A Review on the Role of Pilocarpine on the Management of Xerostomia and the Importance of the Topical Administration Systems Development
Abstract
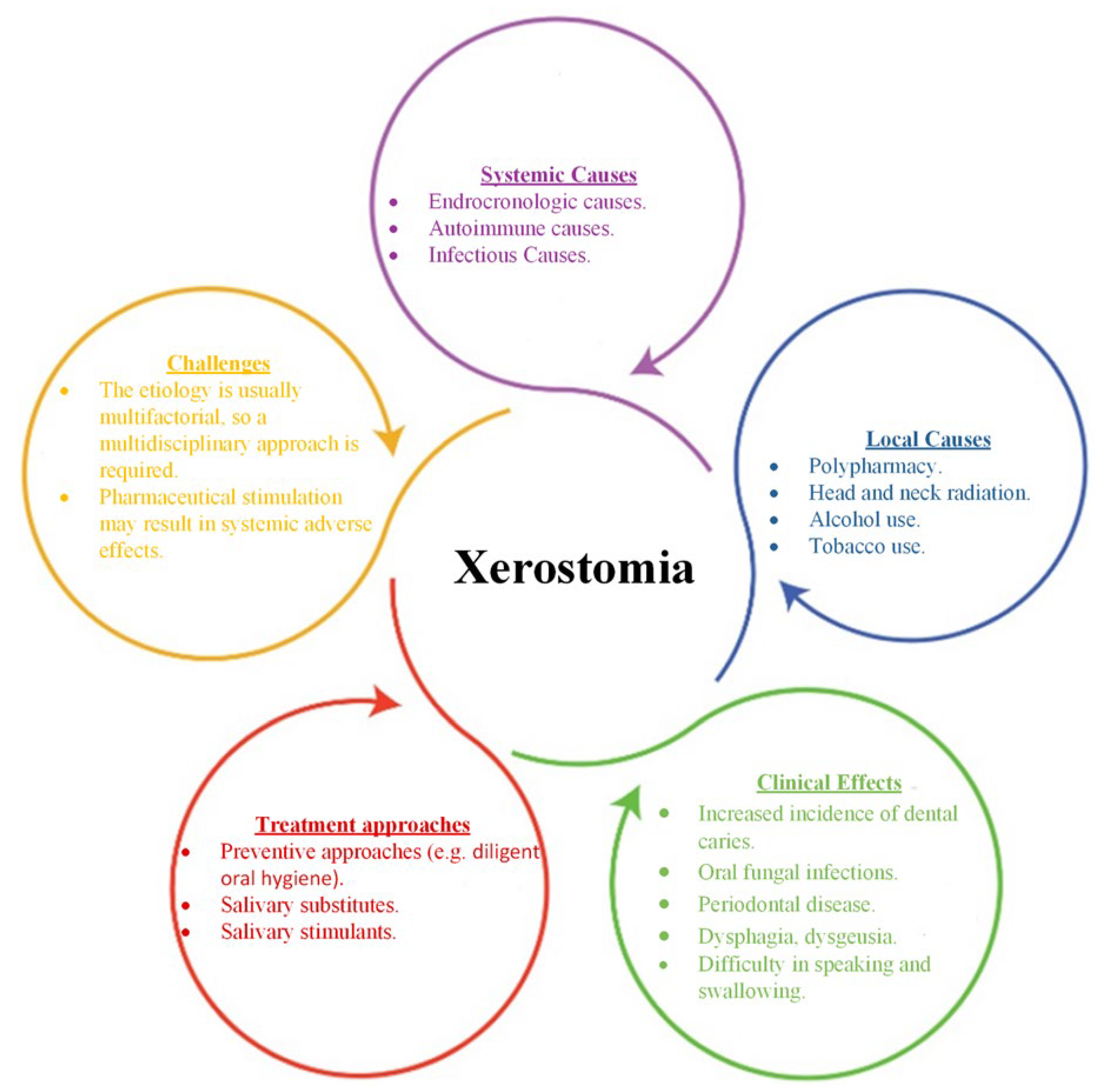
:1. General
2. Approach of the Review
3. General Information on Pilocarpine
Mechanism of Action
4. Systemic Administration of Pilocarpine for the Management of Xerostomia
Adverse Effects
5. Topical Administration of Pilocarpine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stankeviciene, I.; Puriene, A.; Mieliauskaite, D.; Stangvaltaite-Mouhat, L.; Aleksejuniene, J. Detection of xerostomia, Sicca, and Sjogren’s syndromes in a national sample of adults. BMC Oral Health 2021, 21, 552. [Google Scholar] [CrossRef] [PubMed]
- Bustillos, H.; Indorf, A.; Alwan, L.; Thompson, J.; Jung, L. Xerostomia: An immunotherapy-related adverse effect in cancer patients. Supportive Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 1681–1687. [Google Scholar] [CrossRef]
- Bulthuis, M.S.; Jan Jager, D.H.; Brand, H.S. Relationship among perceived stress, xerostomia, and salivary flow rate in patients visiting a saliva clinic. Clin. Oral Investig. 2018, 22, 3121–3127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napeñas, J.J.; Brennan, M.T.; Fox, P.C. Diagnosis and treatment of xerostomia (dry mouth). Odontology 2009, 97, 76–83. [Google Scholar] [CrossRef]
- Epstein, J.B.; Beier Jensen, S. Management of Hyposalivation and Xerostomia: Criteria for Treatment Strategies. Compend. Contin. Educ. Dent. 2015, 36, 600–603. [Google Scholar]
- Kapourani, A.; Kontogiannopoulos, K.N.; Manioudaki, A.-E.; Poulopoulos, A.K.; Tsalikis, L.; Assimopoulou, A.N.; Barmpalexis, P. A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches. Polymers 2022, 14, 850. [Google Scholar] [CrossRef] [PubMed]
- Soutome, S.; Yanamoto, S.; Nishii, M.; Kojima, Y.; Hasegawa, T.; Funahara, M.; Akashi, M.; Saito, T.; Umeda, M. Risk factors for severe radiation-induced oral mucositis in patients with oral cancer. J. Dent. Sci. 2021, 16, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Wang, E.A.; Fazel, N. Etiology, evaluation, and management of xerostomia. Clin. Dermatol. 2017, 35, 468–476. [Google Scholar] [CrossRef]
- Marín, C.; Díaz-de-Valdés, L.; Conejeros, C.; Martínez, R.; Niklander, S. Interventions for the treatment of xerostomia: A randomized controlled clinical trial. J. Clin. Exp. Dent. 2021, 13, e104–e111. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, T.; Costa, K.; Guerra, L. Current alternatives in the prevention and treatment of xerostomia in cancer therapy. RGO-Rev. Gaúcha Odontol. 2020, 68, 1–12. [Google Scholar] [CrossRef]
- Nadig, S.D.; Ashwathappa, D.T.; Manjunath, M.; Krishna, S.; Annaji, A.G.; Shivaprakash, P.K. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J. Oral Maxillofac. Pathol. 2017, 21, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K.; Kim, H.-N. Effects of oral health programmes on xerostomia in community-dwelling elderly: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2020, 18, 52–61. [Google Scholar] [CrossRef]
- Quilici, D.; Zech, K.N. Prevention and treatment options for medication-induced xerostomia. Gen. Dent. 2019, 67, 52–57. [Google Scholar]
- Fleming, M.; Craigs, C.L.; Bennett, M.I. Palliative care assessment of dry mouth: What matters most to patients with advanced disease? Support. Care Cancer 2020, 28, 1121–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salum, F.G.; Medella-Junior, F.d.A.C.; Figueiredo, M.A.Z.; Cherubini, K. Salivary hypofunction: An update on therapeutic strategies. Gerodontology 2018, 35, 305–316. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, X.; Tan, X.; Miao, R.; Tian, W.; Jing, W. Efficacy of a 1% malic acid spray for xerostomia treatment: A systematic review and meta-analysis. Oral Dis. 2021, 1–11. [Google Scholar] [CrossRef]
- Hu, J.; Andablo-Reyes, E.; Mighell, A.; Pavitt, S.; Sarkar, A. Dry mouth diagnosis and saliva substitutes-A review from a textural perspective. J. Texture Stud. 2021, 52, 141–156. [Google Scholar] [CrossRef]
- Niklander, S.; Fuentes, F.; Sanchez, D.; Araya, V.; Chiappini, G.; Martinez, R.; Marshall, M. Impact of 1% malic acid spray on the oral health-related quality of life of patients with xerostomia. J. Oral Sci. 2018, 60, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Ozen, N.; Aydin Sayilan, A.; Mut, D.; Sayilan, S.; Avcioglu, Z.; Kulakac, N.; Ecder, T.; Akyolcu, N. The effect of chewing gum on dry mouth, interdialytic weight gain, and intradialytic symptoms: A prospective, randomized controlled trial. Hemodial. Int. Int. Symp. Home Hemodial. 2021, 25, 94–103. [Google Scholar] [CrossRef]
- Alhejoury, H.A.; Mogharbel, L.F.; Al-Qadhi, M.A.; Shamlan, S.S.; Alturki, A.F.; Babatin, W.M.; Mohammed Alaishan, R.A.; Pullishery, F. Artificial Saliva for Therapeutic Management of Xerostomia: A Narrative Review. J. Pharm. Bioallied Sci. 2021, 13, S903–S907. [Google Scholar] [CrossRef]
- Cifuentes, M.; Del Barrio-Díaz, P.; Vera-Kellet, C. Pilocarpine and artificial saliva for the treatment of xerostomia and xerophthalmia in Sjögren syndrome: A double-blind randomized controlled trial. Br. J. Dermatol. 2018, 179, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Heise, N.; Merzweiler, K.; Deigner, H.-P.; Al-Harrasi, A.; Csuk, R. Concise Synthesis of Both Enantiomers of Pilocarpine. Molecules 2021, 26, 3676. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Bishnoi, A.; Shilpa; Kamboj, P.; Arora, A.K.; Pal, A.; Mahajan, R.; Handa, S. Effectiveness of topical pilocarpine in refractory oral lesions of pemphigus vulgaris: Results from an open-label, prospective, pilot study. Dermatol. Ther. 2022, 35, e15449. [Google Scholar] [CrossRef] [PubMed]
- Codding, P.W.; James, M.N.G. The structure of pilocarpine hydrochloride, C11H17N2O2+.Cl−: A muscarinic alkaloid. Acta Crystallogr. Sect. B 1984, 40, 429–434. [Google Scholar] [CrossRef]
- Treijtel, N.; Groenendaal-van de Meent, D.; Michon, I.; Korstanje, C.; Meijer, J.; Francke, K.; Grossmann, M.; de Vries, M. Pilocarpine-Induced Effects on Salivary Secretion as a Pharmacological Biomarker for Cholinergic Parasympathetic Activation. Clin. Pharmacol. Drug Dev. 2022, 11, 25–33. [Google Scholar] [CrossRef]
- Kaga, K.; Kamasako, T.; Kaga, M.; Fuse, M.; Ishizuka, M.; Yamanishi, T. Efficacy and Safety of Pilocarpine Hydrochloride in the Treatment of Voiding Difficulty in Patients with Detrusor Underactivity. Clin. Surg. 2016, 5, 1–5. [Google Scholar] [CrossRef]
- Minagi, H.O.; Ikai, K.; Araie, T.; Sakai, M.; Sakai, T. Benefits of long-term pilocarpine due to increased muscarinic acetylcholine receptor 3 in salivary glands. Biochem. Biophys. Res. Commun. 2018, 503, 1098–1102. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Birdsall, N.J.M. International Union of Pharmacology. XVII. Classification of Muscarinic Acetylcholine Receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar]
- Abrams, P.; Andersson, K.-E.; Buccafusco, J.J.; Chapple, C.; de Groat, W.C.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J.; et al. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharm. 2006, 148, 565–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pronin, A.N.; Wang, Q.; Slepak, V.Z. Teaching an Old Drug New Tricks: Agonism, Antagonism, and Biased Signaling of Pilocarpine through M3 Muscarinic Acetylcholine Receptor. Mol. Pharmacol. 2017, 92, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, A.B.; Kraus, G.P. Physiology, Cholinergic Receptors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Panarese, V.; Moshirfar, M. Pilocarpine. In StatPearls; Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Taniguchi, A.; Susa, T.; Kogo, H.; Iizuka-Kogo, A.; Yokoo, S.; Matsuzaki, T. Long-term Pilocarpine Treatment Improves Salivary Flow in Irradiated Mice. Acta Histochem. Cytochem. 2019, 52, 45–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, J.; Barros, S.; Carvalho de Almeida, W.; Pereira, R. World Overview of Xerostomia Therapeutics: Clinical Trials Analysis. IOSR J. Dent. Med. Sci. 2020, 19, 59–62. [Google Scholar] [CrossRef]
- Theunissen, M.; Rideaux-Seferina, S.; Magdelijns, F.J.; Janssen, D.J.A.; van den Beuken-van Everdingen, M.H.J. Local Oral Pilocarpine Drops for Relieving Xerostomia (Dry Mouth) in the Elderly: A Pilot Study. J. Am. Med. Dir. Assoc. 2021, 22, 185–186. [Google Scholar] [CrossRef]
- Frydrych, A.; Davies, G.; Slack-Smith, L.; Heywood, J. An Investigation into the Use of Pilocarpine as a Sialagogue in Patients With Radiation Induced Xerostomia. Aust. Dent. J. 2002, 47, 249–253. [Google Scholar] [CrossRef]
- Wiseman, L.R.; Faulds, D. Oral Pilocarpine: A Review of its Pharmacological Properties and Clinical Potential in Xerostomia. Drugs 1995, 49, 143–155. [Google Scholar] [CrossRef]
- Hendrickson, R.G.; Morocco, A.P.; Greenberg, M.I. Pilocarpine toxicity and the treatment of xerostomia. J. Emerg. Med. 2004, 26, 429–432. [Google Scholar] [CrossRef]
- Vissink, A.; Spijkervet, F.K.L.; Jensen, S.B.; Brennan, M.T. 23—Xerostomia. In Supportive Oncology; Davis, M.P., Feyer, P.C., Ortner, P., Zimmermann, C., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2011; pp. 232–242. [Google Scholar]
- Fox, P.C.; van der Ven, P.F.; Baum, B.J.; Mandel, I.D. Pilocarpine for the treatment of xerostomia associated with salivary gland dysfunction. Oral Surg. Oral Med. Oral Pathol. 1986, 61, 243–248. [Google Scholar] [CrossRef]
- Greenspan, D.; Daniels, T.E. Effectiveness of pilocarpine in postradiation xerostomia. Cancer 1987, 59, 1123–1125. [Google Scholar] [CrossRef]
- Schuller, D.E.; Stevens, P.; Clausen, K.P.; Olsen, J.; Gahbauer, R.; Martin, M. Treatment of radiation side effects with oral pilocarpine. J. Surg. Oncol. 1989, 42, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.C.; Atkinson, J.C.; Macynski, A.A.; Wolff, A.; Kung, D.S.; Valdez, I.H.; Jackson, W.; Delapenha, R.A.; Shiroky, J.; Baum, B.J. Pilocarpine treatment of salivary gland hypofunction and dry mouth (xerostomia). Arch. Intern. Med. 1991, 151, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- LeVeque, F.G.; Montgomery, M.; Potter, D.; Zimmer, M.B.; Rieke, J.W.; Steiger, B.W.; Gallagher, S.C.; Muscoplat, C.C. A multicenter, randomized, double-blind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation-induced xerostomia in head and neck cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Valdez, I.H.; Wolff, A.; Atkinson, J.C.; Macynski, A.A.; Fox, P.C. Use of pilocarpine during head and neck radiation therapy to reduce xerostomia and salivary dysfunction. Cancer 1993, 71, 1848–1851. [Google Scholar] [CrossRef]
- Johnson, J.T.; Ferretti, G.A.; Nethery, W.J.; Valdez, I.H.; Fox, P.C.; Ng, D.; Muscoplat, C.C.; Gallagher, S.C. Oral Pilocarpine for Post-Irradiation Xerostomia in Patients with Head and Neck Cancer. N. Engl. J. Med. 1993, 329, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Rieke, J.W.; Hafermann, M.D.; Johnson, J.T.; LeVeque, F.G.; Iwamoto, R.; Steiger, B.W.; Muscoplat, C.; Gallagher, S.C. Oral pilocarpine for radiation-induced xerostomia: Integrated efficacy and safety results from two prospective randomized clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 661–669. [Google Scholar] [CrossRef]
- Zimmerman, R.P.; Mark, R.J.; Tran, L.M.; Juillard, G.F. Concomitant pilocarpine during head and neck irradiation is associated with decreased posttreatment xerostomia. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 571–575. [Google Scholar] [CrossRef]
- Vivino, F.B.; Al-Hashimi, I.; Khan, Z.; LeVeque, F.G.; Salisbury, P.L., 3rd; Tran-Johnson, T.K.; Muscoplat, C.C.; Trivedi, M.; Goldlust, B.; Gallagher, S.C. Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjögren syndrome: A randomized, placebo-controlled, fixed-dose, multicenter trial. P92-01 Study Group. Arch. Intern. Med. 1999, 159, 174–181. [Google Scholar] [CrossRef]
- Horiot, J.C.; Lipinski, F.; Schraub, S.; Maulard-Durdux, C.; Bensadoun, R.J.; Ardiet, J.M.; Bolla, M.; Coscas, Y.; Baillet, F.; Coche-Dequéant, B.; et al. Post-radiation severe xerostomia relieved by pilocarpine: A prospective French cooperative study. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2000, 55, 233–239. [Google Scholar] [CrossRef]
- Mateos, J.J.; Setoain, X.; Ferre, J.; Rovirosa, A.; Navalpotro, B.; Martin, F.; Ortega, M.; Lomeña, F.; Fuster, D.; Pavia, J.; et al. Salivary scintigraphy for assessing the protective effect of pilocarpine in head and neck irradiated tumours. Nucl. Med. Commun. 2001, 22, 651–656. [Google Scholar] [CrossRef]
- Haddad, P.; Karimi, M. A randomized, double-blind, placebo-controlled trial of concomitant pilocarpine with head and neck irradiation for prevention of radiation-induced xerostomia. Radiother. Oncol. 2002, 64, 29–32. [Google Scholar] [CrossRef]
- Warde, P.; O’Sullivan, B.; Aslanidis, J.; Kroll, B.; Lockwood, G.; Waldron, J.; Payne, D.; Bayley, A.; Ringash, J.; Kim, J.; et al. A Phase III placebo-controlled trial of oral pilocarpine in patients undergoing radiotherapy for head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 9–13. [Google Scholar] [CrossRef]
- Fisher, J.; Scott, C.; Scarantino, C.W.; Leveque, F.G.; White, R.L.; Rotman, M.; Hodson, D.I.; Meredith, R.F.; Foote, R.; Bachman, D.G.; et al. Phase III quality-of-life study results: Impact on patients’ quality of life to reducing xerostomia after radiotherapy for head-and-neck cancer—RTOG 97-09. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 832–836. [Google Scholar] [CrossRef]
- Gornitsky, M.; Shenouda, G.; Sultanem, K.; Katz, H.; Hier, M.; Black, M.; Velly, A.M. Double-blind randomized, placebo-controlled study of pilocarpine to salvage salivary gland function during radiotherapy of patients with head and neck cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Papas, A.S.; Sherrer, Y.S.; Charney, M.; Golden, H.E.; Medsger, T.A., Jr.; Walsh, B.T.; Trivedi, M.; Goldlust, B.; Gallagher, S.C. Successful Treatment of Dry Mouth and Dry Eye Symptoms in Sjögren’s Syndrome Patients with Oral Pilocarpine: A Randomized, Placebo-Controlled, Dose-Adjustment Study. JCR J. Clin. Rheumatol. 2004, 10, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Taweechaisupapong, S.; Pesee, M.; Aromdee, C.; Laopaiboon, M.; Khunkitti, W. Efficacy of pilocarpine lozenge for post-radiation xerostomia in patients with head and neck cancer. Aust. Dent. J. 2006, 51, 333–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarantino, C.; LeVeque, F.; Swann, R.S.; White, R.; Schulsinger, A.; Hodson, D.I.; Meredith, R.; Foote, R.; Brachman, D.; Lee, N. Effect of pilocarpine during radiation therapy: Results of RTOG 97-09, a phase III randomized study in head and neck cancer patients. J. Supportive Oncol. 2006, 4, 252–258. [Google Scholar]
- Wu, C.H.; Hsieh, S.C.; Lee, K.L.; Li, K.J.; Lu, M.C.; Yu, C.L. Pilocarpine hydrochloride for the treatment of xerostomia in patients with Sjögren's syndrome in Taiwan--a double-blind, placebo-controlled trial. J. Formos. Med. Assoc. Taiwan Yi Zhi 2006, 105, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Chitapanarux, I.; Kamnerdsupaphon, P.; Tharavichitkul, E.; Sumitsawan, Y.; Sittitrai, P.; Pattarasakulchai, T.; Lorvidhaya, V.; Sukthomya, V.; Pukanhaphan, N.; Traisatit, P. Effect of oral pilocarpine on post-irradiation xerostomia in head and neck cancer patients: A single-center, single-blind clinical trial. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2008, 91, 1410–1415. [Google Scholar]
- Abueva, C.D.G. Photobiomodulation Therapy in the Treatment of Salivary Dysfunction. Med. Lasers 2022, 11, 15–20. [Google Scholar] [CrossRef]
- Porter, S.R.; Scully, C.; Hegarty, A.M. An update of the etiology and management of xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.C.; Grisius, M.; Massey, W. Salivary Hypofunction and Xerostomia: Diagnosis and Treatment. Dent. Clin. N. Am. 2005, 49, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Tarzia, O.; Bergamaschi, C.; Santos, F.; Andrade, E.; Groppo, F. Comparison of the effects of pilocarpine and cevimeline on salivary flow. Int. J. Dent. Hyg. 2009, 7, 126–130. [Google Scholar] [CrossRef]
- Casson, R.J. Medical therapy for glaucoma: A review. Clin. Exp. Ophthalmol. 2022, 50, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Criswell, L.A. Primary Sjögren's Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef]
- Pakala, R.S.; Brown, K.N.; Preuss, C.V. Cholinergic Medications. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fox, R.I.; Fox, C.M. Management of Sjögren's. In Dubois' Lupus Erythematosus and Related Syndromes, 9th ed.; Wallace, D.J., Hahn, B.H., Eds.; Elsevier: London, UK, 2019; pp. 745–758. [Google Scholar]
- Barbe, A.G. Medication-Induced Xerostomia and Hyposalivation in the Elderly: Culprits, Complications, and Management. Drugs Aging 2018, 35, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E. Efficacy and economic evaluation of pilocarpine in treating radiation-induced xerostomia. Expert Opin. Pharmacother. 2003, 4, 1489–1497. [Google Scholar] [CrossRef]
- Farag, A.M.; Holliday, C.; Cimmino, J.; Roomian, T.; Papas, A. Comparing the effectiveness and adverse effects of pilocarpine and cevimeline in patients with hyposalivation. Oral Dis. 2019, 25, 1937–1944. [Google Scholar] [CrossRef]
- Nakamura, N.; Sasano, N.; Yamashita, H.; Igaki, H.; Shiraishi, K.; Terahara, A.; Asakage, T.; Nakao, K.; Ebihara, Y.; Ohtomo, K.; et al. Oral pilocarpine (5mg t.i.d.) used for xerostomia causes adverse effects in Japanese. Auris Nasus Larynx 2009, 36, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Noaiseh, G.; Baker, J.F.; Vivino, F.B. Comparison of the discontinuation rates and side-effect profiles of pilocarpine and cevimeline for xerostomia in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2014, 32, 575–577. [Google Scholar]
- Chainani-Wu, N.; Gorsky, M.; Mayer, P.; Bostrom, A.; Epstein, J.B.; Jr., S.S. Assessment of the use of sialogogues in the clinical management of patients with xerostomia. Spec. Care Dent. 2006, 26, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nakano, H.; Yoneto, K.; Yoneto, C.; Furubayashi, T.; Suzuki, K.; Okae, A.; Ueno, T.; Sakane, T. Topical Xerostomia Treatment with Hyaluronate Sheets Containing Pilocarpine. Biol. Pharm. Bull. 2022, 45, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Schuh, M.J. Effects of pilocarpine on salivary flow in patients with Sjögren's syndrome. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 545–549. [Google Scholar] [CrossRef]
- Davies, A.N.; Singer, J. A comparison of artificial saliva and pilocarpine in radiation induced xerostomia. J. Laryngol. Otol. 1994, 108, 663–665. [Google Scholar] [CrossRef]
- Bernardi, R.; Perin, C.; Becker, F.L.; Ramos, G.Z.; Gheno, G.Z.; Lopes, L.R.; Pires, M.; Barros, H.M. Effect of pilocarpine mouthwash on salivary flow. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2002, 35, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Ahn, H.J.; Choi, J.H.; Jung, D.W.; Kwon, J.S. Effect of 0.1% pilocarpine mouthwash on xerostomia: Double-blind, randomised controlled trial. J. Oral Rehabil. 2014, 41, 226–235. [Google Scholar] [CrossRef]
- Tanigawa, T.; Yamashita, J.-i.; Sato, T.; Shinohara, A.; Shibata, R.; Ueda, H.; Sasaki, H. Efficacy and safety of pilocarpine mouthwash in elderly patients with xerostomia. Spec. Care Dent. 2015, 35, 164–169. [Google Scholar] [CrossRef]
- Park, J.-E.; Song, C.-W.; Kim, K.-S. Comparison of the Effects of Pilocarpine Solution and Tablet on Salivary Flow Rate. J. Oral Med. Pain 2015, 40, 10–16. [Google Scholar] [CrossRef]
- Song, J.-I.; Park, J.-E.; Kim, H.-K.; Kim, M.-E.; Kim, K.-S. Dose- and Time-Related Effects of Pilocarpine Mouthwash on Salivation. J. Oral Med. Pain 2017, 42, 72–80. [Google Scholar] [CrossRef]
- Vera Beatris, M.; Cielo; Grassi, M.; Paim, E.; Mt, H. The Effects of Pilocarpine Mouthwash on Vocal Quality. IOSR J. Pharm. Biol. Sci. 2018, 13, 30–35. [Google Scholar] [CrossRef]
- Hamlar, D.D.; Schuller, D.E.; Gahbauer, R.A.; Buerki, R.A.; Staubus, A.E.; Hall, J.; Altman, J.S.; Elzinga, D.J.; Martin, M.R. Determination of the efficacy of topical oral pilocarpine for postirradiation xerostomia in patients with head and neck carcinoma. Laryngoscope 1996, 106, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Santos Polvora, T.L.; De Sousa Pereira, R.M.; Macedo, A.P.; De Macedo, L.D.; Motta, A.C.F.; Tirapelli, C.; Pedrazzi, V. Pilocarpine Spray for the Treatment of Xerostomia: A Randomized, Double-Blind, Placebo-Controlled Trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, e47. [Google Scholar] [CrossRef]
- Pereira, R.M.d.S.; Bastos, M.D.R.; Ferreira, M.P.; de Freitas, O.; de Macedo, L.D.; de Oliveira, H.F.; Ricz, H.M.A.; Motta, A.C.F.; Macedo, A.P.; Tirapelli, C.; et al. Topical pilocarpine for xerostomia in patients with head and neck cancer treated with radiotherapy. Oral Dis. 2020, 26, 1209–1218. [Google Scholar] [CrossRef]
- Dawes, C.; Wood, C.M. The contribution of oral minor mucous gland secretions to the volume of whole saliva in man. Arch. Oral Biol. 1973, 18, 337–342. [Google Scholar] [CrossRef]
- Neyraud, E.; Palicki, O.; Schwartz, C.; Nicklaus, S.; Feron, G. Variability of human saliva composition: Possible relationships with fat perception and liking. Arch. Oral Biol. 2012, 57, 556–566. [Google Scholar] [CrossRef]
- Gibson, J.; Halliday, J.A.; Ewert, K.; Robertson, S. A controlled release pilocarpine buccal insert in the treatment of Sjögren’s syndrome. Br. Dent. J. 2007, 202, E17; discussion 404–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthumariappan, S.; Ng, W.C.; Adine, C.; Ng, K.K.; Davoodi, P.; Wang, C.H.; Ferreira, J.N. Localized Delivery of Pilocarpine to Hypofunctional Salivary Glands through Electrospun Nanofiber Mats: An Ex Vivo and In Vivo Study. Int. J. Mol. Sci. 2019, 20, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Yamada, C.; Komagata, Y.; Kikuchi, H.; Hosono, H.; Itagaki, F. New low-dose liquid pilocarpine formulation for treating dry mouth in Sjögren's syndrome: Clinical efficacy, symptom relief, and improvement in quality of life. J. Pharm. Health Care Sci. 2018, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Sarideechaigul, W.; Priprem, A.; Limsitthichaikoon, S.; Phothipakdee, P.; Chaijit, R.; Jorns, T.P.; Lungruammit, N.; Chaiya, K. Efficacy and safety of two artificial saliva-based polymers containing 0.1% pilocarpine for treatment of xerostomia: A randomized clinical pilot trial. J. Clin. Exp. Dent. 2021, 13, e994–e1000. [Google Scholar] [CrossRef]
- Gusmão, G.; Borba, P.M.; Galindo, M.A.; Souto Maior, L.F.; Gueiros, L.A.M.; Leão, J.C.; Soares Sobrinho, J.L.; Carvalho, A.A.T. Evaluation of a mucoadhesive pilocarpine tablet for the treatment of xerostomia: A randomized, double-blind, crossover clinical trial. Gen. Dent. 2021, 69, 19–26. [Google Scholar] [PubMed]






| Reference | Year of Publishment | Number of Patients | Diagnosis | Administrated Dose of Pilocarpine and Duration | Outcome/Results |
|---|---|---|---|---|---|
| Fox et al. [42] | 1986 | 6 | Inflammatory exocrinopathy. | 5.0 mg once a day (o.d) for 2 days. | Reduction in oral dryness and increased salivation in all patients. |
| Creenspan et al. [43] | 1987 | 12 | Radiation-induced xerostomia. | 5.0 or 7.5 mg three times a day (t.i.d) or four times a day (q.i.d) for 90 days. | 75% of the patients treated with pilocarpine experienced significant improvement of the mean stimulated whole salivary and parotid salivary flow rate. |
| Schuller et al. [44] | 1989 | 14 | Radiation-induced xerostomia. | 3.0 mg t.i.d | 60% of the patients treated with pilocarpine presented increased whole salivary flow rate after 6 weeks. |
| Fox et al. [45] | 1991 | 39 | Sjögren’s syndrome (21 patients), radiation-induced xerostomia (12 patients), idiopathic salivary gland dysfunction (6 patients). | 5.0 mg t.i.d for 5 months. | ~68% of the patients treated with pilocarpine (i.e., 21/31) presented significantly increased salivary flow. Moreover, 27/31 participants reported a subjective amelioration in oral dryness feeling, as well as in the processes of speaking, chewing and swallowing. |
| LeVeque et al. [46] | 1993 | 156 | Radiation-induced xerostomia. | 2.5 mg t.i.d increased up to 10 mg t.i.d for 12 weeks. | Pilocarpine significantly ameliorated overall global assessments as compared to placebo. |
| Valdez et al. [47] | 1993 | 9 | Radiation-induced xerostomia. | 5.0 mg q.i.d for 3 months. | A lower frequency of oral symptoms during treatment was reported in the pilocarpine-treated group, as compared to the placebo-treated group. |
| Johnson et al. [48] | 1993 | 207 | Radiation-induced xerostomia. | 5.0 mg or 10.0 mg tablets t.i.d for 12 weeks. | 44 and 46% of patients treated with 5 and 10 mg, respectively, claimed that their feeling of oral dryness was improved. Moreover, 31 and 37% of patients treated with 5 and 10 mg, respectively, referred improved mouth and tongue comfort. |
| Rieke et al. [49] | 1995 | 369 | Radiation-induced xerostomia. | 5.0 mg or 10.0 mg tablets t.i.d for 12 weeks. | Statistically significant improvements in salivary flow in pilocarpine treatment groups. |
| Zimmerman et al. [50] | 1997 | 44 | Radiation-induced xerostomia. | 5.0 mg q.i.d for 18 months. | Oral pilocarpine usage during and 3 months thereafter radiotherapy, appeared to have contributed to a significantly less subjective xerostomia, as compared to patients excluded from pilocarpine treatment. |
| Vivino et al. [51] | 1999 | 373 | Primary or secondary Sjögren’s syndrome. | 2.5 mg or 5.0 mg q.i.d for 12 weeks. | Patients administrated with 5 mg pilocarpine 4 times daily reported a decent tolerance, as well as an important improvement in dry mouth symptoms. |
| Horiot et al. [52] | 2000 | 145 | Radiation-induced xerostomia. | 5.0 mg t.i.d for 12 weeks. | 97 patients (~67%) mentioned a significant alleviation of xerostomia’s symptoms at 12 weeks. 38 patients (26%) stopped treatment because of acute intolerance (sweating, nausea, vomiting) or no response. |
| Mateos et al. [53] | 2001 | 49 | Radiation-induced xerostomia. | 5.0 mg t.i.d (started the day before radiation treatment and continued throughout the first year of follow-up). | The differencies between patients treated with pilocarpine and those receiving placebo was not statistically significant. |
| Haddad et al. [54] | 2002 | 39 | Radiation-induced xerostomia. | 5.0 mg t.i.d (started with radiation and continued until 3 months after the end of radiotherapy. | As compared to placebo, pilocarpine administrated at radiotherapy was capable of resulting in a remarkable alleviation of xerostomia. |
| Warde et al. [55] | 2002 | 130 | Radiation-induced xerostomia. | 5.0 mg t.i.d (starting from day 1 of the radiation and continuing for 1 month after the end of the treatment). | No therapeutic effect of pilocarpine on radiation-induced xerostomia was pointed out. |
| Fisher et al. [56] (ClinicalTrials.gov ID: NCT00003139) | 2003 | 213 | Radiation-induced xerostomia. | 5.0 mg q.i.d (starting before the radiation treatment until 3 or 6 months after treatment). | Concomitant use of pilocarpine maintained and protected unstimulated salivary flow. |
| Gornitsky et al. [57] | 2004 | 58 | Radiation-induced xerostomia. | 1st study phase: 5.0 mg five times a day (from the first to the last day of radiation treatment) 2nd study phase (post-radiation): 5.0 mg q.i.d. | Pilocarpine reduced discomfort and pain symptoms as well. An improved global quality of life was reported only at the conclusion of the first study phase. |
| Papas et al. [58] (ClinicalTrials.gov ID: NCT04470479) | 2004 | 256 | Sjögren’s-related xerostomia. | 5.0 mg q.i.d for six weeks and then 7.5 mg q.i.d for the next 6 weeks. | A remarkable relief in dry mouth symptoms was noted at 20 mg/day. |
| Taweechaisupapong et al. [59] | 2006 | 33 | Radiation-induced xerostomia. | 3.0 or 5.0 mg every ten days. | There was statistically significant increase in salivary production in pilocarpine treatment groups vs. placebo. |
| Scarantino et al.[60] (ClinicalTrials.gov ID: NCT00003139) | 2006 | 245 | Radiation-induced xerostomia. | 5.0 mg q.i.d. | The overall results for salivary function at 3 and 6 months demonstrated statistically significant differences in favor of the pilocarpine arm for unstimulated salivary flow. |
| Wu et al. [61] | 2006 | 44 | Sjögren’s syndrome. | 5.0 mg q.i.d for 12 weeks. | Pilocarpine treatment managed a significant amelioration of mouth dryness-related symptoms and saliva production, as compared to placebo. |
| Chitapanarux et al. [62] | 2008 | 33 | Radiation-induced xerostomia. | 5.0 mg t.i.d (starting from the 1st of the radiation and continuing for 3 month after the end of the treatment). | Improvement of xerostomia symptoms was observed, with a mean total subjective xerostomia score improvement at the first 4 weeks of oral pilocarpine treatment. |
| Cifuentes et al. [23] (ClinicalTrials.gov ID: NCT04470479) | 2018 | 72 | Sjögren’s syndrome. | 5.0 mg t.i.d for 12 weeks. | Patients treated with pilocarpine showed a statistically significant improvement in their salivary flow, lachrymal flow, as well as their subjective global assessment, as compared to the patients administrated artificial saliva |
| Reference | Year of Publishment | Volunteers | Drug Formulation for Topical Administration | Reference/Outcome |
|---|---|---|---|---|
| Rhodus et al. [78] | 1992 | 18 | Pilocarpine ophthalmic solution | Both whole unstimulated salivary flow and parotid stimulated salivary flow presented a significant improvement in the pilocarpine group, compared to those of the placebo group. |
| Davies et al. [79] | 1994 | 20 | Mouthwash | The increased pilocarpine mouthwash effectiveness, compared to that of the artificial saliva in relieving the patients’ symptoms was noted by patients. |
| Hamlar et al. [86] | 1996 | 40 | Candy-like pastilles | The alleviation of subjective xerostomia’s symptoms was reported by 74% of patients. Moreover, the topical pilocarpine administration approached the same level of efficacy compared to previous delivery methods for radiation-induced xerostomia yet presenting the comparative advantage of a significantly improved patient tolerance. |
| Bernardi et al. [80] | 2002 | 40 | Mouthwash | The results of pilocarpine mouthwash solutions in increasing salivary flow in healthy participants was proved, with no adverse effects. |
| Frydrych et al. [38] | 2002 | 23 | Mouth spray | All patients treated with pilocarpine demonstrated improvement in stimulated and unstimulated salivary flow rates. Candida counts decreased among the cases. |
| Taweechaisupapong et al. [59] | 2006 | 33 | Lozenges | Salivary production in pilocarpine treatment group, as compared to that of the placebo group, appeared a statistically significant improvement. The 5 mg pilocarpine lozenge claims the top spot, as far as the clinical results are concerned. |
| Gibson et al. [91] | 2007 | 8 | Hydrogel buccal inserts | Better oral and ocular scores, along with a generally ameliorated saliva production were noted, while all patients, with the exception of one who wore dentures, agreed on the decent tolerance of the inserts. |
| Kim et al. [81] | 2014 | 60 | Mouthwash | The unstimulated whole salivary flow rate was increased. |
| Tanigawa et al. [82] | 2015 | 40 | Mouthwash | 47% of patients treated with pilocarpine reported an overall improvement. Moreover, following pilocarpine mouthwash treatment, the stimulated salivary flow rate appeared to be significantly increased, along with a predominant attenuation of side effects referred after pilocarpine mouthwash use to oral discomfort. |
| Park et al. [83] | 2015 | 12 | Mouthwash | The examined 2% pilocarpine solution as mouthwash increased salivary flow rate, compared to the placebo solution. Its efficacy was comparable to pilocarpine tablet, yet with the comparative advantage of presenting reduced side effects in healthy subjects. |
| Song et al. [84] | 2017 | 30 | Mouthwash | Pilocarpine mouthwash with at least 1.0% concentration and at a more-than- a-minute application might be clinically effective without any serious side effects. |
| Beatris et al. [85] | 2018 | 36 | Mouthwash | Treatment with pilocarpine mouthwash provided an increased salivation, without being followed any significant clinical adverse effect. |
| Watanabe et al.[93] | 2018 | 24 | Mouthwash | This new, low-dose pilocarpine formulation was well-tolerated and resulted to significant improvements in dry mouth symptoms and other xerostomic conditions in patients with Sjögren’s syndrome. |
| Santos Polvora et al. [87] | 2018 | 28 | Mouth spray | The pilocarpine spray significantly increased the salivary flow and alleviated xerostomia symptoms. |
| Pereira et al. [88] | 2020 | 40 | Mouth spray | The pilocarpine spray presented no significant differences as compared to placebo. |
| Sarideechaigul et al. [94] | 2021 | 31 | Artificial saliva | The evaluated formulations with were regarded as safe with minimum referred adverse effects. Specifically, while some adverse effects were in fact mentioned, they were not regarded as severe. |
| Gusmão et al. [95] | 2021 | 25 | Mucoadhesive tablets | The mucoadhesive tablets resulted to higher salivary concentrations of pilocarpine as compared to the conventional oral tablet. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapourani, A.; Kontogiannopoulos, K.N.; Barmpalexis, P. A Review on the Role of Pilocarpine on the Management of Xerostomia and the Importance of the Topical Administration Systems Development. Pharmaceuticals 2022, 15, 762. https://doi.org/10.3390/ph15060762
Kapourani A, Kontogiannopoulos KN, Barmpalexis P. A Review on the Role of Pilocarpine on the Management of Xerostomia and the Importance of the Topical Administration Systems Development. Pharmaceuticals. 2022; 15(6):762. https://doi.org/10.3390/ph15060762
Chicago/Turabian StyleKapourani, Afroditi, Konstantinos N. Kontogiannopoulos, and Panagiotis Barmpalexis. 2022. "A Review on the Role of Pilocarpine on the Management of Xerostomia and the Importance of the Topical Administration Systems Development" Pharmaceuticals 15, no. 6: 762. https://doi.org/10.3390/ph15060762
APA StyleKapourani, A., Kontogiannopoulos, K. N., & Barmpalexis, P. (2022). A Review on the Role of Pilocarpine on the Management of Xerostomia and the Importance of the Topical Administration Systems Development. Pharmaceuticals, 15(6), 762. https://doi.org/10.3390/ph15060762








