Discrepancy between the Actions of Glucagon-like Peptide-1 Receptor Ligands in the Protection of the Heart against Ischemia Reperfusion Injury
Abstract
:1. Introduction
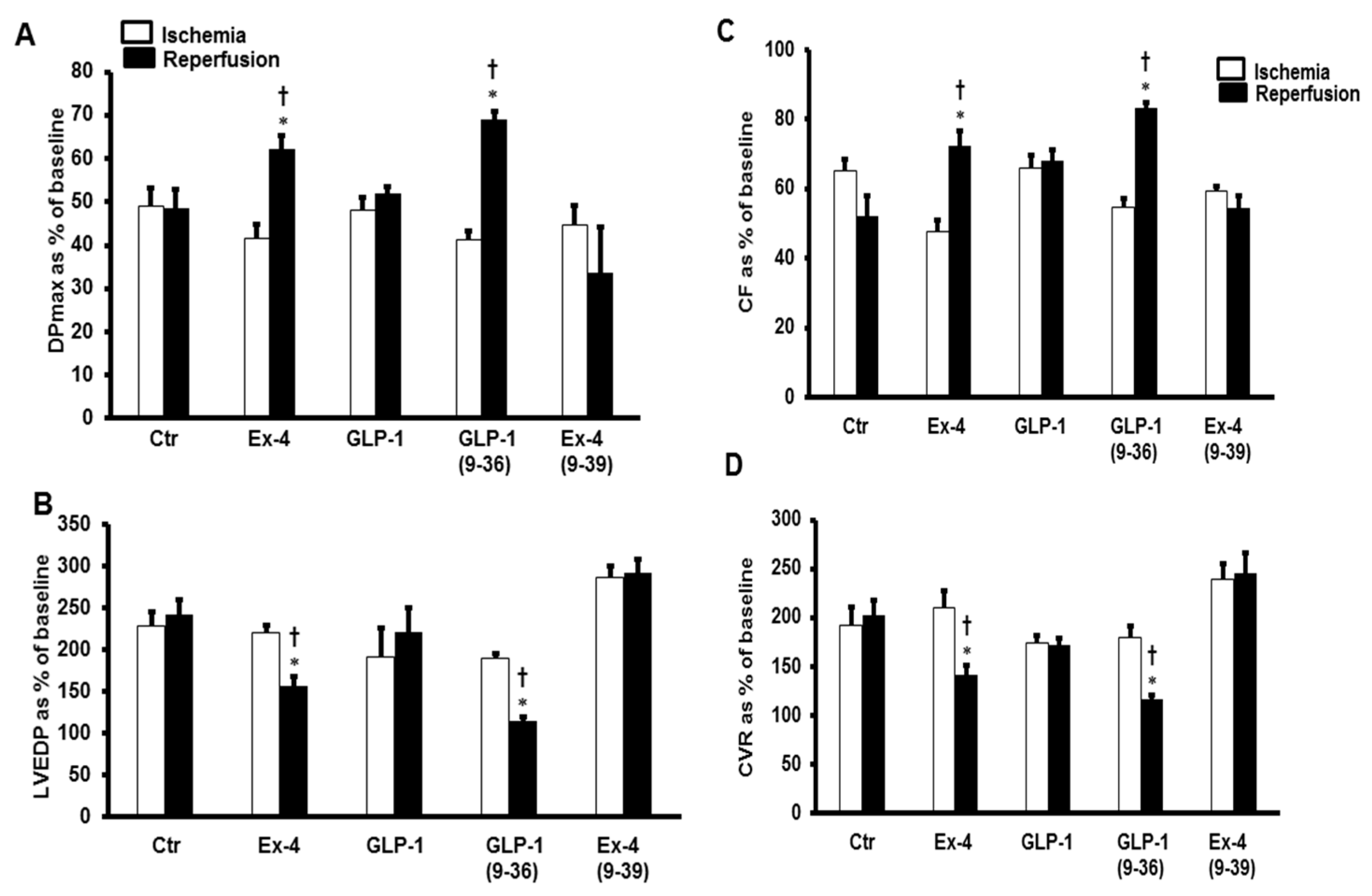
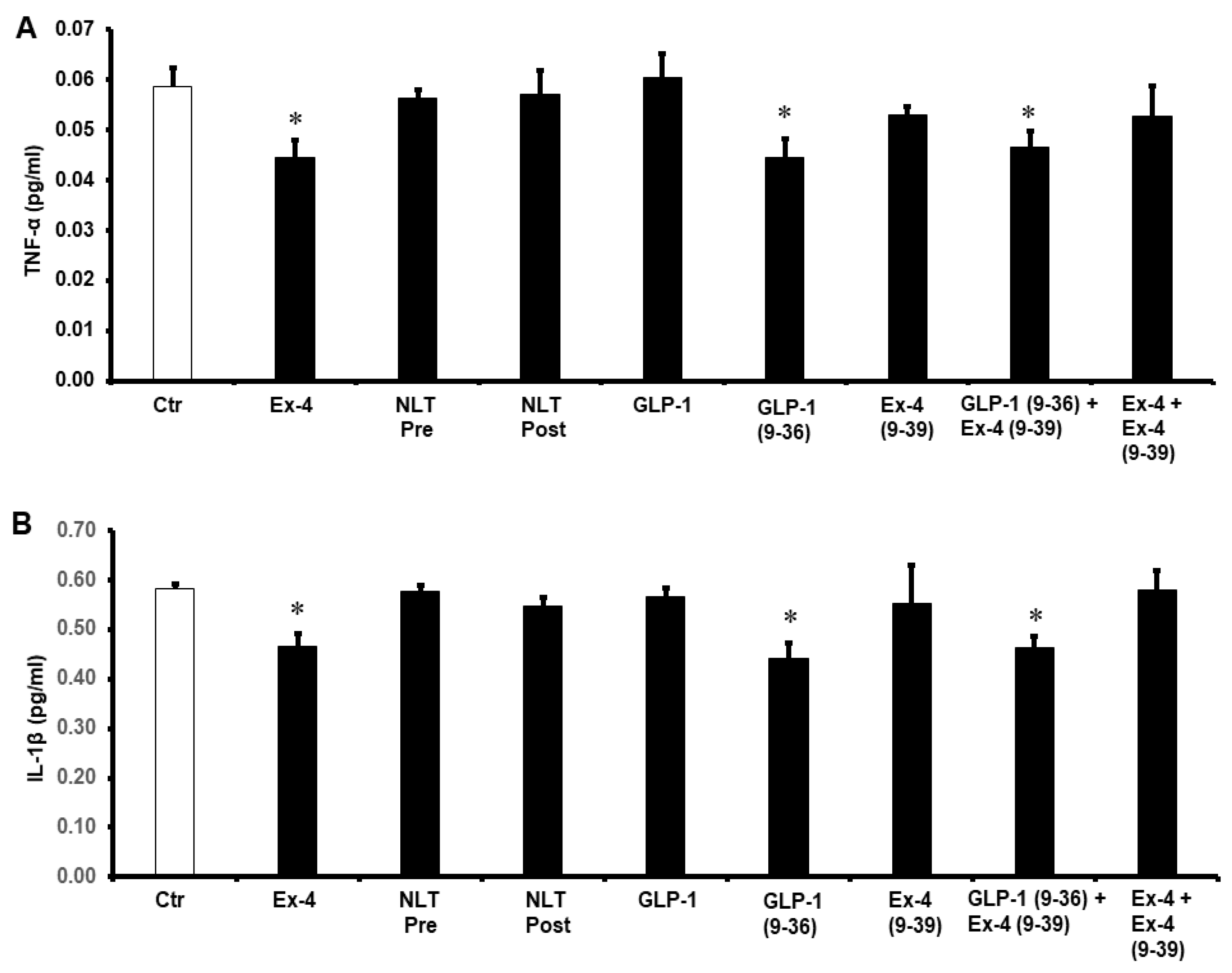
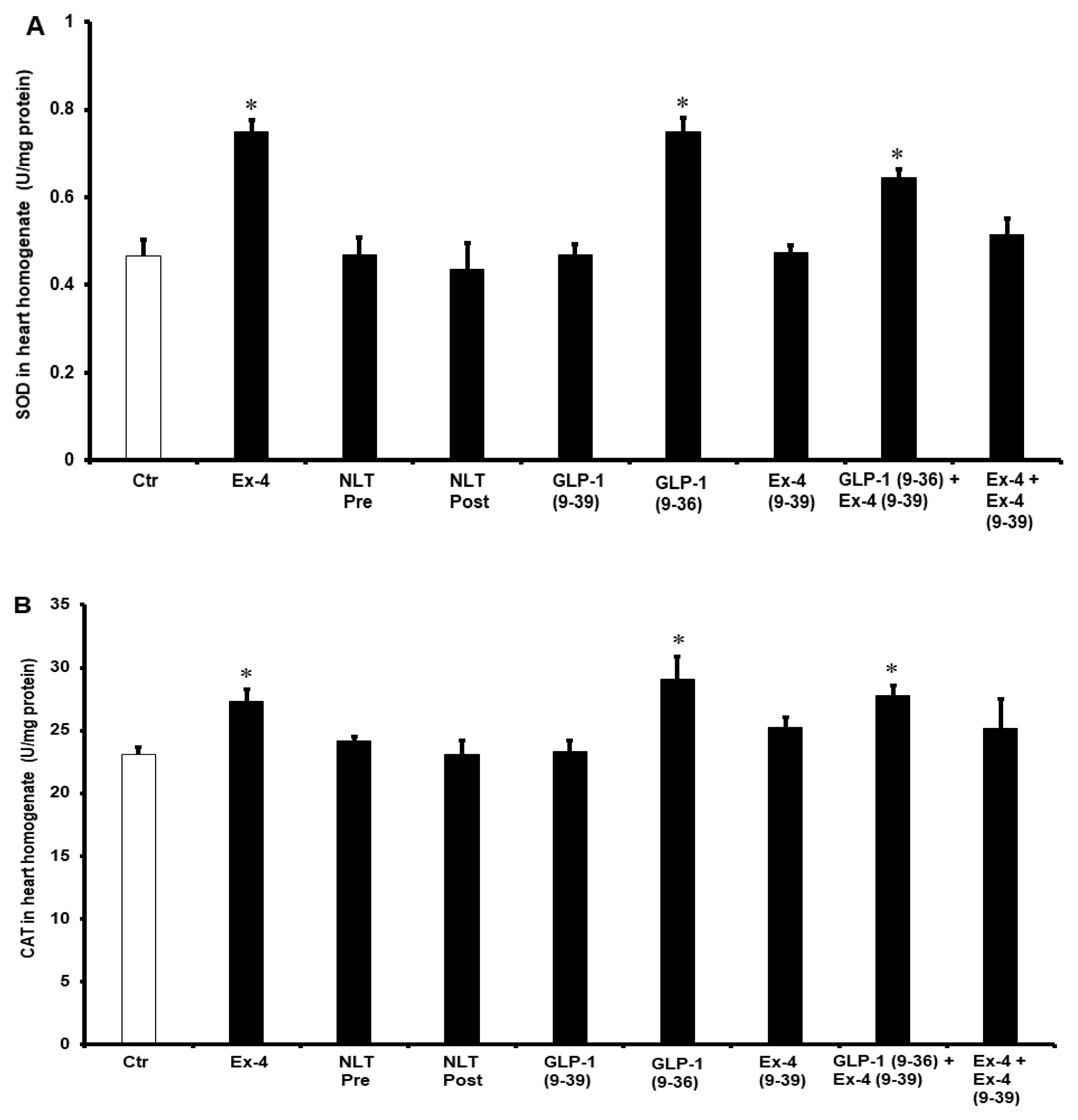
2. Results
3. Discussion
4. Materials and Methods
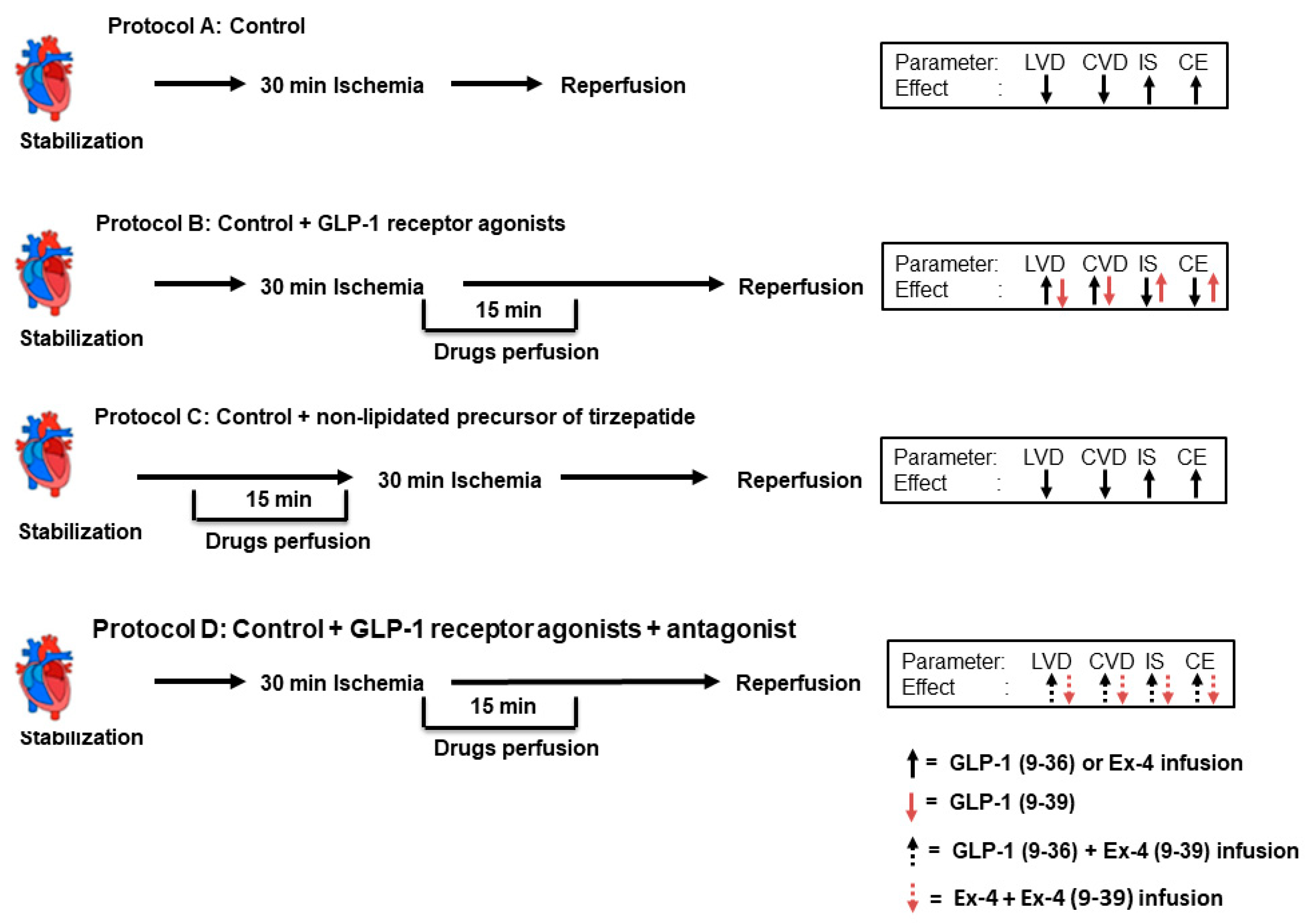
4.1. Ischemia Reperfusion Study
4.2. Study Protocol and Study Groups
4.3. Data Collection and Processing
4.4. Sample Collection and Storage
4.5. Infarct-Size Evaluation
4.6. Protein Extraction from the Hearts
4.7. Antioxidant Enzyme Superoxide Dismutase Status
4.8. Estimation of Inflammatory Cytokines
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- GBD 2016 Mortality Collaborators. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1084–1150. [Google Scholar] [CrossRef] [Green Version]
- Cung, T.T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guerin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. The mitochondrial permeability transition pore: Its fundamental role in mediating cell death during ischaemia and reperfusion. J. Mol. Cell Cardiol. 2003, 35, 339–341. [Google Scholar] [CrossRef] [Green Version]
- Limalanathan, S.; Andersen, G.O.; Klow, N.E.; Abdelnoor, M.; Hoffmann, P.; Eritsland, J. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI results of the POSTEMI (POstconditioning in ST-Elevation Myocardial Infarction) randomized trial. J. Am. Heart Assoc. 2014, 3, e000679. [Google Scholar] [CrossRef] [Green Version]
- Tsang, A.; Hausenloy, D.J.; Yellon, D.M. Myocardial postconditioning: Reperfusion injury revisited. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2–H7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Meng, J.; Li, X.; Zhou, S.; Qu, D.; Wang, N.; Jia, M.; Ma, X.; Luo, X. Pro-GLP-1, a Pro-drug of GLP-1, is neuroprotective in cerebral ischemia. Eur. J. Pharm. Sci. 2015, 70, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Herz, W.; Babiker, F. Acute Intravenous Infusion of Immunoglobulins Protects Against Myocardial Ischemia-Reperfusion Injury Through Inhibition of Caspase-3. Cell. Physiol. Biochem. 2017, 42, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Freixa, X.; Bellera, N.; Ortiz-Perez, J.T.; Jimenez, M.; Pare, C.; Bosch, X.; De Caralt, T.M.; Betriu, A.; Masotti, M. Ischaemic postconditioning revisited: Lack of effects on infarct size following primary percutaneous coronary intervention. Eur. Heart J. 2012, 33, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abwainy, A.; Babiker, F.; Akhtar, S.; Benter, I.F. Endogenous angiotensin-(1-7)/Mas receptor/NO pathway mediates the cardioprotective effects of pacing postconditioning. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H104–H112. [Google Scholar] [CrossRef] [Green Version]
- Babiker, F.A. Pacing Postconditioning: Recent Insights of Mechanism of Action and Probable Future Clinical Application. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2016, 25 (Suppl. S1), 22–28. [Google Scholar] [CrossRef] [PubMed]
- Babiker, F.A.; van Golde, J.; Vanagt, W.Y.; Prinzen, F.W. Pacing postconditioning: Impact of pacing algorithm, gender, and diabetes on its myocardial protective effects. J. Cardiovasc. Transl. Res. 2012, 5, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Al-Sabah, S. Molecular Pharmacology of the Incretin Receptors. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2016, 25 (Suppl. S1), 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 In Vitro and In Vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef]
- Thorens, B.; Porret, A.; Buhler, L.; Deng, S.P.; Morel, P.; Widmann, C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes 1993, 42, 1678–1682. [Google Scholar] [CrossRef] [Green Version]
- Ban, K.; Kim, K.H.; Cho, C.K.; Sauve, M.; Diamandis, E.P.; Backx, P.H.; Drucker, D.J.; Husain, M. Glucagon-like peptide (GLP)-1(9-36) amide-mediated cytoprotection is blocked by exendin (9-39) yet does not require the known GLP-1 receptor. Endocrinology 2010, 151, 1520–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frias, J.P.; Davies, M.J.; Rosenstock, J.; Perez Manghi, F.C.; Lando, F.L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; Investigators, S. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Sheahan, K.H.; Wahlberg, E.A.; Gilbert, M.P. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad. Med. J. 2020, 96, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Sivertsen, J.; Rosenmeier, J.; Holst, J.J.; Vilsboll, T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat. Rev. Cardiol. 2012, 9, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Drucker, D.J. Cardiovascular biology of the incretin system. Endocr. Rev. 2012, 33, 187–215. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Yusta, B.; Mulvihill, E.E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 Receptor Expression within the Human Heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef] [Green Version]
- Ban, K.; Noyan-Ashraf, M.H.; Hoefer, J.; Bolz, S.S.; Drucker, D.J.; Husain, M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008, 117, 2340–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, E.; Stanojevic, V.; Habener, J.F. GLP-1-derived nonapeptide GLP-1(28-36) amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul. Pept. 2011, 167, 177–184. [Google Scholar] [CrossRef]
- Matsubara, M.; Kanemoto, S.; Leshnower, B.G.; Albone, E.F.; Hinmon, R.; Plappert, T.; Gorman, J.H., 3rd; Gorman, R.C. Single dose GLP-1-Tf ameliorates myocardial ischemia/reperfusion injury. J. Surg. Res. 2011, 165, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Wang, S.X.; Sha, W.W.; Zhou, X.; Wang, W.L.; Han, L.P.; Li, D.Q.; Yu, D.M. Effects and mechanism of glucagon-like peptide-1 on injury of rats cardiomyocytes induced by hypoxia-reoxygenation. Chin. Med. J. 2008, 121, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H. Cardiovascular effects of glucagonlike peptide-1 agonists. Am. J. Cardiol. 2011, 108, 33B–41B. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; Henriques, J.P.; de Kleijn, D.P.; Devries, J.H.; Kemperman, H.; Steendijk, P.; Verlaan, C.W.; Kerver, M.; Piek, J.J.; Doevendans, P.A.; et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J. Am. Coll. Cardiol. 2009, 53, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, L.B.; Pridal, L. Glucagon-like peptide-1-(9-36) amide is a major metabolite of glucagon-like peptide-1-(7-36) amide after in vivo administration to dogs, and it acts as an antagonist on the pancreatic receptor. Eur. J. Pharmacol. 1996, 318, 429–435. [Google Scholar] [CrossRef]
- Li, N.; Lu, J.; Willars, G.B. Allosteric modulation of the activity of the glucagon-like peptide-1 (GLP-1) metabolite GLP-1 9-36 amide at the GLP-1 receptor. PLoS ONE 2012, 7, e47936. [Google Scholar] [CrossRef] [Green Version]
- Montrose-Rafizadeh, C.; Yang, H.; Rodgers, B.D.; Beday, A.; Pritchette, L.A.; Eng, J. High potency antagonists of the pancreatic glucagon-like peptide-1 receptor. J. Biol. Chem. 1997, 272, 21201–21206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauve, M.; Ban, K.; Momen, M.A.; Zhou, Y.Q.; Henkelman, R.M.; Husain, M.; Drucker, D.J. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 2010, 59, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Wang, W.; Ding, H.; Yang, Q.; Dong, Q.; Cui, M. The glucagon-like peptide-1 receptor agonist exendin-4 ameliorates warfarin-associated hemorrhagic transformation after cerebral ischemia. J. Neuroinflamm. 2016, 13, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeNicola, M.; Du, J.; Wang, Z.; Yano, N.; Zhang, L.; Wang, Y.; Qin, G.; Zhuang, S.; Zhao, T.C. Stimulation of glucagon-like peptide-1 receptor through exendin-4 preserves myocardial performance and prevents cardiac remodeling in infarcted myocardium. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E630–E643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.A.; Kolachala, V.L.; Jiang, R.; Abramowsky, C.; Romero, R.; Fifadara, N.; Anania, F.; Knechtle, S.; Kirk, A. The glucagon-like peptide-1 receptor agonist Exendin 4 has a protective role in ischemic injury of lean and steatotic liver by inhibiting cell death and stimulating lipolysis. Am. J. Pathol. 2012, 181, 1693–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, A.K.; Mocanu, M.M.; Carr, R.D.; Yellon, D.M. Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc. Drugs Ther. 2007, 21, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.; Gopal, K.; Greenwell, A.A.; Young, A.; Gill, R.; Aburasayn, H.; Al Batran, R.; Chahade, J.J.; Gandhi, M.; Eaton, F.; et al. The GLP-1 Receptor Agonist Liraglutide Increases Myocardial Glucose Oxidation Rates via Indirect Mechanisms and Mitigates Experimental Diabetic Cardiomyopathy. Can. J. Cardiol. 2021, 37, 140–150. [Google Scholar] [CrossRef]
- Aravindhan, K.; Bao, W.; Harpel, M.R.; Willette, R.N.; Lepore, J.J.; Jucker, B.M. Cardioprotection Resulting from Glucagon-Like Peptide-1 Administration Involves Shifting Metabolic Substrate Utilization to Increase Energy Efficiency in the Rat Heart. PLoS ONE 2015, 10, e0130894. [Google Scholar] [CrossRef] [Green Version]
- Ussher, J.R.; Baggio, L.L.; Campbell, J.E.; Mulvihill, E.E.; Kim, M.; Kabir, M.G.; Cao, X.; Baranek, B.M.; Stoffers, D.A.; Seeley, R.J.; et al. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol. Metab. 2014, 3, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, P.; Linz, W.; Hubschle, T.; Linz, D.; Huber, J.; Hess, S.; Crowther, D.; Werner, U.; Ruetten, H. Cardioprotective effects of lixisenatide in rat myocardial ischemia-reperfusion injury studies. J. Transl. Med. 2013, 11, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babiker, F.A.; Joseph, S.; Juggi, J.S. What’s new in salvage of the ischemic myocardium: Estrogen postconditioning. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2011, 20, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Bunck, M.C.; Diamant, M.; Eliasson, B.; Corner, A.; Shaginian, R.M.; Heine, R.J.; Taskinen, M.R.; Yki-Jarvinen, H.; Smith, U. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010, 33, 1734–1737. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, A.E.; Gaoatswe, G.; Lynch, L.; Corrigan, M.A.; Woods, C.; O’Connell, J.; O’Shea, D. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia 2014, 57, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Gouda, E.; Babiker, F. Micronized flavonoid fraction Daflon 500 protects heart against ischemia-reperfusion injury: An old medicine for a new target. All Life 2020, 13, 556–568. [Google Scholar] [CrossRef]
- Ridker, P.M.; Howard, C.P.; Walter, V.; Everett, B.; Libby, P.; Hensen, J.; Thuren, T.; Group, C.P.I. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A phase IIb randomized, placebo-controlled trial. Circulation 2012, 126, 2739–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Murtuza, B.; Smolenski, R.T.; Sammut, I.A.; Suzuki, N.; Kaneda, Y.; Yacoub, M.H. Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation 2001, 104, I-308–I-313. [Google Scholar] [CrossRef] [PubMed]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujadas, G.; Drucker, D.J. Vascular Biology of Glucagon Receptor Superfamily Peptides: Mechanistic and Clinical Relevance. Endocr. Rev. 2016, 37, 554–583. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.Y.; You, Y.H.; Ham, D.S.; Lee, S.H.; Yang, H.K.; Jeong, I.K.; Ko, S.H.; Yoon, K.H. Suppression of ROS Production by Exendin-4 in PSC Attenuates the High Glucose-Induced Islet Fibrosis. PLoS ONE 2016, 11, e0163187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Du, X.; Carratu, A.; Gerfen, G.J.; D’Apolito, M.; Giardino, I.; Rasola, A.; Marin, O.; Divakaruni, A.S.; Murphy, A.N.; et al. GLP-1 Cleavage Product Reverses Persistent ROS Generation after Transient Hyperglycemia by Disrupting an ROS-Generating Feedback Loop. Diabetes 2015, 64, 3273–3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuamnaichati, N.; Mangmool, S.; Chattipakorn, N.; Parichatikanond, W. Stimulation of GLP-1 Receptor Inhibits Methylglyoxal-Induced Mitochondrial Dysfunctions in H9c2 Cardiomyoblasts: Potential Role of Epac/PI3K/Akt Pathway. Front. Pharmacol. 2020, 11, 805. [Google Scholar] [CrossRef]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.M.; Tsutsumi, R.; Hamaguchi, E.; Sakai, Y.; Kasai, A.; Ishikawa, Y.; Yokoyama, U.; Tanaka, K. Exendin-4 ameliorates cardiac ischemia/reperfusion injury via caveolae and caveolins-3. Cardiovasc. Diabetol. 2014, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Babiker, F.; Al-Kouh, A.; Kilarkaje, N. Lead exposure induces oxidative stress, apoptosis, and attenuates protection of cardiac myocytes against ischemia-reperfusion injury. Drug Chem. Toxicol. 2019, 42, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Babiker, F.A.; Al-Jarallah, A.; Joseph, S. Understanding pacing postconditioning-mediated cardiac protection: A role of oxidative stress and a synergistic effect of adenosine. J. Physiol. Biochem. 2017, 73, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.; Babiker, F. Discrepancy in calcium release from the sarcoplasmic reticulum and intracellular acidic stores for the protection of the heart against ischemia/reperfusion injury. J. Physiol. Biochem. 2016, 72, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Babiker, F.A.; Joseph, S.; Juggi, J. The protective effects of 17beta-estradiol against ischemia-reperfusion injury and its effect on pacing postconditioning protection to the heart. J. Physiol. Biochem. 2014, 70, 151–162. [Google Scholar] [CrossRef]
- Ferrera, R.; Benhabbouche, S.; Bopassa, J.C.; Li, B.; Ovize, M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc. Drugs Ther. 2009, 23, 327–331. [Google Scholar] [CrossRef] [PubMed]






| Treatment | +dP/dt | −dP/dt | ||
|---|---|---|---|---|
| Ischemia | Reperfusion | Ischemia | Reperfusion | |
| Ctr | 57.30 ± 4.81 | 55.62 ± 5.05 | 55.41 ± 3.68 | 48.27 ± 4.03 |
| Ctr + EX-4 | 46.65 ± 1.38 | 73.53 ± 3.20 * † | 42.73 ± 1.83 | 71.36 ± 3.61 * † |
| Ctr + NLT Rep | 52.90 ± 4.82 | 48.55 ± 2.22 | 51.16 ± 2.66 | 44.17 ± 1.27 |
| Ctr + NLT pre | 54.18 ± 3.75 | 44.90 ± 5.41 | 56.94 ± 4.11 | 54.65 ± 4.71 |
| Ctr + GLP-1 | 46.63 ± 2.44 | 51.13 ± 2.96 | 44.90 ± 1.66 | 51.68 ± 3.71 |
| Ctr + GLP-1 (9-36) | 48.33 ± 4.12 | 71.28 ± 1.98 * † | 48.10 ± 2.79 | 66.11 ± 1.91 * † |
| Ctr + Ex-4 (9-39) | 65.56 ± 5.36 | 42.22 ± 7.56 | 59.58 ± 2.04 | 51.10 ± 2.83 |
| Ctr + Ex-4 + Ex-4 (9-39) | 63.44 ± 5.10 | 68.32 ± 4.61 | 51.22 ± 6.05 | 55.13 ± 8.33 |
| Ctr + GLP-1 (9-36) + Ex-4 (9-39) | 60.86 ± 3.60 | 76.04 ± 1.88 * † | 59.02 ± 3.52 | 73.63 ± 2.32 * † |
| Treatment | CK (IU/L) | p Value | LDH (IU/L) | p Value |
|---|---|---|---|---|
| Ctr | 15.78 ± 0.46 | - | 10.95 ± 1.02 | - |
| Ctr + EX-4 | 10.73 ± 0.56 * † | 0.001 | 7.45 ± 0.47 * † | 0.01 |
| Ctr + NLT Rep | 10.11 ± 0.46 | 0.369 | 10.40 ± 0.17 | 0.558 |
| Ctr + NLT pre | 14.95 ± 0.84 | 0.448 | 15.02 ± 0.76 | 0.471 |
| Ctr + GLP-1 | 13.78 ± 1.40 | 0.215 | 9.43 ± 0.52 | 0.131 |
| Ctr + GLP-1 (9-36) | 10.58 ± 0.85 * † | 0.001 | 6.25 ± 0.82 * † | 0.01 |
| Ctr + Ex-4 (9-39) | 15.33 ± 0.95 | 0.635 | 10.88 ± 0.37 | 0.937 |
| Ctr + Ex-4 + Ex-4 (9-39) | 14.70 ± 1.59 | 0.427 | 10.33 ± 0.44 | 0.487 |
| Ctr + GLP-1 (9-36) + Ex-4 (9-39) | 10.48 ± 0.78 * † | 0.01 | 6.28 ± 0.49 * † | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismaeil, A.; Babiker, F.; Al-Sabah, S. Discrepancy between the Actions of Glucagon-like Peptide-1 Receptor Ligands in the Protection of the Heart against Ischemia Reperfusion Injury. Pharmaceuticals 2022, 15, 720. https://doi.org/10.3390/ph15060720
Ismaeil A, Babiker F, Al-Sabah S. Discrepancy between the Actions of Glucagon-like Peptide-1 Receptor Ligands in the Protection of the Heart against Ischemia Reperfusion Injury. Pharmaceuticals. 2022; 15(6):720. https://doi.org/10.3390/ph15060720
Chicago/Turabian StyleIsmaeil, Ali, Fawzi Babiker, and Suleiman Al-Sabah. 2022. "Discrepancy between the Actions of Glucagon-like Peptide-1 Receptor Ligands in the Protection of the Heart against Ischemia Reperfusion Injury" Pharmaceuticals 15, no. 6: 720. https://doi.org/10.3390/ph15060720
APA StyleIsmaeil, A., Babiker, F., & Al-Sabah, S. (2022). Discrepancy between the Actions of Glucagon-like Peptide-1 Receptor Ligands in the Protection of the Heart against Ischemia Reperfusion Injury. Pharmaceuticals, 15(6), 720. https://doi.org/10.3390/ph15060720






