A More Biomimetic Cell Migration Assay with High Reliability and Its Applications
Abstract
:1. Introduction
2. Results
2.1. Design Principle for the Microfluidic Chip
2.2. Characterization of Stamping Controlled by the Electromagnetic System
2.3. Simulation Study of Shear Stress in the Microfluidic Chip
2.4. Microfluidic Cell Migration Assay
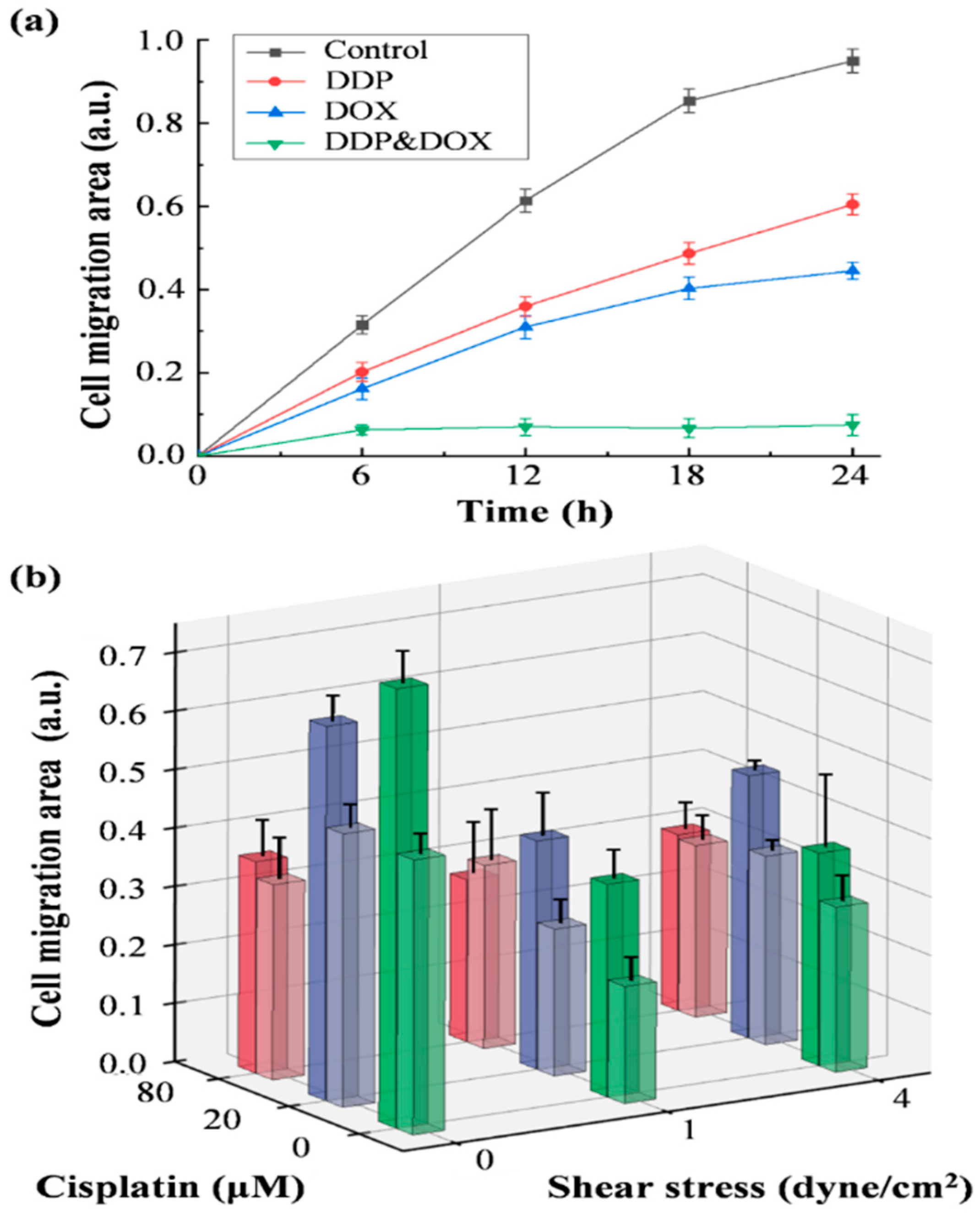
2.5. Shear Stress and Drug Effect on Cell Migration
3. Discussion
4. Materials and Methods
4.1. Design and Fabrication of Device
4.2. Cell Culturing and Fluorescent Image Acquisition
4.3. Experimental Setup of Cell Exclusion and Cell Depletion
4.4. Measurement of Stamping Pillars
4.5. Simulation of the Flow Conditions in the Culture Chamber
4.6. Quantification of Cell Migration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kratz, S.R.A.; Eilenberger, C.; Schuller, P.; Bachmann, B.; Spitz, S.; Ertl, P.; Rothbauer, M. Characterization of four functional biocompatible pressure-sensitive adhesives for rapid prototyping of cell-based lab-on-a-chip and organ-on-a-chip systems. Sci. Rep. 2019, 9, 9287. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G.; Co, C.C.; Ho, C.C. Directing cell migration in continuous microchannels by topographical amplification of natural directional persistence. Biomaterials 2013, 34, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirure, V.S.; Lam, S.F.; Shergill, B.; Chu, Y.E.; Ng, N.R.; George, S.C. Quantitative design strategies for fine control of oxygen in microfluidic systems. Lab Chip 2020, 20, 3036–3050. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, Q.; Qin, L.; Cai, J.; Du, B. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the Akt pathway. Biomed. Res. Int. 2014, 2014, 418624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, A.; Slama, Z.M.; Morford, R.K.; Madden, S.A.; Provenzano, P.P. Enhanced Directional Migration of Cancer Stem Cells in 3D Aligned Collagen Matrices. Biophys. J. 2017, 112, 1023–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Serra-Picamal, X.; Tambe, D.T.; Zhou, E.H.; Park, C.Y.; Sadati, M.; Park, J.A.; Krishnan, R.; Gweon, B.; Millet, E.; et al. Propulsion and navigation within the advancing monolayer sheet. Nat. Mater. 2013, 12, 856–863. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Si, H.; Xing, T.; Ding, Y.; Zhang, H.; Yin, R.; Zhang, W. 3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels. Polymers 2019, 11, 1584. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Zhang, H.; Yang, S.; Xi, Z.; Tang, T.; Yin, R.; Zhang, W. Electrospun PLGA membrane incorporated with andrographolide-loaded mesoporous silica nanoparticles for sustained antibacterial wound dressing. Nanomedicine 2018, 13, 2881–2899. [Google Scholar] [CrossRef]
- Barbu, A.; Neamtu, B.; Zăhan, M.; Iancu, G.M.; Bacila, C.; Mireșan, V. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Marsh, S.; O’Shaughnessy, R.; Camera, E.; Picardo, M.; Aumailley, M.; O’Toole, E.; Caley, M. 274 junctional epidermolysis bullosa: Bottom up control of the skin barrier? J. Investig. Dermatol. 2019, 139, S261. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, N.D.; Grimmer, J.A.; Tanwar, V.; Schwieterman, N.; Mohler, P.J.; Wold, L.E. Cardiovascular risk of electronic cigarettes: A review of preclinical and clinical studies. Cardiovasc. Res. 2020, 116, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Du, G.; Zhang, B.; Zhang, H.; Yin, R.; Zhang, W.; Yang, S.M. Efficient Drug Screening and Nephrotoxicity Assessment on Co-culture Microfluidic Kidney Chip. Sci. Rep. 2020, 10, 6568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergir, E.; Bachmann, B.; Redl, H.; Forte, G.; Ertl, P. Small Force, Big Impact: Next Generation Organ-on-a-Chip Systems Incorporating Biomechanical Cues. Front. Physiol. 2018, 9, 1417. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Liddle, R.A. Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J. Biol. Chem. 2021, 296, 100171. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Shyy, J.Y.P. Shear Stress Regulation of Endothelium: A Double-edged Sword. J. Transl. Int. Med. 2018, 6, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Molladavoodi, S.; Robichaud, M.; Wulff, D.; Gorbet, M. Corneal epithelial cells exposed to shear stress show altered cytoskeleton and migratory behaviour. PLoS ONE 2017, 12, e0178981. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, H.; Zhan, Y.; Fan, S. An emerging tumor invasion mechanism about the collective cell migration. Am. J. Transl. Res. 2019, 11, 5301–5312. [Google Scholar]
- Charras, G.; Sahai, E. Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 2014, 15, 813–824. [Google Scholar] [CrossRef]
- Yang, S.M.; Lin, Q.; Zhang, H.; Yin, R.; Zhang, W.; Zhang, M.; Cui, Y. Dielectrophoresis assisted high-throughput detection system for multiplexed immunoassays. Biosens. Bioelectron. 2021, 180, 113148. [Google Scholar] [CrossRef] [PubMed]
- Pijuan, J.; Barcelo, C.; Moreno, D.F.; Maiques, O.; Siso, P.; Marti, R.M.; Macia, A.; Panosa, A. In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.Y.; Lo, K.Y.; Sun, Y.S. A microfluidics-based wound-healing assay for studying the effects of shear stresses, wound widths, and chemicals on the wound-healing process. Sci. Rep. 2019, 9, 20016. [Google Scholar] [CrossRef]
- Sticker, D.; Lechner, S.; Jungreuthmayer, C.; Zanghellini, J.; Ertl, P. Microfluidic Migration and Wound Healing Assay Based on Mechanically Induced Injuries of Defined and Highly Reproducible Areas. Anal. Chem. 2017, 89, 2326–2333. [Google Scholar] [CrossRef]
- Shabestani Monfared, G.; Ertl, P.; Rothbauer, M. An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure. Sci. Rep. 2020, 10, 16192. [Google Scholar] [CrossRef]
- Vedula, S.R.; Ravasio, A.; Lim, C.T.; Ladoux, B. Collective cell migration: A mechanistic perspective. Physiology 2013, 28, 370–379. [Google Scholar] [CrossRef]
- Riahi, R.; Yang, Y.; Zhang, D.D.; Wong, P.K. Advances in wound-healing assays for probing collective cell migration. J. Lab. Autom. 2012, 17, 59–65. [Google Scholar] [CrossRef]
- Gojova, A.; Barakat, A.I. Vascular endothelial wound closure under shear stress: Role of membrane fluidity and flow-sensitive ion channels. J. Appl. Physiol. (1985) 2005, 98, 2355–2362. [Google Scholar] [CrossRef] [Green Version]
- van der Meer, A.D.; Vermeul, K.; Poot, A.A.; Feijen, J.; Vermes, I. A microfluidic wound-healing assay for quantifying endothelial cell migration. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H719–H725. [Google Scholar] [CrossRef] [Green Version]
- Shih, H.C.; Lee, T.A.; Wu, H.M.; Ko, P.L.; Liao, W.H.; Tung, Y.C. Microfluidic Collective Cell Migration Assay for Study of Endothelial Cell Proliferation and Migration under Combinations of Oxygen Gradients, Tensions, and Drug Treatments. Sci. Rep. 2019, 9, 8234. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.E.; Lee, K.L.; Wei, P.K.; Cheng, J.Y. Screening anti-metastasis drugs by cell adhesion-induced color change in a biochip. Lab Chip 2021, 21, 2955–2970. [Google Scholar] [CrossRef] [PubMed]
- Poujade, M.; Grasland-Mongrain, E.; Hertzog, A.; Jouanneau, J.; Chavrier, P.; Ladoux, B.; Buguin, A.; Silberzan, P. Collective migration of an epithelial monolayer in response to a model wound. Proc. Natl. Acad. Sci. USA 2007, 104, 15988–15993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montiel, M.; Urso, L.; de la Blanca, E.P.; Marsigliante, S.; Jimenez, E. Cisplatin reduces endothelial cell migration via regulation of type 2-matrix metalloproteinase activity. Cell. Physiol. Biochem. 2009, 23, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The effect of pH on the extracellular matrix and biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Chang, W.W.; Chang, C.P.; Liu, T.Y.; Chuang, C.Y.; Qian, K.; Zheng, Y.G.; Li, C. Downregulation of PRMT1 promotes the senescence and migration of a non-MYCN amplified neuroblastoma SK-N-SH cells. Sci. Rep. 2019, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- White, F.M. Viscous Fluid Flow; McGraw-Hill: New York, NY, USA, 2006; Volume 3. [Google Scholar]
- Bagri, A.; Kouros-Mehr, H.; Leong, K.G.; Plowman, G.D. Use of anti-VEGF adjuvant therapy in cancer: Challenges and rationale. Trends Mol. Med. 2010, 16, 122–132. [Google Scholar] [CrossRef]
- Shirmanova, M.V.; Druzhkova, I.N.; Lukina, M.M.; Dudenkova, V.V.; Ignatova, N.I.; Snopova, L.B.; Shcheslavskiy, V.I.; Belousov, V.V.; Zagaynova, E.V. Chemotherapy with cisplatin: Insights into intracellular pH and metabolic landscape of cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8911. [Google Scholar] [CrossRef] [Green Version]
- Laurencot, C.M.; Kennedy, K.A. Influence of pH on the cytotoxicity of cisplatin in EMT6 mouse mammary tumor cells. Oncol. Res. 1995, 7, 371–379. [Google Scholar]
- Yuan, C.; Tony, A.; Yin, R.; Wang, K.; Zhang, W. Tactile and thermal sensors built from carbon–polymer nanocomposites—A critical review. Sensors 2021, 21, 1234. [Google Scholar] [CrossRef]
- Barbu, A.; Neamțu, M.B.; Zăhan, M.; Mireșan, V. Trends in alginate-based films and membranes for wound healing. Rom. Biotechnol. Lett. 2020, 25, 1683–1689. [Google Scholar]
- Papaioannou, T.G.; Stefanadis, C. Vascular wall shear stress: Basic principles and methods. Hell. J. Cardiol. 2005, 46, 9–15. [Google Scholar]
- Shikatani, E.A.; Trifonova, A.; Mandel, E.R.; Liu, S.T.; Roudier, E.; Krylova, A.; Szigiato, A.; Beaudry, J.; Riddell, M.C.; Haas, T.L. Inhibition of proliferation, migration and proteolysis contribute to corticosterone-mediated inhibition of angiogenesis. PLoS ONE 2012, 7, e46625. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, D.; Zhang, H.; Yang, C.; Zhang, W.; Yang, S. A More Biomimetic Cell Migration Assay with High Reliability and Its Applications. Pharmaceuticals 2022, 15, 695. https://doi.org/10.3390/ph15060695
Yin D, Zhang H, Yang C, Zhang W, Yang S. A More Biomimetic Cell Migration Assay with High Reliability and Its Applications. Pharmaceuticals. 2022; 15(6):695. https://doi.org/10.3390/ph15060695
Chicago/Turabian StyleYin, Di, Hongbo Zhang, Chun Yang, Wenjun Zhang, and Shihmo Yang. 2022. "A More Biomimetic Cell Migration Assay with High Reliability and Its Applications" Pharmaceuticals 15, no. 6: 695. https://doi.org/10.3390/ph15060695
APA StyleYin, D., Zhang, H., Yang, C., Zhang, W., & Yang, S. (2022). A More Biomimetic Cell Migration Assay with High Reliability and Its Applications. Pharmaceuticals, 15(6), 695. https://doi.org/10.3390/ph15060695








