The Synthesis, Characterization, Molecular Docking and In Vitro Antitumor Activity of Benzothiazole Aniline (BTA) Conjugated Metal-Salen Complexes as Non-Platinum Chemotherapeutic Agents
Abstract
:1. Introduction
2. Results and Discussion
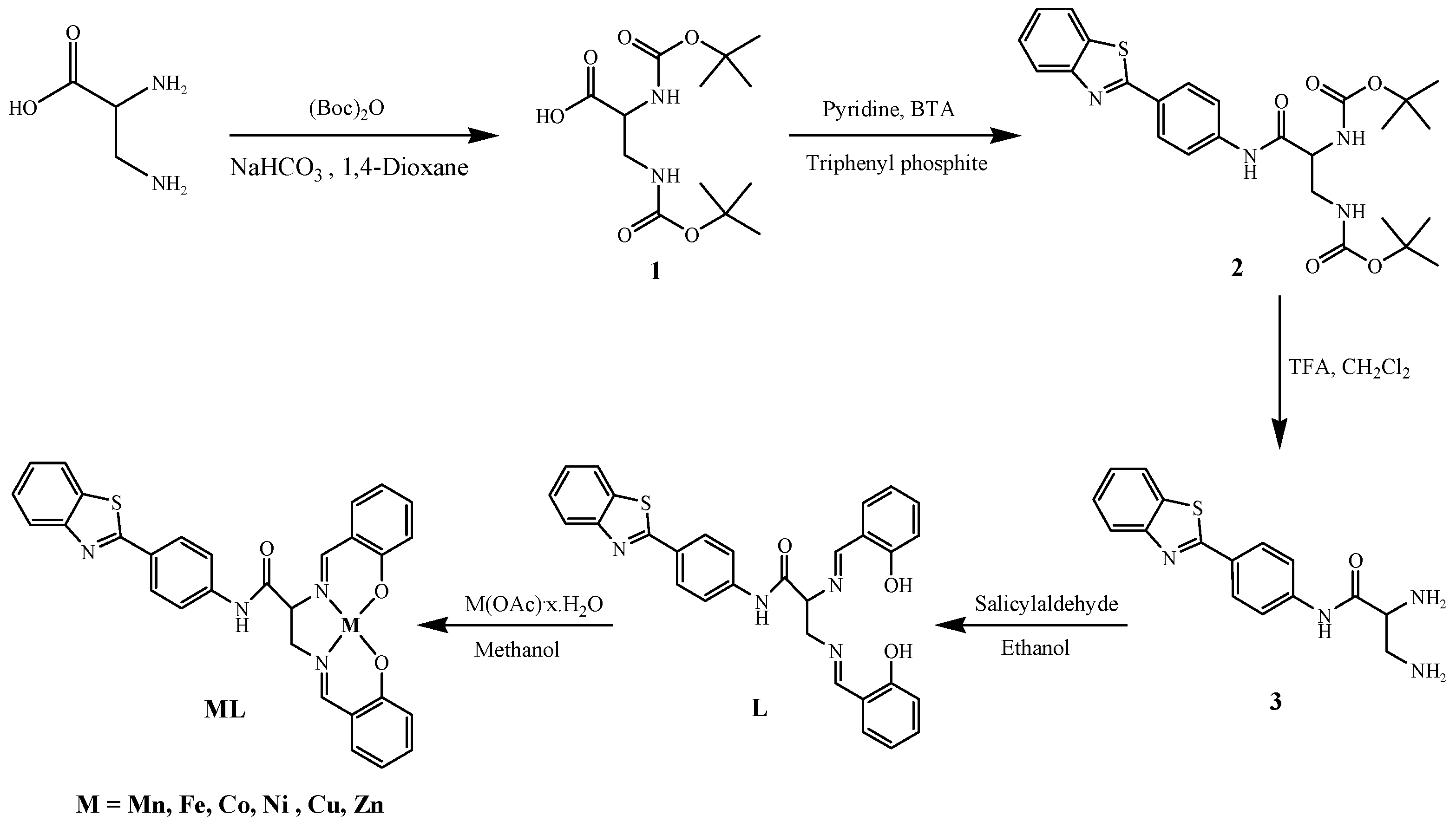
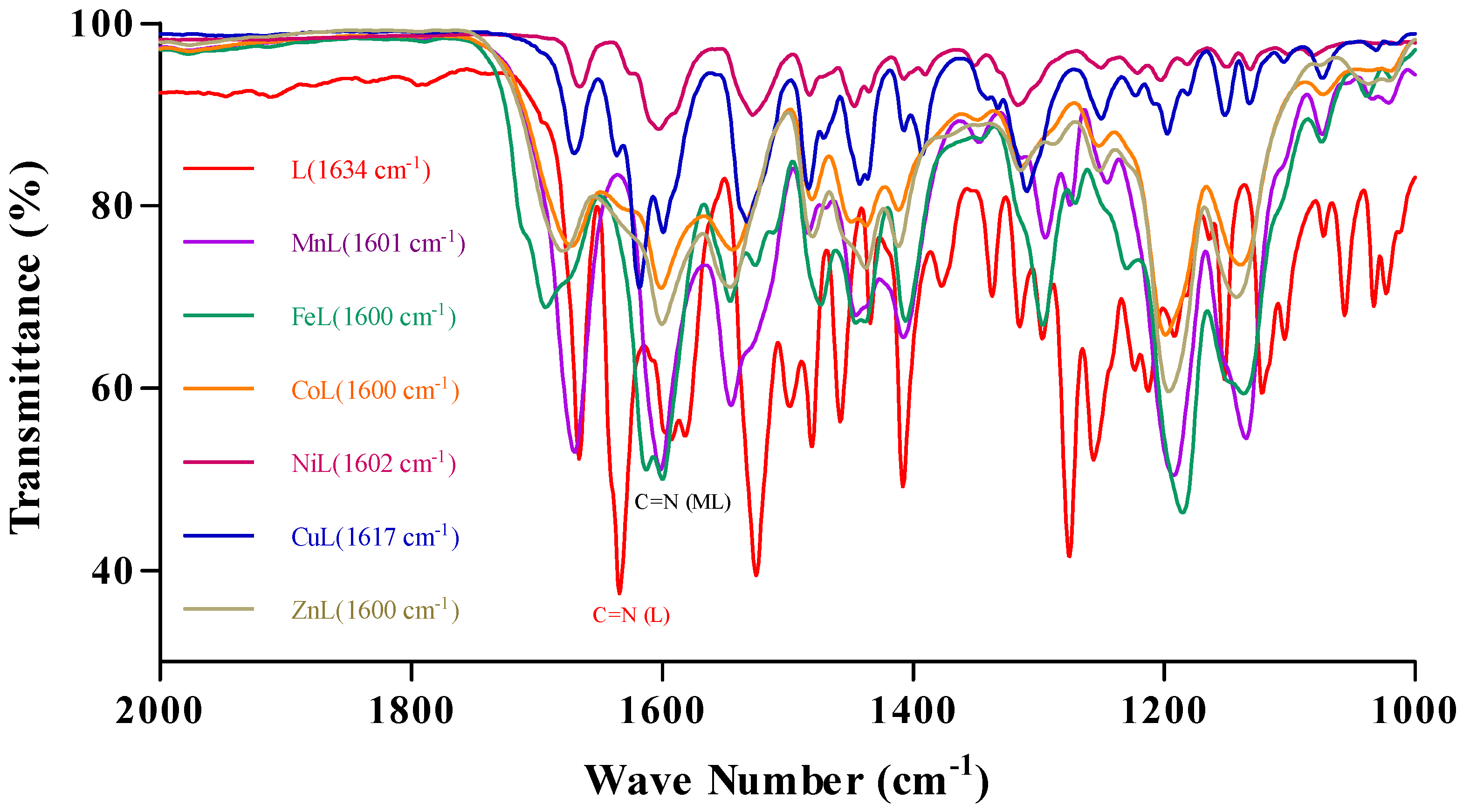
2.1. Synthesis and Characterization
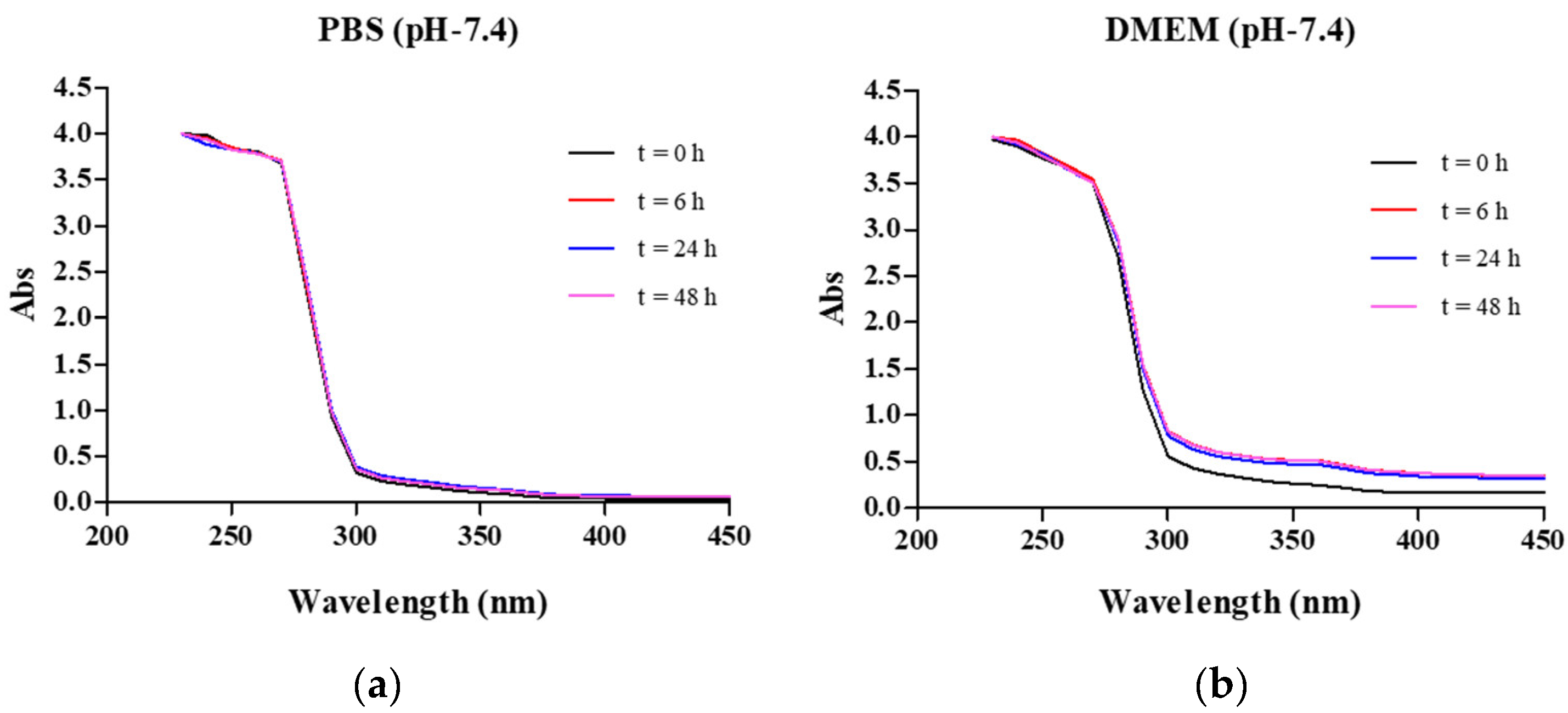
2.2. Complex Stability
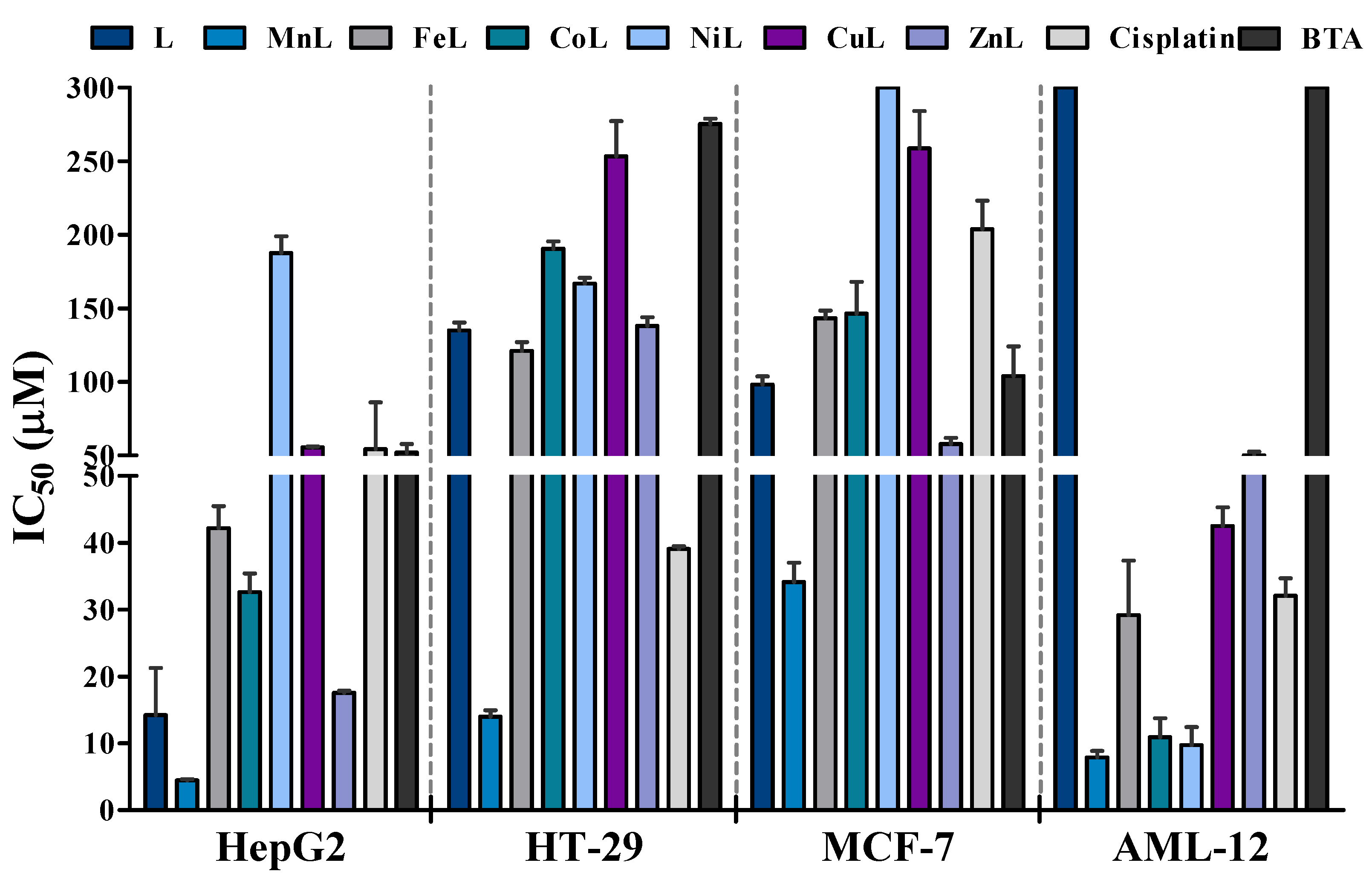
2.3. Antiproliferative Activity
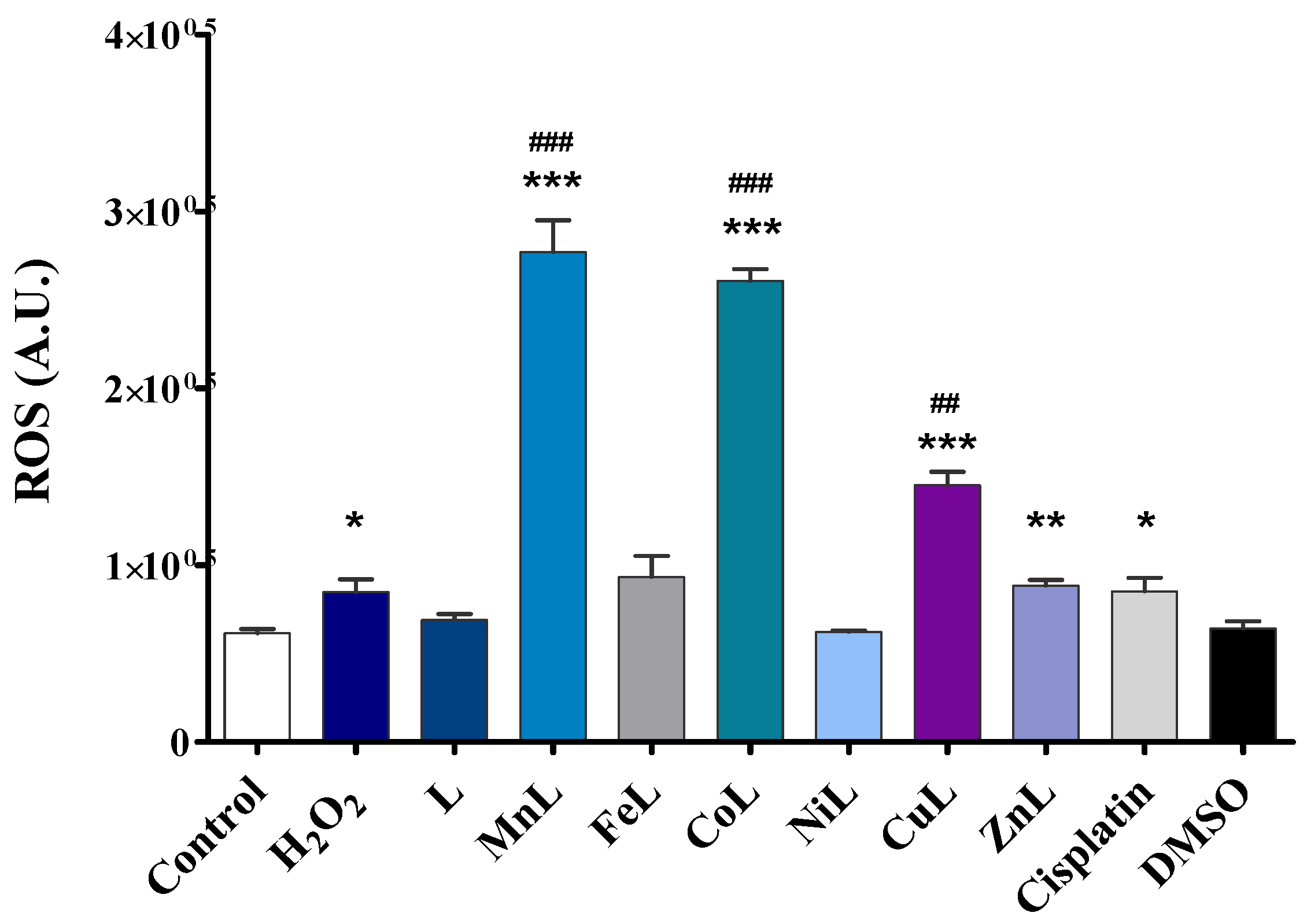
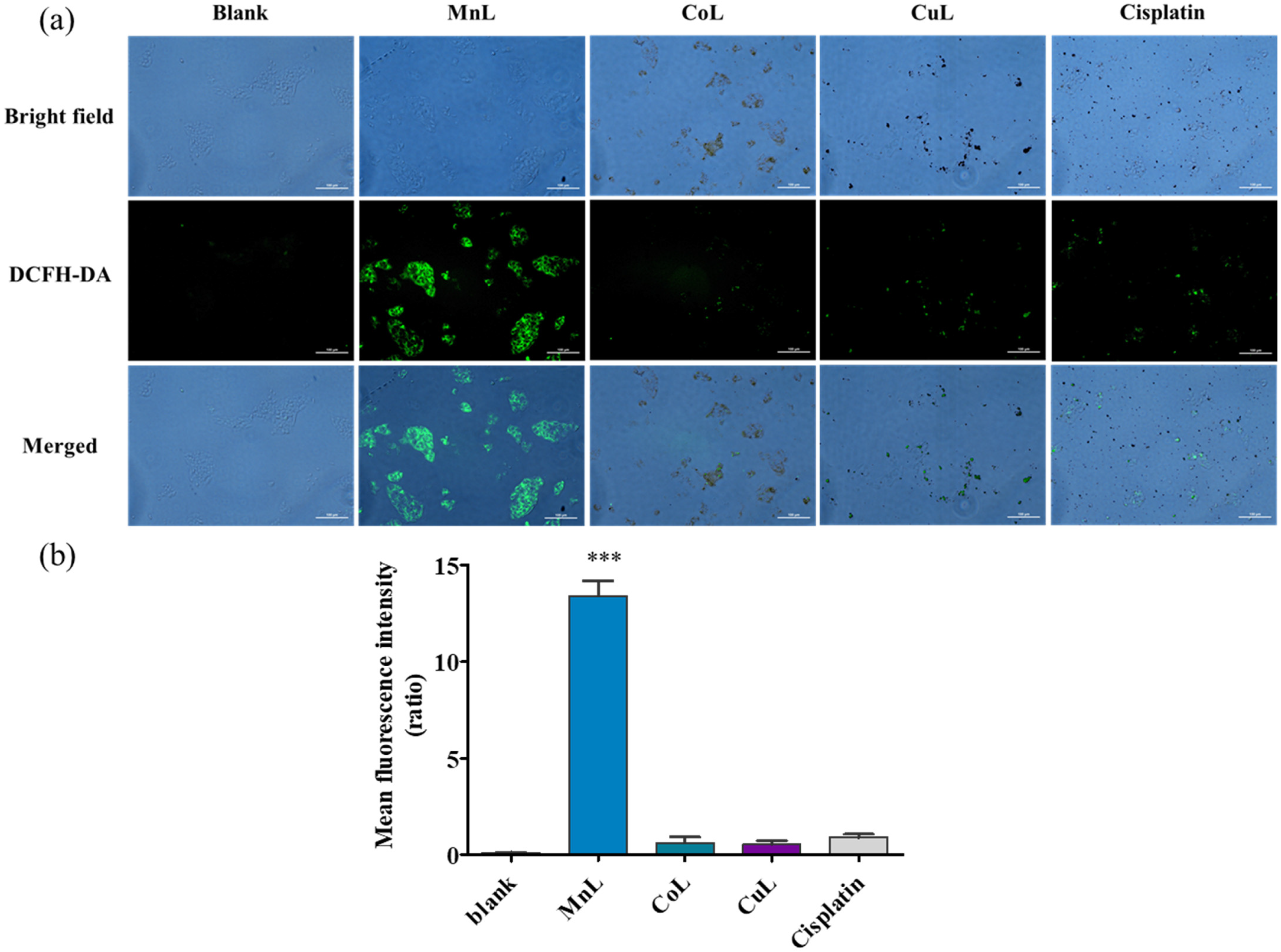
2.4. Intracellular ROS Generation
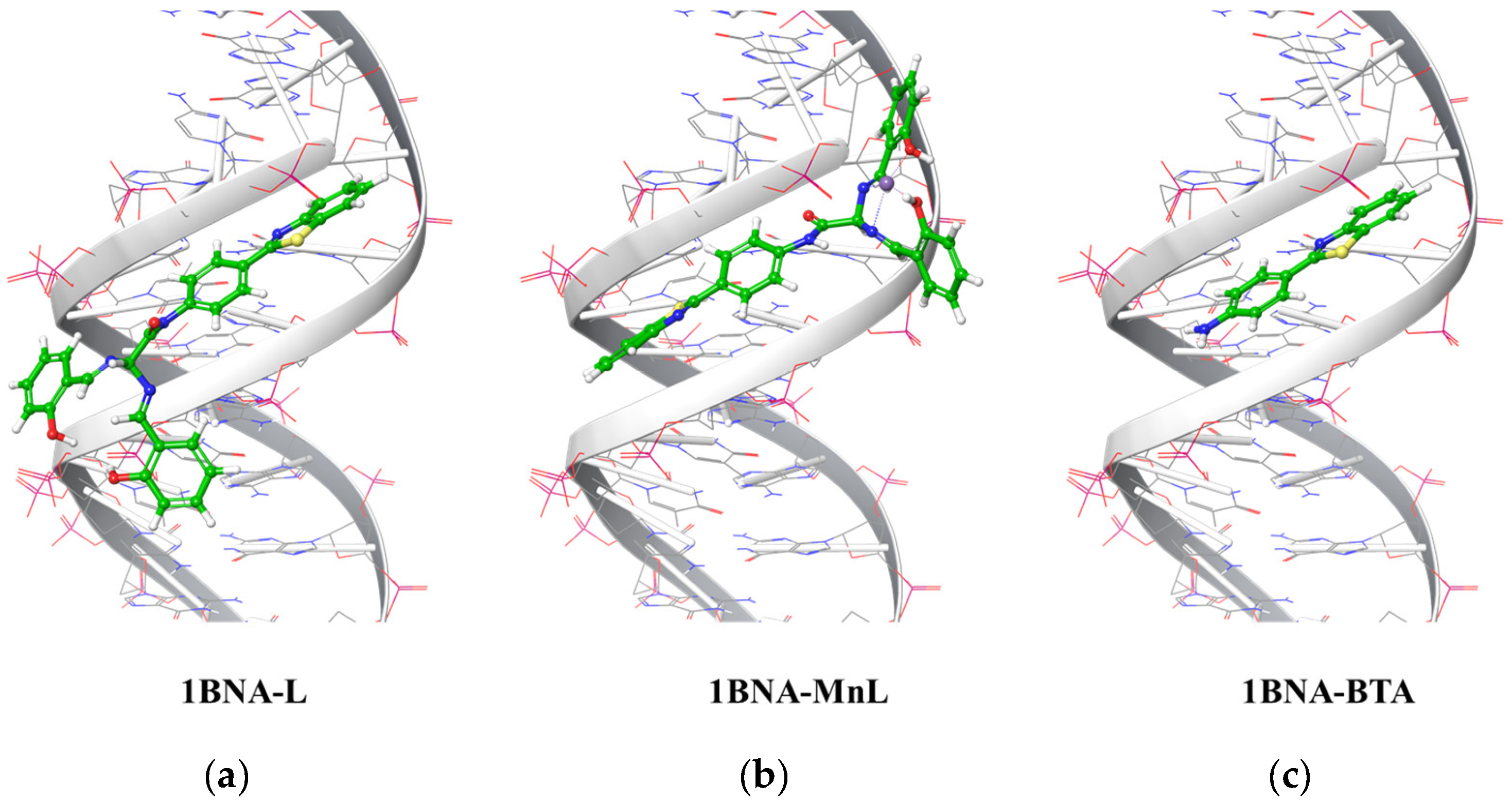
2.5. Ligand Docking Simulation of L, MnL, and BTA
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Synthesis and Characterization of Ligand
N-(4-Benzothiazol-2-yl-phenyl)-2,3-bis-[(2-hydroxy-benzylidene)-amino]-propionamide (L)
3.3. Synthesis and Characterization of Metal Complexes
3.3.1. Synthesis of MnL
3.3.2. Synthesis of FeL
3.3.3. Synthesis of CoL
3.3.4. Synthesis of NiL
3.3.5. Synthesis of CuL
3.3.6. Synthesis of ZnL
3.4. Stability of the Lead Complex
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. Intracellular ROS Analysis
3.8. Computational Method for Predicting Ligand-DNA Complexes
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, B.; VanCamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Muggia, F.M.; Bonetti, A.; Hoeschele, J.D.; Rozencweig, M.; Howell, S.B. Platinum Antitumor Complexes: 50 Years Since Barnett Rosenberg’s Discovery. J. Clin. Oncol. 2015, 33, 4219–4226. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.; Blasiak, J. Recognition and repair of DNA-cisplatin adducts. Acta Biochim. Pol. 2002, 49, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Markman, M. Toxicities of the platinum antineoplastic agents. Expert Opin. Drug Saf. 2003, 2, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Valente, A.; Podolski-Renic, A.; Poetsch, I.; Filipovic, N.; Lopez, O.; Turel, I.; Heffeter, P. Metal- and metalloid-based compounds to target and reverse cancer multidrug resistance. Drug Resist. Updates 2021, 58, 100778. [Google Scholar] [CrossRef]
- Kanat, O.; Ertas, H.; Caner, B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J. Clin. Oncol. 2017, 8, 329–335. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum neurotoxicity pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.A.; Patil, S.A.; Patil, R.; Keri, R.S.; Budagumpi, S.; Balakrishna, G.R.; Tacke, M. N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs. Future Med. Chem. 2015, 7, 1305–1333. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gust, R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Li, J.; Zhang, S.; Guo, L.; He, X.; Kong, D.; Zhang, H.; Liu, Z. Half-sandwich ruthenium(ii) complexes containing N^N-chelated imino-pyridyl ligands that are selectively toxic to cancer cells. Chem. Commun. 2017, 53, 12810–12813. [Google Scholar] [CrossRef] [PubMed]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, K.L.; Franz, K.J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef] [Green Version]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Dou, Q.P. Novel metals and metal complexes as platforms for cancer therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Loginova, N.V.; Harbatsevich, H.I.; Osipovich, N.P.; Ksendzova, G.A.; Koval’chuk, T.V.; Polozov, G.I. Metal Complexes as Promising Agents for Biomedical Applications. Curr. Med. Chem. 2020, 27, 5213–5249. [Google Scholar] [CrossRef]
- Hille, A.; Ott, I.; Kitanovic, A.; Kitanovic, I.; Alborzinia, H.; Lederer, E.; Wolfl, S.; Metzler-Nolte, N.; Schafer, S.; Sheldrick, W.S.; et al. [N,N′-Bis(salicylidene)-1,2-phenylenediamine]metal complexes with cell death promoting properties. J. Biol. Inorg. Chem. 2009, 14, 711–725. [Google Scholar] [CrossRef]
- Bouche, M.; Hognon, C.; Grandemange, S.; Monari, A.; Gros, P.C. Recent advances in iron-complexes as drug candidates for cancer therapy: Reactivity, mechanism of action and metabolites. Dalton Trans. 2020, 49, 11451–11466. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadele, K.T.; Tsega, T.W. Schiff Bases and their Metal Complexes as Potential Anticancer Candidates: A Review of Recent Works. Anticancer Agents Med. Chem. 2019, 19, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kamra, M.; Bhan, A.; Mandal, S.S.; Bhattacharya, S. New Fe(iii) and Co(ii) salen complexes with pendant distamycins: Selective targeting of cancer cells by DNA damage and mitochondrial pathways. Dalton Trans. 2016, 45, 9345–9353. [Google Scholar] [CrossRef]
- Damercheli, M.; Mahdi, M.; Mehravi, B.; Shafiee Ardestani, M. Bioactive Salen-type Schiff Base Transition Metal Complexes as Possible Anticancer Agents. Iran J. Pharm. Res. 2019, 18, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.S. Metallo-salen complexes show promise towards treatment of leukemia. Leuk. Res. 2011, 35, 571–572. [Google Scholar] [CrossRef] [Green Version]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hille, A.; Kitanovic, I.; Jesse, P.; Henze, G.; Wolfl, S.; Gust, R.; Prokop, A. [Fe(III)(salophene)Cl], a potent iron salophene complex overcomes multiple drug resistance in lymphoma and leukemia cells. Leuk. Res. 2011, 35, 387–393. [Google Scholar] [CrossRef]
- Gust, R.; Ott, I.; Posselt, D.; Sommer, K. Development of cobalt(3,4-diarylsalen) complexes as tumor therapeutics. J. Med. Chem. 2004, 47, 5837–5846. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hille, A.; Frias, C.; Kater, B.; Bonitzki, B.; Wolfl, S.; Scheffler, H.; Prokop, A.; Gust, R. [NiII(3-OMe-salophene)]: A potent agent with antitumor activity. J. Med. Chem. 2010, 53, 6064–6070. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, X.; Zhang, L.; Zhang, J.; Li, C.; Zhang, N.; Xu, H.; Li, Y. A new Schiff base coordinated copper(II) compound induces apoptosis and inhibits tumor growth in gastric cancer. Cancer Cell Int. 2019, 19, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Jia, J.; Li, F.; Liu, W.; Yang, C.; Jin, B.; Shi, Q.; Wang, X.; He, D.; Guo, P. Salen-Mn compounds induces cell apoptosis in human prostate cancer cells through promoting AMPK activity and cell autophagy. Oncotarget 2017, 8, 86277–86286. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.D.; Stevens, M.F.; Westwell, A.D. The discovery of the potent and selective antitumour agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203) and related compounds. Curr. Med. Chem. 2001, 8, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.F.; Bradshaw, T.D.; Wrigley, S.; McCall, C.J.; Lelieveld, P.; Fichtner, I.; Stevens, M.F. Antitumor benzothiazoles. 3. Synthesis of 2-(4-aminophenyl)benzothiazoles and evaluation of their activities against breast cancer cell lines in vitro and in vivo. J. Med. Chem. 1996, 39, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.D.; Westwell, A.D. The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate. Curr. Med. Chem. 2004, 11, 1009–1021. [Google Scholar] [CrossRef]
- Noolvi, M.N.; Patel, H.M.; Kaur, M. Benzothiazoles: Search for anticancer agents. Eur. J. Med. Chem. 2012, 54, 447–462. [Google Scholar] [CrossRef]
- Zhang, Y.; Chakraborty, M.; Cerda-Smith, C.G.; Bratton, R.N.; Maurer, N.E.; Senser, E.M.; Novak, M. Chemistry of ring-substituted 4-(benzothiazol-2-yl)phenylnitrenium ions from antitumor 2-(4-aminophenyl)benzothiazoles. J. Org. Chem. 2013, 78, 6992–7000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, T.D.; Shi, D.F.; Schultz, R.J.; Paull, K.D.; Kelland, L.; Wilson, A.; Garner, C.; Fiebig, H.H.; Wrigley, S.; Stevens, M.F. Influence of 2-(4-aminophenyl)benzothiazoles on growth of human ovarian carcinoma cells in vitro and in vivo. Br. J. Cancer 1998, 78, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Kashiyama, E.; Hutchinson, I.; Chua, M.S.; Stinson, S.F.; Phillips, L.R.; Kaur, G.; Sausville, E.A.; Bradshaw, T.D.; Westwell, A.D.; Stevens, M.F. Antitumor benzothiazoles. 8. Synthesis, metabolic formation, and biological properties of the C- and N-oxidation products of antitumor 2-(4-aminophenyl)benzothiazoles. J. Med. Chem. 1999, 42, 4172–4184. [Google Scholar] [CrossRef]
- Leong, C.O.; Gaskell, M.; Martin, E.A.; Heydon, R.T.; Farmer, P.B.; Bibby, M.C.; Cooper, P.A.; Double, J.A.; Bradshaw, T.D.; Stevens, M.F. Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo. Br. J. Cancer 2003, 88, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Kang, M.K.; Jung, K.H.; Kang, S.H.; Kim, Y.H.; Jung, J.C.; Lee, G.H.; Chang, Y.; Kim, T.J. Gadolinium complex of DO3A-benzothiazole aniline (BTA) conjugate as a theranostic agent. J. Med. Chem. 2013, 56, 8104–8111. [Google Scholar] [CrossRef] [PubMed]
- Mavroidi, B.; Sagnou, M.; Stamatakis, K.; Paravatou-Petsotas, M.; Pelecanou, M.; Methenitis, C. Palladium(II) and platinum(II) complexes of derivatives of 2-(4′-aminophenyl)benzothiazole as potential anticancer agents. Inorg. Chim. Acta 2016, 444, 63–75. [Google Scholar] [CrossRef]
- Islam, M.K.; Baek, A.R.; Sung, B.; Yang, B.W.; Choi, G.; Park, H.J.; Kim, Y.H.; Kim, M.; Ha, S.; Lee, G.H.; et al. Synthesis, Characterization, and Anticancer Activity of Benzothiazole Aniline Derivatives and Their Platinum (II) Complexes as New Chemotherapy Agents. Pharmaceuticals 2021, 14, 832. [Google Scholar] [CrossRef] [PubMed]
- Lenis-Rojas, O.A.; Robalo, M.P.; Tomaz, A.I.; Carvalho, A.; Fernandes, A.R.; Marques, F.; Folgueira, M.; Yanez, J.; Vazquez-Garcia, D.; Lopez Torres, M.; et al. Ru(II)(p-cymene) Compounds as Effective and Selective Anticancer Candidates with No Toxicity in Vivo. Inorg. Chem. 2018, 57, 13150–13166. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.T.T.; Pham, V.; Huynh, H.V. Mixed Arylolefin/NHC Complexes of Platinum(II): Syntheses, Characterizations, and In Vitro Cytotoxicities. Organometallics 2020, 39, 3505–3513. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Lenis-Rojas, O.A.; Carvalho, B.; Cabral, R.; Silva, M.; Friaes, S.; Roma-Rodrigues, C.; Meireles, M.S.H.; Gomes, C.S.B.; Fernandez, J.A.A.; Vila, S.F.; et al. Manganese(I) tricarbonyl complexes as potential anticancer agents. J. Biol. Inorg. Chem. 2021, 27, 49–64. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Jiang, G.B.; Zheng, X.; Yao, J.H.; Han, B.J.; Li, W.; Wang, J.; Huang, H.L.; Liu, Y.J. Ruthenium(II) polypyridyl complexes induce BEL-7402 cell apoptosis by ROS-mediated mitochondrial pathway. J. Inorg. Biochem. 2014, 141, 170–179. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.A.; Parce, J.W.; Dechatelet, L.R.; Szejda, P.; Seeds, M.C.; Thomas, M. Flow cytometric studies of oxidative product formation by neutrophils: A graded response to membrane stimulation. J. Immunol. 1983, 130, 1910–1917. [Google Scholar] [PubMed]
- Dehkhodaei, M.; Sahihi, M.; Amiri Rudbari, H.; Momenbeik, F. DNA and HSA interaction of Vanadium (IV), Copper (II), and Zinc (II) complexes derived from an asymmetric bidentate Schiff-base ligand: Multi spectroscopic, viscosity measurements, molecular docking, and ONIOM studies. J. Biol. Inorg. Chem. 2018, 23, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, C.; Singh, S.K. Computational and Experimental Binding Mechanism of DNA-drug Interactions. Curr. Pharm. Des. 2018, 24, 3739–3757. [Google Scholar] [CrossRef]
- De, B.; Bhandari, K.; Mendonca, F.J.B.; Scotti, M.T.; Scotti, L. Computational Studies in Drug Design against Cancer. Anticancer Agents Med. Chem. 2019, 19, 587–591. [Google Scholar] [CrossRef]
- Islam, M.K.; Kim, S.; Kim, H.K.; Park, S.; Lee, G.H.; Kang, H.J.; Jung, J.C.; Park, J.S.; Kim, T.J.; Chang, Y. Manganese Complex of Ethylenediaminetetraacetic Acid (EDTA)-Benzothiazole Aniline (BTA) Conjugate as a Potential Liver-Targeting MRI Contrast Agent. J. Med. Chem. 2017, 60, 2993–3001. [Google Scholar] [CrossRef]
- Chang, Y.M.; Kim, Y.H.; Chang, S.J.; Islam, M.K. Compound Having Novel Structure, Complex Comprising Same, Anti-Cancer Pharmaceutical Composition, and Anti-Cancer Drug. US20220024911A1, 27 January 2022. [Google Scholar]
- Kim, T.J.J.; Yong, M. Benzothiozole-Containing Ligand-Metal Complex, and Method for the Preparation Thereof. KR2018055135A, 25 May 2018. [Google Scholar]
- Schrödinger Release, 2021-3, Glide; Schrödinger, LLC: New York, NY, USA, 2021.
| Compounds | IC50 (μM) | Selectivity Index (SI) a | |||
|---|---|---|---|---|---|
| Human Liver Cancer (HepG2) | Human Colon Cancer (HT-29) | Human Breast Cancer (MCF-7) | Mouse Liver Hepatocyte (AML-12) | ||
| L | 14.2 ± 7.0 | 135.0 ± 4.4 | 98.1 ± 7.9 | >300 | 21.13 |
| MnL | 5.8 ± 0.3 | 15.1 ± 1.1 | 34.5 ± 4.0 | 9.4 ± 0.8 | 1.62 |
| FeL | 41.2 ± 3.8 | 110.3 ± 17.6 | 142.0 ± 8.9 | 48.1 ± 0.7 | 1.17 |
| CoL | 32.1 ± 4.4 | 181.8 ± 16.4 | 146.5 ± 30.5 | 14.7 ± 0.5 | 0.46 |
| NiL | 193.6 ± 17.7 | 167.1 ± 5.8 | >300 | 12.4 ± 0.6 | 0.06 |
| CuL | 55.5 ± 0.6 | 135.1 ± 10.0 | 271.2 ± 37.0 | 46.5 ± 0.8 | 0.84 |
| ZnL | 17.6 ± 0.4 | 254.8 ± 33.8 | 57.6 ± 5.8 | 52.6 ± 0.5 | 2.99 |
| Cisplatin b | 54.2 ± 31.8 | 39.1 ± 0.6 | 203.8 ± 27.3 | 32.1 ± 4.4 | 0.59 |
| BTA b | 52.0 ± 5.7 | 275.3 ± 5.0 | 103.9 ± 28.6 | >300 | 5.77 |
| Compounds | Docking Binding Energy (kcal/mol) | ||
|---|---|---|---|
| 1BNA a | 1LU5 b | 3CO3 c | |
| L | −7.239 | −6.307 | −7.718 |
| MnL | −5.894 | −4.253 | −3.340 |
| BTA | −6.618 | −4.259 | −5.941 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.K.; Ha, S.; Baek, A.-R.; Yang, B.-W.; Kim, Y.-H.; Park, H.-J.; Kim, M.; Nam, S.-W.; Lee, G.-H.; Chang, Y. The Synthesis, Characterization, Molecular Docking and In Vitro Antitumor Activity of Benzothiazole Aniline (BTA) Conjugated Metal-Salen Complexes as Non-Platinum Chemotherapeutic Agents. Pharmaceuticals 2022, 15, 751. https://doi.org/10.3390/ph15060751
Islam MK, Ha S, Baek A-R, Yang B-W, Kim Y-H, Park H-J, Kim M, Nam S-W, Lee G-H, Chang Y. The Synthesis, Characterization, Molecular Docking and In Vitro Antitumor Activity of Benzothiazole Aniline (BTA) Conjugated Metal-Salen Complexes as Non-Platinum Chemotherapeutic Agents. Pharmaceuticals. 2022; 15(6):751. https://doi.org/10.3390/ph15060751
Chicago/Turabian StyleIslam, Md. Kamrul, Seongmin Ha, Ah-Rum Baek, Byeong-Woo Yang, Yeoun-Hee Kim, Hyun-Jin Park, Minsup Kim, Sung-Wook Nam, Gang-Ho Lee, and Yongmin Chang. 2022. "The Synthesis, Characterization, Molecular Docking and In Vitro Antitumor Activity of Benzothiazole Aniline (BTA) Conjugated Metal-Salen Complexes as Non-Platinum Chemotherapeutic Agents" Pharmaceuticals 15, no. 6: 751. https://doi.org/10.3390/ph15060751