± 9.6 AB | 37.8 ± 6.6 AB | 17.6 ± 7.9 B | 2.90 * |
| PGE2 | 40.6 ± 4.8 A | 32.9 ± 2.6 A | 26.5 ± 4.7 A | 25.7 ± 3.3 AB | 1.03 * |
| Bacterial Strains | G. alypum | G. punctata | G. cordifolia | G. meridionalis | Gentamicin a | Norfloxacin b |
|---|---|---|---|---|---|---|
| Gram-Positive | ||||||
| Bacillus cereus ATCC 11778 | 8.3 ± 0.6 | 9.3 ± 1.2 | 9.7 ± 0.6 | 8.0 ± 0.0 | 22.3 ± 0.6 | 26.6 ± 0.9 |
| Bacillus subtilis ATCC 6633 | 7.7 ± 0.6 | n.d. | 8.3 ± 1.5 | n.d. | 24.7 ± 0.6 | 41.0 ± 0.0 |
| Enterococcus faecalis ATCC 29212 | n.d. | 13.0 ± 0.0 | n.d. | n.d. | 15.0 ± 0.0 | 23.8 ± 0.8 |
| Staphylococcus aureus ATCC 6538 | 25.0 ± 0.0 | 21.3 ± 0.6 | 23.0 ± 0.0 | 23.7 ± 1.5 | 24.0 ± 0.0 | 33.8 ± 0.8 |
| Gram-Negative | ||||||
| Escherichia coli ATCC 10536 | n.d. | 24.0 ± 1.7 | n.d. | n.d. | 23.3 ± 0.6 | 44.7 ± 1.0 |
| Klebsiella pneumoniae MFBF 10402 | n.d. | n.d. | n.d. | n.d. | 21.0 ± 0.0 | 7.3 ± 0.5 |
| Pseudomonas aeruginosa ATCC 27853 | 9.7 ± 0.6 | n.d. | n.d. | n.d. | 24.3 ± 0.6 | 30.7 ± 2.8 |
| Bacterial Strains | G. alypum | G. punctata | G. cordifolia | G. meridionalis | Gentamicin a | Verbascoside b |
|---|---|---|---|---|---|---|
| Gram-Positive | ||||||
| Bacillus cereus ATCC 11778 | 11.36 c 11.36 d | 2.84 c 2.84 d | 11.36 c 11.36 d | 11.36 c n.d. d | 0.0015 c 0.0015 d | n.d. c n.d. d |
| Bacillus subtilis ATCC 6633 | n.d. c n.d. d | 1.89 c 2.84 d | n.d. c n.d. d | n.d. c n.d. d | < 0.0001 c 0.0002 d | n.d. c n.d. d |
| Enterococcus faecalis ATCC 29212 | n.d. c * n.d. d | n.d. c * 5.68 d | n.d. c * n.d. d | n.d. c * n.d. d | n.d. c * 0.0046 d | n.d. c * n.d. d |
| Methicillin-Susceptible Staphylococcus aureus (MSSA) | ||||||
| Staphylococcus aureus ATCC 6538 | 1.42 c 1.89 d | 1.42 c 1.42 d | 2.84 c 3.79 d | 2.84 c 2.84 d | 0.0006 c 0.0008 d | 0.2272 c n.d. d |
| Staphylococcus aureus ATCC 29213 | 4.73 c 4.73 d | 11.36 c 11.36 d | 7.58 c 11.36 d | 11.36 c 11.36 d | 0.0003 c 0.0007 d | 0.2272 c 0.2272 c |
| Staphylococcus aureus MFBF 505 | 2.37 c 2.37 d | 1.42 c 1.42 d | 2.84 c 2.84 d | 2.84 c 2.84 d | n.m. n.m. | n.m. n.m. |
| Staphylococcus aureus MFBF 10661 | 1.42 c 1.42 d | 1.42 c 1.42 d | 1.89 c 2.37 d | 1.42 c 1.89 d | n.m. n.m. | n.m. n.m. |
| Staphylococcus aureus MFBF 10666 | 1.42 c 1.42 d | 1.89 c 1.89 d | 1.89 c 1.89 d | 1.42 c 1.42 d | n.m. n.m. | n.m. n.m. |
| Methicillin-Resistant Staphylococcus aureus (MRSA) | ||||||
| MRSA MFBF 101 | 1.89 c 2.84 d | 1.42 c 1.42 d | 2.84 c 2.84 d | 2.84 c 2.84 d | n.m. n.m. | n.m. n.m. |
| MRSA MFBF 124 | 2.84 c 2.84 d | 2.84 c 2.84 d | 3.31 c 3.79 d | 2.84 c 2.84 d | n.m. n.m. | n.m. n.m. |
| MRSA MFBF 154 | 2.84 c 2.84 d | 1.89 c 2.37 d | 2.84 c 2.84 d | 2.84 c 2.84 d | n.m. n.m. | n.m. n.m. |
| MRSA MFBF 164 | 2.84 c 2.84 d | 1.42 c 1.89 d | 2.84 c 2.84 d | 3.79 c 3.79 d | n.m. n.m. | n.m. n.m. |
| MRSA MFBF 177 | 2.84 c 2.84 d | 2.37 c 2.84 d | 3.79 c 3.79 d | 2.84 c 2.84 d | n.m. n.m. | n.m. n.m. |
| Gram-Negative | ||||||
| Escherichia coli ATCC 10536 | n.d. c n.d. d | 1.42 c 2.84 d | n.d. c n.d. d | n.d. c n.d. d | 0.0001 c 0.0015 d | 0.2272 c n.d. d |
| Klebsiella pneumoniae MFBF 10402 | n.d. c n.d. d | n.d. c n.d. d | n.d. c n.d. d | n.d. c n.d. d | 0.0003 c 0.0008 d | n.d. c n.d. d |
| Pseudomonas aeruginosa ATCC 27853 | 22.73 c 22.73 d | n.d. c n.d. d | n.d. c n.d. d | n.d. c n.d. d | 0.0011 c 0.0030 d | 0.2272 c n.d. d |
| G. alypum | G. punctata | G. cordifolia | G. meridionalis |
|---|---|---|---|
| 231.43 | 140.54 | 180.42 | 129.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friščić, M.; Petlevski, R.; Kosalec, I.; Madunić, J.; Matulić, M.; Bucar, F.; Hazler Pilepić, K.; Maleš, Ž. Globularia alypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential. Pharmaceuticals 2022, 15, 506. https://doi.org/10.3390/ph15050506
Friščić M, Petlevski R, Kosalec I, Madunić J, Matulić M, Bucar F, Hazler Pilepić K, Maleš Ž. Globularia alypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential. Pharmaceuticals. 2022; 15(5):506. https://doi.org/10.3390/ph15050506
Chicago/Turabian StyleFriščić, Maja, Roberta Petlevski, Ivan Kosalec, Josip Madunić, Maja Matulić, Franz Bucar, Kroata Hazler Pilepić, and Željan Maleš. 2022. "Globularia alypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential" Pharmaceuticals 15, no. 5: 506. https://doi.org/10.3390/ph15050506
APA StyleFriščić, M., Petlevski, R., Kosalec, I., Madunić, J., Matulić, M., Bucar, F., Hazler Pilepić, K., & Maleš, Ž. (2022). Globularia alypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential. Pharmaceuticals, 15(5), 506. https://doi.org/10.3390/ph15050506