Safety and Tolerability of Ivermectin and Albendazole Mass Drug Administration in Lymphatic Filariasis Endemic Communities of Tanzania: A Cohort Event Monitoring Study
Abstract
:1. Introduction
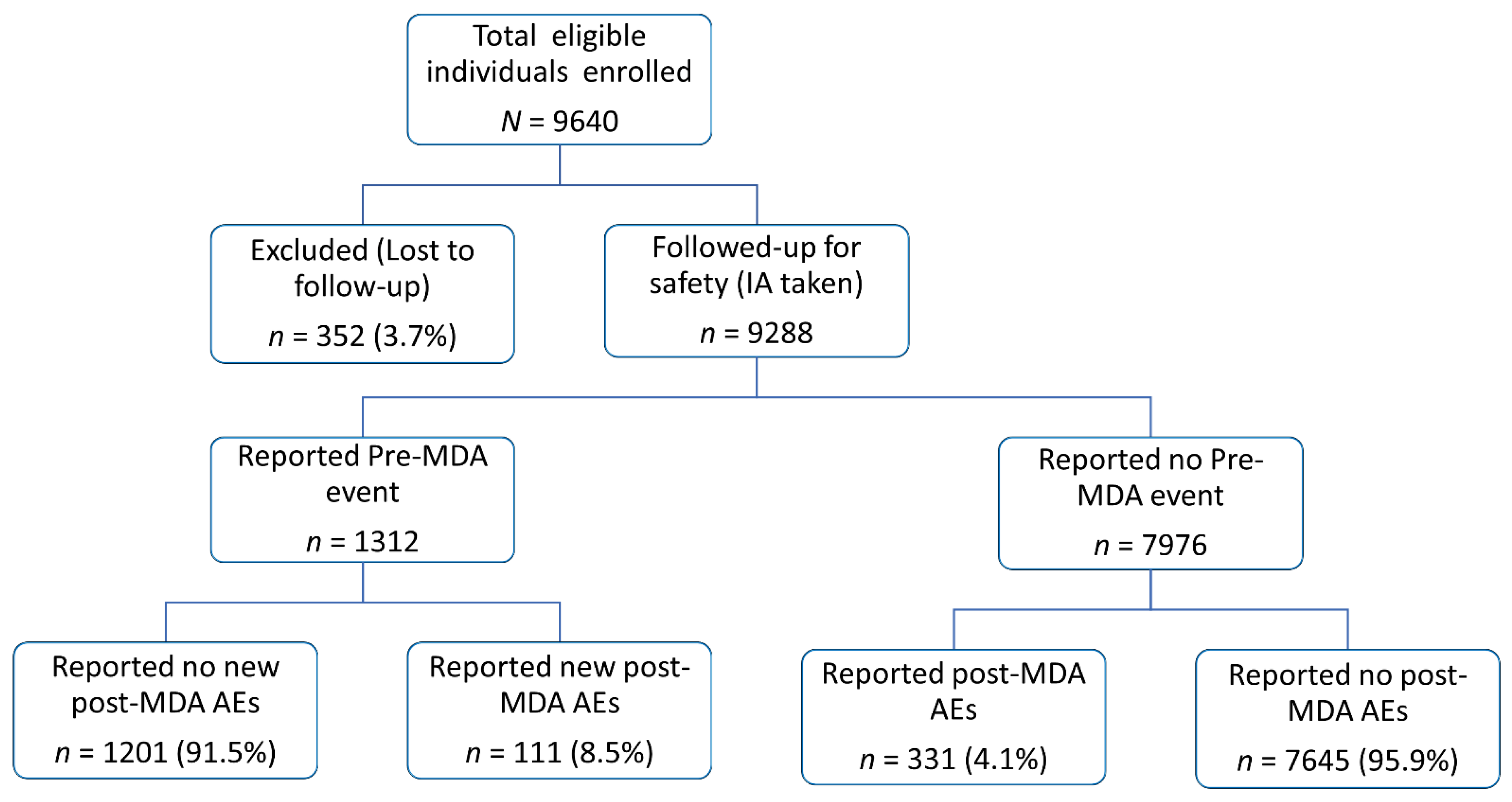
2. Results
2.1. Baseline Characteristics of the Study Population
2.2. Incidence of Adverse Events Following Ivermectin and Albendazole MDA
2.3. Inicdence Strafied by Types of MDA-Associated Adverse Events
2.4. Severity Grading of MDA-Associated Adverse Events
2.5. Correlates and Predictors of Adverse Events Following MDA
2.6. Chronic Clinical Conditions and Their Association with Adverse Events
3. Discussion
4. Materials and Methods
4.1. Study Setting and Population
4.2. Study Design, Enrolment, and Sample Size
4.3. Treatment and Safety Follow Up
4.4. Assessment and Severity Grading of Adverse Events
- Grade 1: Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated.
- Grade 2: Moderate; minimal, local, or non-invasive intervention indicated; limiting age-appropriate Instrumental Activities of Daily Living (ADL).
- Grade 3: Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL.
- Grade 4: Life-threatening consequences; urgent intervention indicated.
- Grade 5: Death related to AE.
4.5. Data Management and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Guideline: Alternative Mass Drug Administration Regimens to Eliminate Lymphatic Filariasis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- WHO. Lymphatic Filariasis. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 1 April 2022).
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic filariasis and onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef]
- Shenoy, R.K. Clinical and pathological aspects of filarial lymphedema and its management. Korean J. Parasitol. 2008, 46, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Preventive Chemotherapy in Human Helminthiasis. Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Available online: https://www.who.int/publications/i/item/9241547103 (accessed on 7 February 2022).
- World Health Organization. Accelerating Work to Overcome the Global Impact of NTDs: 2011–2020 Progress Dashboard. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/overview/progress-dashboard-2011-2020 (accessed on 2 March 2022).
- Budge, P.J.; Herbert, C.; Andersen, B.J.; Weil, G.J. Adverse events following single dose treatment of lymphatic filariasis: Observations from a review of the literature. PLoS Negl. Trop. Dis. 2018, 12, e0006454. [Google Scholar] [CrossRef]
- World Health Organization. Safety in Administering Medicines for Neglected Tropical Diseases. Geneva. Available online: https://www.who.int/publications/i/item/9789240024144 (accessed on 12 April 2022).
- Neglected Tropical Diseases Control Program Tanzania. Lymphatic Filariasis. Available online: https://www.ntdcp.go.tz/diseases/lf (accessed on 2 April 2022).
- Kiguba, R.; Olsson, S.; Waitt, C. Pharmacovigilance in low- and middle-income countries: A review with particular focus on Africa. Br. J. Clin. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.; Olsson, S.; Minzi, O.; Bienvenu, E.; Msakonnen, E.; Kamuhabwa, A.; Oluka, M.; Guantai, A.; Bergman, U.; van Puijenbroek, E.; et al. Comparative Assessment of the National Pharmacovigilance Systems in East Africa: Ethiopia, Kenya, Rwanda and Tanzania. Drug Saf. 2020, 43, 339–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, A.; Olsson, S.; Khaemba, C.; Kabatende, J.; Dires, T.; Fimbo, A.; Minzi, O.; Bienvenu, E.; Makonnen, E.; Kamuhabwa, A.; et al. Comparative Assessment of the Pharmacovigilance Systems within the Neglected Tropical Diseases Programs in East Africa-Ethiopia, Kenya, Rwanda, and Tanzania. Int. J. Environ. Res. Public Health 2021, 18, 1941. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Practical Handbook on the Pharmacovigilance of Antiretroviral Medicines. Available online: https://apps.who.int/iris/bitstream/handle/10665/44236/9789241547949_eng.pdf?sequence=1&isAllowed=y (accessed on 12 April 2022).
- World Health Organization. A Practical Handbook on the Pharmacovigilance of Medicines Used in the Treatment of Tuberculosis: Enhancing the Safety of the TB Patient, World Health Organization, Geneva. Available online: https://www.who.int/medicines/publications/Pharmaco_TB_web_v3.pdf (accessed on 2 April 2022).
- WHO. The Safety of Medicines in Public Health Programmes. Geneva. Available online: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/Pharmacovigilance_B.pdf (accessed on 8 February 2022).
- de Souza, D.K.; Dorlo, T.P.C. Safe mass drug administration for neglected tropical diseases. Lancet Glob. Health 2018, 6, e1054–e1055. [Google Scholar] [CrossRef] [Green Version]
- Campillo, J.T.; Boussinesq, M.; Bertout, S.; Faillie, J.L.; Chesnais, C.B. Serious adverse reactions associated with ivermectin: A systematic pharmacovigilance study in sub-Saharan Africa and in the rest of the World. PLoS Negl. Trop. Dis. 2021, 15, e0009354. [Google Scholar] [CrossRef]
- van der Heijden, P.G.; van Puijenbroek, E.P.; van Buuren, S.; van der Hofstede, J.W. On the assessment of adverse drug reactions from spontaneous reporting systems: The influence of under-reporting on odds ratios. Stat. Med. 2002, 21, 2027–2044. [Google Scholar] [CrossRef]
- Khaemba, C.; Barry, A.; Omondi, W.P.; Bota, K.; Matendechero, S.; Wandera, C.; Siyoi, F.; Kirui, E.; Oluka, M.; Nambwa, P.; et al. Safety and Tolerability of Mass Diethylcarbamazine and Albendazole Administration for the Elimination of Lymphatic Filariasis in Kenya: An Active Surveillance Study. Pharmaceuticals 2021, 14, 264. [Google Scholar] [CrossRef]
- Minzi, O.M.; Mnkugwe, R.H.; Ngaimisi, E.; Kinung’hi, S.; Hansson, A.; Pohanka, A.; Kamuhabwa, A.; Aklillu, E. Effect of Dihydroartemisinin-Piperaquine on the Pharmacokinetics of Praziquantel for Treatment of Schistosoma mansoni Infection. Pharmaceuticals 2021, 14, 400. [Google Scholar] [CrossRef] [PubMed]
- Mnkugwe, R.H.; Minzi, O.; Kinung’hi, S.; Kamuhabwa, A.; Aklillu, E. Effect of Pharmacogenetics Variations on Praziquantel Plasma Concentrations and Schistosomiasis Treatment Outcomes Among Infected School-Aged Children in Tanzania. Front. Pharmacol. 2021, 12, 712084. [Google Scholar] [CrossRef] [PubMed]
- Aklillu, E.; Engidawork, E. The impact of catha edulis (vahl) forssk. ex endl. (celestraceae) (khat) on pharmacokinetics of clinically used drugs. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Bedada, W.; de Andres, F.; Engidawork, E.; Hussein, J.; LLerena, A.; Aklillu, E. Effects of Khat (Catha edulis) use on catalytic activities of major drug-metabolizing cytochrome P450 enzymes and implication of pharmacogenetic variations. Sci. Rep. 2018, 8, 12726. [Google Scholar] [CrossRef] [PubMed]
- Fimbo, A.M.; Minzi, O.M.S.; Mmbando, B.P.; Barry, A.; Nkayamba, A.F.; Mwamwitwa, K.W.; Malishee, A.; Seth, M.D.; Makunde, W.H.; Gurumurthy, P.; et al. Prevalence and Correlates of Lymphatic Filariasis Infection and Its Morbidity Following Mass Ivermectin and Albendazole Administration in Mkinga District, North-Eastern Tanzania. J. Clin. Med. 2020, 9, 1550. [Google Scholar] [CrossRef]
- Palmeirim, M.S.; Hurlimann, E.; Knopp, S.; Speich, B.; Belizario, V., Jr.; Joseph, S.A.; Vaillant, M.; Olliaro, P.; Keiser, J. Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: A systematic review, meta-analysis and individual patient data analysis. PLoS Negl. Trop. Dis. 2018, 12, e0006458. [Google Scholar] [CrossRef] [Green Version]
- Patel, C.; Hurlimann, E.; Keller, L.; Hattendorf, J.; Sayasone, S.; Ali, S.M.; Ame, S.M.; Coulibaly, J.T.; Keiser, J. Efficacy and safety of ivermectin and albendazole co-administration in school-aged children and adults infected with Trichuris trichiura: Study protocol for a multi-country randomized controlled double-blind trial. BMC Infect. Dis. 2019, 19, 262. [Google Scholar] [CrossRef] [Green Version]
- Speich, B.; Ali, S.M.; Ame, S.M.; Bogoch, I.I.; Alles, R.; Huwyler, J.; Albonico, M.; Hattendorf, J.; Utzinger, J.; Keiser, J. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: A four-arm, randomised controlled trial. Lancet Infect. Dis. 2015, 15, 277–284. [Google Scholar]
- Awadzi, K.; Edwards, G.; Duke, B.O.; Opoku, N.O.; Attah, S.K.; Addy, E.T.; Ardrey, A.E.; Quartey, B.T. The co-administration of ivermectin and albendazole--safety, pharmacokinetics and efficacy against Onchocerca volvulus. Ann. Trop. Med. Parasitol. 2003, 97, 165–178. [Google Scholar] [CrossRef]
- Tavul, L.; Laman, M.; Howard, C.; Kotty, B.; Samuel, A.; Bjerum, C.; O’Brian, K.; Kumai, S.; Amuga, M.; Lorry, L.; et al. Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua New Guinea: An open-label, cluster-randomised trial. PLoS Negl. Trop. Dis. 2022, 16, e0010096. [Google Scholar] [CrossRef]
- Edi, C.; Bjerum, C.M.; Ouattara, A.F.; Chhonker, Y.S.; Penali, L.K.; Meite, A.; Koudou, B.G.; Weil, G.J.; King, C.L.; Murry, D.J. Pharmacokinetics, safety, and efficacy of a single co-administered dose of diethylcarbamazine, albendazole and ivermectin in adults with and without Wuchereria bancrofti infection in Cote d’Ivoire. PLoS Negl. Trop. Dis. 2019, 13, e0007325. [Google Scholar] [CrossRef] [Green Version]
- Weil, G.J.; Bogus, J.; Christian, M.; Dubray, C.; Djuardi, Y.; Fischer, P.U.; Goss, C.W.; Hardy, M.; Jambulingam, P.; King, C.L.; et al. The safety of double- and triple-drug community mass drug administration for lymphatic filariasis: A multicenter, open-label, cluster-randomized study. PLoS Med. 2019, 16, e1002839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rademaker, M. Do women have more adverse drug reactions? Am. J. Clin. Dermatol. 2001, 2, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.; Ekman, E.; Blomgren, K.J. Influence of age, sex and seriousness on reporting of adverse drug reactions in Sweden. Pharmacoepidemiol. Drug Saf. 2017, 26, 335–343. [Google Scholar]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kajiwara, A. Female sex as a risk factor for adverse drug reactions. Nihon Rinsho 2015, 73, 581–585. [Google Scholar] [PubMed]
- Watson, S.; Caster, O.; Rochon, P.A.; den Ruijter, H. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine 2019, 17, 100188. [Google Scholar] [CrossRef] [Green Version]
- Andersen, B.J.; Kumar, J.; Curtis, K.; Sanuku, N.; Satofan, S.; King, C.L.; Fischer, P.U.; Weil, G.J. Changes in Cytokine, Filarial Antigen, and DNA Levels Associated With Adverse Events Following Treatment of Lymphatic Filariasis. J. Infect. Dis. 2018, 217, 280–287. [Google Scholar] [CrossRef]
- Vaqas, B.; Ryan, T.J. Lymphoedema: Pathophysiology and management in resource-poor settings—Relevance for lymphatic filariasis control programmes. Filaria J. 2003, 2, 4. [Google Scholar] [CrossRef]
- Magnussen, P.; Makunde, W.; Simonsen, P.E.; Meyrowitsch, D.; Jakubowski, K. Chronic pulmonary disorders, including tropical pulmonary eosinophilia, in villages with endemic lymphatic filariasis in Tanga region and in Tanga town, Tanzania. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 406–409. [Google Scholar] [CrossRef]
- Chaccour, C.; Hammann, F.; Rabinovich, N.R. Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar. J. 2017, 16, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyer, G.; Ottesen, E.A.; Galdino, E.; Andrade, L.; Rocha, A.; Medeiros, Z.; Moura, I.; Casimiro, I.; Beliz, F.; Coutinho, A. Renal abnormalities in microfilaremic patients with Bancroftian filariasis. Am. J. Trop. Med. Hyg. 1992, 46, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Layton, D.; Hazell, L.; Shakir, S.A. Modified prescription-event monitoring studies: A tool for pharmacovigilance and risk management. Drug Saf. 2011, 34, e1–e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf (accessed on 14 February 2022).

| Variables (N = 9288) | Statistics |
|---|---|
| Age (years) median (Interquartile range) | 26.0 (13.0–45.6) |
| Female sex, n (%) | 4816 (51.9) |
| Body weight (kg), mean (SD) * | 50.93 (19.63) |
| Height (cm), mean (SD) | 149.24 (18.31) |
| Swollen arm, n (%) | 52 (0.56) |
| Swollen leg, n (%) | 122 (1.13) |
| Swollen breast (Males and Females), n (%) | 18 (0.19) |
| Swollen scrotum (Males) n (%) ** | 148 (3.31) |
| Testicles/scrotum pain (Males), n (%) | 119 (2.66) |
| Chronic manifestation of LF n (%) | 389 (4.19) |
| Joint or muscle pain, n (%) | 428 (4.61) |
| General body weakness, n (%) | 216 (2.33) |
| Swelling/pain of armpit/groin, n (%) | 61 (0.66) |
| Skin itching, n (%) | 246 (2.65) |
| Skin rash, n (%) | 102 (1.10) |
| Chronic illness | 831 (8.95) |
| Use of bed nets | 7804 (84.0) |
| Use of traditional medicines | 139 (1.50) |
| Adverse Event | Total Number of Events | Severity Grading | ||
|---|---|---|---|---|
| Grade 1 (Mild) | Grade 2 (Moderate) | Grade3 (Severe) | ||
| Headache | 114 | 91 (79.8) | 23 (20.2) | |
| Drowsiness | 107 | 93 (86.9) | 13 (12.1) | 1 (0.9) |
| Fever | 104 | 84 (80.8) | 20 (19.2) | |
| Dizziness | 98 | 86 (87.8) | 11 (11.2) | 1 (1.0) |
| Stomach pain | 82 | 61 (74.4) | 21 (25.6) | |
| Nausea | 62 | 59 (95.2) | 3 (4.8) | |
| Loss of appetite | 45 | 42 (93.3) | 3 (6.7) | |
| Diarrhea | 43 | 33 (76.7) | 10 (23.3) | |
| Coughing | 32 | 30 (93.7) | 2 (6.3) | |
| Breathing difficulty | 14 | 10 (71.4) | 4 (28.6) | |
| Vomiting | 11 | 8 (72.7) | 3 (27.3) | |
| Confusion | 6 | 5 (83.3) | 1 (16.7) | |
| Total | 718 | 602 (83.8) | 114 (15.9) | 2 (0.3) |
| Variable | Any MDA Associated Adverse Event (%) | χ2 | p-Value | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| Sex | Male | 4305 (96.3) | 167 (3.7) | 19.97 | <0.001 |
| Female | 4541 (94.3) | 275 (5.7) | |||
| Age group (years) | 5–9 | 1913 (95.51) | 90 (4.49) | 1.73 | 0.63 |
| 10–17 | 1413 (95.73) | 63 (4.27) | |||
| 18–64 | 4752 (95) | 250 (5) | |||
| 65+ | 752 (95.19) | 38 (4.81) | |||
| Used bed net | No | 1680 (94.9) | 90 (5.1) | 0.52 | 0.47 |
| Yes | 7153 (95.3) | 351 (4.7) | |||
| Used MDA last round | No | 4341(95.4) | 209 (4.6) | 0.66 | 0.42 |
| Yes | 4235(95.0) | 221(5.0) | |||
| Use of traditional medicines | No | 8703 (95.2) | 439 (4.8) | 2.51 | 0.11 |
| Yes | 132 (98.5) | 2 (1.5) | |||
| Chronic illness | No | 8105 (95.0) | 382 (4.5) | 14.03 | <0.001 |
| Yes | 716 (92.5) | 58 (7.5) | |||
| Chronic LF manifestation | No | 8502 (95.4) | 413 (4.6) | 7.80 | 0.005 |
| Yes | 344 (92.2) | 29 (7.8) | |||
| Number of IA tablets taken | One | 827 (95.4) | 40 (4.6) | 2.50 | 0.48 |
| Two | 1397 (95.6) | 64 (4.4) | |||
| Three | 4336 (94.8) | 238 (5.2) | |||
| Four | 1941 (95.5) | 92 (4.5) | |||
| Ever used IA | Yes | 4725 (95.3) | 234 (4.7) | 0.339 | 0.56 |
| No | 2401(95.0) | 127(5.0) | |||
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | ||
| Age group | 18–64 | 1 | |||
| 59 | 0.85 (0.64–1.12) | 0.25 | |||
| 10–17 | 0.89 (0.70–1.14) | 0.38 | |||
| 18–64 | 0.96 (0.68–1.36) | 0.821 | |||
| Sex | Male | 1 | |||
| Female | 1.56 (1.28–1.90) | <0.001 | 1.55 (1.27–1.89) | <0.001 | |
| Used bed net | Yes | 1 | |||
| No | 1.09 (0.86–1.39) | 0.47 | |||
| Used MDA last round | Yes | 1 | |||
| No | 0.92 (0.76–1.12) | 0.42 | |||
| Used traditional medicines | No | 1 | |||
| Yes | 0.300 (0.07–1.22) | 0.07 | 0.26 (0.06–1.06) | 0.06 | |
| Chronic illness | No | 1 | |||
| Yes | 1.72 (1.29–2.29) | <0.001 | 1.61 (1.20–2.16) | 0.001 | |
| Chronic LF manifestation | No | 1 | |||
| Yes | 1.74 (1.17–2.57) | 0.006 | 1.76 (1.18–2.62) | 0.005 | |
| Number of ivermectin tablets taken | One | 1 | |||
| Two | 0.95 (0.63–1.42) | 0.79 | |||
| Three | 1.13 (0.81–1.60) | 0.47 | |||
| Four | 0.98 (0.67–1.43) | 0.92 | |||
| Ever used IA | No | 1 | |||
| Yes | 0.93 (0.75–1.16) | 0.56 | |||
| Use IA last MDA distribution | No | 1 | |||
| Yes | 1.09 (0.89–1.32) | 0.42 | |||
| Variable | Proportion, n/N (%) | OR | 95%CI | p-Value | |
|---|---|---|---|---|---|
| Hypertension | No | 412/8974 (4.6) | 1 | ||
| Yes | 28/287 (9.8) | 2.11 | (1.40–3.18) | <0.001 | |
| Asthma | No | 430/9159 (4.7) | 1 | ||
| Yes | 10/102 (9.8) | 1.98 | (1.02–3.86) | 0.045 | |
| Kidney problems | No | 438/9239 (4.7) | 1 | ||
| Yes | 2/22 (9.1) | 1.30 | (0.29–5.76) | 0.73 | |
| Diabetes | No | 439/9238 (4.7) | 1 | ||
| Yes | 1/23 (4.3) | 0.84 | (0.11–6.31) | 0.87 | |
| Tuberculosis | No | 439/9240 (4.7) | 1 | ||
| Yes | 1/21 (4.8) | 1.04 | (0.14–7.78) | 0.97 | |
| Other chronic condition * | No | 413/8943 (4.6) | 1 | ||
| Yes | 29/345 (8.4) | 1.78 | (1.19–2.66) | 0.005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fimbo, A.M.; Minzi, O.M.; Mmbando, B.P.; Gurumurthy, P.; Kamuhabwa, A.A.R.; Aklillu, E. Safety and Tolerability of Ivermectin and Albendazole Mass Drug Administration in Lymphatic Filariasis Endemic Communities of Tanzania: A Cohort Event Monitoring Study. Pharmaceuticals 2022, 15, 594. https://doi.org/10.3390/ph15050594
Fimbo AM, Minzi OM, Mmbando BP, Gurumurthy P, Kamuhabwa AAR, Aklillu E. Safety and Tolerability of Ivermectin and Albendazole Mass Drug Administration in Lymphatic Filariasis Endemic Communities of Tanzania: A Cohort Event Monitoring Study. Pharmaceuticals. 2022; 15(5):594. https://doi.org/10.3390/ph15050594
Chicago/Turabian StyleFimbo, Adam M., Omary Mashiku Minzi, Bruno P. Mmbando, Parthasarathi Gurumurthy, Appolinary A. R. Kamuhabwa, and Eleni Aklillu. 2022. "Safety and Tolerability of Ivermectin and Albendazole Mass Drug Administration in Lymphatic Filariasis Endemic Communities of Tanzania: A Cohort Event Monitoring Study" Pharmaceuticals 15, no. 5: 594. https://doi.org/10.3390/ph15050594
APA StyleFimbo, A. M., Minzi, O. M., Mmbando, B. P., Gurumurthy, P., Kamuhabwa, A. A. R., & Aklillu, E. (2022). Safety and Tolerability of Ivermectin and Albendazole Mass Drug Administration in Lymphatic Filariasis Endemic Communities of Tanzania: A Cohort Event Monitoring Study. Pharmaceuticals, 15(5), 594. https://doi.org/10.3390/ph15050594






