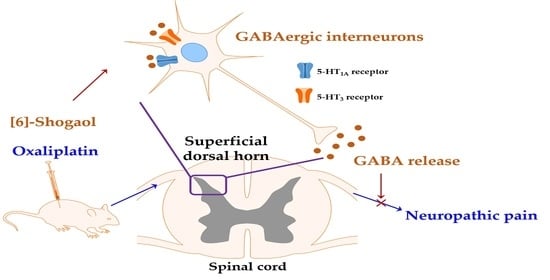
[6]-Shogaol Attenuates Oxaliplatin-Induced Allodynia through Serotonergic Receptors and GABA in the Spinal Cord in Mice
Abstract
:1. Introduction
2. Results
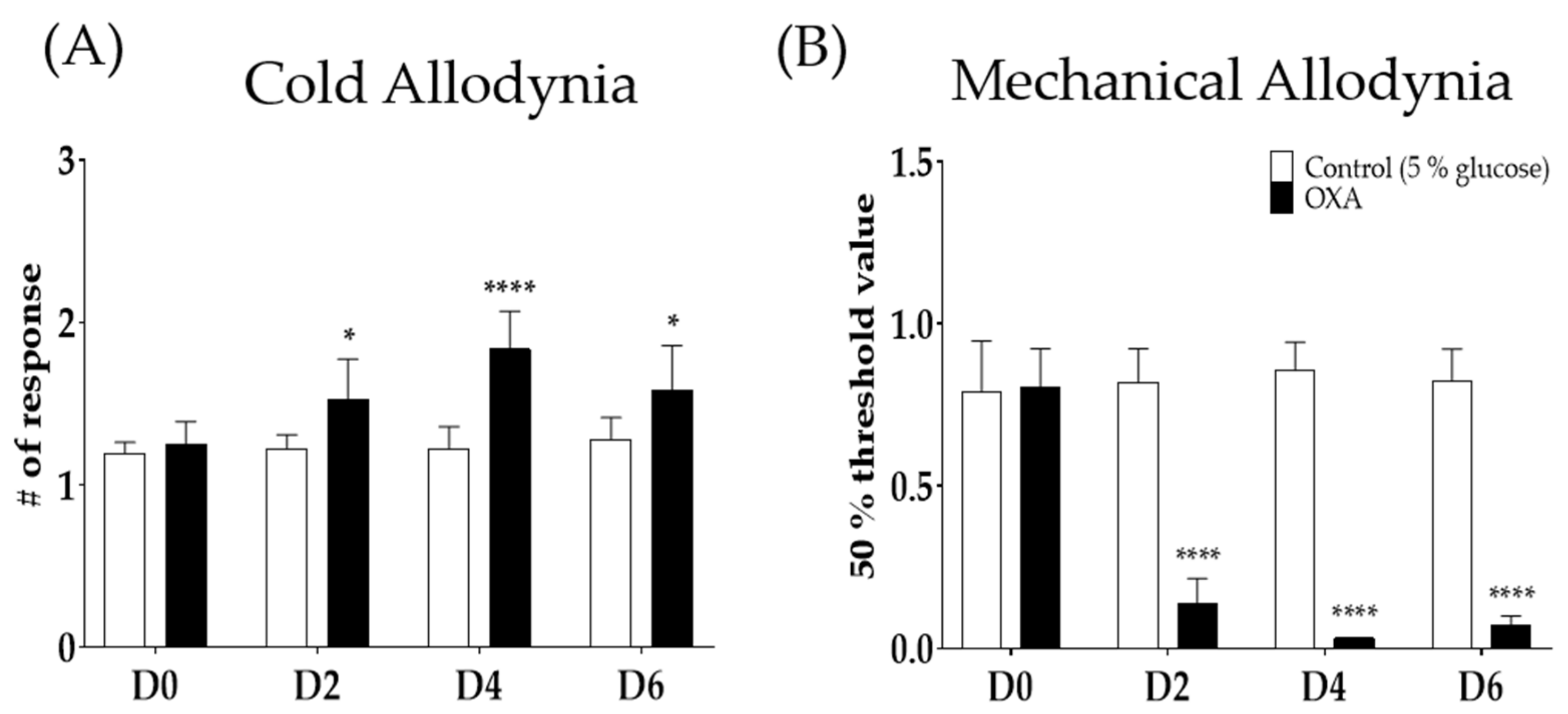
2.1. Single Oxaliplatin Injection Induced Cold and Mechanical Allodynia in Mice
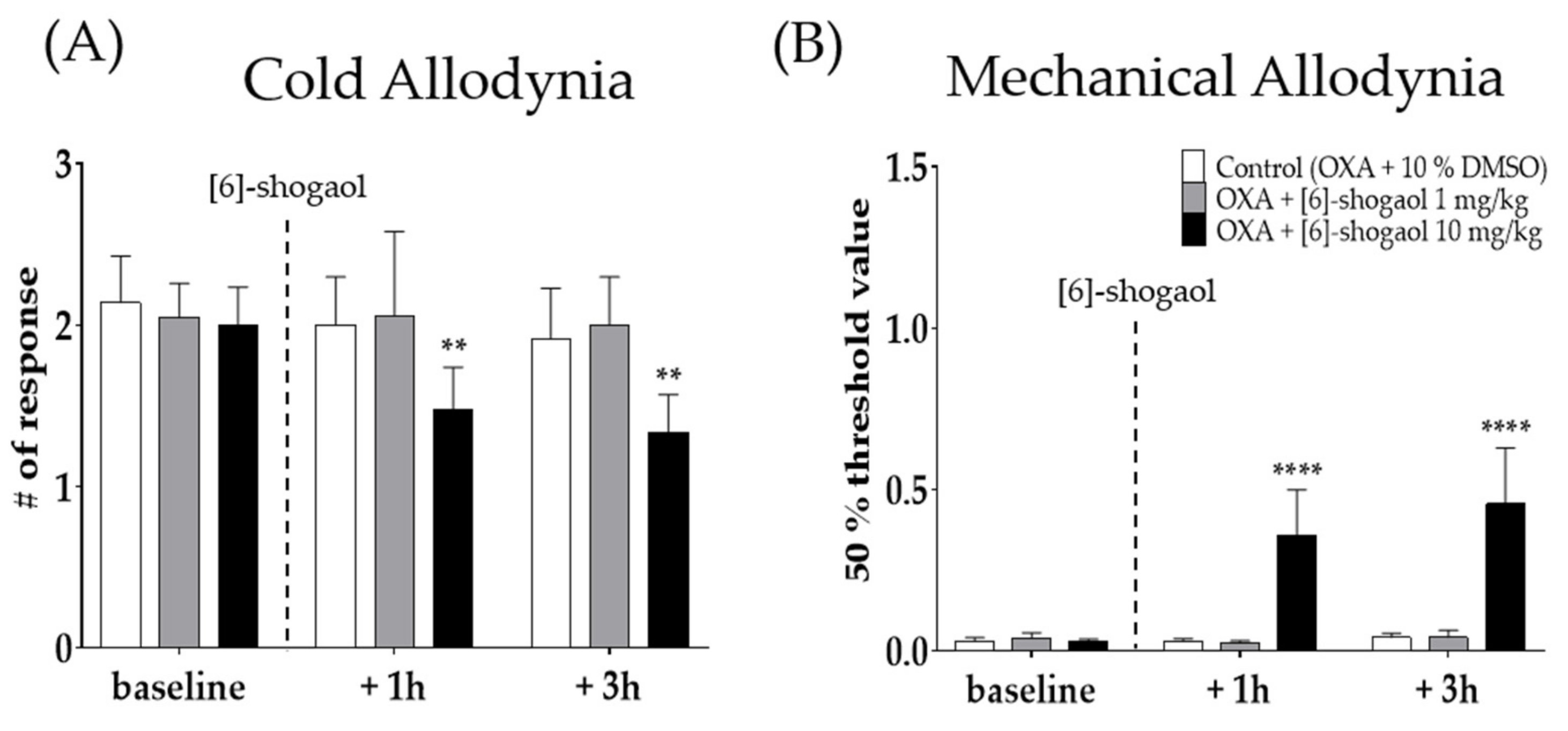
2.2. Intraperitoneal Injection of [6]-Shogaol Decreased Cold and Mechanical Alloydnia in Mice
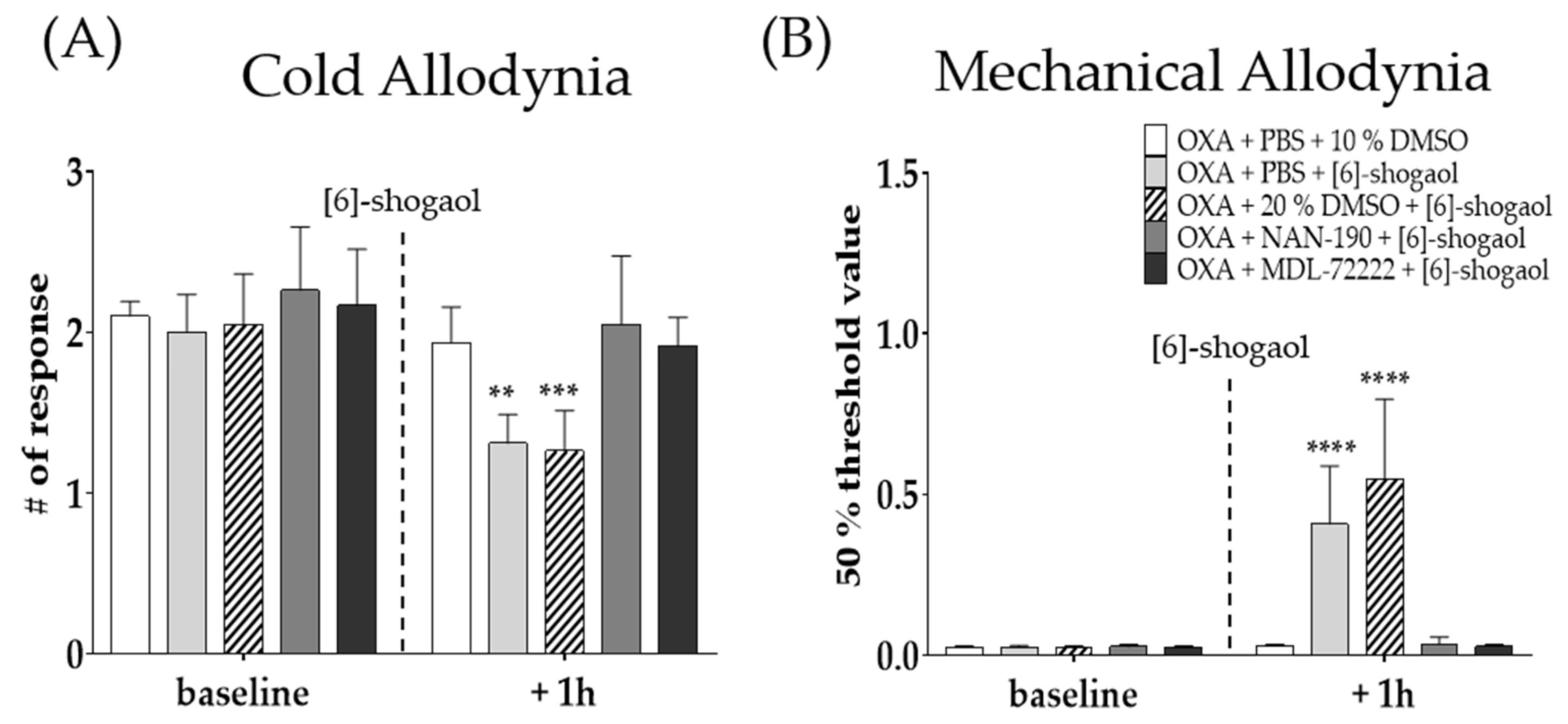
2.3. Intrathecal Serotonergic Receptor Antagonists Completely Block the Effect of [6]-Shogaol
2.4. GABAB Receptor Antagonist Prevented the Analgesic Effect of [6]-Shogaol
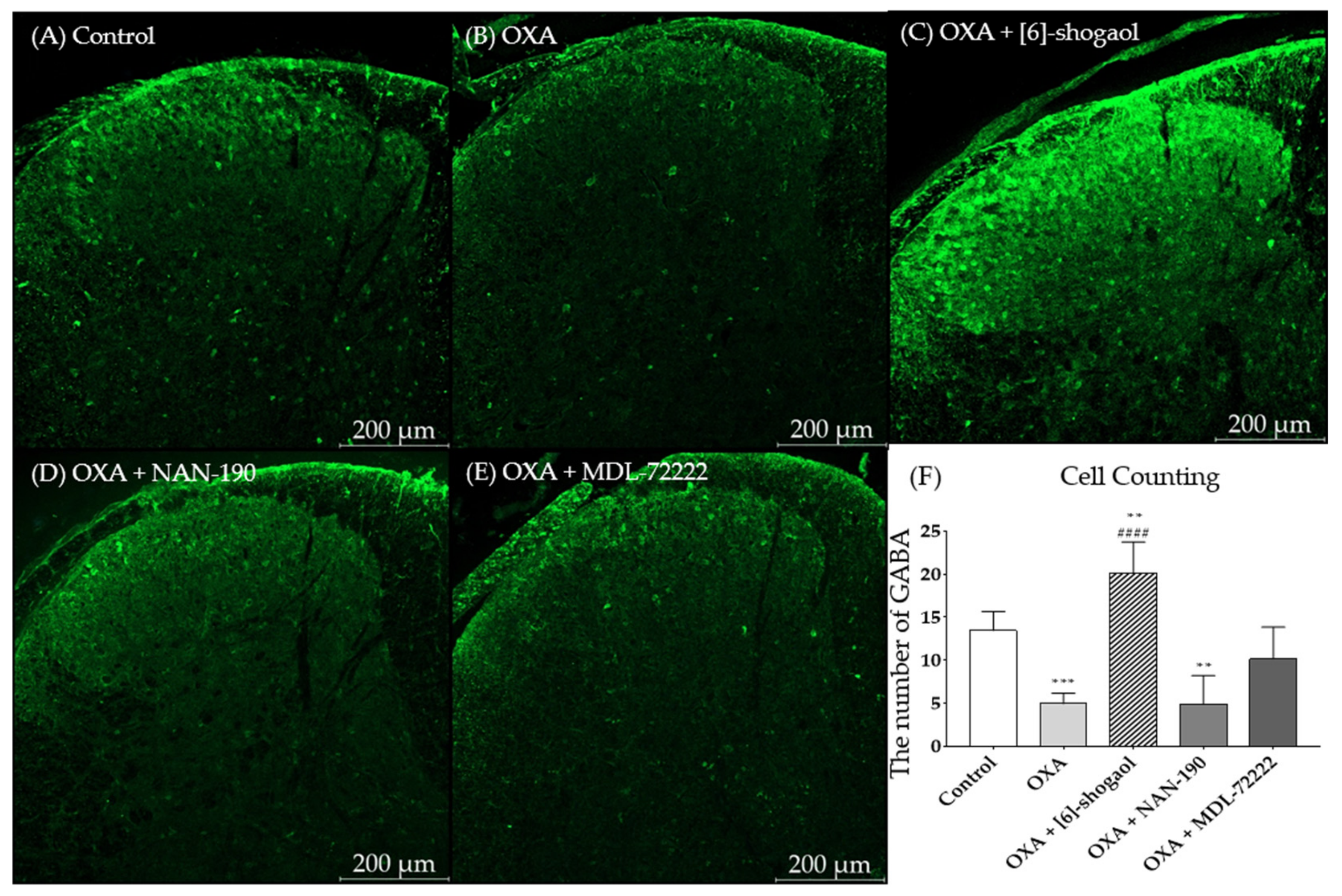
2.5. [6]-Shogaol Increased GABA Concentration in the Superficial Laminae of the Dorsal Horn of the Mice Spinal Cord
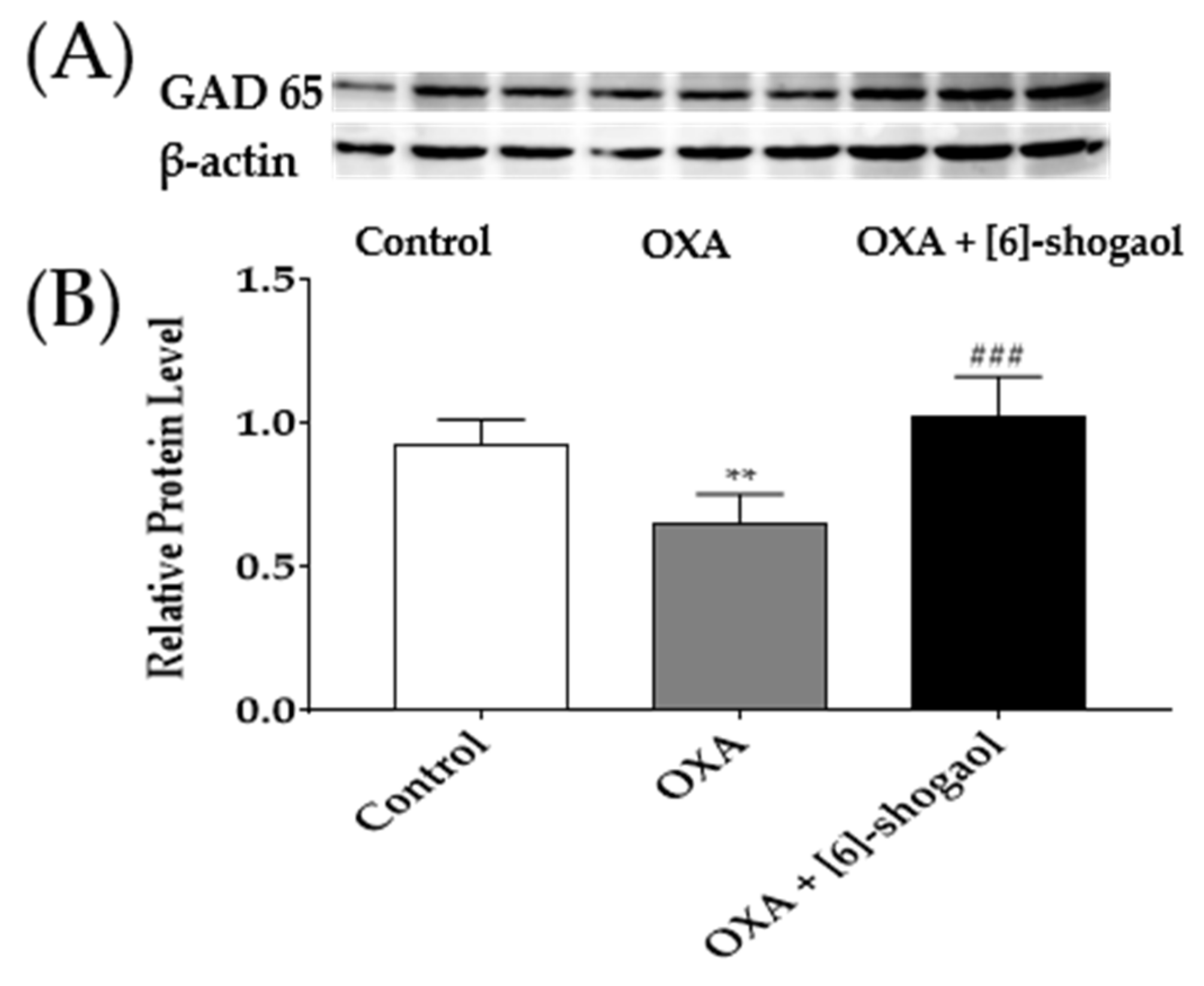
2.6. Administration of [6]-Shogaol Increased GAD65 Protein in the Spinal Cord in Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Oxaliplatin-Induced Neuropathic Pain
4.3. Adminsitration of [6]-Shogaol
4.4. Intrathecal Injection of 5-HT and GABAB Receptor Antagonists
4.5. Behavioral Assessments
4.6. Immunohistochemistry (IHC)
4.7. Western Blots
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, M.A.; Lockwood, G.F.; Greenslade, D.; Brienza, S.; Bayssas, M.; Gamelin, E. Clinical pharmacokinetics of oxaliplatin: A critical review. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 1205–1218. [Google Scholar]
- Pasetto, L.M.; D’Andrea, M.R.; Rossi, E.; Monfardini, S. Oxaliplatin-related neurotoxicity: How and why? Crit. Rev. Oncol. Hematol. 2006, 59, 159–168. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [Green Version]
- Ta, L.E.; Low, P.A.; Windebank, A.J. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol. Pain 2009, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebremedhn, E.G.; Shortland, P.J.; Mahns, D.A. The incidence of acute oxaliplatin-induced neuropathy and its impact on treatment in the first cycle: A systematic review. BMC Cancer 2018, 18, 410. [Google Scholar] [CrossRef] [Green Version]
- Lehky, T.; Leonard, G.; Wilson, R.; Grem, J.; Floeter, M. Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve 2004, 29, 387–392. [Google Scholar] [CrossRef]
- Voscopoulos, C.; Lema, M. When does acute pain become chronic? Br. J. Anaesth. 2010, 105, i69–i85. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Perahia, D.G.; Kajdasz, D.K.; Walker, D.J.; Raskin, J.; Tylee, A. Duloxetine 60 mg once daily in the treatment of milder major depressive disorder. Int. J. Clin. Pract. 2006, 60, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Sommer, C. Serotonin in pain and analgesia. Mol. Neurobiol. 2004, 30, 117–125. [Google Scholar] [CrossRef]
- Dogrul, A.; Ossipov, M.H.; Porreca, F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009, 1280, 52–59. [Google Scholar] [CrossRef]
- Bardin, L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011, 22, 390–404. [Google Scholar] [CrossRef]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar] [PubMed]
- Qi, Y.X.; Xia, R.Y.; Wu, Y.S.; Stanley, D.; Huang, J.; Ye, G.Y. Larvae of the small white butterfly, Pieris rapae, express a novel serotonin receptor. J. Neurochem. 2014, 131, 767–777. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Ali, Z.; Wu, G.; Kozlov, A.; Barasi, S. The actions of 5-HT1 agonists and antagonists on nociceptive processing in the rat spinal cord: Results from behavioural and electrophysiological studies. Brain Res. 1994, 661, 83–90. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernandez, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Meyerson, B.A.; Linderoth, B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 2011, 152, 1666–1673. [Google Scholar] [CrossRef]
- Hu, B.; Doods, H.; Treede, R.D.; Ceci, A. Duloxetine and 8-OH-DPAT, but not fluoxetine, reduce depression-like behaviour in an animal model of chronic neuropathic pain. Neurosci. Lett. 2016, 619, 162–167. [Google Scholar] [CrossRef]
- Kayser, V.; Elfassi, I.E.; Aubel, B.; Melfort, M.; Julius, D.; Gingrich, J.A.; Hamon, M.; Bourgoin, S. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A-/-, 5-HT1B-/-, 5-HT2A-/-, 5-HT3A-/- and 5-HTT-/- knock-out male mice. Pain 2007, 130, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.F.; Coull, J.A.; Chery, N.; Poisbeau, P.; De Koninck, Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 7871–7880. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Xu, E. The role and the mechanism of gamma-aminobutyric acid during central nervous system development. Neurosci. Bull. 2008, 24, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megumu, Y.; Hidemasa, F. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J. Pharmacol. Sci. 2006, 101, 107–117. [Google Scholar]
- Meisner, J.G.; Marsh, A.D.; Marsh, D.R. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J. Neurotrauma 2010, 27, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Hulsebosch, C.E. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology 2011, 60, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Soghomonian, J.J.; Martin, D.L. Two isoforms of glutamate decarboxylase: Why? Trends Pharmacol. Sci. 1998, 19, 500–505. [Google Scholar] [CrossRef]
- Pinal, C.S.; Tobin, A. Uniqueness and redundancy in GABA production. Perspect. Dev. Neurobiol. 1998, 5, 109–118. [Google Scholar] [PubMed]
- Nashawi, H.; Masocha, W.; Edafiogho, I.O.; Kombian, S.B. Paclitaxel Causes Electrophysiological Changes in the Anterior Cingulate Cortex via Modulation of the γ-Aminobutyric Acid-ergic System. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2016, 25, 423–428. [Google Scholar] [CrossRef]
- Lee, J.H.; Min, D.; Lee, D.; Kim, W. Zingiber officinale roscoe rhizomes attenuate oxaliplatin-induced neuropathic pain in mice. Molecules 2021, 26, 548. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Zhang, Y. Research progress on chemical constituents of Zingiber officinale Roscoe. Bio. Med. Res. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef] [Green Version]
- McCarson, K.E.; Enna, S. GABA pharmacology: The search for analgesics. Neurochem. Res. 2014, 39, 1948–1963. [Google Scholar] [CrossRef]
- Ling, B.; Coudoré-Civiale, M.A.; Balayssac, D.; Eschalier, A.; Coudoré, F.; Authier, N. Behavioral and immunohistological assessment of painful neuropathy induced by a single oxaliplatin injection in the rat. Toxicology 2007, 234, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Bobylev, I.; Joshi, A.R.; Barham, M.; Neiss, W.F.; Lehmann, H.C. Depletion of mitofusin-2 causes mitochondrial damage in cisplatin-induced neuropathy. Mol. Neurobiol. 2018, 55, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-induced neurotoxicity and preventive strategies: Past, present, and future. Oncologist 2015, 20, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannelli, L.D.C.; Pacini, A.; Micheli, L.; Tani, A.; Zanardelli, M.; Ghelardini, C. Glial role in oxaliplatin-induced neuropathic pain. Exp. Neurol. 2014, 261, 22–33. [Google Scholar] [CrossRef]
- Webster, R.G.; Brain, K.L.; Wilson, R.H.; Grem, J.L.; Vincent, A. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. Br. J. Pharmacol. 2005, 146, 1027–1039. [Google Scholar] [CrossRef]
- Siau, C.; Bennett, G.J. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth. Analg. 2006, 102, 1485. [Google Scholar] [CrossRef]
- Marmitt, D.J.; Shahrajabian, M.H. Plant species used in Brazil and Asia regions with toxic properties. Phytother. Res. 2021, 35, 4703–4726. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.L.; Campbell, R.; Pak, S.C.; Golombick, T.; Manoharan, A.; Ramakrishna, R.; Badmaev, V.; Schloss, J. Is 6-Shogaol an Effective Phytochemical for Patients With Lower-risk Myelodysplastic Syndrome? A Narrative Review. Integr. Cancer Ther. 2021, 20, 1–12. [Google Scholar] [CrossRef]
- White, B. Ginger: An overview. Am. Fam. Physician 2007, 75, 1689–1691. [Google Scholar] [PubMed]
- Simon, A.; Darcsi, A.; Kéry, Á.; Riethmüller, E. Blood-brain barrier permeability study of ginger constituents. J. Pharm. Biomed. Anal. 2020, 177, 112820. [Google Scholar] [CrossRef]
- Fajrin, F.A.; Nugroho, A.E.; Nurrochmad, A.; Susilowati, R. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J. Ethnopharmacol. 2020, 249, 112396. [Google Scholar] [CrossRef]
- Rydelek-Fitzgerald, L.; Tietler, M.; Fletcher, P.W.; Ismaiel, A.M.; Glennon, R.A. NAN-190: Agonist and antagonist interactions with brain 5-HT1A receptors. Brain Res. 1990, 532, 191–196. [Google Scholar] [CrossRef]
- Fozard, J. MDL 72222: A potent and highly selective antagonist at neuronal 5-hydroxytryptamine receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1984, 326, 36–44. [Google Scholar] [CrossRef]
- Skyba, D.; Radhakrishnan, R.; Rohlwing, J.; Wright, A.; Sluka, K. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain 2003, 106, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, R.; King, E.W.; Dickman, J.K.; Herold, C.A.; Johnston, N.F.; Spurgin, M.L.; Sluka, K.A. Spinal 5-HT2 and 5-HT3 receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain 2003, 105, 205–213. [Google Scholar] [CrossRef]
- Marlier, L.; Teilhac, J.R.; Cerruti, C.; Privat, A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991, 550, 15–23. [Google Scholar] [CrossRef]
- Avila-Rojas, S.H.; Velazquez-Lagunas, I.; Salinas-Abarca, A.B.; Barragan-Iglesias, P.; Pineda-Farias, J.B.; Granados-Soto, V. Role of spinal 5-HT5A, and 5-HT1A/1B/1D, receptors in neuropathic pain induced by spinal nerve ligation in rats. Brain Res. 2015, 1622, 377–385. [Google Scholar] [CrossRef]
- Barnes, N.M.; Hales, T.G.; Lummis, S.C.; Peters, J.A. The 5-HT3 receptor–the relationship between structure and function. Neuropharmacology 2009, 56, 273–284. [Google Scholar] [CrossRef]
- Brady, S. Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Laporte, A.; Koscielniak, T.; Ponchant, M.; Verge, D.; Hamon, M.; Gozlan, H. Quantitative autoradiographic mapping of 5-HT3 receptors in the rat CNS using [125I] iodo-zacopride and [3H] zacopride as radioligands. Synapse 1992, 10, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Glaum, S.R.; Proudfit, H.K.; Anderson, E.G. Reversal of the antinociceptive effects of intrathecally administered serotonin in the rat by a selective 5-HT3 receptor antagonist. Neurosci. Lett. 1988, 95, 313–317. [Google Scholar] [CrossRef]
- Alhaider, A.A.; Lei, S.Z.; Wilcox, G.L. Spinal 5-HT3 receptor-mediated antinociception: Possible release of GABA. J. Neurosci. 1991, 11, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Petroff, O.A. Book review: GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Li, C.; Lei, Y.; Tian, Y.; Xu, S.; Shen, X.; Wu, H.; Bao, S.; Wang, F. The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain. Mol. Pain 2019, 15, 1744806919847366. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, H.; Gao, H.; Zhao, H.; Liu, D.; Li, J. Participation of pro-inflammatory cytokines in neuropathic pain evoked by chemotherapeutic oxaliplatin via central GABAergic pathway. Mol. Pain 2018, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Malcangio, M. GABAB receptors and pain. Neuropharmacology 2018, 136, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudet, C.; Magnaghi, V.; Landry, M.; Nagy, F.; Gereau IV, R.W.; Pin, J.-P. Metabotropic receptors for glutamate and GABA in pain. Brain Res. Rev. 2009, 60, 43–56. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wei, Y.-Y.; Huang, J.; Wang, W.; Tamamaki, N.; Li, Y.-Q.; Wu, S.-X. Expression patterns of 5-HT receptor subtypes 1A and 2A on GABAergic neurons within the spinal dorsal horn of GAD67-GFP knock-in mice. J. Chem. Neuroanat. 2009, 38, 75–81. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.-Y.; Wang, W.; Li, Y.-Q.; Tamamaki, N.; Wu, S.-X. 5-HT3A receptor subunit is expressed in a subpopulation of GABAergic and enkephalinergic neurons in the mouse dorsal spinal cord. Neurosci. Lett. 2008, 441, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Gao, X.; Ji, G.-C.; Huang, Y.-L.; Wu, G.-C.; Zhao, Z.-Q. Expression of 5-HT1A receptor mRNA in rat lumbar spinal dorsal horn neurons after peripheral inflammation. Pain 2002, 98, 287–295. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Li, D.; Lee, J.H. The Analgesic Effect of Venlafaxine and Its Mechanism on Oxaliplatin-Induced Neuropathic Pain in Mice. Int. J. Mol. Sci. 2019, 20, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.H.; Kim, W.; Li, D.; Kim, Y.; Lee, K.; Kim, S.K. The Suppressive Effects of Cinnamomi Cortex and Its Phytocompound Coumarin on Oxaliplatin-Induced Neuropathic Cold Allodynia in Rats. Molecules 2016, 21, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Gang, J.; Lee, J.-H.; Yang, H.; Cheon, C.; Ko, S.-G.; Bae, H.; Kim, W. [6]-Shogaol Attenuates Oxaliplatin-Induced Allodynia through Serotonergic Receptors and GABA in the Spinal Cord in Mice. Pharmaceuticals 2022, 15, 726. https://doi.org/10.3390/ph15060726
Kim S, Gang J, Lee J-H, Yang H, Cheon C, Ko S-G, Bae H, Kim W. [6]-Shogaol Attenuates Oxaliplatin-Induced Allodynia through Serotonergic Receptors and GABA in the Spinal Cord in Mice. Pharmaceuticals. 2022; 15(6):726. https://doi.org/10.3390/ph15060726
Chicago/Turabian StyleKim, Suyong, Juan Gang, Ji-Hwan Lee, Hyejin Yang, Chunhoo Cheon, Seong-Gyu Ko, Hyunsu Bae, and Woojin Kim. 2022. "[6]-Shogaol Attenuates Oxaliplatin-Induced Allodynia through Serotonergic Receptors and GABA in the Spinal Cord in Mice" Pharmaceuticals 15, no. 6: 726. https://doi.org/10.3390/ph15060726