Development of Photoremovable Linkers as a Novel Strategy to Improve the Pharmacokinetics of Drug Conjugates and Their Potential Application in Antibody–Drug Conjugates for Cancer Therapy
Abstract
:1. Introduction
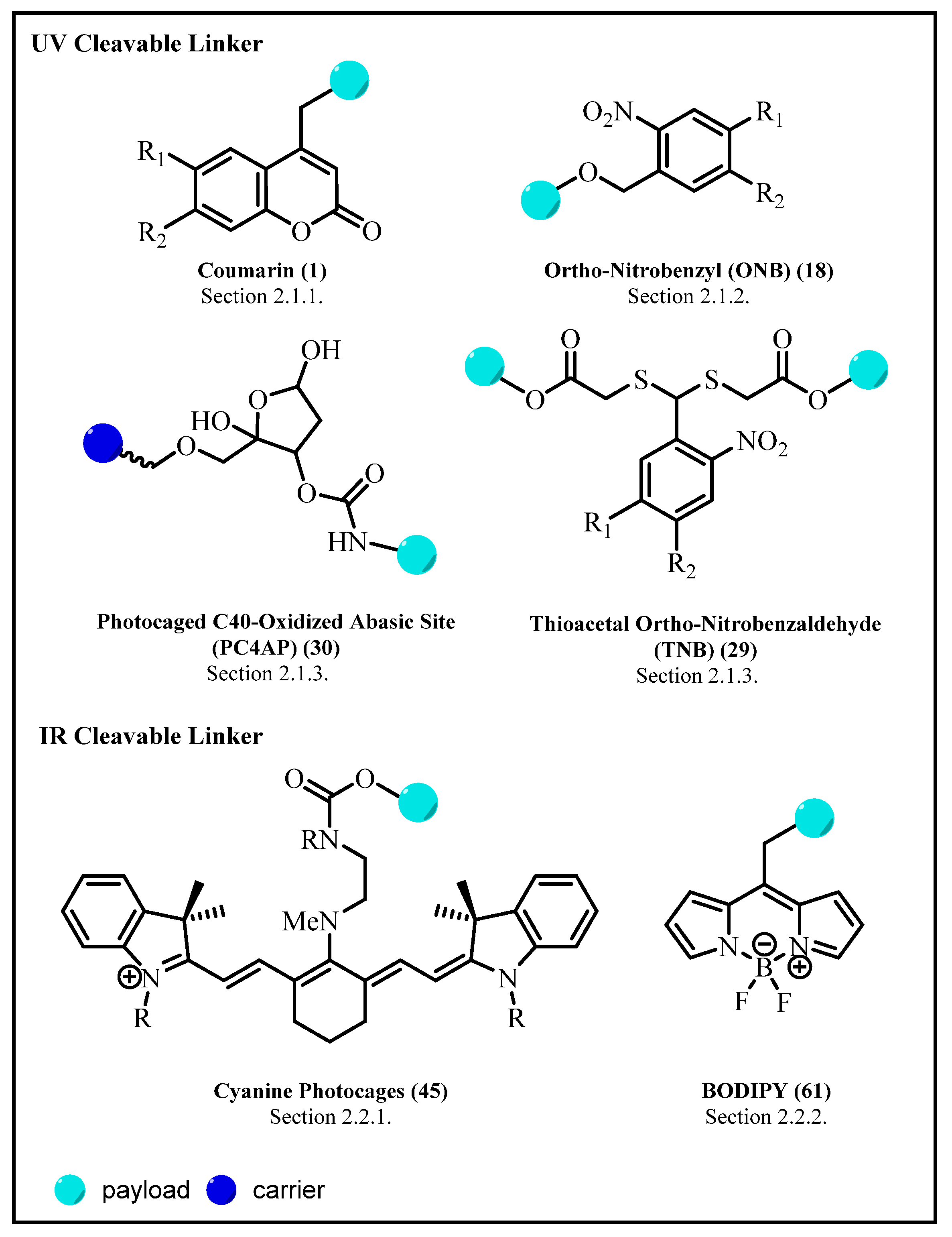
2. Photoresponsive Linker
2.1. Ultraviolet/Visible (UV/Vis)-Triggered Linker
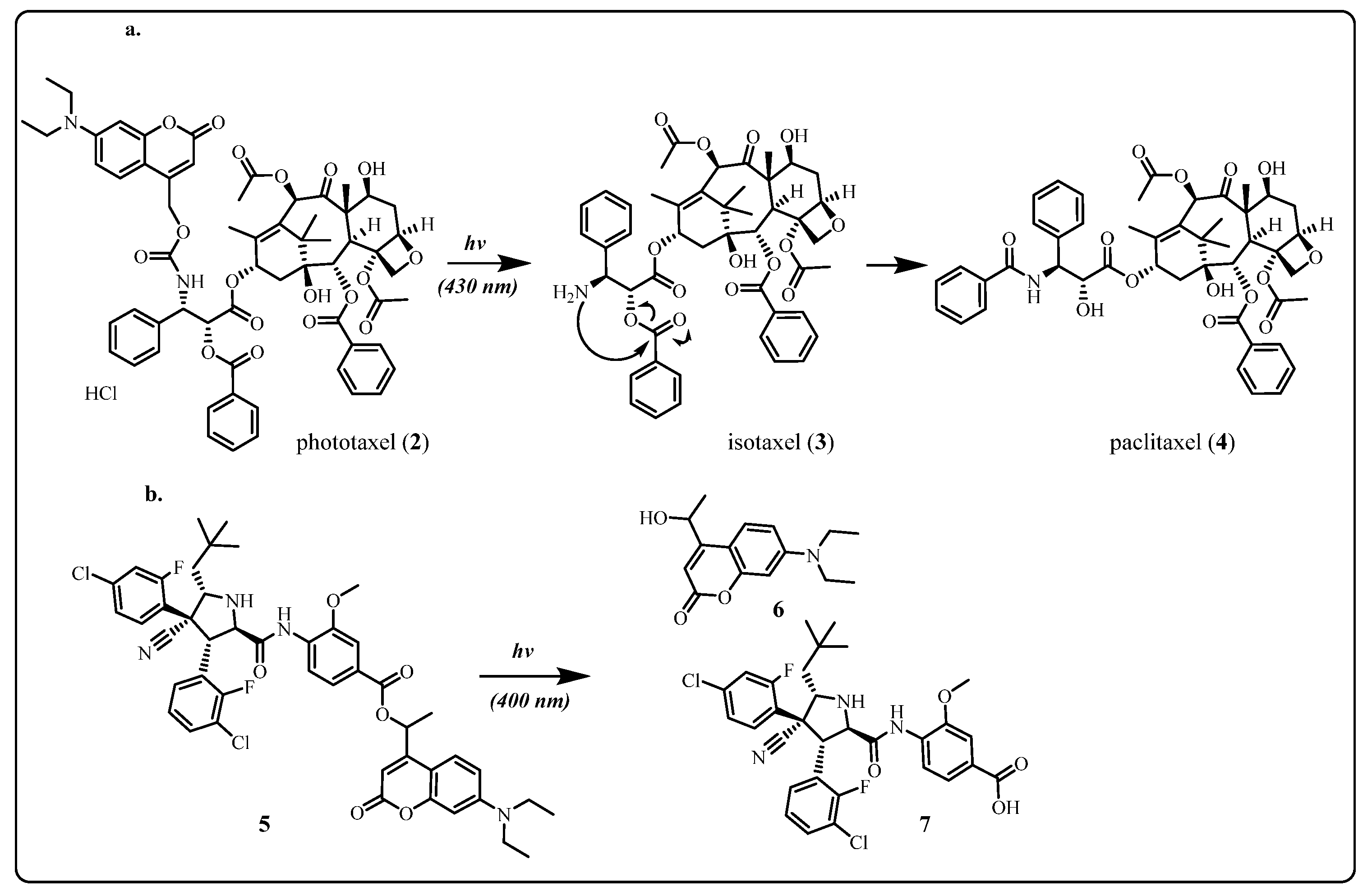
2.1.1. Coumarin and Its Derivatives
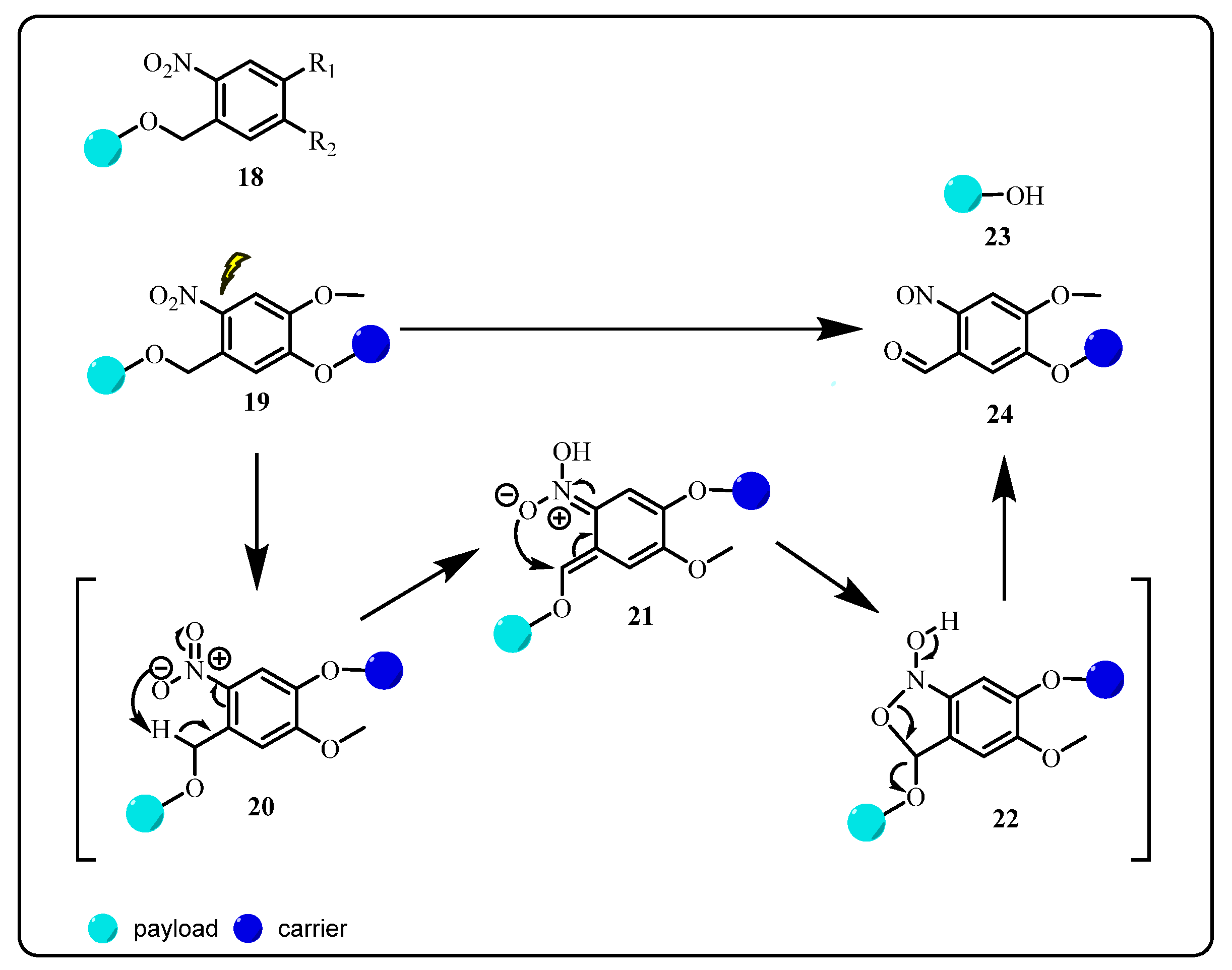
2.1.2. Ortho-Nitrobenzyl (ONB) Group
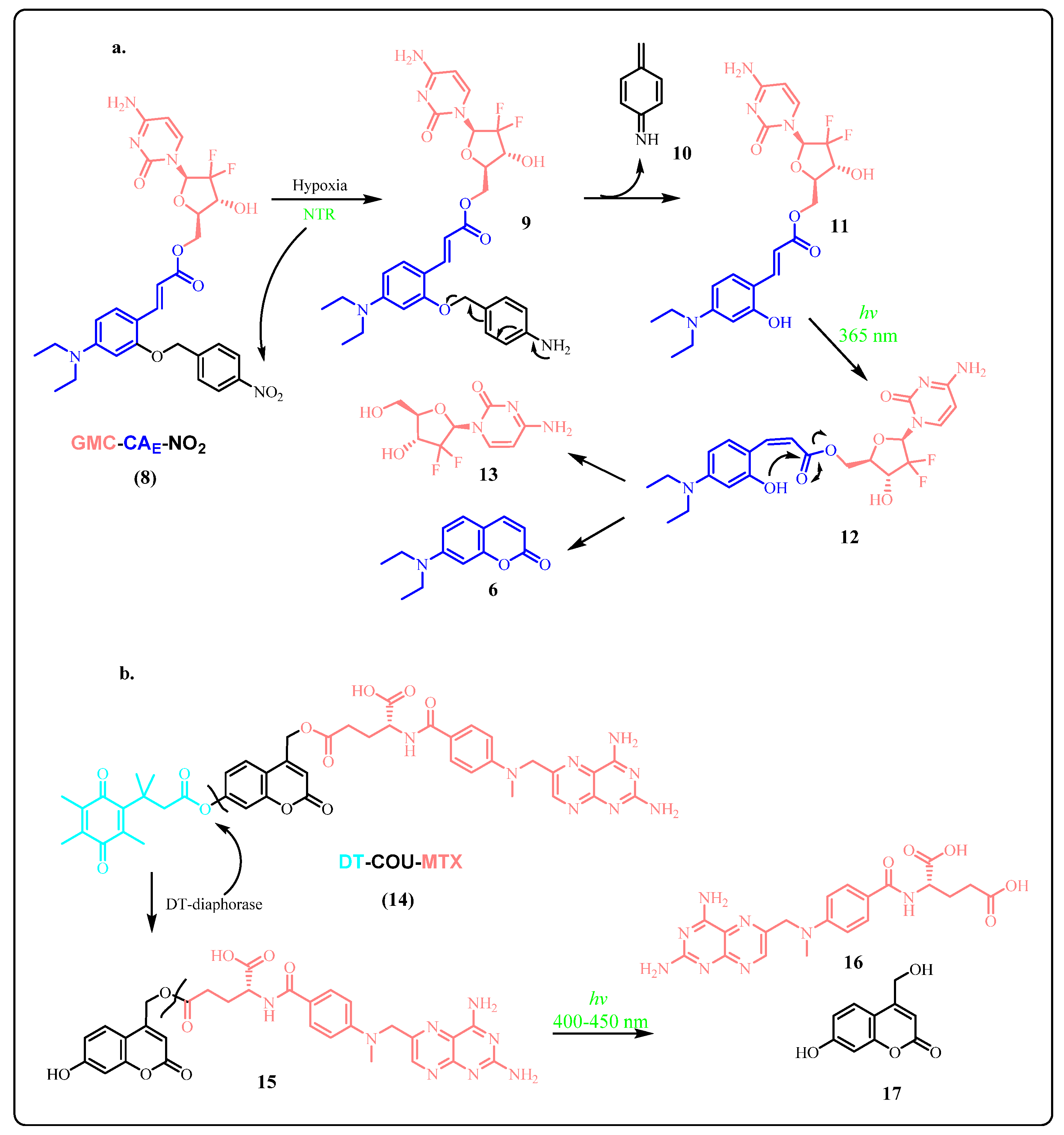
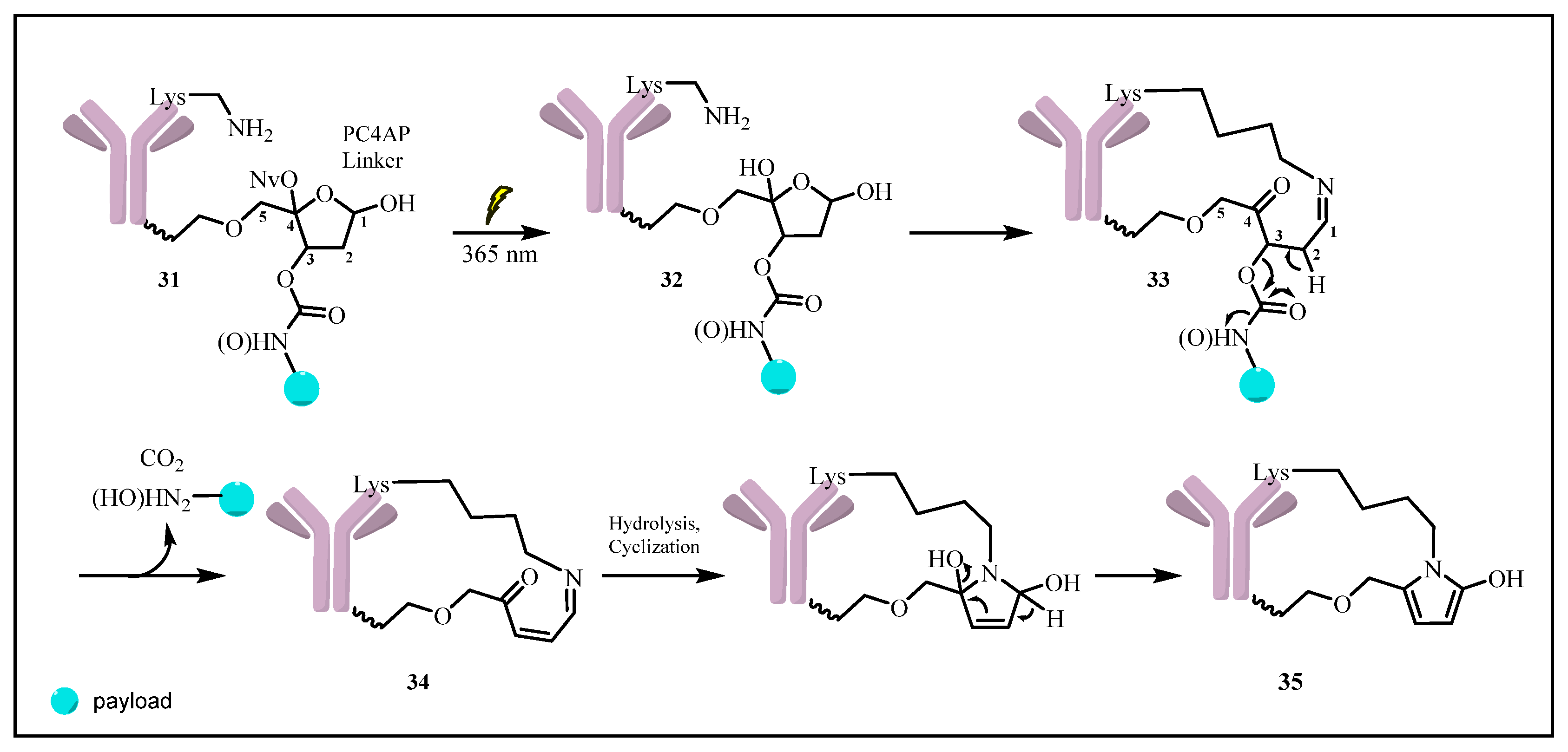
2.1.3. Novel Photo-Triggered Linkers—Thioacetal Ortho-Nitrobenzaldehyde (TNB) Group and Photocaged C40-Oxidized Abasic Site (PC4AP)
Photocaged C40-Oxidized Abasic Site (PC4AP)
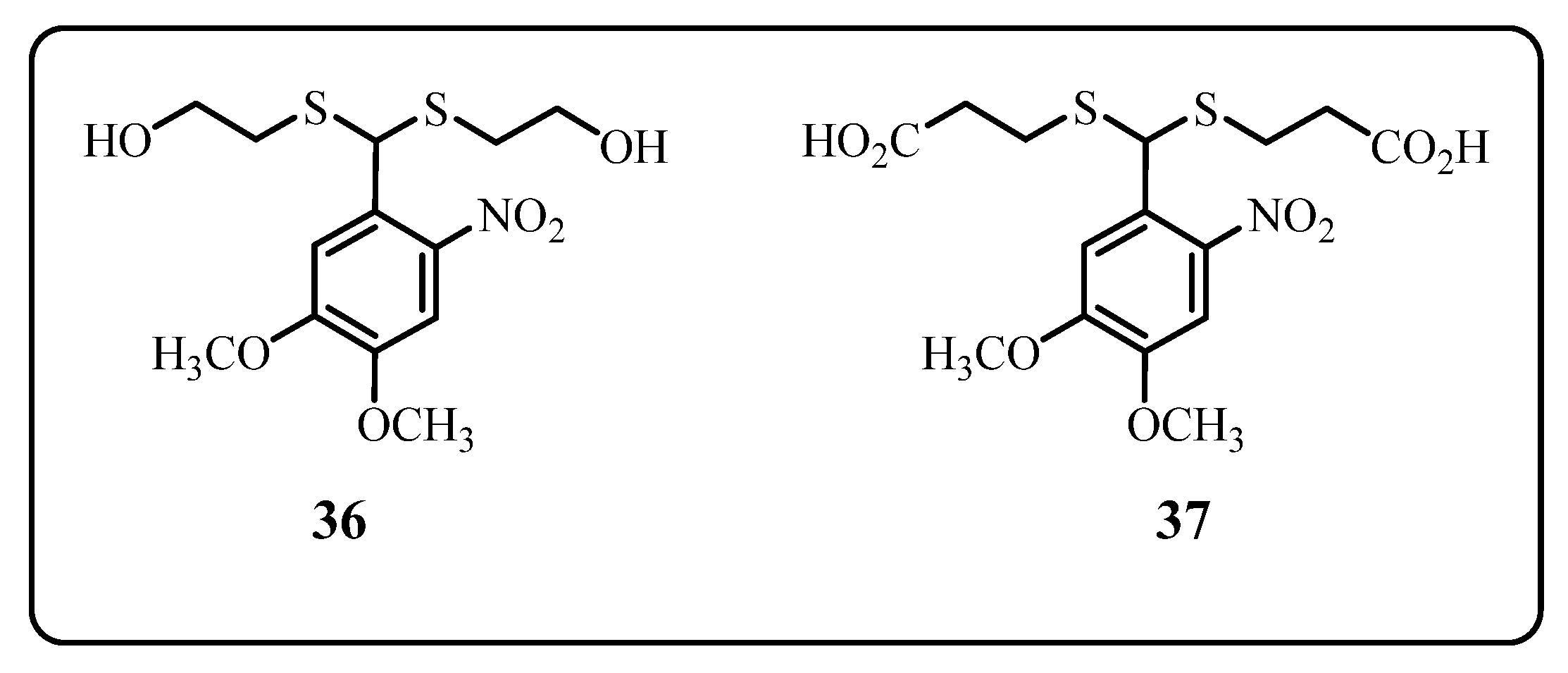
Thioacetal Ortho-Nitrobenzaldehyde (TNB)
2.1.4. The Limitation of UV-Active Linker and the Optional Solutions
2.2. Near-Infrared (NIR), Visible-Light-Triggered Linker Strategy
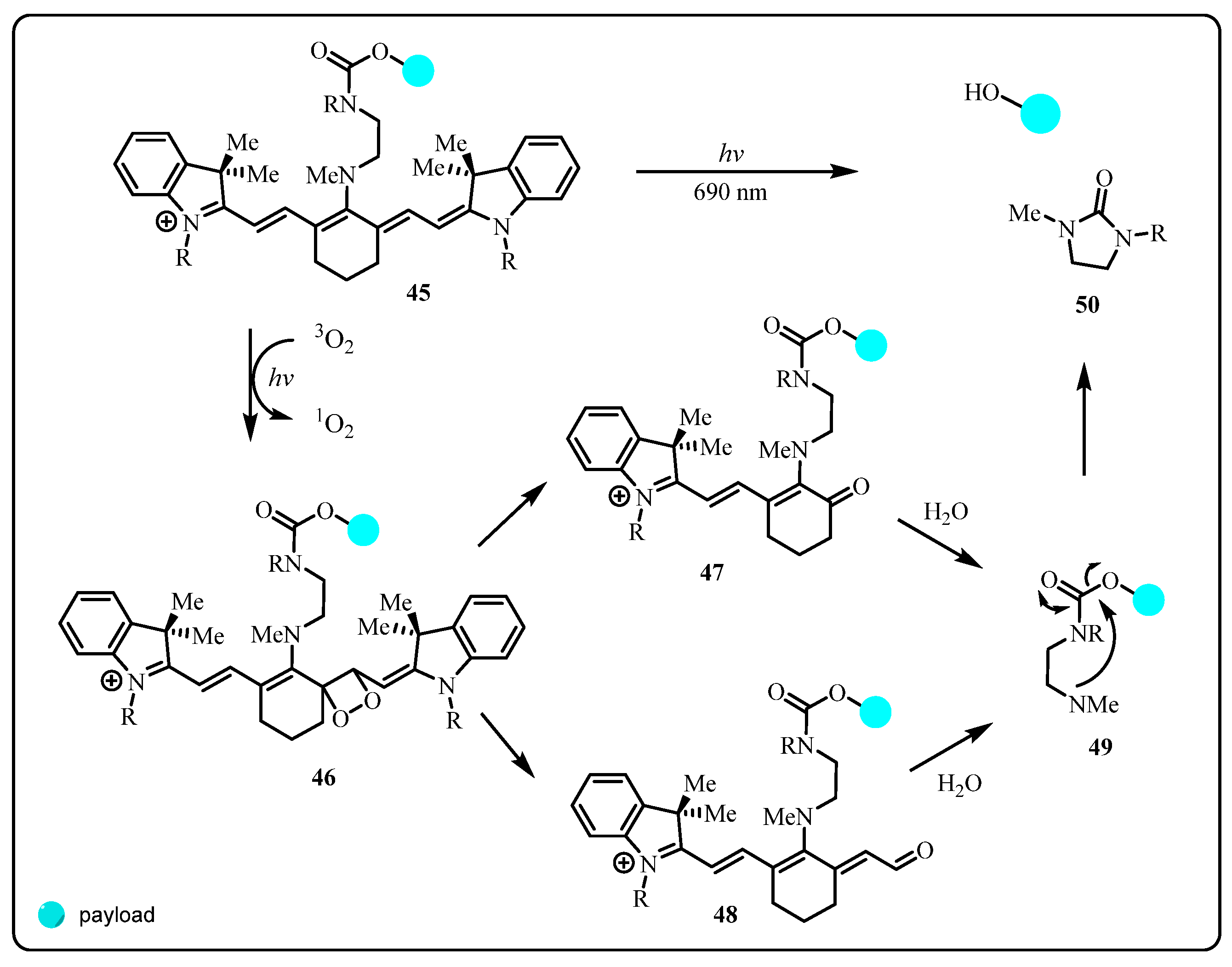
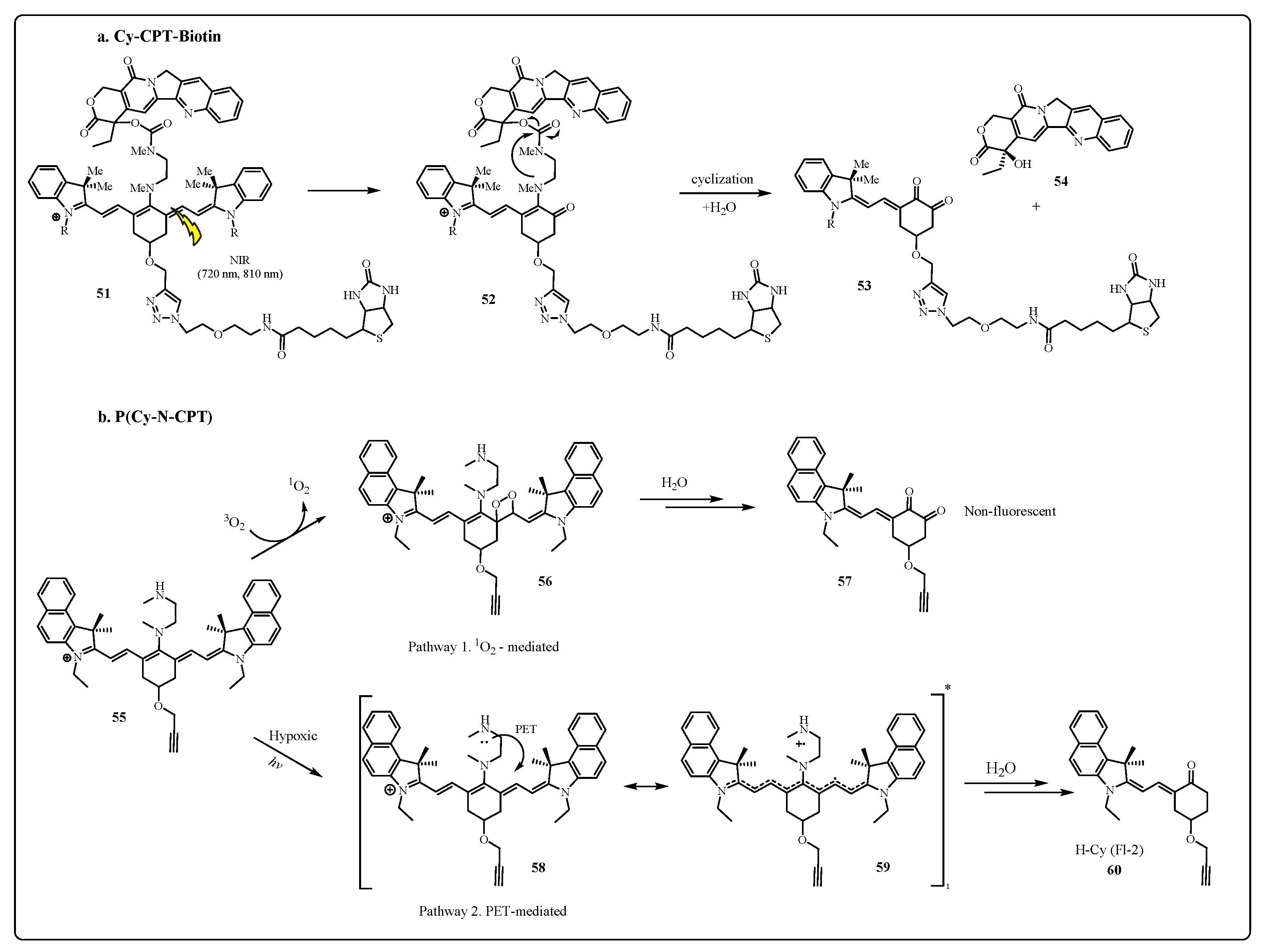
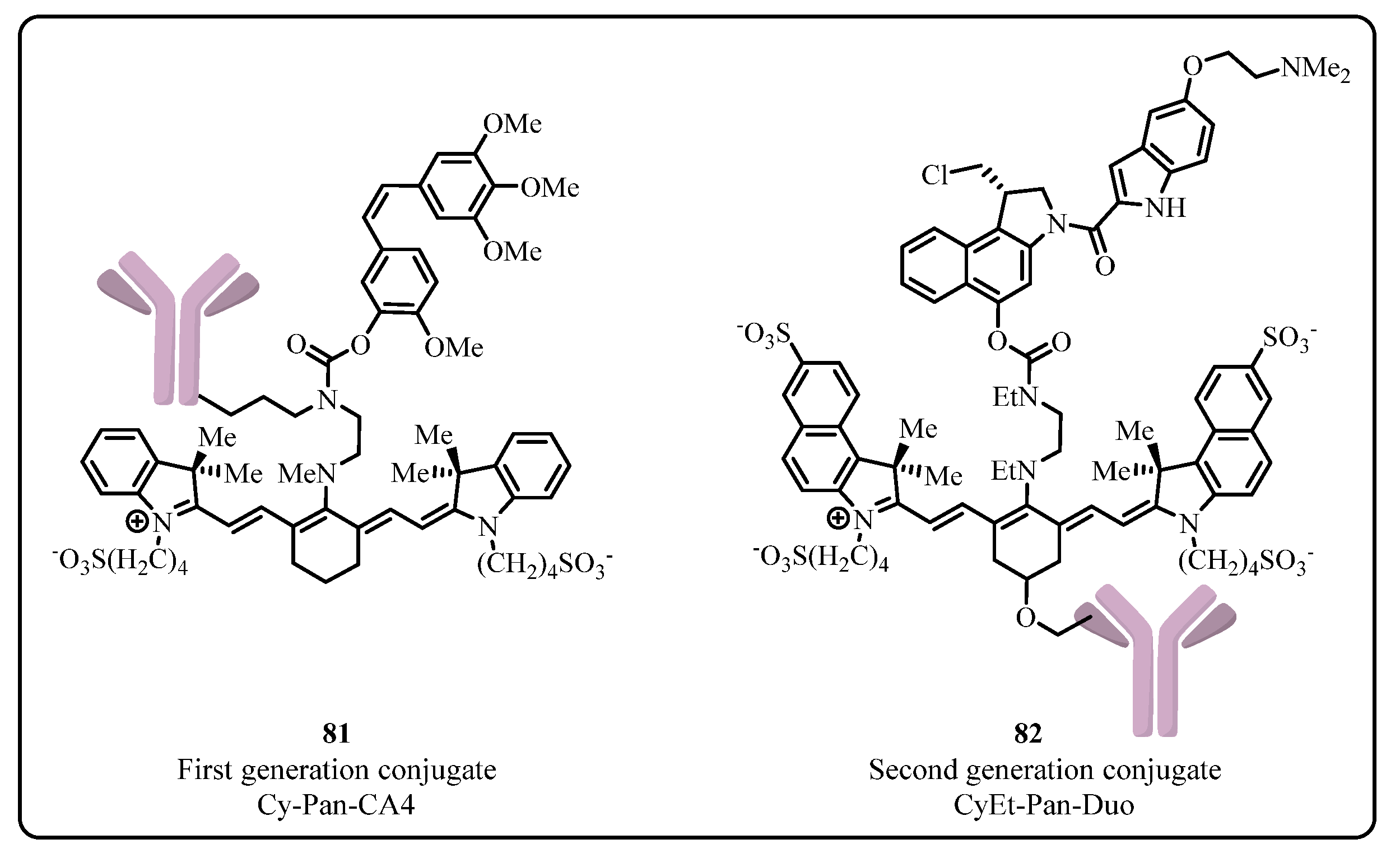
2.2.1. Cyanine Photocages
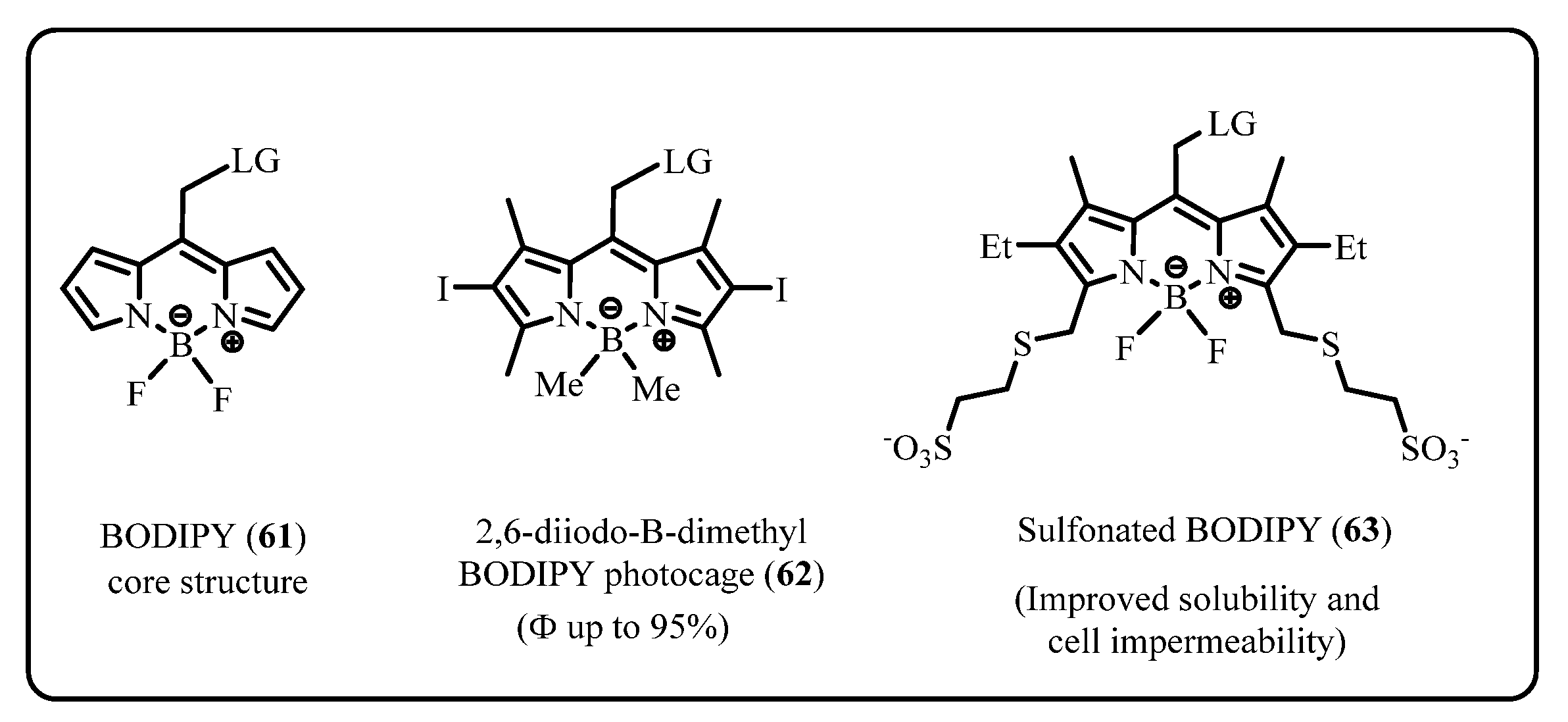
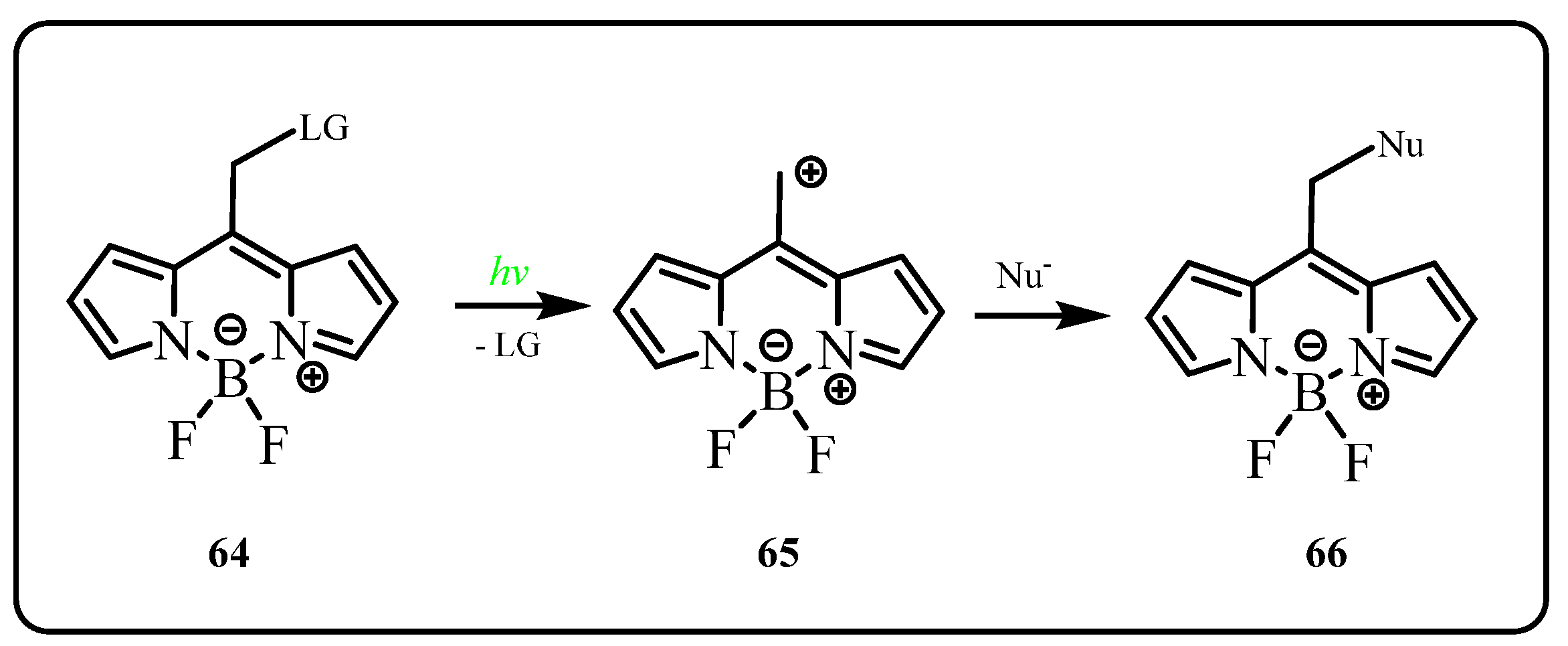
2.2.2. BODIPY
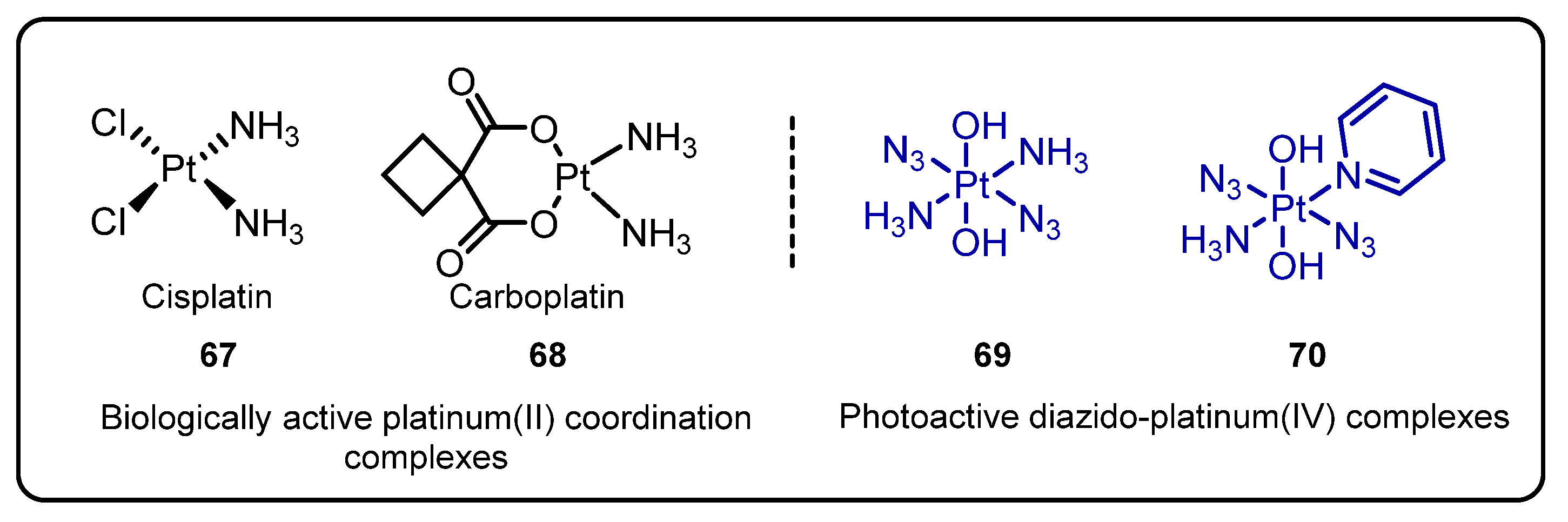
2.3. Diazido Platinum(IV) Complexes for Photoactivated Anticancer Chemotherapy(PACT)
3. Application in Antibody–Drug Conjugate (ADC)
3.1. ADC in Cancer Therapy
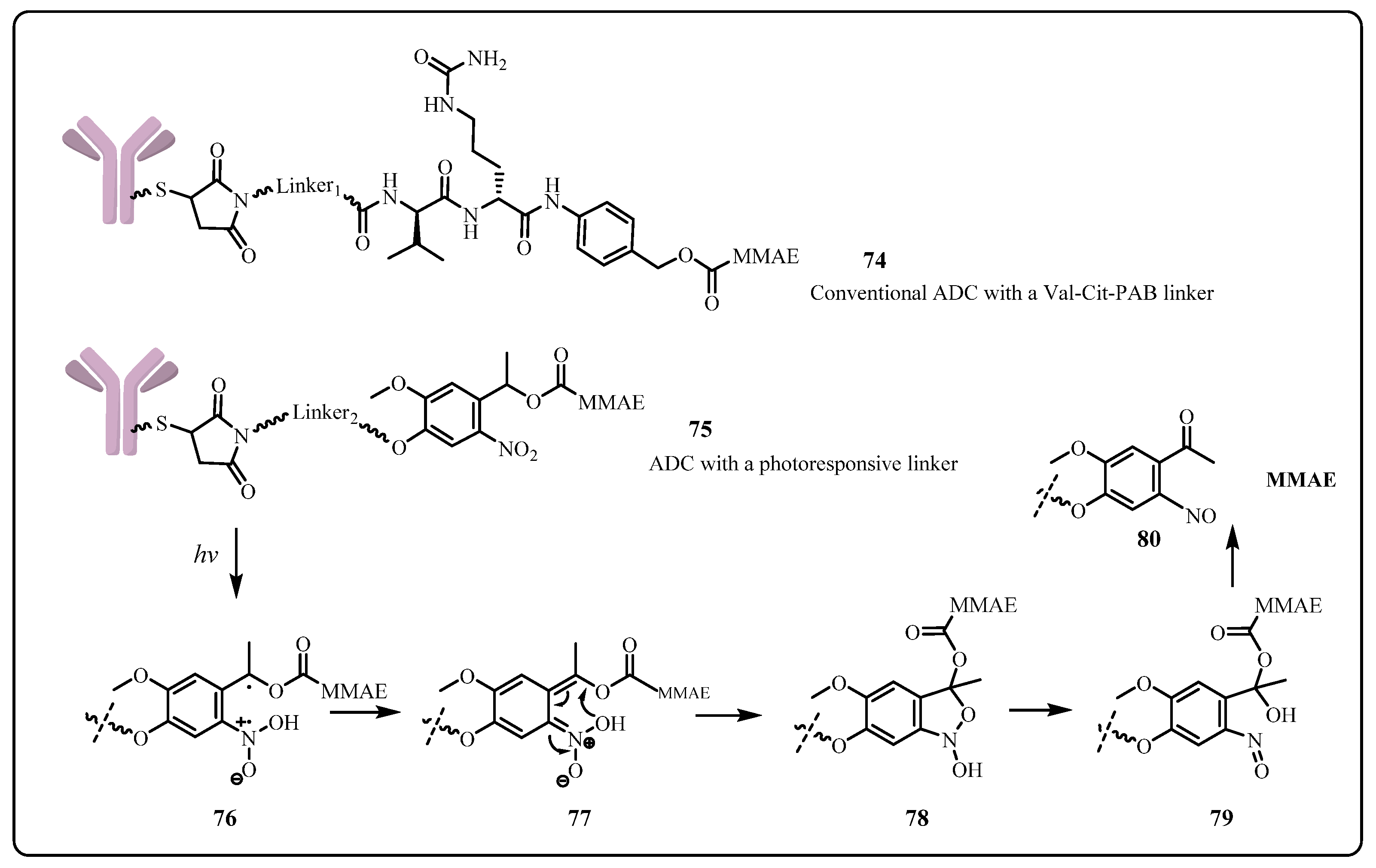
3.2. Application of Photoresponsive Linker in ADC
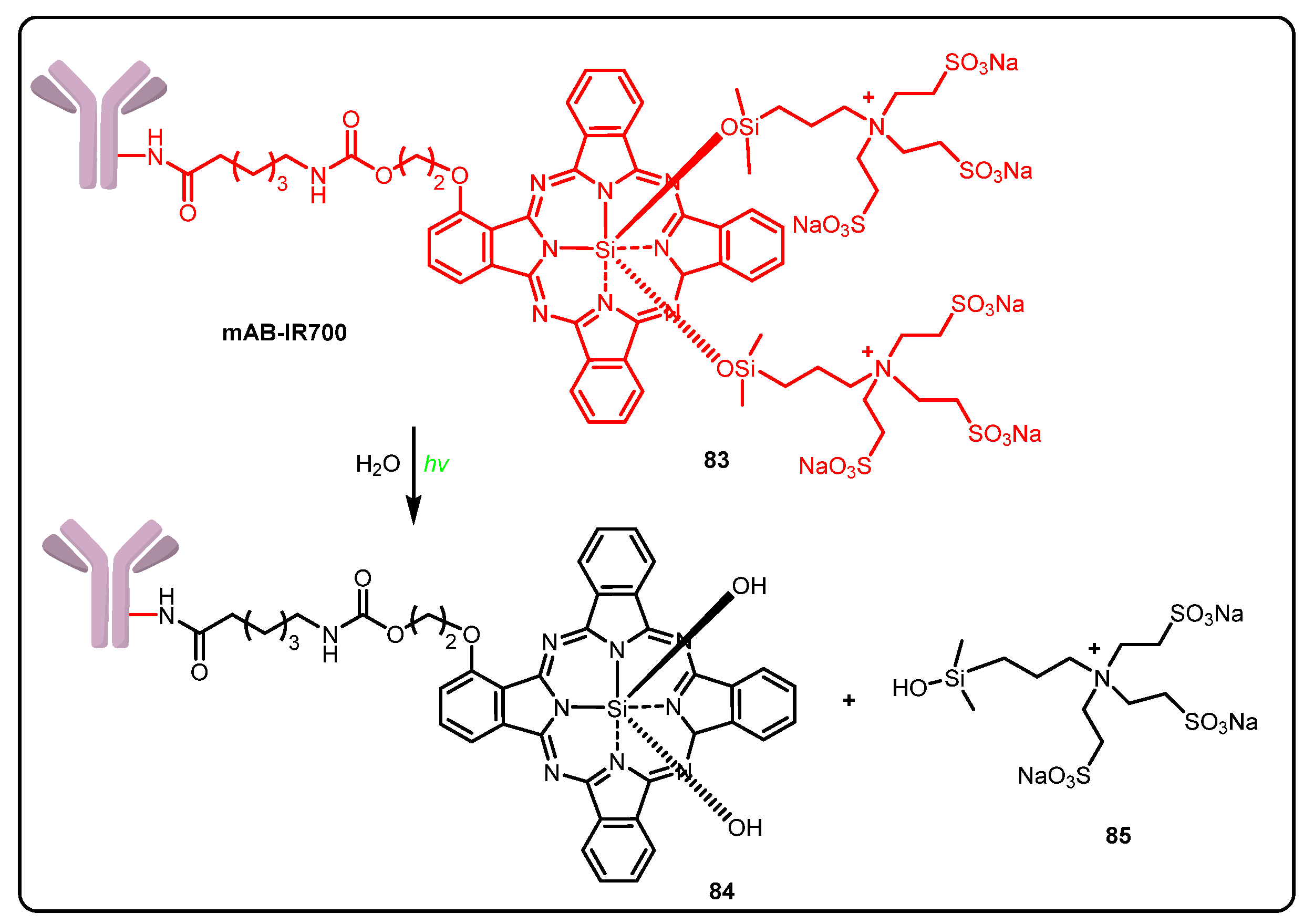
3.3. Photoimmunotherapy (PIT)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaghoubi, S.; Karimi, M.H.; Lotfinia, M.; Gharibi, T.; Mahi-Birjand, M.; Kavi, E.; Hosseini, F.; Sineh Sepehr, K.; Khatami, M.; Bagheri, N.; et al. Potential drugs used in the antibody–drug conjugate (ADC) architecture for cancer therapy. J. Cell. Physiol. 2020, 235, 31–64. [Google Scholar] [CrossRef]
- World Health Organization. Estimated Number of New Cases in 2020, Worldwide, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1 (accessed on 3 August 2021).
- Dembic, Z. Antitumor Drugs and Their Targets. Molecules 2020, 25, 5776. [Google Scholar] [CrossRef] [PubMed]
- Thurston, D. Cytotoxic Payloads for Antibody–Drug Conjugates; Royal Society of Chemistry: London, UK, 2019; pp. 1–6. [Google Scholar]
- Haman, P.R. Monoclonal Antibody-Drug Conjugates. Expert Opin. Ther. Pat. 2005, 15, 1087–1103. [Google Scholar] [CrossRef]
- Dal Corso, A.; Pignataro, L.; Belvisi, L.; Gennari, C. Innovative Linker Strategies for Tumor-Targeted Drug Conjugates. Chem. Eur. J. 2019, 25, 14740–14757. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiao, D.; Xie, F.; Liu, L.; Wang, Y.; Fan, S.; Zhou, X.; Li, S. Antibody-drug conjugates: Recent advances in linker chemistry. Acta Pharm. Sin. B 2021, 11, 3889–3907. [Google Scholar] [CrossRef]
- Timko, B.P.; Kohane, D.S. Materials to clinical devices: Technologies for remotely triggered drug delivery. Clin. Ther. 2012, 34, S25–S35. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Kauscher, U.; Holme, M.N.; Bjornmalm, M.; Stevens, M.M. Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes. Adv. Drug. Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
- Rossin, R.; van Duijnhoven, S.M.; Ten Hoeve, W.; Janssen, H.M.; Kleijn, L.H.; Hoeben, F.J.; Versteegen, R.M.; Robillard, M.S. Triggered Drug Release from an Antibody-Drug Conjugate Using Fast “Click-to-Release” Chemistry in Mice. Bioconjug. Chem. 2016, 27, 1697–1706. [Google Scholar] [CrossRef]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Vaghari, H.; Anarjan, N.; Ahmadi, O.; Berenjian, A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017, 37, 492–509. [Google Scholar] [CrossRef]
- Li, J.; Xiao, D.; Xie, F.; Li, W.; Zhao, L.; Sun, W.; Yang, X.; Zhou, X. Novel antibody-drug conjugate with UV-controlled cleavage mechanism for cytotoxin release. Bioorg. Chem. 2021, 111, 104475. [Google Scholar] [CrossRef]
- Barhoumi, A.; Liu, Q.; Kohane, D.S. Ultraviolet light-mediated drug delivery: Principles, applications, and challenges. J. Controlled Release 2015, 219, 31–42. [Google Scholar] [CrossRef]
- Yan, Q.; Han, D.; Zhao, Y. Main-chain photoresponsive polymers with controlled location of light-cleavable units: From synthetic strategies to structural engineering. Polym. Chem. 2013, 4, 5026–5037. [Google Scholar] [CrossRef]
- Ben Mihoub, A.; Larue, L.; Moussaron, A.; Youssef, Z.; Colombeau, L.; Baros, F.; Frochot, C.; Vanderesse, R.; Acherar, S. Use of Cyclodextrins in Anticancer Photodynamic Therapy Treatment. Molecules 2018, 23, 1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, S.; Park, N.; Han, J.H.; Jeon, H.M.; Lee, J.H.; Bhuniya, S.; Kang, C.; Kim, J.S. Gemcitabine-coumarin-biotin conjugates: A target specific theranostic anticancer prodrug. J. Am. Chem. Soc. 2013, 135, 4567–4572. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, J.; Muthukrishnan, S.; Gudmundsdottir, A.D. Photoremovable protecting groups based on photoenolization. Adv. Phys. Org. Chem. 2009, 43, 39–77. [Google Scholar]
- Cho, H.J.; Chung, M.; Shim, M.S. Engineered photo-responsive materials for near-infrared-triggered drug delivery. J. Ind. Eng. Chem. 2015, 31, 15–25. [Google Scholar] [CrossRef]
- Olejniczak, J.; Carling, C.J.; Almutairi, A. Photocontrolled release using one-photon absorption of visible or NIR light. J. Controlled Release 2015, 219, 18–30. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Molnar, M.; Lončarić, M.; Kovač, M. Green Chemistry Approaches to the Synthesis of Coumarin Derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Loncaric, M.; Gaso-Sokac, D.; Jokic, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Cazin, I.; Rossegger, E.; Guedes de la Cruz, G.; Griesser, T.; Schlogl, S. Recent Advances in Functional Polymers Containing Coumarin Chromophores. Polymers 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending Topics on Coumarin and Its Derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef]
- Dong-Wei, C.; Yuan, Z.; Xiao-Yi, D.; Yu, Z.; Guo-Hui, L.; Xue-Song, F. Progress in Pretreatment and Analytical Methods of Coumarins: An Update since 2012—A Review. Crit. Rev. Anal. Chem. 2020, 51, 1–24. [Google Scholar] [CrossRef]
- Sharma, M.; Friedman, S.H. The Issue of Tissue: Approaches and Challenges to the Light Control of Drug Activity. ChemPhotoChem 2021, 5, 611–618. [Google Scholar] [CrossRef]
- Tasior, M.; Kim, D.; Singha, S.; Krzeszewski, M.; Ahn, K.H.; Gryko, D.T. π-Expanded coumarins: Synthesis, optical properties and applications. J. Mater. Chem. C 2015, 3, 1421–1446. [Google Scholar] [CrossRef]
- Choi, S.K. Photocleavable linkers: Design and applications in nanotechnology. In Photonanotechnology for Therapeutics and Imaging; Choi, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–275. [Google Scholar]
- Shen, W.; Zheng, J.; Zhou, Z.; Zhang, D. Approaches for the synthesis of o-nitrobenzyl and coumarin linkers for use in photocleavable biomaterials and bioconjugates and their biomedical applications. Acta Biomater. 2020, 115, 75–91. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Noguchi, M.; Hirota, S.; Sohma, Y.; Kimura, T.; Hayashi, Y.; Kiso, Y. Development of first photoresponsive prodrug of paclitaxel. Bioorg. Med. Chem. Lett. 2006, 16, 4492–4496. [Google Scholar] [CrossRef]
- Hansen, M.J.; Feringa, F.M.; Kobauri, P.; Szymanski, W.; Medema, R.H.; Feringa, B.L. Photoactivation of MDM2 Inhibitors: Controlling Protein-Protein Interaction with Light. J. Am. Chem. Soc. 2018, 140, 13136–13141. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Gao, C.; Liu, W.; Ren, H.; Wang, C.; Ge, K.; Li, S.; Zhou, G.; Li, H.; Wang, S.; et al. A novel anticancer theranostic pro-prodrug based on hypoxia and photo sequential control. Chem. Commun. 2016, 52, 9434–9437. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, B.; Xie, X.; Zeng, F.; Wu, S. A sequential enzyme-activated and light-triggered pro-prodrug nanosystem for cancer detection and therapy. J. Mater. Chem. B 2018, 6, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Klan, P.; Solomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef]
- Mewes, J.M.; Dreuw, A. On the role of singlet versus triplet excited states in the uncaging of ortho-nitrobenzyl caged compounds. Phys. Chem. Chem. Phys. 2013, 15, 6691–6698. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Zhu, L.; Lin, Q.; Tian, H. Building biomedical materials using photochemical bond cleavage. Adv. Mater. 2015, 27, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Agasti, S.S.; Chompoosor, A.; You, C.C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated release of caged anticancer drugs from gold nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.K.; Thomas, T.P.; Li, M.H.; Desai, A.; Kotlyar, A.; Baker, J.R., Jr. Photochemical release of methotrexate from folate receptor-targeting PAMAM dendrimer nanoconjugate. Photochem. Photobiol. Sci. 2012, 11, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.S.; Han, J.; Kumar, R.; Lee, G.G.; Sessler, J.L.; Kim, J.H.; Kim, J.S. Programmed activation of cancer cell apoptosis: A tumor-targeted phototherapeutic topoisomerase I inhibitor. Sci. Rep. 2016, 6, 29018. [Google Scholar] [CrossRef] [Green Version]
- Ibsen, S.; Zahavy, E.; Wrasdilo, W.; Berns, M.; Chan, M.; Esener, S. A novel Doxorubicin prodrug with controllable photolysis activation for cancer chemotherapy. Pharm. Res. 2010, 27, 1848–1860. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Hatta, H.; Ito, T.; Nishimoto, S. Synthesis and photochemical properties of photoactivated antitumor prodrugs releasing 5-fluorouracil. Org. Biomol. Chem. 2005, 3, 592–596. [Google Scholar] [CrossRef]
- Zang, C.; Wang, H.; Li, T.; Zhang, Y.; Li, J.; Shang, M.; Du, J.; Xi, Z.; Zhou, C. A light-responsive, self-immolative linker for controlled drug delivery via peptide- and protein-drug conjugates. Chem. Sci. 2019, 10, 8973–8980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.T.; Tang, S.; Cannon, J.; Mukherjee, J.; Isham, D.; Gam, K.; Payne, M.; Yanik, S.A.; Baker, J.R., Jr.; Choi, S.K. A Thioacetal Photocage Designed for Dual Release: Application in the Quantitation of Therapeutic Release by Synchronous Reporter Decaging. Chembiochem 2017, 18, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.; Tang, S.; Cannon, J.; Chen, D.; Sun, R.; Lee, J.; Phan, J.; Tao, K.; Sun, K.; Chen, B.; et al. Photocontrolled Release of Doxorubicin Conjugated through a Thioacetal Photocage in Folate-Targeted Nanodelivery Systems. Bioconjug. Chem. 2017, 28, 3016–3028. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Werner, S.; Weiß, T.; Liefeith, K.; Hoffmann, C. Ortho-Nitrobenzyl alcohol based two-photon excitation controlled drug release system. RSC Adv. 2012, 2, 156–160. [Google Scholar] [CrossRef]
- Momotake, A.; Lindegger, N.; Niggli, E.; Barsotti, R.J.; Ellis-Davies, G.C. The nitrodibenzofuran chromophore: A new caging group for ultra-efficient photolysis in living cells. Nat. Methods 2006, 3, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Fan, G.; Lin, Q.; Li, B.; Cheng, S.; Huang, Q.; Zhu, L. Styryl Conjugated Coumarin Caged Alcohol: Efficient Photorelease by Either One-Photon Long Wavelength or Two-Photon NIR Excitation. Org. Lett. 2012, 14, 572–575. [Google Scholar] [CrossRef]
- Wylie, R.G.; Shoichet, M.S. Two-photon micropatterning of amines within an agarose hydrogel. J. Mater. Chem. 2008, 18, 2716–2721. [Google Scholar] [CrossRef]
- Nani, R.R.; Gorka, A.P.; Nagaya, T.; Kobayashi, H.; Schnermann, M.J. Near-IR Light-Mediated Cleavage of Antibody-Drug Conjugates Using Cyanine Photocages. Angew. Chem. Int. Ed. 2015, 54, 13635–13638. [Google Scholar] [CrossRef] [Green Version]
- Vorobev, A.Y.; Moskalensky, A.E. Long-wavelength photoremovable protecting groups: On the way to in vivo application. Comput. Struct. Biotechnol. J. 2020, 18, 27–34. [Google Scholar] [CrossRef]
- Luciano, M.P.; Nourian, S.; Gorka, A.P.; Nani, R.R.; Nagaya, T.; Kobayashi, H.; Schnermann, M.J. A near-infrared light-mediated cleavable linker strategy using the heptamethine cyanine chromophore. In Methods in Enzymology; 2020/07/28 ed.; Chenoweth, D.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 641, pp. 245–275. [Google Scholar]
- Rosenthal, E.L.; Warram, J.M.; de Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer. Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, E.L.; Moore, L.S.; Tipirneni, K.; de Boer, E.; Stevens, T.M.; Hartman, Y.E.; Carroll, W.R.; Zinn, K.R.; Warram, J.M. Sensitivity and Specificity of Cetuximab-IRDye800CW to Identify Regional Metastatic Disease in Head and Neck Cancer. Clin. Cancer. Res. 2017, 23, 4744–4752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; van den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neuro-Oncol. 2018, 139, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Ma, Y.; Liu, Y.; Yan, C.; Shi, P.; Tian, H.; Zhu, W.-H. Photocaged prodrug under NIR light-triggering with dual-channel fluorescence: In Vivo real-time tracking for precise drug delivery. Sci. China Chem. 2018, 61, 1293–1300. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Zheng, Q.; Jia, Q.; Wang, Z.; Shi, P.; Guo, Z. Harnessing Hypoxia-Dependent Cyanine Photocages for In Vivo Precision Drug Release. Angew. Chem. Int. Ed. 2021, 60, 9553–9561. [Google Scholar] [CrossRef] [PubMed]
- Nani, R.R.; Gorka, A.P.; Nagaya, T.; Yamamoto, T.; Ivanic, J.; Kobayashi, H.; Schnermann, M.J. In Vivo Activation of Duocarmycin-Antibody Conjugates by Near-Infrared Light. ACS Cent. Sci. 2017, 3, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Wijesooriya, C.; Gehrmann, E.J.; Mahoney, K.M.; Goswami, P.P.; Albright, T.R.; Syed, A.; Dutton, A.S.; Smith, E.A.; Winter, A.H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140, 7343–7346. [Google Scholar] [CrossRef]
- Slanina, T.; Shrestha, P.; Palao, E.; Kand, D.; Peterson, J.A.; Dutton, A.S.; Rubinstein, N.; Weinstain, R.; Winter, A.H.; Klan, P. In Search of the Perfect Photocage: Structure-Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups. J. Am. Chem. Soc. 2017, 139, 15168–15175. [Google Scholar] [CrossRef]
- Kand, D.; Liu, P.; Navarro, M.X.; Fischer, L.J.; Rousso-Noori, L.; Friedmann-Morvinski, D.; Winter, A.H.; Miller, E.W.; Weinstain, R. Water-Soluble BODIPY Photocages with Tunable Cellular Localization. J. Am. Chem. Soc. 2020, 142, 4970–4974. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.K. A Histochemical Approach to the Mechanism of Action of Cisplatin and Its Analogues. J. Histochem. Cytochem. 1993, 41, 1053–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, P.; Shi, D.; Zhang, S.; Zhu, Y.; Zhou, J. Cardamonin enhances the anti-proliferative effect of cisplatin on ovarian cancer. Oncol. Lett. 2018, 15, 3991–3997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchetti, G.; Rimoldi, I. Anticancer platinum(II) complexes bearing N-heterocycle rings. Bioorganic Med. Chem. Lett. 2019, 29, 1257–1263. [Google Scholar] [CrossRef]
- Misset, J.L.; Bleiberg, H.; Sutherland, W.; Bekradda, M.; Cvitkovic, E. Oxaliplatin clinical activity: A review. Crit. Rev. Oncol. Hematol. 2000, 35, 75–93. [Google Scholar] [CrossRef]
- Chaaban, M.; Zhou, C.; Lin, H.; Chyi, B.; Ma, B. Platinum(ii) binuclear complexes: Molecular structures, photophysical properties, and applications. J. Mater. Chem. C 2019, 7, 5910–5924. [Google Scholar] [CrossRef]
- Choi, S.; Filotto, C.; Bisanzo, M.; Delaney, S.; Lagasee, D.; Whitworth, J.L.; Jusko, A.; Li, C.; Wood, N.A.; Willingham, J.; et al. Reduction and Anticancer Activity of Platinum(IV) Complexes. Inorg. Chem. 1998, 37, 2500–2504. [Google Scholar] [CrossRef]
- Ling, X.; Tu, J.; Wang, J.; Shajii, A.; Kong, N.; Feng, C.; Zhang, Y.; Yu, M.; Xie, T.; Bharwani, Z.; et al. Glutathione-Responsive Prodrug Nanoparticles for Effective Drug Delivery and Cancer Therapy. ACS Nano 2019, 13, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Wang, Z. Photoactivatable Platinum-Based Anticancer Drugs: Mode of Photoactivation and Mechanism of Action. Molecules 2020, 25, 5167. [Google Scholar] [CrossRef]
- Shi, H.; Imberti, C.; Sadler, P.J. Diazido platinum(iv) complexes for photoactivated anticancer chemotherapy. Inorg. Chem. Front. 2019, 6, 1623–1638. [Google Scholar] [CrossRef] [Green Version]
- Mu, M.; Zhan, J.; Dai, X.; Gao, H. Research progress of azido-containing Pt(IV) antitumor compounds. Eur. J. Med. Chem. 2022, 227, 113927. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, P.J.; Korpis, K.; Westendorf, A.F.; Perfahl, S.; Grunert, R. Effects of light-activated diazido-PtIV complexes on cancer cells In Vitro. Philos. Trans. A Math. Phys. Eng. Sci. 2013, 371, 20120118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagyal, L.; Kumar, A.; Sharma, R.; Yadav, R.; Chaudhary, P.; Singh, R. Bioinorganic Chemistry of Platinum (IV) Complexes as Platforms for Anticancer Agents. Curr. Bioact. Compd. 2020, 16, 726–737. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Tian, H. Current Developments in Pt(IV) Prodrugs Conjugated with Bioactive Ligands. Bioinorg. Chem. Appl. 2018, 2018, 8276139. [Google Scholar] [CrossRef] [Green Version]
- Shaili, E. Platinum anticancer drugs and photochemotherapeutic agents: Recent advances and future developments. Sci. Prog. 2014, 97, 20–40. [Google Scholar] [CrossRef]
- Zhao, Y.; Woods, J.A.; Farrer, N.J.; Robinson, K.S.; Pracharova, J.; Kasparkova, J.; Novakova, O.; Li, H.; Salassa, L.; Pizarro, A.M.; et al. Diazido mixed-amine platinum(IV) anticancer complexes activatable by visible-light form novel DNA adducts. Chemistry 2013, 19, 9578–9591. [Google Scholar] [CrossRef] [Green Version]
- Bednarski, P.J.; Grunert, R.; Zielzki, M.; Wellner, A.; Mackay, F.S.; Sadler, P.J. Light-activated destruction of cancer cell nuclei by platinum diazide complexes. Chem. Biol. 2006, 13, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Mu, M.; Gao, H. DFT Study on the Substituent Effect of Anticancer Picoline-Diazido-Pt(IV) Compounds. Front. Oncol. 2021, 11, 749178. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Wu, K.; Gao, F.; Cheng, Y.; Zou, T.; Wu, X.; Zhao, Y.; Wang, F. Photoactivatable diazido Pt(IV) anticancer complex can bind to and oxidize all four nucleosides. Dalton Trans. 2020, 49, 17157–17163. [Google Scholar] [CrossRef]
- Blanco, N.G.; Maldonado, C.R.; Mareque-Rivas, J.C. Effective photoreduction of a Pt(IV) complex with quantum dots: A feasible new light-induced method of releasing anticancer Pt(II) drugs. Chem. Commun. 2009, 45, 5257–5259. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Cheng, S.-C.; Xu, K.; Deng, Z.; Chen, S.; Xu, Z.; Xie, K.; Tse, M.-K.; Shi, P.; et al. Phorbiplatin, a Highly Potent Pt(IV) Antitumor Prodrug That Can Be Controllably Activated by Red Light. Chem 2019, 5, 3151–3165. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, N.; Liu, Y.; Xu, Z.; Wang, Z.; Lau, T.C.; Zhu, G. A Photocaged, Water-Oxidizing, and Nucleolus-Targeted Pt(IV) Complex with a Distinct Anticancer Mechanism. J. Am. Chem. Soc. 2020, 142, 7803–7812. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, X.; Zhang, C.; Huang, W.; Zhou, Y.; Yan, D. Oxygen and Pt(II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat. Commun. 2018, 9, 2053. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-D.; Xiang, H.-J.; An, L.; Yang, S.-P.; Liu, J.-G. Targeted delivery of photoactive diazido PtIV complexes conjugated with fluorescent carbon dots. New J. Chem. 2015, 39, 800–804. [Google Scholar] [CrossRef]
- Knutson, S.; Raja, E.; Bomgarden, R.; Nlend, M.; Chen, A.; Kalyanasundaram, R.; Desai, S. Development and Evaluation of a Fluorescent Antibody-Drug Conjugate for Molecular Imaging and Targeted Therapy of Pancreatic Cancer. PLoS ONE 2016, 11, e0157762. [Google Scholar] [CrossRef] [Green Version]
- Parslow, A.C.; Parakh, S.; Lee, F.T.; Gan, H.K.; Scott, A.M. Antibody-Drug Conjugates for Cancer Therapy. Biomedicines 2016, 4, 14. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Li, W.; Jeanty, C.; Xiang, G.; Dong, Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm. Sin. B 2020, 10, 1589–1600. [Google Scholar] [CrossRef]
- Gauzy-Lazo, L.; Sassoon, I.; Brun, M.-P. Advances in Antibody-Drug Conjugate design: Current clinical lanscape and future innovation. Sage 2020, 25, 843–865. [Google Scholar] [CrossRef]
- Ricart, A.D. Antibody-drug conjugates of calicheamicin derivative: Gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin. Cancer Res. 2011, 17, 6417–6427. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaia, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug. Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.S.; Tice, D.A.; Soria, J.C. Antibody-Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rao, J.; Wang, M.; Li, X.; Liu, K.; Naylor, M.F.; Nordquist, R.E.; Chen, W.R.; Zhou, F. Cancer photo-immunotherapy: From bench to bedside. Theranostics 2021, 11, 2218–2231. [Google Scholar] [CrossRef]
- Kato, T.; Wakiyama, H.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy. Cancers 2021, 13, 2535. [Google Scholar] [CrossRef] [PubMed]
- Paraboschi, I.; Turnock, S.; Kramer-Marek, G.; Musleh, L.; Barisa, M.; Anderson, J.; Giuliani, S. Near-InfraRed PhotoImmunoTherapy (NIR-PIT) for the local control of solid cancers: Challenges and potentials for human applications. Crit. Rev. Oncol. Hematol. 2021, 161, 103325. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, C.; Yang, X. Endoscopic Molecular Imaging plus Photoimmunotherapy: A New Strategy for Monitoring and Treatment of Bladder Cancer. Mol. Ther. Oncolytics 2020, 18, 409–418. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Chang, R.; Yan, X. Supramolecular cancer photoimmunotherapy based on precise peptide self-assembly design. Chem. Commun. 2022, 58, 2247–2258. [Google Scholar] [CrossRef]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [Green Version]
- Maruoka, Y.; Wakiyama, H.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy for cancers: A translational perspective. EBioMedicine 2021, 70, 103501. [Google Scholar] [CrossRef]
- Wakiyama, H.; Kato, T.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy of cancer; possible clinical applications. Nanophotonics 2021, 10, 3135–3151. [Google Scholar] [CrossRef]
- Jin, J.; Sivakumar, I.; Mironchik, Y.; Krishnamachary, B.; Wildes, F.; Barnett, J.D.; Hung, C.F.; Nimmagadda, S.; Kobayashi, H.; Bhujwalla, Z.M.; et al. PD-L1 near Infrared Photoimmunotherapy of Ovarian Cancer Model. Cancers 2022, 14, 619. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johan, A.N.; Li, Y. Development of Photoremovable Linkers as a Novel Strategy to Improve the Pharmacokinetics of Drug Conjugates and Their Potential Application in Antibody–Drug Conjugates for Cancer Therapy. Pharmaceuticals 2022, 15, 655. https://doi.org/10.3390/ph15060655
Johan AN, Li Y. Development of Photoremovable Linkers as a Novel Strategy to Improve the Pharmacokinetics of Drug Conjugates and Their Potential Application in Antibody–Drug Conjugates for Cancer Therapy. Pharmaceuticals. 2022; 15(6):655. https://doi.org/10.3390/ph15060655
Chicago/Turabian StyleJohan, Audrey Nathania, and Yi Li. 2022. "Development of Photoremovable Linkers as a Novel Strategy to Improve the Pharmacokinetics of Drug Conjugates and Their Potential Application in Antibody–Drug Conjugates for Cancer Therapy" Pharmaceuticals 15, no. 6: 655. https://doi.org/10.3390/ph15060655
APA StyleJohan, A. N., & Li, Y. (2022). Development of Photoremovable Linkers as a Novel Strategy to Improve the Pharmacokinetics of Drug Conjugates and Their Potential Application in Antibody–Drug Conjugates for Cancer Therapy. Pharmaceuticals, 15(6), 655. https://doi.org/10.3390/ph15060655





