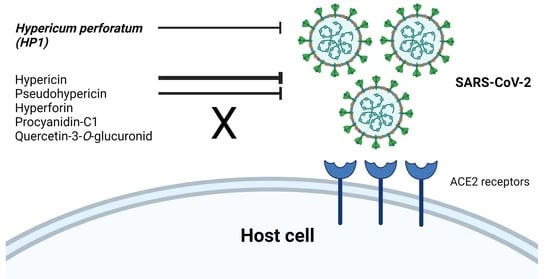
Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2
Abstract
:1. Introduction
2. Results
2.1. The Hypericum perforatum Extract (HP1) Inhibits Infection of Cells by the Pseudo-Typed VSV SARS-CoV-2 S Protein-d21-Carrying Virus
2.2. The Naphtodianthrones Hypericin and Pseudohypericin from HP1 Are Active against the Pseudo-Typed VSV SARS-CoV-2 S Protein-d21-Carrying Virus
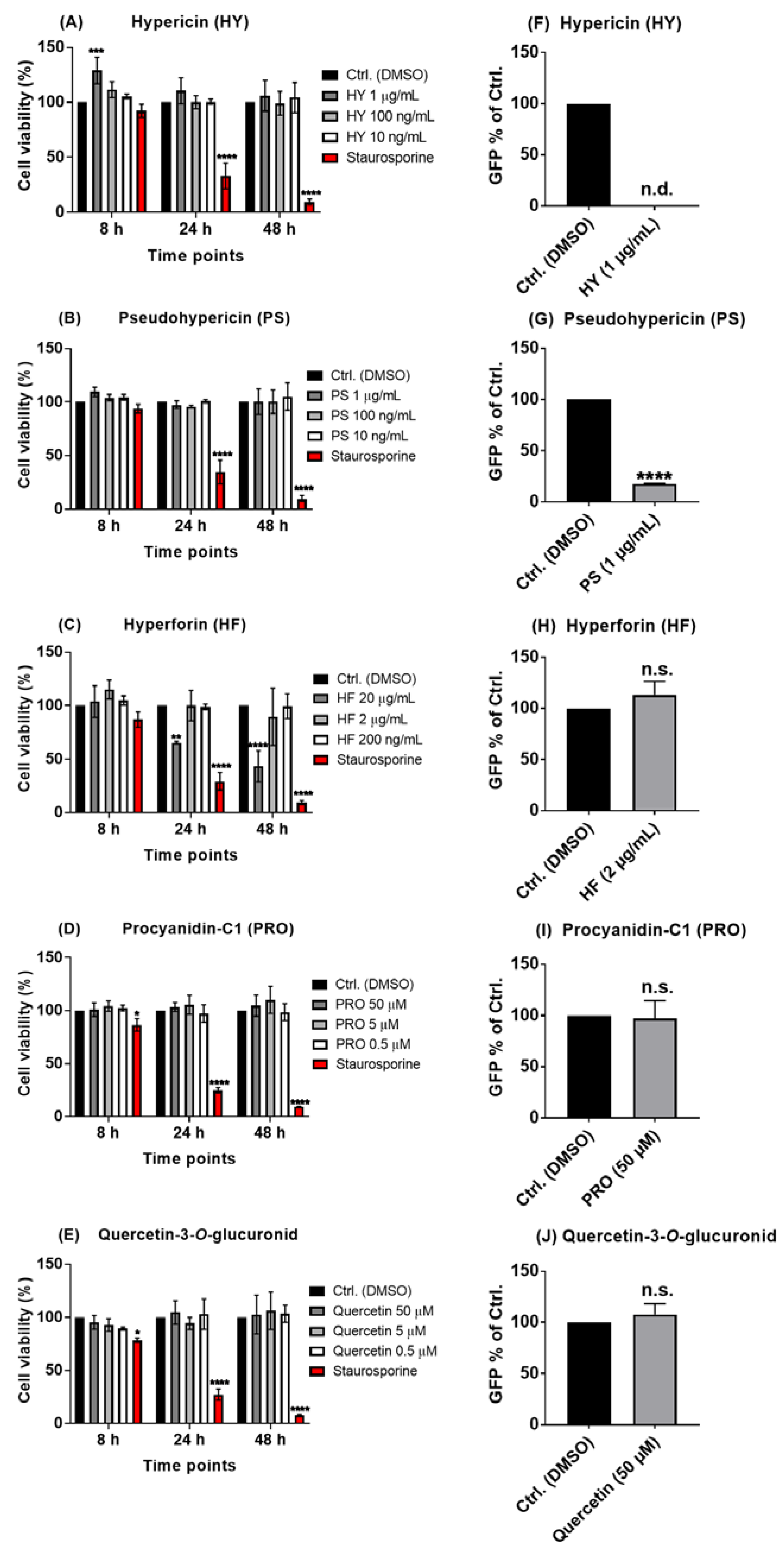
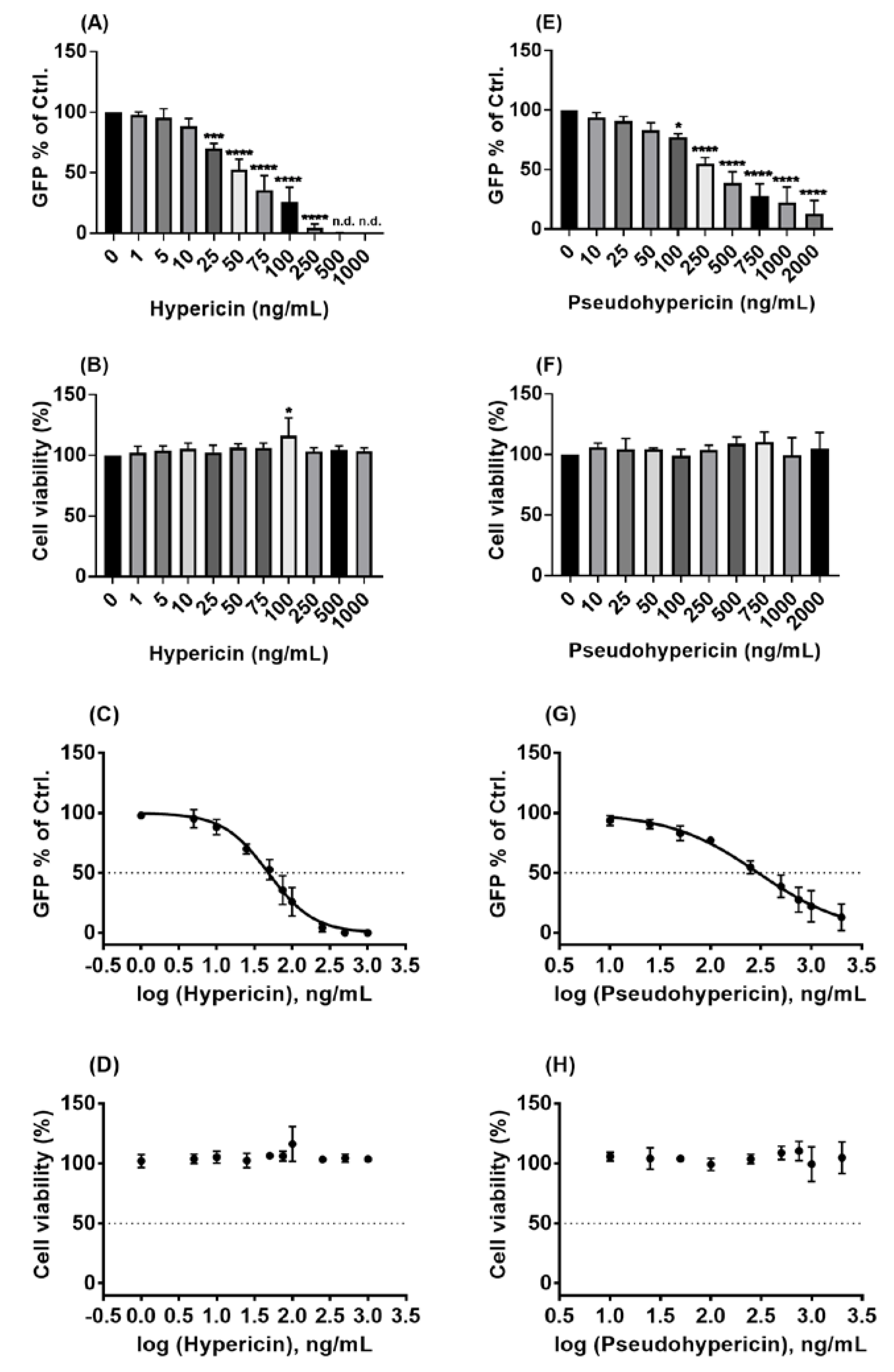
2.3. Hypericin and Pseudohypericin Exhibit a Strong Antiviral Activity against the Pseudo-Typed VSV SARS-CoV-2 S Protein-d21-Carrying Virus
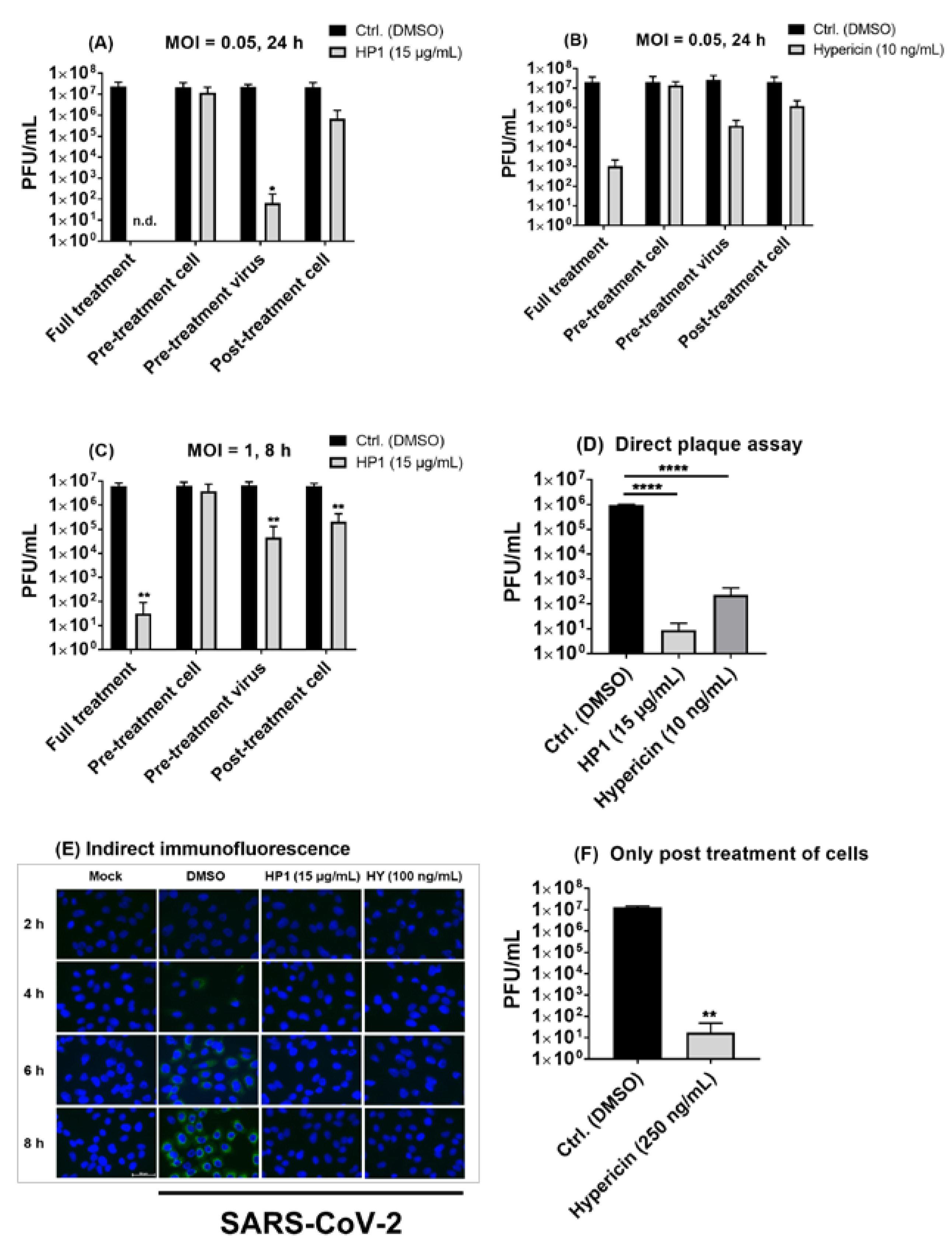
2.4. Hypericum perforatum (HP1) and Its Ingredients, Hypericin and Pseudohypericin, Are Antivirally Active against SARS-CoV-2
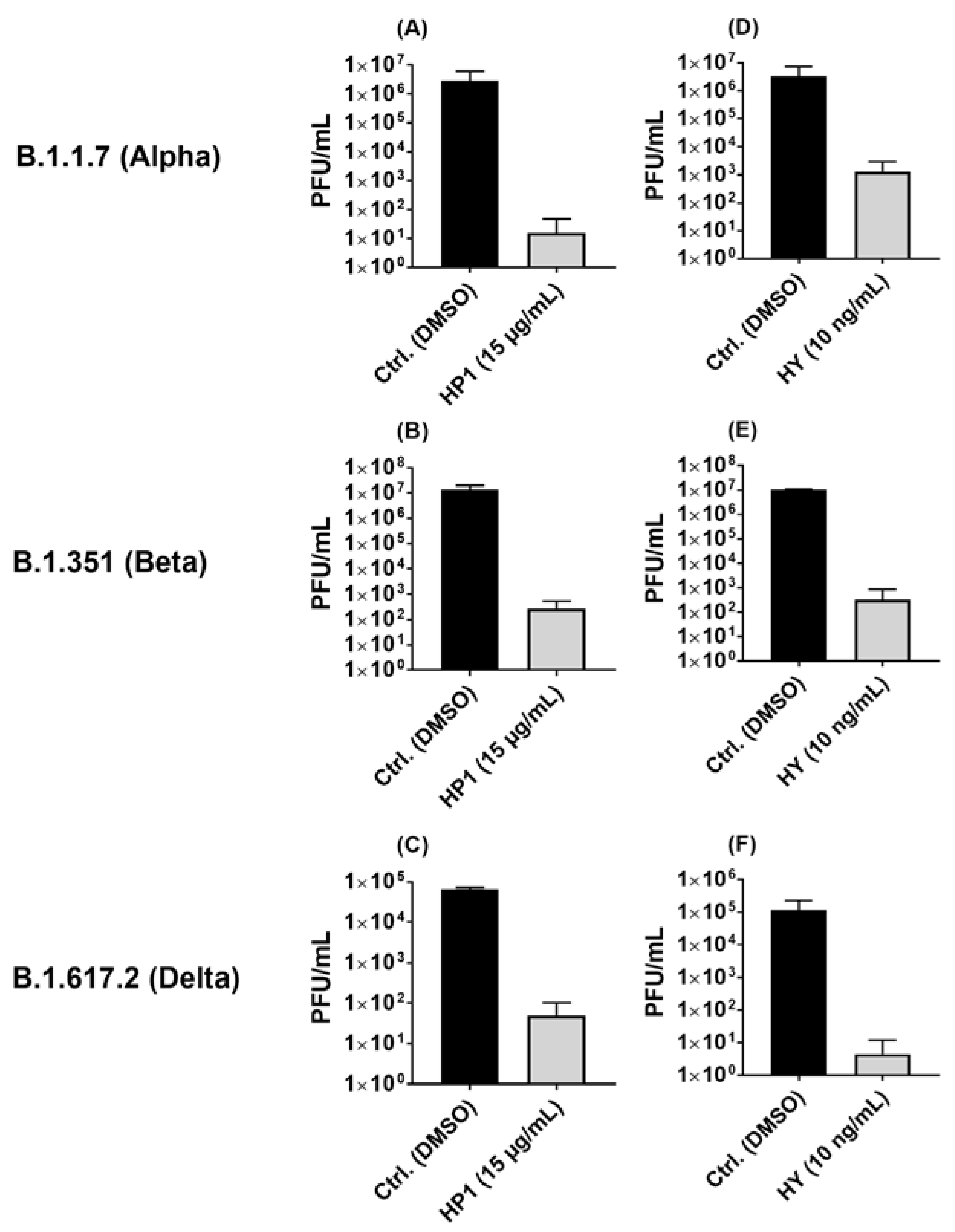
2.5. Hypericum perforatum (HP1) and Hypericin Displayed an Antiviral Activity against SARS-CoV-2 Variants
2.6. Pre-Treatment of SARS-CoV-2 Virus Particles Prior to Infection with Hypericum perforatum (HP1) or Hypericin Is Mostly Effective in Blocking Virus Infection
2.7. The Antiviral Effect of Hypericum perforatum (HP1) and Hypericin Is Not Mediated by Blocking Specific SARS-CoV-2 S Protein Functions
2.8. The Antiviral Activity of Hypericum perforatum (HP1) and Hypericin against the VSV Pseudo-Typed Virus Carrying the Omicron S Protein
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Compounds
4.3. Production of VSV-ΔG+G Virus
4.4. C-Terminal Truncation of the Full-Length SARS-CoV-2 S Protein (d21)
4.5. Production of the Pseudo-Typed VSV-ΔG SARS-CoV-2 S Protein (d21) Virus
4.6. Cell Cytotoxicity Assay (MTT Assay)
4.7. SARS-CoV-2 Infection
4.8. Testing of Substances under Investigation against the Pseudo-Typed VSV-ΔG SARS-CoV-2 S Protein (d21) Virus or SARS-CoV-2 Virus
4.9. Plaque Assay
4.10. Indirect Immunofluorescence
4.11. hACE2-RBD Surrogate Virus-Neutralization Assay (sVNT)
4.12. Virus-Free Cell–Cell Fusion Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drosten, C.; Günther, S.; Preiser, W.; Van Der Werf, S.; Brodt, H.-R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (C.S.G). The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Neuman, B.W.; Kiss, G.; Kunding, A.H.; Bhella, D.; Baksh, M.F.; Connelly, S.; Droese, B.; Klaus, J.P.; Makino, S.; Sawicki, S.G. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011, 174, 11–22. [Google Scholar] [CrossRef]
- Ruch, T.R.; Machamer, C.E. The coronavirus E protein: Assembly and beyond. Viruses 2012, 4, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Risco, C.; Antón, I.M.; Enjuanes, L.; Carrascosa, J.L. The transmissible gastroenteritis coronavirus contains a spherical core shell consisting of M and N proteins. J. Virol. 1996, 70, 4773–4777. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Batra, J.; Srinivasan, S. COVID-19: Targeting proteases in viral invasion and host immune response. Front. Mol. Biosci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Koelle, K.; Martin, M.A.; Antia, R.; Lopman, B.; Dean, N.E. The changing epidemiology of SARS-CoV-2. Science 2022, 375, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Jukič, M.; Kores, K.; Janežič, D.; Bren, U. Repurposing of Drugs for SARS-CoV-2 Using Inverse Docking Fingerprints. Front. Chem. 2021, 9, 757826. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.C.; de Castro, A.A.; de Jesus, J.; Nepovimova, E.; Kuca, K.; Ramalho, T.C.; La Porta, F.A. Computational evidence for nitro derivatives of quinoline and quinoline N-oxide as low-cost alternative for the treatment of SARS-CoV-2 infection. Sci. Rep. 2021, 11, 6397. [Google Scholar] [CrossRef] [PubMed]
- Llivisaca-Contreras, S.A.; Naranjo-Morán, J.; Pino-Acosta, A.; Pieters, L.; Vanden Berghe, W.; Manzano, P.; Vargas-Pérez, J.; León-Tamariz, F.; Cevallos-Cevallos, J.M. Plants and natural products with activity against various types of coronaviruses: A review with focus on SARS-CoV-2. Molecules 2021, 26, 4099. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Hrincius, E.R.; Korte, V.; Mazur, I.; Droebner, K.; Poetter, A.; Dreschers, S.; Schmolke, M.; Planz, O.; Ludwig, S. A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antivir. Res. 2007, 76, 38–47. [Google Scholar] [CrossRef]
- Derksen, A.; Hensel, A.; Hafezi, W.; Herrmann, F.; Schmidt, T.J.; Ehrhardt, C.; Ludwig, S.; Kühn, J. 3-O-galloylated procyanidins from Rumex acetosa L. inhibit the attachment of influenza A virus. PLoS ONE 2014, 9, e110089. [Google Scholar] [CrossRef]
- Klemow, K.M.; Bartlow, A.; Crawford, J.; Kocher, N.; Shah, J.; Ritsick, M. Medical Attributes of St. John’s Wort (Hypericum perforatum). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; Chapter 11. Available online: https://www.ncbi.nlm.nih.gov/books/NBK92750/ (accessed on 11 April 2022).
- Weber, N.; Murray, B.; North, J.; Wood, S. The antiviral agent hypericin has in vitro activity against HSV-1 through non-specific association with viral and cellular membranes. Antivir. Chem. Chemother. 1994, 5, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Axarlis, S.; Mentis, A.; Demetzos, C.; Mitaku, S.; Skaltsounis, A.; Marselos, M.; Malamas, M. Antiviral in vitro activity of Hypericum perforatum L. extract on the human cytomegalovirus (HCMV). Phytother. Res. 1998, 12, 507–511. [Google Scholar] [CrossRef]
- Pang, R.; Tao, J.; Zhang, S.; Zhu, J.; Yue, X.; Zhao, L.; Ye, P.; Zhu, Y. In vitro anti-hepatitis B virus effect of Hypericum perforatum L. J. Huazhong Univ. Sci. Technol. 2010, 30, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.-Y.; Liang, J.-P.; Wang, X.-H.; Xu, T.; Hua, L.-Y.; Shang, R.-F.; Liu, Y.; Xing, Y.-M. Anti-influenza A virus effect of Hypericum perforatum L. extract. Virol. Sin. 2009, 24, 19–27. [Google Scholar] [CrossRef]
- Xiuying, P.; Jianping, L.; Ruofeng, S.; Liye, Z.; Xuehong, W.; Yan, L. Therapeutic efficacy of Hypericum perforatum L. extract for mice infected with an influenza A virus. Can. J. Physiol. Pharmacol. 2012, 90, 123–130. [Google Scholar] [CrossRef]
- Maury, W.; Price, J.P.; Brindley, M.A.; Oh, C.; Neighbors, J.D.; Wiemer, D.F.; Wills, N.; Carpenter, S.; Hauck, C.; Murphy, P. Identification of light-independent inhibition of human immunodeficiency virus-1 infection through bioguided fractionation of Hypericum perforatum. Virol. J. 2009, 6, 101. [Google Scholar] [CrossRef] [Green Version]
- Pu, X.-y.; Liang, J.-p.; Shang, R.-f.; Wang, X.-h.; Wang, Z.-X.; Hua, L.-Y.; Yu, L. Influence of Hypericum perforatum extract on piglet infected with porcine respiratory and reproductive syndrome virus. Agric. Sci. China 2009, 8, 730–739. [Google Scholar] [CrossRef]
- Shang, R.; He, C.; Chen, J.; Pu, X.; Liu, Y.; Hua, L.; Wang, L.; Liang, J. Hypericum perforatum extract therapy for chickens experimentally infected with infectious bursal disease virus and its influence on immunity. Can. J. Vet. Res. 2012, 76, 180–185. [Google Scholar] [PubMed]
- Chen, H.; Muhammad, I.; Zhang, Y.; Ren, Y.; Zhang, R.; Huang, X.; Diao, L.; Liu, H.; Li, X.; Sun, X. Antiviral activity against infectious bronchitis virus and bioactive components of Hypericum perforatum L. Front. Pharmacol. 2019, 10, 1272. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zou, M.; Oerlemans, R.; Shao, C.; Ren, Y.; Zhang, R.; Huang, X.; Li, G.; Cong, Y. Hypericin Inhibit Alpha-Coronavirus Replication by Targeting 3CL Protease. Viruses 2021, 13, 1825. [Google Scholar] [CrossRef]
- St. John’s Wort Dry Extract, Quantified (01/2017:1874). European Pharmacopoeia 10th Edition-European Directorate for the Quality of Medicines & Healthcare (EDQM). Available online: https://extranet.edqm.eu/4DLink1/pdfs/chromatos/1874.pdf (accessed on 11 April 2022).
- Reaxys Database. Available online: https://www.reaxys.com/ (accessed on 11 April 2022).
- Harder, T.; Koch, J.; Vygen-Bonnet, S.; Külper-Schiek, W.; Pilic, A.; Reda, S.; Scholz, S.; Wichmann, O. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: Interim results of a living systematic review, 1 January to 14 May 2021. Eurosurveillance 2021, 26, 2100563. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Henderson, J.P.; Joyner, M.J.; Pirofski, L.-A. SARS-CoV-2 variants and convalescent plasma: Reality, fallacies, and opportunities. J. Clin. Investig. 2021, 131, e148832. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Shawa, I.; De La Torre, I.; Pustizzi, J.M.; Haustrup, N.; Patel, D.R.; Huhn, G. A narrative review of the clinical practicalities of Bamlanivimab and Etesevimab antibody therapies for SARS-CoV-2. Infect. Dis. Ther. 2021, 10, 1933–1947. [Google Scholar] [CrossRef] [PubMed]
- Kmietowicz, Z. COVID-19: Monoclonal Antibodies Authorised in US as Alternative to Vaccines for Certain Groups. BMJ 2021, 375, n3064. [Google Scholar] [CrossRef]
- Stebbing, J.; Nievas, G.S.; Falcone, M.; Youhanna, S.; Richardson, P.; Ottaviani, S.; Shen, J.X.; Sommerauer, C.; Tiseo, G.; Ghiadoni, L. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci. Adv. 2021, 7, eabe4724. [Google Scholar] [CrossRef]
- Jordan, S.C.; Zakowski, P.; Tran, H.P.; Smith, E.A.; Gaultier, C.; Marks, G.; Zabner, R.; Lowenstein, H.; Oft, J.; Bluen, B. Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin. Infect. Dis. 2020, 71, 3168–3173. [Google Scholar] [CrossRef]
- Fernández-Cruz, A.; Ruiz-Antorán, B.; Muñoz-Gómez, A.; Sancho-López, A.; Mills-Sánchez, P.; Centeno-Soto, G.A.; Blanco-Alonso, S.; Javaloyes-Garachana, L.; Galán-Gómez, A.; Valencia-Alijo, Á. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob. Agents Chemother. 2020, 64, e01168-20. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef]
- Indari, O.; Jakhmola, S.; Manivannan, E.; Jha, H.C. An update on antiviral therapy against SARS-CoV-2: How far have we come? Front. Pharmacol. 2021, 12, 632677. [Google Scholar] [CrossRef]
- Kumar, S.; Çalışkan, D.M.; Janowski, J.; Faist, A.; Conrad, B.C.G.; Lange, J.; Ludwig, S.; Brunotte, L. Beyond vaccines: Clinical status of prospective COVID-19 therapeutics. Front. Immunol. 2021, 12, 752227. [Google Scholar] [CrossRef]
- Fischer, W.A.; Eron, J.J.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; Sheahan, T.P.; Baric, R.; Mollan, K.R.; Wolfe, C.R.; et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 2022, 14, eabl7430. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. COVID antiviral pills: What scientists still want to know. Nature 2021, 599, 358–359. [Google Scholar] [CrossRef]
- Wanzala, W.; Zessin, K.; Kyule, N.; Baumann, M.; Mathia, E.; Hassanali, A. Ethnoveterinary Medicine: A Critical Review of its Evolution, Perception, Understanding and the Way Forward. Livest. Res. Rural. Dev. 2005, 17. [Google Scholar]
- Wei, F.; Ma, S.-C.; Ma, L.-Y.; But, P.P.-H.; Lin, R.-C.; Khan, I.A. Antiviral Flavonoids from the Seeds of Aesculus c hinensis. J. Nat. Prod. 2004, 67, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, C.; Zhang, H.; Guo, H.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.; Yu, J.; Xiao, P. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Kaczmarek, J.N.; Leivers, S.; Ghebremariam, Y.T.; Kulkarni, A.P.; Bauer, G.; De Beer, C.; Preiser, W.; Mohamed, A.R. Anti-HIV, Anti-Poxvirus, and Anti-SARS Activity of a Nontoxic, Acidic Plant Extract from the Trifollium Species Secomet-V/anti-Vac Suggests That It Contains a Novel Broad-Spectrum Antiviral. Ann. N. Y. Acad. Sci. USA 2005, 1056, 293–302. [Google Scholar] [CrossRef]
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral potentials of medicinal plants. Virus Res. 2008, 131, 111–120. [Google Scholar] [CrossRef]
- Benarba, B.; Pandiella, A. Medicinal plants as sources of active molecules against COVID-19. Front. Pharmacol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef]
- Mensah, M.L.; Komlaga, G.; Forkuo, A.D.; Firempong, C.; Anning, A.K.; Dickson, R.A. Toxicity and Safety Implications of Herbal Medicines Used in Africa. In Herbal Medicine; IntechOpen: London, UK, 2019; Chapter 5. [Google Scholar] [CrossRef] [Green Version]
- Bajrai, L.H.; El-Kafrawy, S.A.; Alnahas, R.S.; Azhar, E.I. In vitro screening of anti-viral and virucidal effects against SARS-CoV-2 by Hypericum perforatum and Echinacea. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lenard, J.; Rabson, A.; Vanderoef, R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: Inhibition of fusion and syncytia formation. Proc. Natl. Acad. Sci. USA 1993, 90, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Colacino, J.M.; Larsen, S.H.; Spitzer, W. Virucidal activity of hypericin against enveloped and non-enveloped DNA and RNA viruses. Antivir. Res. 1990, 13, 313–325. [Google Scholar] [CrossRef]
- Nayak, D.P.; Hui, E.K.-W.; Barman, S. Assembly and budding of influenza virus. Virus Res. 2004, 106, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Cortese, M.; Winter, S.L.; Wachsmuth-Melm, M.; Neufeldt, C.J.; Cerikan, B.; Stanifer, M.L.; Boulant, S.; Bartenschlager, R.; Chlanda, P. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun. 2020, 11, 5885. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C. Budding events in herpesvirus morphogenesis. Virus Res. 2004, 106, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kocanova, S.; Hornakova, T.; Hritz, J.; Jancura, D.; Chorvat Jr, D.; Mateasik, A.; Ulicny, J.; Refregiers, M.; Maurizot, J.C.; Miskovsky, P. Characterization of the Interaction of Hypericin with Protein Kinase C in U-87 MG Human Glioma Cells. Photochem. Photobiol. 2006, 82, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sarma, S.; Katiyar, S.P.; Das, M.; Bhardwaj, R.; Sundar, D.; Dubey, V.K. Probing the molecular mechanism of hypericin-induced parasite death provides insight into the role of spermidine beyond redox metabolism in Leishmania donovani. Antimicrob. Agents Chemother. 2015, 59, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Yalçın, S.; Yalçınkaya, S.; Ercan, F. Determination of Potential Drug Candidate Molecules of the Hypericum perforatum for COVID-19 Treatment. Curr. Pharmacol. Rep. 2021, 7, 42–48. [Google Scholar] [CrossRef]
- Matos, A.D.R.; Caetano, B.C.; Martins, J.S.C.D.C.; Oliveira, M.G.P.d.; Sousa, T.d.C.; Horta, M.A.P.; Siqueira, M.M.; Fernandez, J.H. Identification of Hypericin as a candidate repurposed therapeutic agent for COVID-19 and its potential anti-SARS-CoV-2 activity. Front. Microbiol. 2022, 13, 828984. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Balmeh, N.; Mohammadi, N.; Sadeghian-Rizi, T. The Novel Drug Discovery to Combat COVID-19 by Repressing Important Virus Proteins Involved in Pathogenesis Using Medicinal Herbal Compounds. Avicenna J. Med. Biotechnol. 2021, 13, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bahun, M.; Jukić, M.; Oblak, D.; Kranjc, L.; Bajc, G.; Butala, M.; Bozovičar, K.; Bratkovič, T.; Podlipnik, Č.; Ulrih, N.P. Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols. Food Chem. 2022, 373, 131594. [Google Scholar] [CrossRef]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.; Nguyen, H. StatPearls; St. John’s Wort: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557465/ (accessed on 23 March 2022).
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Eatemadnia, A.; Ansari, S.; Abedi, P.; Najar, S. The effect of Hypericum perforatum on postmenopausal symptoms and depression: A randomized controlled trial. Complementary Ther. Med. 2019, 45, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.-U.; Schürer, M.; Bässler, D.; Weiser, D. Investigation of the bioavailability of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin following single and multiple oral dosing of a hypericum extract containing tablet. Arzneimittelforschung 2005, 55, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerb, R.; Brockmöller, J.; Staffeldt, B.; Ploch, M.; Roots, I. Single-dose and steady-state pharmacokinetics of hypericin and pseudohypericin. Antimicrob. Agents Chemother. 1996, 40, 2087–2093. [Google Scholar] [CrossRef] [Green Version]
- Lavie, G.; Mazur, Y.; Lavie, D.; Prince, A.; Pascual, D.; Liebes, L.; Levin, B.; Meruelo, D. Hypericin as an inactivator of infectious viruses in blood components. Transfusion 1995, 35, 392–400. [Google Scholar] [CrossRef]
- Miskovsky, P.; Hritz, J.; Sanchez-Cortes, S.; Fabriciova, G.; Ulicny, J.; Chinsky, L. Interaction of Hypericin with Serum Albumins: Surface-enhanced Raman Spectroscopy, Resonance Raman Spectroscopy and Molecular Modeling Study. Photochem. Photobiol. 2001, 74, 172–183. [Google Scholar] [CrossRef]
- Masiello, P.; Novelli, M.; Beffy, P.; Menegazzi, M. Can Hypericum perforatum (SJW) prevent cytokine storm in COVID-19 patients? Phytother. Res. 2020, 34, 1471–1473. [Google Scholar] [CrossRef]
- Havranek, K.E.; Jimenez, A.R.; Acciani, M.D.; Lay Mendoza, M.F.; Reyes Ballista, J.M.; Diaz, D.A.; Brindley, M.A. SARS-CoV-2 Spike Alterations Enhance Pseudoparticle Titers and Replication-Competent VSV-SARS-CoV-2 Virus. Viruses 2020, 12, 1465. [Google Scholar] [CrossRef]
- Zettl, F.; Meister, T.L.; Vollmer, T.; Fischer, B.; Steinmann, J.; Krawczyk, A.; V’kovski, P.; Todt, D.; Steinmann, E.; Pfaender, S. Rapid quantification of SARS-CoV-2-neutralizing antibodies using propagation-defective vesicular stomatitis virus pseudotypes. Vaccines 2020, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, F.F.; Anhlan, D.; Schöfbänker, M.; Schreiber, A.; Classen, N.; Hensel, A.; Hempel, G.; Scholz, W.; Kühn, J.; Hrincius, E.R.; et al. Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals 2022, 15, 530. https://doi.org/10.3390/ph15050530
Mohamed FF, Anhlan D, Schöfbänker M, Schreiber A, Classen N, Hensel A, Hempel G, Scholz W, Kühn J, Hrincius ER, et al. Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals. 2022; 15(5):530. https://doi.org/10.3390/ph15050530
Chicago/Turabian StyleMohamed, Fakry F., Darisuren Anhlan, Michael Schöfbänker, André Schreiber, Nica Classen, Andreas Hensel, Georg Hempel, Wolfgang Scholz, Joachim Kühn, Eike R. Hrincius, and et al. 2022. "Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2" Pharmaceuticals 15, no. 5: 530. https://doi.org/10.3390/ph15050530
APA StyleMohamed, F. F., Anhlan, D., Schöfbänker, M., Schreiber, A., Classen, N., Hensel, A., Hempel, G., Scholz, W., Kühn, J., Hrincius, E. R., & Ludwig, S. (2022). Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals, 15(5), 530. https://doi.org/10.3390/ph15050530