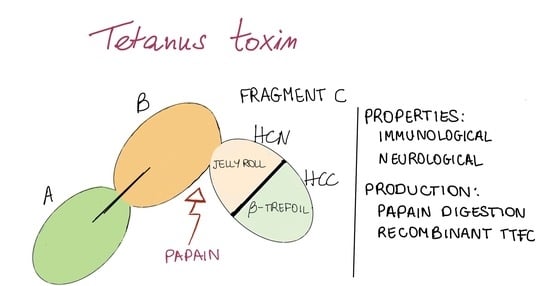
Tetanus Toxin Fragment C: Structure, Drug Discovery Research and Production
Abstract
:1. Introduction
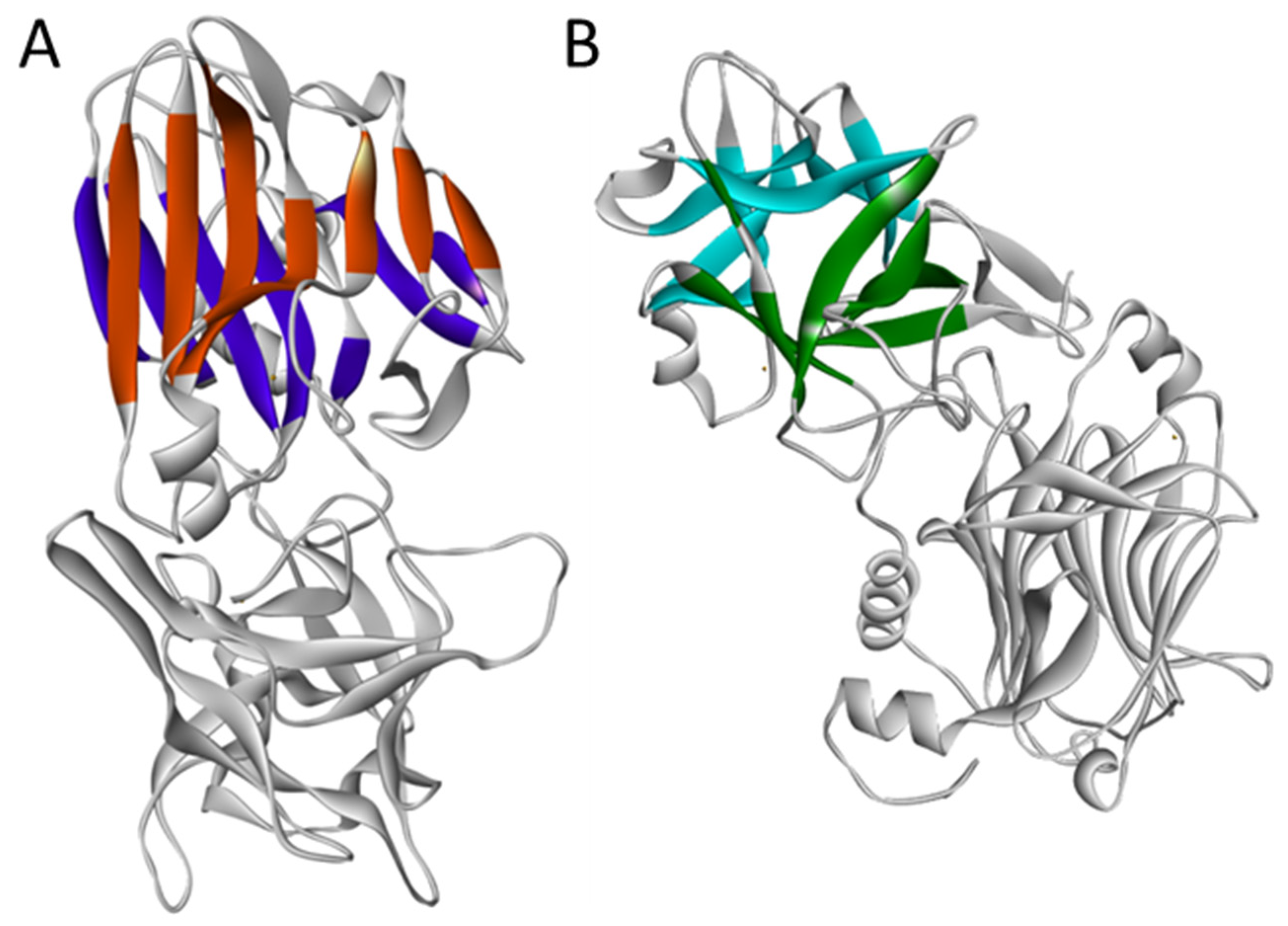
2. Structure and Association with Receptors
3. TTFC Properties and Uses: CNS Delivery and Immunogenicity
3.1. TTFC Neurological Properties
3.1.1. Permeability and CNS Delivery
3.1.2. Intrinsic Neuronal Protection
3.1.3. Overview of the Uses of TTFC for Its Neurological Properties
3.2. Immunological Properties
3.2.1. Immunological Properties against Tetanus
3.2.2. TTFC as a Fusion Protein: Enhancement of Immunogenicity
3.2.3. In Silico Design of Epitope-Based Vaccines
3.2.4. TTFC Uses for Its Immunological Properties
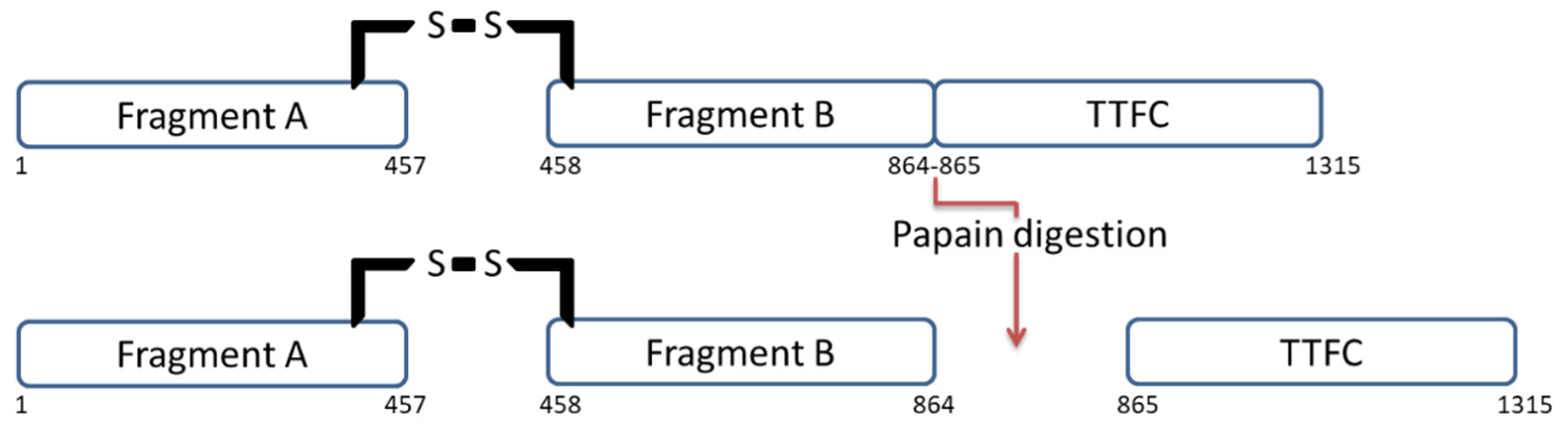
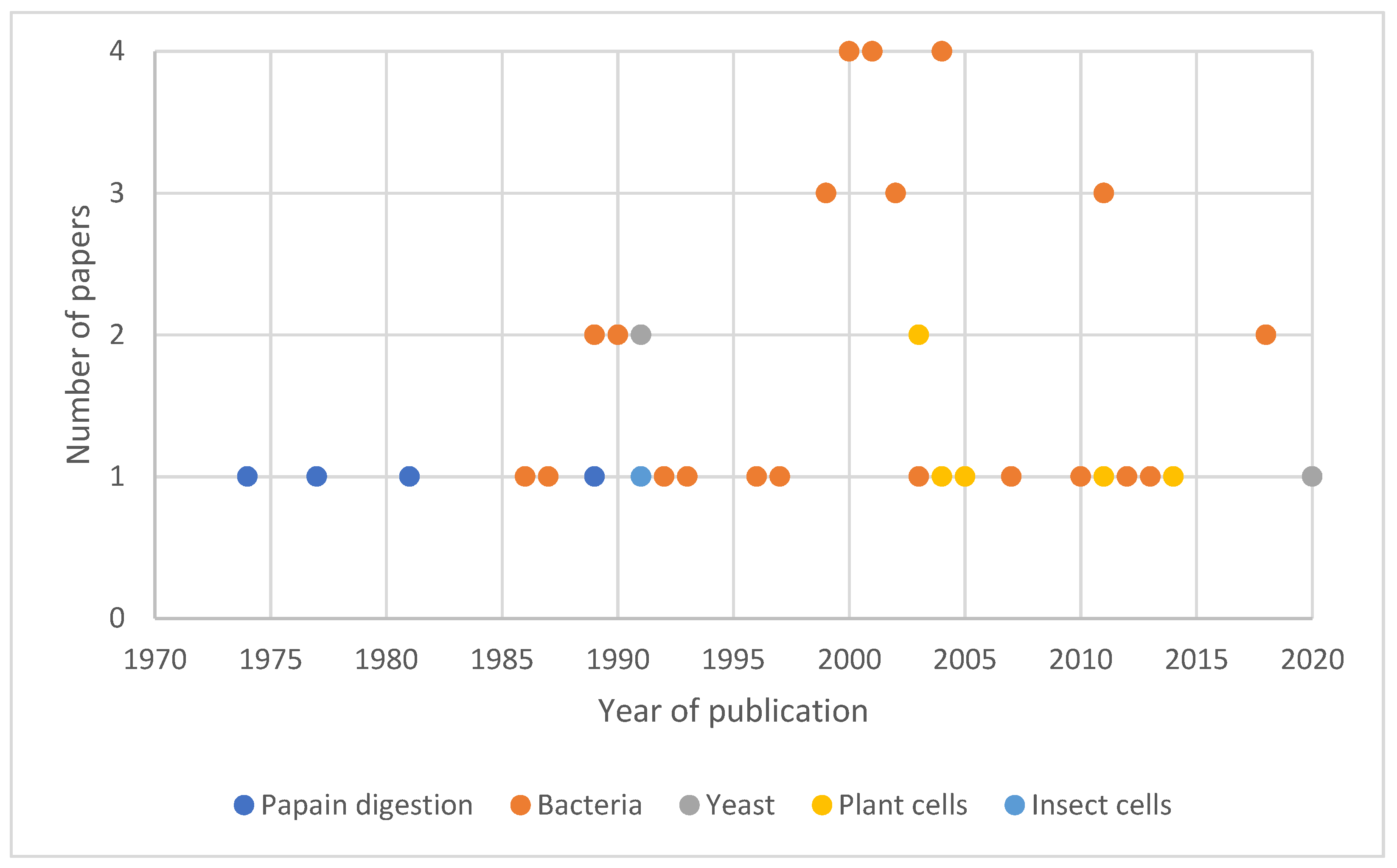
4. TTFC Production
4.1. Papain Digestion
4.2. TTFC Production in Recombinant Systems
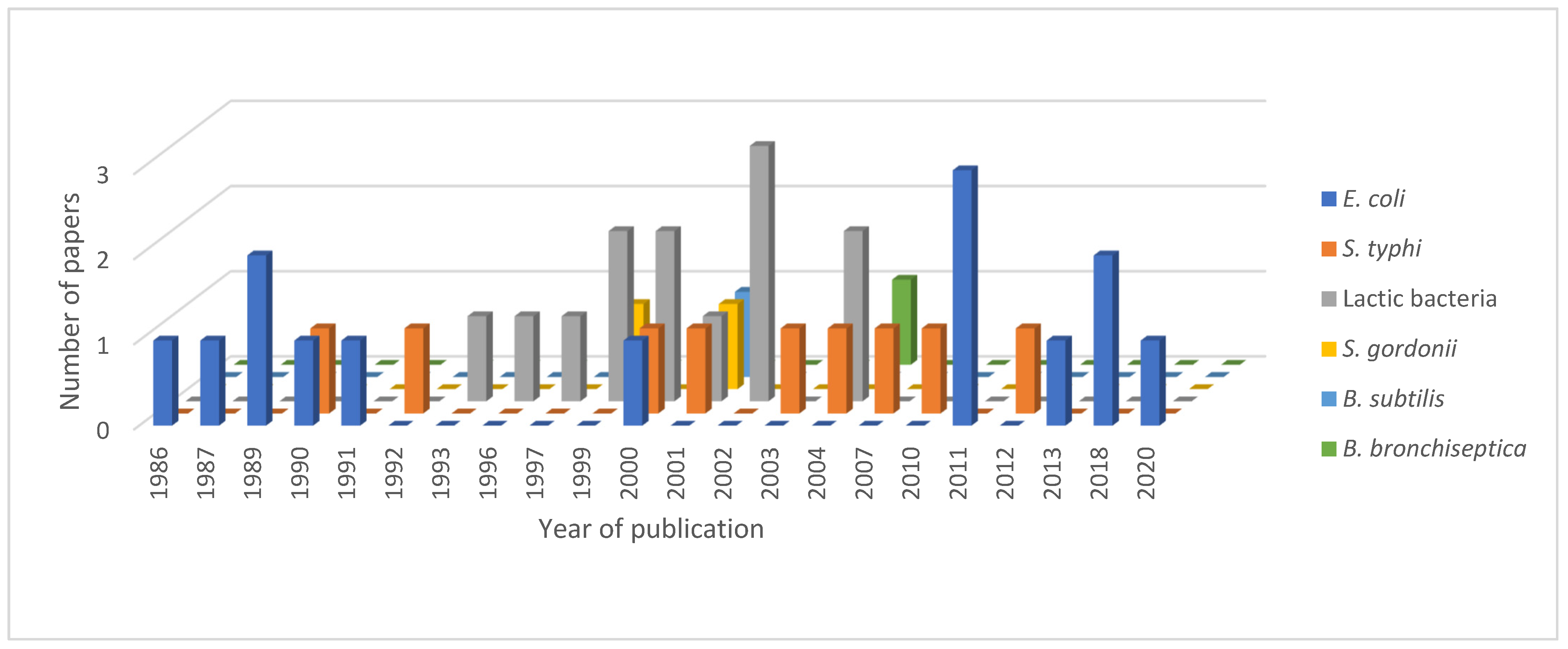
4.2.1. Escherichia coli as a Host for TTFC Production
4.2.2. TTFC Expression and Delivery in Other Bacterial Host Strains
4.2.3. Recombinant TTFC Expression in Yeast and Plant Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| BBB | blood brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CFU | colony-forming unit |
| CNS | central nervous system |
| CoaR | coagulase R domain |
| CTL | cytotoxic T lymphocyte |
| DA | dopamine |
| DOM | domain |
| EDL | extensor digitorum longus |
| FlaB | Flagellin B |
| FVB | Friend virus B |
| GDNF | glial cell line-derived neurotrophic factor |
| GFP | green fluorescence protein |
| GRAS | generally recognized as safe |
| HLA | human leukocyte antigen |
| HPV | human papillomavirus |
| HTL | helper T lymphocyte |
| IGF-1 | insulin-like growth factor-1 |
| LPS | lipopolysaccharide |
| mAb | monoclonal antibody |
| METH | methamphetamine |
| MEV | multiepitope vaccine |
| MN | motor neurons |
| NMJ | neuromuscular junction |
| NP | nanoparticles |
| NPY-Cre | neuropeptide Y-Cre recombinase |
| OPS | O-specific polysaccharide |
| PD | Parkinson’s disease |
| PEISH | thiolated poly(ethylene imine) |
| PI3K | phosphoinositide 3-kinase |
| PS | polysaccharide |
| PSMA | prostate-specific membrane antigen |
| SC | spinal cord |
| SOD | superoxide dismutase |
| SOL | soleus |
| Tem 1 | tumor endothelial marker 1 |
| TEVp | tobacco etch virus protease |
| TLR5 | Toll-like receptor 5 |
| TPC | total protein content |
| TTd | tetanus toxoid |
| TTFC | tetanus toxin fragment C |
| Trx | thioredoxin |
References
- Tetanus. Available online: https://ourworldindata.org/tetanus (accessed on 8 May 2022).
- Emsley, P.; Fotinou, C.; Black, I.; Fairweather, N.F.; Charles, I.G.; Watts, C.; Hewitt, E.; Isaacs, N.W. The structures of the H(C) fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. J. Biol. Chem. 2000, 275, 8889–8894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montecucco, C.; Schiavo, G. Mechanism of action of tetanus and botulinum neurotoxins. Mol. Microbiol. 1994, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deinhardt, K.; Berninghausen, O.; Willison, H.J.; Hopkins, C.R.; Schiavo, G. Tetanus toxin is internalized by a sequential clathrin-dependent mechanism initiated within lipid microdomains and independent of epsin1. J. Cell Biol. 2006, 174, 459–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisel, U.; Jarousch, W.; Goretzki, K.; Henschen, A.; Engels, J.; Weller, U.; Hudel, M.; Habermann, E.; Niemman, H. Tetanus toxin: Primary structure, expression in E. coli, and homology with botulinum toxins. EMBO J. 1986, 5, 2495–2502. [Google Scholar] [CrossRef]
- Matsuda, M.; Lei, D.L.; Sugimoto, N.; Ozutsumi, K.; Okabe, T. Isolation, purification, and characterization of fragment B, the NH2-terminal half of the heavy chain of tetanus toxin. Infect. Immun. 1989, 57, 3588–3593. [Google Scholar] [CrossRef] [Green Version]
- Helting, T.; Zwister, O. Enzymatic breakdown of tetanus toxin. Biochem. Biophys. Res. Commun. 1974, 57, 1263–1270. [Google Scholar] [CrossRef]
- Umland, T.C.; Wingert, L.M.; Swaminathan, S.; Furey, W.F.; Schmidt, J.J.; Sax, M. Structure of the receptor binding fragment HC of tetanus neurotoxin. Nat. Struct. Biol. 1997, 4, 788–792. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank. Available online: https://www.rcsb.org (accessed on 9 April 2022).
- Louch, H.A.; Buczko, E.S.; Woody, M.A.; Venable, R.M.; Vann, W.F. Identification of a binding site for ganglioside on the receptor binding domain of tetanus toxin. Biochemistry 2002, 41, 13644–13652. [Google Scholar] [CrossRef]
- Calvo, A.C.; Oliván, S.; Manzano, R.; Zaragoza, P.; Aguilera, J.; Osta, R. Fragment C of tetanus toxin: New insights into its neuronal signaling pathway. Int. J. Mol. Sci. 2012, 13, 6883–6901. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, S.; Eswaramoorthy, S.; Kumaran, D.; Swaminathan, S. Common binding site for disialyllactose and tri-peptide in C-fragment of tetanus neurotoxin. Proteins 2005, 61, 288–295. [Google Scholar] [CrossRef]
- Sinha, K.; Box, M.; Lalli, G.; Schiavo, G.; Schneider, H.; Groves, M.; Siligardi, G.; Fairweather, N.F. Analysis of mutants of tetanus toxin HC fragment: Ganglioside binding, cell binding and retrograde axonal transport properties. Mol. Microbiol. 2000, 37, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fu, Z.; Kim, J.-J.P.; Barbieri, J.T.; Baldwin, M.R. Gangliosides as high affinity receptors for tetanus neurotoxin. J. Biol. Chem. 2009, 284, 26569–26577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Baldwin, M.R.; Barbieri, J.T. Molecular basis for tetanus toxin coreceptor interactions. Biochemistry 2008, 47, 7179–7186. [Google Scholar] [CrossRef] [PubMed]
- Fotinou, C.; Emsley, P.; Black, I.; Ando, H.; Ishida, H.; Kiso, M.; Sinha, K.A.; Fairweather, N.F.; Isaacs, N.W. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J. Biol. Chem. 2001, 276, 32274–32281. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, C.R.; Hirama, T.; Lee, K.K.; Altman, E.; Young, N.M. Quantitative analysis of bacterial toxin affinity and specificity for glycolipid receptors by surface plasmon resonance. J. Biol. Chem. 1997, 272, 5533–5538. [Google Scholar] [CrossRef] [Green Version]
- Montecucco, C. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem. Sci. 1986, 11, 314–317. [Google Scholar] [CrossRef]
- Herreros, J.; Lalli, G.; Schiavo, G. C-terminal half of tetanus toxin fragment C is sufficient for neuronal binding and interaction with a putative protein receptor. Biochem. J. 2000, 347 Pt 1, 199–204. [Google Scholar] [CrossRef]
- Vajn, K.; Viljetić, B.; Degmečić, I.V.; Schnaar, R.L.; Heffer, M. Differential distribution of major brain gangliosides in the adult mouse central nervous system. PLoS ONE 2013, 8, e75720. [Google Scholar] [CrossRef] [Green Version]
- Toivonen, J.M.; Oliván, S.; Osta, R. Tetanus toxin C-fragment: The courier and the cure? Toxins 2010, 2, 2622–2644. [Google Scholar] [CrossRef] [Green Version]
- Gramlich, P.A.; Remington, M.P.; Amin, J.; Betenbaugh, M.J.; Fishman, P.S. Tat-tetanus toxin fragment C: A novel protein delivery vector and its use with photochemical internalization. J. Drug Target. 2013, 21, 662–674. [Google Scholar] [CrossRef]
- Francis, J.W.; Bastia, E.; Matthews, C.C.; Parks, D.A.; Schwarzschild, M.A.; Brown, R.H.; Fishman, P.S. Tetanus toxin fragment C as a vector to enhance delivery of proteins to the CNS. Brain Res. 2004, 1011, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Benn, S.C.; Ay, I.; Bastia, E.; Chian, R.-J.; Celia, S.A.; Pepinsky, R.B.; Fishman, P.S.; Brown, R.H.; Francis, J.W. Tetanus toxin fragment C fusion facilitates protein delivery to CNS neurons from cerebrospinal fluid in mice. J. Neurochem. 2005, 95, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Bordet, T.; Castelnau-Ptakhine, L.; Fauchereau, F.; Friocourt, G.; Kahn, A.; Haase, G. Neuronal targeting of cardiotrophin-1 by coupling with tetanus toxin C fragment. Mol. Cell. Neurosci. 2001, 17, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Kissa, K.; Mordelet, E.; Soudais, C.; Kremer, E.J.; Demeneix, B.A.; Brûlet, P.; Coen, L. In vivo neuronal tracing with GFP-TTC gene delivery. Mol. Cell. Neurosci. 2002, 20, 627–637. [Google Scholar] [CrossRef]
- Miana-Mena, F.J.; Roux, S.; Benichou, J.-C.; Osta, R.; Brûlet, P. Neuronal activity-dependent membrane traffic at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2002, 99, 3234–3239. [Google Scholar] [CrossRef] [Green Version]
- Kassa, R.; Monterroso, V.; David, L.L.; Tshala-Katumbay, D. Diagnostic and therapeutic potential of tetanus toxin-derivatives in neurological diseases. J. Mol. Neurosci. 2013, 51, 788–791. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Igoa, M.; Calvo, A.C.; Penas, C.; Manzano, R.; Oliván, S.; Muñoz, M.J.; Mancuso, R.; Zaragoza, P.; Aguilera, J.; Navarro, X.; et al. Fragment C of tetanus toxin, more than a carrier. Novel perspectives in non-viral ALS gene therapy. J. Mol. Med. 2010, 88, 297–308. [Google Scholar] [CrossRef]
- Mendieta, L.; Bautista, E.; Sánchez, A.; Guevara, J.; Herrando-Grabulosa, M.; Moran, J.; Martínez, R.; Aguilera, J.; Limón, I.D. The C-terminal domain of the heavy chain of tetanus toxin given by intramuscular injection causes neuroprotection and improves the motor behavior in rats treated with 6-hydroxydopamine. Neurosci. Res. 2012, 74, 156–167. [Google Scholar] [CrossRef]
- Chaïb-Oukadour, I.; Gil, C.; Rodríguez-Alvarez, J.; Ortega, A.; Aguilera, J. Tetanus toxin HC fragment reduces neuronal MPP+ toxicity. Mol. Cell. Neurosci. 2009, 41, 297–303. [Google Scholar] [CrossRef]
- Chaïb-Oukadour, I.; Gil, C.; Aguilera, J. The C-terminal domain of the heavy chain of tetanus toxin rescues cerebellar granule neurones from apoptotic death: Involvement of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. J. Neurochem. 2004, 90, 1227–1236. [Google Scholar] [CrossRef]
- Mendieta, L.; Venegas, B.; Moreno, N.; Patricio, A.; Martínez, I.; Aguilera, J.; Limón, I.D. The carboxyl-terminal domain of the heavy chain of tetanus toxin prevents dopaminergic degeneration and improves motor behavior in rats with striatal MPP+-lesions. Neurosci. Res. 2009, 65, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.; Chaib-Oukadour, I.; Aguilera, J. C-terminal fragment of tetanus toxin heavy chain activates Akt and MEK/ERK signalling pathways in a Trk receptor-dependent manner in cultured cortical neurons. Biochem. J. 2003, 373 Pt 2, 613–620. [Google Scholar] [CrossRef]
- Cubí, R.; Candalija, A.; Ortega, A.; Gil, C.; Aguilera, J. Tetanus toxin Hc fragment induces the formation of ceramide platforms and protects neuronal cells against oxidative stress. PLoS ONE 2013, 8, e68055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radenovic, L.; Selakovic, V.; Olivan, S.; Calvo, A.C.; Rando, A.; Janac, B.; Osta, R. Neuroprotective efficiency of tetanus toxin C fragment in model of global cerebral ischemia in Mongolian gerbils. Brain Res. Bull. 2014, 101, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Martinez, L.; de la Torre, M.; Muñoz, M.J.; Zaragoza, P.; Aguilera, J.; Calvo, A.C.; Osta, R. Neuroprotective fragment C of tetanus toxin modulates IL-6 in an ALS mouse model. Toxins 2020, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Getachew, B.; Mendieta, L.; Csoka, A.B.; Aguilera, J.; Tizabi, Y. Antidepressant effects of C-terminal domain of the heavy chain of tetanus toxin in a rat model of depression. Behav. Brain Res. 2019, 370, 111968. [Google Scholar] [CrossRef] [PubMed]
- Netzahualcoyotzi, C.; Tapia, R. Tetanus toxin C-fragment protects against excitotoxic spinal motoneuron degeneration in vivo. Sci. Rep. 2018, 8, 16584. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Galarza, N.; Mendieta, L.; Palafox-Sánchez, V.; Herrando-Grabulosa, M.; Gil, C.; Limón, D.I.; Aguilera, J. Peripheral administration of tetanus toxin Hc fragment prevents MPP+ toxicity In vivo. Neurotox. Res. 2018, 34, 47–61. [Google Scholar] [CrossRef]
- Patricio-Martínez, A.; Mendieta, L.; Martínez, I.; Aguilera, J.; Limón, I.D. The recombinant C-terminal fragment of tetanus toxin protects against cholinotoxicity by intraseptal injection of β-amyloid peptide (25–35) in rats. Neuroscience 2016, 315, 18–30. [Google Scholar] [CrossRef]
- Mendieta, L.; Granado, N.; Aguilera, J.; Tizabi, Y.; Moratalla, R. Fragment C domain of tetanus toxin mitigates methamphetamine neurotoxicity and its motor consequences in mice. Int. J. Neuropsychopharmacol. 2016, 19, pyw021. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-González, A.; Mendieta, L.; Palafox, V.; Candalija, A.; Luna, F.; Aguilera, J.; Limón, I.D. The restorative effect of intramuscular injection of tetanus toxin C-fragment in hemiparkinsonian rats. Neurosci. Res. 2014, 84, 1–9. [Google Scholar] [CrossRef]
- Bohne, P.; Schwarz, M.K.; Herlitze, S.; Mark, M.D. A new projection from the deep cerebellar nuclei to the hippocampus via the ventrolateral and laterodorsal thalamus in mice. Front. Neural Circuits 2019, 13, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Igoa, M.; Calvo, A.C.; Jesús, C.; Muñoz, M.J.; Zaragoza, P.; Rosario, O. Non-viral gene delivery of the GDNF, either alone or fused to the C-fragment of tetanus toxin protein, prolongs survival in a mouse ALS model. Restor. Neurol. Neurosci. 2012, 30, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bráz, J.M.; Basbaum, A.I. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience 2009, 163, 1220–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.M.; Messi, M.L.; Zheng, Z.; Delbono, O. Motor neuron targeting of IGF-1 attenuates age-related external Ca2+-dependent skeletal muscle contraction in senescent mice. Exp. Gerontol. 2007, 42, 309–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, M.C.; Bernier, A.P.; Renaud, J.S.; Roux, S.; Glover, J.C. C fragment of tetanus toxin hybrid proteins evaluated for muscle-specific transsynaptic mapping of spinal motor circuitry in the newborn mouse. Neuroscience 2006, 141, 803–816. [Google Scholar] [CrossRef]
- Lee, P.J.; Kennedy, Z.; Wang, Y.; Lu, Y.; Cefaliello, C.; Uyan, Ö.; Song, C.Q.; da Cruz Godinho, B.M.; Xu, Z.; Rusckowski, M.; et al. Imaging net retrograde axonal transport in vivo: A physiological biomarker. Ann. Neurol. 2022, 91, 716–729. [Google Scholar] [CrossRef]
- Lopes, C.D.; Oliveira, H.; Estevão, I.; Pires, L.R.; Pêgo, A.P. In vivo targeted gene delivery to peripheral neurons mediated by neurotropic poly(ethylene imine)-based nanoparticles. Int. J. Nanomed. 2016, 11, 2675–2683. [Google Scholar] [CrossRef] [Green Version]
- Larsen, K.E.; Benn, S.C.; Ay, I.; Chian, R.-J.; Celia, S.A.; Remington, M.P.; Bejarano, M.; Liu, M.; Ross, J.; Carmillo, P.; et al. A glial cell line-derived neurotrophic factor (GDNF): Tetanus toxin fragment C protein conjugate improves delivery of GDNF to spinal cord motor neurons in mice. Brain Res. 2006, 1120, 1–12. [Google Scholar] [CrossRef]
- Reece, J.C.; Geysen, H.M.; Rodda, S.J. Mapping the major human T helper epitopes of tetanus toxin. The emerging picture. J. Immunol. 1993, 151, 6175–6184. [Google Scholar]
- Diethelm-Okita, B.M.; Okita, D.K.; Banaszak, L.; Conti-Fine, B.M. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J. Infect. Dis. 2000, 181, 1001–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diethelm-Okita, B.M.; Raju, R.; Okita, D.K.; Contl-Fine, B.M. Epitope repertoire of human CD4+ T cells on tetanus toxin: Identification of immunodominant sequence segments. J. Infect. Dis. 1997, 175, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valmori, D.; Pessi, A.; Bianchi, E.; Corradin, G. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J. Immunol. 1992, 149, 717–721. [Google Scholar] [PubMed]
- Panina-Bordignon, P.; Tan, A.; Termijtelen, A.; Demotz, S.; Corradin, G.; Lanzavecchia, A. Universally immunogenic T cell epitopes: Promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 1989, 19, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Biradhar, N.; Nimmagadda, S.V.; Aavula, S.M.; Parthasarathy, S.; Sula, S.; Maithal, K. Identification and characterization of novel binding epitope of tetanus toxoid by phage display peptide library. Curr. Trends Biotechnol. Pharm. 2015, 9, 49–58. [Google Scholar]
- Nezafat, N.; Ghasemi, Y.; Javadi, G.; Khoshnoud, M.J.; Omidinia, E. A novel multi-epitope peptide vaccine against cancer: An in silico approach. J. Theor. Biol. 2014, 349, 121–134. [Google Scholar] [CrossRef]
- James, E.A.; Bui, D.; Berger, J.; Huston, L.; Roti, M.; Kwok, W.W. Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. Int. Immunol. 2007, 19, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Ghafari-Khamene, M.; Torabi-Goudarzi, S.; Hosseini, M.; Haji-Fatahaliha, M.; Sadreddini, S.; Seyfi-Najmi, M.; Majidi, J.; Yousefi, M. Response of human T cells to tetanus neurotoxin HCC sub-domain: T cell cytokine production and activation marker induced by HCC. Iran. J. Allergy Asthma Immunol. 2015, 14, 519–525. [Google Scholar]
- Yousefi, M.; Younesi, V.; Bayat, A.A.; Jadidi-Niaragh, F.; Abbasi, E.; Razavi, A.; Khosravi-Eghbal, R.; Asgarin-Omran, H.; Shokri, F. Comparative human and mouse antibody responses against tetanus toxin at clonal level. J. Immunotoxicol. 2016, 13, 243–248. [Google Scholar] [CrossRef]
- Volk, W.A.; Bizzini, B.; Snyder, R.M.; Bernhard, E.; Wagner, R.R. Neutralization of tetanus toxin by distinct monoclonal antibodies. Binding to multiple epitopes on the toxin molecule. Infect. Immun. 1984, 45, 604–609. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Kamei, M.; Sugimoto, N.; Ma, Y.; Hashizume, S. Characteristics of toxin-neutralization by anti-tetanus human monoclonal antibodies directed against the three functional domains [A], [B] and [C] of the tetanus toxin molecule and a reliable method for evaluating the protective effects of monoclonal antibodies. Eur. J. Epidemiol. 1992, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, C.; Yu, J.; Lin, S.; Liu, T.; Zan, L.; Li, N.; Hong, P.; Wang, X.; Jia, Z.; et al. Structural basis of tetanus toxin neutralization by native human monoclonal antibodies. Cell Rep. 2021, 35, 109070. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Marinković, E.; Filipović, A.; Krnjaja, O.; Kosanović, D.; Inić-Kanada, A.; Stojanović, M. Key protection factors against tetanus: Anti-tetanus toxin antibody affinity and its ability to prevent tetanus toxin—Ganglioside interaction. Toxicon 2015, 103, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ghotloo, S.; Golsaz-Shirazi, F.; Amiri, M.M.; Jeddi-Tehrani, M.; Shokri, F. Epitope mapping of tetanus toxin by monoclonal antibodies: Implication for immunotherapy and vaccine design. Neurotox. Res. 2020, 37, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, N.F.; Lyness, V.A.; Maskell, D.J. Immunization of mice against tetanus with fragments of tetanus toxin synthesized in Escherichia coli. Infect. Immun. 1987, 55, 2541–2545. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Qin, L.; Mao, X.; Chen, L.; Yu, S.; Li, Q.; Liu, W.; Zhang, W.; Gu, J.; Zou, Q. Identification of a novel linear epitope in tetanus toxin recognized by a protective monoclonal antibody: Implications for vaccine design. Vaccine 2012, 30, 6449–6455. [Google Scholar] [CrossRef]
- Johnston, L.; Mawas, F.; Tierney, R.; Qazi, O.; Fairweather, N.F.; Sesardic, D. Transcutaneous delivery of tetanus toxin Hc fragment induces superior tetanus toxin neutralizing antibody response compared to tetanus toxoid. Hum. Vaccin. 2009, 5, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Qazi, O.; Sesardic, D.; Tierney, R.; Soderback, Z.; Crane, D.; Bolgiano, B.; Fairweather, N.F. Reduction of the ganglioside binding activity of the tetanus toxin HC fragment destroys immunogenicity: Implications for development of novel tetanus vaccines. Infect. Immun. 2006, 74, 4884–4891. [Google Scholar] [CrossRef] [Green Version]
- Amuguni, J.H.; Lee, S.; Kerstein, K.O.; Brown, D.W.; Belitsky, B.R.; Herrmann, J.E.; Keusch, G.T.; Sonenshein, A.L.; Tzipori, S. Sublingually administered Bacillus subtilis cells expressing tetanus toxin C fragment induce protective systemic and mucosal antibodies against tetanus toxin in mice. Vaccine 2011, 29, 4778–4784. [Google Scholar] [CrossRef]
- Fishman, P.S.; Matthews, C.C.; Parks, D.A.; Box, M.; Fairweather, N.F. Immunization does not interfere with uptake and transport by motor neurons of the binding fragment of tetanus toxin. J. Neurosci. Res. 2006, 83, 1540–1543. [Google Scholar] [CrossRef]
- Ramakrishnan, G.; Wright, M.; Alam, M.; Naylor, C.; Kabir, M.; Zerin, A.; Ferdous, T.; Pedersen, K.; Hennig, B.J.; Donowitz, J.R.; et al. Rapid assessment of tetanus vaccine-induced immunity in Bangladesh and the Gambia. Diagn. Microbiol. Infect. Dis. 2017, 87, 272–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, W.W.; Baker, M.C.; Berenberg, T.L.; Lu, M.C.; Yannie, P.J.; Udey, M.C. Enhancement of DNA tumor vaccine efficacy by gene gun-mediated codelivery of threshold amounts of plasmid-encoded helper antigen. Blood 2009, 113, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henken, F.E.; Oosterhuis, K.; Öhlschläger, P.; Bosch, L.; Hooijberg, E.; Haanen, J.B.A.G.; Steenbergen, R.D.M. Preclinical safety evaluation of DNA vaccines encoding modified HPV16 E6 and E7. Vaccine 2012, 30, 4259–4266. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, K.; Öhlschläger, P.; Van den Berg, J.H.; Teebs, M.; Gomez, R.; Schumacher, T.N.; Haanen, J.B. Preclinical development of highly effective and safe DNA vaccines directed against HPV 16 E6 and E7. Int. J. Cancer 2011, 129, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Jahouh, F.; Xu, P.; Vann, W.F.; Kováč, P.; Banoub, J.H. Mapping the glycation sites in the neoglycoconjugate from hexasaccharide antigen of Vibrio cholerae, serotype Ogawa and the recombinant tetanus toxin C-fragment carrier: Glycations sites of rTT-Hc neoglycoconjugates. J. Mass Spectrom. 2013, 48, 1083–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, P.C.; Saksena, R.; Peterson, D.C.; Lee, C.H.; An, Y.; Cipollo, J.F.; Vann, W.F. Chemoenzymatic synthesis of immunogenic meningococcal group C polysialic acid-tetanus Hc fragment glycoconjugates. Glycoconj. J. 2013, 30, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.E.; Ngugi, S.A.; Laws, T.R.; Corser, D.; Lonsdale, C.L.; D’Elia, R.V.; Titball, R.W.; Williamson, E.D.; Atkins, T.P.; Prior, J.L. Protection against experimental melioidosis following immunisation with a lipopolysaccharide-protein conjugate. J. Immunol. Res. 2014, 2014, 392170. [Google Scholar] [CrossRef] [Green Version]
- Sayeed, M.A.; Bufano, M.K.; Xu, P.; Eckhoff, G.; Charles, R.C.; Alam, M.M.; Sultana, T.; Rashu, M.R.; Berger, A.; Gonzalez-Escobedo, G.; et al. A Cholera conjugate vaccine containing O-specific polysaccharide (OSP) of V. cholerae O1 Inaba and recombinant fragment of tetanus toxin heavy chain (OSP:rTTHc) induces serum, memory and lamina proprial responses against OSP and is protective in mice. PLoS Negl. Trop. Dis. 2015, 9, e0003881. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Kelly, M.; Vann, W.F.; Qadri, F.; Ryan, E.T.; Kováč, P. Conjugate vaccines from bacterial antigens by squaric acid chemistry: A closer look. ChemBioChem 2017, 18, 799–815. [Google Scholar] [CrossRef]
- Karkhah, A.; Saadi, M.; Nouri, H.R. In silico analyses of heat shock protein 60 and calreticulin to designing a novel vaccine shifting immune response toward T helper 2 in atherosclerosis. Comput. Biol. Chem. 2017, 67, 244–254. [Google Scholar] [CrossRef]
- Saadi, M.; Karkhah, A.; Nouri, H.R. Development of a multi-epitope peptide vaccine inducing robust T cell responses against brucellosis using immunoinformatics based approaches. Infect. Genet. Evol. 2017, 51, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Validi, M.; Karkhah, A.; Prajapati, V.K.; Nouri, H.R. Immuno-informatics based approaches to design a novel multi epitope-based vaccine for immune response reinforcement against leptospirosis. Mol. Immunol. 2018, 104, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Kefayat, A.; Abiri, A.; Mahdevar, E.; Behnia, A.H.; Ghahremani, F. In silico analysis of transmembrane protein 31 (TMEM31) antigen to design novel multiepitope peptide and DNA cancer vaccines against melanoma. Mol. Immunol. 2019, 112, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nezafat, N.; Sadraeian, M.; Rahbar, M.R.; Khoshnoud, M.J.; Mohkam, M.; Gholami, A.; Banihashemi, M.; Ghasemi, Y. Production of a novel multi-epitope peptide vaccine for cancer immunotherapy in TC-1 tumor-bearing mice. Biologicals 2015, 43, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Ji, C.; Xu, J.; Wang, D.; Fang, T.; Jing, Y.; Kwang-Fu Shen, C.; Chen, W. The immunogenicity of the C fragment of tetanus neurotoxin in production of tetanus antitoxin. Biomed. Res. Int. 2018, 2018, 6057348. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.; Zhao, T.; Li, R.; Yang, Q.; Yu, R.; Yin, Y.; Zai, X.; Li, Y.; Zhang, J.; Xu, J.; et al. Targeting the R domain of coagulase by active vaccination protects mice against lethal Staphylococcus aureus infection. Microbes Infect. 2019, 21, 163–169. [Google Scholar] [CrossRef]
- Lee, S.E.; Nguyen, C.T.; Kim, S.Y.; Thi, T.N.; Rhee, J.H. Tetanus toxin fragment C fused to flagellin makes a potent mucosal vaccine. Clin. Exp. Vaccine Res. 2015, 4, 59–67. [Google Scholar] [CrossRef] [Green Version]
- McCann, K.J.; Godeseth, R.; Chudley, L.; Mander, A.; Di Genova, G.; Lloyd-Evans, P.; Kerr, J.P.; Malykh, V.B.; Jenner, M.W.; Orchard, K.H.; et al. Idiotypic DNA vaccination for the treatment of multiple myeloma: Safety and immunogenicity in a phase I clinical study. Cancer Immunol. Immunother. 2015, 64, 1021–1032. [Google Scholar] [CrossRef] [Green Version]
- Facciponte, J.G.; Ugel, S.; De Sanctis, F.; Li, C.; Wang, L.; Nair, G.; Sehgal, S.; Raj, A.; Matthaiou, E.; Coukos, G.; et al. Tumor endothelial marker 1–specific DNA vaccination targets tumor vasculature. J. Clin. Investig. 2014, 124, 1497–1511. [Google Scholar] [CrossRef] [Green Version]
- Chudley, L.; McCann, K.; Mander, A.; Tjelle, T.; Campos-Perez, J.; Godeseth, R.; Creak, A.; Dobbyn, J.; Johnson, B.; Bass, P.; et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8+ T-cell responses and increases PSA doubling time. Cancer Immunol. Immunother. 2012, 61, 2161–2170. [Google Scholar] [CrossRef] [Green Version]
- Iurescia, S.; Fioretti, D.; Pierimarchi, P.; Signori, E.; Zonfrillo, M.; Tonon, G.; Fazio, V.M.; Rinaldi, M. Genetic immunization with CDR3-based fusion vaccine confers protection and long-term tumor-free survival in a mouse model of lymphoma. J. Biomed. Biotechnol. 2010, 2010, 316069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez, A.J.; McNair, N.; Mead, J.R. Oral immunization with attenuated Salmonella enterica serovar Typhimurium encoding Cryptosporidium parvum Cp23 and Cp40 antigens induces a specific immune response in mice. Clin. Vaccine Immunol. 2009, 16, 1272–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, R.; Xu, J.; Hu, T.; Chen, W. The pneumococcal polysaccharide-tetanus toxin native C-fragment conjugate vaccine: The carrier effect and immunogenicity. Mediators Inflamm. 2020, 2020, e9596129. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, R.; Yang, X.; Liu, S.; Fang, T.; Song, X.; Hou, L.; Yu, C.; Xu, J.; Fu, L.; et al. Protection against Staphylococcus aureus and tetanus infections by a combined vaccine containing SasA and TeNT-Hc in mice. Mol. Med. Rep. 2017, 15, 2369–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotprakaikiat, W.; Allen, A.; Bui-Minh, D.; Harden, E.; Jobsri, J.; Cavallo, F.; Gleba, Y.; Stevenson, F.K.; Ottensmeier, C.; Klimyuk, V.; et al. A plant-expressed conjugate vaccine breaks CD4(+) tolerance and induces potent immunity against metastatic Her2(+) breast cancer. Oncoimmunology 2016, 5, e1166323. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.E.; Christ, W.J.; George, A.J.; Stokes, M.G.; Lohman, G.J.; Guo, Y.; Jones, M.; Titball, R.W.; Atkins, T.P.; Campbell, A.S.; et al. Protection against experimental melioidosis with a synthetic manno-heptopyranose hexasaccharide glycoconjugate. Bioconjug. Chem. 2016, 27, 1435–1446. [Google Scholar] [CrossRef] [Green Version]
- Tierney, R.; Nakai, T.; Parkins, C.J.; Caposio, P.; Fairweather, N.F.; Sesardic, D.; Jarvis, M.A. A single-dose cytomegalovirus-based vaccine encoding tetanus toxin fragment C induces sustained levels of protective tetanus toxin antibodies in mice. Vaccine 2012, 30, 3047–3052. [Google Scholar] [CrossRef]
- Murzello, K.; Kaundinya, J.O.; Dandekar, S. Simplified method for purification of C-fragment from tetanus toxin and toxoid by enzymatic fragmentation and chromatography. Indo Am. J. Pharm. Res. 2014, 4, 4060–4066. [Google Scholar]
- Weller, U.; Dauzenroth, M.E.; Meyer zu Reindorf, D.; Habermann, E. Chains and fragments of tetanus toxin. Separation, reassociation and pharmacological properties. Eur. J. Biochem. 1989, 182, 649–656. [Google Scholar] [CrossRef]
- Neubauer, V.; Helting, T.B. Structure of tetanus toxin: The arrangement of papain digestion products within the heavy chain-light chain framework of extracellular toxin. Biochim. Biophys. Acta 1981, 668, 141–148. [Google Scholar] [CrossRef]
- Helting, T.B.; Zwisler, O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J. Biol. Chem. 1977, 252, 187–193. [Google Scholar] [CrossRef]
- Makoff, A.J.; Ballantine, S.P.; Smallwood, A.E.; Fairweather, N.F. Expression of tetanus toxin fragment C in E. coli: Its purification and potential use as a vaccine. Nat. Biotechnol. 1989, 7, 1043–1046. [Google Scholar] [CrossRef]
- Halpern, J.L.; Habig, W.H.; Neale, E.A.; Stibitz, S. Cloning and expression of functional fragment C of tetanus toxin. Infect. Immun. 1990, 58, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, N.F.; Lyness, V.A.; Pickard, D.J.; Allen, G.; Thomson, R.O. Cloning, nucleotide sequencing, and expression of tetanus toxin fragment C in Escherichia coli. J. Bacteriol. 1986, 165, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefi, M.; Khosravi-Eghbal, R.; Hemmati, A.; Shokri, F. Production and characterization of recombinant light chain and carboxyterminal heavy chain fragments of tetanus toxin. Avicenna J. Med. Biotechnol. 2013, 5, 220–226. [Google Scholar]
- Yu, Y.Z.; Gong, Z.W.; Ma, Y.; Zhang, S.M.; Zhu, H.Q.; Wang, W.B.; Du, Y.; Wang, S.; Yu, W.Y.; Sun, Z.W. Co-expression of tetanus toxin fragment C in Escherichia coli with thioredoxin and its evaluation as an effective subunit vaccine candidate. Vaccine 2011, 29, 5978–5985. [Google Scholar] [CrossRef]
- Motamedi, H.; Seyfiabad Shapouri, M.R.; Ghorbanpour Najafabadi, M.; Arefzadeh, N. Cloning and expression of tetanus toxin C fragment (Fc) in prokaryotic vector for constructing recombinant protein based vaccine for tetanus. Iran. J. Vet. Res. 2011, 12, 107–112. [Google Scholar] [CrossRef]
- Yu, R.; Hou, L.; Yu, C.; Liu, S.; Ren, J.; Fang, T.; Zhang, X.; Chen, W. Enhanced expression of soluble recombinant tetanus neurotoxin Hc in Escherichia coli as a tetanus vaccine candidate. Immunobiology 2011, 216, 485–490. [Google Scholar] [CrossRef]
- Makoff, A.J.; Romanos, M.A.; Oxer, M.D.; Fairweather, N.F.; Ballantine, S.P. Expression of tetanus toxin fragment C in E. coli: High level expression by removing rare codons. Nucleic Acids Res. 1989, 17, 10191–10202. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A.V.; Ho, P.L.; Tanizaki, M.M.; Raw, I.; Nascimento, A.L.T.O. High-level expression of tetanus toxin fragment C–thioredoxin fusion protein in Escherichia coli. Biotechnol. Appl. Biochem. 2000, 31, 91–94. [Google Scholar] [CrossRef]
- Aghayipour, K.; Teymourpour, R. High-level expression of tetanus toxin fragment C in Escherichia coli. Arch. Razi Inst. 2018, 73, 27–38. [Google Scholar] [CrossRef]
- Koopaei, N.N.; Khadiv-Parsi, P.; Khoshayand, M.R.; Mazlomi, M.A.; Kebriaeezadeh, A.; Moloudian, H.; Solhi, R.; Aminian, M. Optimization of rPDT fusion protein expression by Escherichia coli in pilot scale fermentation: A statistical experimental design approach. AMB Express 2018, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Betterle, N.; Hidalgo Martinez, D.; Melis, A. Recombinant protein stability in cyanobacteria. ACS Synth. Biol. 2021, 10, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo Martinez, D.; Betterle, N.; Melis, A. Phycocyanin fusion constructs for heterologous protein expression accumulate as functional heterohexameric complexes in cyanobacteria. ACS Synth. Biol. 2022, 11, 1152–1166. [Google Scholar] [CrossRef]
- Levine, M.M. Immunogenicity and efficacy of oral vaccines in developing countries: Lessons from a live cholera vaccine. BMC Biol. 2010, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Maassen, C.B.; Laman, J.D.; den Bak-Glashouwer, M.J.; Tielen, F.J.; van Holten-Neelen, J.C.; Hoogteijling, L.; Antonissen, C.; Leer, R.J.; Pouwels, P.H.; Boersma, W.J.; et al. Instruments for oral disease-intervention strategies: Recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine 1999, 17, 2117–2128. [Google Scholar] [CrossRef]
- Reveneau, N.; Geoffroy, M.C.; Locht, C.; Chagnaud, P.; Mercenier, A. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 2002, 20, 1769–1777. [Google Scholar] [CrossRef]
- Robinson, K.; Chamberlain, L.M.; Schofield, K.M.; Wells, J.M.; Le Page, R.W. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 1997, 15, 653–657. [Google Scholar] [CrossRef]
- Grangette, C.; Muller-Alouf, H.; Hols, P.; Goudercourt, D.; Delcour, J.; Turneer, M.; Mercenier, A. Enhanced mucosal delivery of antigen with cell wall mutants of lactic acid bacteria. Infect. Immun. 2004, 72, 2731–2737. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Chamberlain, L.M.; Lopez, M.C.; Rush, C.M.; Marcotte, H.; Le Page, R.W.F.; Wells, J.M. Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect. Immun. 2004, 72, 2753–2761. [Google Scholar] [CrossRef] [Green Version]
- Grangette, C.; Müller-Alouf, H.; Geoffroy, M.; Goudercourt, D.; Turneer, M.; Mercenier, A. Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: Impact of strain viability and in vivo persistence. Vaccine 2002, 20, 3304–3309. [Google Scholar] [CrossRef]
- Shaw, D.M.; Gaerthé, B.; Leer, R.J.; Van Der Stap, J.G.M.M.; Smittenaar, C.; Heijne Den Bak-Glashouwer, M.J.; Thole, J.E.R.; Tielen, F.J.; Pouwels, P.H.; Havenith, C.E.G. Engineering the microflora to vaccinate the mucosa: Serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 2000, 100, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Norton, P.M.; Brown, H.W.; Wells, J.M.; Macpherson, A.M.; Wilson, P.W.; Le Page, R.W. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol. Med. Microbiol. 1996, 14, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Wilson, P.W.; Norton, P.M.; Gasson, M.J.; Le Page, R.W. Lactococcus lactis: High-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol. Microbiol. 1993, 8, 1155–1162. [Google Scholar] [CrossRef]
- Yang, X.Q.; Zhao, Y.G.; Chen, X.Q.; Jiang, B.; Sun, D.Y. The protective effect of recombinant Lactococcus lactis oral vaccine on a Clostridium difficile-infected animal model. BMC Gastroenterol. 2013, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.I.; Kim, J.S.; Eom, J.S.; Kim, H.G.; Kim, B.H.; Lim, S.; Bang, I.S.; Park, Y.K. Expression and delivery of tetanus toxin fragment C fused to the N-terminal domain of SipB enhances specific immune responses in mice. Microbiol. Immunol. 2012, 56, 595–604. [Google Scholar] [CrossRef]
- Mazzantini, R.P.; Miyaji, E.N.; Dias, W.O.; Sakauchi, D.; Nascimento, A.L.T.O.; Raw, I.; Winter, N.; Gicquel, B.; Rappuoli, R.; Leite, L.C.C. Adjuvant activity of Mycobacterium bovis BCG expressing CRM197 on the immune response induced by BCG expressing tetanus toxin fragment C. Vaccine 2004, 22, 740–746. [Google Scholar] [CrossRef]
- Medaglini, D.; Ciabattini, A.; Spinosa, M.R.; Maggi, T.; Marcotte, H.; Oggioni, M.R.; Pozzi, G. Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine 2001, 19, 1931–1939. [Google Scholar] [CrossRef]
- Corinti, S.; Medaglini, D.; Cavani, A.; Rescigno, M.; Pozzi, G.; Ricciardi-Castagnoli, P.; Girolomoni, G. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 1999, 163, 3029–3036. [Google Scholar]
- Amuguni, H.; Tzipori, S. Bacillus subtilis: A temperature resistant and needle free delivery system of immunogens. Hum. Vaccines Immunother. 2012, 8, 979–986. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Belitsky, B.R.; Brown, D.W.; Brinker, J.P.; Kerstein, K.O.; Herrmann, J.E.; Keusch, G.T.; Sonenshein, A.L.; Tzipori, S. Efficacy, heat stability and safety of intranasally administered Bacillus subtilis spore or vegetative cell vaccines expressing tetanus toxin fragment C. Vaccine 2010, 28, 6658–6665. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, E.M.F.; Cangiano, G.; Maurano, F.; Saggese, V.; De Felice, M.; Rossi, M.; Ricca, E. Germination-independent induction of cellular immune response by Bacillus subtilis spores displaying the C fragment of the tetanus toxin. Vaccine 2007, 25, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Parigi, R.; Isticato, R.; Oggioni, M.R.; Pozzi, G. Oral priming of mice by recombinant spores of Bacillus subtilis. Vaccine 2004, 22, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, C.; Castaldi, S.; Lanzilli, M.; Saggese, A.; Donadio, G.; Baccigalupi, L.; Ricca, E.; Isticato, R. The temperature of growth and sporulation modulates the efficiency of spore-display in Bacillus subtilis. Microb. Cell Factories 2020, 19, 185. [Google Scholar] [CrossRef]
- Stevenson, A.; Roberts, M. Intranasal immunisation against tetanus with an attenuated Bordetella bronchiseptica vector expressing FrgC: Improved immunogenicity using a Bvg-regulated promoter to express FrgC. Vaccine 2004, 22, 4300–4305. [Google Scholar] [CrossRef]
- Dunstan, S.J.; Simmons, C.P.; Strugnell, R.A. In vitro and in vivo stability of recombinant plasmids in a vaccine strain of Salmonella enterica var. Typhimurium. FEMS Immunol. Med. Microbiol. 2003, 37, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Orr, N.; Galen, J.E.; Levine, M.M. Novel use of anaerobically induced promoter, dmsA, for controlled expression of fragment C of tetanus toxin in live attenuated Salmonella enterica serovar Typhi strain CVD 908-htrA. Vaccine 2001, 19, 1694–1700. [Google Scholar] [CrossRef]
- Chatfield, S.N.; Charles, I.G.; Makoff, A.J.; Oxer, M.D.; Dougan, G.; Pickard, D.; Slater, D.; Fairweather, N.F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: Development of a single-dose oral tetanus vaccine. Biotechnology 1992, 10, 888–892. [Google Scholar] [CrossRef]
- Roberts, M.; Li, J.; Bacon, A.; Chatfield, S. Oral vaccination against tetanus: Comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters. Infect. Immun. 1998, 66, 3080–3087. [Google Scholar] [CrossRef] [Green Version]
- Romanos, M.A.; Makoff, A.J.; Fairweather, N.F.; Beesley, K.M.; Salter, D.E.; Rayment, F.B.; Payne, M.M.; Clare, J.J. Expression of tetanus toxin fragment C in yeast: Gene synthesis is required to eliminate fortuitous polyadenylation sites in AT-rich DNA. Nucleic Acids Res. 1991, 19, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Clare, J.J.; Rayment, F.B.; Ballantine, S.P.; Sreekrishna, K.; Romanos, M.A. High-level expression of tetanus toxin fragment C in Pichia pastoris strains containing multiple tandem integrations of the gene. Biotechnology 1991, 9, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, K.Y.; Xu, F.; Li, G.; Liu, D. The effect of N-glycosylation on the expression of the tetanus toxin fragment C in Pichia pastoris. Protein Expr. Purif. 2020, 166, 105503. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Nixon, P.; Kuroda, H.; Svab, Z.; Clare, S.; Bowe, F.; Fairweather, N.; Ytterberg, J.; van Wijk, K.J.; Dougan, G.; et al. Expression of tetanus toxin fragment C in tobacco chloroplasts. Nucleic Acids Res. 2003, 31, 1174–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tregoning, J.S.; Maliga, P.; Dougan, G.; Nixon, P.J. New advances in the production of edible plant vaccines: Chloroplast expression of a tetanus vaccine antigen, TetC. Phytochemistry 2004, 65, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Yagi, Y.; Shiina, T. Recent advances in the study of chloroplast gene expression and its evolution. Front. Plant Sci. 2014, 5, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.K.-C.; Drake, P.M.W.; Christou, P. Genetic modification: The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003, 4, 794–805. [Google Scholar] [CrossRef]
- Michoux, F.; Ahmad, N.; McCarthy, J.; Nixon, P.J. Contained and high-level production of recombinant protein in plant chloroplasts using a temporary immersion bioreactor. Plant Biotechnol. J. 2011, 9, 575–584. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Clare, S.; Bowe, F.; Edwards, L.; Fairweather, N.F.; Qazi, O.; Nixon, P.J.; Maliga, P.; Dougan, G.; Hussell, T. Protection against tetanus toxin using a plant-based vaccine. Eur. J. Immunol. 2005, 35, 1320–1326. [Google Scholar] [CrossRef]
- Charles, I.G.; Rodgers, B.C.; Makoff, A.J.; Chatfield, S.N.; Salter, D.E.; Fairweather, N.F. Synthesis of tetanus toxin fragment C in insect cells by use of a baculovirus expression system. Infect. Immun. 1991, 59, 1627–1632. [Google Scholar] [CrossRef] [Green Version]
- Bayart, C.; Peronin, S.; Jean, E.; Paladino, J.; Talaga, P.; Borgne, M.L. The combined use of analytical tools for exploring tetanus toxin and tetanus toxoid structures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1054, 80–92. [Google Scholar] [CrossRef]
- Chai, P.; Pu, X.; Li, J.; Xia, X.; Ge, J.; Luo, A.; Su, H.; Zhang, W.; Ma, J. Expression and purification of tetanus toxin fragment C in Escherichia coli BL21(DE3). Protein Pept. Lett. 2020, 27, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Szabat-Iriaka, B.; Le Borgne, M. Brain safety concerns of nanomedicines: The need for a specific regulatory framework. Drug Discov. Today 2021, 26, 2502–2507. [Google Scholar] [CrossRef] [PubMed]
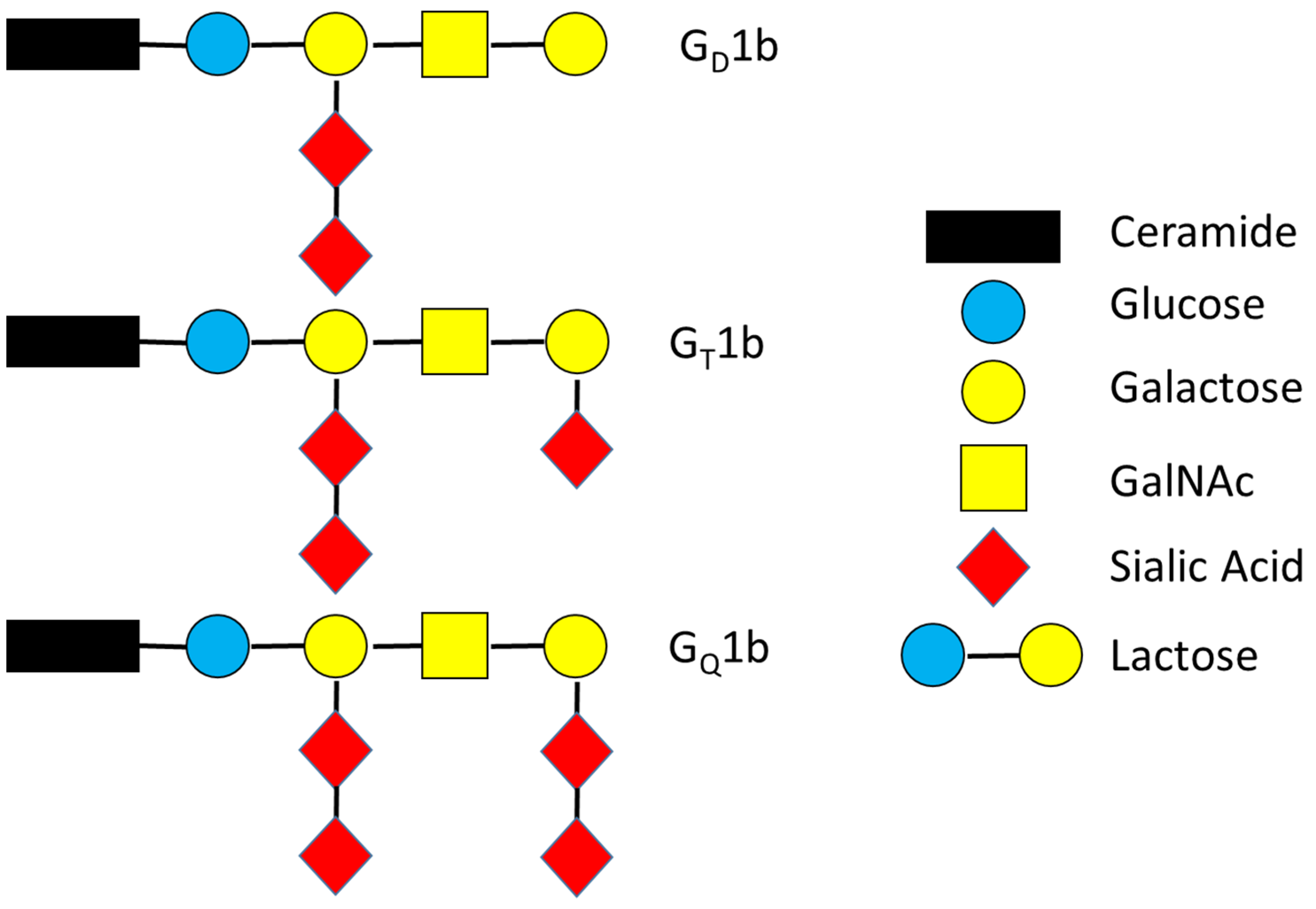
| Key Terms Used | Abbreviations |
|---|---|
| Tetanus toxin fragment C | TTFC or TTFrC or TtxFC |
| Tetanus toxin C fragment | TTFC or TTC or TetC or TCF or TTCF |
| Tetanus toxin heavy C fragment Non-toxic carboxylic fragment of tetanus toxin | TeTx Hc or Hc-TeTx |
| Recombinant tetanus toxin fragment C | rTT-Hc |
| Tetanus toxin native heavy C-fragment | TeNT-Hc |
| Heavy C fragment wild-type | HcWT |
| Carboxylic fragment of tetanus toxin | HC |
| C-terminal fragment of tetanus toxin Fragment C ot tetanus toxin | FrgC or FrC |
| Medicinal Product | Biological Interest | Administration and Dose | Experimental Model | Observed Effects | Ref. |
|---|---|---|---|---|---|
| TTFC used alone | |||||
| TTFC | neuronal protection (ALS) | Intramuscular 1 μg | male and female SOD1-G93A mice |
| [37] |
| TTFC | neuropsychiatric disorders (depression) | intramuscular 20–60 μg/kg | adult male Wistar-Kyoto rats |
| [38] |
| TTFC | neuronal protection (spinal MN degeneration) | direct spinal infusion (total amount of ~42 ng/rat) intramuscular (total amount of ~400 ng/rat) | adult male Wistar rats |
| [39] |
| TTFC | neuronal protection (PD) | intraperitoneal 0.5 mg/kg | male 8-week-old Sprague–Dawley rats |
| [40] |
| TTFC | neuronal protection (AD, effect on learning and memory) | medial septum (local administration) 100 ng | adult male Wistar rats |
| [41] |
| TTFC | neuronal protection (post-methamphetamine treatment) | intramuscular 40 μg/kg | adult male C57BL/6J mice |
| [42] |
| TTFC | neuronal protection (restorative effect) | intramuscular 20 µg/kg | adult male Wistar rats |
| [43] |
| Naked DNA encoding for TTFC | neuronal protection (cerebral ischemia) | intramuscular 200 µg | adult male Mongolian gerbils |
| [36] |
| Naked DNA encoding for TTFC | neuronal protection (ALS disease) | intramuscular 300 µg | SOD1-G93A mice |
| [29] |
| TTFC used as a fusion protein | |||||
| TTFC fused with rAAV8, CMV and eGFP | tracing study (connectivity map) | hippocampal injection 1 µL | adult male and female tdTomatoJ mice |
| [44] |
| TTFC fused with GDNF | neuronal protection (ALS disease) | intramuscular 300 µg | SODG93A mice |
| [45] |
| TTFC fused with GFP | study of neuronal network (study of nerve injury) | / | transgenic mice (NPY-Cre, ZWX) |
| [46] |
| TTFC fused with IGF-1 | neuronal protection(age related nerve alteration) | intramuscular 10 µg | old control FVB and DBA mice |
| [47] |
| TTFC fused with GFP or β-galactosidase | study of neuronal network (muscle specific spinal motor circuitry) | intramuscular 10.57–19.2 µg/mL | new born BalbC/J mice |
| [48] |
| TTFC fused with SOD1 | neuronal delivery (protein) | intra- cerebroventricular | adult male C57BL6 mice |
| [24] |
| Other forms of TTFC (analog, complex, conjugate) | |||||
| 125I-TTFC | retrograde transport (spinal cord) | intramuscular 10 µg of radiolabeled TTC | transgenic mice (C57BL6, SOD193A) |
| [49] |
| PEISH-based NP with HC | neuronal delivery (gene therapy) | subcutaneous 150 μL of dispersion (conc. 7.5 µg pegylated HC per 2 µg of pDNA) | male 4-month old Wistar rats |
| [50] |
| Synthetic analog of TTFC, Tet1-peptide | neuronal delivery (small molecules) | intramuscular 1 µL/g of body weight) | young adult male heterozygous rats |
| [28] |
| TTFC chemically coupled to GDNF | neuronal delivery (therapeutics) | intramuscular 60–100 µg | adult male mice |
| [51] |
| Medicinal Product | Biological Interest | Administration and Dose | Experimental Model | Observed Effects | Ref. |
|---|---|---|---|---|---|
| TTFC used alone | |||||
| TTFC | tetanus antitoxin | Intramuscular 0.625–15 mg | horses |
| [87] |
| TTFC | vaccine (tetanus) | / | mAbs obtained after BALB/c mice immunization with TT |
| [68] |
| 0.1 mg | BALB/c mice | ||||
| TTFC | vaccine (tetanus) | transcutaneous 30 µg | BALB/c mice |
| [69] |
| TTFC used as fusion protein | |||||
| TTFC fused with S. aureus coagulase R domain | vaccine (S. aureus) | intramuscular 30 µg of TTFC-CoaR | BALB/c mice |
| [88] |
| TTFC fused with several epitopes | cancer vaccine (HPV-induced cancer) | subcutaneous 1.5 nmol of MEV (100 µL) | C57BL/6 mice |
| [86] |
| TTFC fused to flagellin | mucosal vaccine (tetanus) | intranasal 2.75 μg | female BALB/c mice |
| [89] |
| TTFC fused with DNA | cancer vaccine (multiple myeloma) | intramuscular 6 times 1 mg fusion vaccine | clinical trial—phase I 14 patients with multiple myeloma |
| [90] |
| TTFC fused with Tem 1 cDNA | cancer vaccine (tumor vasculature) | intramuscular 50 µg of plasmid in saline | C57BL/6 and BALB/c mice |
| [91] |
| TTFC domain fused with DNA (PSMA27–35) | cancer vaccine (prostate) | intramuscular 5 times 400–3200 µg of fusion vaccine | clinical trial—phase I/II 32 HLA-A2+ patients and 32 HLA-A2− control patients |
| [92] |
| TTFC fused with naked DNA (VHCDR3109–116) | cancer vaccine (lymphoma) | intramuscular 50 µg DNA plasmid | male C3H/HeN mice |
| [93] |
| TTFC fused with DNA | DNA vaccine (HPV 16 E6 and E7) | intradermal tattoo vaccination 20 µg | C57BL/6 mice |
| [76] |
| TTFC fused with Cryptosporidium parvum antigens | vaccine (Cryptosporidium parvum) | per os single dose 5 × 109 CFU | female C57BL/6 and IL18-KO mice |
| [94] |
| Other forms of TTFC (conjugate, bacteria) | |||||
| TTFC conjugated to pneumococcal polysaccharide | vaccine (Pneumococcus) | intraperitoneal 2 µg/mL of PS per vaccine | female BALB/c mice |
| [95] |
| TTFC and S. aureus surface protein A (SasA) | combined vaccine (tetanus and S. aureus) | intraperitoneal 10 µg SasA + 10 µg TTFC | female BALB/c mice |
| [96] |
| TTFC conjugated to Her2 protein fragment | cancer vaccine (Her2+ breast cancer) | subcutaneous 50 µg of conjugate, 4 boosters of 25 µg | female BALB-neuT mice |
| [97] |
| TTFC conjugated to Burkholderia pseudomallei PS | vaccine (melioidosis) | intraperitoneal 66 µg of conjugate per dose | female BALB/c mice |
| [98] |
| TTFC conjugate to Vibrio cholerae OPS | conjugate vaccine (cholera) | intramuscular and intradermal 10 µg of OPS per animal (5:1 conjugate molar ratio OPS:TTFC) | female Swiss- Webster mice |
| [80] |
| Cytomegalovirus expressing TTFC | vaccine (tetanus) | intraperitoneal 5 × 106 pfu | age-matched female 129S1/SvlmJ/Cr mice |
| [99] |
| Bacillus subtilis expressing TTFC | vaccine (tetanus) | sublingual and intranasal 1 × 109 cells of died TTFC-expressing B. subtilis | weaned piglets |
| [71] |
| Expression Conditions | Fairweather et al. 1986 [106] | Makkof et al. 1989 [104] | Makkof et al. 1989 [111] | Halpern et al. 1990 [105] | Ribas et al. 2000 [112] | Motamedi et al. 2011 [109] | Yu et al. 2011 [108] | Yu et al. 2011 [110] | Yousefi et al. 2013 [107] | Aghayipour et al. 2018 [113] |
|---|---|---|---|---|---|---|---|---|---|---|
| host | DH1 | E. coli | E. coli | DH5α | BL21 | DH5α | BL21 | BL21 | BL21 | BL21pLys |
| TTFC DNA origin | C. tetani | synthetic for end of TTFC | synthetic (optimized codons for TTFC) | C. tetani | C. tetani | C. tetani | synthetic | synthetic (optimized AT: 72.50% to 52.47%) | C. tetani | C. tetani |
| recombinant protein | TrpE-TTFC (trpE: anthranilate synthetase) | 1: met-3AA INFγ-TTFB(537–864)-TTFC(865–1315) 2: met-TTFC | met-TTFC | fusion with 8AA from vector and 9AA from fragment B | 112AA Trx-45AA TTFC- His-tag | MBP-TTFC (MBP:maltose binding protein) | Trx-TTFC-6His tag | no tag | Cterm of TTFC (25 kDa)-6His tag | 6His-tagged fusion protein |
| plasmid | pWRL507 | pTET-Tact1 pTET-Tact2 | pTET-Tact2 | pTTQ8 | pET32a | pMalc2x | pTIG-Trx | pET32a+ | pET28b+ | pET28a pET22a |
| promotor | trpE | tac (derived from trp and lac UV5) | tac | tac | T7 | tac | T7 | T7 | T7 | T7 |
| inducer | indoylacrylic acid | IPTG 60 µg/mL | IPTG | IPTG 0.67 mM | IPTG 1 mM | IPTG | IPTG 0.4 mM | IPTG 0.2 mM | IPTG 1 mM | IPTG (optimized protocol) |
| quantity | low amount of fusion protein /trpE protein | 2: 12 mg TTFC/L (3–4% TPC) | 11–14% TPC (with optimized promotor) | 1 mg/L (0.5% TPC) | 35 mg/L | un-specified | 15–30% TPC (20–35 mg/mL after purification) | 333 mg/L 42 L fermentor (46% TPC) | 35% TCP | pET28a: 38 mg/mL pET22a: 32 mg/mL |
| solubility | soluble | 1: low solubilty 2: soluble | soluble | soluble | soluble | soluble | soluble | soluble | soluble | soluble |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayart, C.; Mularoni, A.; Hemmani, N.; Kerachni, S.; Jose, J.; Gouet, P.; Paladino, J.; Le Borgne, M. Tetanus Toxin Fragment C: Structure, Drug Discovery Research and Production. Pharmaceuticals 2022, 15, 756. https://doi.org/10.3390/ph15060756
Bayart C, Mularoni A, Hemmani N, Kerachni S, Jose J, Gouet P, Paladino J, Le Borgne M. Tetanus Toxin Fragment C: Structure, Drug Discovery Research and Production. Pharmaceuticals. 2022; 15(6):756. https://doi.org/10.3390/ph15060756
Chicago/Turabian StyleBayart, Caroline, Angélique Mularoni, Nada Hemmani, Soumeya Kerachni, Joachim Jose, Patrice Gouet, Joseph Paladino, and Marc Le Borgne. 2022. "Tetanus Toxin Fragment C: Structure, Drug Discovery Research and Production" Pharmaceuticals 15, no. 6: 756. https://doi.org/10.3390/ph15060756
APA StyleBayart, C., Mularoni, A., Hemmani, N., Kerachni, S., Jose, J., Gouet, P., Paladino, J., & Le Borgne, M. (2022). Tetanus Toxin Fragment C: Structure, Drug Discovery Research and Production. Pharmaceuticals, 15(6), 756. https://doi.org/10.3390/ph15060756