Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview
Abstract
1. Introduction
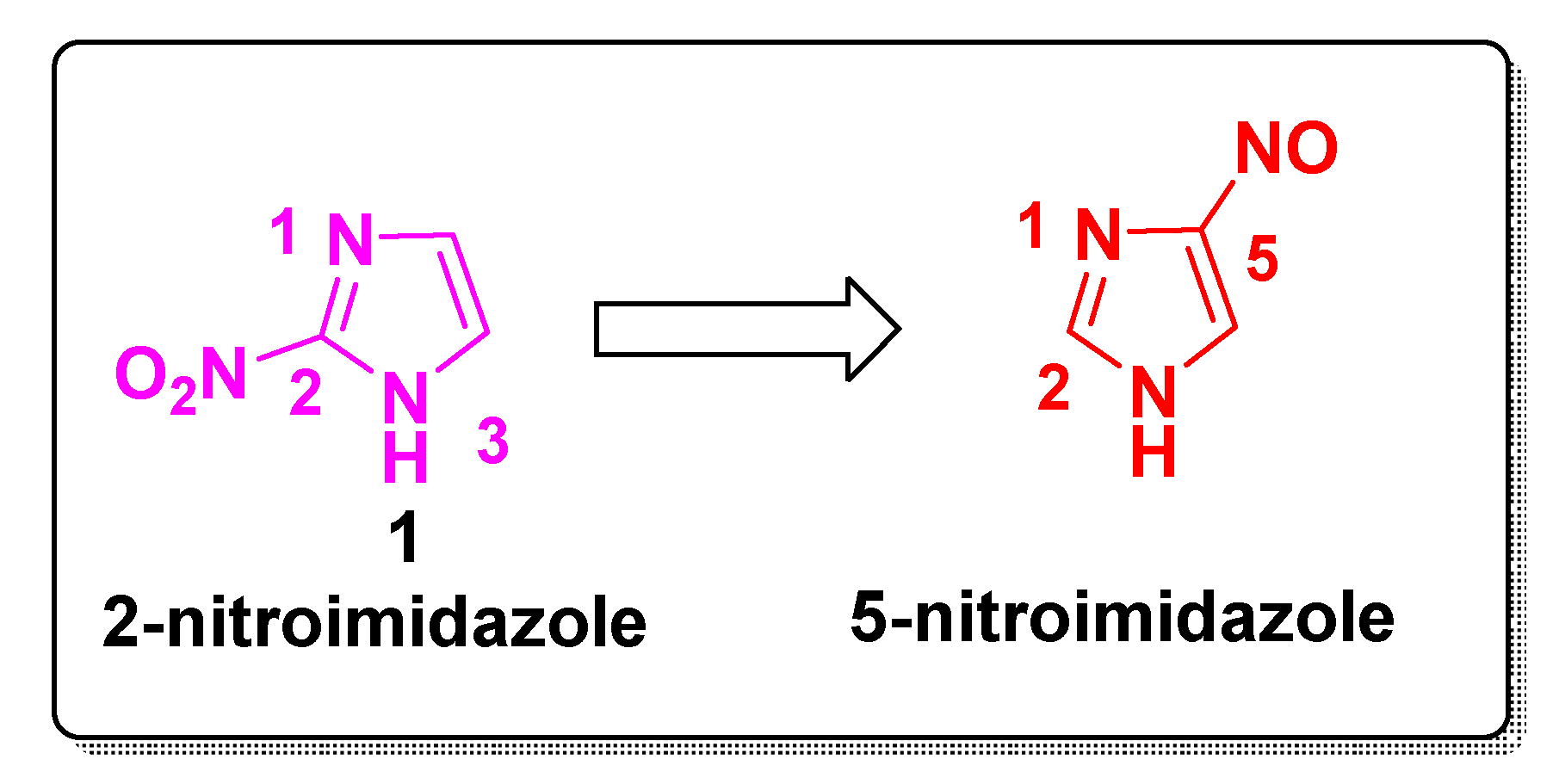
Mechanism of Action of Nitroimidazole Derivatives at a Glance
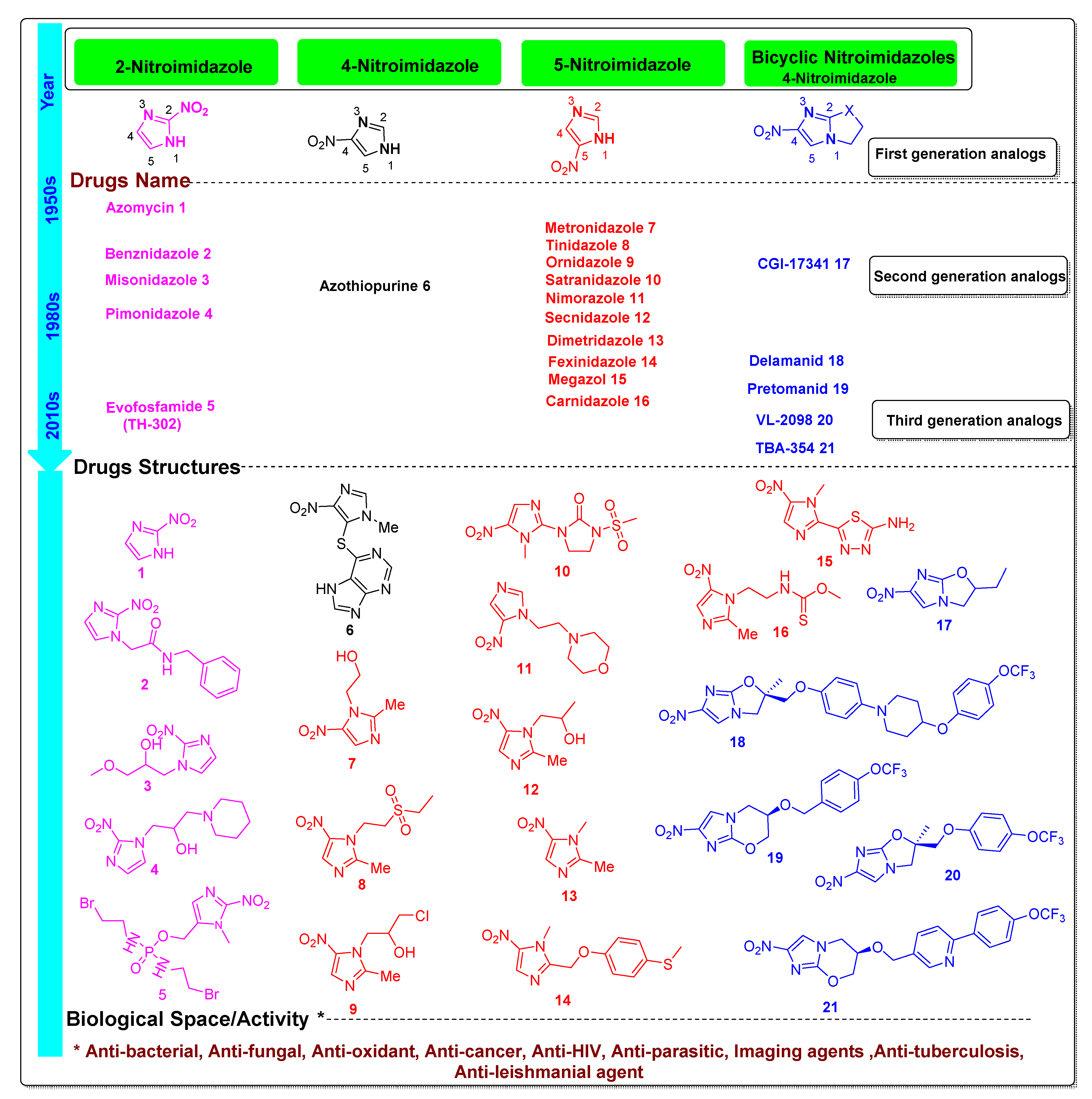
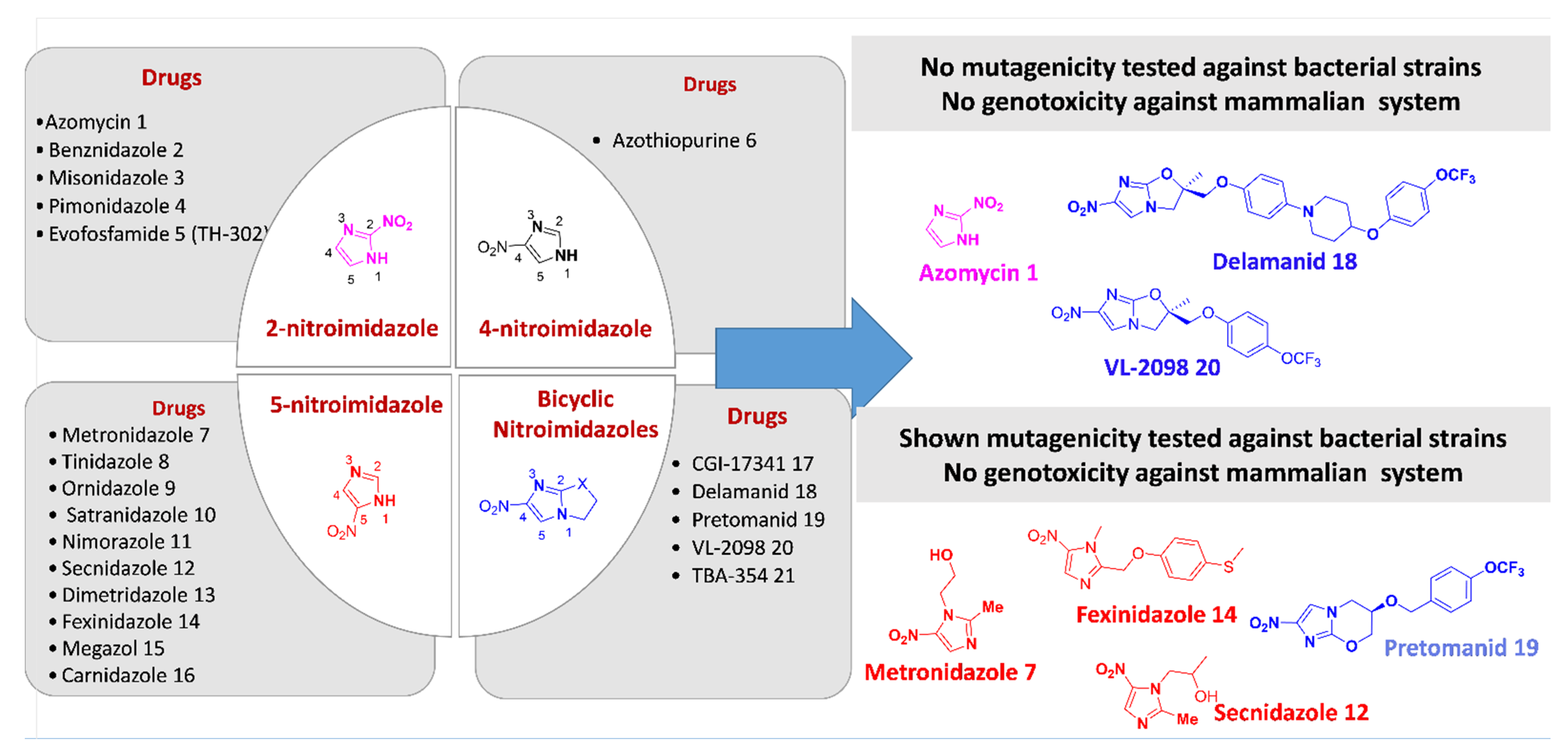
2. Activity Profile and Synthetic Pathways Developed to Construct Functionalized Nitroimidazole Derivatives
2.1. Functionalized 2-Nitroimidazole Scaffold
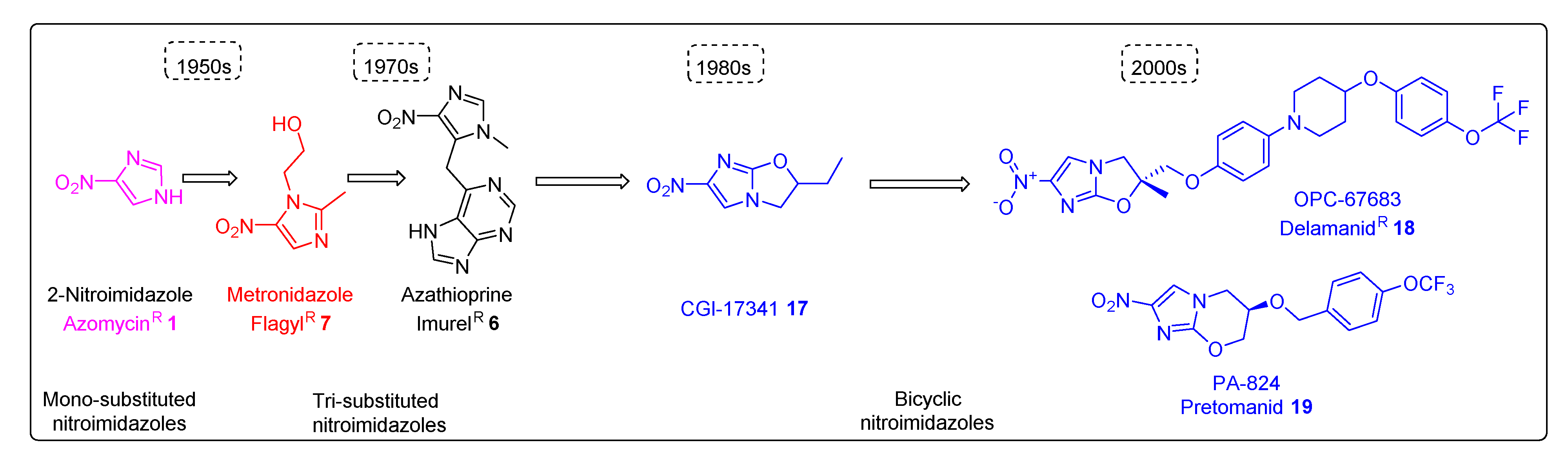
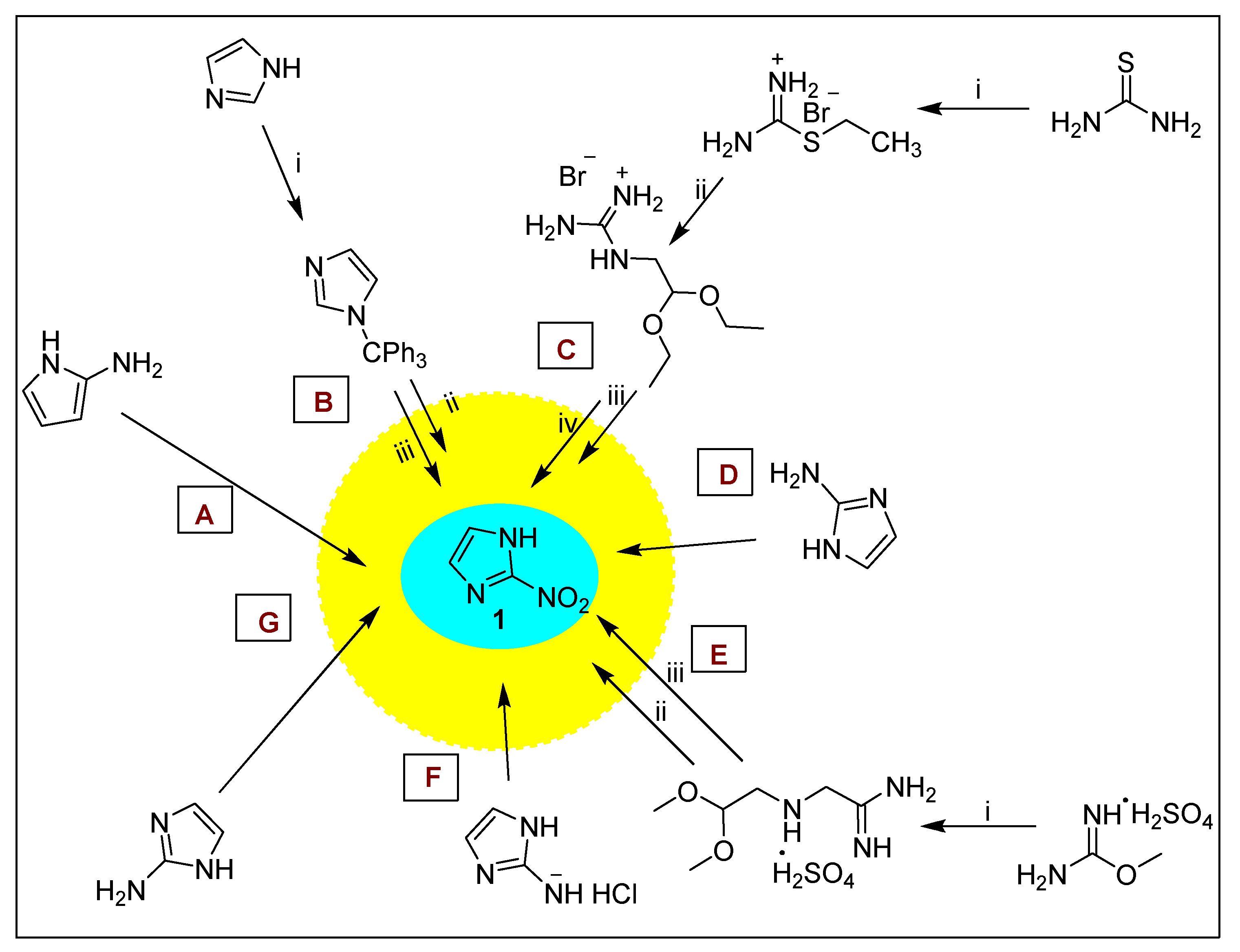
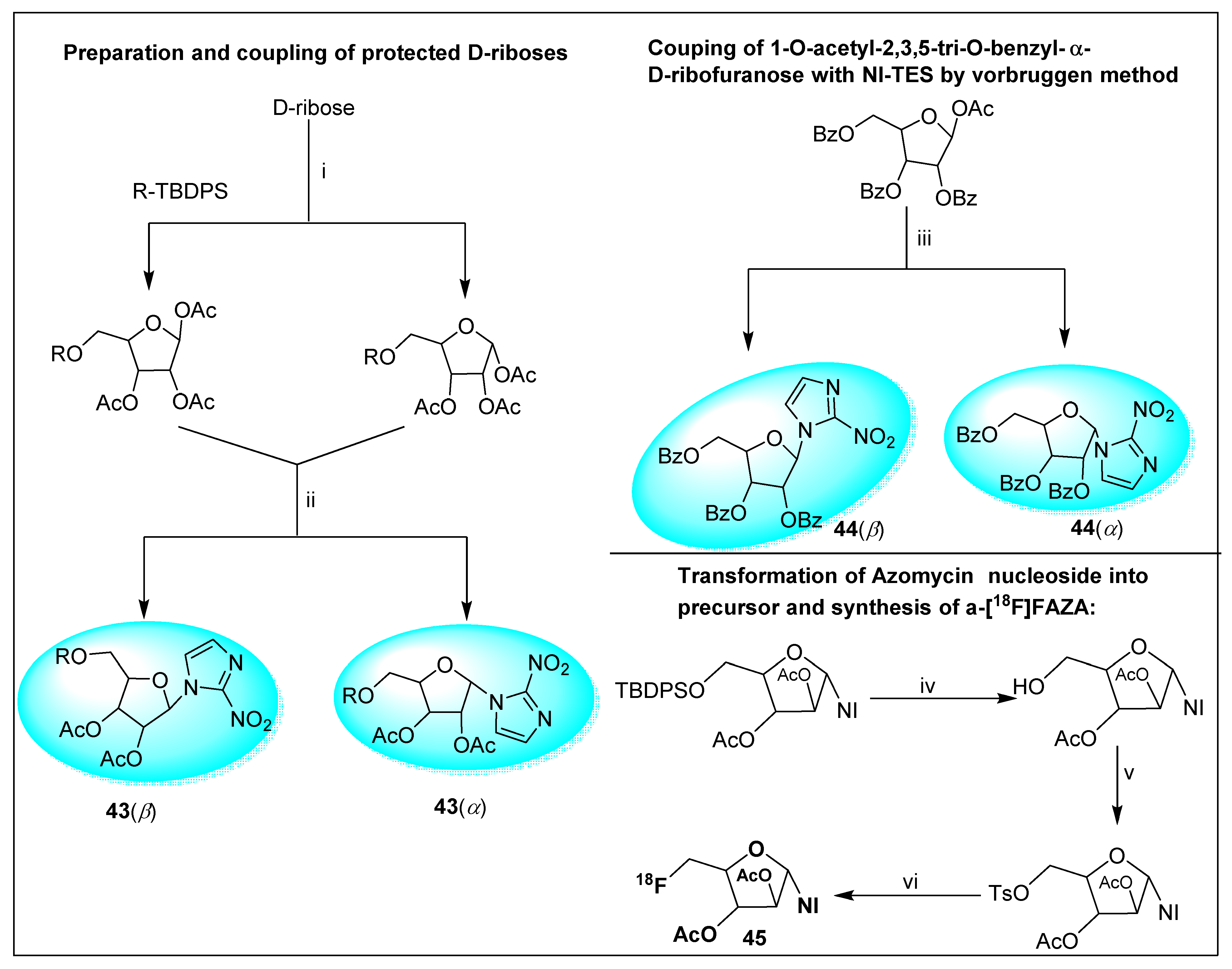
2.1.1. Azomycin 1
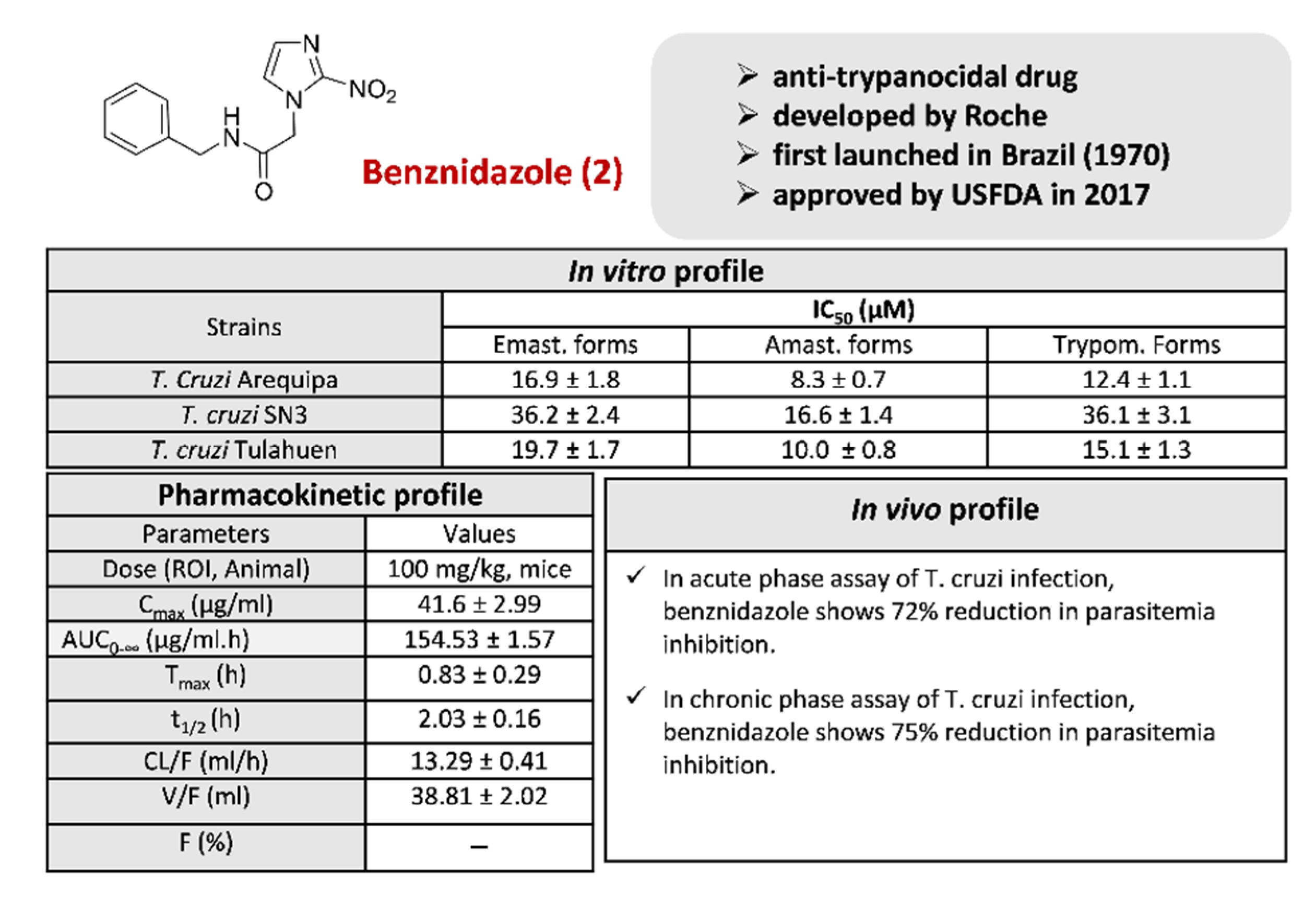
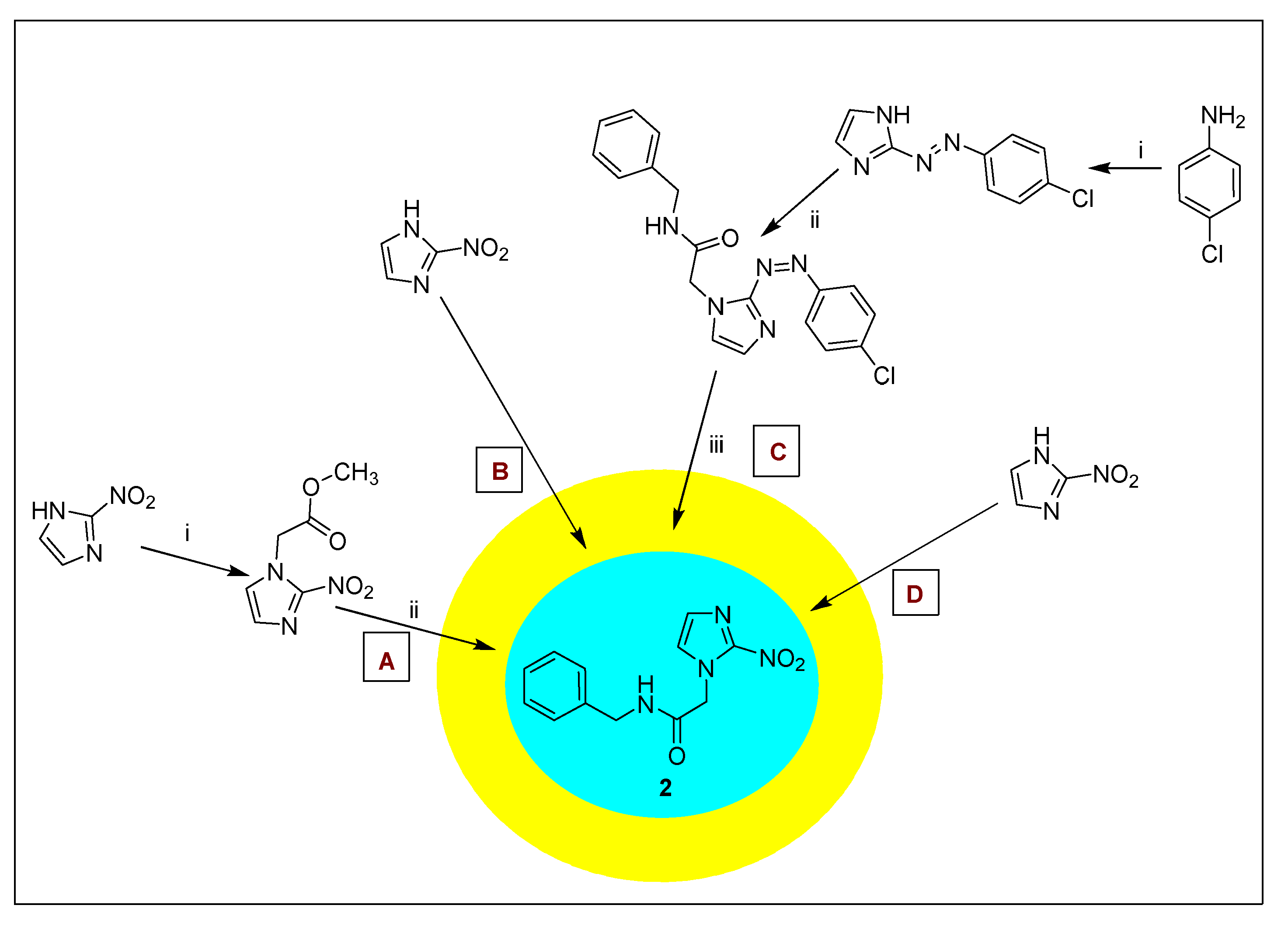
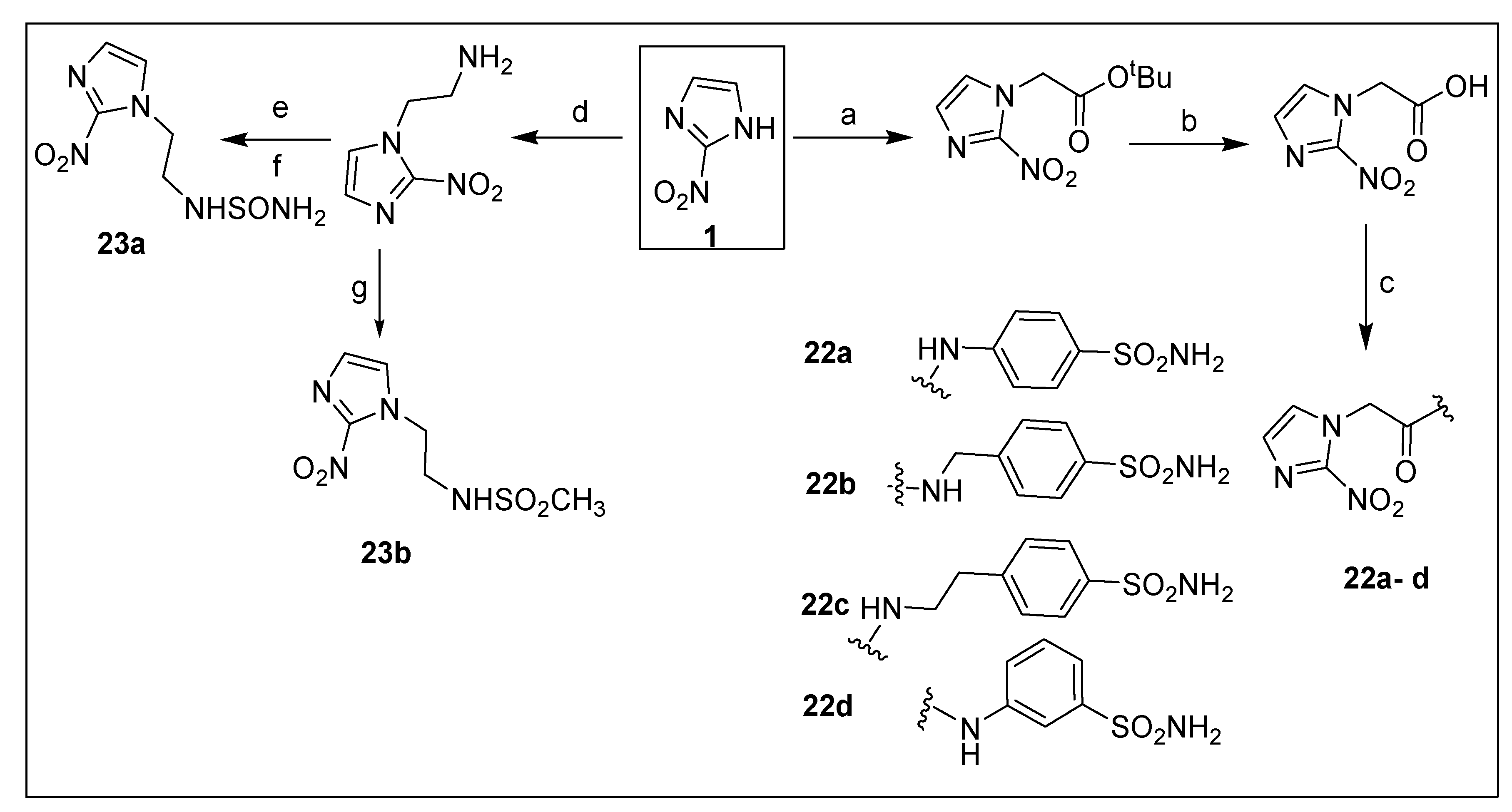
2.1.2. Benznidazole 2 and Its Derivatives
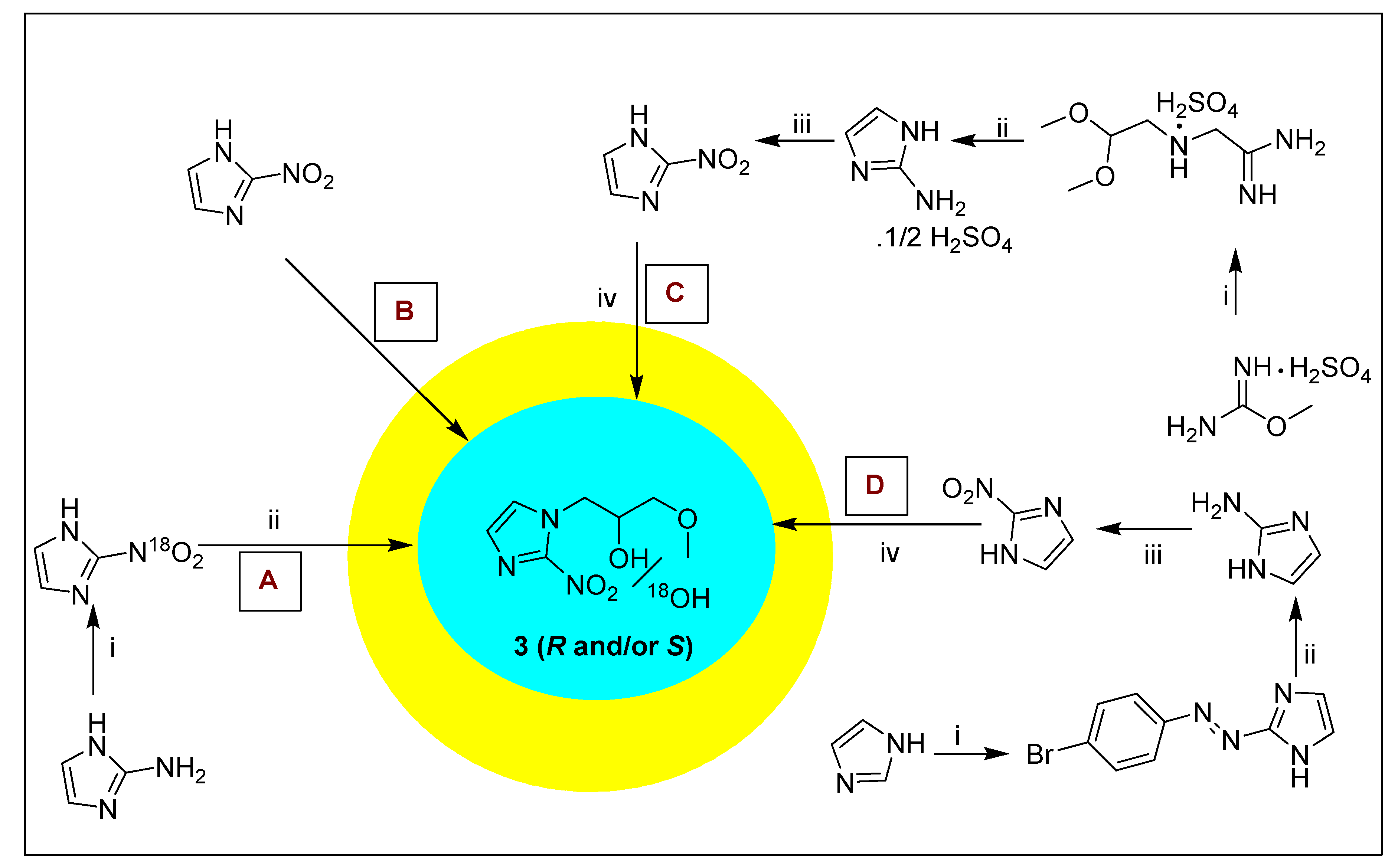
2.1.3. Misonidazole 3 and Its Derivatives
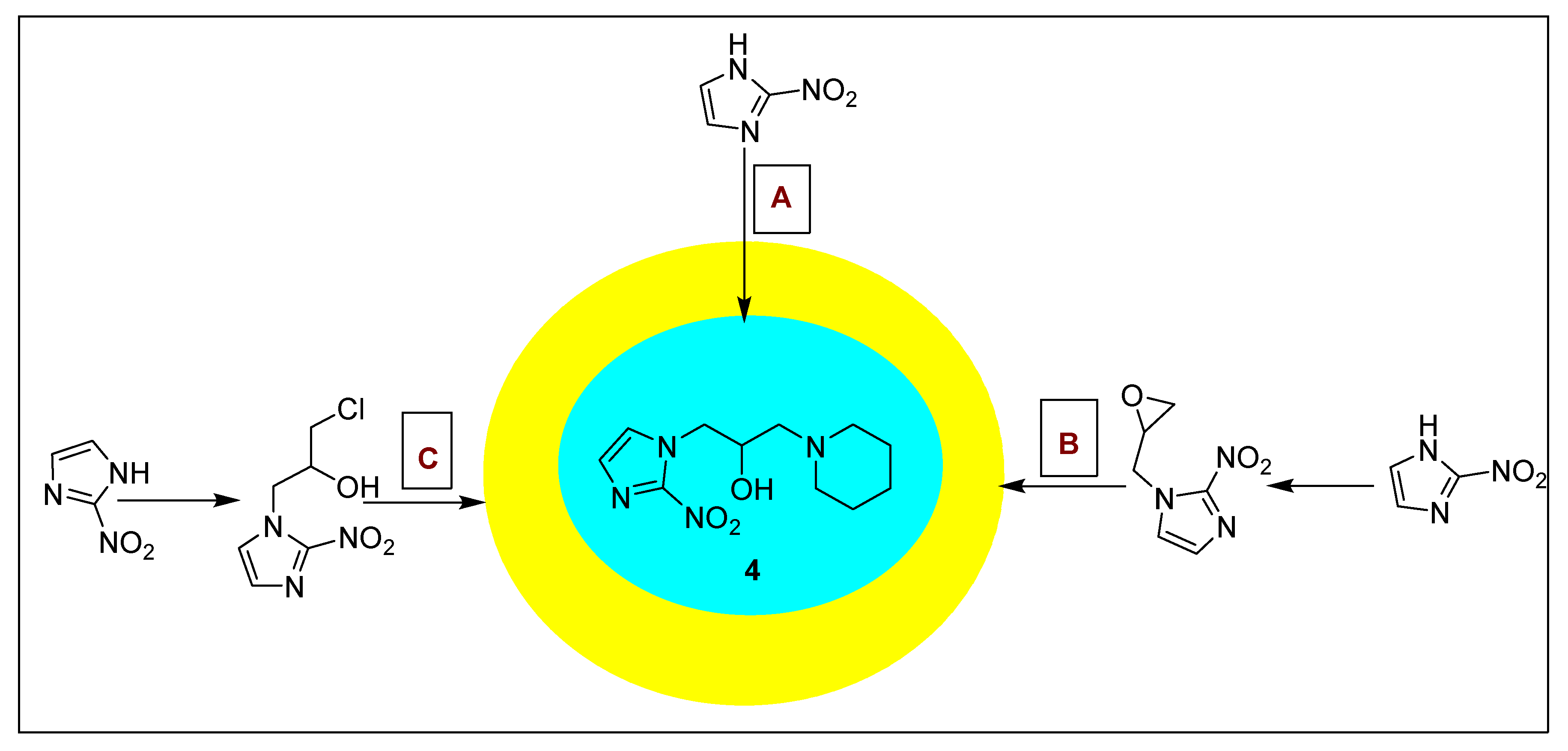
2.1.4. Pimonidazole 4 and Its Derivatives
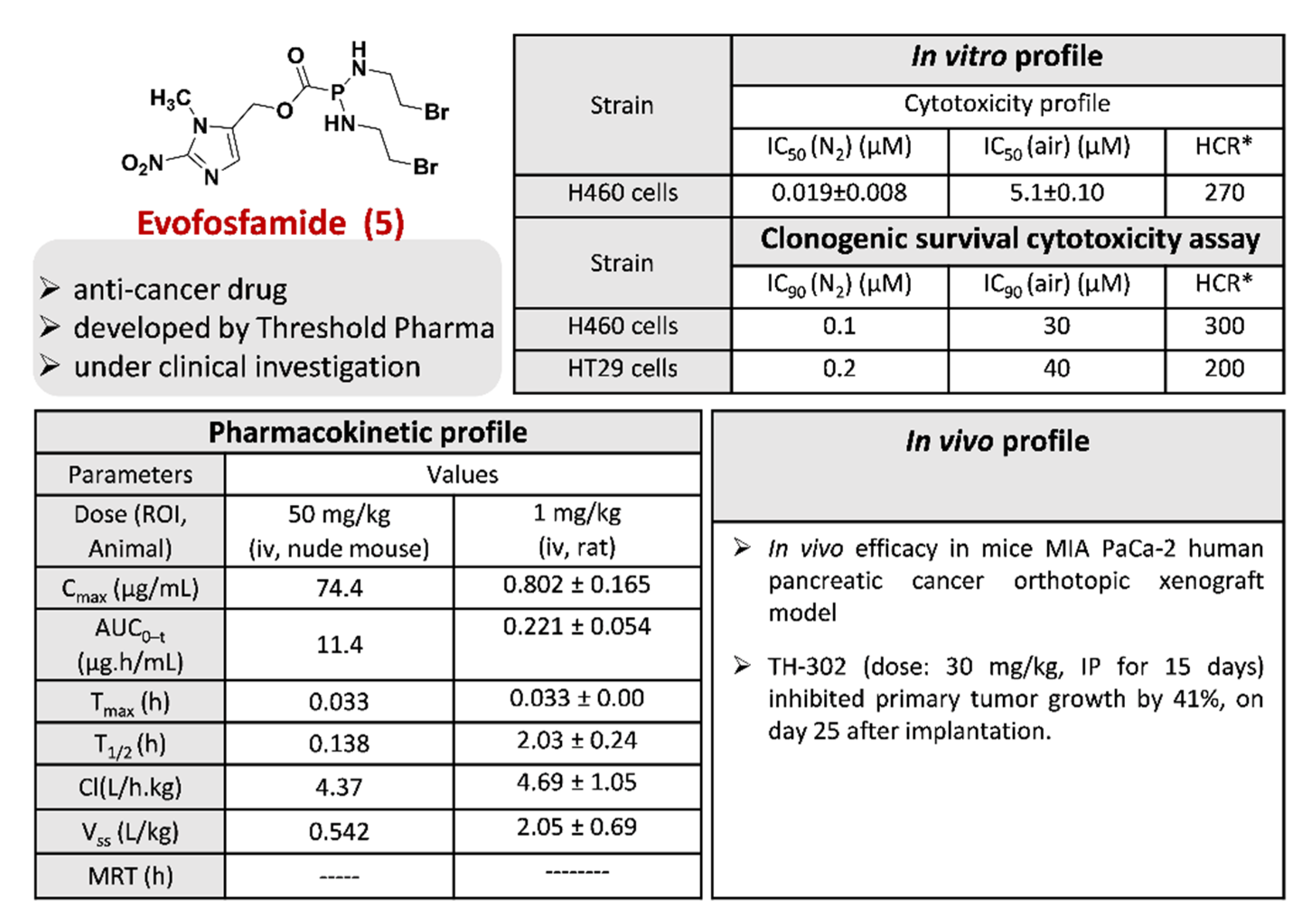
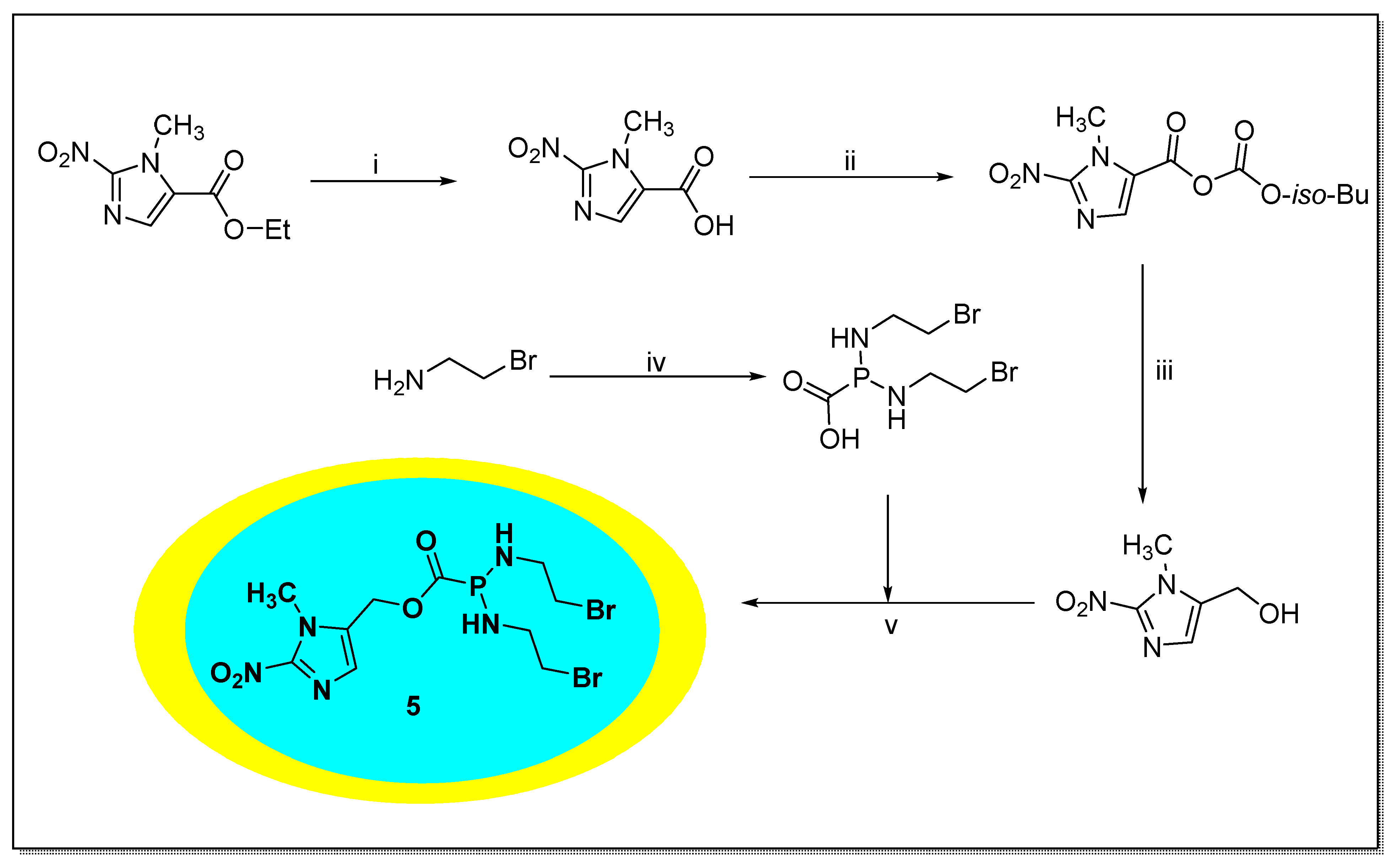
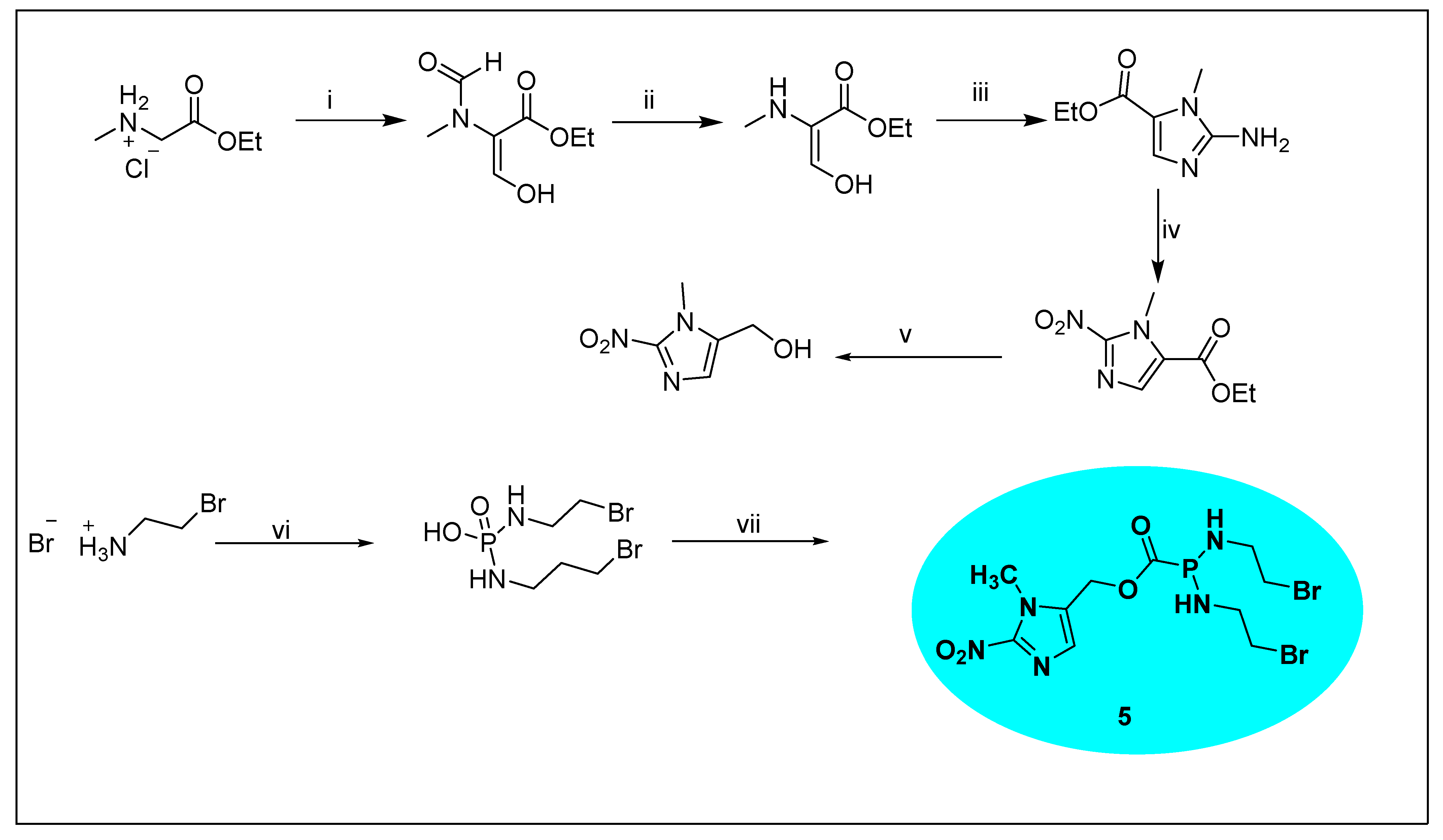
2.1.5. Evofosfamide 5
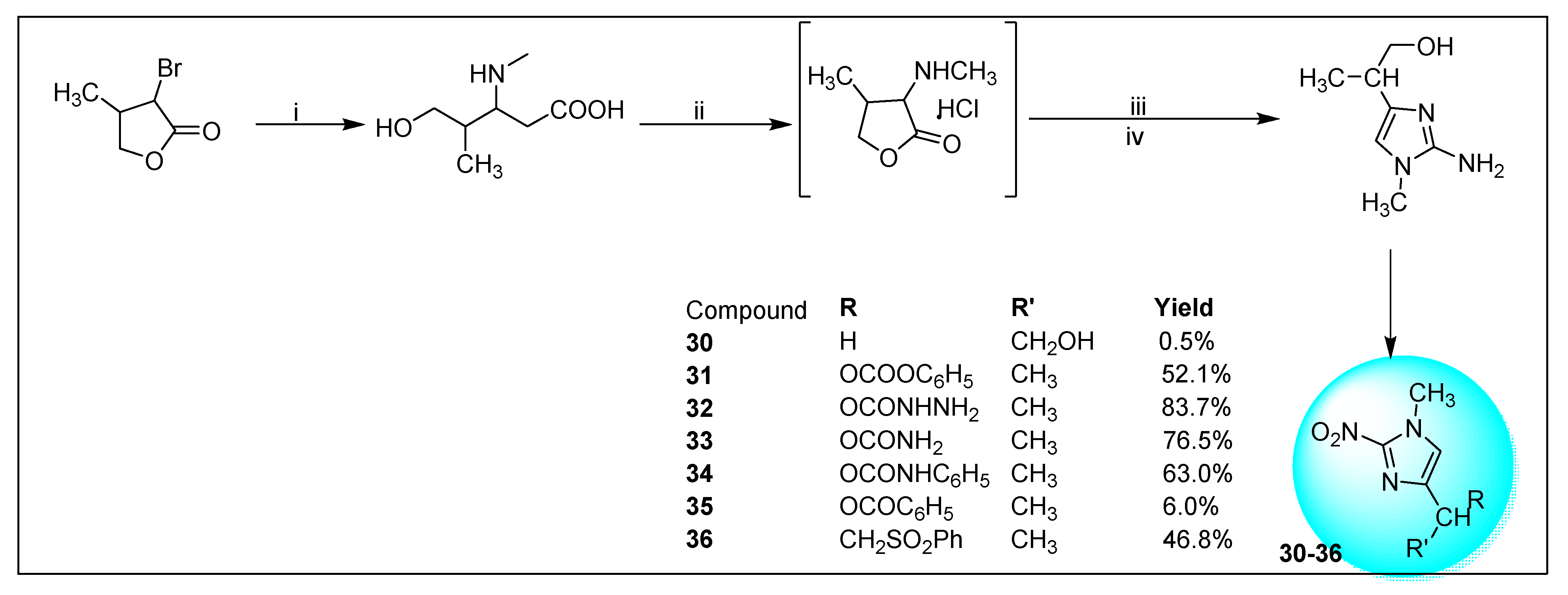
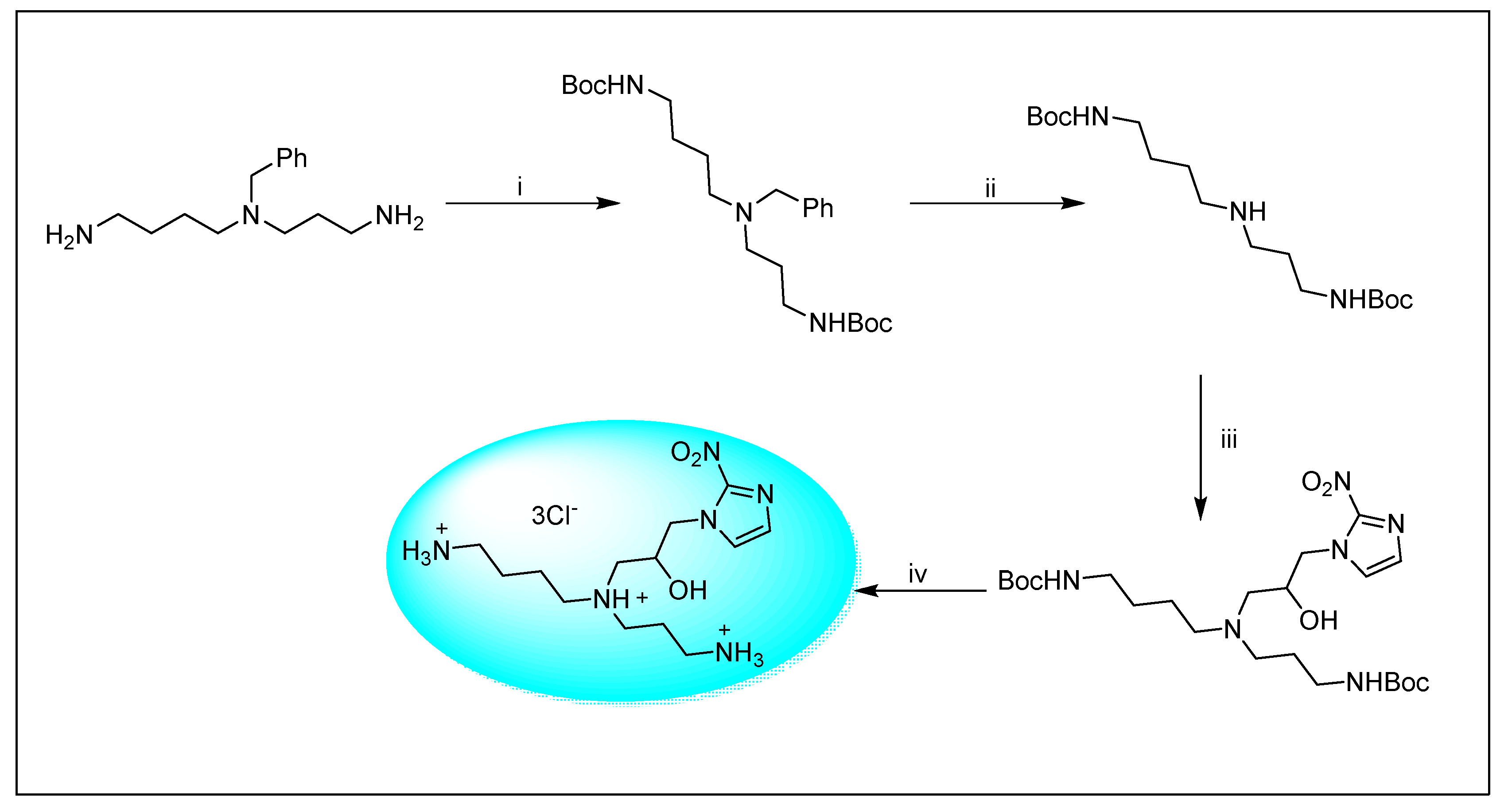
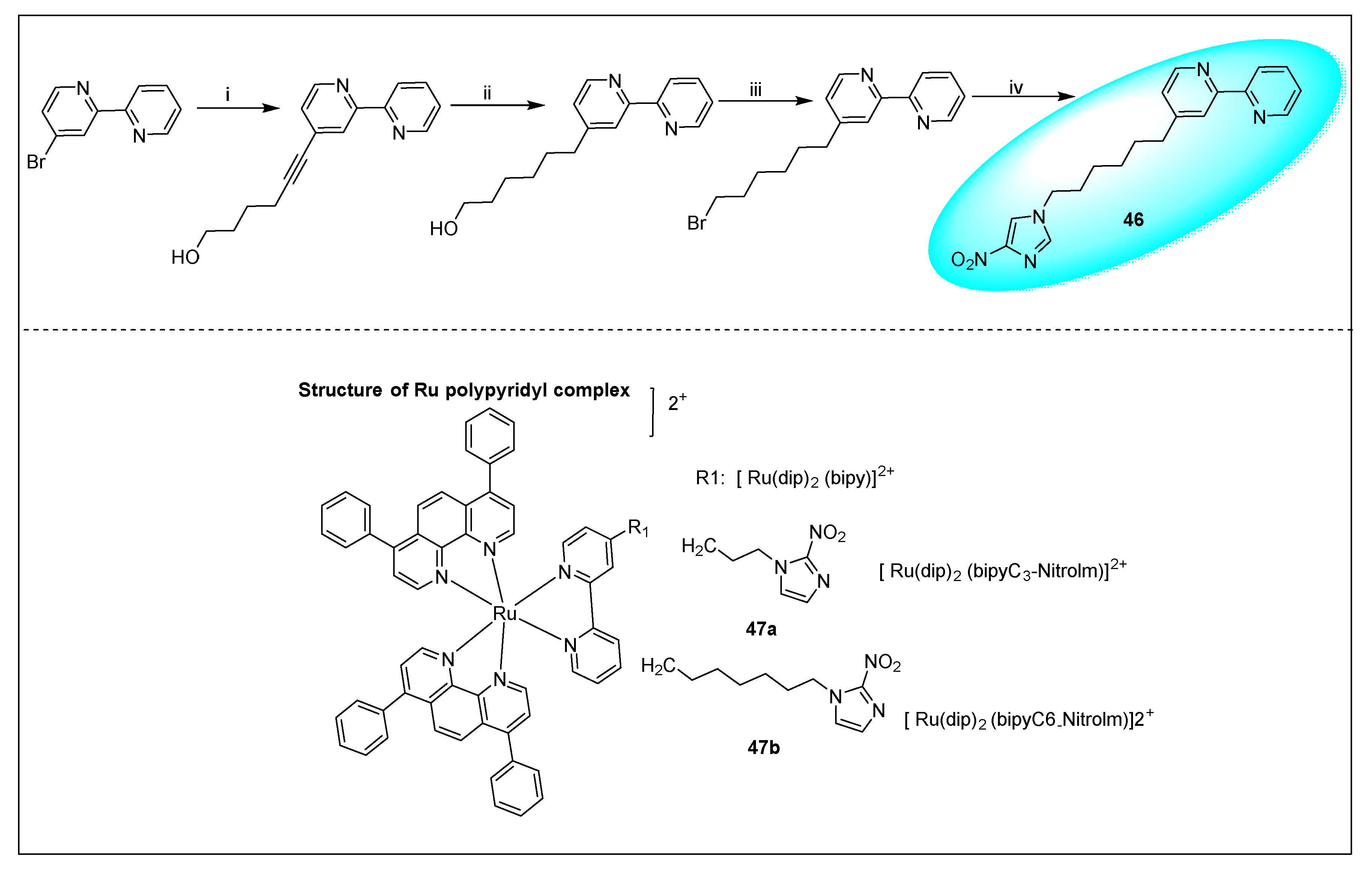
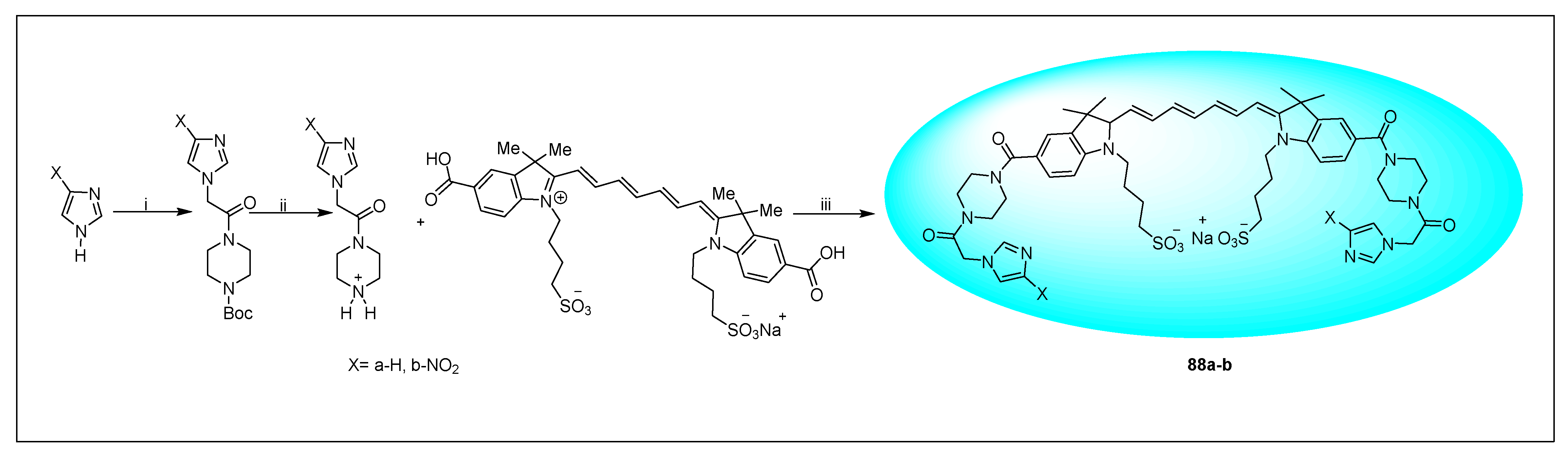
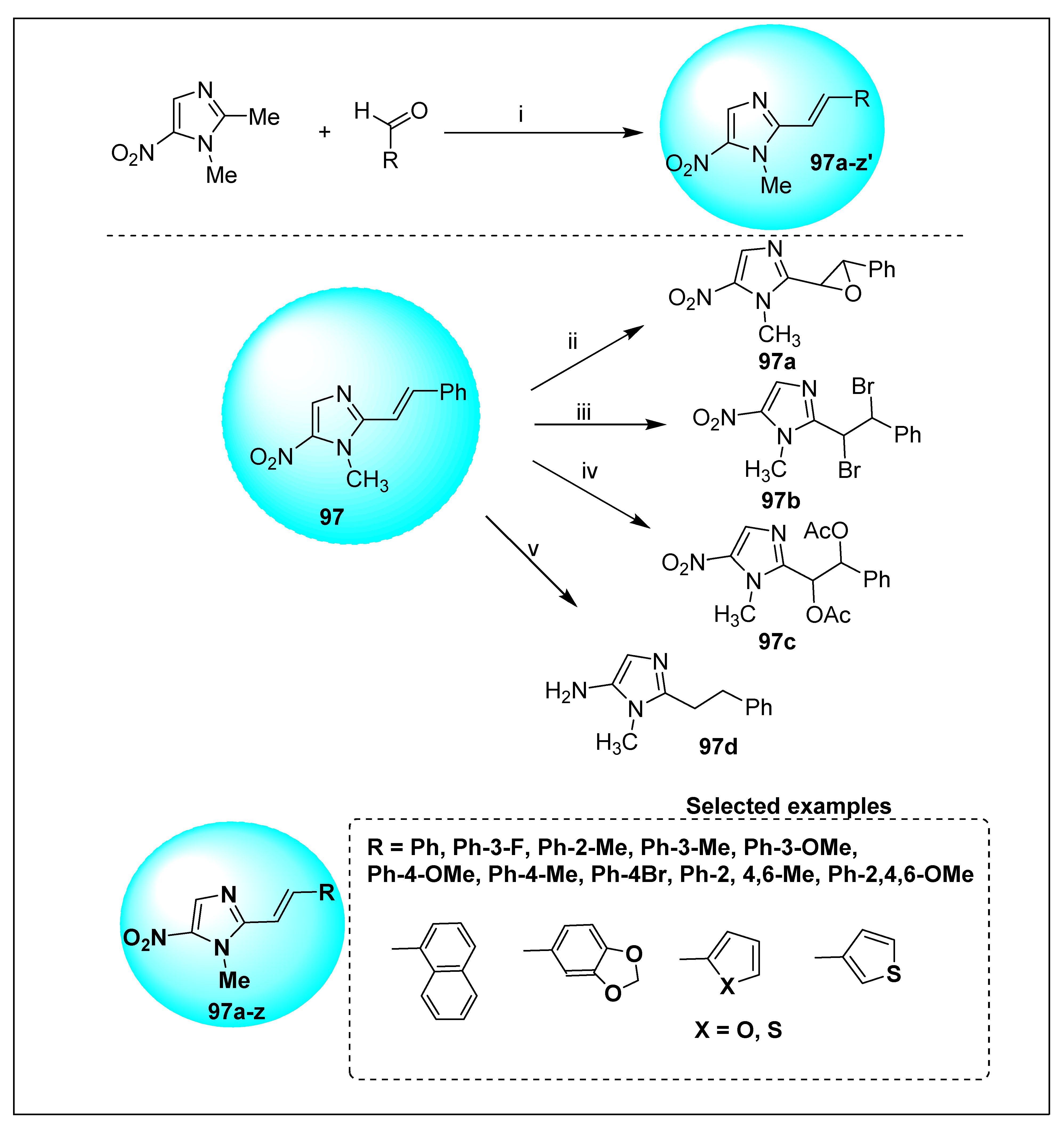
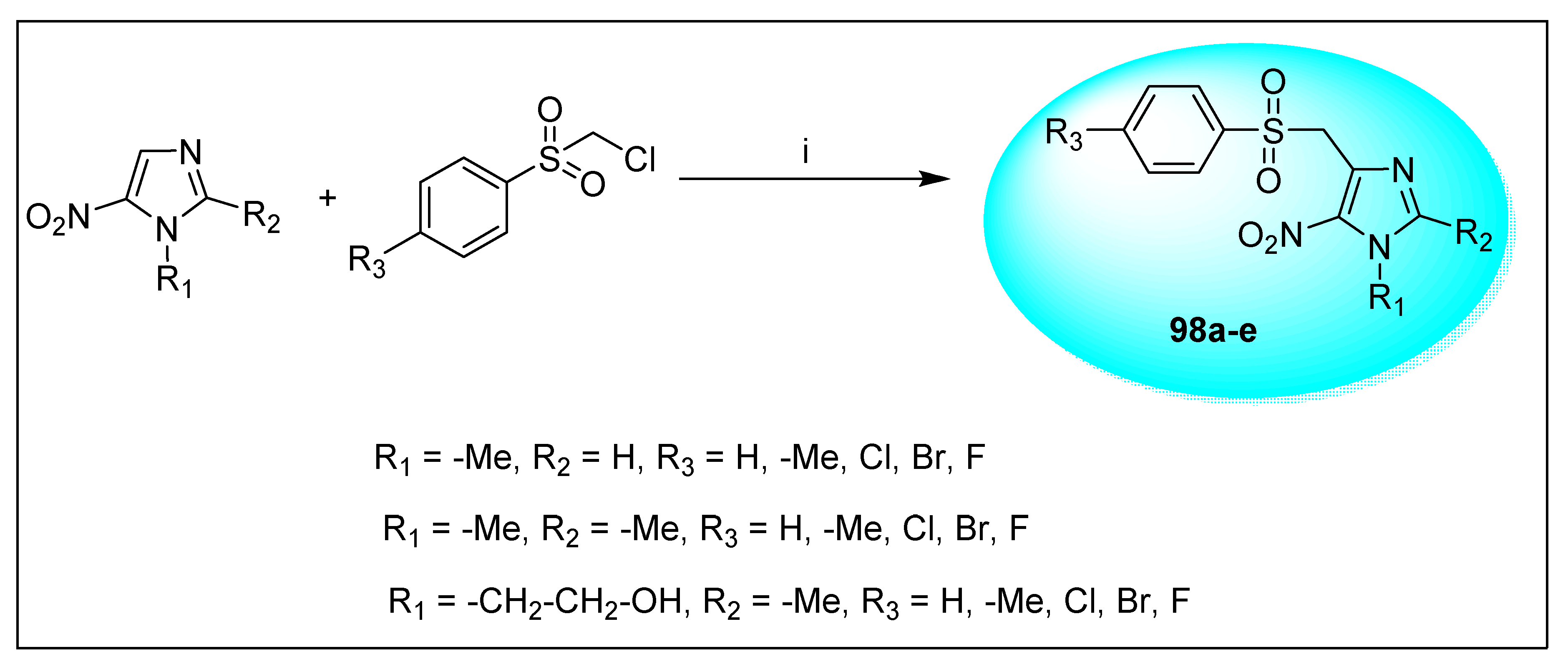
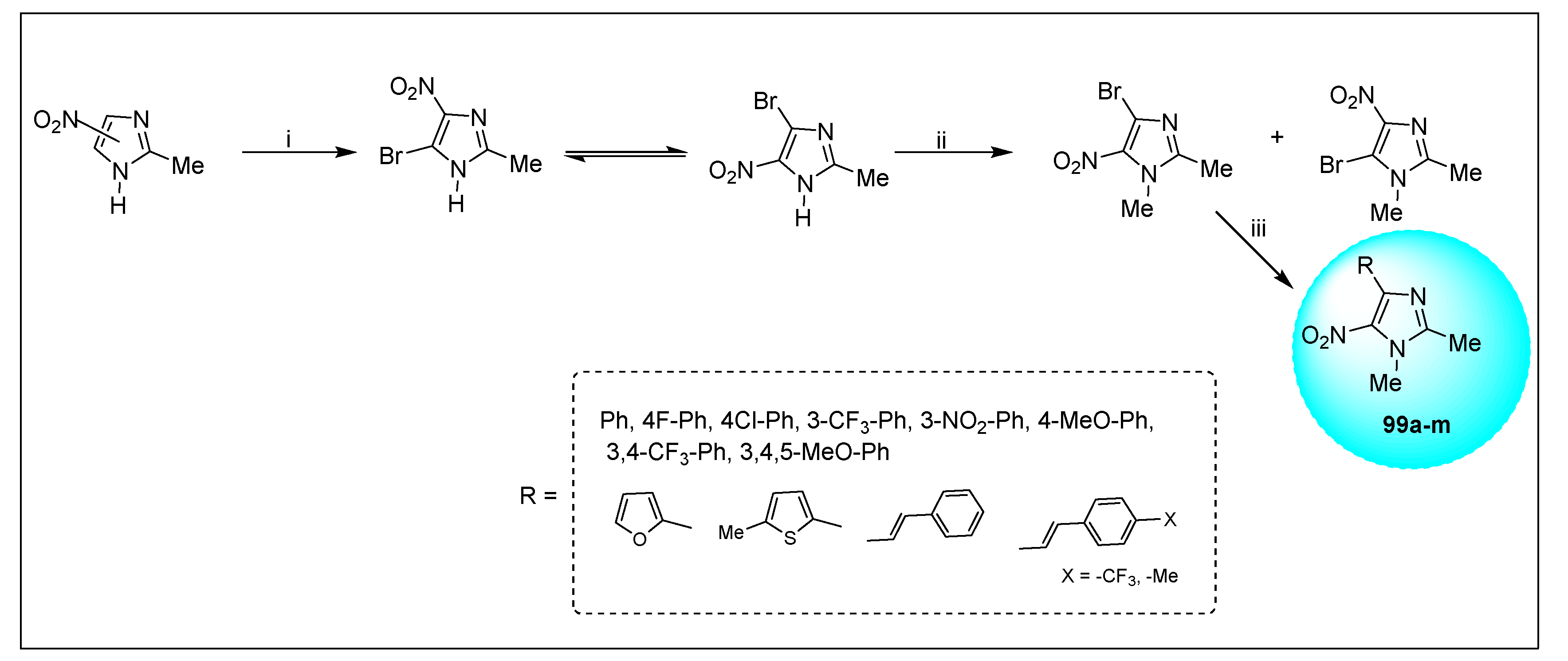
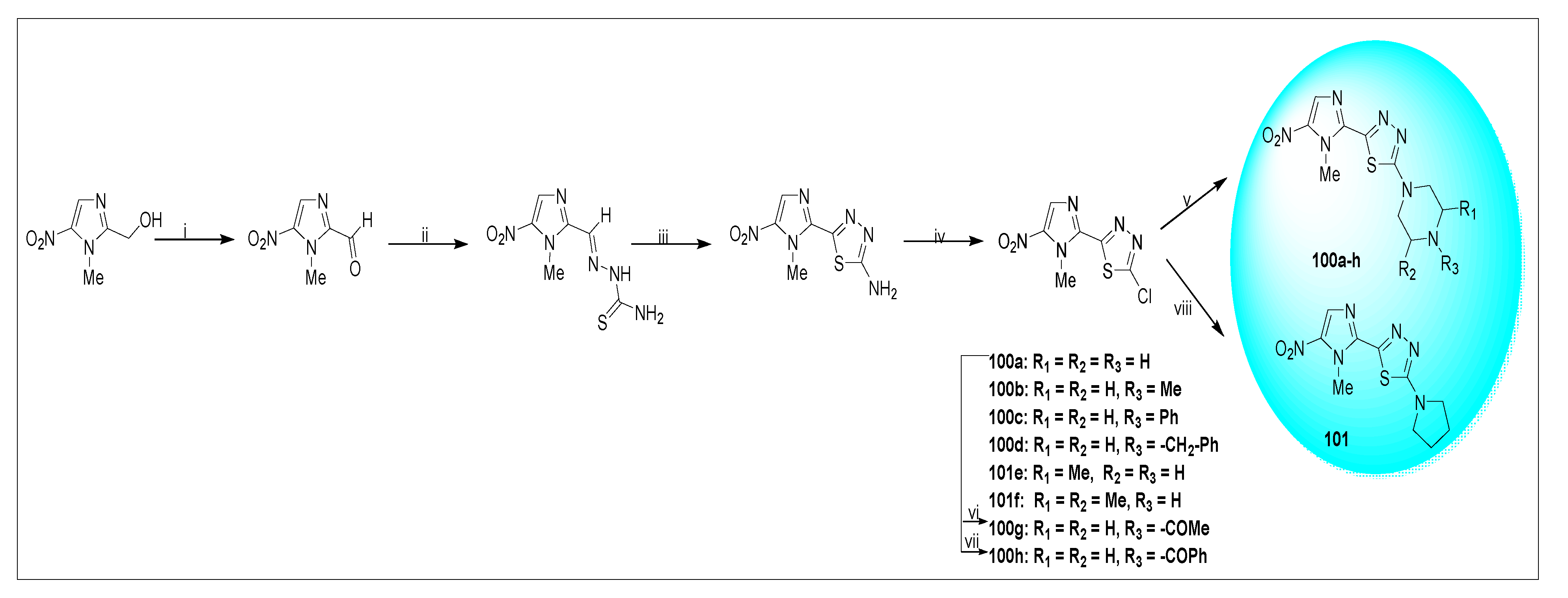
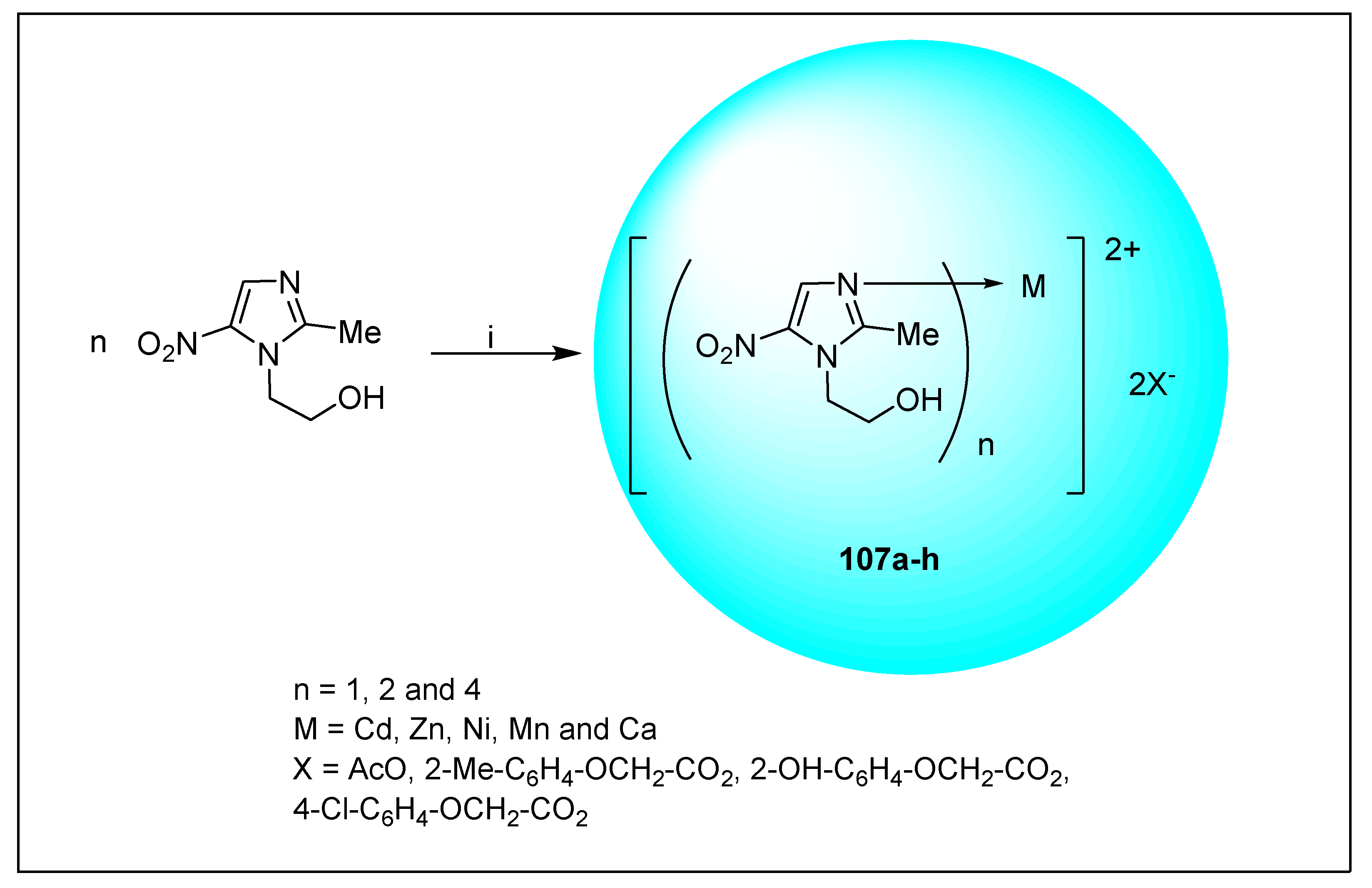
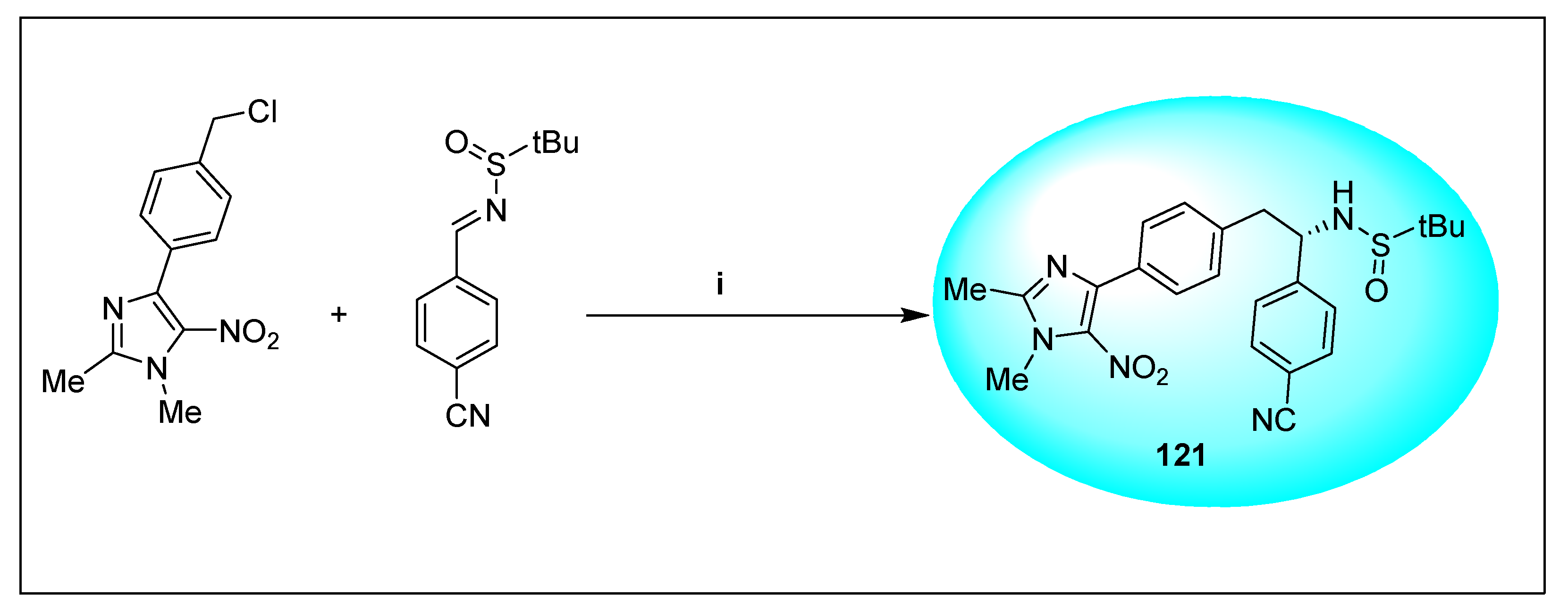
2.1.6. Miscellaneous 2-Nitroimidazole Derivatives
2.2. Functionalized 4-Nitroimidazole Scaffold
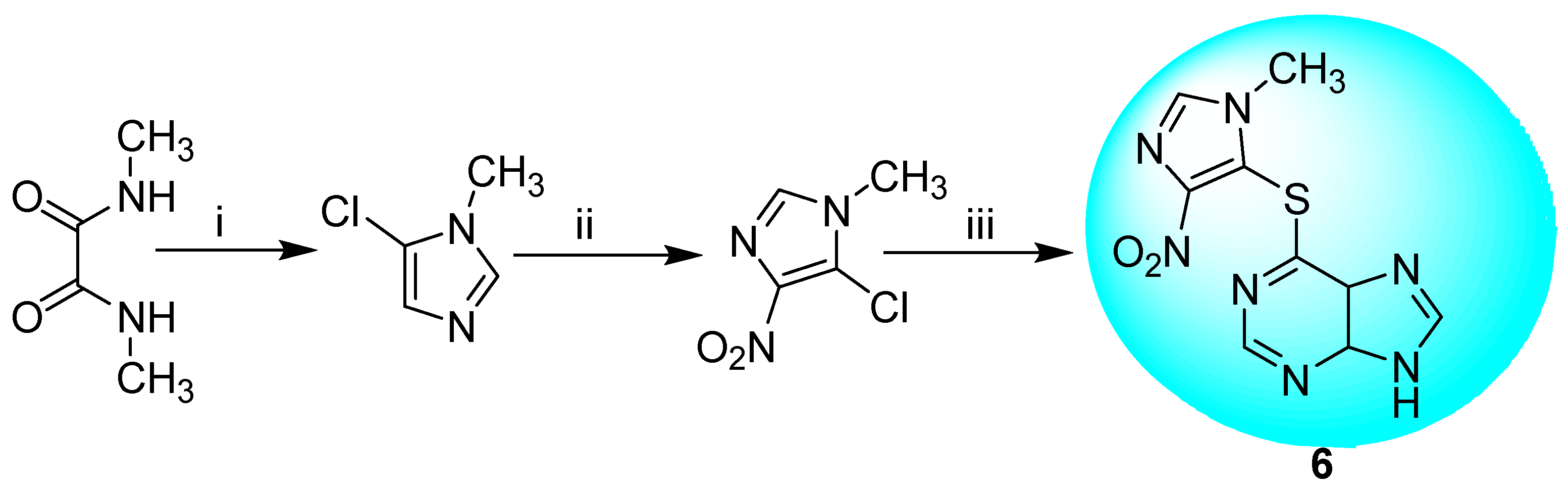
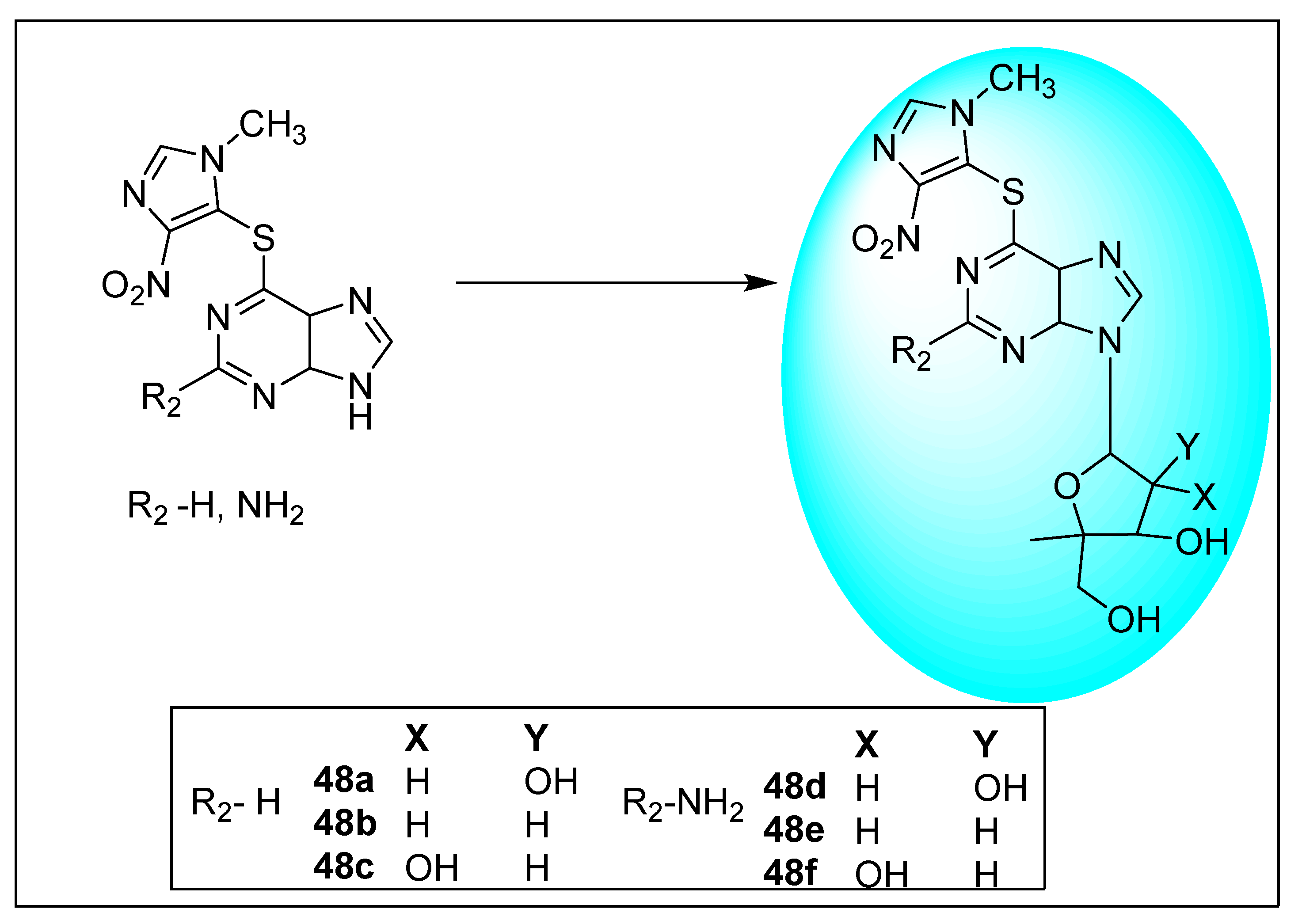
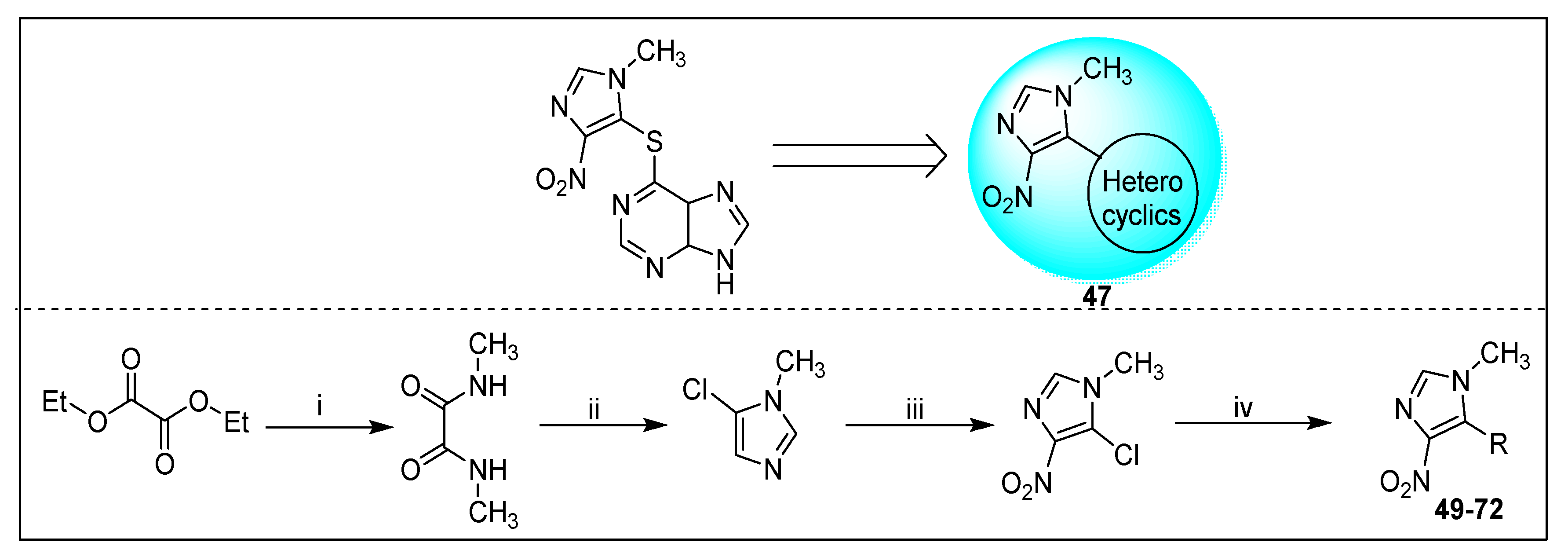
2.2.1. Azathioprine 6 and Its Derivatives
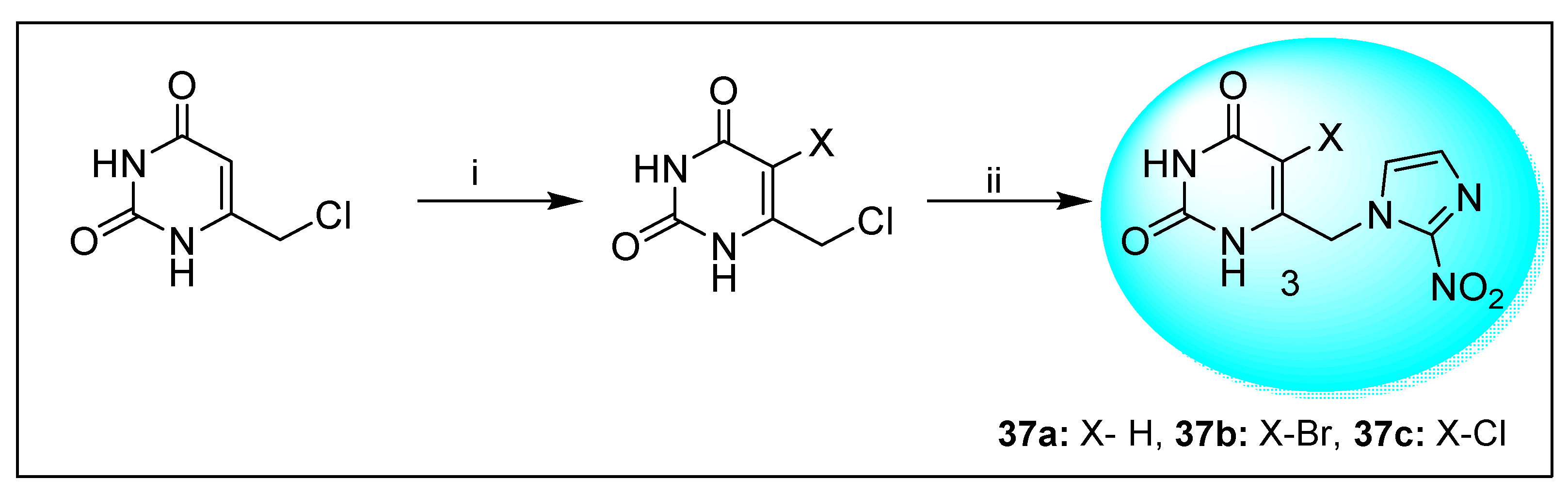
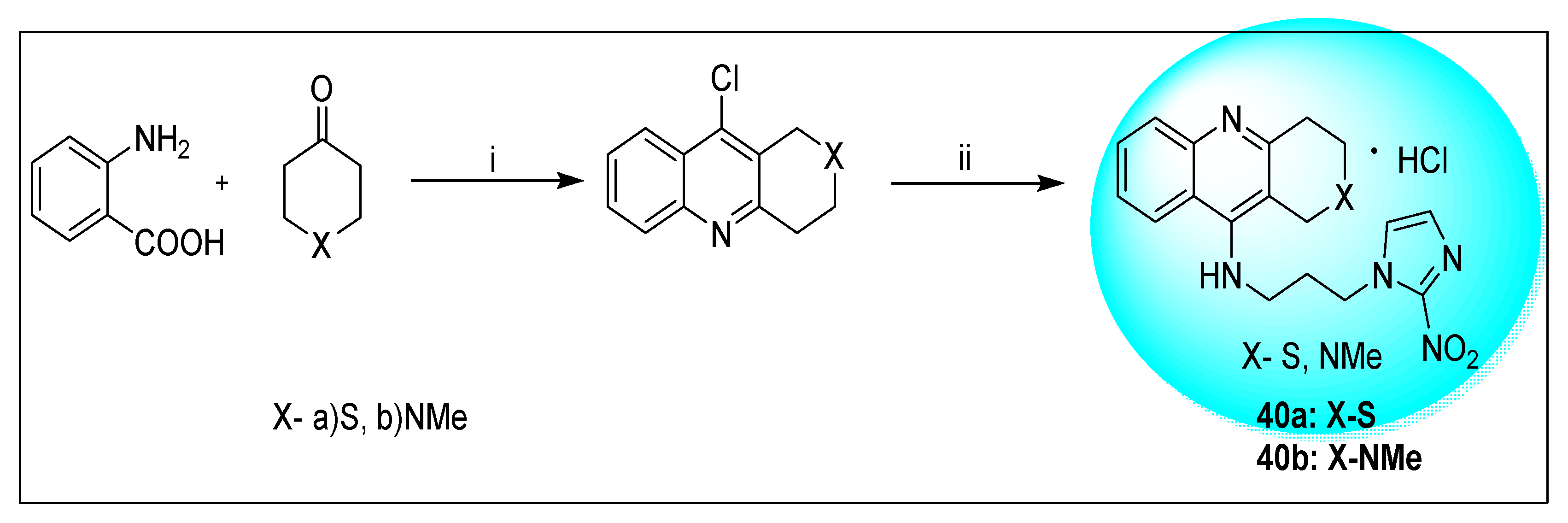
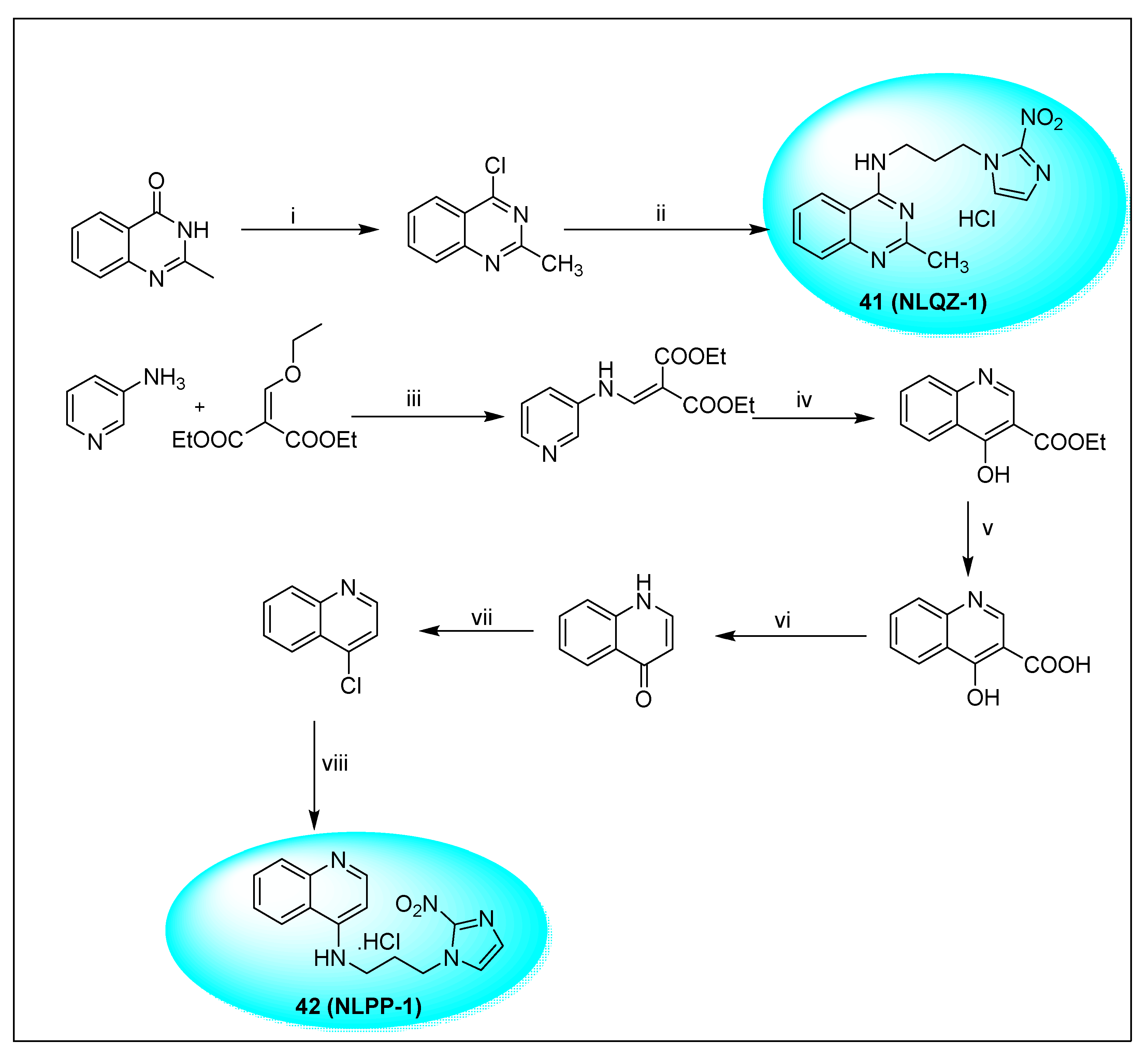
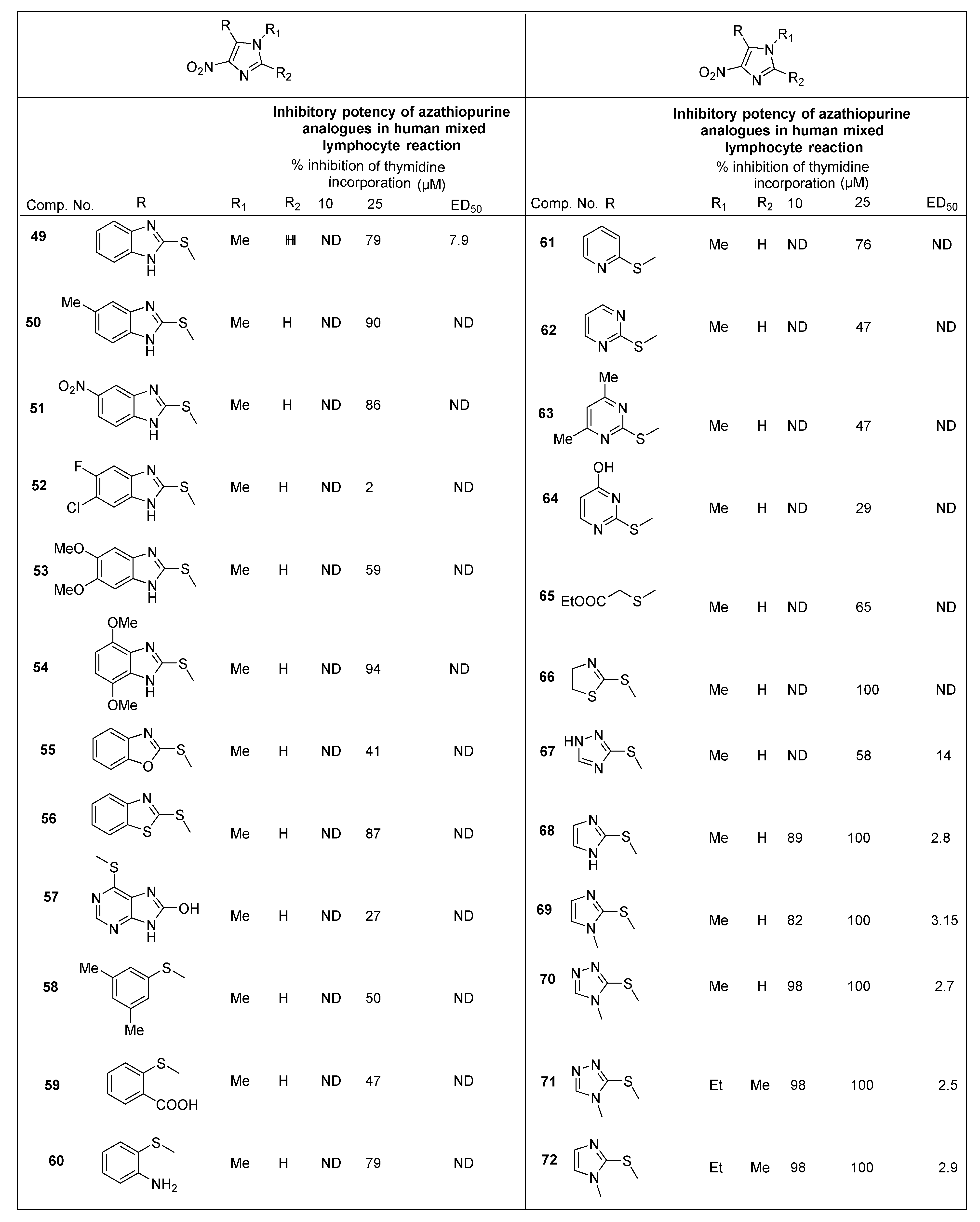
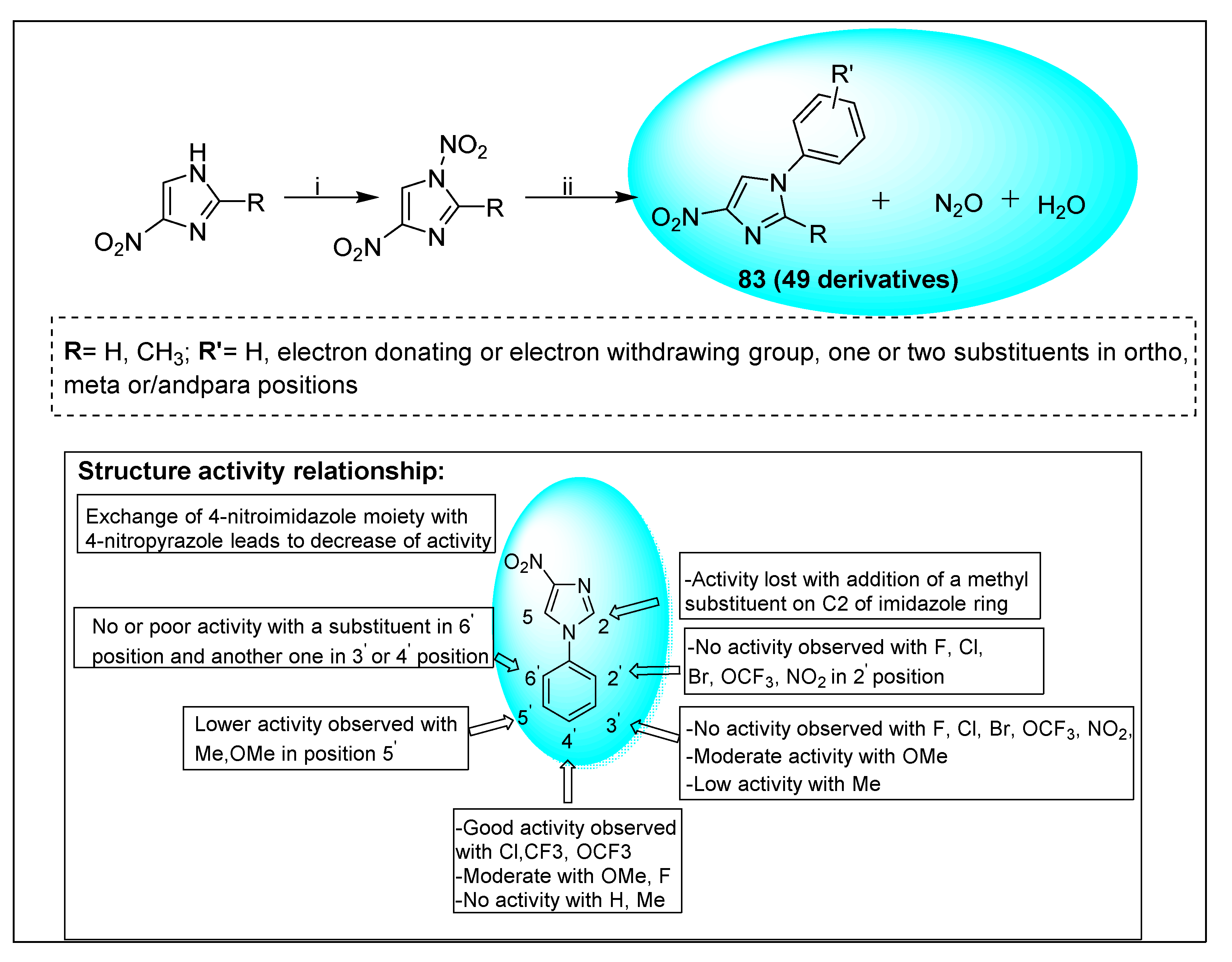
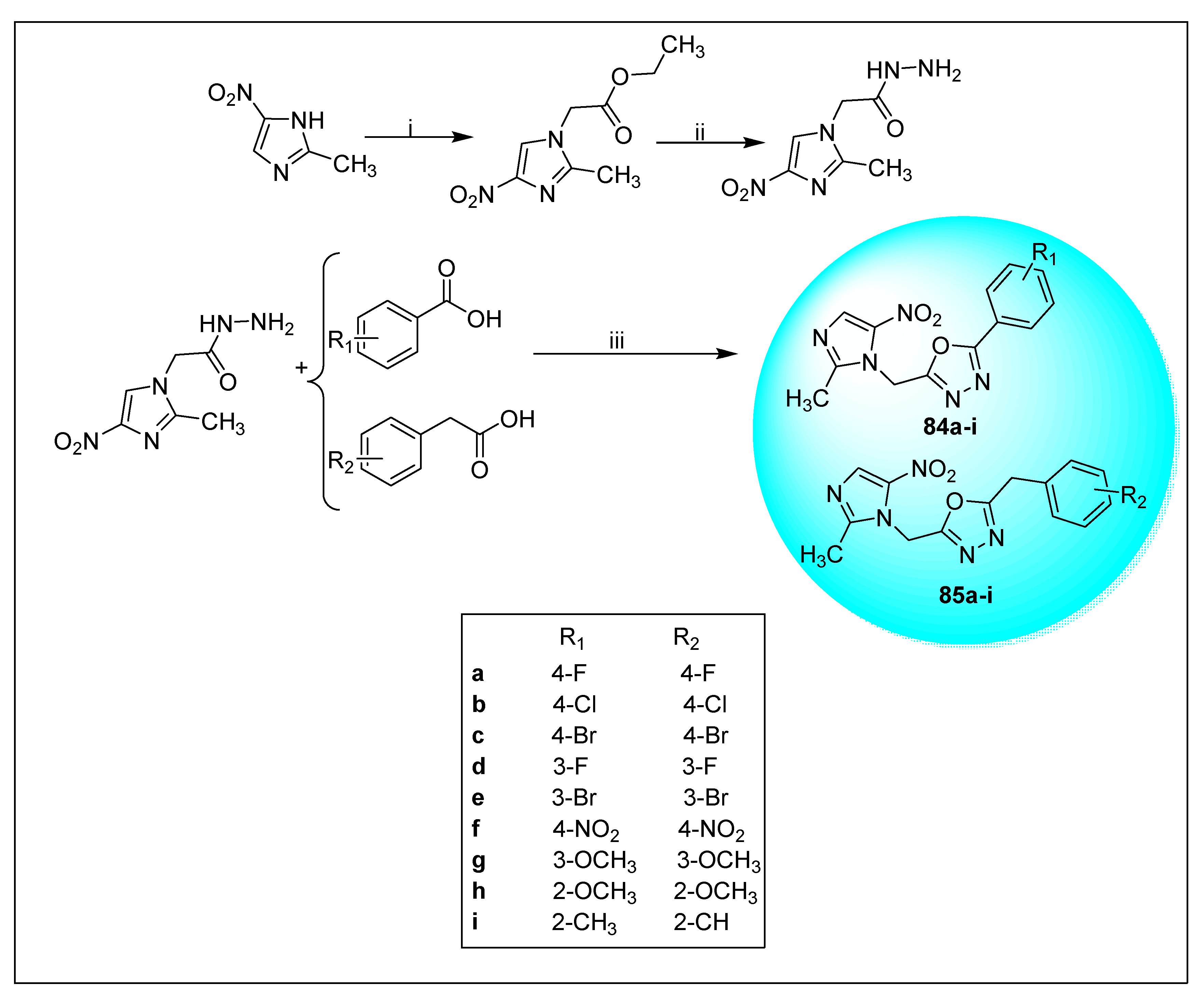
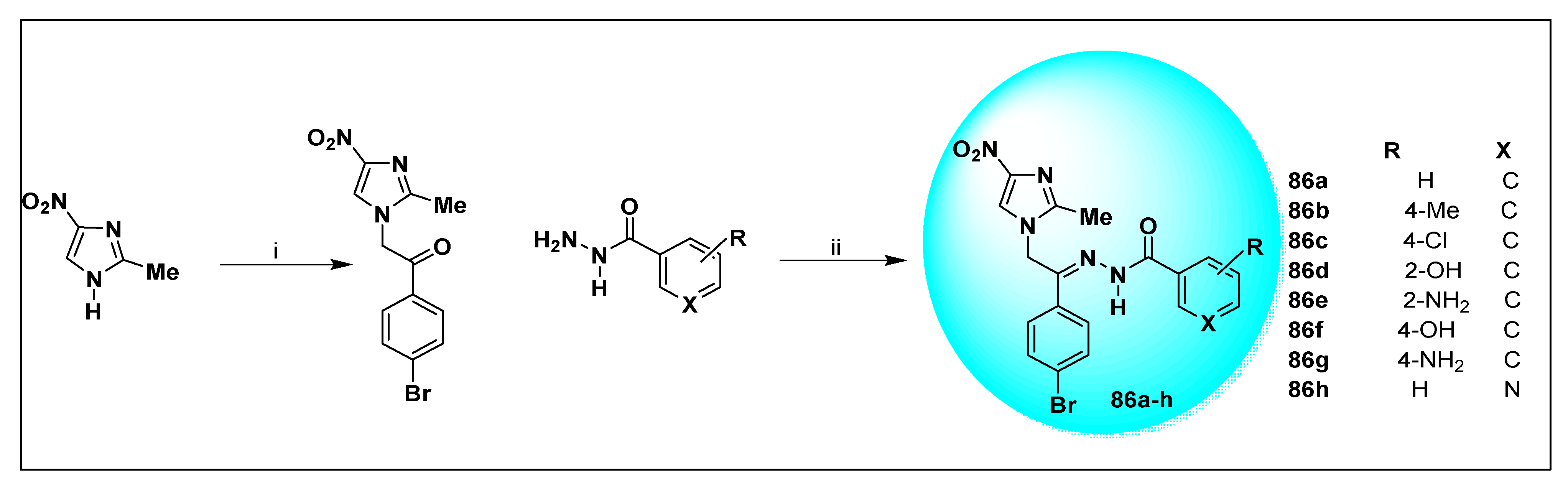
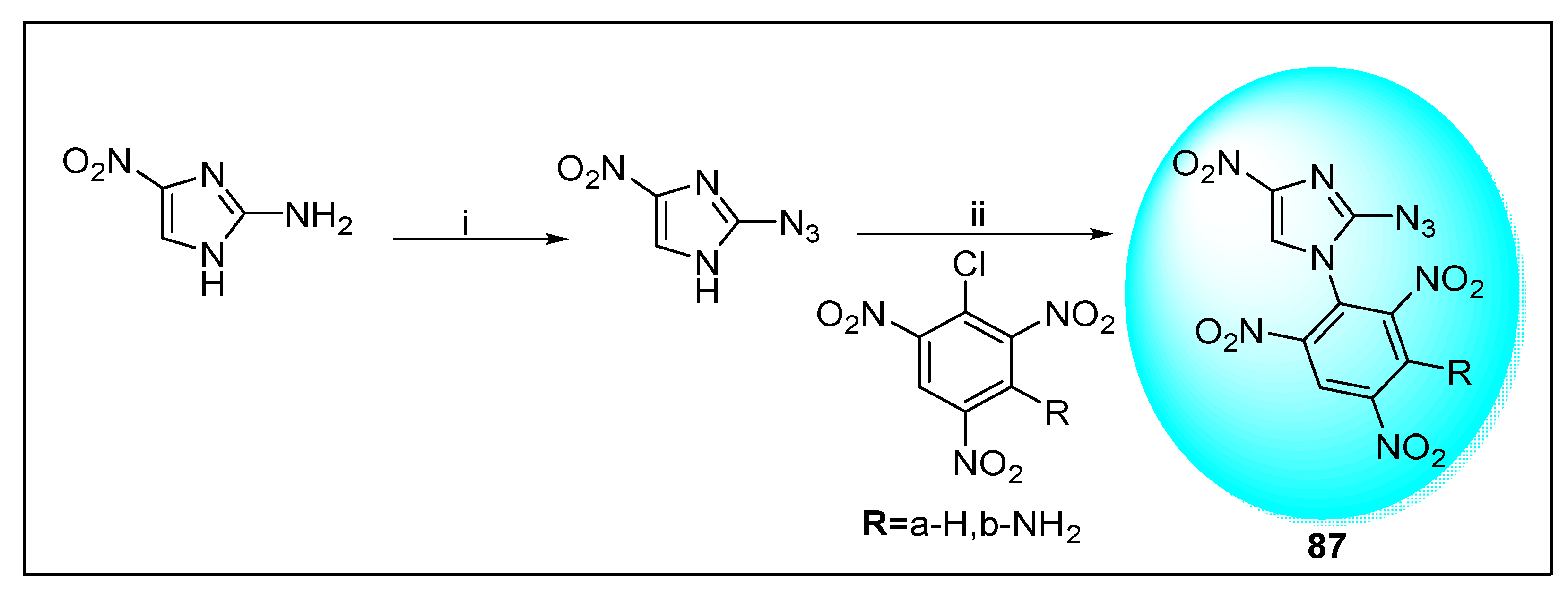
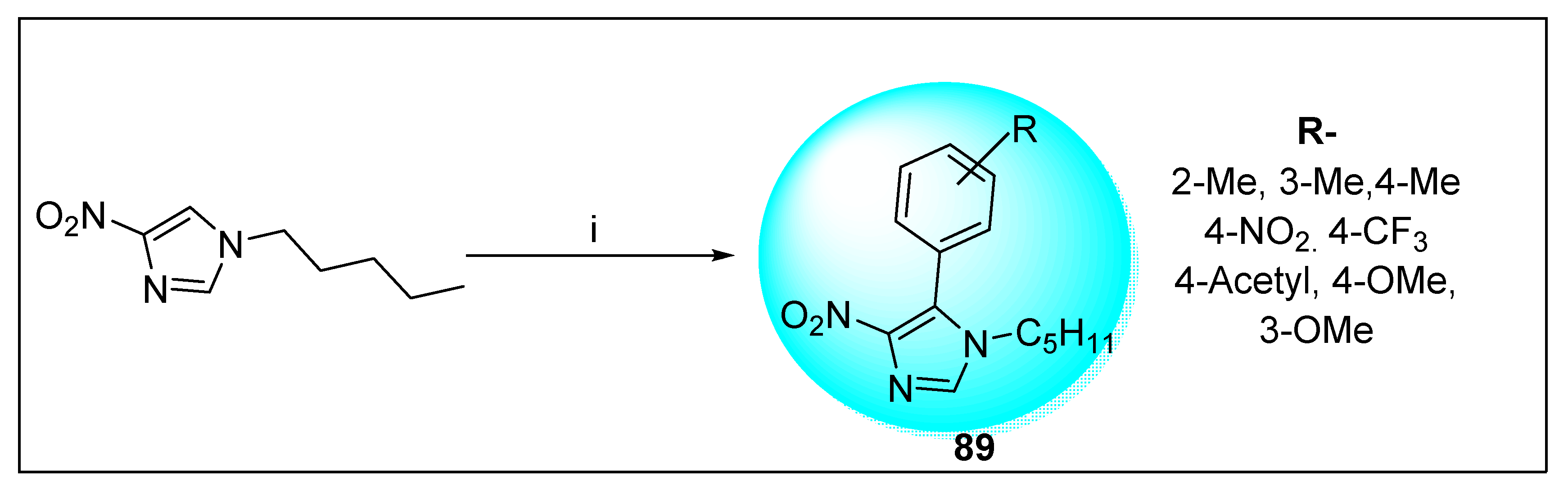
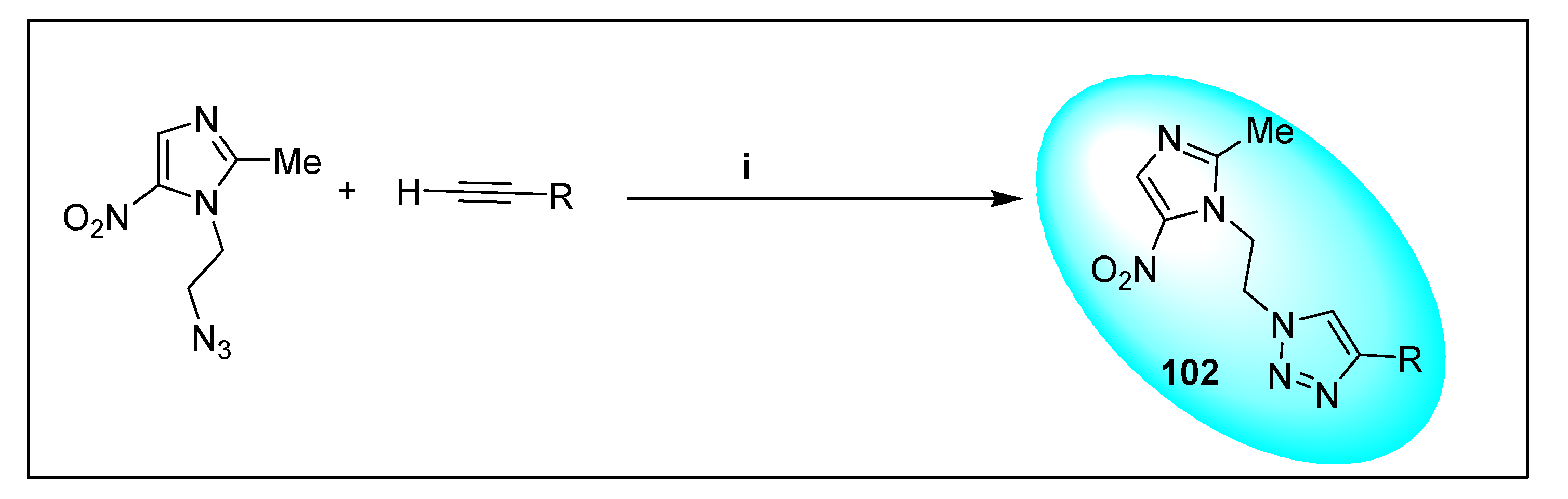
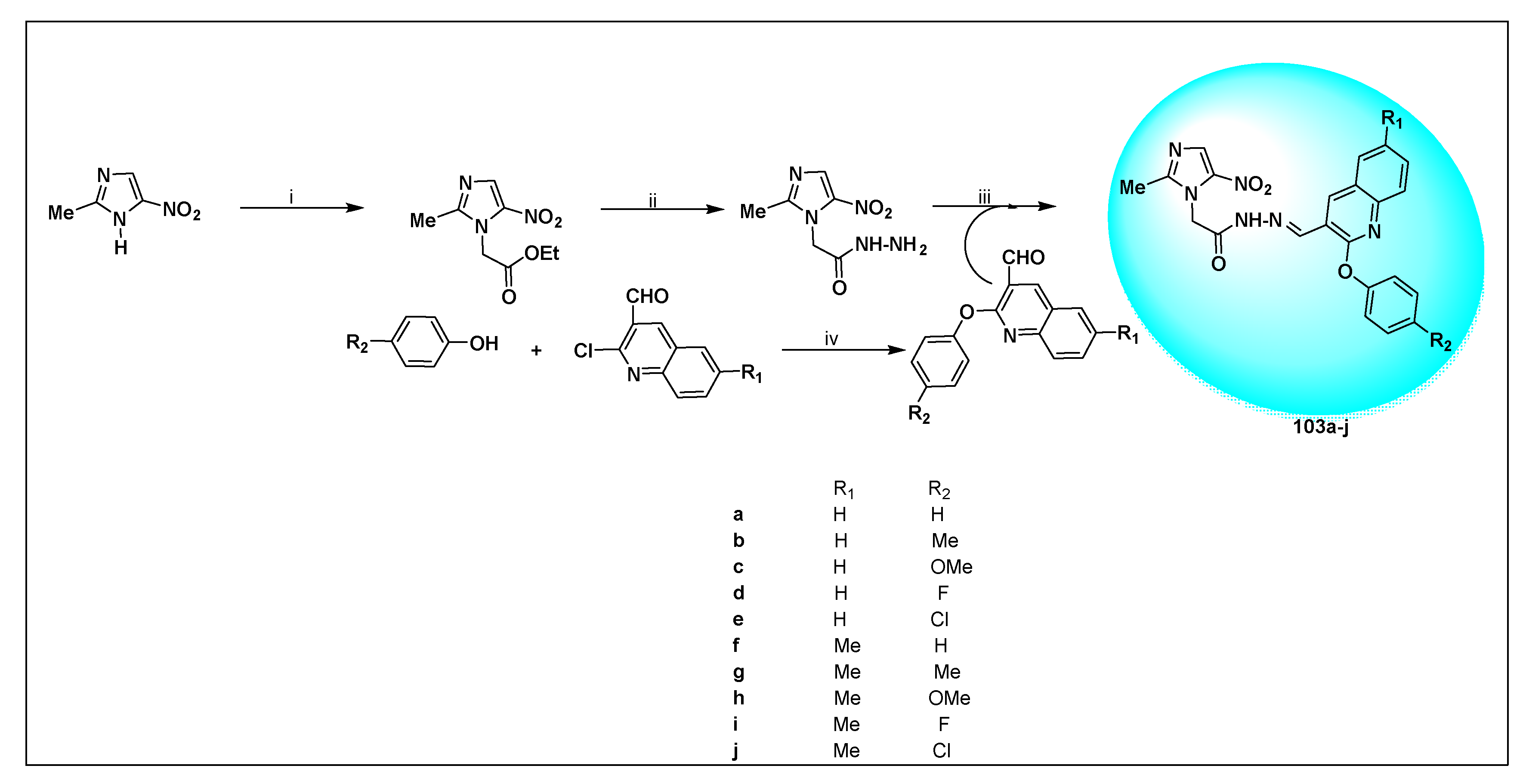
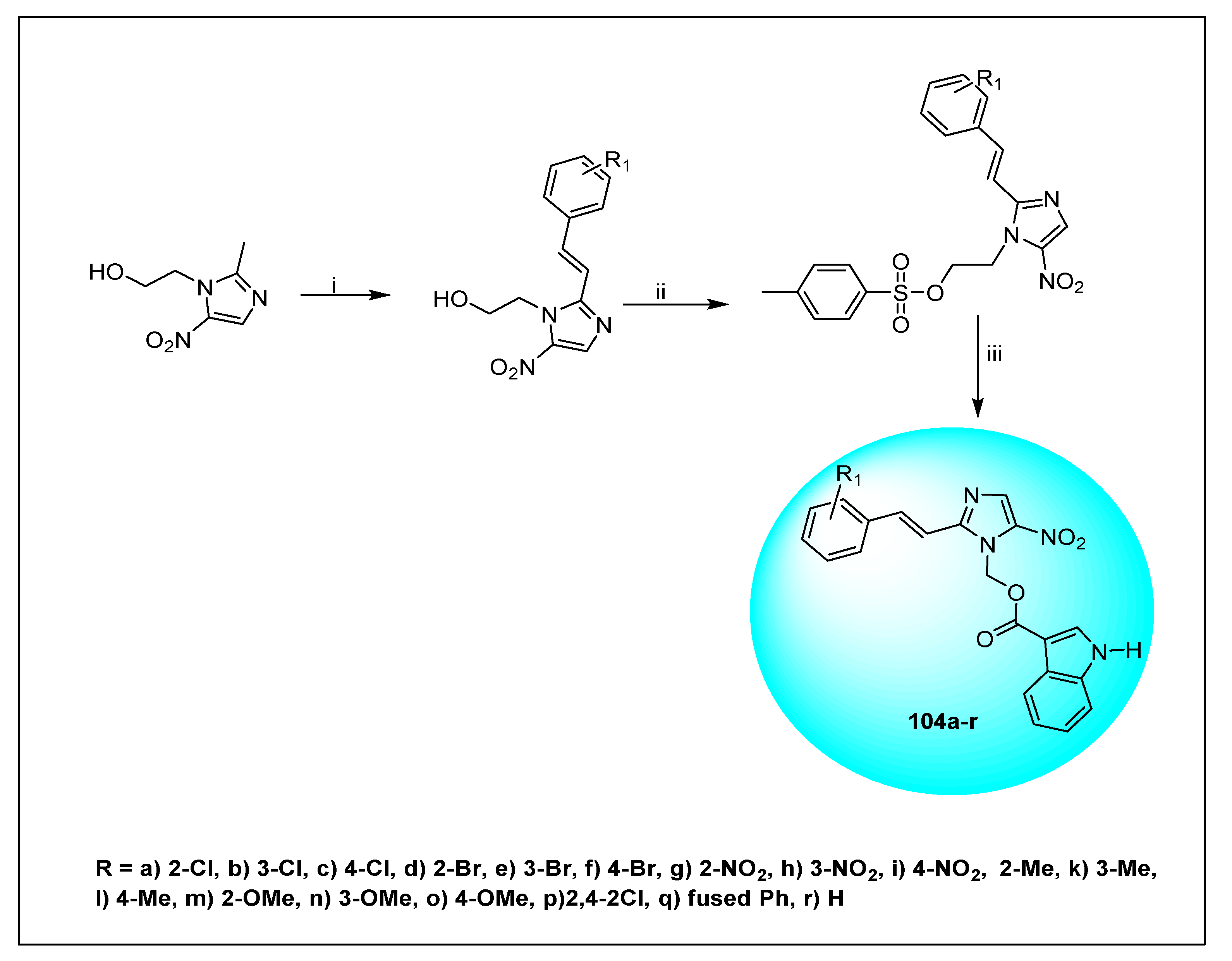
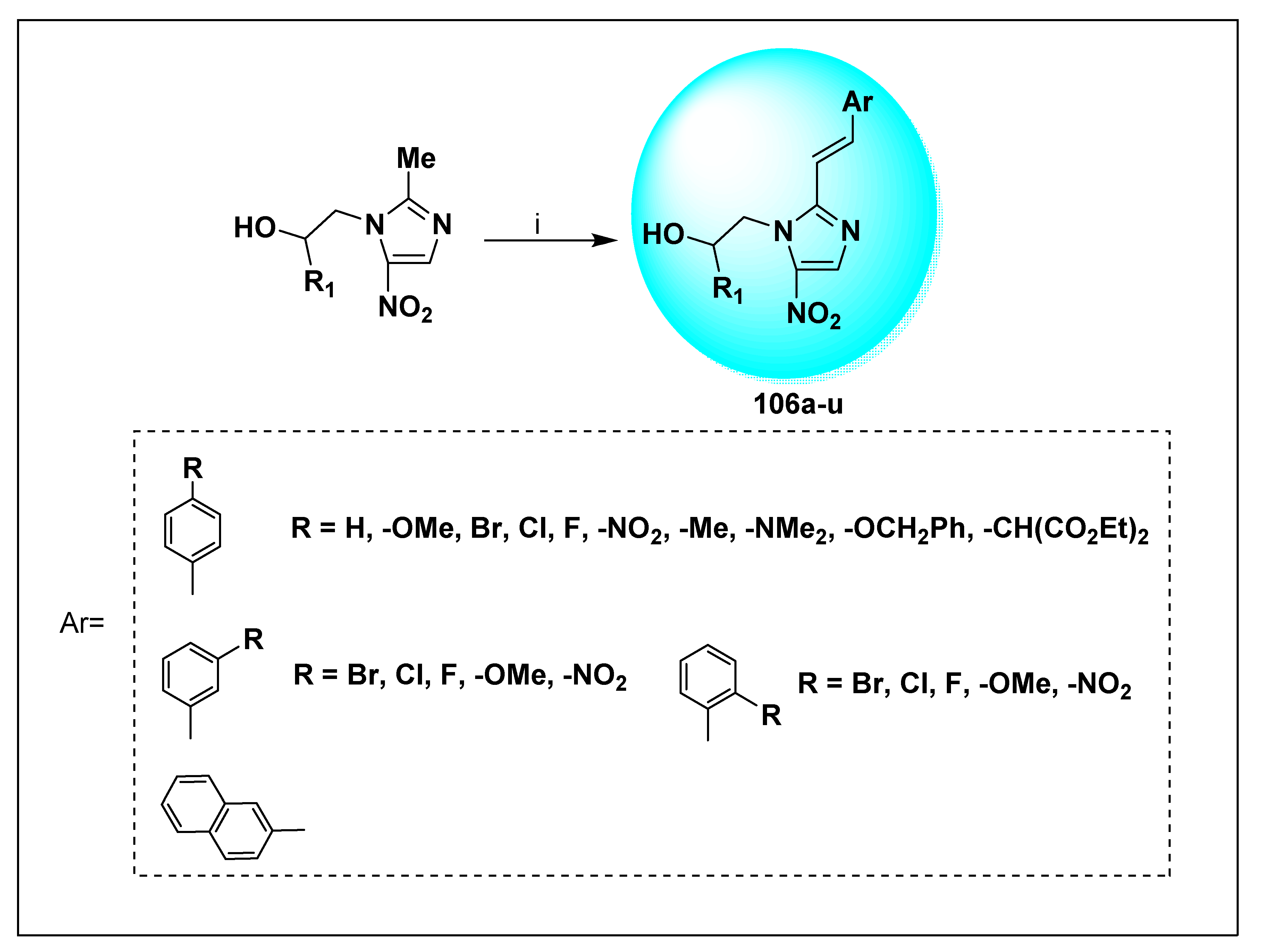
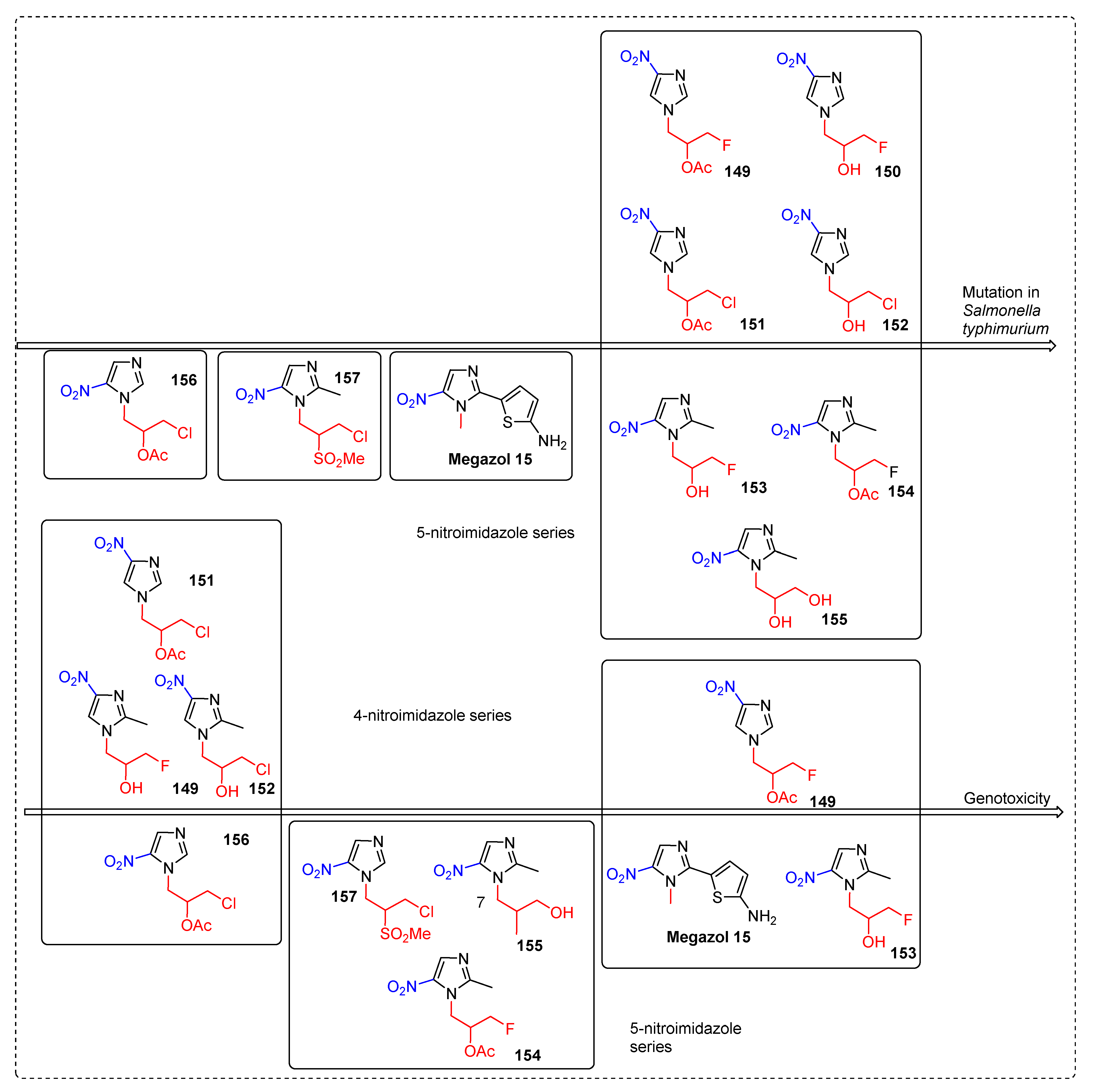
2.2.2. Miscellaneous 4-Nitroimidazole Derivatives
2.3. Functionalized 5-Nitroimidazole Scaffold
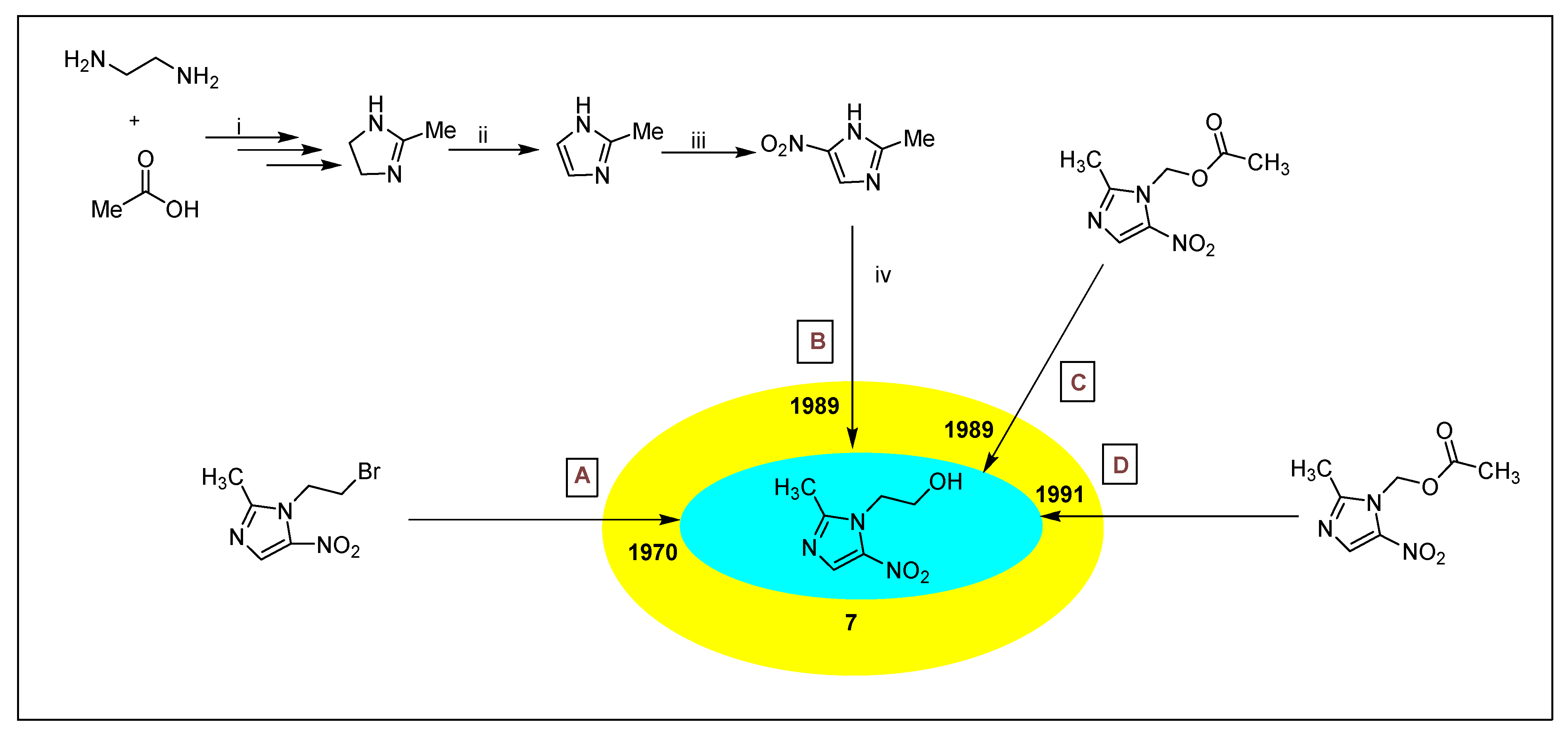
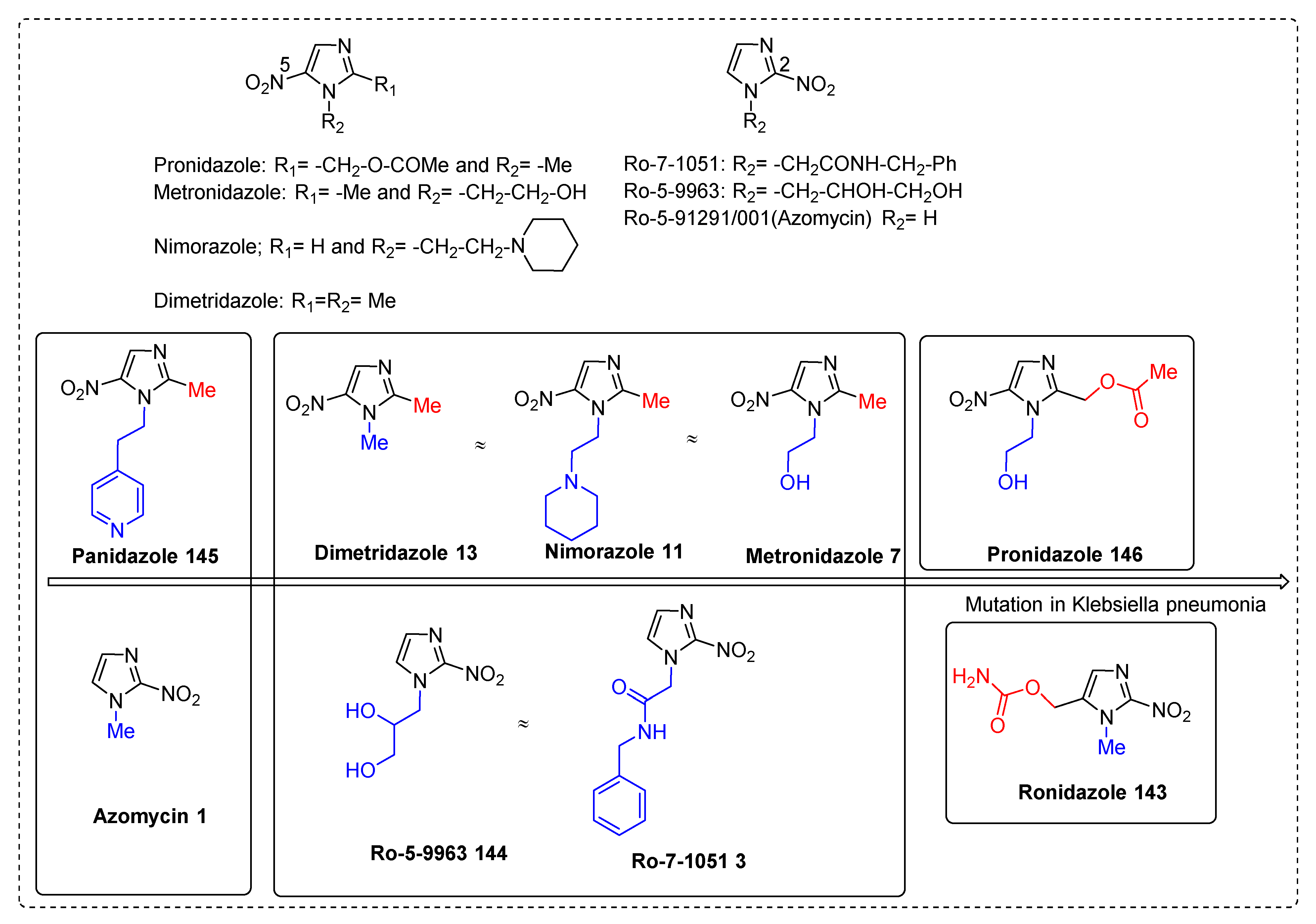
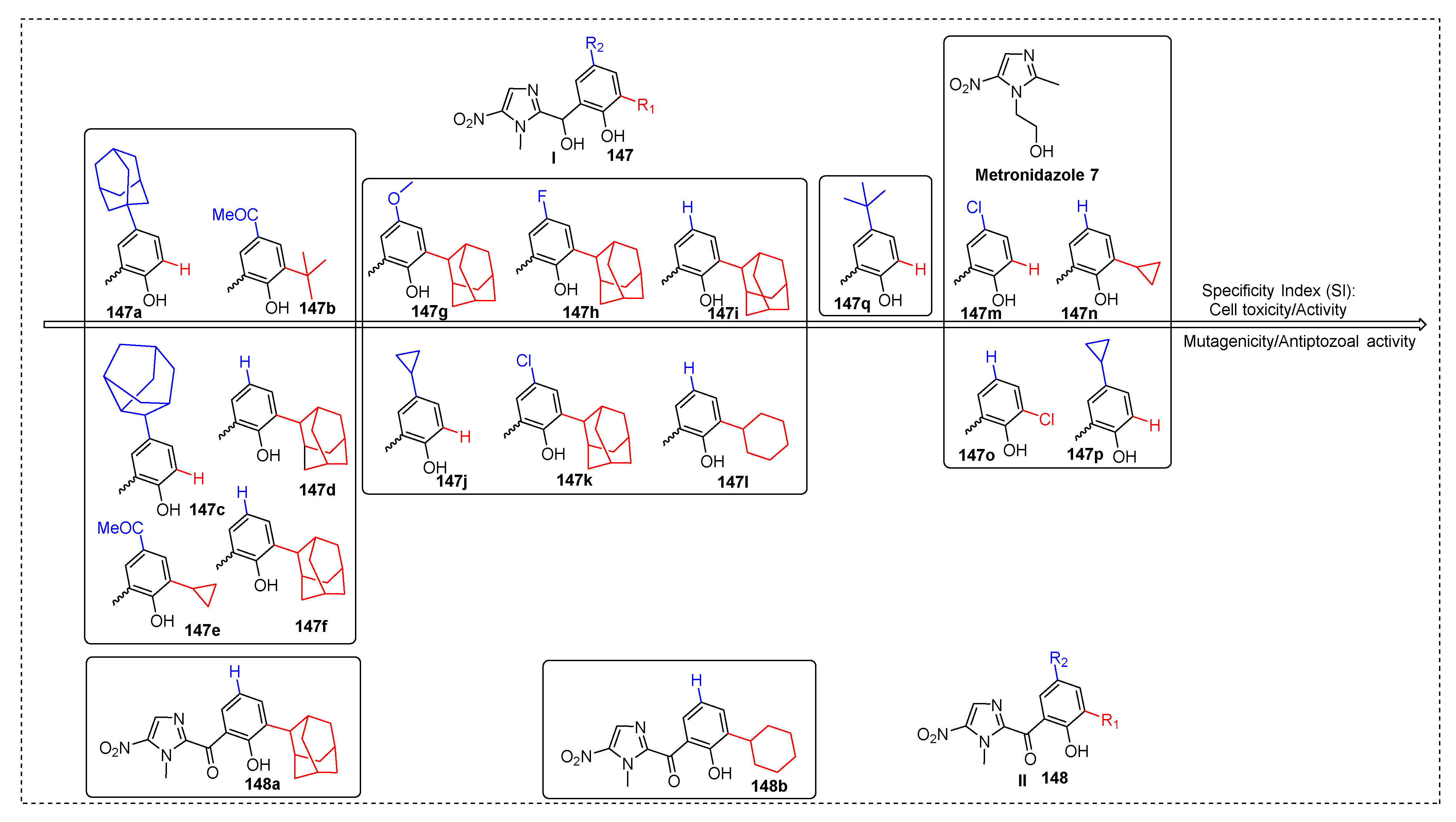
2.3.1. Metronidazole 7
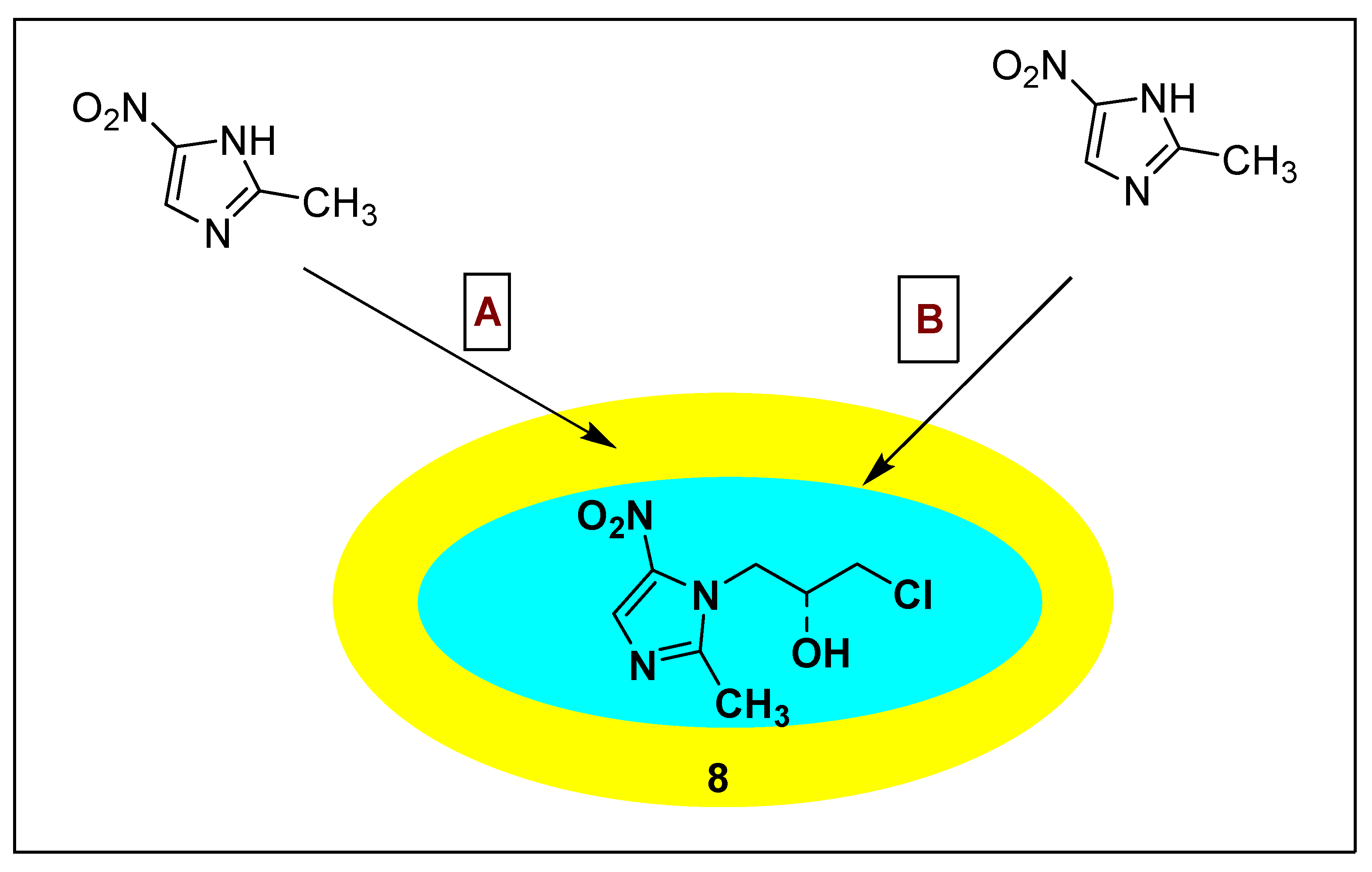
2.3.2. Ornidazole 8
2.3.3. Tinidazole 9
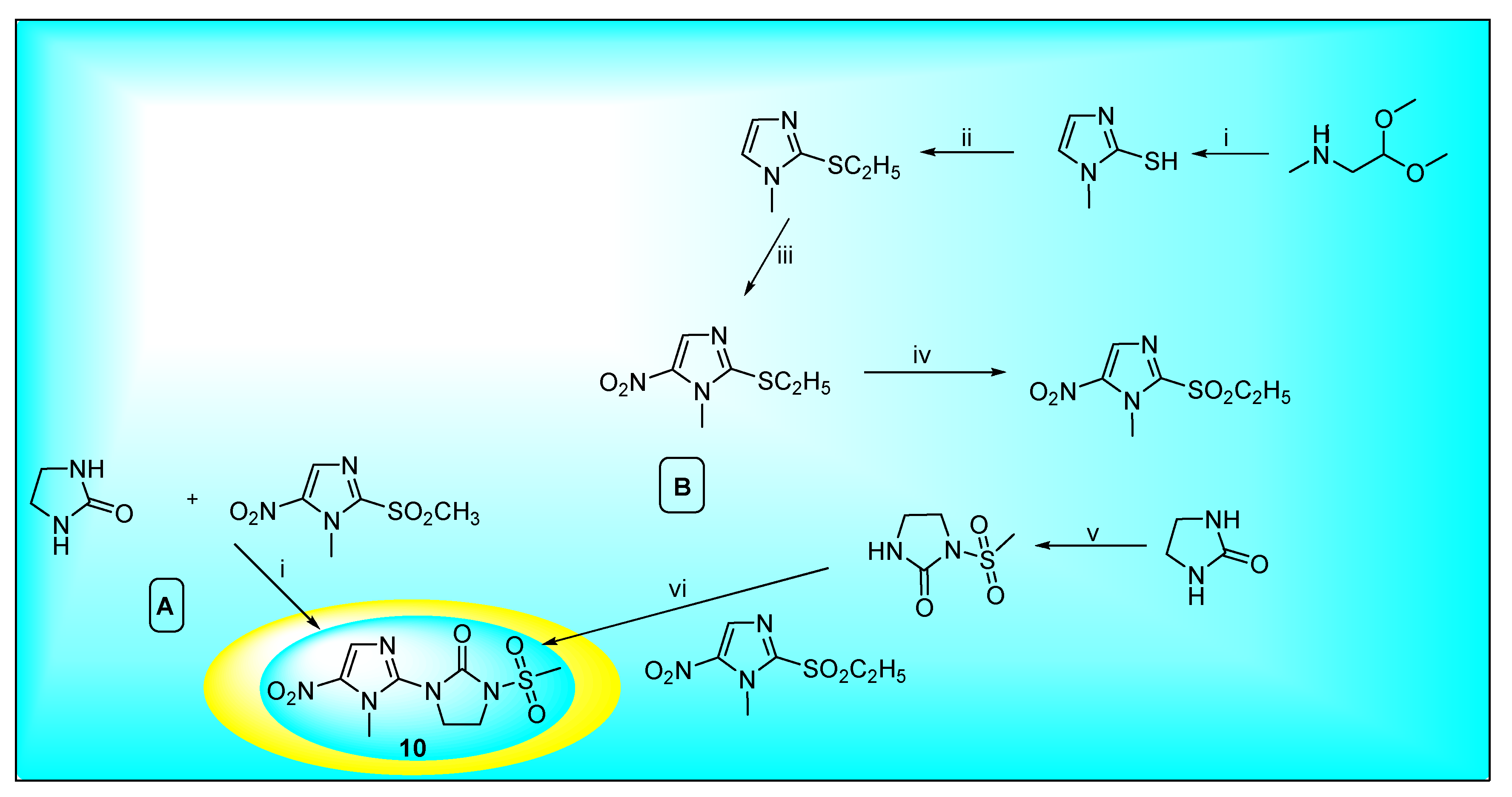
2.3.4. Satranidazole 10
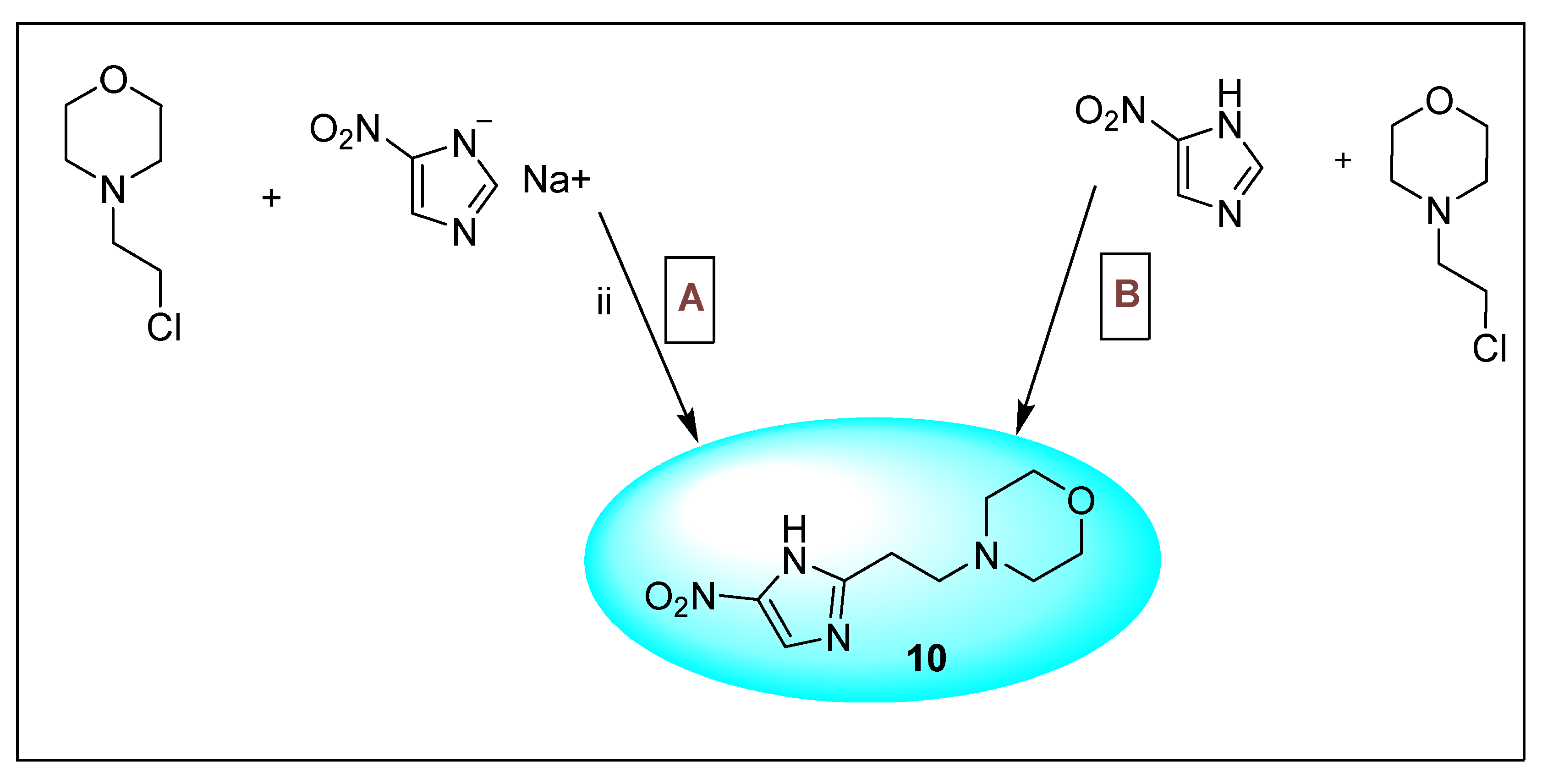
2.3.5. Nimorazole 11
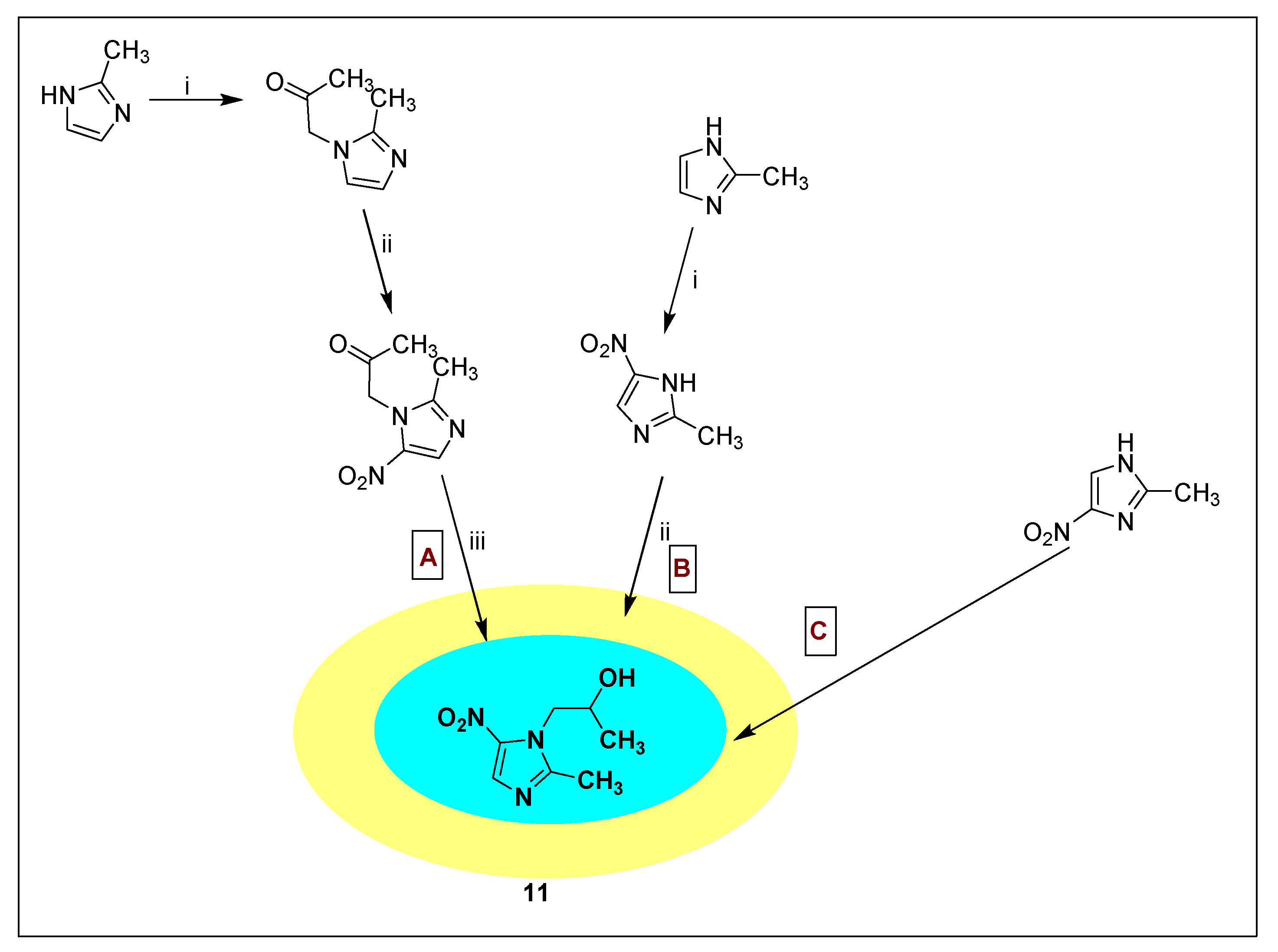
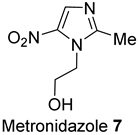
2.3.6. Secnidazole 12
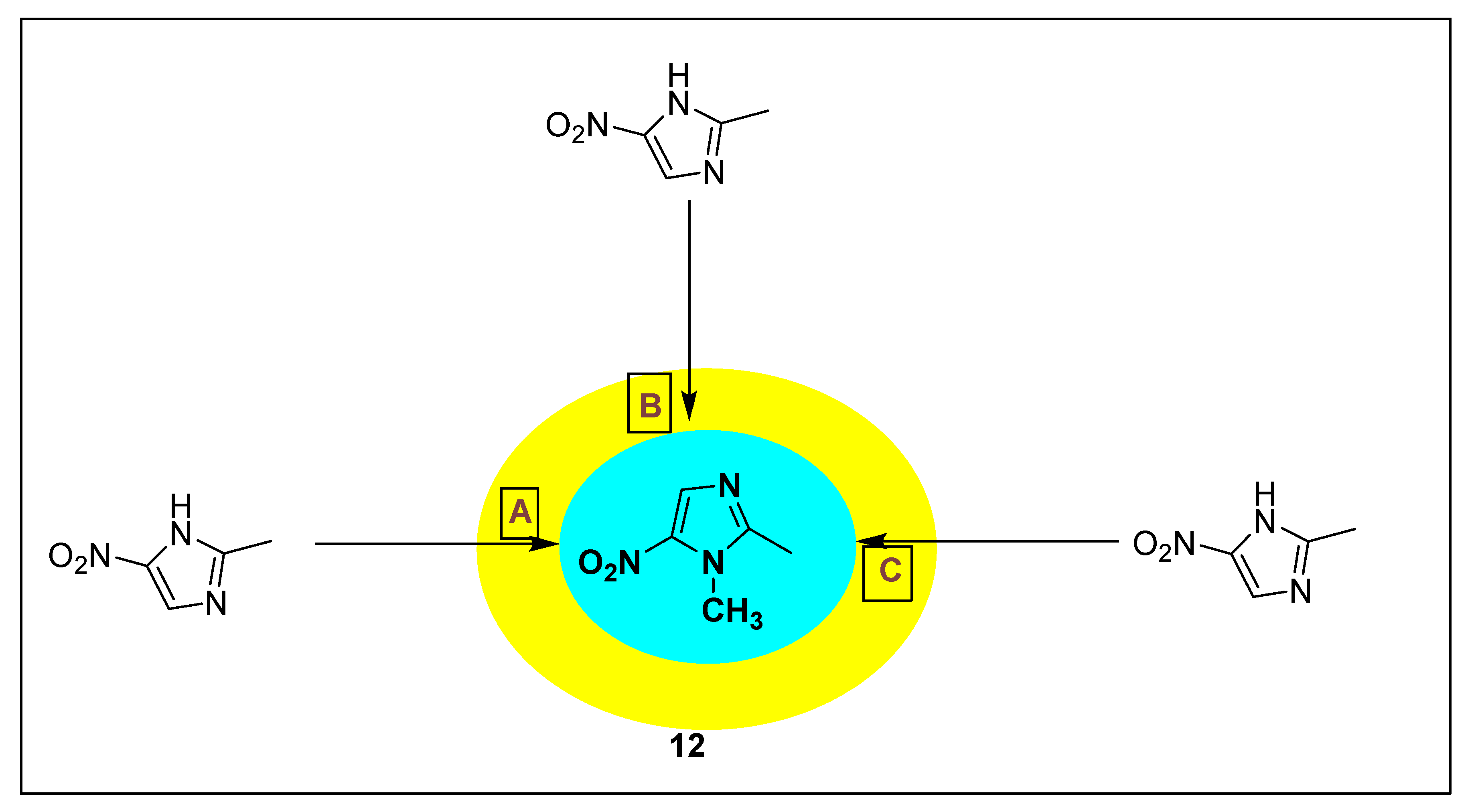
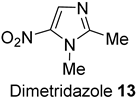
2.3.7. Dimetridazole 13
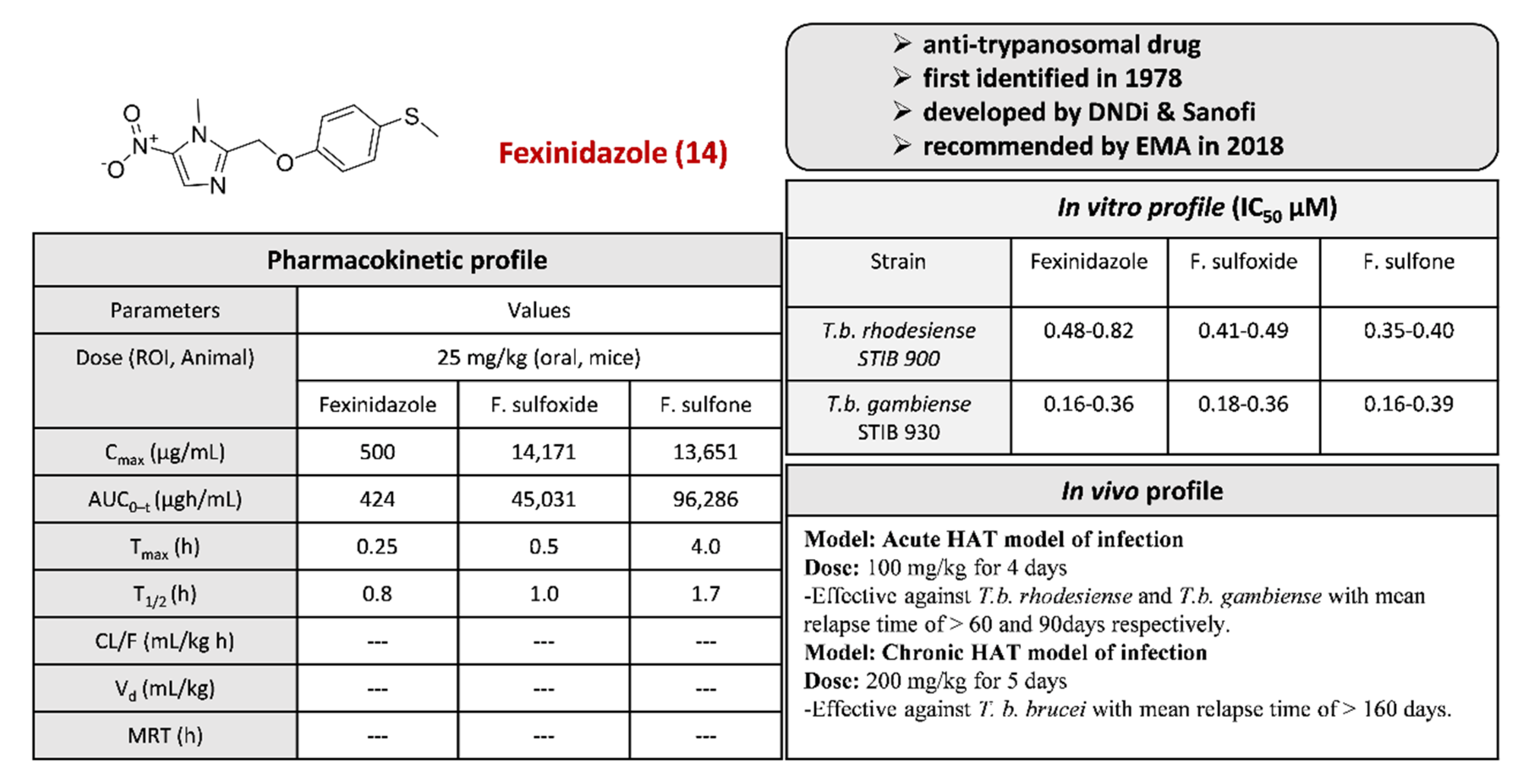
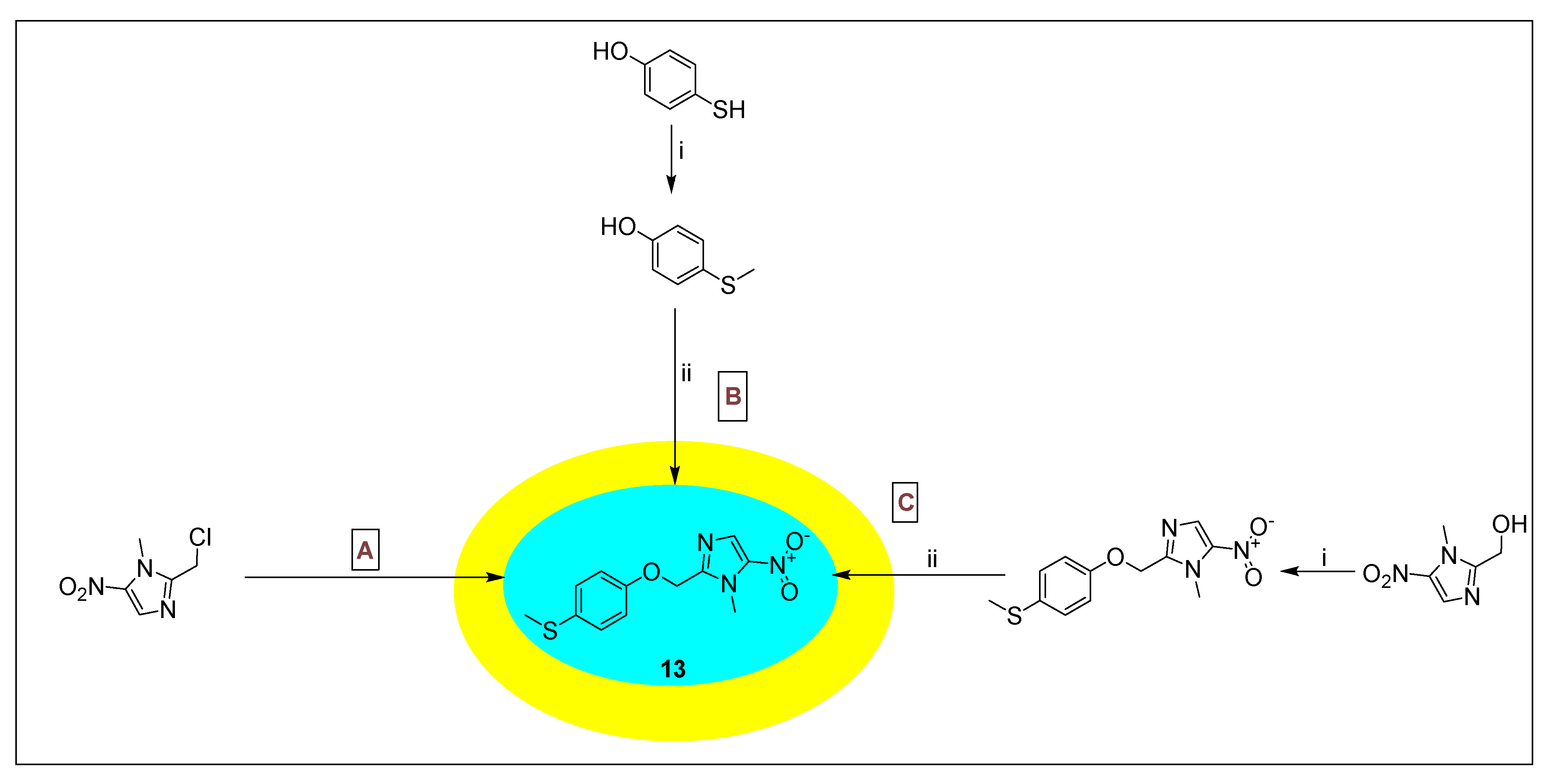
2.3.8. Fexinidazole 14
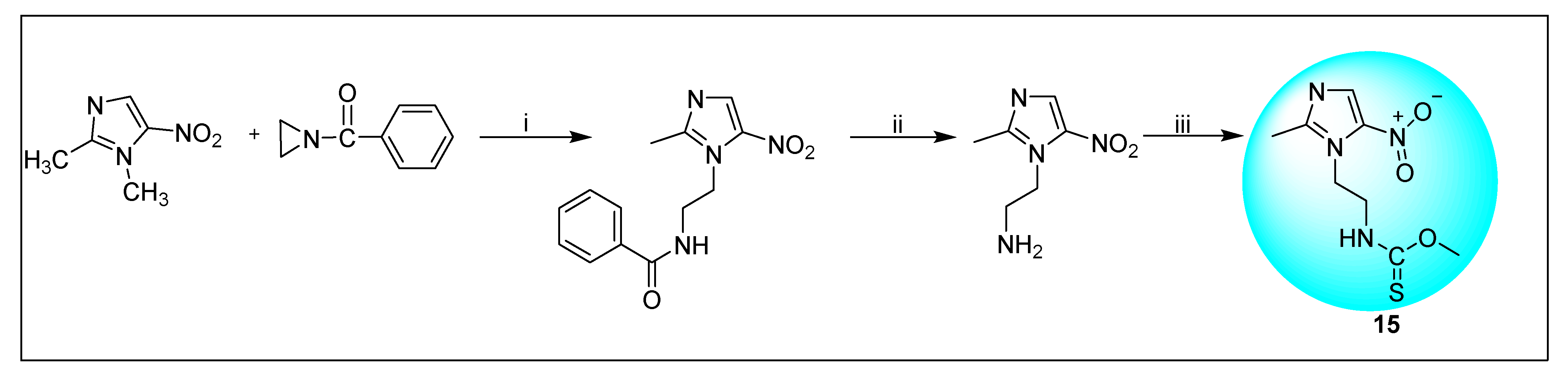
2.3.9. Megazol 15
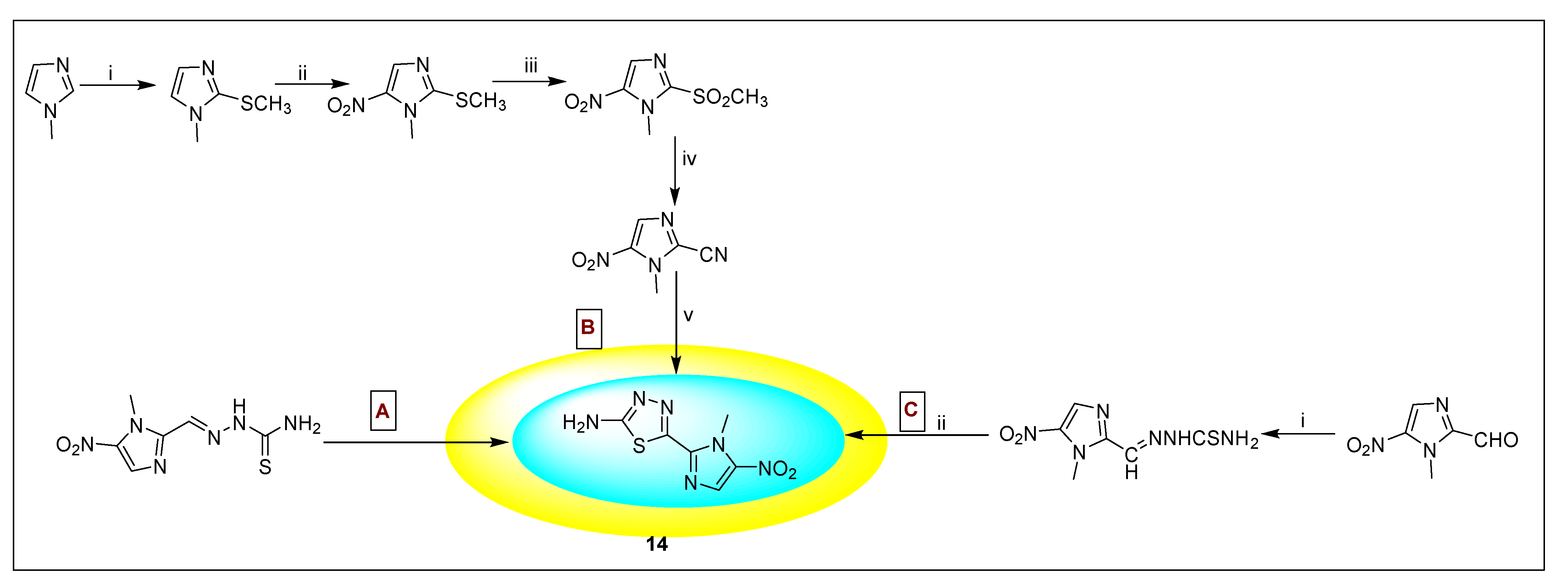
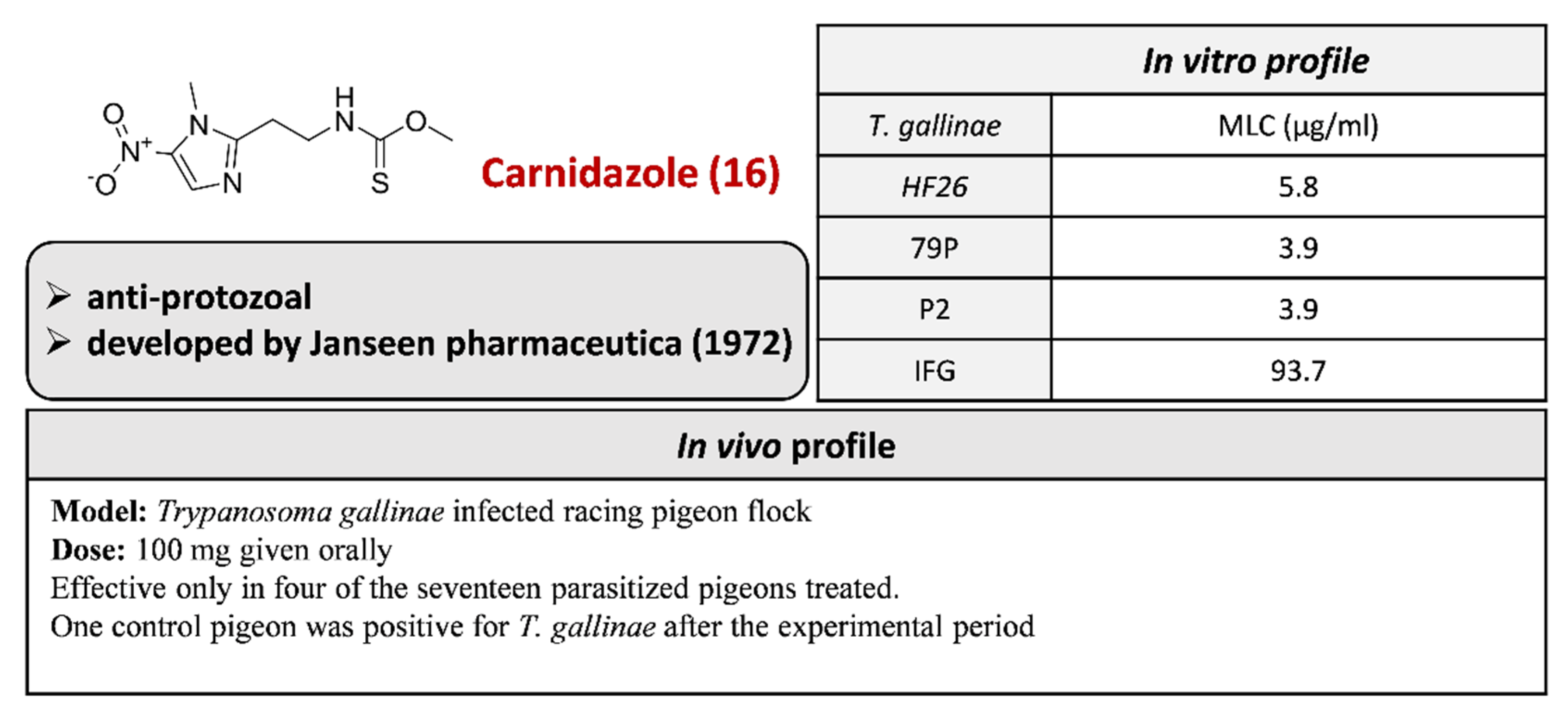
2.3.10. Carnidazole 16
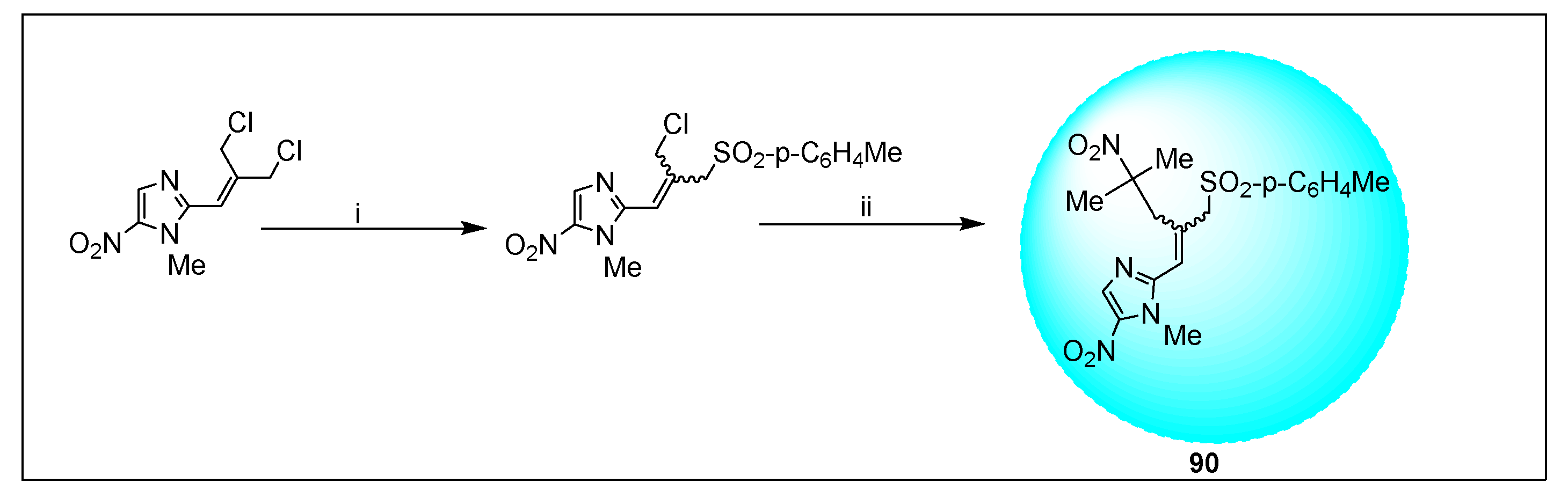
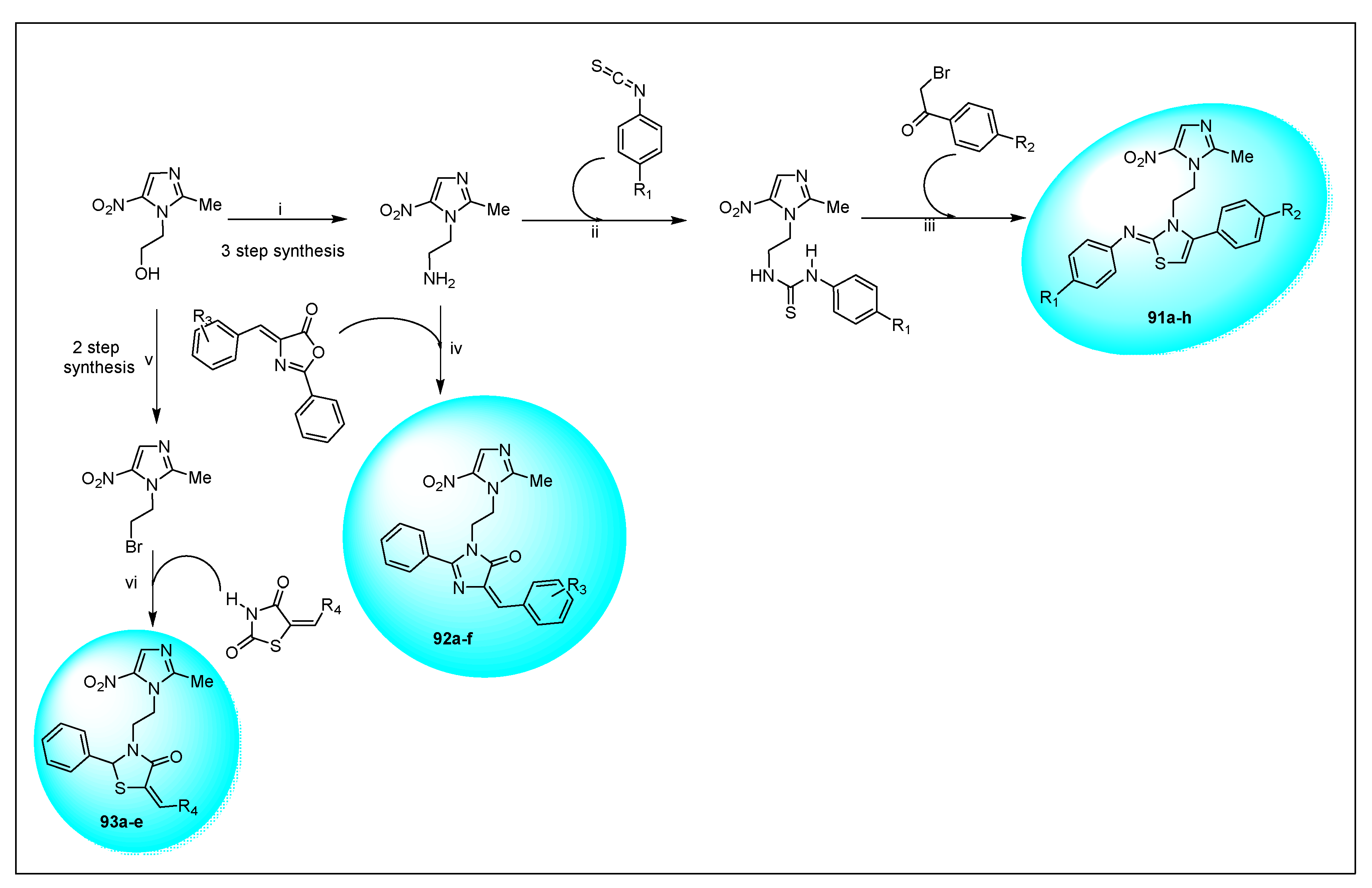
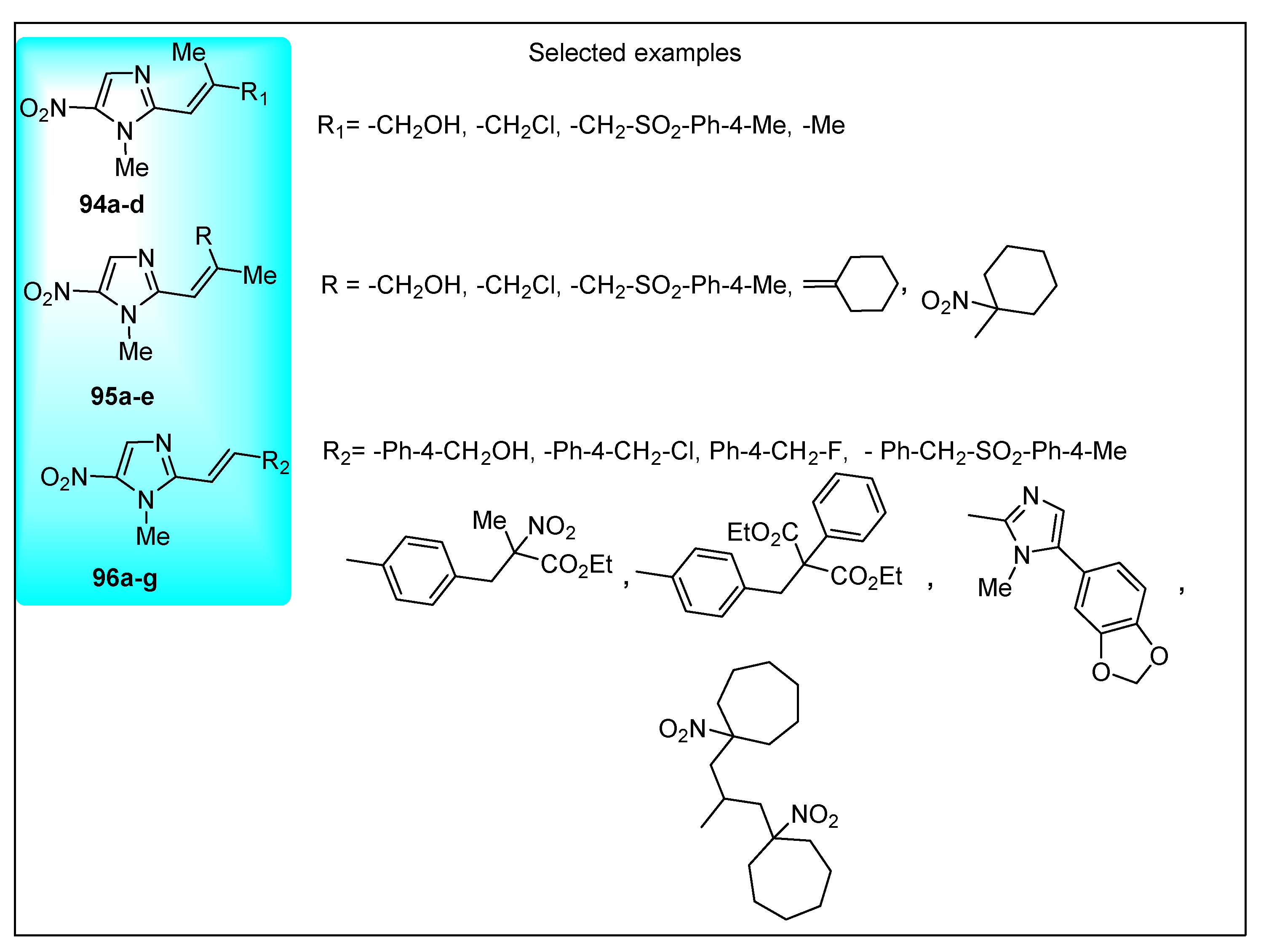
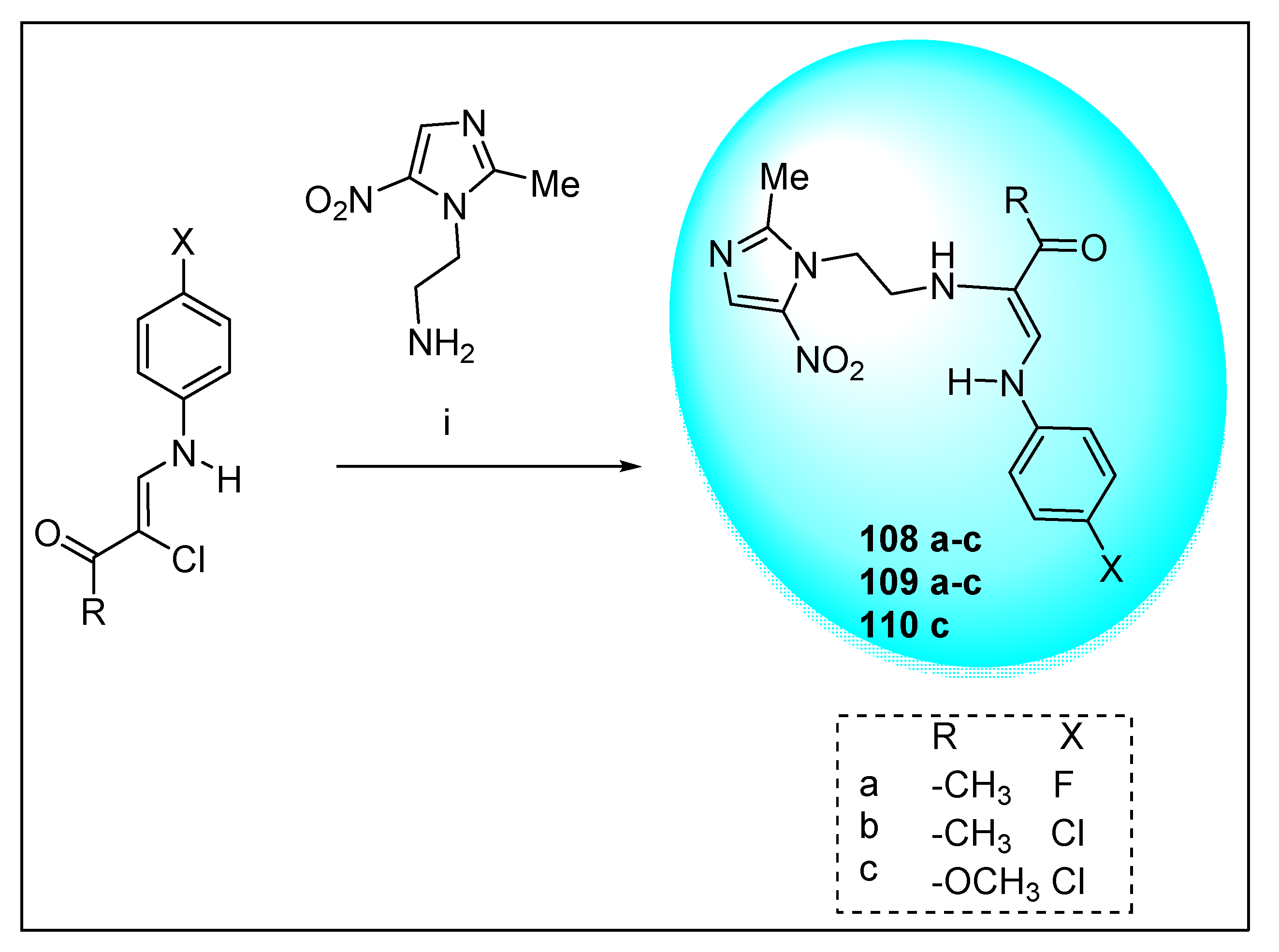
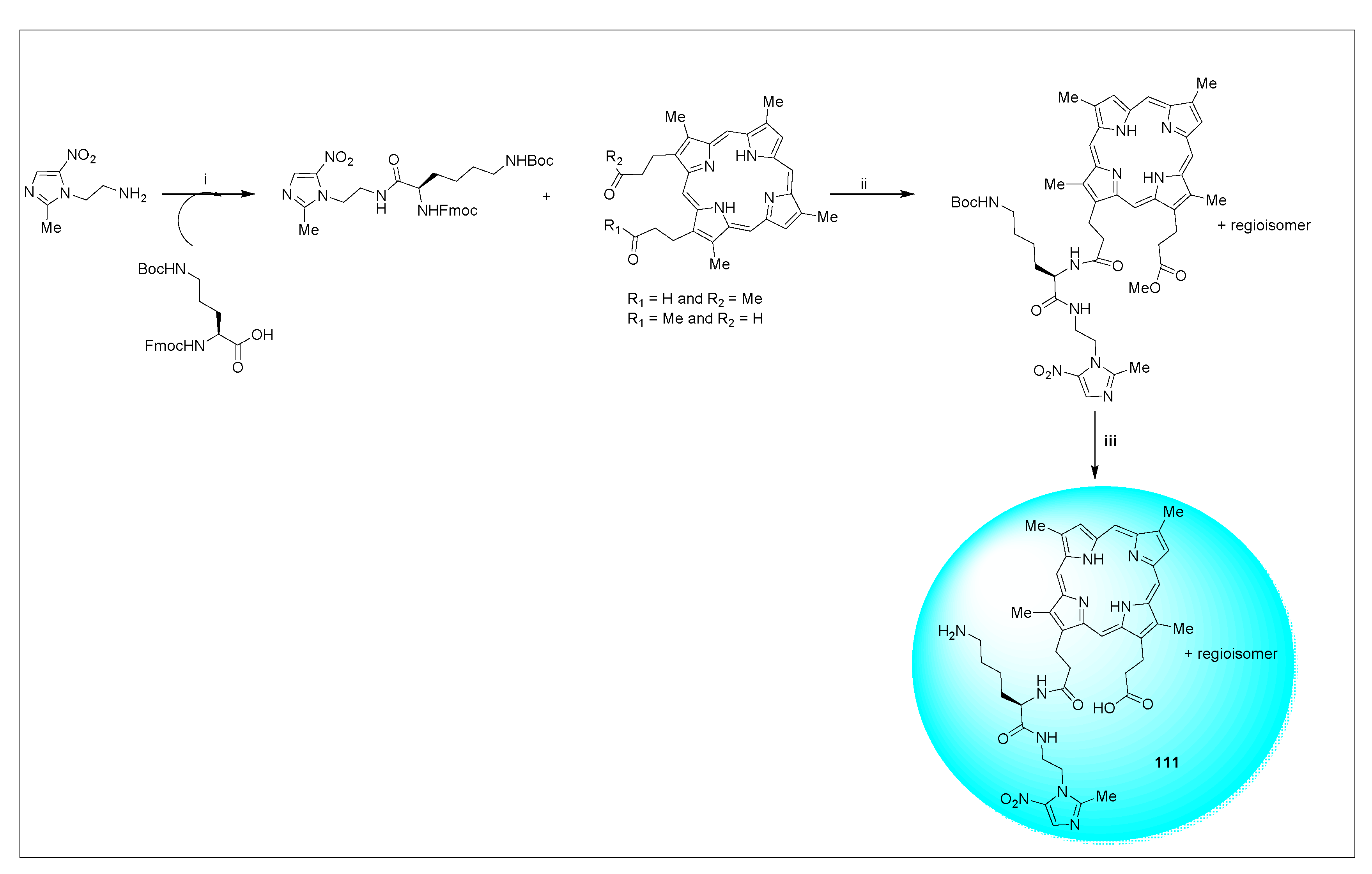
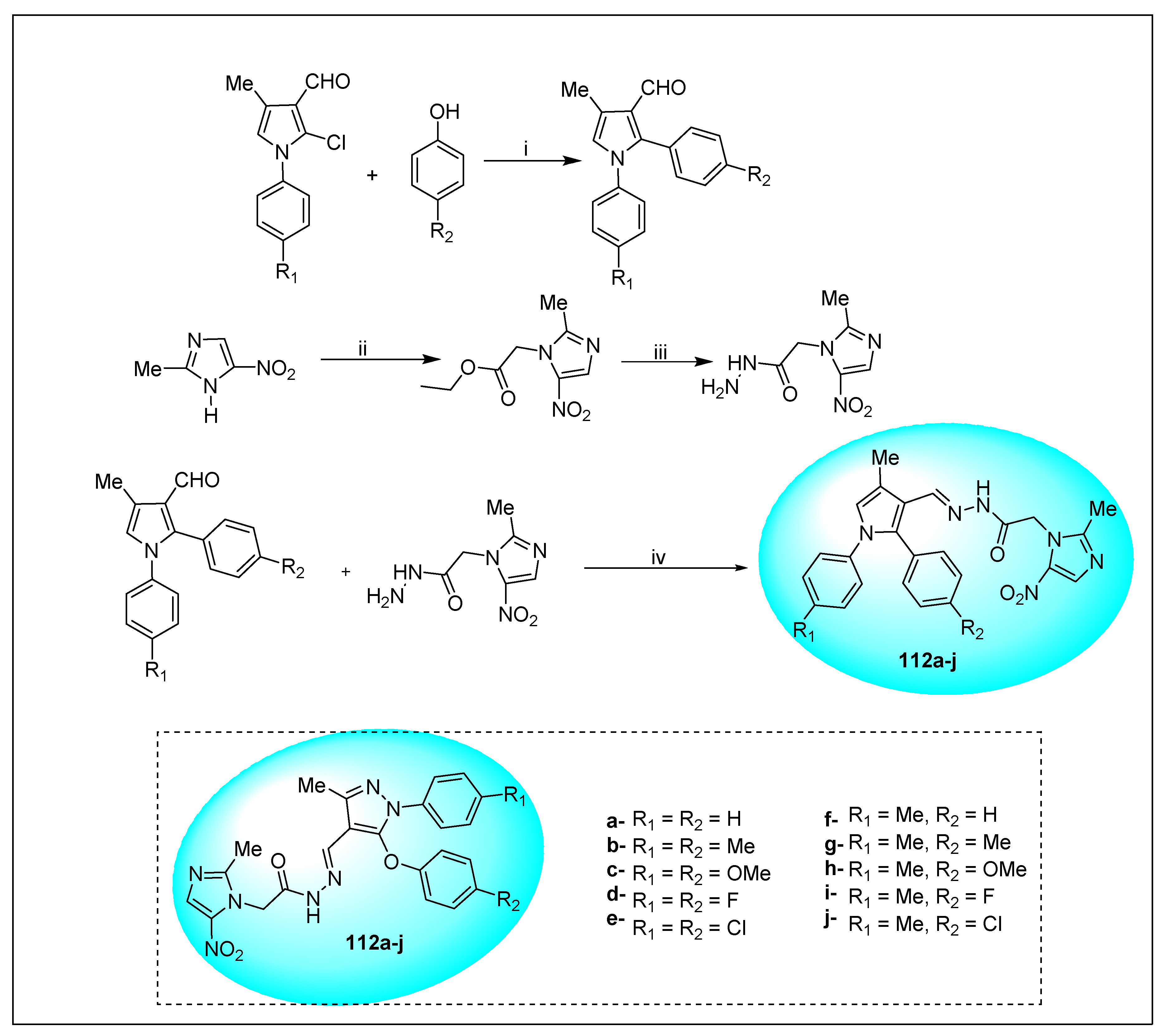
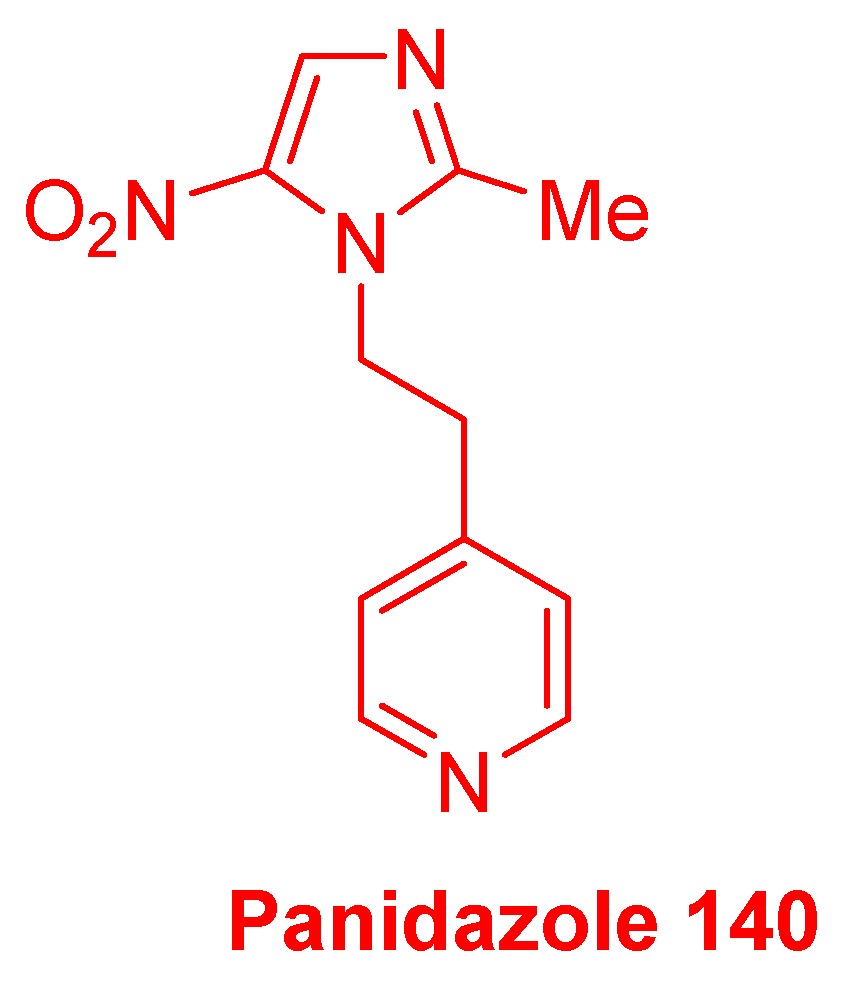
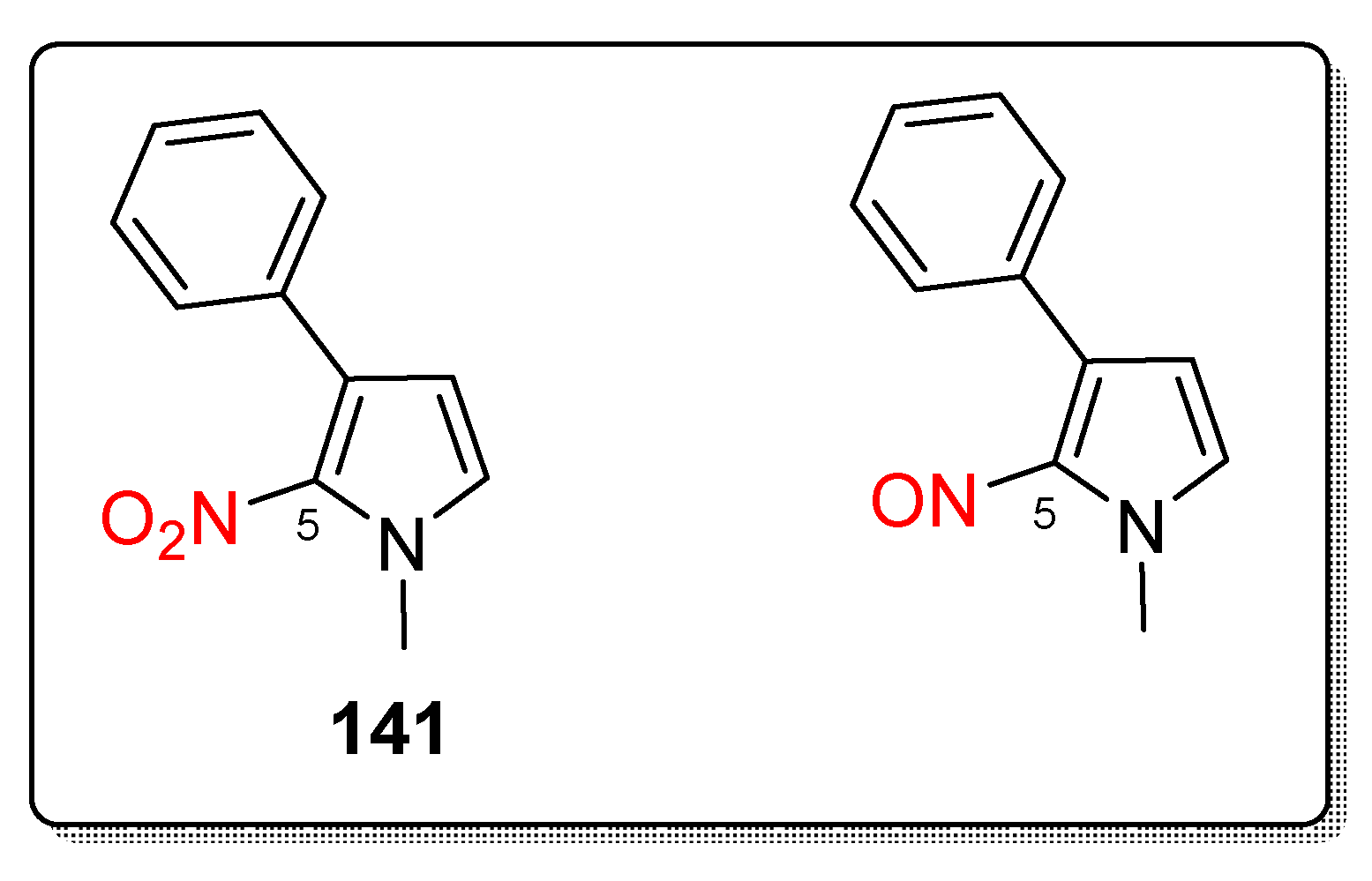
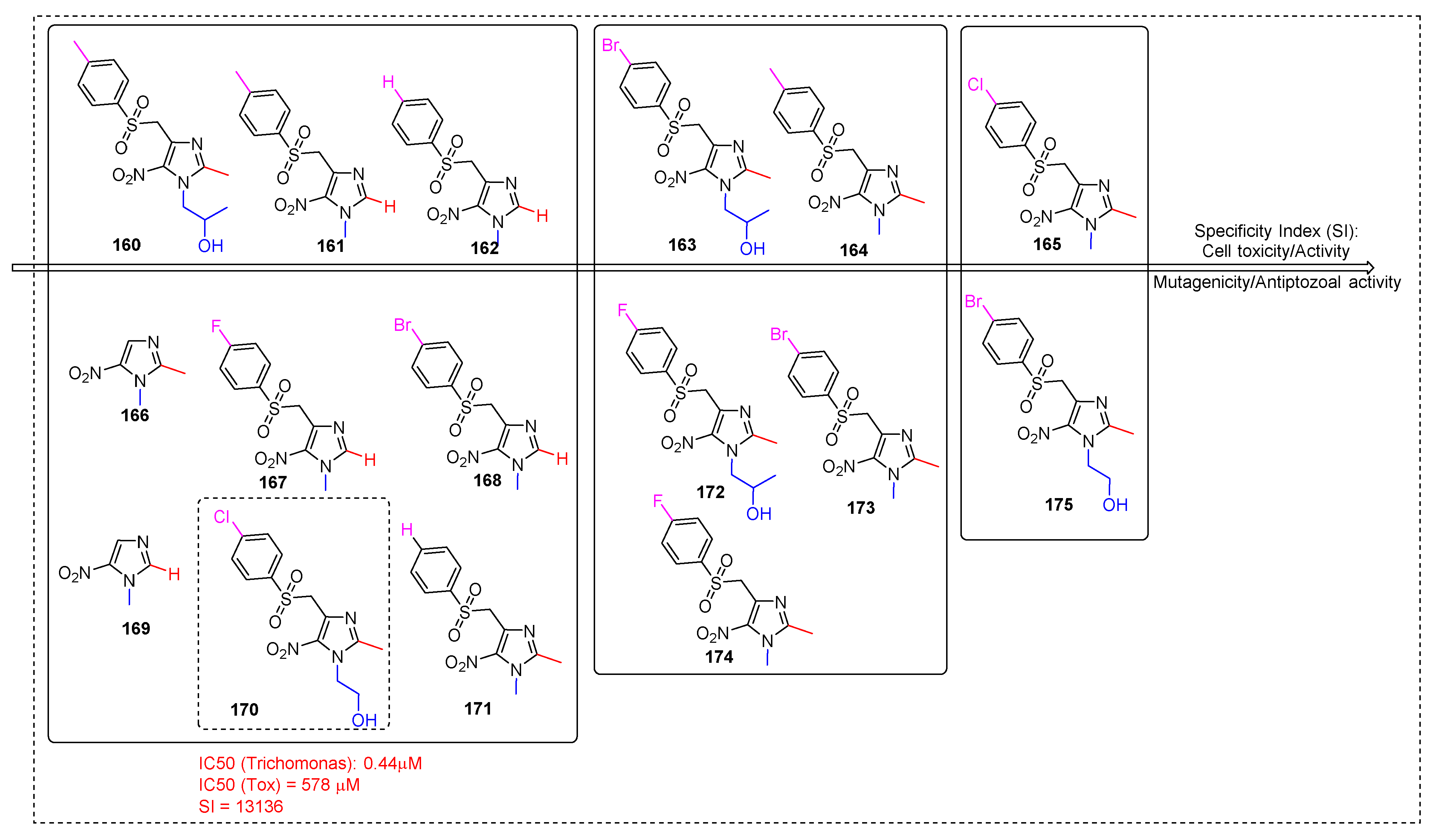
2.3.11. Miscellaneous 5-Nitroimidazole Derivatives
2.4. Scaffold
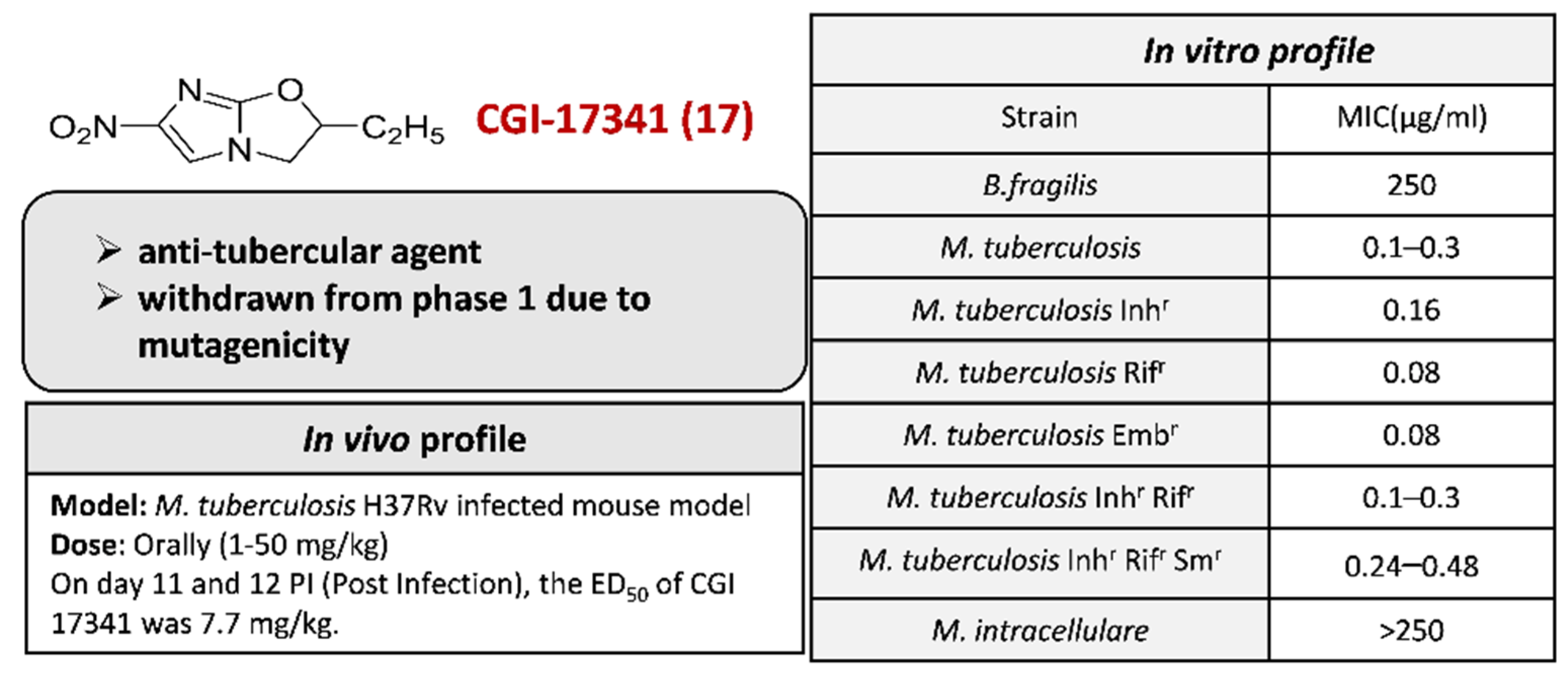
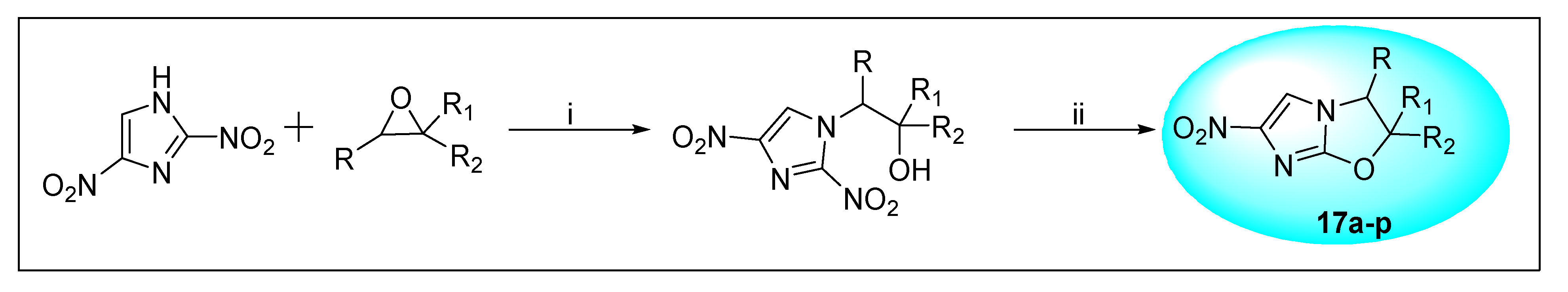
2.4.1. CGI-17341 17 and Its Derivatives
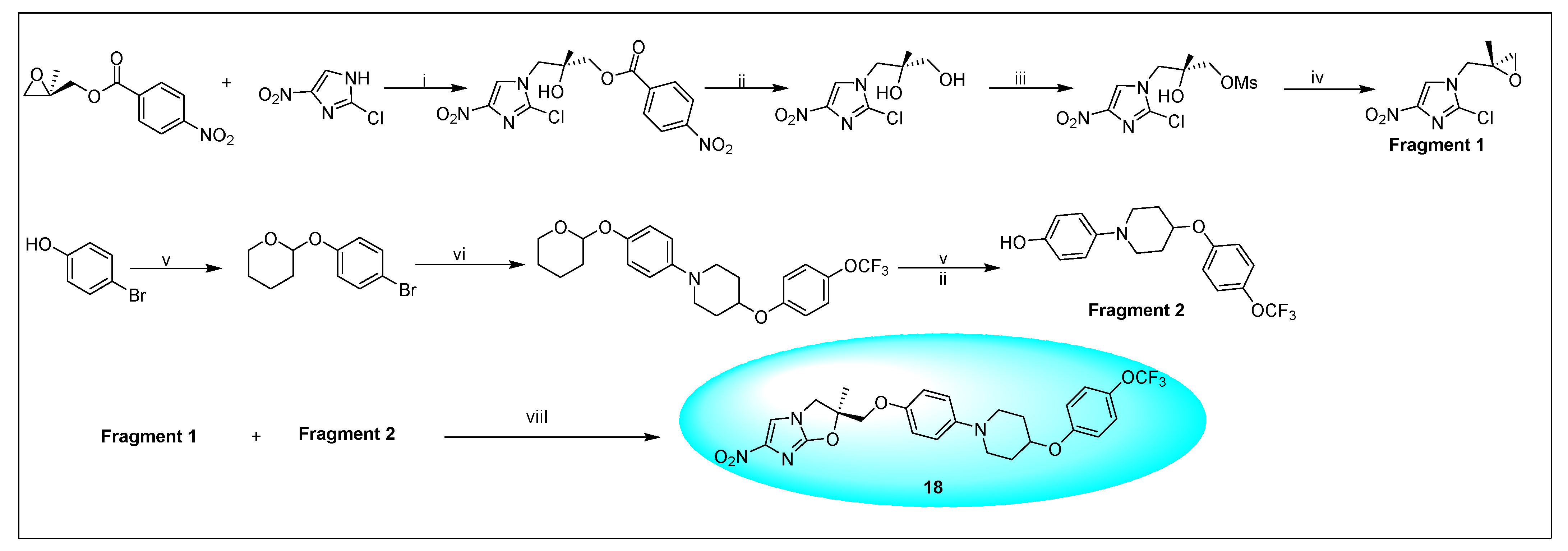
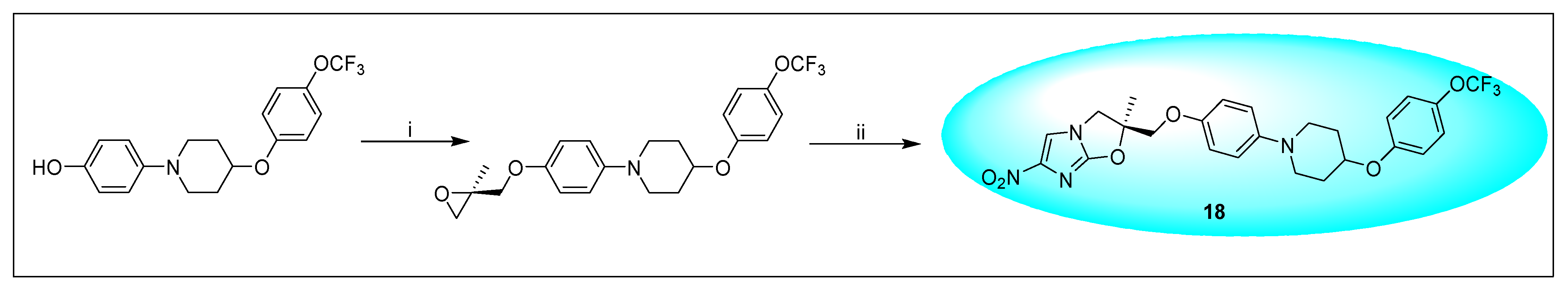
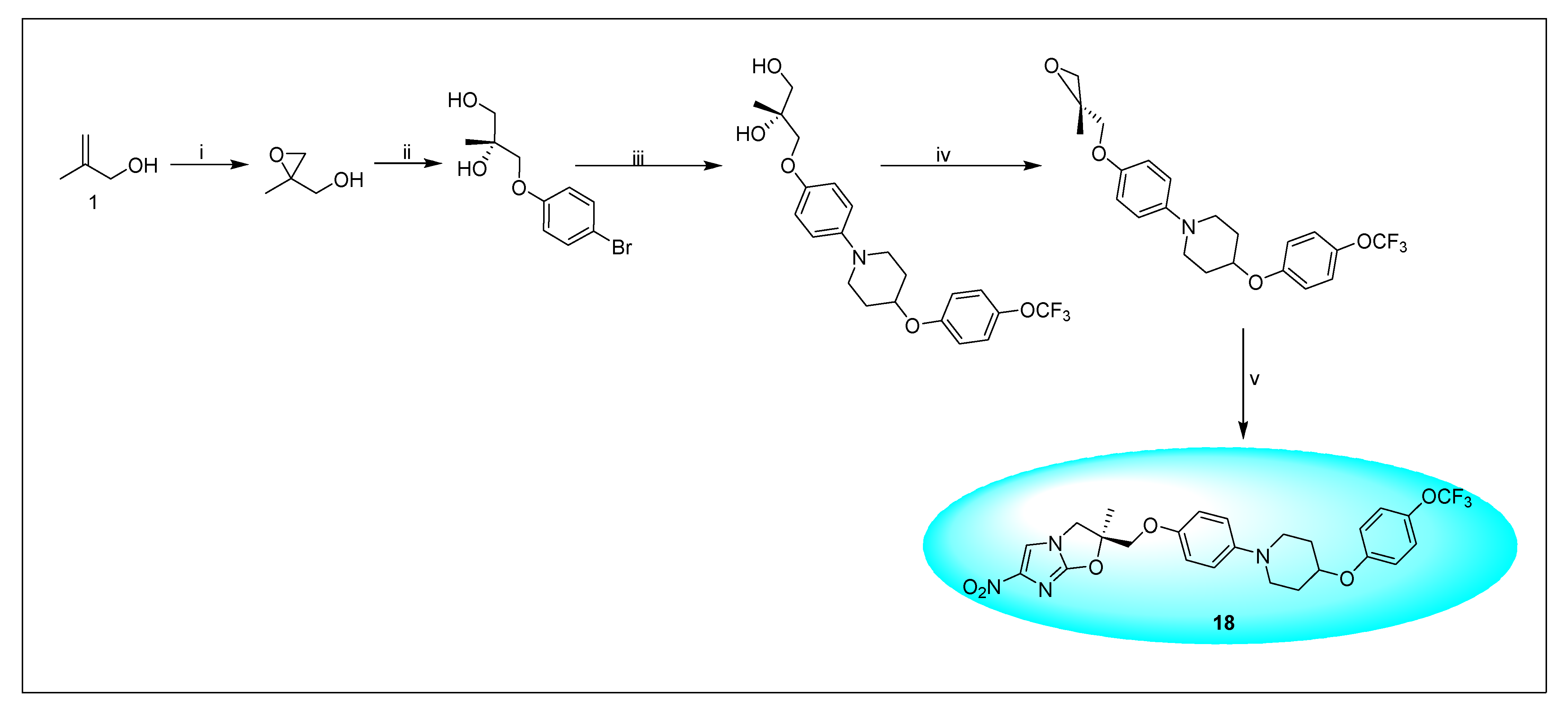
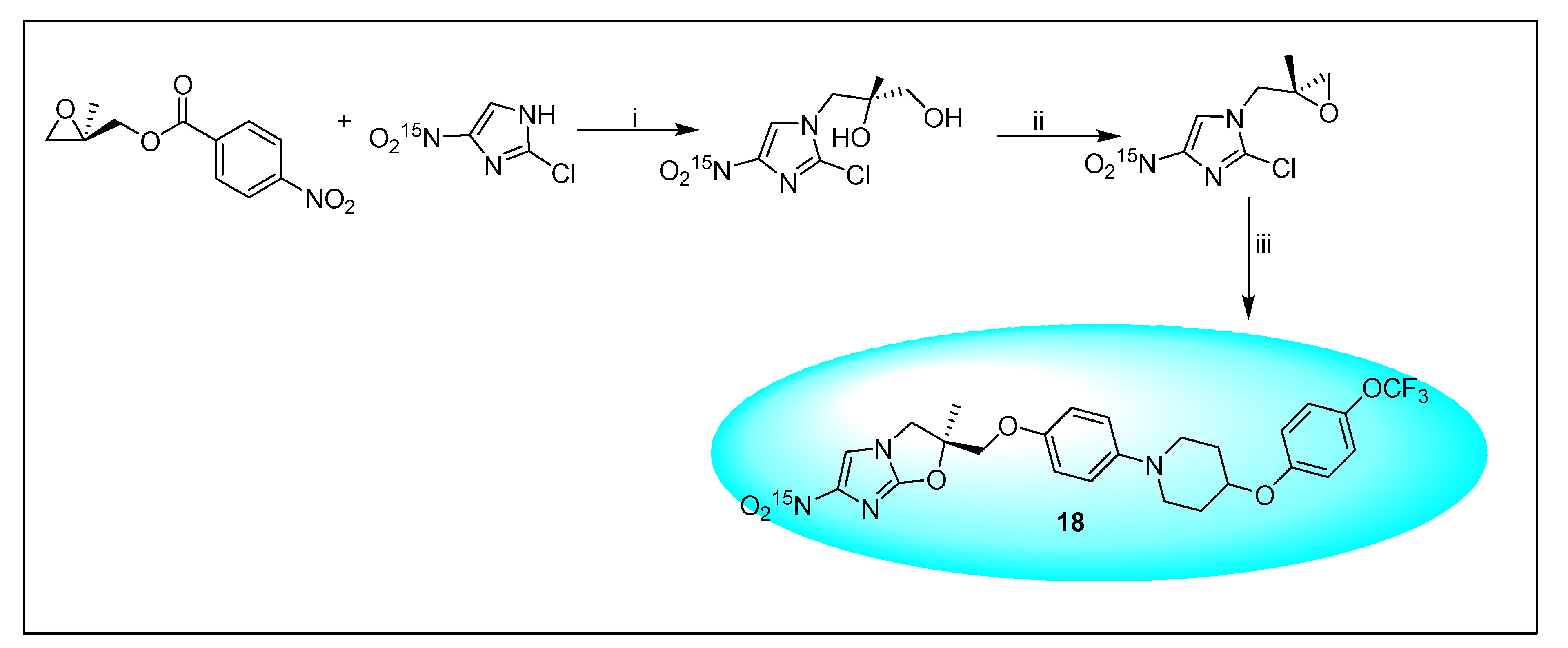
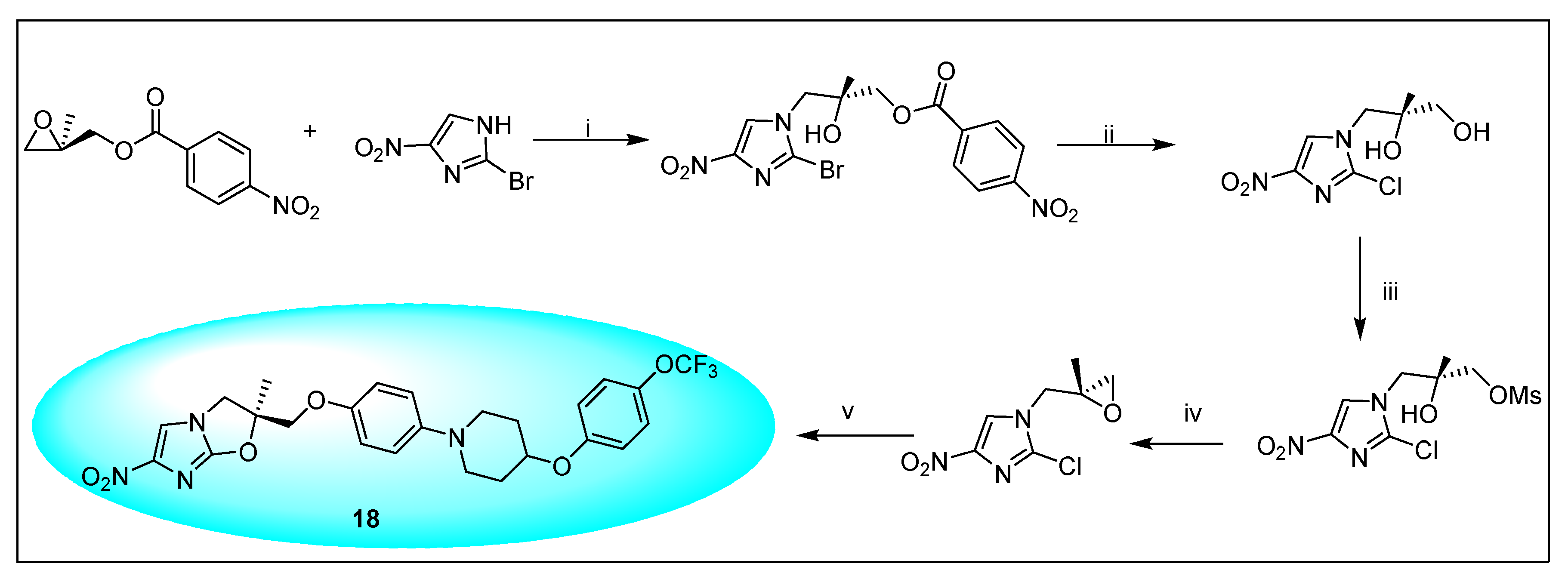
2.4.2. Delamanid 18 and Its Derivatives
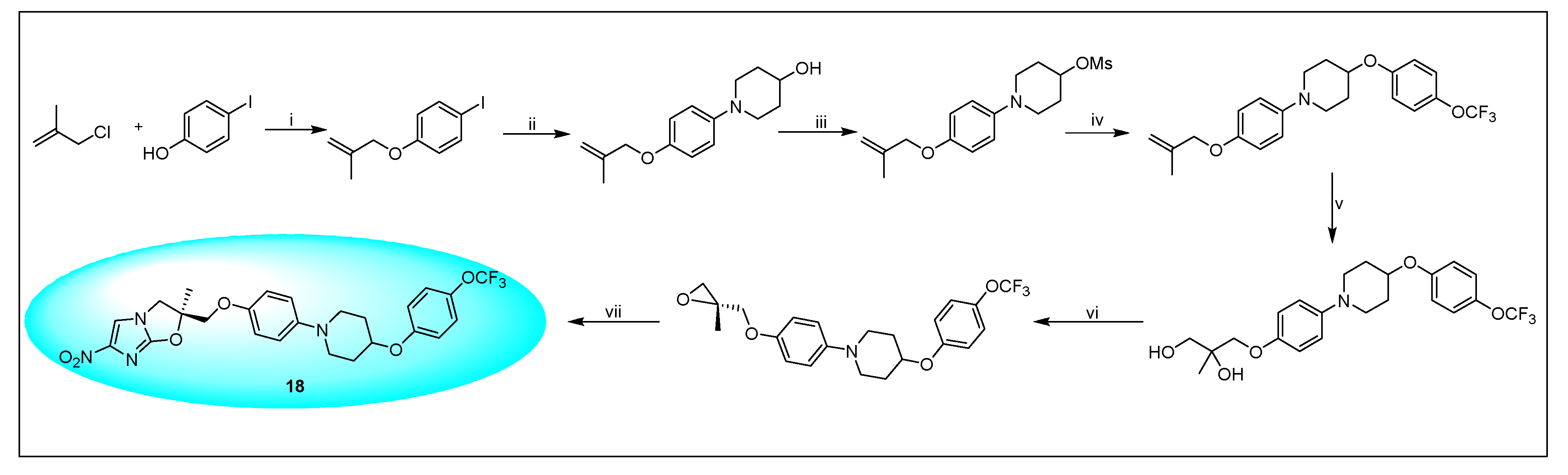
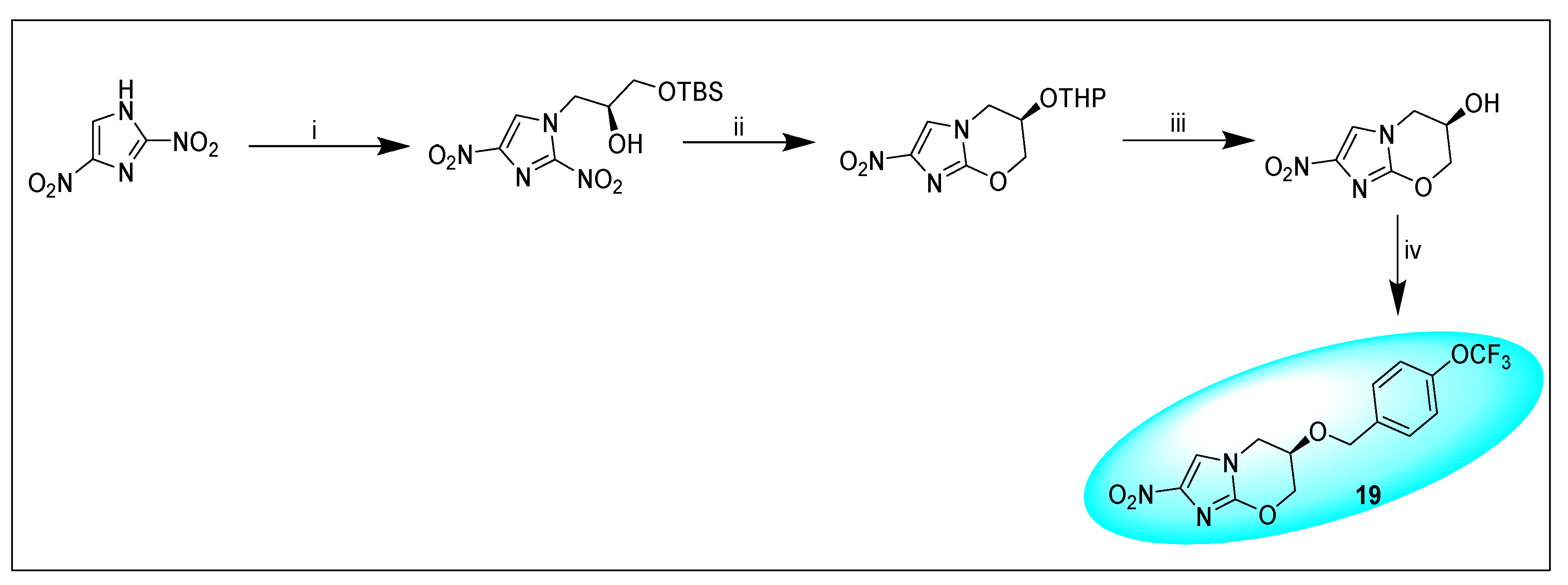
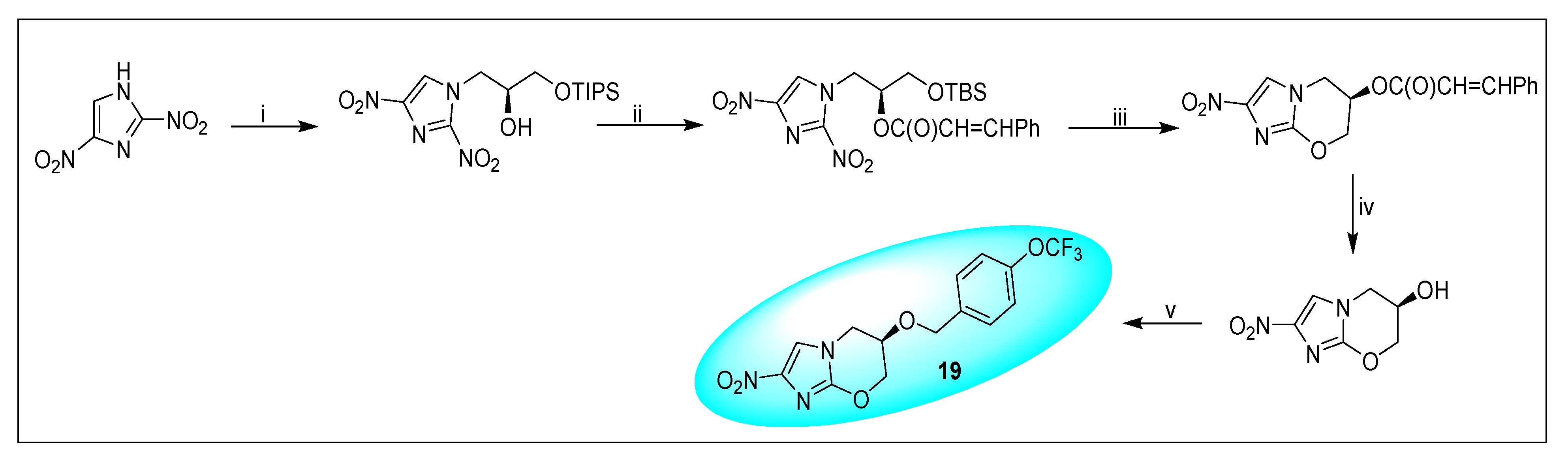
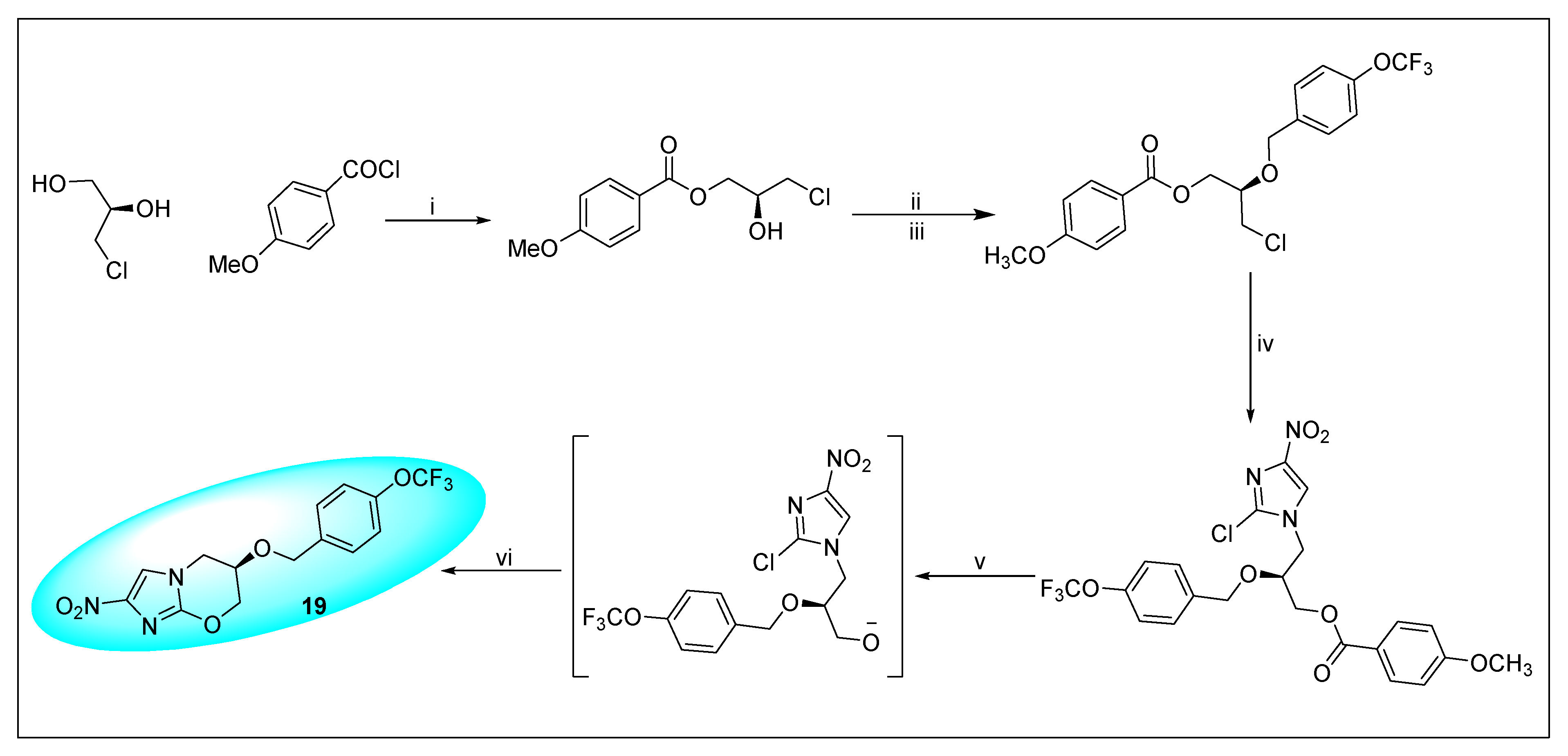
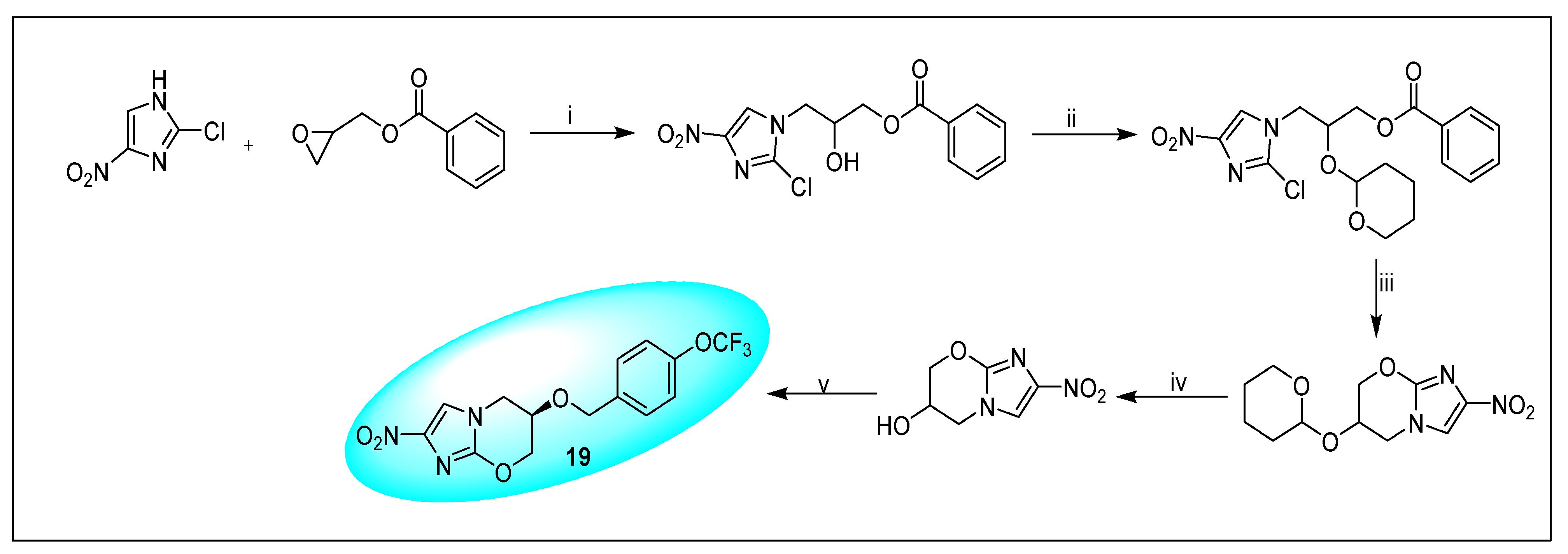
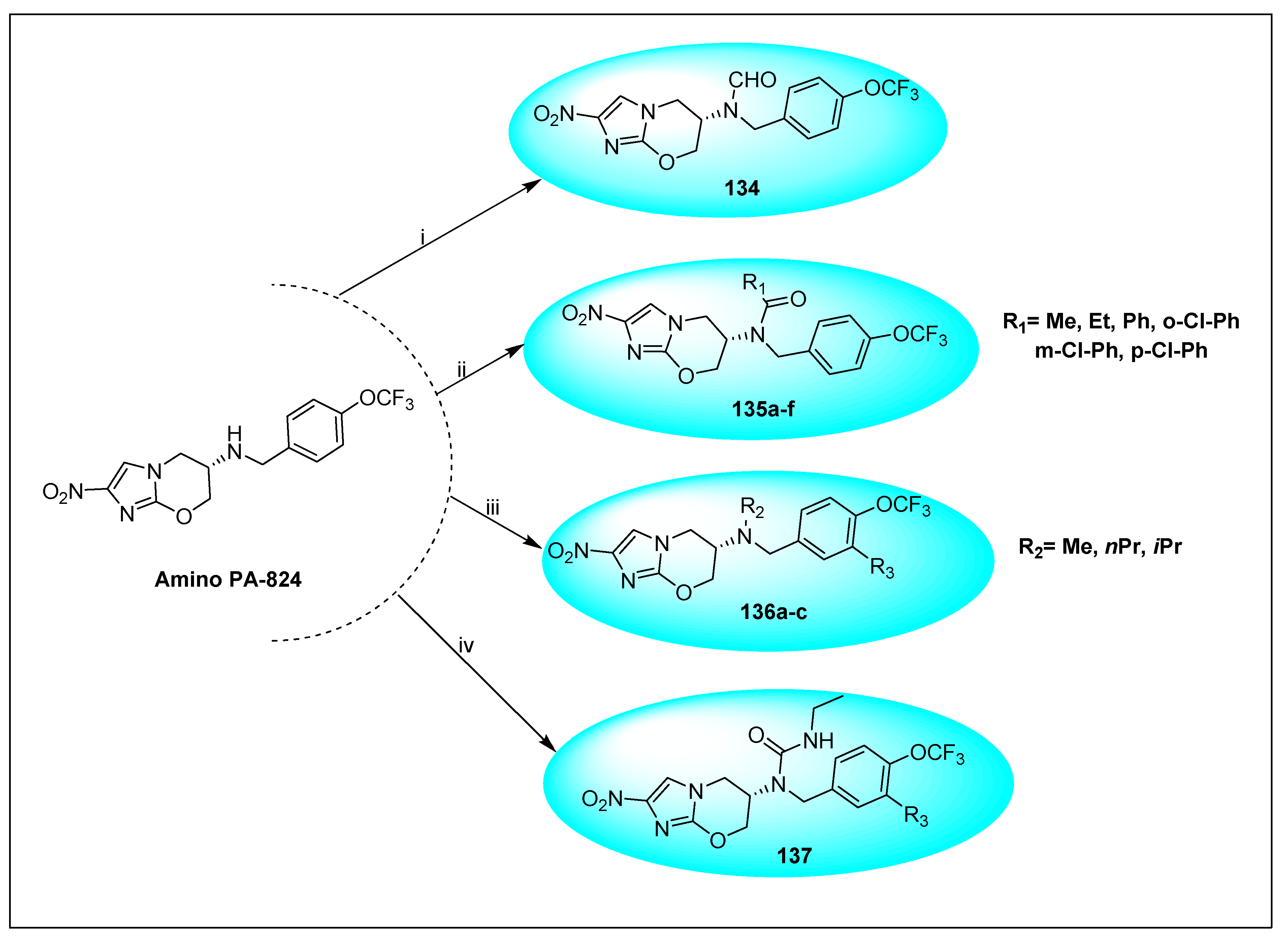
2.4.3. Pretonamid (PA-824) (19)
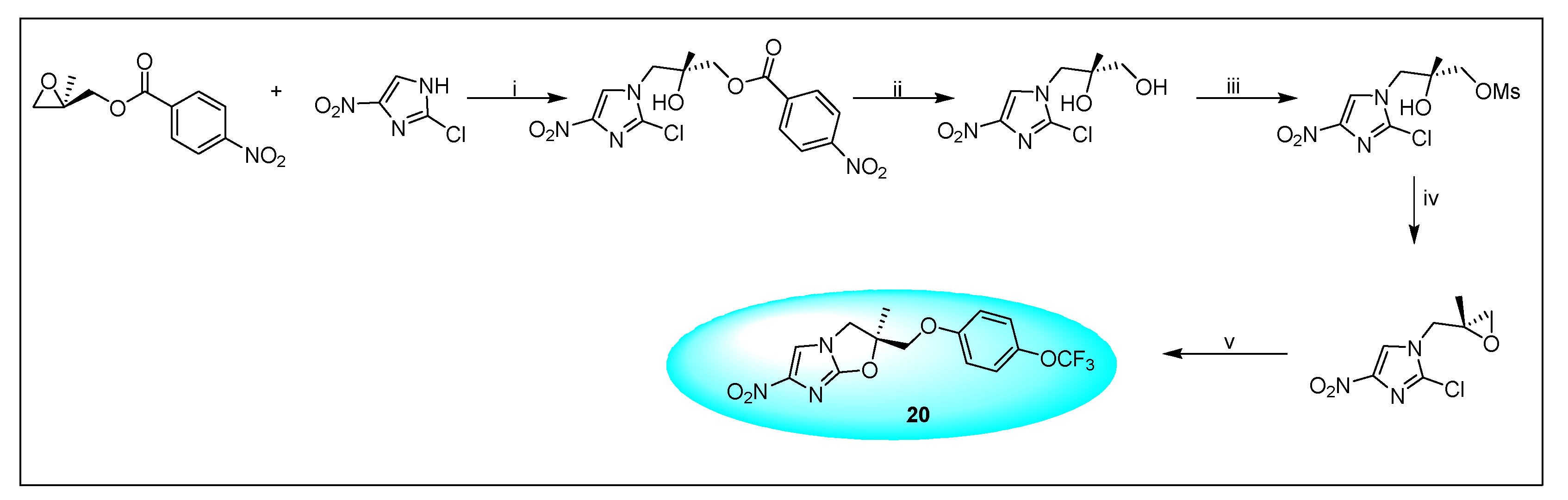
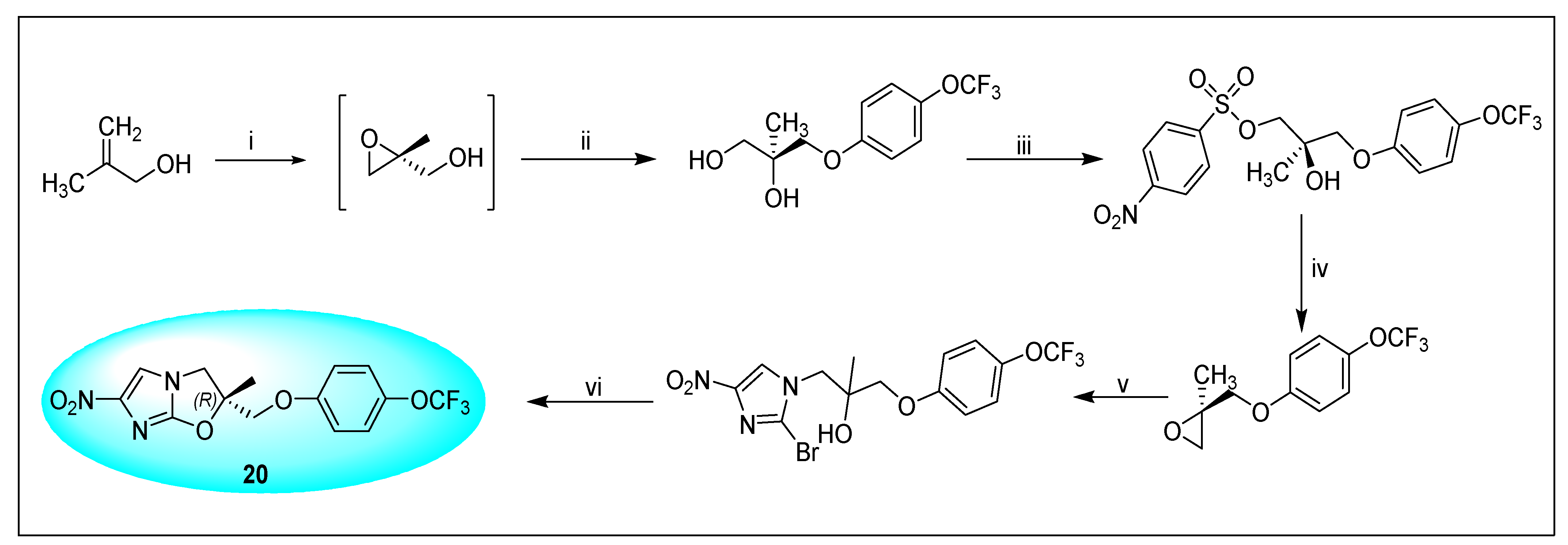
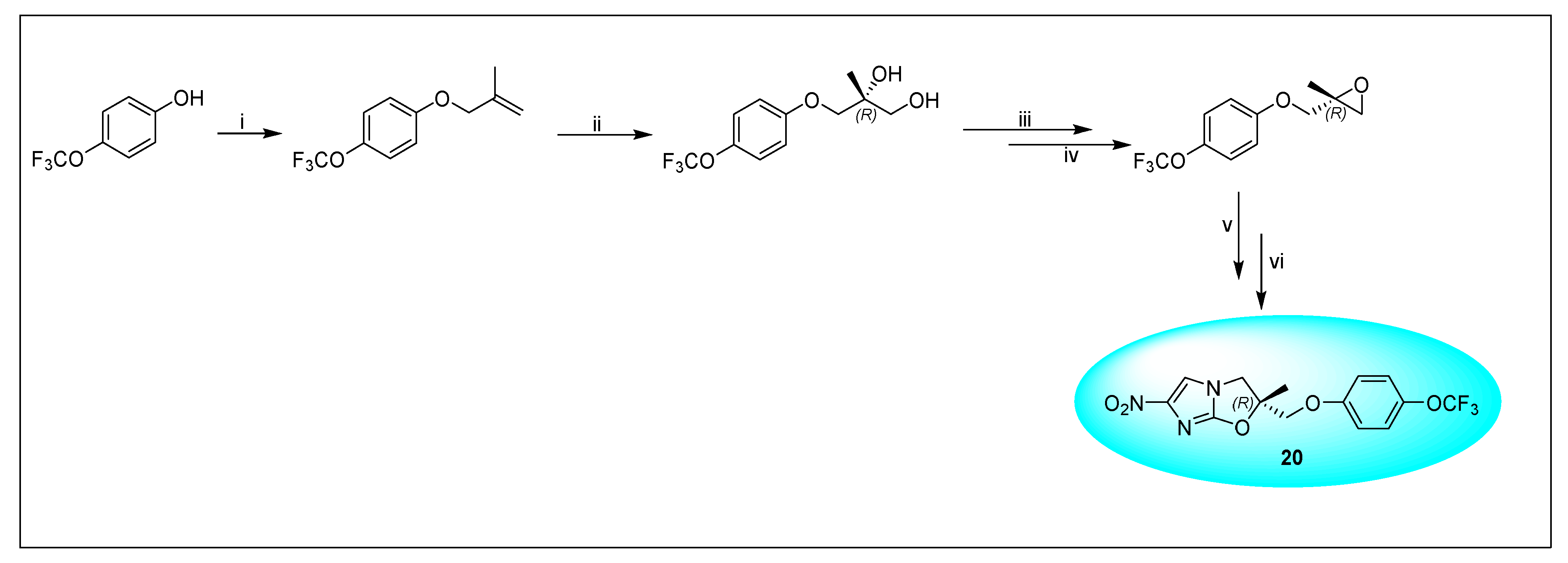
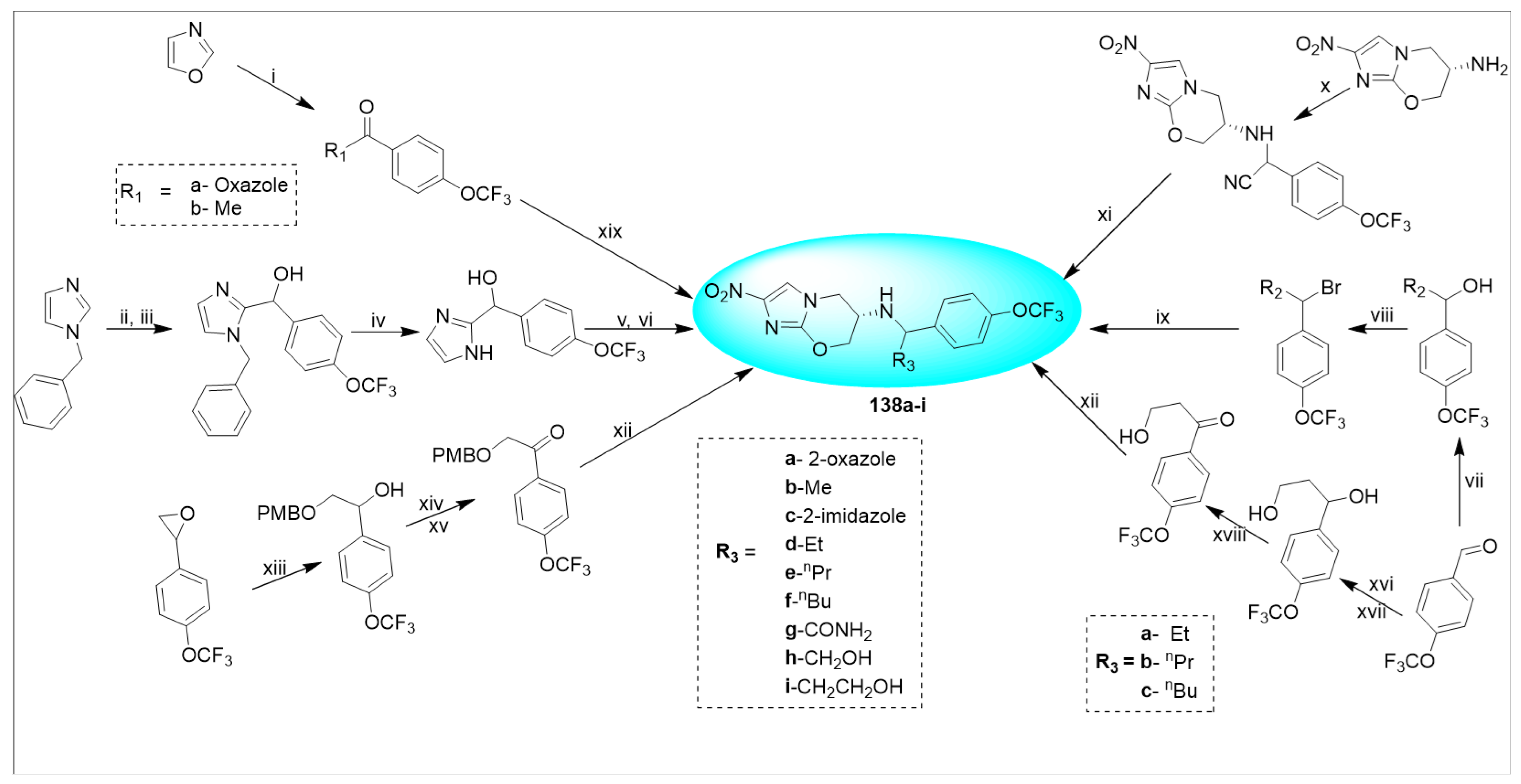
2.4.4. VL-2098 (20) and Its Derivatives
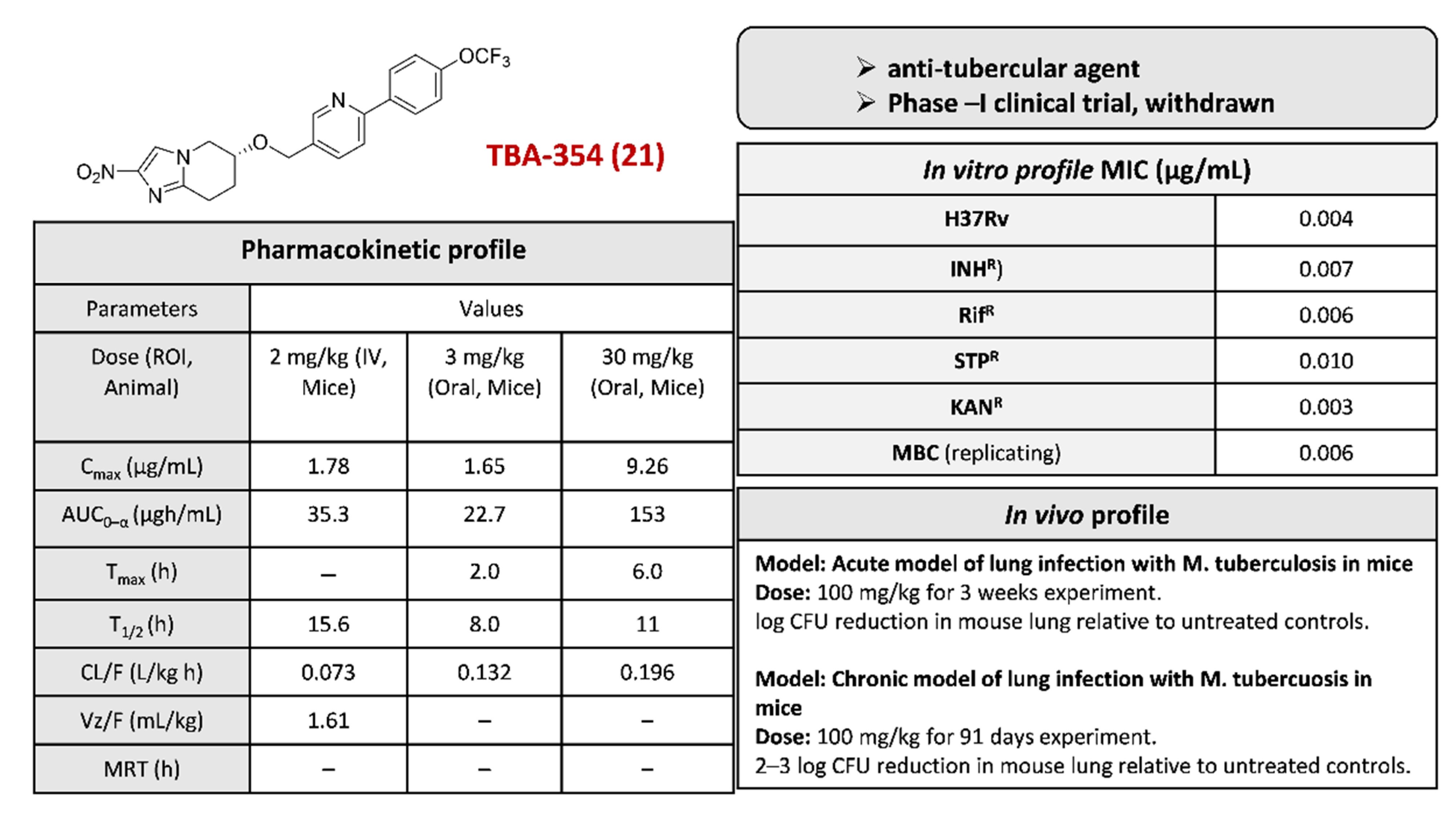
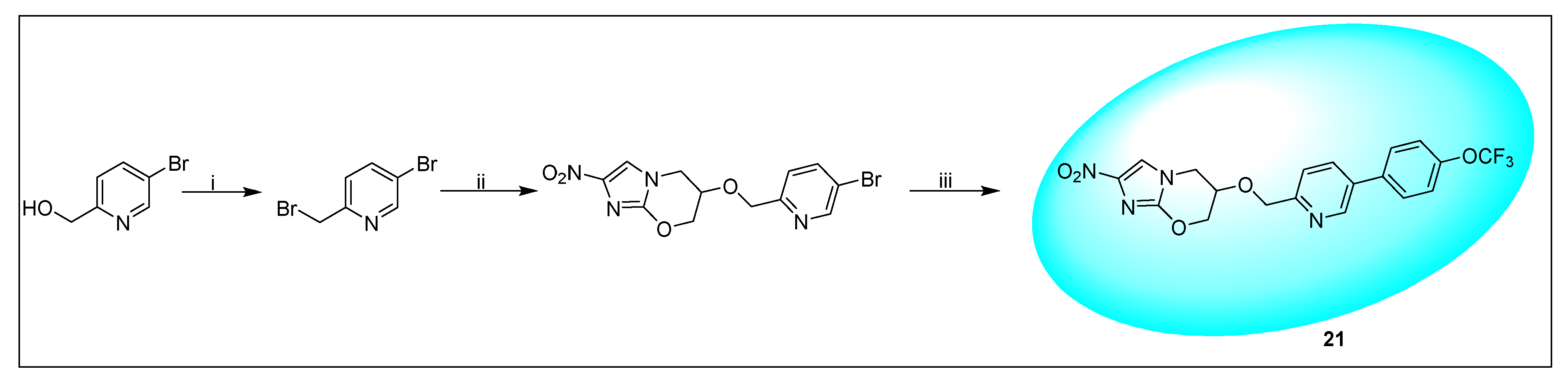
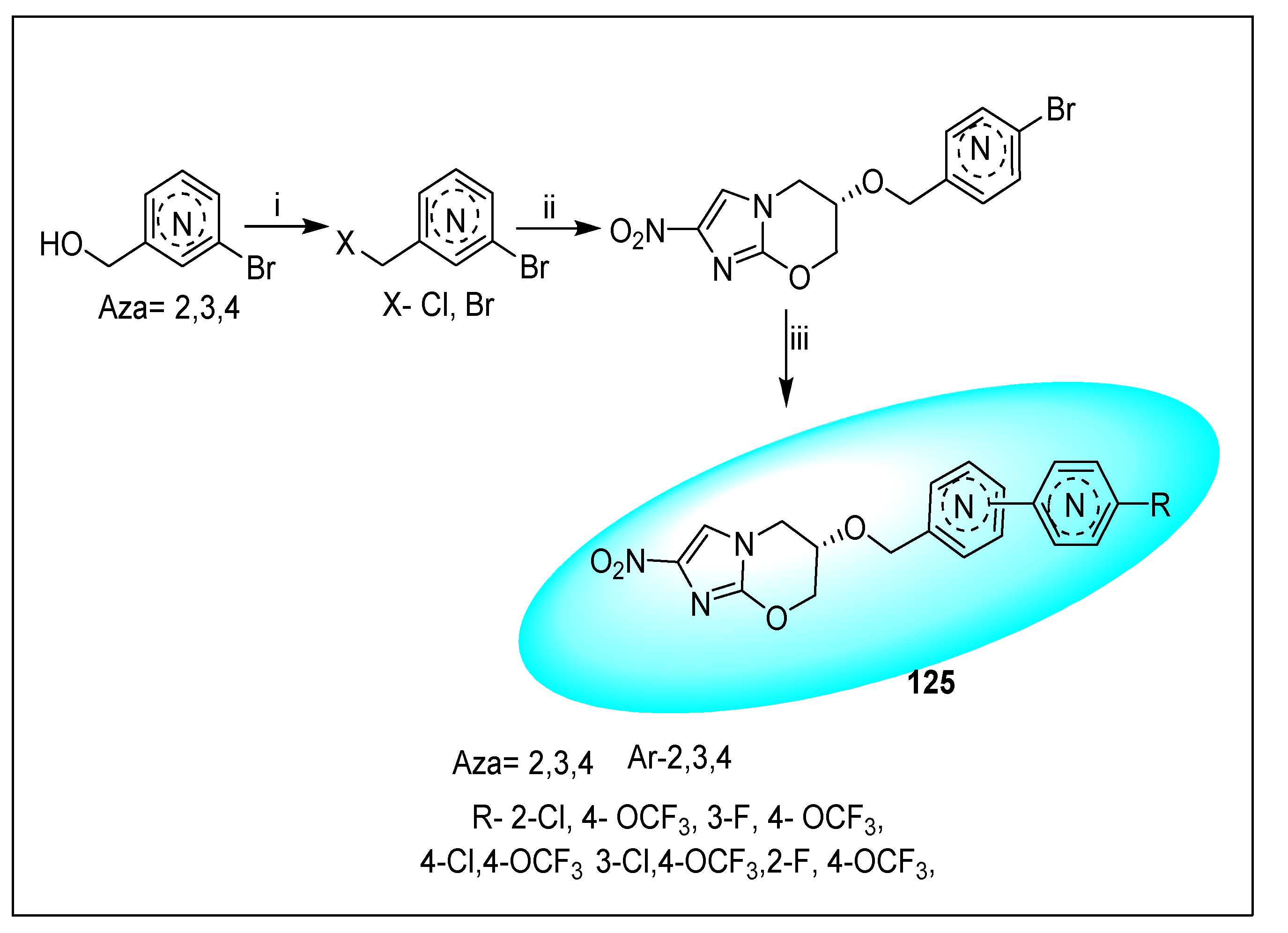
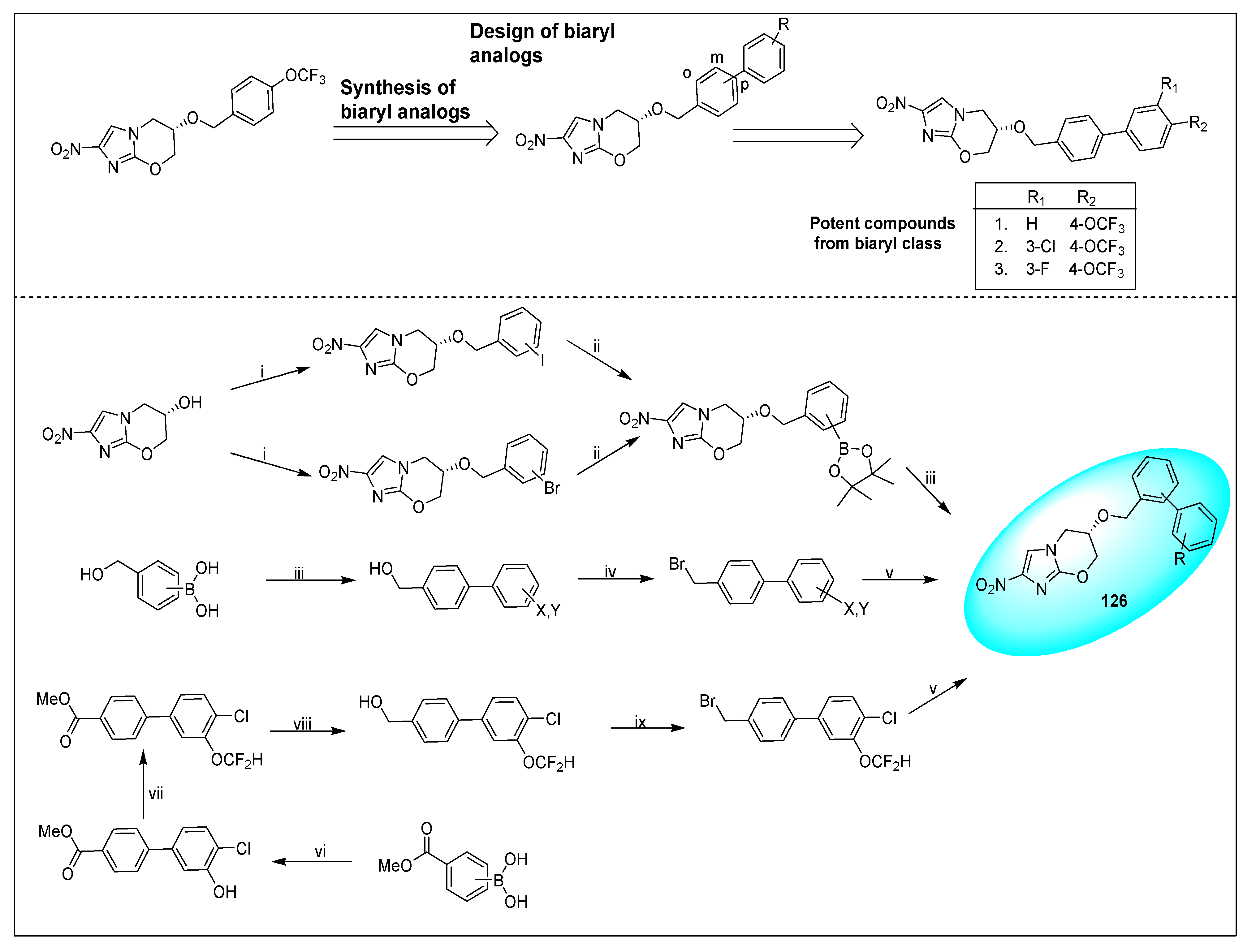
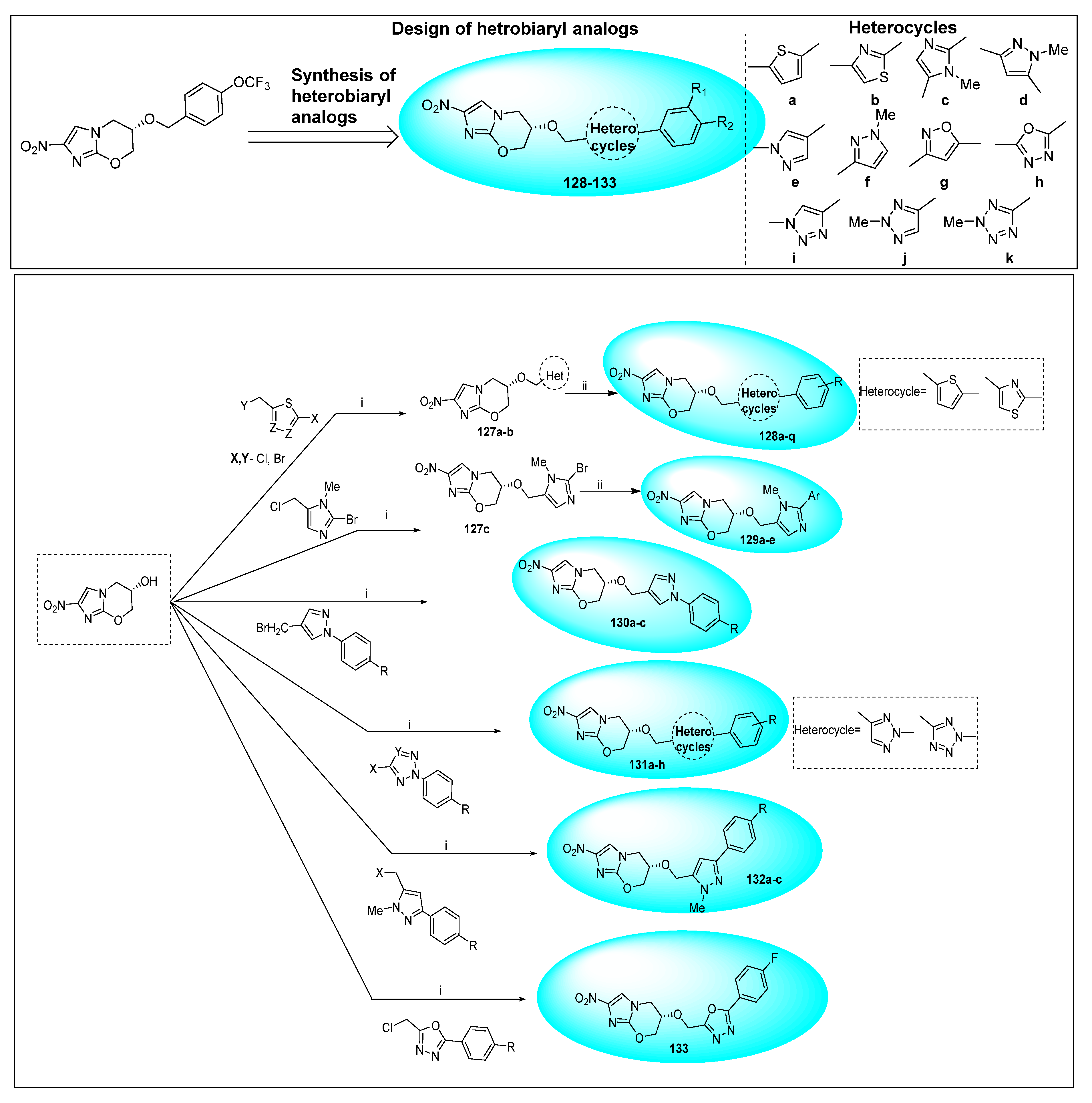
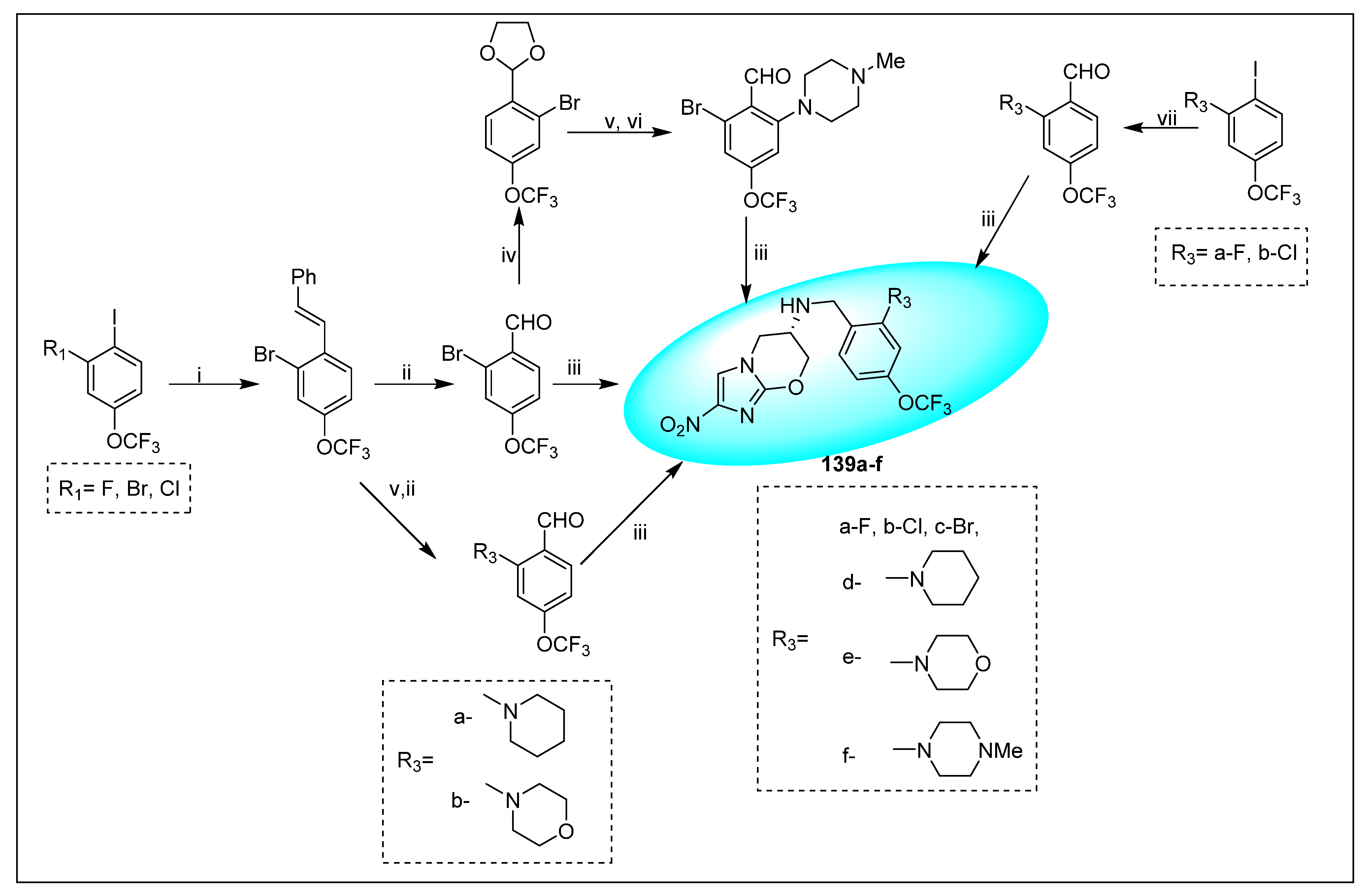
2.4.5. TBA-354 (21) and Its Derivatives
2.4.6. Miscellaneous Fused Nitroimidazoles
3. Mutagenicity and Genotoxicity of Selected Nitroimidazole Derivatives
3.1. Azomycin 1
3.2. Benznidazole 2
3.3. Misonidazole 3
3.4. Azathioprine 6
3.5. Metronidazole 7
3.6. Ornidazole 8
3.7. Tinidazole 9
3.8. Nimorazole 11
3.9. Secnidazole 12
3.10. Dimetridazole 13
3.11. Fexinidazole 14
3.12. Megazol 15
3.13. CGI-17341 17
3.14. Delamanid (OPC-67683) 18
3.15. Pretomanid (PA-824) 19
3.16. VL-2098 20
3.17. Nitroimidazole Derivatives Mutagenicity and Genotoxicity SARS: Selected Examples
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADMET | absorption, distribution, metabolism, elimination, and toxicity |
| AUC0-t | area under the concentration–time curve from 0 to the last quantifiable concentration estimated by the trapezoidal rule |
| AUC0-∞ | area under the curve extrapolated to infinity, after a single dose |
| BSA | body surface area |
| Cmax | maximum (or peak) serum concentration that a drug achieves |
| CL/F | oral clearance |
| Cl | rate at which the active drug is removed from the body |
| ELISA | enzyme-linked immunosorbent assay |
| FDA | Food and Drug Administration |
| F(%) | oral bioavailability |
| IC90 | concentrations leading to relative effects of 10% |
| KI | inhibitory constant |
| MIC50 | minimum inhibitory concentration (inhibition of 50%) |
| MIC90 | minimum inhibitory concentration (inhibition of 90%) |
| MRT | mean residence time of drug in the body |
| PK | drug pharmacokinetics |
| R&D | research and drug development |
| Tmax | amount of time that a drug is present at the maximum concentration in serum |
| t1/2 | time taken for levels of drug to decrease by half (a measure of the rate of elimination of the drug from plasma) |
| V/F | ratio of volume of distribution/bioavailability |
| Vd | olume of distribution is the theoretical volume that would be necessary to contain the total amount of an administered drug at the same concentration that it is observed in the blood plasma |
References
- Ang, C.W.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A.T. Nitroimidazoles: Molecular fireworks that combat a broad spectrum of infectious diseases. J. Med. Chem. 2017, 60, 7636–7657. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; Andricopulo, A.D. ADMET modeling approaches in drug discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, E.; Nelson, G.M.; Haynes, M.; Sargeant, F. Success rates for product development strategies in new drug development. J. Clin. Pharm. Ther. 2016, 41, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. A new antibiotic, azomycin. J. Antibiot. 1953, 6, 182. [Google Scholar]
- Nakamura, S. Structure of Azomycin, a new antibiotic. Pharm. Bull. 1955, 3, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Crozet, M.; Terme, T.; Vanelle, P. Designing new 5-nitroimidazoles: Towards safer anti-infectious agents. Lett. Drug Des. Discov. 2014, 11, 29. [Google Scholar] [CrossRef]
- Cosar, C.; Julou, L. Activity of 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole (8823 RP) against experimental. Trichomonas Vaginalis Ann. De L’institut Pasteur 1959, 96, 238–241. [Google Scholar]
- Freeman, C.D.; Klutman, N.E.; Lamp, K.C. Metronidazole. a therapeutic review and update. Drugs 1997, 54, 679–708. [Google Scholar] [CrossRef]
- Dunn, L.A.; Burgess, A.G.; Krauer, K.G.; Eckmann, L.; Vanelle, P.; Crozet, M.D.; Gillin, F.D.; Upcroft, P.; Upcroft, J.A. A new-generation 5-nitroimidazole can induce highly metronidazole-resistant Giardia lamblia in vitro. Int. J. Antimicrob. Agents 2010, 36, 37–42. [Google Scholar] [CrossRef]
- Torreele, E.; Trunz, B.B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazue, G.; Bray, M.A.; Pecoul, B. Fexinidazole-A new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e923. [Google Scholar] [CrossRef]
- Cerecetto, H.; González, M. Synthetic medicinal chemistry in Chagas’ disease: Compounds at the final stage of “Hit-to-Lead” phase. Pharmaceuticals 2010, 3, 810–838. [Google Scholar] [CrossRef]
- Via, L.E.; Ling Lin, P.L.; Ray, S.M.; Carrillo, J.; Allen, S.S.; Eum, S.Y.; Taylor, K.; Klein, E.; Manjunatha, U.; Gonzales, J.; et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 2008, 76, 2333–2340. [Google Scholar] [CrossRef]
- Duan, J.-X.; Jiao, H.; Kaizerman, J.; Stanton, T.; Evans, J.W.; Lan, L.; Lorente, G.; Banica, M.; Jung, D.; Wang, J. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J. Med. Chem. 2008, 51, 2412–2420. [Google Scholar] [CrossRef]
- The nitroimidazole family of derugs. Br. J. Vener. Dis. 1978, 54, 69–71.
- Rode, H.B.; Lade, D.M.; Grée, R.; Mainkar, P.S.; Chandrasekhar, S. Strategies towards the synthesis of anti-tuberculosis drugs. Org. Biomol. Chem. 2019, 17, 5428–5459. [Google Scholar] [CrossRef]
- Ashtekar, D.R.; Costa-Perira, R.; Nagrajan, K.; Vishvanathan, N.; Bhatt, A.D.; Rittel, W. In vitro and in vivo activities of the nitroimidazole CGI 17341 against Mycobacterium tuberculosis. Antimicrob. Agents. Chemother. 1993, 37, 183–186. [Google Scholar] [CrossRef]
- Nagarajan, K.; Shankar, R.G.; Rajappa, S.; Shenoy, S.J.; Costa-Pereira, R. Nitroimidazoles XXI 2,3-dihydro-6-nitroimidazo [2,1-b]oxazoles with antitubercular activity. Eur. J. Med. Chem. 1989, 24, 631–633. [Google Scholar] [CrossRef]
- Kapoor, V.K.; Chadha, R.; Venisetty, P.K.; Prasanth, S. Medicinal significance of nitroimidazoles-Some recent advances. J. Sci. Ind. Res. India 2003, 62, 659–665. [Google Scholar]
- Xavier, A.S.; Lakshmanan, M. Delamanid: A new armor in combating drug-resistant tuberculosis. J. Pharmacol. Pharmacother. 2014, 5, 222–224. [Google Scholar] [CrossRef]
- Low, M. The Tuberculosis Treatment Pipeline: A Breakthrough Year for the Treatment of XDR-TB. Pipeline Report. 2017. Available online: https://www.treatmentactiongroup.org/wp-content/uploads/2017/07/2017_pipeline_report_tb_treatment.pdf (accessed on 20 July 2017).
- Gupta, S.; Yardley, V.; Vishwakarma, P.; Shivahare, R.; Sharma, B.; Launay, D.; Puri, S.K. Nitroimidazo-oxazole compound DNDI-VL-2098: An orally effective preclinical drug candidate for the treatment of visceral leishmaniasis. J. Antimicrob. Chemother. 2014, 70, 518–527. [Google Scholar] [CrossRef]
- Mukherjee, T.; Boshoff, H. Nitroimidazoles for the treatment of TB: Past, present and future. Future Med. Chem. 2011, 3, 1427–1454. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.; Escobedo, A.A.; Deshpande, A.; Thota, P.; Roman, Y.; Hernandez, A.V. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: A systematic review of randomized controlled trials. PLoS Negl. Trop. Dis. 2014, 8, e2733. [Google Scholar] [CrossRef] [PubMed]
- Mital, A. Synthetic nitroimidazoles: Biological activities and mutagenicity relationships. Sci. Pharm. 2009, 77, 497–520. [Google Scholar] [CrossRef]
- Edwards, D.I. Nitroimidazole drugs-Action and resistance mechanisms. I. Mechanisms of action. J. Antimicrob. Chemother. 1993, 31, 9–20. [Google Scholar] [CrossRef]
- Davis, D.P.; Kirk, K.L.; Cohen, L.A. New synthesis of 2-nitroimidazoles. J. Heterocycl. Chem. 1982, 19, 253–256. [Google Scholar] [CrossRef]
- Larina, L.; Lopyrev, V. Nitroazoles: Synthesis, Structure and Applications; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Kalinin, S.; Vedekhina, T.; Paramonova, P.; Krasavin, M. Antimicrobial Activity of 5-membered Nitroheteroaromatic Compounds beyond Nitrofurans and Nitroimidazoles: Recent Progress. Curr. Med. Chem. 2021, 28, 5926–5982. [Google Scholar] [CrossRef]
- Rice, A.M.; Long, Y.; King, S.B. Nitroaromatic Antibiotics as Nitrogen Oxide Sources. Biomolecules 2021, 11, 267. [Google Scholar] [CrossRef]
- Boechat, N.; Carvalho, A.S.; Salomão, K.; de Castro, S.L.; Araujo-Lima, C.F.; Mello, F.V.C.; Felzenszwalb, I.; Aiub, C.A.F.; Conde, T.R.; Zamith, H.P.S.; et al. Studies of genotoxicity and mutagenicity of nitroimidazoles: Demystifying this critical relationship with the nitro group. Mem. Inst. Oswaldo Cruz. 2015, 110, 492–499. [Google Scholar] [CrossRef]
- Rajão, M.A.; Furtado, C.; Alves, C.L.; Passos-Silva, D.G.; de Moura, M.B.; Schamber-Reis, B.L.; Kunrath-Lima, M.; Zuma, A.A.; Vieira-da-Rocha, J.P.; Garcia, J.B.F.; et al. Unveiling Benznidazole’s mechanism of action through overexpression of DNA repair proteins in Trypanosoma cruzi. Environ. Mol. Mutagenesis 2014, 55, 309–321. [Google Scholar] [CrossRef]
- Mutschler, E.; Geisslinger, G.; Kroemer, H.K.; Ruth, P.; Schäfer-Korting, M. Arzneimittelwirkungen-Lehrbuch der Pharmakologie und Toxikologie. Endo-Praxis 2008, 24, 29. [Google Scholar] [CrossRef]
- Dinnendahl, V.; Fricke, U. Arzneistoff-Profile, 25th ed.; Govi Pharmazeutischer Verlag: Eschborn, Germany, 2011; ISBN 978-3-7741-9846-3. [Google Scholar]
- Steinhilber, D.; Schubert-Zsilavecz, M.; Roth, H.J. Medizinische Chemie; Deutscher Apotheker Verlag: Stuttgart, Germany, 2005; ISBN 978-3-7692-3483-1. [Google Scholar]
- Maltzman, J.S.; Koretzky, G.A. Azathioprine: Old drug, new actions. J. Clin. Investig. 2003, 111, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Mudde, S.E.; Upton, A.M.; Lenaerts, A.; Bax, H.I.; De Steenwinkel, J.E.M. Delamanid or pretomanid? A Solomonic judgement! J. Antimicrob. Chemother. 2022, 77, 880–902. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Groundwater, P.W.; Todd, A.; Worsley, A.J. Antibacterial Agents: Chemistry, Mode of Action, Mechanisms of Resistance and Clinical Applications; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Jenks, P.J.; Edwards, D.I. Metronidazole resistance in Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 19, 1–7. [Google Scholar] [CrossRef]
- Beaman, A.G.; Tautz, W.; Gabriel, T.; Duschinsky, R. The Synthesis of Azomycin. J. Am. Chem. Soc. 1965, 87, 389–390. [Google Scholar] [CrossRef]
- Qing, X.; Yu, Y.D.Y.; Ping, L.; Yuguo, Z. Improved synthesis of 2-Nitroimidazole. Chin. J. Pharm. Ind. 2001, 32, 557–558. [Google Scholar]
- Phukan, K.; Devi, N. Greener and Versatile Synthesis of Bioactive 2-Nitroimidazoles using Microwave Irradiation. J. Chem. Pharm. Res. 2011, 3, 1037–1044. [Google Scholar]
- Wilde, F.; Chamseddin, C.; Lemmerhirt, H.; Bednarski, P.J.; Jira, T.; Link, A. Evaluation of (S)-and (R)-M isonidazole as GPX Inhibitors: Synthesis, Characterization Including Circular Dichroism and In Vitro Testing on Bovine GP x-1. Arch. Der Pharm. 2014, 347, 153–160. [Google Scholar] [CrossRef]
- Hui, L.; Qiao-Juan, G.; Wei-Bing, W.; Chen-Zhong, Y.; Qiu-Ping, H. Improvement on the synthesis of 2-nitroimidazole. Appl. Chem. Ind. 2014, 3, 425–426. [Google Scholar]
- Zhao, X.X.; Zhang, J.C.; Li, S.H.; Yang, Q.P.; Li, Y.C.; Pang, S.P. A green and facile approach for synthesis of nitro heteroaromatics in water. Org. Process Res. Dev. 2014, 18, 886–890. [Google Scholar] [CrossRef]
- Castor, J.; Roberts, P.J. Novel imidazole derivatives and a process for manufacture therof. Drug Future 1977, 2, 568. [Google Scholar]
- Varia, M.A.; Calkins-Adams, D.P.; Rinker, L.H.; Kennedy, A.S.; Novotny, D.B.; Fowler, W.C.; Raleigh, J.A. Pimonidazole: A Novel Hypoxia Marker for Complementary Study of Tumor Hypoxia and Cell Proliferation in Cervical Carcinoma. Gynecol. Oncol. 1998, 71, 270–277. [Google Scholar] [CrossRef]
- Martin-Escolano, R.; Molina-Carreno, D.; Delgado-Pinar, E.; Martin-Montes, Á.; Clares, M.P.; Medina-Carmona, E.; Pitarch-Jarque, J.; Martín-Escolano, J.; Rosales, M.J.; García-España, E. New polyamine drugs as more effective antichagas agents than benznidazole in both the acute and chronic phases. Eur. J. Med. Chem. 2019, 164, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Perin, L.; Silva, R.M.; Fonsec, K.S.; Cardoso, J.M.O.; Mathias, F.A.S.; Reis, L.E.S.; Molin, I.; Oliveira, R.C.; Vieira, P.M.A.; Carneiro, C.M. Pharmacokinetics and Tissue Distribution of Benznidazole after Oral Administration in Mice. Antimicrob. Agents Chemother 2017, 61, e02410–e02416. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.G.J.; Batista, M.M.; Oliveira, G.M.; Britto, C.C.; Rodrigues, A.C.M.; Stephens, C.E.; Boykin, D.W.; Soeiro, M.N.C. Combined Treatment of Heterocyclic Analogues and Benznidazole upon Trypanosoma cruzi In Vivo. PLoS ONE 2011, 6, e2215. [Google Scholar] [CrossRef] [PubMed]
- Gamaliel, B.A.; Robert, D.; Paul, T.W. Novel Imidazole Derivatives and a Process for the Manufacture Thereof. GB Patent GB1138529A, 12 September 1969. [Google Scholar]
- Handal, V.E.; Arias, R.C.E.; Cuchilla, D.M.E.K. Production Method for Producing N-Benzyl-2-(2-Nitro-1H-Imidazole-1-yl)acetamide. Internatinal Patent WO2015/076760A1, 28 May 2015. [Google Scholar]
- Donadio, G.; Mariana, L.G.; Checura, C.; Santos, C.; Marina, C.; Julieta, M. A Method for Preparation of 2-Nitroimidazoles and Their Intermediates. Argentina Patent AR 97991A1, 2016. [Google Scholar]
- Lynsey, W. Method of Making Benznidazole. Internatinal Patent WO2017/205622Al, 30 November 2017. [Google Scholar]
- Rami, M.; Dubois, L.; Parvathaneni, N.-K.; Alterio, V.; van Kuijk, S.J.A.; Monti, S.M.; Lambin, P.; De Simone, G.; Supuran, C.T.; Winum, J.-Y. Hypoxia-Targeting Carbonic Anhydrase IX Inhibitors by a New Series of Nitroimidazole-Sulfonamides/Sulfamides/Sulfamates. J. Med. Chem. 2013, 56, 8512–8520. [Google Scholar] [CrossRef]
- Josephy, P.D.; Palcic, B.; Skarsgard, L.D. In vitro metabolism of misonidazole. Br. J. Cancer 1981, 43, 443–450. [Google Scholar] [CrossRef]
- Wardman, P. Nitroimidazoles as hypoxic cell radiosensitizers and hypoxia probes: Misonidazole, myths and mistakes. Br. J. Radiol. 2019, 92, 20170915. [Google Scholar] [CrossRef]
- Workman, P.; Brown, J.M. Structure-pharmacokinetic relationships for misonidazole analogues in mice. Cancer Chemother. Pharmacol. 1981, 6, 39–49. [Google Scholar] [CrossRef]
- Cowan, D.S.; Matejovic, J.F.; McClelland, R.A.; Rauth, A.M. DNA-targeted 2-nitroimidazoles: In vitro and in vivo studies. Br. J. Cancer 1994, 70, 1067–1074. [Google Scholar] [CrossRef][Green Version]
- Overgaard, J.; Overgaard, M.; Nielsen, O.S.; Pedersen, A.K.; Timothy, A.R. A comparative investigation of nimorazole and misonidazole as hypoxic radiosensitizers in a C3H mammary carcinoma in vivo. Br. J. Cancer 1982, 46, 904–911. [Google Scholar] [CrossRef]
- Yang, C.-C.; Goldberg, I.H. Synthesis of 1-([18O2]-2-nitro-1-imidazolyl)-3-methoxy-2-propanol ([18O2]-misonidazole). J. Label. Compd. Radiopharm. 1989, 27, 423–434. [Google Scholar] [CrossRef]
- Jin, C.-Z.; Nagasawa, H.; Shimamura, M.; Uto, Y.; Inayama, S.; Takeuchi, Y.; Kirk, K.L.; Hori, H. Angiogenesis inhibitor TX-1898: Syntheses of the enantiomers of sterically diverse haloacetylcarbamoyl-2-nitroimidazole hypoxic cell radiosensitizers. Bioorg. Med. Chem. 2004, 12, 4917–4927. [Google Scholar] [CrossRef] [PubMed]
- Kleiter, M.M.; Thrall, D.E.; Malarkey, D.E.; Ji, X.; Lee, D.Y.W.; Chou, S.-C.; Raleigh, J.A. A comparison of oral and intravenous pimonidazole in canine tumors using intravenous CCI-103F as a control hypoxia marker. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Min, D.I.; Monaco, A.P. Complications associated with immunosuppressive therapy and their management. Pharmacotherapy 1991, 11, 119s–125s. [Google Scholar] [PubMed]
- Castar, J.; Prous, J. Ro-038799. Drug Future 1986, 11, 580. [Google Scholar]
- Webb, P.; Threadgill, M.D. Labelled compounds of interest as antitumour agents. Part II (1). Synthesis of 2H and 3H isotopomers of RSU 1069 and Ro 03-8799 (pimonidazole). J. Label. Compd. Radiopharm. 1990, 28, 257–264. [Google Scholar] [CrossRef]
- Pourmorteza, M.; Rahman, Z.U.; Young, M. Evofosfamide, a new horizon in the treatment of pancreatic cancer. Anti-Cancer Drugs 2016, 27, 723–725. [Google Scholar] [CrossRef]
- Jung, D.; Lin, L.; Jiao, H.; Cai, X.; Duan, J.-X.; Matteucci, M. Pharmacokinetics of TH-302: A hypoxically activated prodrug of bromo-isophosphoramide mustard in mice, rats, dogs and monkeys. Cancer Chemother. Pharmacol. 2012, 69, 643–654. [Google Scholar] [CrossRef]
- Haynes, J.; McKee, T.D.; Haller, A.; Wang, Y.; Leung, C.; Gendoo, D.M.A.; Lima-Fernandes, E.; Kreso, A.; Wolman, R.; Szentgyorgyi, E.; et al. Administration of Hypoxia-Activated Prodrug Evofosfamide after Conventional Adjuvant Therapy Enhances Therapeutic Outcome and Targets Cancer-Initiating Cells in Preclinical Models of Colorectal Cancer. Clin. Cancer Res. 2018, 24, 2116. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, Y.; Zhao, Y.; Xue, C.; Zhan, J.; Liu, L.; He, X.; Zhang, L. Efficacy of the hypoxia-activated prodrug evofosfamide (TH-302) in nasopharyngeal carcinoma in vitro and in vivo. Cancer Commun. 2018, 38, 15. [Google Scholar] [CrossRef]
- Russell, J.; Carlin, S.; Burke, S.A.; Wen, B.; Yang, K.M.; Ling, C.C. Immunohistochemical Detection of Changes in Tumor Hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1177–1186. [Google Scholar] [CrossRef]
- O’Connor, L.J.; Cazares-Körner, C.; Saha, J.; Evans, C.N.; Stratford, M.R.; Hammond, E.M.; Conway, S.J. Efficient synthesis of 2-nitroimidazole derivatives and the bioreductive clinical candidate Evofosfamide (TH-302). Org. Chem. Front. 2015, 2, 1026–1029. [Google Scholar] [CrossRef]
- Cavalleri, B. Nitroimidazoles, Nitroimidazole Chemistry I: Synthetic Methods; NATO Advanced Study Institutes Series book series NSSA 42; Springer: Berlin/Heidelberg, Germany, 1933; pp. 9–33. [Google Scholar]
- Cole, C.; Reigan, P.; Gbaj, A.; Edwards, P.N.; Douglas, K.T.; Stratford, I.J.; Freeman, S.; Jaffar, M. Potential Tumor-Selective Nitroimidazolylmethyluracil Prodrug Derivatives: Inhibitors of the Angiogenic Enzyme Thymidine Phosphorylase. J. Med. Chem. 2003, 46, 207–209. [Google Scholar] [CrossRef]
- Papadopoulou, M.V.; Rosenzweig, H.S.; Bloomer, W.D. Synthesis of a novel nitroimidazole-spermidine derivative as a tumor-targeted hypoxia-selective cytotoxin. Bioorg. Med. Chem. Lett. 2004, 14, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.V.; Rosenzweig, H.S.; Bloomer, W.D. Synthesis of novel 2-nitroimidazole-tethered tricyclic quinolines, bearing a second heteroatom, and their in vitro evaluation as hypoxia-selective cytotoxins and radiosensitizers. Bioorg. Med. Chem. Lett. 2004, 14, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.V.; Bloomer, W.D. Nitroimidazole-based bioreductive compounds bearing a quinazoline or a naphthyridine chromophore. Anti-Cancer Drugs 2009, 20, 493–502. [Google Scholar] [CrossRef]
- Schweifer, A.; Hammerschmidt, F. Preparation of Nucleosides Derived from 2-Nitroimidazole and d-Arabinose, d-Ribose, and d-Galactose by the Vorbrüggen Method and Their Conversion to Potential Precursors for Tracers to Image Hypoxia. J. Org. Chem. 2011, 76, 8159–8167. [Google Scholar] [CrossRef]
- Mazuryk, O.; Krysiak-Foria, O.; Żak, A.; Suzenet, F.; Ptak-Belowska, A.; Brzozowski, T.; Stochel, G.; Brindell, M. Nitroimidazole derivatives of polypyridyl ruthenium complexes: Towards understanding their anticancer activity and mode of action. Eur. J. Pharm. Sci. 2017, 101, 43–55. [Google Scholar] [CrossRef]
- Elion, G.B.; Goodman, I.; Lange, W.; Hitchings, G.H. Condensed Pyrimidine Systems. XX. Purines Related to 6-Mercaptopurine and Thioguanine. J. Am. Chem. Soc. 1959, 81, 1898–1902. [Google Scholar] [CrossRef]
- Delmonico, F.L. KDIGO guideline for the care of kidney transplant recipients will be resource challenged. Kidney Int. 2010, 77, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef] [PubMed]
- Singer, O.; McCune, W.J. Update on maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis. Curr. Opin. Rheumatol. 2017, 29, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.; D’Cruz, D. Current and emerging treatment options in the management of lupus. Immunotargets Ther. 2016, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Model List of Essential Medicines; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Nath, B.; Nath, L.K. Formulation development and in-vitro/in-vivo correlation for a novel sterculia gum-based oral colon-targeted drug delivery system of azathioprine. Drug Dev. Ind. Pharm. 2013, 39, 1765–1773. [Google Scholar] [CrossRef]
- Elion, G.B.; Hitchings, G.H. 9-nucleoside-6-thioheterocycles and Method of Making. U.S. Patent 3176005, 30 March 1965. [Google Scholar]
- Krenitsky, T.A.; Hall, W.W.; Selph, J.L.; Truax, J.F.; Vinegar, R. Nucleosides of azathioprine and thiamiprine as antiarthritics. J. Med. Chem. 1989, 32, 1471–1475. [Google Scholar] [CrossRef]
- Crawford, D.J.K.; Maddocks, J.L.; Jones, D.N.; Szawlowski, P. Rational design of novel immunosuppressive drugs: analogues of azathioprine lacking the 6-mercaptopurine substituent retain or have enhanced immunosuppressive effects. J. Med. Chem. 1996, 39, 2690–2695. [Google Scholar] [CrossRef]
- Donskaya, O.V.; Elokhina, V.N.; Nakhmanovich, A.S.; Vakul’skaya, T.I.; Larina, L.I.; Vokin, A.I.; Albanov, A.I.; Lopyrev, V.A. Vicarious C-amination of 1-methyl-4-nitroimidazole. Tetrahedron Lett. 2002, 43, 6613–6616. [Google Scholar] [CrossRef]
- Al-Masoudi, N.A.; Al-Soud, Y.A.; De Clercq, E.; Pannecouque, C. Nitroimidazoles Part 6. Synthesis, structure and in vitro anti-HIV Activity of new 5-substituted piperazinyl-4-nitroimidazole derivatives. Antivir. Chem. Chemother. 2007, 18, 191–200. [Google Scholar] [CrossRef]
- Al-Soud, Y.A.; Al-Masoudi, N.A.; De Clercq, E.; Paneccoque, C. Nitroimidazoles, part 4: Synthesis and anti-HIV activity of new 5-alkylsulfanyl and 5-(4′-arylsulfonyl)piperazinyl-4-nitroimidazole derivatives. Heteroat. Chem. 2007, 18, 333–340. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, S.; Yun, M.-H.; Lee, Y.S.; Cho, S.N.; Oh, T.; Kim, P. Synthesis and antitubercular activity of monocyclic nitroimidazoles: Insights from econazole. Bioorg. Med. Chem. Lett. 2011, 21, 1515–1518. [Google Scholar] [CrossRef]
- Trunz, B.B.; Jędrysiak, R.; Tweats, D.; Brun, R.; Kaiser, M.; Suwiński, J.; Torreele, E. 1-Aryl-4-nitro-1H-imidazoles, a new promising series for the treatment of human African trypanosomiasis. Eur. J. Med. Chem. 2011, 46, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, Y.; Hu, Y.; Zhu, D.-D.; Zhang, S.; Liu, Z.-J.; Gong, H.-B.; Zhu, H.-L. Design, synthesis and antimicrobial activities of nitroimidazole derivatives containing 1,3,4-oxadiazole scaffold as FabH inhibitors. Bioorg. Med. Chem. 2012, 20, 4316–4322. [Google Scholar] [CrossRef]
- Makawana, J.A.; Sun, J.; Zhu, H.-L. Schiff’s base derivatives bearing nitroimidazole moiety: New class of antibacterial, anticancer agents and potential EGFR tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 6264–6268. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Ma, C.; Liu, Z. Synthesis of 2-azido-4-nitroimidazole and its derivatives for high-energy materials. Chin. J. Chem. 2013, 31, 1539–1545. [Google Scholar] [CrossRef]
- Abuteen, A.; Zhou, F.; Dietz, C.; Mohammad, I.; Smith, M.B.; Zhu, Q. Synthesis of a 4-nitroimidazole indocyanine dye-conjugate and imaging of tumor hypoxia in BALB/c tumor-bearing female mice. Dyes Pigm. 2016, 126, 251–260. [Google Scholar] [CrossRef]
- Woo, H.; Park, J.; Park, J.C.; Park, S.; Leed, J.M.; Park, K.H. Facile synthesis of hybrid Cu2O/Pd–Fe3O4 nanocatalysts for C–H arylation of 4-nitroimidazoles. RSC Adv. 2016, 6, 36211–36217. [Google Scholar] [CrossRef]
- Fung, H.B.; Doan, T.L. Tinidazole: A nitroimidazole antiprotozoal agent. Clin. Ther. 2005, 27, 1859–1884. [Google Scholar] [CrossRef]
- Becker, S.; Hoffman, P.; Houpt, E.R. Efficacy of antiamebic drugs in a mouse model. Am. J. Trop Med. Hyg. 2011, 84, 581–586. [Google Scholar] [CrossRef][Green Version]
- Gowrishankar, R.; Phadke, R.P.; Oza, S.D.; Talwalker, S. Satranidazole: Experimental evaluation of activity against anaerobic bacteria in vitro and in animal models of anaerobic infection. J. Antimicrob. Chemother. 1985, 15, 463–470. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Chen, Y.-F. Pharmacokinetics of metronidazole in rat blood, brain and bile studied by microdialysis coupled to microbore liquid chromatography. J. Chromatogr. A 2003, 987, 277–282. [Google Scholar] [CrossRef]
- Stranz, M.; Bradley, W. Metronidazole (Flagyl IV). Drug Intell. Clin. Pharm. 1981, 15, 838. [Google Scholar] [PubMed]
- Leitsch, D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Fajdiga, T.; Kajfez, F.; Sunjic, V. Process for Preparation of 1-(2′-hydroxyethyl)-2-methyl-5-nitroimidazole. U.S. Patent US3520900DA, 21 July 1970. [Google Scholar]
- Kraft, M.Y.; Kochergin, P.M.; Tsyganova, A.M.; Shlikhunova, V.S. Synthesis of metronidazole from ethylenediamine. Pharm. Chem. J. 1989, 23, 861–863. [Google Scholar] [CrossRef]
- Buforn, A.; Bernadette, M.C.; Massonneau, V.; Mulhauser, M. Method for Preparing 1-hydroxyalkyl-5-nitroimidazole. DK Patent DK14889D0, 13 January 1989. [Google Scholar]
- Lavigne, M.; Bernadette, M.C. Process for Preparing Hydroxyl alkyl-1-nitro-5 Imidazoles. European Patent EP19910906063, 13 September 1991. [Google Scholar]
- Wilcox, M.H. Nitroimidazoles, Metronidazole, Ornidazole and Tinidazole; and Fidaxomicin. Infect. Dis. 2017, 2, 1261–1263. [Google Scholar]
- Zhang, L.; Zhang, Z.; Wu, K. In vivo and real time determination of ornidazole and tinidazole and pharmacokinetic study by capillary electrophoresis with microdialysis. J. Pharm. Biomed. Anal. 2006, 41, 1453–1457. [Google Scholar] [CrossRef]
- Hizarcioğlu, S.Y.; Zeynep, A.Y.; Özyazici, M. Bioavailability File: Ornidazole. FABAD J. Pharm. Sci. 2004, 29, 133–144. [Google Scholar]
- Hu, J.; Zhang, J.; Wu, S.; Zhu, D.; Huang, H.; Chen, Y.; Yang, Y.; Zhang, Y. Evaluation of the in vitro activity of levornidazole, its metabolites and comparators against clinical anaerobic bacteria. Int. J. Antimicrob. Agents 2014, 44, 514–519. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mut. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Skupin, R.; Cooper, T.G.; Fröhlich, R.; Prigge, J.; Haufe, G. Lipase-catalyzed resolution of both enantiomers of Ornidazole and some analogues. Tetrahedron Asymmetry 1997, 8, 2453–2464. [Google Scholar] [CrossRef]
- Mandalapu, D.; Kushwaha, B.; Gupta, S.; Singh, N.; Shukla, M.; Kumar, J.; Tanpula, D.K.; Sankhwar, S.N.; Maikhuri, J.P.; Siddiqi, M.I.; et al. 2-Methyl-4/5-nitroimidazole derivatives potentiated against sexually transmitted trichomonas: Design, synthesis, biology and 3D-QSAR study. Eur. J. Med. Chem. 2016, 29, 820–839. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Tanaka, T. Session II: Pharmacology of Nitroimidazoles and Intestinal Amoebiasis. Drugs 1978, 15, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nord, C.E.; Kager, L. Tinidazole—Microbiology, pharmacology and efficacy in anaerobic infections. Infection 1983, 11, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lahnborg, G.; Nord, C.E. Efficacy of tinidazole compared to clindamycin in the treatment of experimentally induced intra-abdominal sepsis. J. Antimicrob. Chemother. 1982, 10, 117–121. [Google Scholar] [CrossRef]
- Dongare, T.D.; Bhalekar, M.R.; Gandhi, S.V. Formulation optimization and pharmacokinetics of tinidazole crystallo-co-agglomerates. MOJ Bioequiv. Availab. 2017, 3, 123–129. [Google Scholar]
- Chandorkar, J.G.; Umbarkar, S.B.; Rode, C.V.; Kotwal, V.B.; Dongare, M.K. Synthesis of tinidazole by condensation–oxidation sequence using MoO3/SiO2 bifunctional catalyst. Catal. Commun. 2007, 8, 1550–1555. [Google Scholar] [CrossRef]
- Nagarajan, K.; Arya, V.; George, T.; Sudarsanam, V.; Shah, R. Nitroimidazoles: Part IV. 1-Sulphonyl (carbamoyl/thiocarbamoyl)-3-(1-methyl-5-nitroimidazol-2-yl)-2-imidazolidinones. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1982, 21, 928–940. [Google Scholar]
- Rao, D.T.R.B. An Improved Method of Synthesising a Molecule Called Satranidazole. European Patent A61K31/41, 22 November 2003. [Google Scholar]
- Nicola, G.P.; Vittorio, M. N-tertiary amino-alkylene 4-or 5-nitroimidazoles and Their Preparation. U.S. Patent US3399193, 27 August 1968. [Google Scholar]
- Naik, A.M. Process for the Preparation of Nimorazole Formulation. Indian Patent Application 2995/MUM/2009, 2012. [Google Scholar]
- Das, S.; Dubey, R.; Roychowdhury, S.; Ghosh, M.; Sinha, B.N.; Kumar Pradhan, K.; Bal, T.; Muthukrishnan, V.; Seijas, J.A.; Pujarid, A. A rapid and sensitive determination of hypoxic radiosensitizer agent nimorazole in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 1575–1580. [Google Scholar] [CrossRef]
- Sugie, C.; Shibamoto, Y.; Ito, M.; Ogino, H.; Suzuki, H.; Uto, Y.; Nagasawa, H.; Hori, H. Reevaluation of the radiosensitizing effects of sanazole and nimorazole in vitro and in vivo. J. Radiat. Res. 2005, 46, 453–459. [Google Scholar] [CrossRef]
- Eke, I.G.; Eze, I.O.; Ezeudu, T.A.; Eze, U.U.; Anaga, A.O.; Onyeyili, P.A. Anti-trypanosomal activity of secnidazole in vitro and in vivo. Trop. J. Pharm. Res. 2017, 16, 535. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Chen, Y.; Liu, D.; Chen, X.; Zhong, D. Enantioselective HPLC determination and pharmacokinetic study of secnidazole enantiomers in rats. J. Chromatogr. B Biomed. Appl. 2014, 965, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Centre for Drug Evaluation and Research. Division of Anti-Infective Products. In Clinical Microbiology and Review; Centre for Drug Evaluation and Research: Silver Spring, MD, USA, 2017. [Google Scholar]
- Jeanmart, C.; Messer, M.N. Process for the Preparation of 5-nitroimidazole Derivatives. GB Patent GB1278758A, 21 June 1972. [Google Scholar]
- Hillier, K. Secnidazole. Drugs Future 1979, 4, 280. [Google Scholar] [CrossRef]
- Kuang, H.; Junhua, T. Synthesis Method of 5-nitroimidazole Drugs. CN Patent CN110922362A, 27 March 2020. [Google Scholar]
- Inghelbrecht, S.; Vermeersch, H.; Ronsmans, S.; Remon, J.-P.; DEBACKER, P.; Vercruysse, J. Pharmacokinetics and anti-trichomonal efficacy of a dimetridazole tablet and water-soluble powder in homing pigeons (Columb a livia). J. Vet. Pharmacol. Ther. 1996, 19, 62–67. [Google Scholar] [CrossRef]
- Callait, M.P.; Granier, C.; Chauve, C.; Zenner, L. In vitro activity of therapeutic drugs against Histomonas meleagridis. Poult. Sci. 2002, 81, 1122–1127. [Google Scholar] [CrossRef]
- Bahnous, M.; Bouraiou, A.; Chelghoum, M.; Bouacida, S.; Roisnel, T.; Smati, F.; Bentchouala, C.; Gros, P.C.; Belfaitah, A. Synthesis, crystal structure and antibacterial activity of new highly functionalized ionic compounds based on the imidazole nucleus. Bioorg. Med. Chem. Lett. 2013, 23, 1274–1278. [Google Scholar] [CrossRef]
- Estrada, A.A.; Feng, J.A.; Lyssikatos, J.P.; Sweeney, Z.K.; Fidalgo, D.V. Preparation of Novel heteroaryl-substituted Pyrimidines as Inhibitors of LRRK2. Internatinal Patent WO 2017156493, 9 September 2018. [Google Scholar]
- Yao, F.; Zhihua, D.; Shasha, Y.; Haozhe, Y.; Yunqu, P.; Wu, T.; Zuo, H.; Yongjun, Z.; Shen, C.; Hongfu, W. Method for synthesizing 1,2-dimethyl-5-nitroimidazole. CN Patent CN 108689941, 8 June 2018. [Google Scholar]
- Gil, C.; Rivas, L. Drug Discovery for Leishmaniasis; Royal Society of Chemistry: London, UK, 2017; Volume 60. [Google Scholar]
- Deeks, E.D. Fexinidazole: First Global Approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef]
- Samant, B.S.; Sukhthankar, M.G. Compounds containing 2-substituted imidazole ring for treatment against human African trypanosomiasis. Bioorg. Med. Chem. Lett. 2011, 21, 1015–1018. [Google Scholar] [CrossRef]
- Fontana, E.; Pignatti, A.; Venegoni, S.; Bray, M.A. Synthesis of 2H- and 14C-labeled fexinidazole and its primary metabolites labeled with 2H. J. Label. Compd. Radiopharm. 2011, 54, 714–719. [Google Scholar] [CrossRef]
- Zsolt, P.; Edit, A.; Zoltan, B.; Marton, H. Process for Praparation of Phenyloxymethyl-nitro-imidazole Derivatives Including Fexinidazole and Medicinal Use of Same. PL Patent PL922822T3, 22 November 2012. [Google Scholar]
- Berkelhammer, G.; Asato, G. 2-Amino-5-(1-methyl-5-nitro-2-imidazolyl)-1,3,4-thiadiazole: A New Antimicrobial Agent. Science 1968, 162, 1146. [Google Scholar] [CrossRef]
- Bouteille, B.; Marie-Daragon, A.; Chauvière, G.; de Albuquerque, C.; Enanga, B.; Dardé, M.-L.; Vallat, J.-M.; Périé, J.; Dumas, M. Effect of megazol on Trypanosoma brucei brucei acute and subacute infections in Swiss mice. Acta Trop. 1995, 60, 73–80. [Google Scholar] [CrossRef]
- Enanga, B.; Boudra, H.; Chauvière, G.; Labat, C.; Bouteille, B.; Dumas, M.; Houin, G. Pharmacokinetics, metabolism and excretion of megazol, a new potent trypanocidal drug in animals. Arzneim. Forsch. 1999, 49, 441–447. [Google Scholar] [CrossRef]
- Chauvière, G.; Bouteille, B.; Enanga, B.; de Albuquerque, C.; Croft, S.L.; Dumas, M.; Périé, J. Synthesis and Biological Activity of Nitro Heterocycles Analogous to Megazol, a Trypanocidal Lead. J. Med. Chem. 2003, 46, 427–440. [Google Scholar] [CrossRef]
- Foroumadi, A.; Rineh, A.; Emami, S.; Siavoshi, F.; Massarrat, S.; Safari, F.; Rajabalian, S.; Falahati, M.; Lotfali, E.; Shafiee, A. Synthesis and anti-Helicobacter pylori activity of 5-(nitroaryl)-1,3,4-thiadiazoles with certain sulfur containing alkyl side chain. Bioorg. Med. Chem. Lett. 2008, 18, 3315–3320. [Google Scholar] [CrossRef]
- Notowicz, A.; Stolz, E.; de Koning, G.A. First experiences with single-dose treatment of vaginal trichomoniasis with carnidazole (R 25831). Br. J. Vener. Dis. 1977, 53, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Munoz, E.; Castella, J.; Gutierrez, J.F. In vivo and in vitro sensitivity of Trichomonas gallinae to some nitroimidazole drugs. Vet. Parasitol. 1998, 78, 239–246. [Google Scholar] [CrossRef]
- N-2-5-nitro-1-imidazolyl-ethyl-carbamates. GB Patent GB1471753A, 27 April 1977.
- Benakli, K.; Terme, T.; Vanelle, P. Synthesis of new active sulfones in the 5-nitroimidazole series. Molecules 2002, 7, 382–385. [Google Scholar] [CrossRef]
- Benkli, K.; Karaburun, A.C.; GündoĞdu-Karaburun, N.; Demirayak, S. Synthesis and antimicrobial activities of some new nitroimidazole derivatives. Arch. Pharm. Res. 2003, 26, 773–777. [Google Scholar] [CrossRef]
- Upcroft, J.A.; Dunn, L.A.; Wright, J.M.; Benakli, K.; Upcroft, P.; Vanelle, P. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob. Agents Chemother. 2006, 50, 344–347. [Google Scholar] [CrossRef]
- Valdez, C.A.; Tripp, J.C.; Miyamoto, Y.; Kalisiak, J.; Hruz, P.; Andersen, Y.S.; Brown, S.E.; Kangas, K.; Arzu, L.V.; Davids, B.J.; et al. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J. Med. Chem. 2009, 52, 4038–4053. [Google Scholar] [CrossRef]
- Crozet, M.D.; Botta, C.; Gasquet, M.; Curti, C.; Rémusat, V.; Hutter, S.; Chapelle, O.; Azas, N.; De Méo, M.; Vanelle, P. Lowering of 5-nitroimidazole's mutagenicity: Towards optimal antiparasitic pharmacophore. Eur. J. Med. Chem. 2009, 44, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Crozet, M.D.; Zink, L.; Remusat, V.; Curti, C.; Vanelle, P. Efficient microwave-assisted palladium-catalyzed Suzuki-Miyaura cross-coupling reactions in 5-nitroimidazole series. Synthesis 2009, 18, 3150–3156. [Google Scholar]
- Moshafi, M.H.; Sorkhi, M.; Emami, S.; Nakhjiri, M.; Yahya-Meymandi, A.; Negahbani, A.S.; Siavoshi, F.; Omrani, M.; Alipour, E.; Vosooghi, M.; et al. 5-Nitroimidazole-based 1,3,4-thiadiazoles: Heterocyclic analogs of metronidazole as anti-helicobacter pylori agents. Arch. Pharm. 2011, 344, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Kalisiak, J.; Korthals, K.; Lauwaet, T.; Cheung, D.Y.; Lozano, R.; Cobo, E.R.; Upcroft, P.; Upcroft, J.A.; Berg, D.E.; et al. Expanded therapeutic potential in activity space of next-generation 5-nitroimidazole antimicrobials with broad structural diversity. Proc. Natl. Acad. Sci. USA 2013, 110, 17564–17569. [Google Scholar] [CrossRef]
- Makawana, J.A.; Sangani, C.B.; Lin, L.; Zhu, H.-L. Schiff’s base derivatives bearing nitroimidazole and quinoline nuclei: New class of anticancer agents and potential EGFR tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1734–1736. [Google Scholar] [CrossRef]
- Duan, Y.T.; Sang, Y.L.; Makawana, J.A.; Teraiya, S.B.; Yao, Y.F.; Tang, D.J.; Tao, X.X.; Zhu, H.L. Discovery and molecular modeling of novel 1-indolyl acetate-5-Nitroimidazole targeting tubulin polymerization as antiproliferative agents. Eur. J. Med. Chem. 2014, 85, 341–351. [Google Scholar] [CrossRef]
- Duana, Y.-T.; Yaoa, Y.-F.; Huang, W.; Makawana, J.A.; Teraiya, S.B.; Thumar, N.J.; Tang, D.-J.; Tao, X.-X.; Wanga, Z.-C.; Jiang, A.-Q.; et al. Synthesis, biological evaluation, and molecular docking studies of novel 2-styryl-5-nitroimidazole derivatives containing 1,4-benzodioxan moiety as FAK inhibitors with anticancer activity. Bioorg. Med. Chem. 2014, 22, 2947–2954. [Google Scholar] [CrossRef]
- Duan, Y.-T.; Wang, Z.-C.; Sang, Y.-L.; Tao, X.-X.; Teraiya, S.B.; Wang, P.-F.; Wen, Q.; Zhou, X.-J.; Ding, L.; Yang, Y.-H.; et al. Design and synthesis of 2-styryl of 5-Nitroimidazole derivatives and antimicrobial activities as FabH inhibitors. Eur. J. Med. Chem. 2014, 76, 387–396. [Google Scholar] [CrossRef]
- Adamovich, S.N.; Ushakov, I.A.; Mirskova, A.N.; Mirskov, R.G.; Voronov, V.K. Novel complexes of 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole with metal acetates and arylchalcogenylacetates. Mendeleev Commun. 2014, 24, 293–294. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Al-Qaoud, K.M.; Abu-Qatouseh, L.F.; Shihab, P.; Kaur, H.; Goyal, K.; Sehgal, R.; Mubarak, M.S. Synthesis and biological activity of novel amidrazones incorporating 5-nitroimidazole, ciprofloxacin, and 7-chloro-4-piperazinylquinoline. Med. Chem. Res. 2015, 24, 2247–2256. [Google Scholar] [CrossRef]
- Dingsdag, S.; Benjamin, C.-M.; Yap, B.C.-M.; Hunter, N.; Crossley, M.J. Amino acid-linked porphyrin-nitroimidazole antibiotics targeting Porphyromonas gingivalis. Org. Biomol. Chem. 2015, 13, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sangani, C.B.; Jia, L.-X.; Gong, P.-X.; Wang, F.; Wang, J.-F.; Zhu, H.-L. Design, synthesis, and antibacterial evaluation of new Schiff’s base derivatives bearing nitroimidazole and pyrazole nuclei as potent E. coli FabH inhibitors. Res. Chem. Intermed. 2015, 41, 10137–10149. [Google Scholar]
- Jarrad, A.M.; Debnath, A.; Miyamoto, Y.; Hansford, K.A.; Pelingon, R.; Butler, M.S.; Bains, T.; Karoli, T.; Blaskovich, M.A.T.; Eckmann, L.; et al. Nitroimidazole carboxamides as antiparasitic agents targeting Giardia lamblia, Entamoeba histolytica and Trichomonas vaginalis. Eur. J. Med. Chem. 2016, 120, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.-X.; Duan, Y.-T.; Chen, L.-W.; Tang, D.-J.; Yang, M.-R.; Wang, P.-F.; Xu, C.; Zhu, H.-L. Design, synthesis and biological evaluation of pyrazolyl-nitroimidazole derivatives as potential EGFR/HER-2 kinase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 677–683. [Google Scholar] [CrossRef]
- Spitz, C.; Mathias, F.; Giuglio-Tonolo, A.G.; Terme, T.; Vanelle, P. Practical and metal-free synthesis of novel enantiopure amides containing the potentially bioactive 5-nitroimidazole moiety. Molecules 2016, 21, 1472. [Google Scholar] [CrossRef]
- Sasahara, K.; Shimokawa, Y.; Hirao, Y.; Koyama, N.; Kitano, K.; Shibata, M.; Umehara, K. Pharmacokinetics and metabolism of delamanid, a novel anti-tuberculosis drug, in animals and humans: Importance of albumin metabolism in vivo. Drug Metab. Dispos. 2015, 43, 1267–1276. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hashizume, H.; Tomishige, T.; Kawasaki, M.; Tsubouchi, H.; Sasaki, H.; Shimokawa, Y.; Komatsu, M. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006, 3, e466. [Google Scholar] [CrossRef]
- Sasaki, H.; Haraguchi, Y.; Itotani, M.; Kuroda, H.; Hashizume, H.; Tomishige, T.; Kawasaki, M.; Matsumoto, M.; Komatsu, M.; Tsubouchi, H. Synthesis and antituberculosis activity of a novel series of optically active 6-nitro-2, 3-dihydroimidazo [2, 1-b] oxazoles. J. Med. Chem. 2006, 49, 7854–7860. [Google Scholar] [CrossRef]
- Hidetsugu, T.; Yoshikazu, H.; Satoshi, H.; Naoto, U.; Shinichi, T.; Yoshihisa, T.; Nobuhisa, F.; Koichi, S.; Kimiyoshi, A.; Takuya, F. Epoxy Compound and Method for Manufacturing the Same. International Patent WO2008/140090 A1, 20 November 2008. [Google Scholar]
- Akihiro, Y.; Koichi, S.; Nobuhisa, F.; Shinji, A.; Shin, O.; Naoto, U. Synthetic Intermediate of Oxazole Compound and Method for Producing the Same. International Patent WO2011093529A1, 4 August 2011. [Google Scholar]
- Timmins, G.; Choi, S.W.; Silks, L.P.A. Rapid Phenotype Tests for Antitubercular Drug Sensitivity and Resistance. U.S. Patent US20170010272A1, 12 January 2017. [Google Scholar]
- Patterson, S.; Wyllie, S.; Norval, S.; Stojanovski, L.; Simeons, R.F.; Auer, J.L.; Osuna-Cabello, M.; Read, K.D.; Fairlamb, A.H. The anti-tubercular drug delamanid as a potential oral treatment for visceral leishmaniasis. Elife 2016, 5, e09744. [Google Scholar] [CrossRef]
- Sharma, S.; Anand, R.; Cham, P.S.; Raina, S.; Vishwakarma, R.A.; Singh, P.P. A concise and sequential synthesis of the nitroimidazooxazole based drug, Delamanid and related compounds. RSC Adv. 2020, 10, 17085–17093. [Google Scholar] [CrossRef]
- Lenaerts, A.J.; Veronica Gruppo, V.; Marietta, K.S.; Johnson, C.M.; Driscoll, D.K.; Tompkins, N.M.; Rose, J.D.; Reynolds, R.C.; Orme, I.M. Preclinical Testing of the Nitroimidazopyran PA-824 for Activity against Mycobacterium tuberculosis in a Series of In Vitro and In Vivo Models. Antimicrob. Agents Chemother. 2005, 49, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, Y.; Duan, H.; Yao, J.; Liang, L.; Zhang, R.; Zhou, X.; Liu, X.; Wang, Q.; Zhang, S. Pharmacokinetics and tissue distribution study of PA-824 in rats by LC–MS/MS. J. Chromat. B 2015, 1006, 194–200. [Google Scholar] [CrossRef]
- Thompson, A.M.; Marshall, A.J.; Maes, L.; Yarlett, N.; Bacchi, C.J.; Gaukel, E.; Wring, S.A.; Launay, D.; Braillard, S.; Chatelain, E.; et al. Assessment of a pretomanid analogue library for African trypanosomiasis: Hit-to-lead studies on 6-substituted 2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine 8-oxides. Bioorg. Med. Chem. Lett. 2018, 28, 207–2013. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.; Wyllie, S.; Stojanovski, L.; Perry, M.R.; Simeons, F.R.C.; Norval, S.; Osuna-Cabello, M.; Rycker, M.D.; Read, K.D.; Fairlamb, A.H. The R Enantiomer of the Antitubercular Drug PA-824 as a Potential Oral Treatment for Visceral Leishmaniasis. Antimicrob. Agents Chemother. 2013, 57, 4699–4706. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.R.; Shaopei, C.; Keeler, E.L. Nitro-[2,1-b]imidazopyran Compounds and Antibacterial Uses Thereof. U.S. Patent US6,087,358, 11 July 2000. [Google Scholar]
- Orita, A.; Miwa, K.; Uehara, G.; Otera, J. Integration of Solventless Reaction in a Multi-Step Process: Application to an Efficient Synthesis of PA-824. Adv. Synth. Catal. 2007, 349, 2136–2144. [Google Scholar] [CrossRef]
- Marsini, M.A.; Reider, P.J.; Sorensen, E.J. A Concise and Convergent Synthesis of PA-824. J. Org. Chem. 2010, 75, 7479–7482. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.R.; Malhotra, G.; Pullela, V.S.; Patil, S.L.; Rajeshirke, R.R. Process for the Preparation of Nitroimidazole Compounds, Especially the Tuberculostatic Pretomanid. IN Patent IN201621026053, 2 February 2018. [Google Scholar]
- Mukkavilli, R.; Pinjari, J.; Patel, B.; Sengottuvelan, S.; Mondal, S.; Gadekar, A.; Verma, M.; Patel, J.; Pothuri, L.; Chandrashekar, G.; et al. In vitro metabolism, disposition, preclinical pharmacokinetics and prediction of human pharmacokinetics of DNDI-VL-2098, a potential oral treatment for Visceral Leishmaniasis. Eur. J. Pharm. Sci. 2014, 65, 147–155. [Google Scholar] [CrossRef]
- Satam, V.S.; Pedada, S.R.; Kamaraj, P.; Antao, N.; Singh, A.; Hindupur, R.M.; Pati, H.N.; Thompson, A.M.; Launay, D.; Martin, D. Development of a Scalable Process for the Synthesis of DNDI-VL-2098: A Potential Preclinical Drug Candidate for the Treatment of Visceral Leishmaniasis. Org. Process Res. Dev. 2017, 21, 52–59. [Google Scholar] [CrossRef]
- Upton, A.M.; Cho, S.; Yang, T.J.; Kim, Y.; Wang, Y.; Lu, Y.; Wang, B.; Xu, J.; Mdluli, K.; Ma, Z.; et al. In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 136–144. [Google Scholar] [CrossRef]
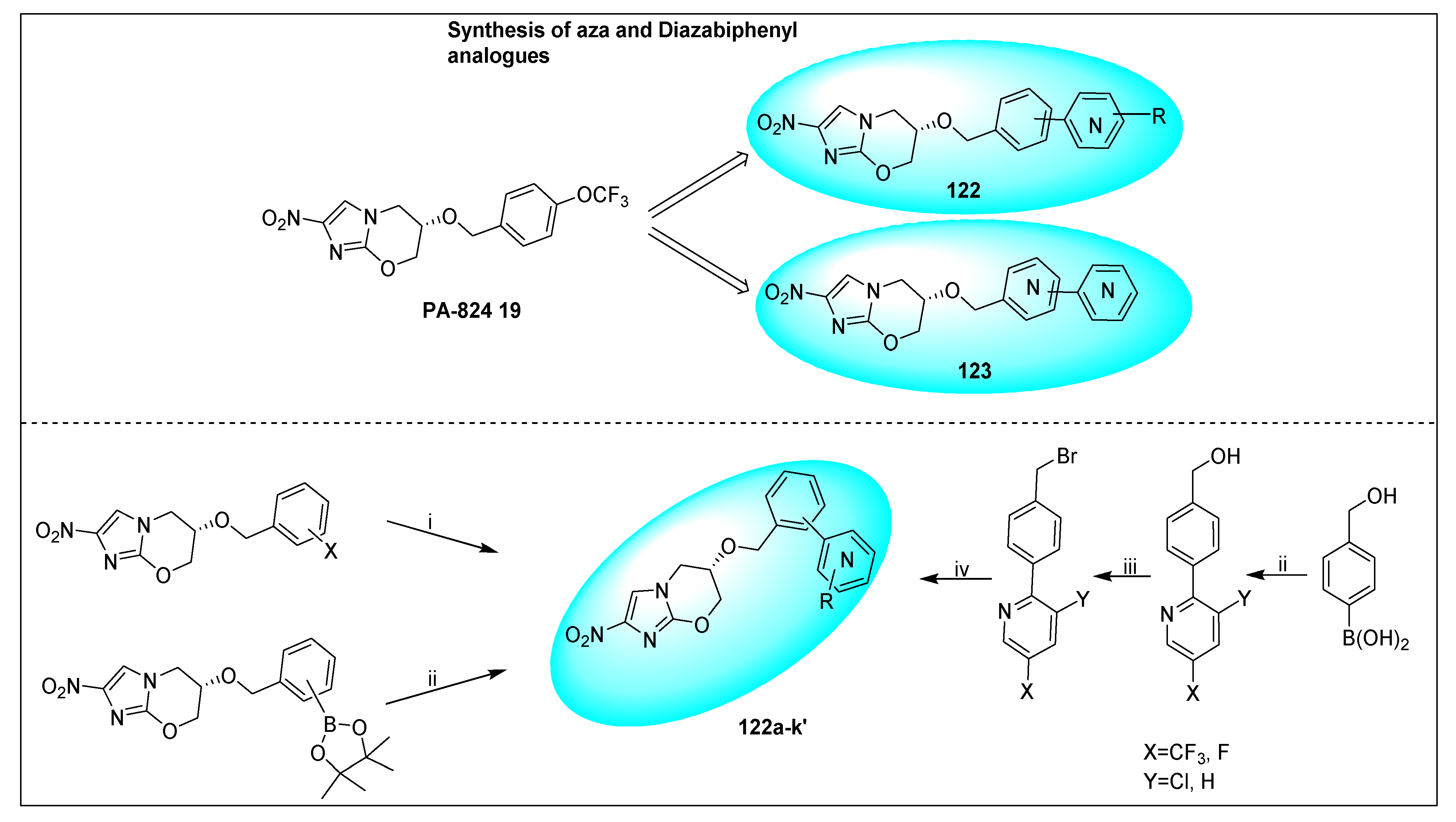
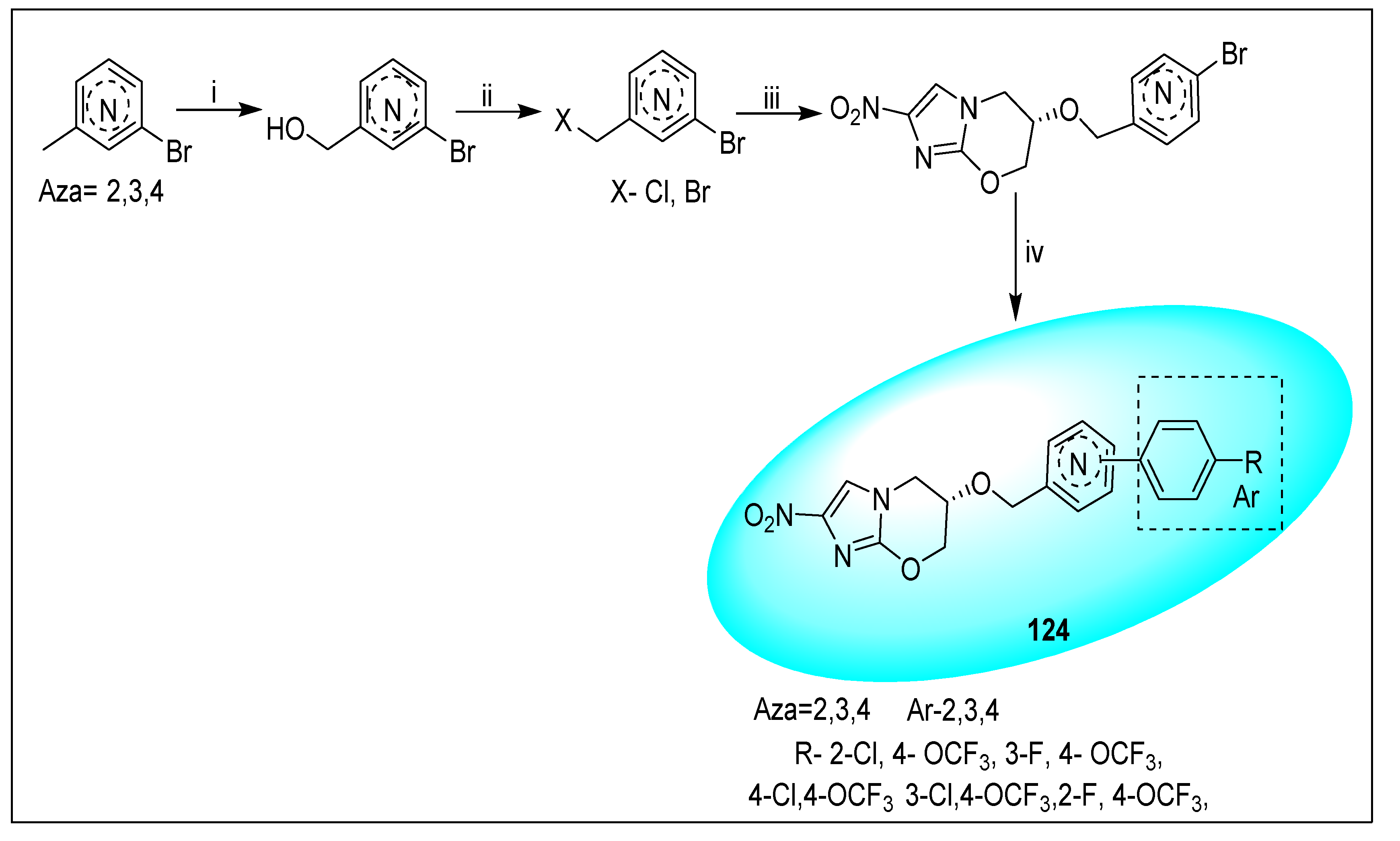
- Kmentova, I.; Sutherland, H.S.; Palmer, B.D.; Blaser, A.; Franzblau, S.G.; Wan, B.; Wang, Y.; Ma, Z.; Denny, W.A.; Thompson, A.M. Synthesis and Structure−Activity Relationships of Aza- and Diazabiphenyl Analogues of the Antitubercular Drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2010, 53, 8421–8439. [Google Scholar] [CrossRef]
- Palmer, B.D.; Thompson, A.M.; Sutherland, H.S.; Blaser, A.; Kmentova, I.; Franzblau, S.G.; Wan, B.; Wang, Y.; Ma, Z.; Denny, W.A. Synthesis and structure-activity studies of biphenyl analogues of the tuberculosis drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2010, 53, 282–294. [Google Scholar] [CrossRef]
- Sutherland, H.S.; Blaser, A.; Kmentova, I.; Franzblau, S.G.; Wan, B.; Wang, Y.; Ma, Z.; Palmer, B.D.; Denny, W.A.; Thompson, A.M. Synthesis and structure−activity relationships of antitubercular 2-nitroimidazooxazines bearing heterocyclic side chains. J. Med. Chem. 2010, 53, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Cherian, C.; Choi, I.; Nayyar, A.; Manjunatha, U.H.; Mukherjee, T.; Lee, Y.S.; Boshoff, H.I.; Singh, R.; Ha, Y.H.; Goodwin, M.; et al. Structure-activity relationships of antitubercular nitroimidazoles. 3. Exploration of the linker and lipophilic tail of ((s)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazin-6-yl)-(4-trifluoromethoxybenzyl)amine (6-amino PA-824). J. Med. Chem. 2011, 54, 5639–5659. [Google Scholar] [CrossRef] [PubMed]
- Roe, R.J. Toxicologic evaluation of metronidazole with particular reference to carcinogenic, mutagenic, and teratogenic potential. Surgery 1983, 93, 158–164. [Google Scholar] [PubMed]
- Roe, F.J. Safety of nitroimidazoles. Scand. J. Infect. Dis. Suppl. 1985, 46, 72–81. [Google Scholar]
- Voogd, C.E. On the mutagenicity of nitroimidazoles. Mutat. Res. 1981, 86, 243–277. [Google Scholar] [CrossRef]
- Moreth, M.; Ornelas, D.; Gomes, C.R.B.; de Souza, M.V.N. Nitroimidazóis- uma promissora classe de substâncias para o tratamento da tuberculose. Rev. Virtual Quim. 2010, 2, 105–117. [Google Scholar]
- Voogd, C.E.; Van Der Stel, J.J.; Jacobs, J.J.J.A.A. The mutagenic action of nitroimidazoles II. Effects of 2-nitroimidazoles. Mutat. Res. 1975, 31, 149–152. [Google Scholar] [CrossRef]
- Ferreira, R.C.C.; Schwarz, U.; Ferreira, L.C.S. Activation of anti-Trypanosoma cruzi drugs to genotoxic metabolites promoted by mammalian microsomal enzymes. Mutat. Res./Genet. Toxicol. 1988, 204, 577–583. [Google Scholar] [CrossRef]
- Voogd, C.E.; Van Der Stel, J.J.; Jacobs, J.J.J.A.A. The mutagenic action of nitroimidazoles: IV. A comparison of the mutagenic action of several nitroimidazoles and some imidazoles. Mutat. Res./Genet. Toxicol. 1979, 66, 207–221. [Google Scholar] [CrossRef]
- Buschini, A.; Ferrarini, L.; Franzoni, S.; Galati, S.; Lazzaretti, M.; Mussi, F.; de Albuquerque, C.N.; Zucchi, T.M.A.D.; Poli, P. Genotoxicity revaluation of three commercial nitroheterocyclic drugs: Nifurtimox, benznidazole, and metronidazole. J. Parasitol. Res. 2009, 2009, 463575. [Google Scholar] [CrossRef] [PubMed]
- Chessin, H.; McLaughlin, T.; Mroczkowski, Z.; Rupp, W.D.; Low, K.B. Radiosensitization, Mutagenicity, and Toxicity of Escherichia coli by Several Nitrofurans and Nitroimidazoles. Radiat. Res. 1978, 75, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Herbold, B.; Buselmaier, W. Induction of point mutations by different chemical mechanisms in the liver microsomal assay. Mutat. Res./Genet. Toxicol. 1976, 40, 73–83. [Google Scholar] [CrossRef]
- Voogd, C.E. Azathioprine, a genotoxic agent to be considered non-genotoxic in man. Mutat. Res./Rev. Genet. Toxicol. 1989, 221, 133–152. [Google Scholar] [CrossRef]
- Van Went, G.F. Investigation into the mutagenic activity of azathioprine (Imuran®) in different test systems. Mutat. Res./Genet. Toxicol. 1979, 68, 153–162. [Google Scholar] [CrossRef]
- Voogd, C.E.; Van Der Stel, J.J.; Jacobs, J.J.J.A.A. The mutagenic action of nitroimidazoles I. Metronidazole, nimorazole, dimetridazole and ronidazole. Mutat. Res. 1974, 26, 483–490. [Google Scholar] [CrossRef]
- Ré, J.L.; De Méo, M.P.; Laget, M.; Guiraud, H.; Castegnaro, M.; Vanelle, P.; Duménil, G. Evaluation of the genotoxic activity of metronidazole and dimetridazole in human lymphocytes by the comet assay. Mutat. Res. 1997, 375, 147–155. [Google Scholar] [CrossRef]
- Voog, C.E.; van der Stel, J.J.; Jacobs, J.J.J.A.A. The mutagenic action of nitroimidazoles. III. Tinidazole, ipronidazole, panidazole and ornidazo. Mutat. Res. 1977, 48, 155–161. [Google Scholar] [CrossRef]
- Nigro, M.M.L.; Carballo, M.A. Genotoxicity and cell death induced by tinidazole (TNZ). Toxicol. Lett. 2008, 180, 46–52. [Google Scholar] [CrossRef]
- Tweats, D.; Trunz, B.B.; Torreele, E. Genotoxicity profile of fexinidazole—a drug candidate in clinical development for human African trypanomiasis (sleeping sickness). Mutagenesis 2012, 27, 523–532. [Google Scholar] [CrossRef]
- Mello, F.V.C.; Carvalho, A.S.; Bastos, M.M.; Boechat, N.; Aiub, C.A.F.; Felzenszwalb, I. Evaluation of Genotoxic Effects of New Molecules with Possible Trypanocidal Activity for Chagas Disease Treatment. Sci. World J. 2013, 2013, 287319. [Google Scholar] [CrossRef] [PubMed]
- Nesslany, F.; Brugier, S.; Mouriès, M.A.; Le Curieux, F.; Marzin, D. In vitro and in vivo chromosomal aberrations induced by megazol. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004, 560, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, E.; Hayashi, M.; Matsumoto, M. Delamanid is not metabolized by Salmonella or human nitroreductases: A possible mechanism for the lack of mutagenicity. Regul. Toxicol. Pharmacol. 2017, 84, 1–8. [Google Scholar] [CrossRef]
- Ehlhardt, W.; Beailieu, B.B.; Goldman, P. Mammlian cell toxicity and bacterial mutagenicity of nitroimidazoles. Biochem. Pharmacol. 1988, 37, 2603–2606. [Google Scholar] [CrossRef]
- Voogd, C.E.; van der Stel, J.J.; van Bruchem, M.C. Increased mutagenicity of some nitroimidazoles by non-mutagenic nitrotoluene on Klebsiella pneumoniae (fluctuation test). Mutat. Res. 1992, 282, 73–77. [Google Scholar] [CrossRef]
- Moreno-Mafias, A.M.; Pleixats, R.; Palacn, C.; Raga, M.M.; Castell, J.M.; Ortiz, J.A. Preparation, Antimicrobial Evaluation, and Mutagenicity of [2-Hydroxyaryl]-[1-methyl-5-nitro-lH-2-imidazolyl]methanols, [5-tert-Butyl-2-methylaminophenyl]-[1-methyl-5-nitro-1H-2-imidazolyl]methanol, and [2-Hydroxyaryl]-[1-methyl-5-nitro-1H-2-imidazolyl] ketones. Bioorg. Med. Chem. 1997, 5, 1959–1968. [Google Scholar]
- De Meo, M.; Vanelle, P.; Bernadini, E.; Laget, M.; Maldonado, J.; Jentzer, O.; Crozet, M.P.; Dumenil, G. Evaluation of the Mutagenic and Genotoxic Activities of 48 Nitroimidazoles and Related lmidazole Derivatives by the Ames Test and the SOS Chromotest. Environ. Mol. Mutagen. 1992, 19, 167–181. [Google Scholar] [CrossRef] [PubMed]

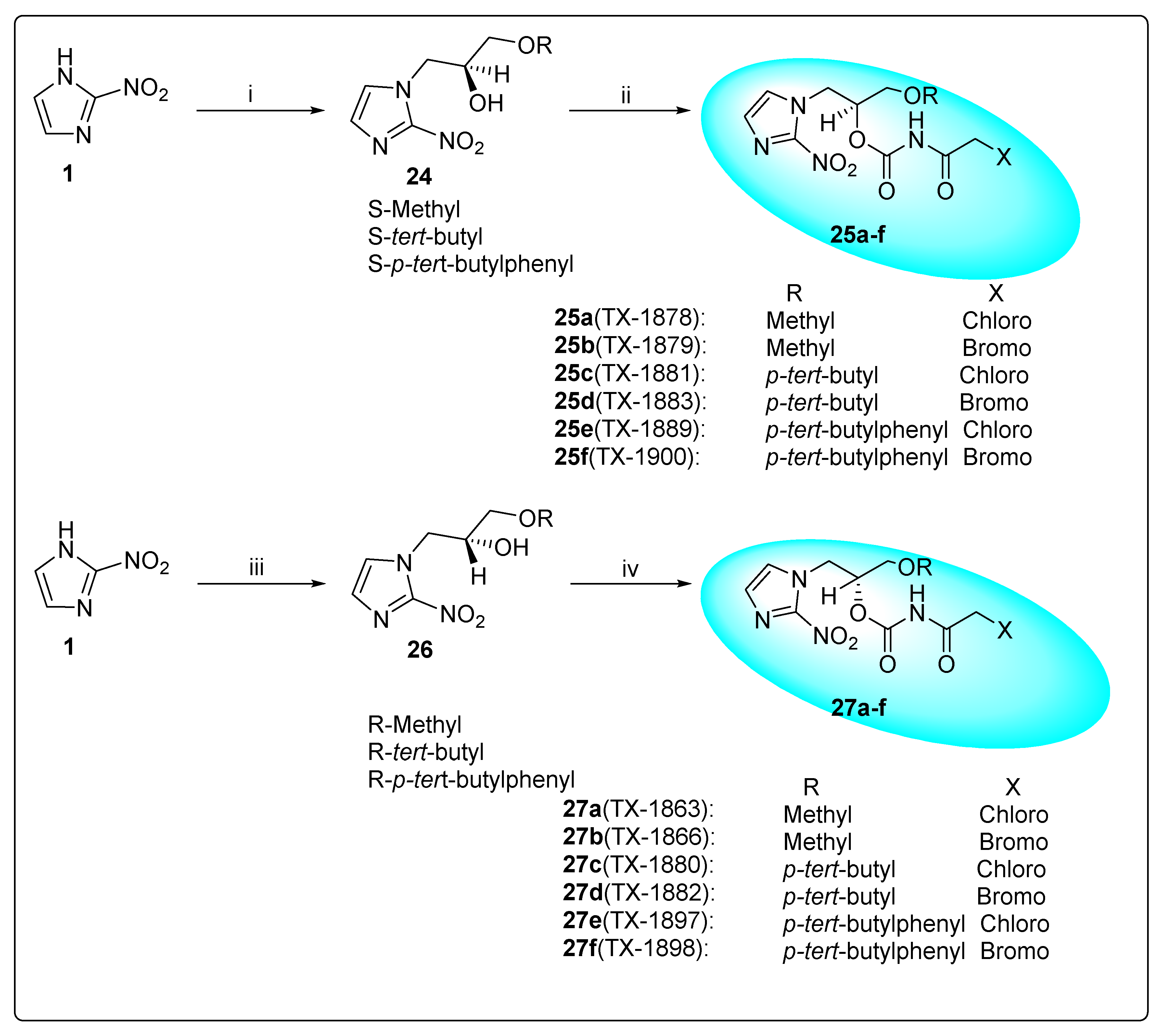


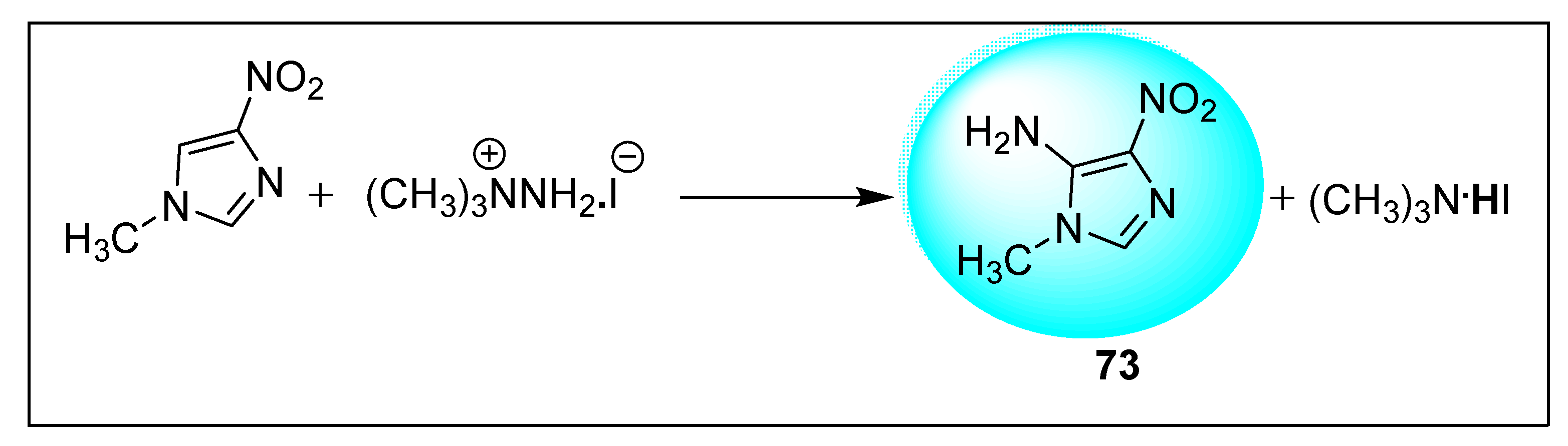
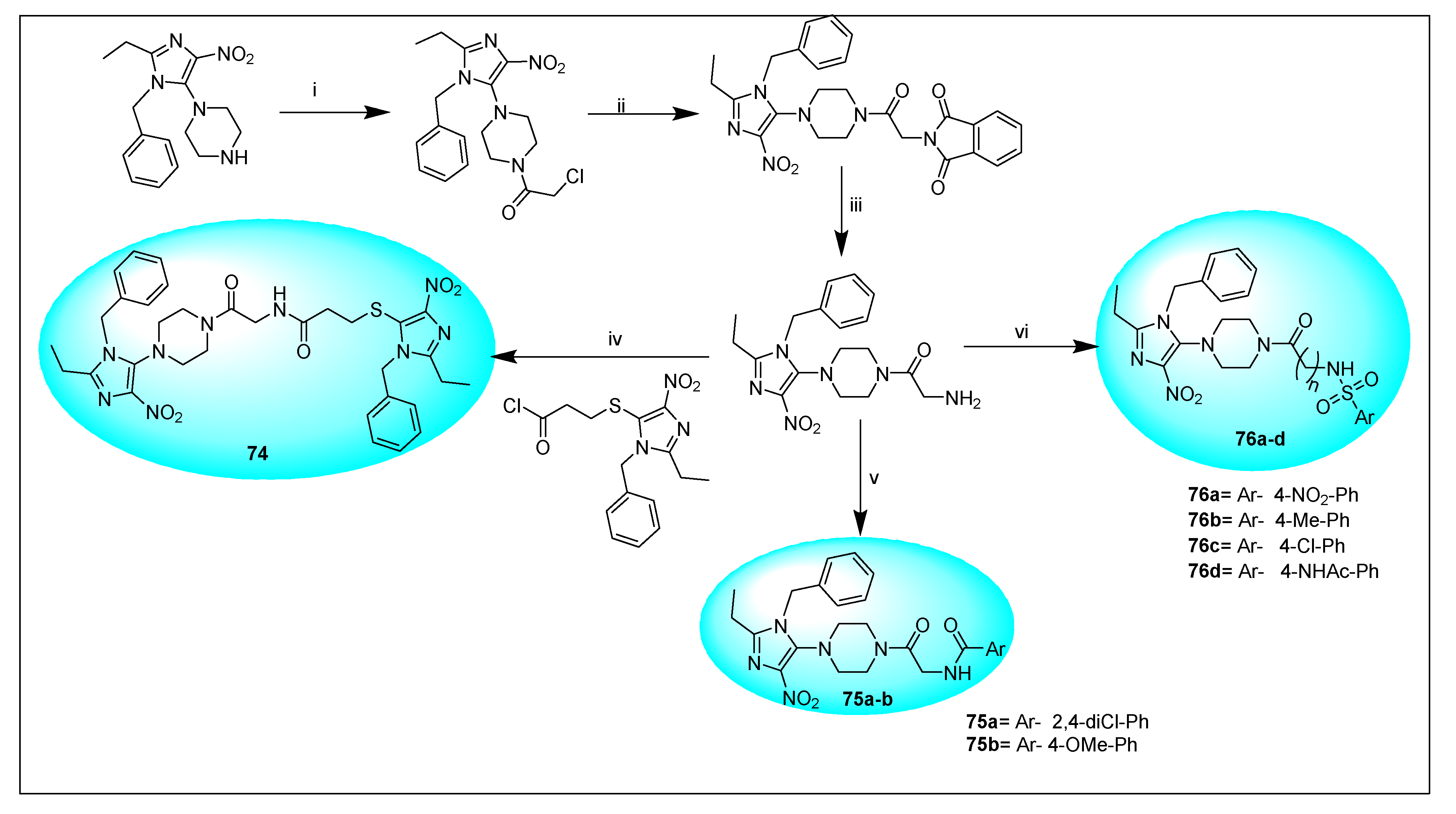
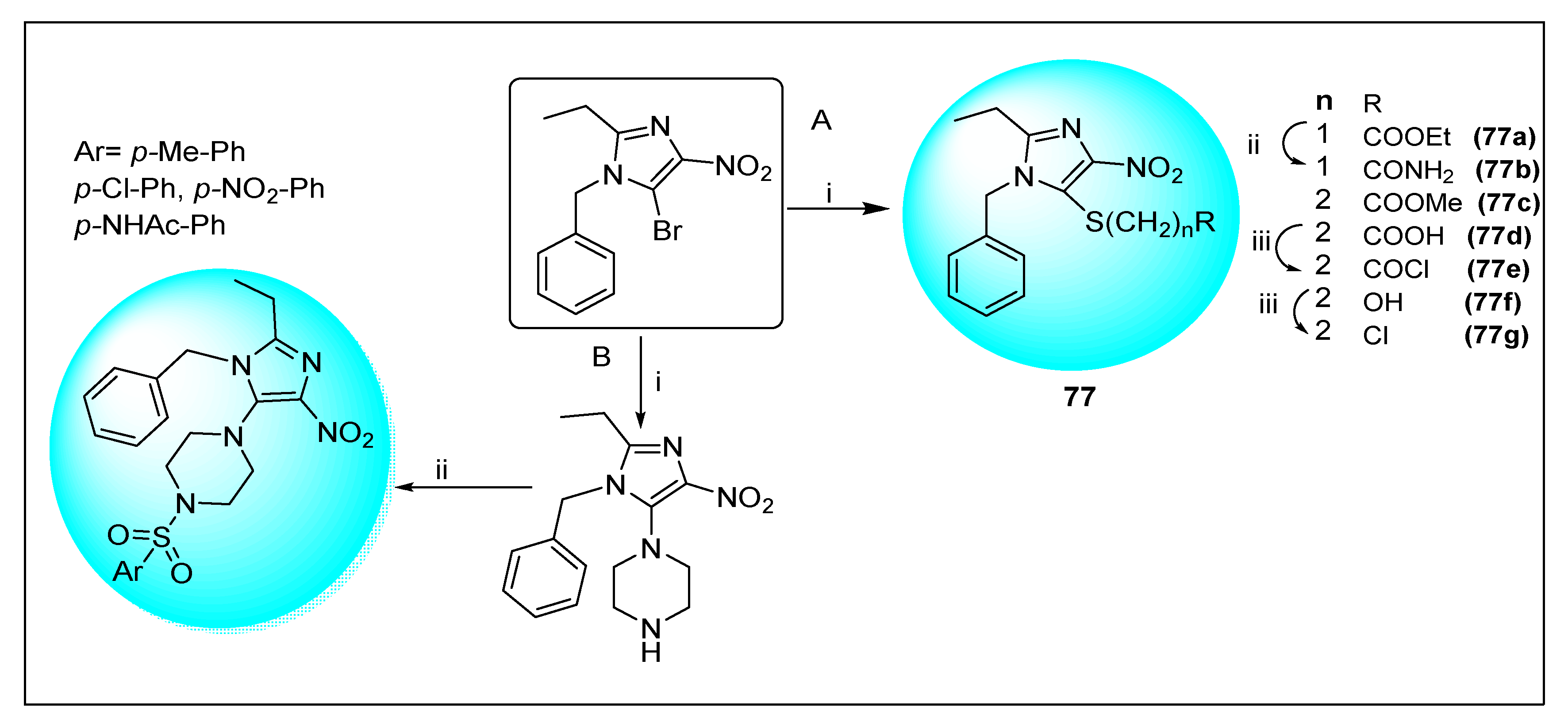
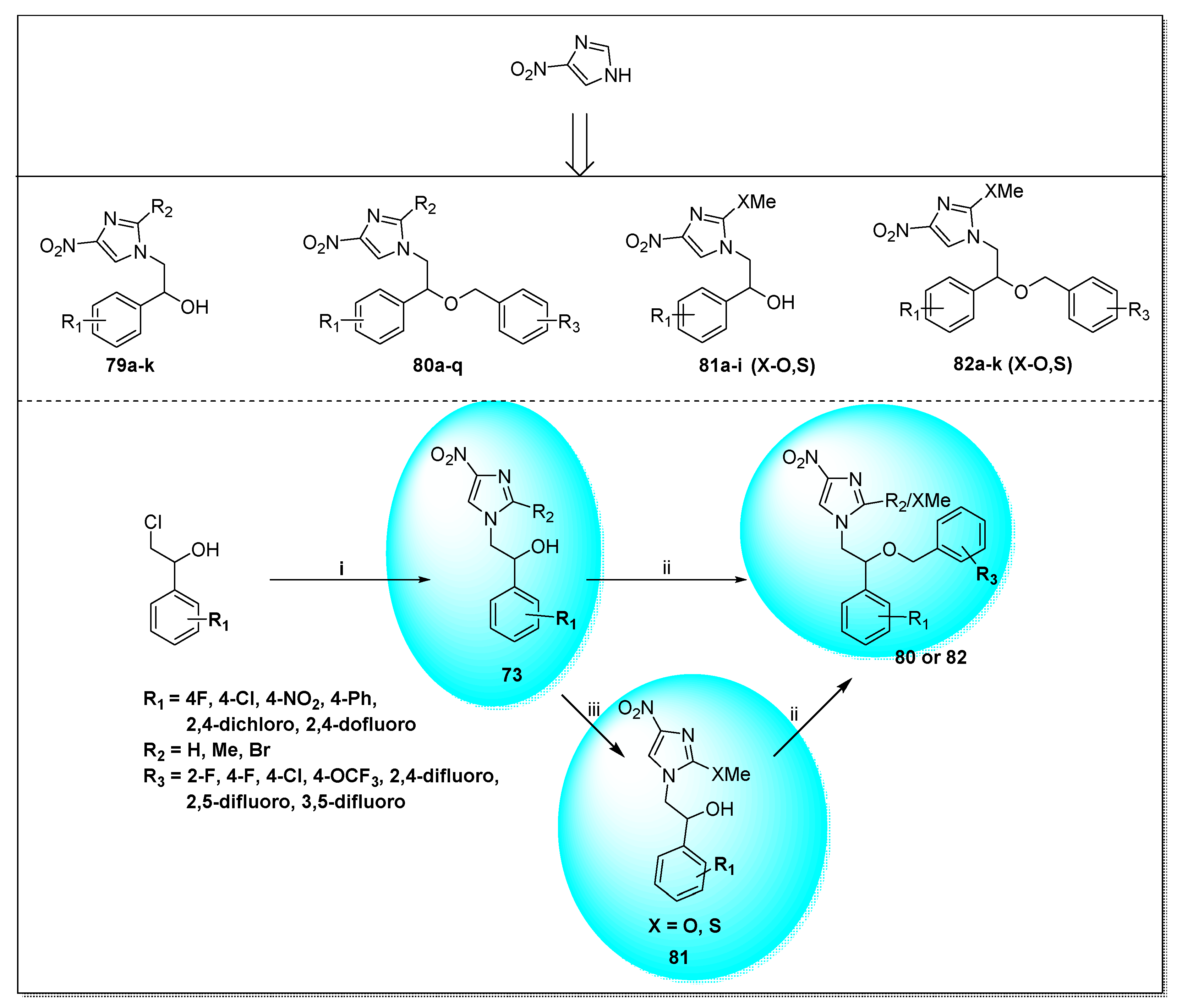


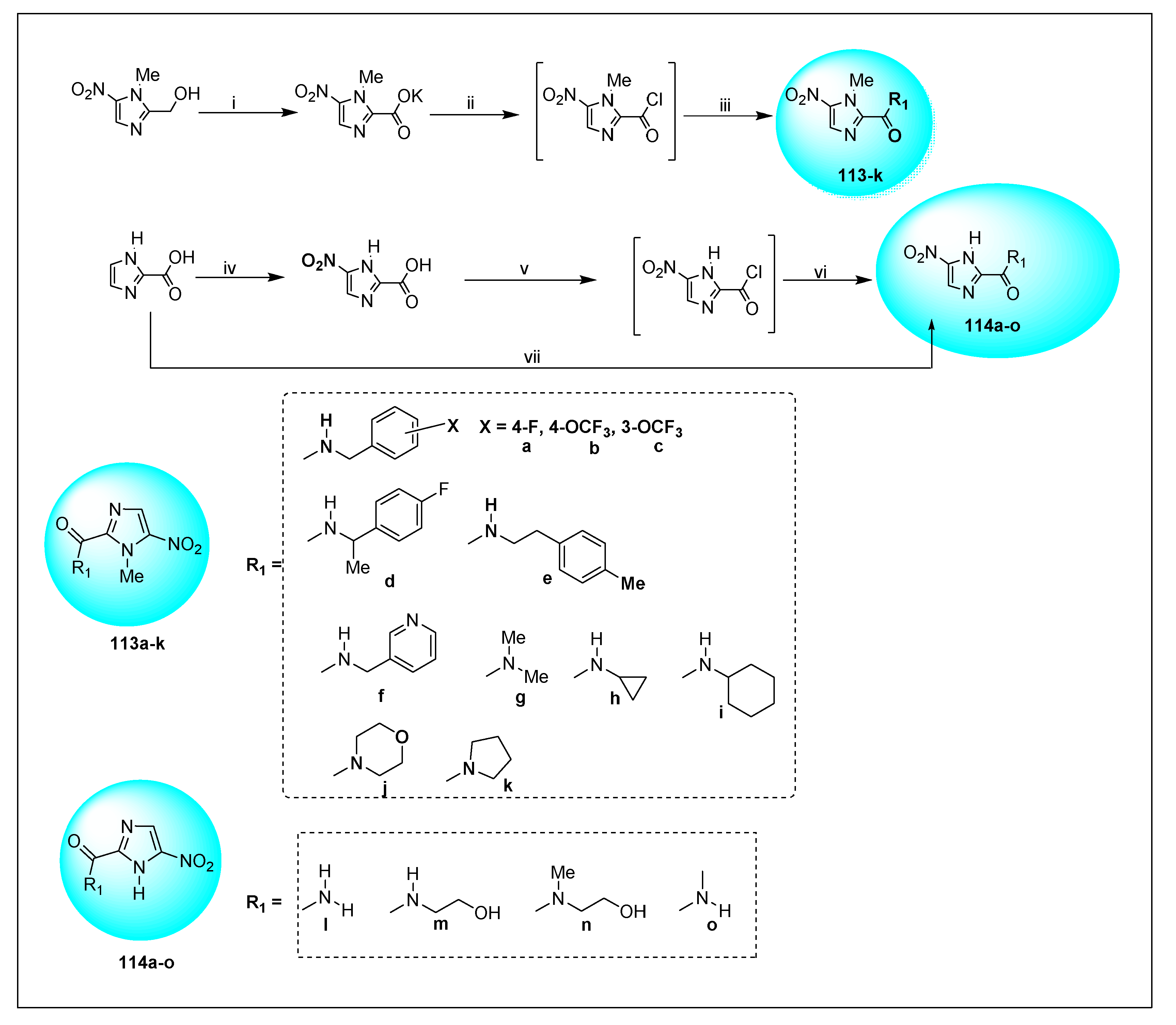
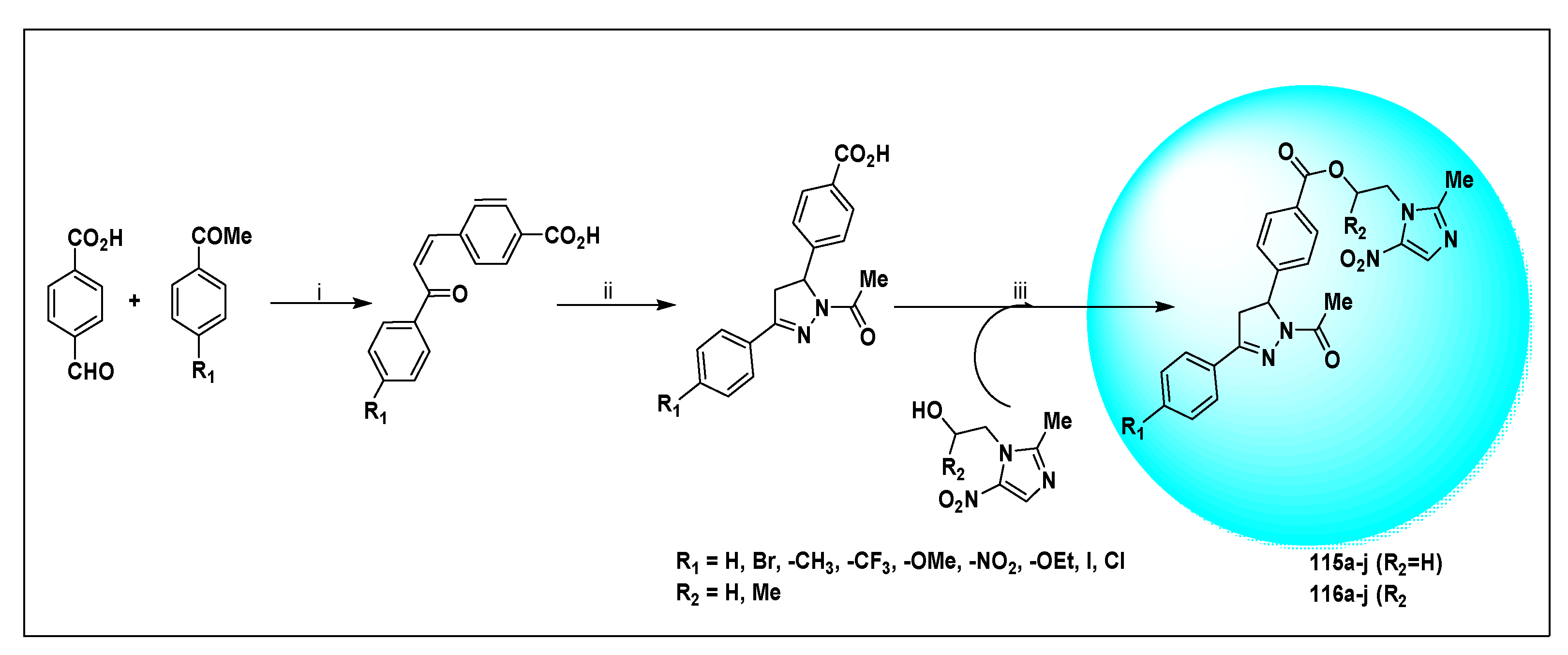
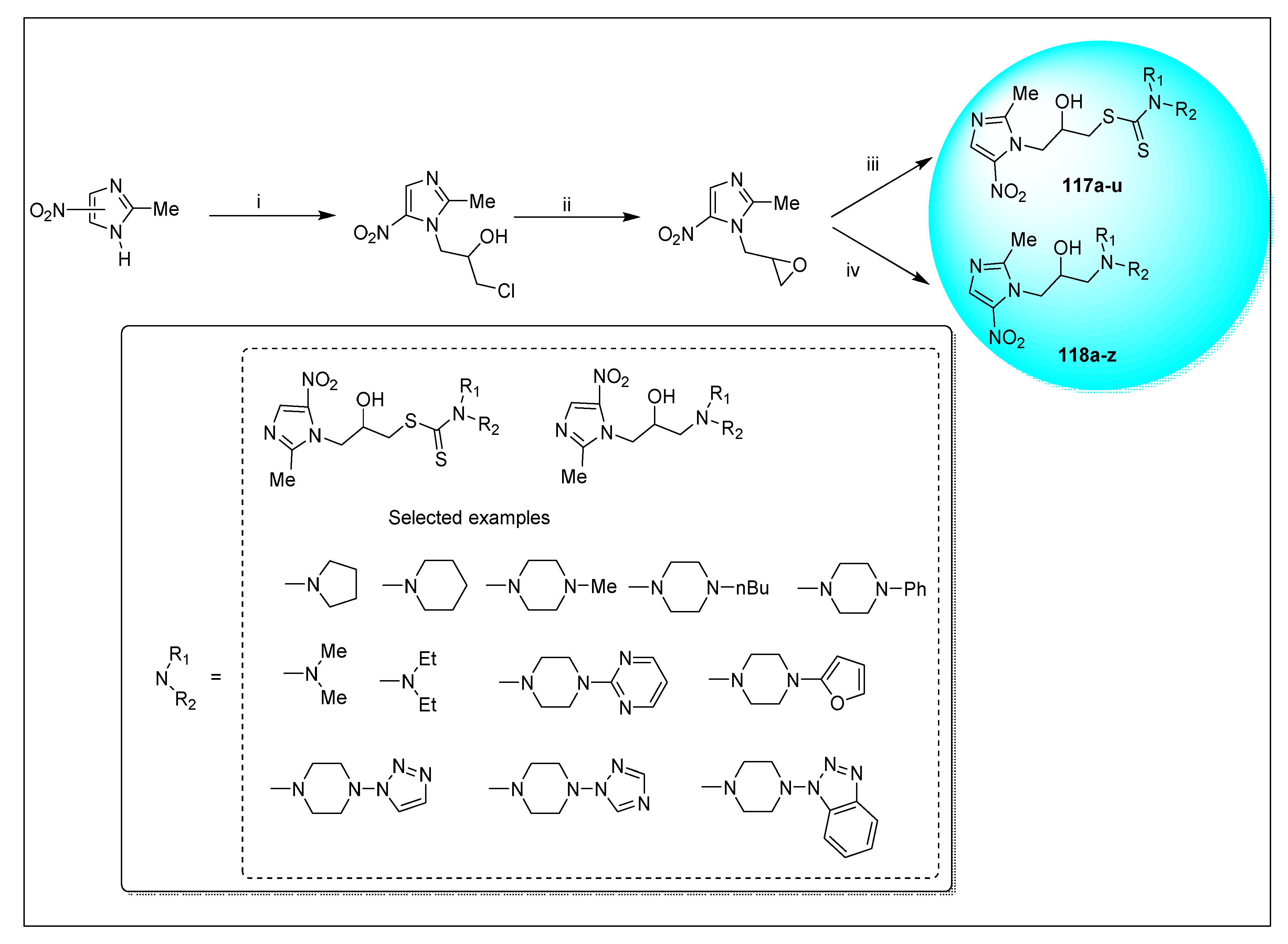
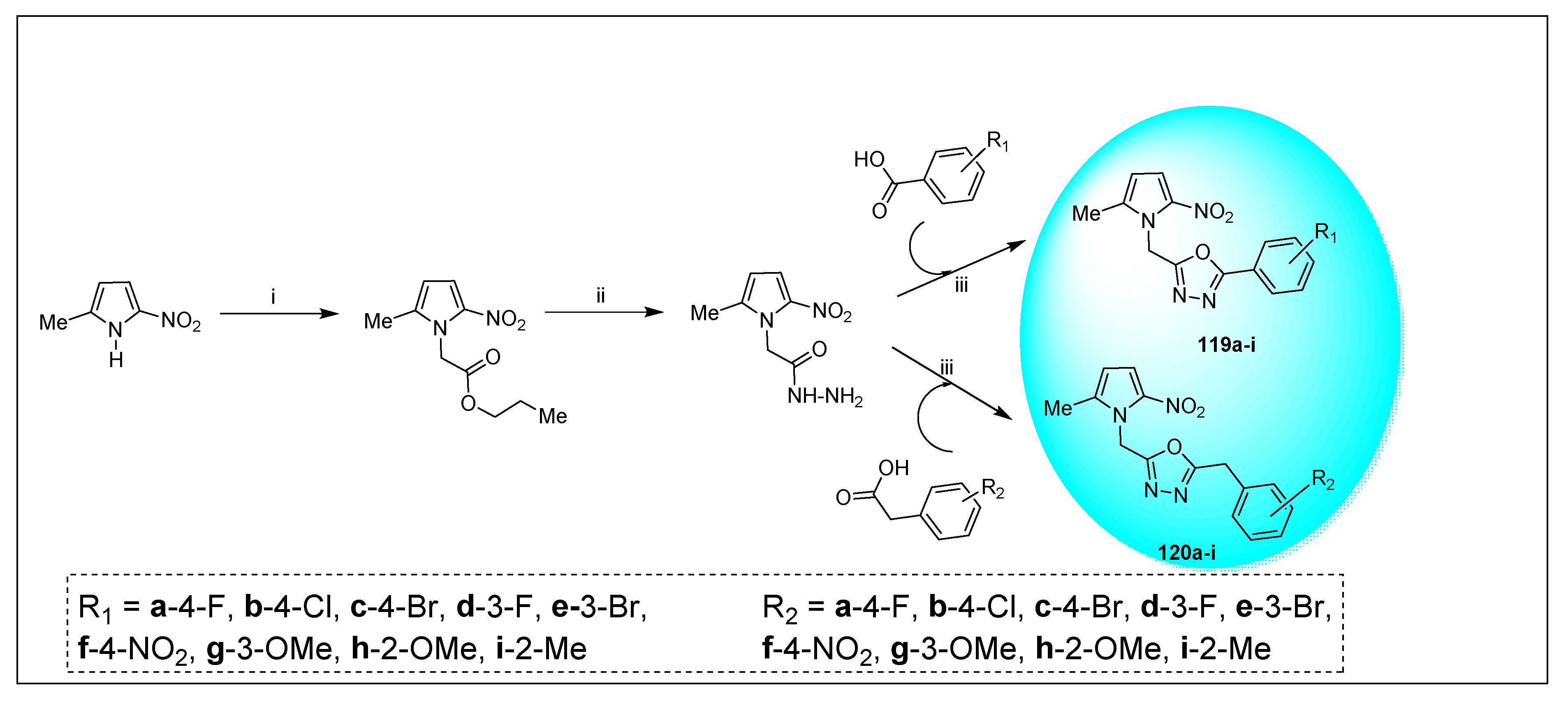

| Anti-Angiogenesis Activity | Radiosensitizing Activity | |||
|---|---|---|---|---|
| Protease Inhibitory Activity | RLE Cell Proliferation Activity | Anti-Angiogenic Activity | Radiosensitizing Effect (10 μM) | |
| Compound ID | KI (μM) | IC50 (μM) | % Inhibition | ER |
| 27a (TX-1863) a | 1180 | 325 | 80 | |
| 27b (TX-1866) a | 66 | 20 | 100 | |
| 27c (TX-1880) a | 260 | 71 | 55 | 1.76 |
| 27d (TX-1882) a | 42 | 6.3 | 61 | 1.82 |
| 27e (TX-1897) a | 30 | 7.5 | 64 | 1.73 |
| 27f (TX-1898) a | 9 | 1.4 | 93 | 1.80 |
| 25a (TX-1878) b | 1750 | 275 | 83 | |
| 25b (TX-1879) b | 220 | 17 | 100 | |
| 25c (TX-1881) b | 557 | 80 | 53 | 1.75 |
| 25d (TX-1883) b | 55 | 6.9 | 77 | 1.83 |
| 25e (TX-1899) b | 56 | 6.5 | 58 | 1.90/1.75 c (1 μM) |
| 25f (TX-1900) b | 14 | 0.9 | 82 | 1.74 |
| In-Vitro Activity against Selected Organism MIC (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | S. aureus | S. pyrogens | C. perfringens | M. gallisepticum | T. vaginalis | In Vivo Activity against T. vaginalis | Rel. ED50 mg/kg | LD50 mg/kg |
| 30 | 25 | 200 | 0.8 | 50 | 25 | >10 | 0.7 | |
| 31 | 100 | 100 | >3.1 | 100 | 2 | 1.65 | 0.28 | 1969 |
| 32 | >100 | 100 | 10 | 25 | 100 | 4.67 | 0.33 | 141 |
| 33 | >100 | >100 | 3.1 | 100 | 10 | 5.74 | 0.40 | 156 |
| 34 | >100 | 100 | 3.1 | 25 | 100 | 28.83 | 2.88 | |
| 35 | 100 | >100 | >100 | 50 | 100 | 16.2 | 1.62 | |
| 36 | >100 | >100 | 5 | 100 | 100 | >40 | 99 | |
| Metronidazole 7 | >300 | >300 | 0.6 | >300 | 5 | 5.77 | 1 | 658 |
| Tinidazole 8 | >300 | >300 | 0.3 | >300 | 3.1 | 2.5 | 0.43 | 1280 |
| Nimorazole 11 | >300 | >300 | 0.0 | >300 | 12.5 | 37.9 | 4.66 | 40 |
| Comp. | IC50(A) a (µmol/L h) | IC50(H) a (µmol/L h) | HS b | C1.6 c (µmol/L) | Ci1.6 d (µmol/L) | ThI e |
|---|---|---|---|---|---|---|
| 41 (NLQZ-1) | 998.6–1486.7 | 73.6–108.4 | 14 | 61.4 | 347.9 ± 6.0 | 16–24 |
| 42 (NLPP-1) (V79) | 1000–962.2 | 86.1–51.9 | 12–19 | 75.0 | 271.9 ± 3.5 | 13 |
| 42 NLPP-1 (A549) | 1668.8–1311.6 | 97.6–59.7 | 17–22 | 44.1 | ND | 30–38 |
| NLCQ-1 | 414.2–958.9 f | 86–14.7 f | 5–65 f | 7.2 | 62.5 ± 1.5 | 57–133 f |
| Sr. No. | R | R1 | R2 | Anti-Tubercular Activity | |
|---|---|---|---|---|---|
| MIC μg/mL | In Vivo Efficacy mg/kg p.o ED50 | ||||
| 17a | H | CH3 | H | 1.95 | 25 |
| 17b (CGI-17341) | H | C2H5 | H | 0.06 | 10 |
| 17c | H | CH2Cl | H | 0.12 | 30–100 |
| 17d | H | Ph | H | 0.95 | Ф at 100 |
| 17e | H | Bu (n) | H | ||
| 17f | H | CH2Br | H | 0.24 | 30–100 |
| 17g | H | CCl3 | H | 31.2 | |
| 17h | H | CH2OPr (iso) | H | 3.9 | Ф at 200 |
| 17i | H | CH2Oallyl | H | 3.9 | Ф at 200 |
| 17j | H | CH2OPh | H | 0.24 | Ф at 200 |
| 17k | H | Bu (n) | CH3 | 0.015 | |
| 17l | H | (C7H15)n | CH3 | 0.0037 | |
| 17m | H | (CH2)5 | H | 0.03 | Ф at 100 |
| 17n | H | 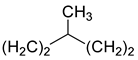 | H | 0.12 | |
| 17o | H | (CH2)6 | 0.05 | Ф at 100 | |
| 17p | H | (CH2)4 | H | 31.2 | -- |
| Compounds | AMES Test Mutagenic Power | SOS Test Induction Power | ||
|---|---|---|---|---|
| −S9 + S9 | −S9 + S9 | |||
 | 10 | 14 | 0.079 | 0.0210 |
 | 12 | 16 | 0.0221 | 0.0724 |
 | 53,117 | 26,830 | 107 | 59.3 |
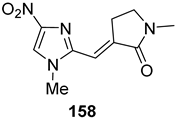 | 11 | 11 | 0.05 | 0.0118 |
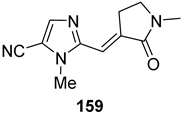 | 0.482 | 1.55 | 0.00426 | <0.0010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, R.; Sharma, S.; Singh, R.; Vishwakarma, R.A.; Mignani, S.; Singh, P.P. Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview. Pharmaceuticals 2022, 15, 561. https://doi.org/10.3390/ph15050561
Gupta R, Sharma S, Singh R, Vishwakarma RA, Mignani S, Singh PP. Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview. Pharmaceuticals. 2022; 15(5):561. https://doi.org/10.3390/ph15050561
Chicago/Turabian StyleGupta, Ria, Sumit Sharma, Rohit Singh, Ram A. Vishwakarma, Serge Mignani, and Parvinder Pal Singh. 2022. "Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview" Pharmaceuticals 15, no. 5: 561. https://doi.org/10.3390/ph15050561
APA StyleGupta, R., Sharma, S., Singh, R., Vishwakarma, R. A., Mignani, S., & Singh, P. P. (2022). Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview. Pharmaceuticals, 15(5), 561. https://doi.org/10.3390/ph15050561



















