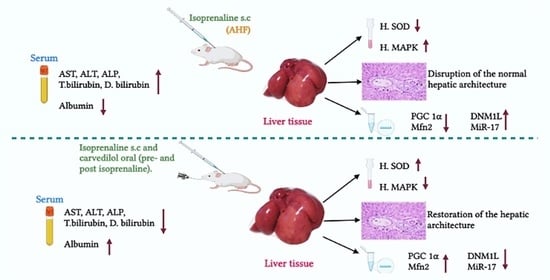
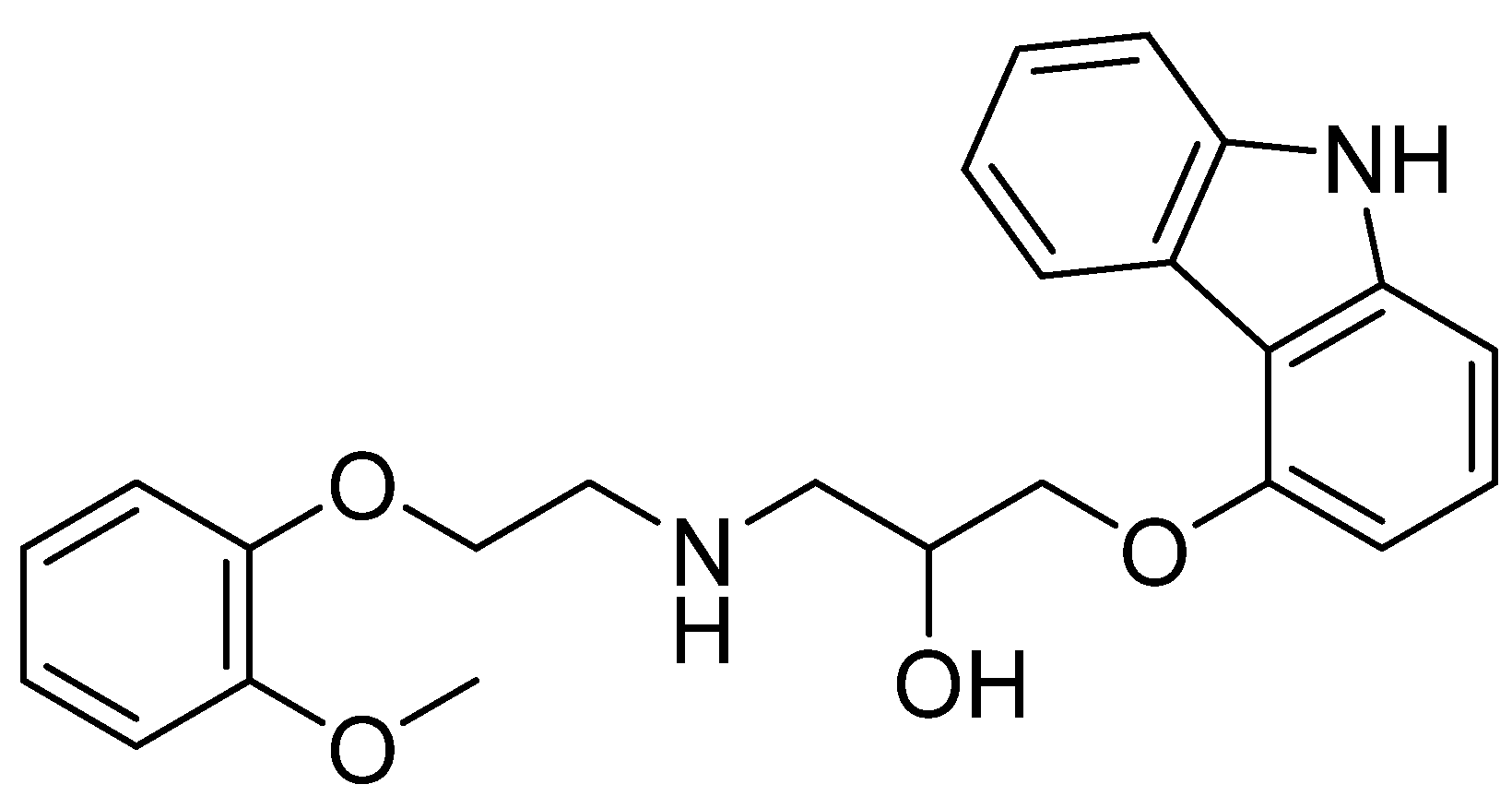
Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting miRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study
Abstract
:1. Introduction
2. Results
2.1. Biochemical Analysis
2.1.1. Effect of Hepatic Ischemia Associated with AHF on Liver Function and Assessment of Carvedilol Administration
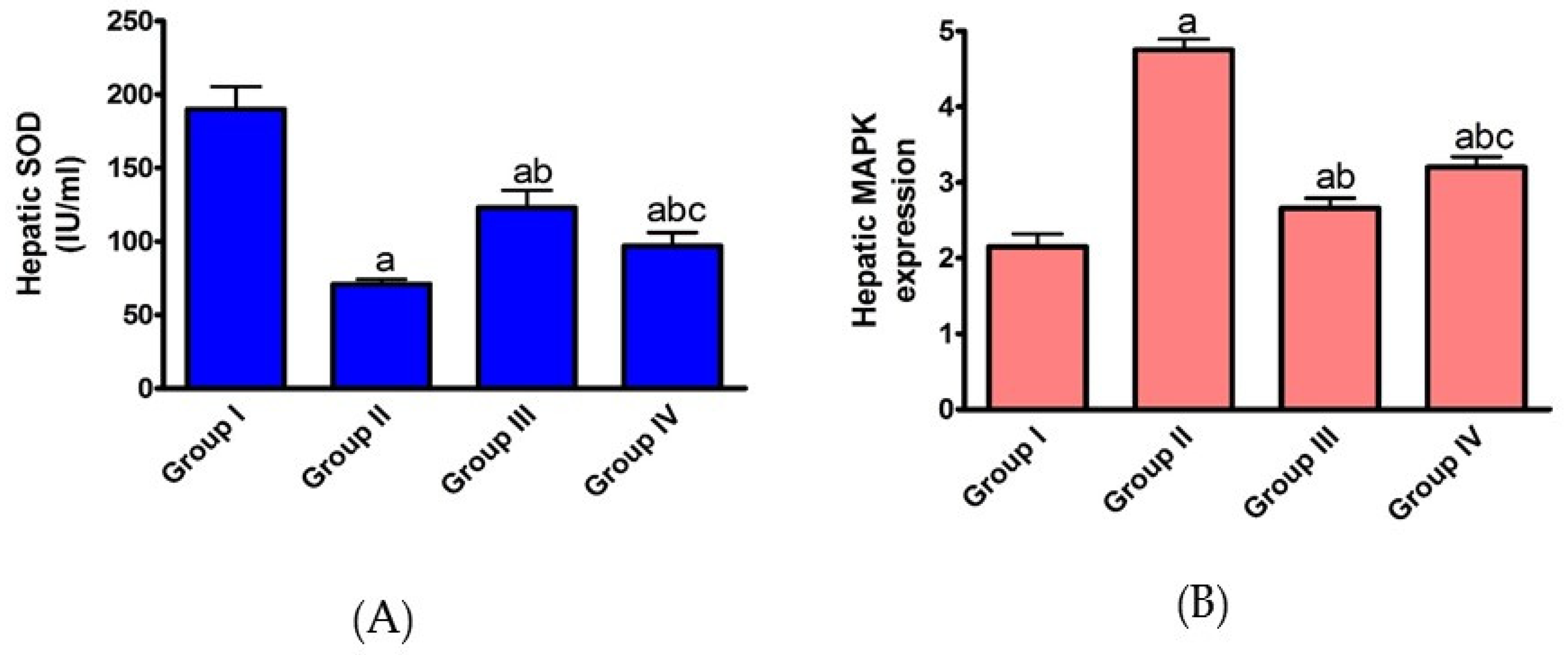
2.1.2. Effect of AHF-Induced Hepatic Ischemia on the Expression of Oxidative Stress Markers and Influence of Carvedilol Administration
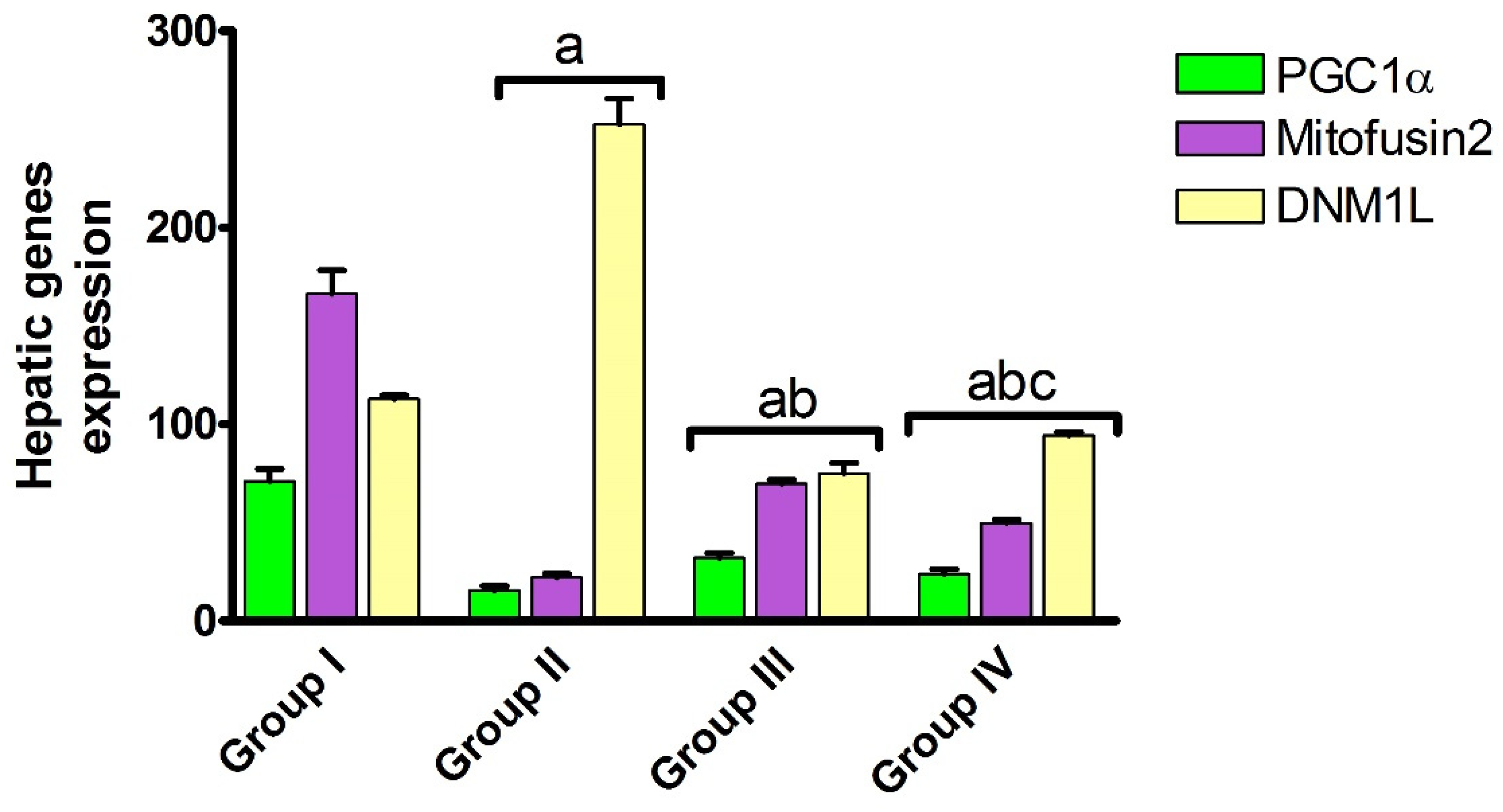
2.1.3. Effect of Carvedilol Treatment on the Expression of PGC-1α, Mitofusin 2, and DNM1L Proteins in Hepatic Ischemia Associated with AHF
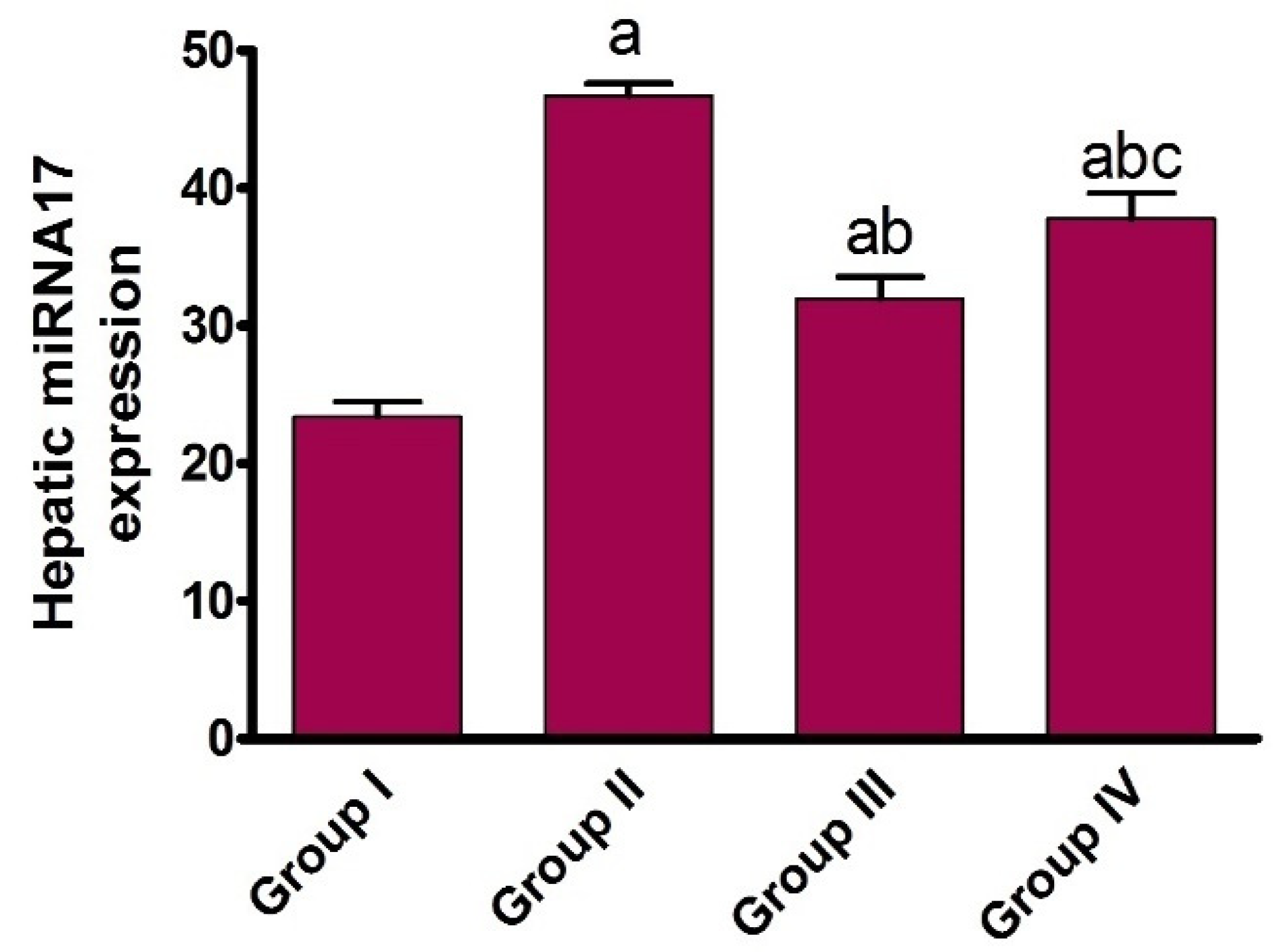
2.1.4. Effect of AHF-Induced Hepatic Ischemia on miRNA-17 Expression and Assessment of Carvedilol Administration
2.2. Histological Analysis
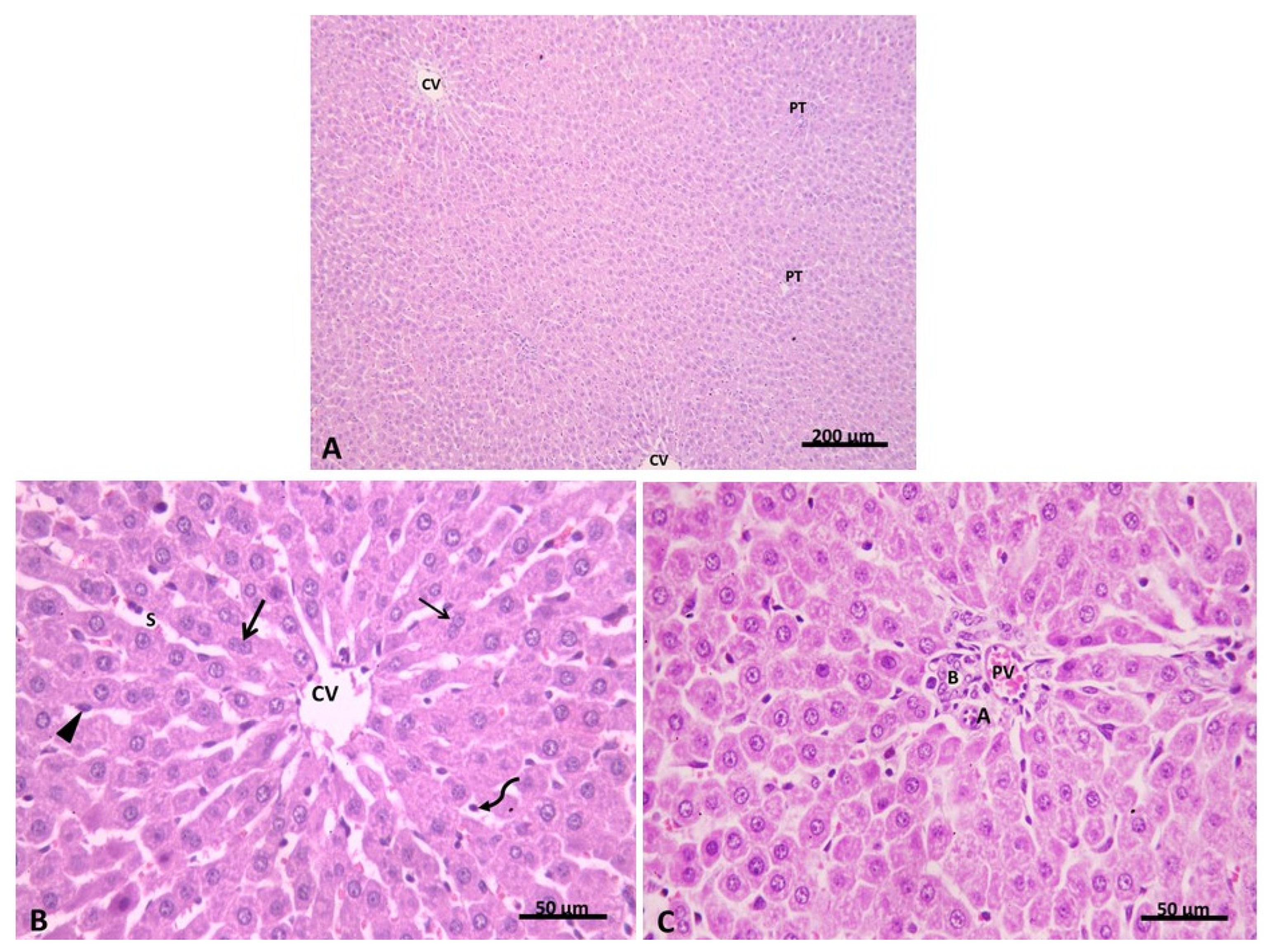
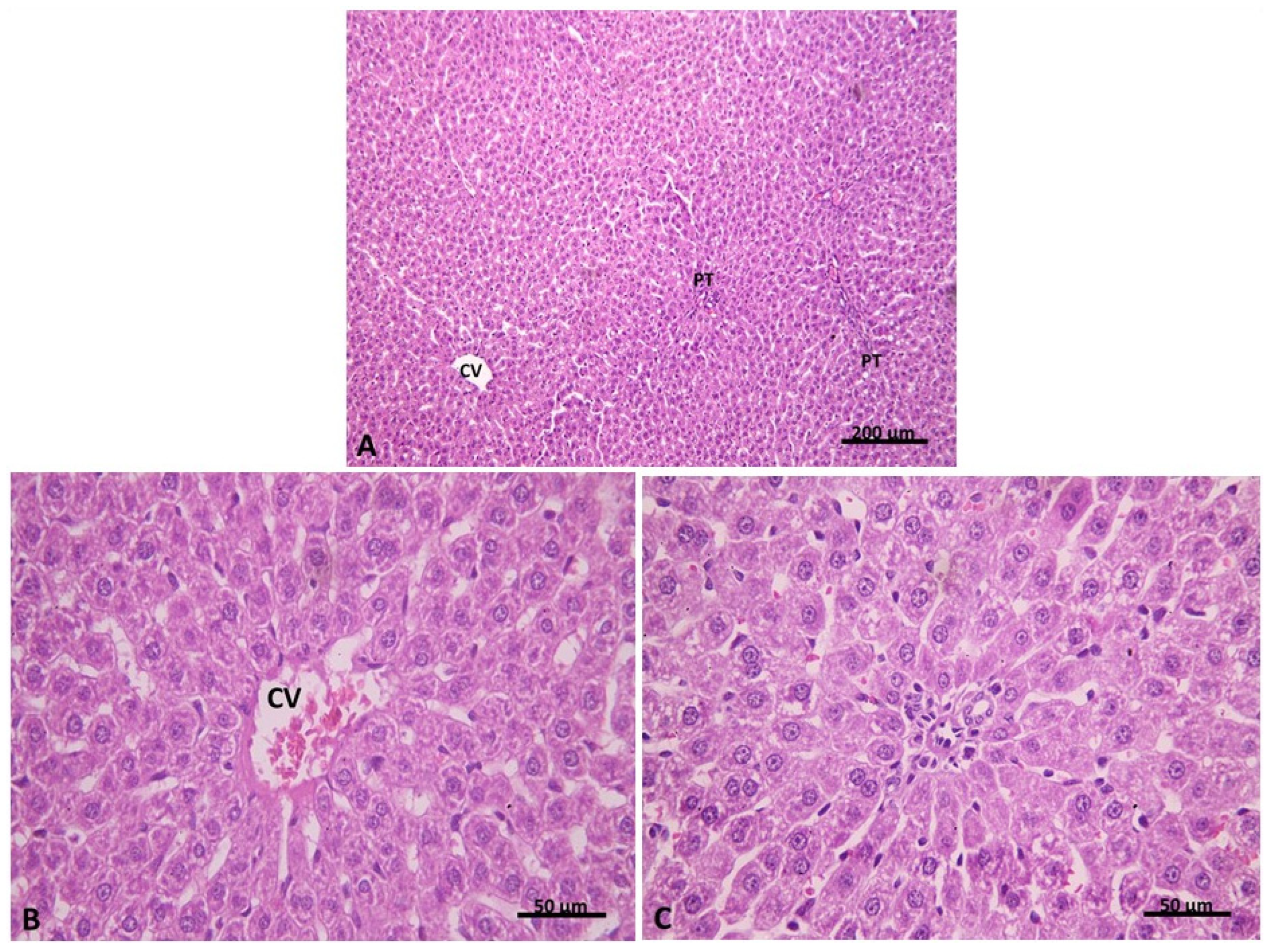
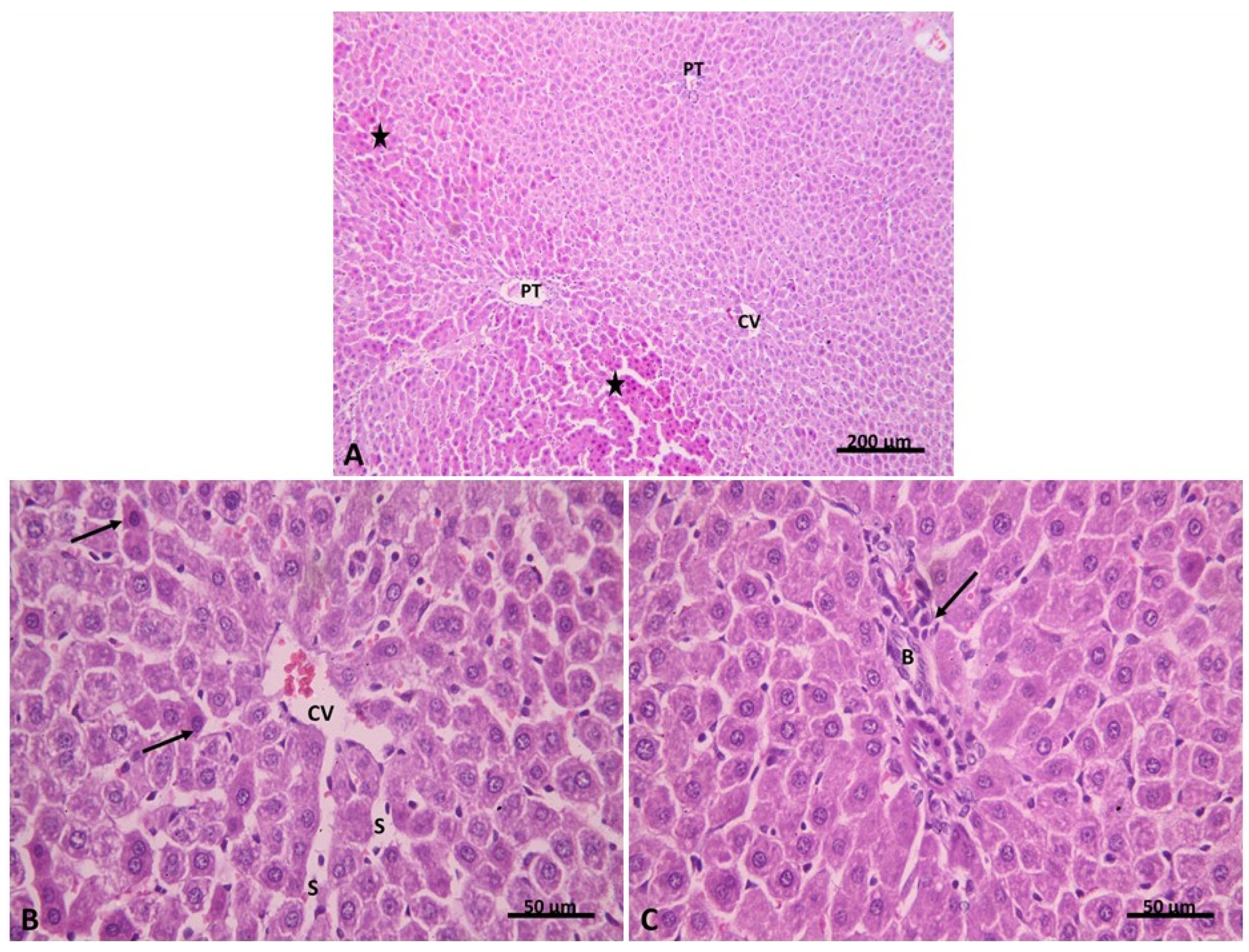
2.2.1. Light Microscopic Analysis
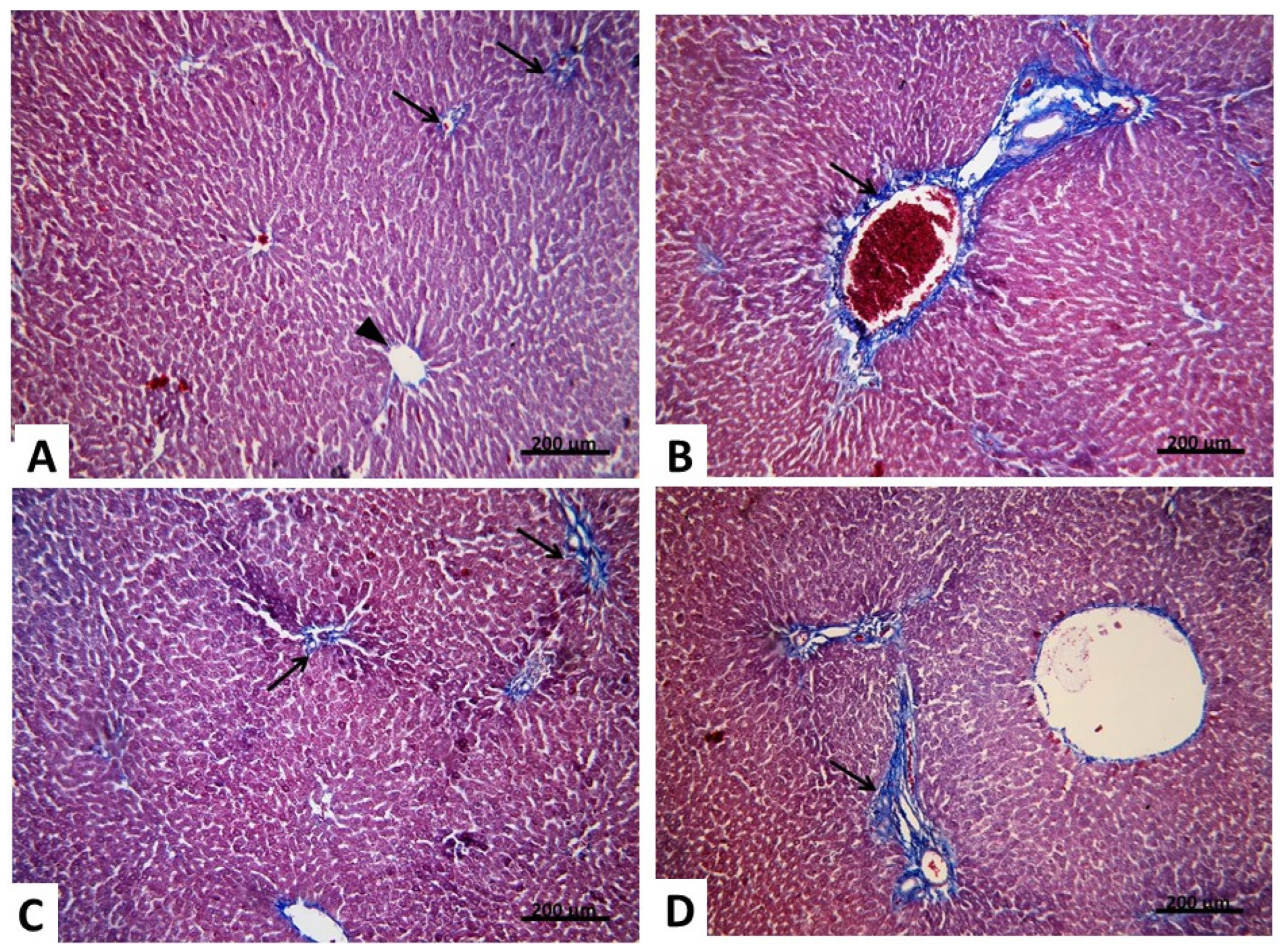
2.2.2. Light Microscopic Analysis (Mallory’s Trichrome Stain)
2.2.3. Morphometric and Statistical Results of Mallory’s Trichrome Stain
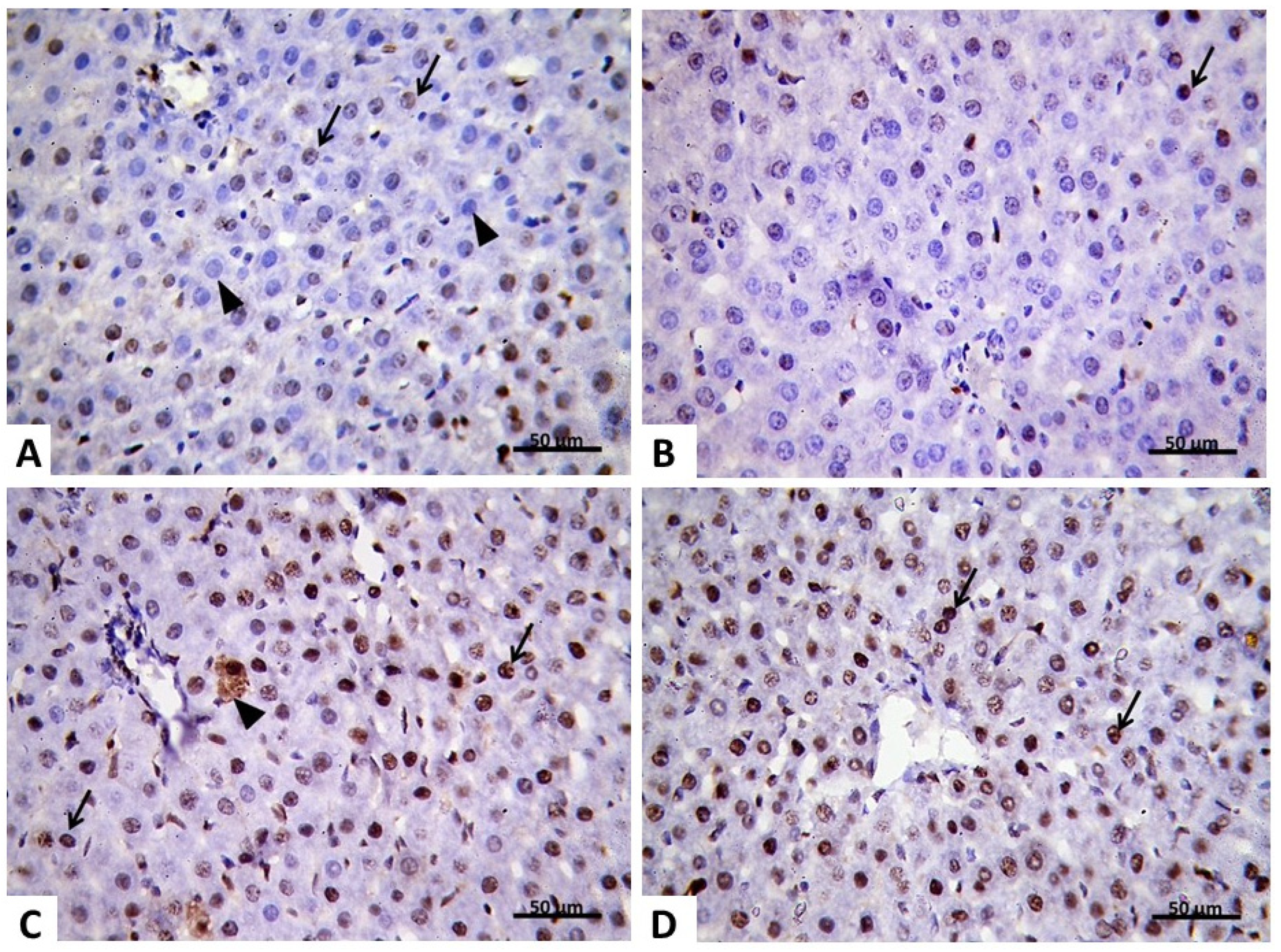
2.2.4. Immunohistochemical Analysis
2.2.5. Morphometric Analysis of PCNA Results
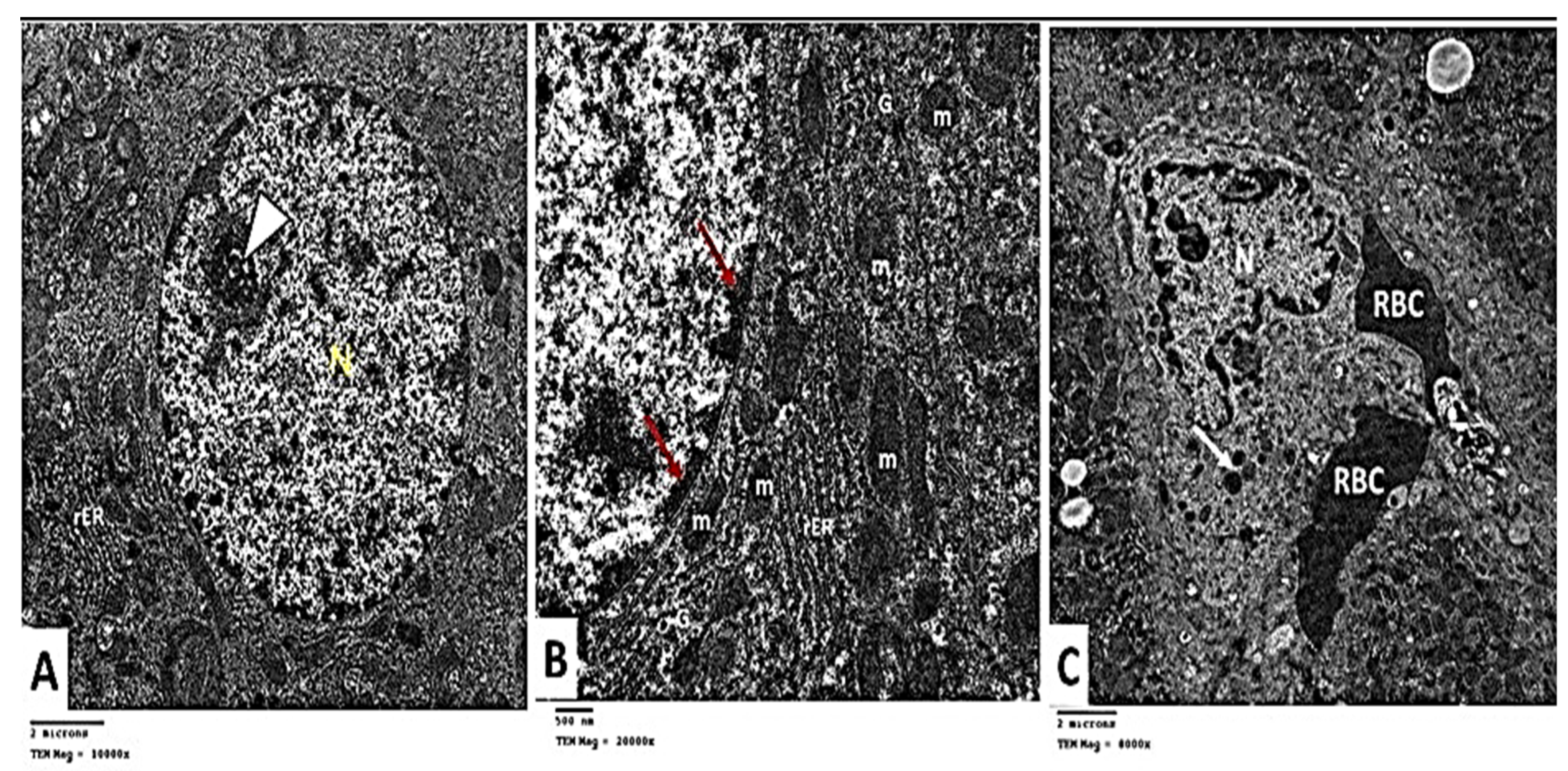
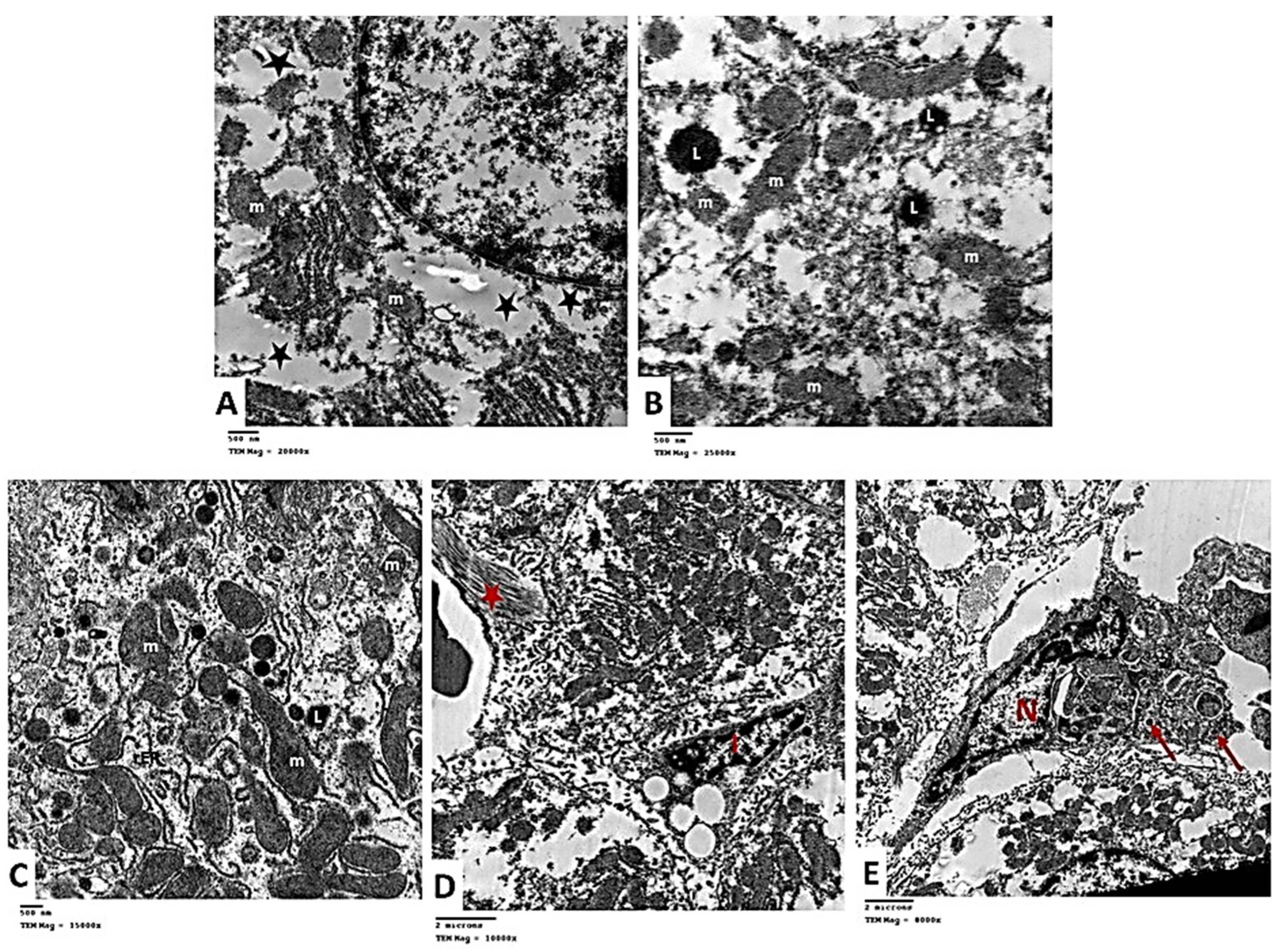
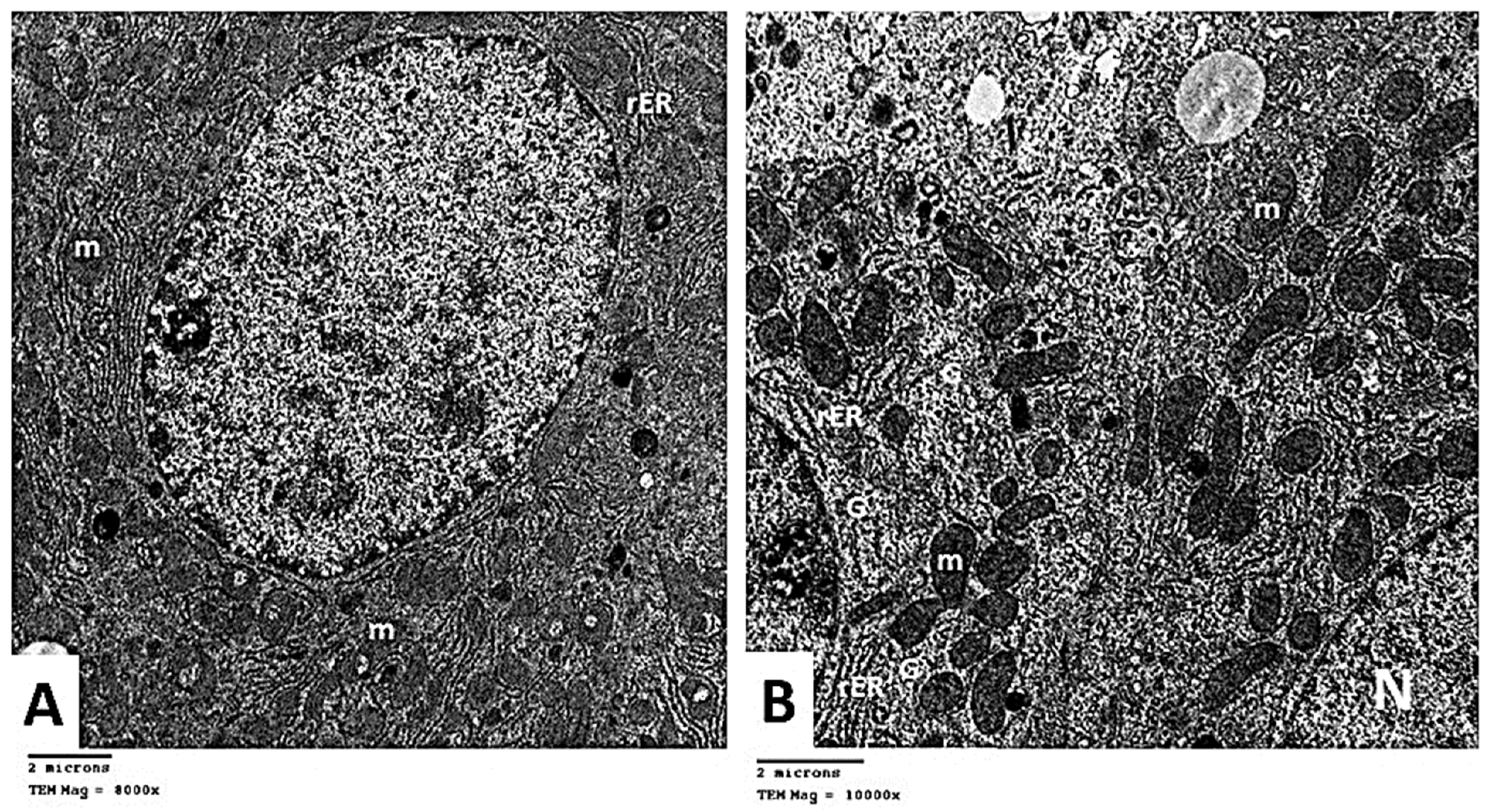
2.2.6. Transmission Electron Microscopic (TEM) Analysis
2.3. In Silico Molecular Docking Study
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Groups and Animals
4.3. Induction of Hepatic Hypoperfusion
4.4. Samples Processing
- Blood samples
- 2.
- Liver tissue samples
4.5. Biochemical Assays
4.5.1. Evaluation of Liver Function
4.5.2. Assessment of the Activity of Hepatic Superoxide Dismutase Enzyme (SOD)
4.5.3. Isolation of miRNA-17
4.5.4. Assessment of miRNA-17, MAPK, PGC 1α, Mitofusin 2, and Dnm1l Expression by qPCR
4.6. Histological Studies
4.6.1. Light Microscopic Study
- Histopathological grading and scoring of liver changes (H&E stain):
4.6.2. Immunohistochemical Study
4.6.3. Morphometric Studies
- The mean proportion of positive immune reactions of PCNA
- The average number of nuclei exhibiting a significant immunological response to PCNA.
- Mean area percentage of collagen fibers stained by Mallory’s trichrome
4.6.4. Transmission Electron Microscopic Study (TEM)
4.7. In Silico Molecular Modelling Study
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- King, J.; Lowery, D.R. Physiology, Cardiac Output. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Alvarez, A.M.; Mukherjee, D. Liver Abnormalities in Cardiac Diseases and Heart Failure. Int. J. Angiol. Off. Publ. Int. Coll. Angiol. Inc. 2011, 20, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Eipel, C.; Abshagen, K.; Vollmar, B. Regulation of Hepatic Blood Flow: The Hepatic Arterial Buffer Response Revisited. World J. Gastroenterol. 2010, 16, 6046–6057. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.P.; Cerini, R.; Sayegh, R.; Moreau, R.; Degott, C.; Lebrec, D.; Lee, S.S. Cardiac Hepatopathy: Clinical, Hemodynamic, and Histologic Characteristics and Correlations. Hepatol. Baltim. Md. 2003, 37, 393–400. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Nakache, R.; Rudick, V.; Fiodorov, D.; Klausner, J.M.; Almogy, N.; Karckevski, E.; Weinbroum, A. Cumulative Damaging Effect of Liver Hypoperfusion and Cyclosporine a on the Peribiliary Capillary Plexus: A Study in an Isolated Dually Perfused Rat Model. Transplantation 1999, 68, 1651–1660. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The Role of Reactive Oxygen Species in the Pathophysiology of Cardiovascular Diseases and the Clinical Significance of Myocardial Redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.-L.; Shin, H.-J.; Feitelson, M.A.; Yu, D.-Y. Oxidative Stress and Antioxidants in Hepatic Pathogenesis. World J. Gastroenterol. WJG 2010, 16, 6035–6043. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and Molecular Mechanisms of Mitochondrial Function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and Reactive Oxygen Species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Tsutsui, H. Role of oxidative stress in heart failure. Nihon Rinsho Jpn. J. Clin. Med. 2003, 61, 756–760. [Google Scholar]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial Fusion, Fission, and Mitochondrial Toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; McStay, G.P. Regulation of Mitochondrial Dynamics by Proteolytic Processing and Protein Turnover. Antioxidants 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandhok, G.; Lazarou, M.; Neumann, B. Structure, Function, and Regulation of Mitofusin-2 in Health and Disease. Biol. Rev. Camb. Philos. Soc. 2018, 93, 933–949. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, B.; Qin, Y.; Li, A.; Gao, M.; Liu, H.; Gong, G. Mitochondrial Fusion Protein Mfn2 and Its Role in Heart Failure. Front. Mol. Biosci. 2021, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Berman, S.; Pineda, F.; Hardwick, J. Mitochondrial Fission and Fusion Dynamics: The Long and Short of It. Cell Death Differ. 2008, 15, 1147–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawan, A.; Bennett, A. Mitogen-Activated Protein Kinase Regulation in Hepatic Metabolism. Trends Endocrinol. Metab. TEM 2017, 28, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Lawan, A.; Zhang, L.; Gatzke, F.; Min, K.; Jurczak, M.J.; Al-Mutairi, M.; Richter, P.; Camporez, J.P.G.; Couvillon, A.; Pesta, D.; et al. Hepatic Mitogen-Activated Protein Kinase Phosphatase 1 Selectively Regulates Glucose Metabolism and Energy Homeostasis. Mol. Cell. Biol. 2015, 35, 26–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabrowska, A.; Venero, J.L.; Iwasawa, R.; Hankir, M.; Rahman, S.; Boobis, A.; Hajji, N. PGC-1α Controls Mitochondrial Biogenesis and Dynamics in Lead-Induced Neurotoxicity. Aging 2015, 7, 629–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, e1452696. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, Q.; Wang, Y.; Wei, S.; Yang, L.; Zhang, J.; Liu, C.; et al. Irisin Alleviates Liver Ischemia-Reperfusion Injury by Inhibiting Excessive Mitochondrial Fission, Promoting Mitochondrial Biogenesis and Decreasing Oxidative Stress. Redox Biol. 2019, 20, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Valinezhad Orang, A.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of MiRNA-Mediated Gene Regulation from Common Downregulation to MRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, e970607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingram, H.; Dogan, M.; Eason, J.D.; Kuscu, C.; Kuscu, C. MicroRNAs: Novel Targets in Hepatic Ischemia–Reperfusion Injury. Biomedicines 2022, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Wang, Z.; Wang, T.; Yu, Y.; He, J.; Zhang, H.; Yang, T.; Shen, Z. MicroRNA-17 Regulates Autophagy to Promote Hepatic Ischemia/Reperfusion Injury via Suppression of Signal Transductions and Activation of Transcription-3 Expression. Liver Transpl. 2016, 22, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Sabet Sarvestani, F.; Azarpira, N.; Al-Abdullah, I.H.; Tamaddon, A.-M. MicroRNAs in Liver and Kidney Ischemia Reperfusion Injury: Insight to Improve Transplantation Outcome. Biomed. Pharmacother. 2021, 133, 110944. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.-H.; Wu, T.; Li, S.; Sun, Y. MicroRNA-17-5p Acts as a Biomarker and Regulates Mitochondrial Dynamics in Trophoblasts and Endothelial Cells by Targeting the Mitofusins Mfn1/Mfn2 in Gestational Diabetes Mellitus. Arch. Med. Sci. 2022. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Gonçalves, L.; Monteiro, P.; Providencia, L.A.; Moreno, A.J. Are the Antioxidant Properties of Carvedilol Important for the Protection of Cardiac Mitochondria? Curr. Vasc. Pharmacol. 2005, 3, 147–158. [Google Scholar] [CrossRef]
- Arozal, W.; Watanabe, K.; Veeraveedu, P.T.; Ma, M.; Thandavarayan, R.A.; Sukumaran, V.; Suzuki, K.; Kodama, M.; Aizawa, Y. Protective Effect of Carvedilol on Daunorubicin-Induced Cardiotoxicity and Nephrotoxicity in Rats. Toxicology 2010, 274, 18–26. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Esteves, T.; Rolo, A.P.; Palmeira, C.M.; Moreno, A.J.M. Carvedilol Inhibits the Mitochondrial Permeability Transition by an Antioxidant Mechanism. Cardiovasc. Toxicol. 2004, 4, 11–20. [Google Scholar] [CrossRef] [Green Version]
- El-Demerdash, E.; Abdel-Sattar, S.A.; El-Bakly, W.M.; Mohamed, E.A. Antifibrotic Effects of Carvedilol and Impact of Liver Fibrosis on Carvedilol Pharmacokinetics in a Rat Model. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 767–779. [Google Scholar] [CrossRef]
- Flesch, M.; Ettelbruck, S.; Rosenkranz, S.; Maack, C.; Cremers, B.; Schluter, K.-D.; Zolk, O.; Bohm, M. Differential Effects of Carvedilol and Metoprolol on Isoprenaline-Induced Changes in b-Adrenoceptor Density and Systolic Function in Rat Cardiac Myocytes. Cardiovasc. Res. 2001, 49, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Kalia, R.; Wang, R.Y.-R.; Yusuf, A.; Thomas, P.V.; Agard, D.A.; Shaw, J.M.; Frost, A. Structural Basis of Mitochondrial Receptor Binding and Constriction by DRP1. Nature 2018, 558, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Wenger, J.; Klinglmayr, E.; Fröhlich, C.; Eibl, C.; Gimeno, A.; Hessenberger, M.; Puehringer, S.; Daumke, O.; Goettig, P. Functional Mapping of Human Dynamin-1-like GTPase Domain Based on x-Ray Structure Analyses. PLoS ONE 2013, 8, e71835. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, C.; Grabiger, S.; Schwefel, D.; Faelber, K.; Rosenbaum, E.; Mears, J.; Rocks, O.; Daumke, O. Structural Insights into Oligomerization and Mitochondrial Remodelling of Dynamin 1-like Protein. EMBO J. 2013, 32, 1280–1292. [Google Scholar] [CrossRef] [Green Version]
- Kishida, H.; Sugio, S. Crystal Structure of GTPase Domain Fused with Minimal Stalks from Human Dynamin-1-like Protein (Dlp1) in Complex with Several Nucleotide Analogues. Curr Top Pept Protein Res 2013, 14, 67–77. [Google Scholar]
- Ma, J.; Zhai, Y.; Chen, M.; Zhang, K.; Chen, Q.; Pang, X.; Sun, F. New Interfaces on MiD51 for Drp1 Recruitment and Regulation. PLoS ONE 2019, 14, e0211459. [Google Scholar] [CrossRef] [Green Version]
- Richter, V.; Palmer, C.S.; Osellame, L.D.; Singh, A.P.; Elgass, K.; Stroud, D.A.; Sesaki, H.; Kvansakul, M.; Ryan, M.T. Structural and Functional Analysis of MiD51, a Dynamin Receptor Required for Mitochondrial Fission. J. Cell Biol. 2014, 204, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [Green Version]
- Allawadhi, P.; Khurana, A.; Sayed, N.; Kumari, P.; Godugu, C. Isoproterenol-Induced Cardiac Ischemia and Fibrosis: Plant-Based Approaches for Intervention. Phytother. Res. PTR 2018, 32, 1908–1932. [Google Scholar] [CrossRef] [Green Version]
- Akter, N.; Chowdhury, F.I.; Selim, S.; Nayan, S.I.; Khan, F.; Subhan, N.; Hossain, H.; Rahman, M.M.; Haque, M.A.; Alam, M.A. Polyphenolics in Ramontchi Protect Cardiac Tissues via Suppressing Isoprenaline-Induced Oxidative Stress and Inflammatory Responses in Long-Evans Rats. J. Funct. Foods 2020, 75, 104250. [Google Scholar] [CrossRef]
- Copik, A.J.; Baldys, A.; Nguyen, K.; Sahdeo, S.; Ho, H.; Kosaka, A.; Dietrich, P.J.; Fitch, B.; Raymond, J.R.; Ford, A.P.D.W.; et al. Isoproterenol Acts as a Biased Agonist of the Alpha-1A-Adrenoceptor That Selectively Activates the MAPK/ERK Pathway. PLoS ONE 2015, 10, e0115701. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanzadeh-Moghadam, M.; Khadem-Ansari, M.H.; Farjah, G.H.; Rasmi, Y. Hepatoprotective Effects of Betaine on Liver Damages Followed by Myocardial Infarction. Vet. Res. Forum 2018, 9, 129–135. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, P.K.; Raina, R.; Sood, S.; Prawez, S.; Manhas, L. Isoprenaline Induced Hepatic Alterations and Modulation by Hydroalcoholic Extract of Juglans Regia Hull in Wistar Rats. Toxicol. Int. 2020, 27, 58–69. [Google Scholar] [CrossRef]
- Long, Y.; Wei, H.; Li, J.; Li, M.; Wang, Y.; Zhang, Z.; Cao, T.; Carlos, C.; German, L.G.; Jiang, D.; et al. Prevention of Hepatic Ischemia-Reperfusion Injury by Carbohydrate-Derived Nanoantioxidants. Nano Lett. 2020, 20, 6510–6519. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhang, Y.; Wang, X.; Gao, B.; Li, Y.; Li, R.; Wang, J. Protective Effects of Fisetin on Hepatic Ischemia-Reperfusion Injury Through Alleviation of Apoptosis and Oxidative Stress. Arch. Med. Res. 2021, 52, 163–173. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. MMBR 2011, 75, 50–83. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Wang, Y.; Zhan, X. The MAPK Pathway-Based Drug Therapeutic Targets in Pituitary Adenomas. Front. Endocrinol. 2019, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Wu, L.; Tashiro, S.; Onodera, S.; Uchiumi, F.; Ikejima, T. Activation of Extracellular Signal-Regulated Kinase during Silibinin-Protected, Isoproterenol-Induced Apoptosis in Rat Cardiac Myocytes Is Tyrosine Kinase Pathway-Mediated and Protein Kinase C-Dependent. Acta Pharmacol. Sin. 2007, 28, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, S.; St-Pierre, J. PGC1α and Mitochondrial Metabolism—Emerging Concepts and Relevance in Ageing and Neurodegenerative Disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpulla, R.C. Metabolic Control of Mitochondrial Biogenesis through the PGC-1 Family Regulatory Network. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldelli, S.; Aquilano, K.; Ciriolo, M.R. PGC-1α Buffers ROS-Mediated Removal of Mitochondria during Myogenesis. Cell Death Dis. 2014, 5, e1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halling, J.F.; Pilegaard, H. PGC-1α-Mediated Regulation of Mitochondrial Function and Physiological Implications. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Lehman, J.J.; Kelly, D.P. Transcriptional Activation Of Energy Metabolic Switches In The Developing And Hypertrophied Heart. Clin. Exp. Pharmacol. Physiol. 2002, 29, 339–345. [Google Scholar] [CrossRef]
- Luan, A.; Tang, F.; Yang, Y.; Lu, M.; Wang, H.; Zhang, Y. Astragalus Polysaccharide Attenuates Isoproterenol-Induced Cardiac Hypertrophy by Regulating TNF-α/PGC-1α Signaling Mediated Energy Biosynthesis. Environ. Toxicol. Pharmacol. 2015, 39, 1081–1090. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, H.; Chen, W.; Cui, Y.; Huang, A.; Qi, X. Perindopril Improves Cardiac Function by Enhancing the Expression of SIRT3 and PGC-1α in a Rat Model of Isoproterenol-Induced Cardiomyopathy. Front. Pharmacol. 2020, 11, 94. [Google Scholar] [CrossRef]
- Hasan, P.; Saotome, M.; Ikoma, T.; Iguchi, K.; Kawasaki, H.; Iwashita, T.; Hayashi, H.; Maekawa, Y. Mitochondrial Fission Protein, Dynamin-Related Protein 1, Contributes to the Promotion of Hypertensive Cardiac Hypertrophy and Fibrosis in Dahl-Salt Sensitive Rats. J. Mol. Cell. Cardiol. 2018, 121, 103–106. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Eros, K.; Szabo, A.; Magyar, K.; Sumegi, B.; Toth, K.; Halmosi, R.; Szabados, E. Doxycycline Protects against ROS-Induced Mitochondrial Fragmentation and ISO-Induced Heart Failure. PLoS ONE 2017, 12, e0175195. [Google Scholar] [CrossRef]
- Chen, X.; Liang, J.; Bin, W.; Luo, H.; Yang, X. Anti-Hyperlipidemic, Anti-Inflammatory, and Ameliorative Effects of DRP1 Inhibition in Rats with Experimentally Induced Myocardial Infarction. Cardiovasc. Toxicol. 2021, 21, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Chen, W.; Pan, F.; Peng, B.; Gong, J. Role of Mitofusin 2 in the Protective Effect of Breviscapine against Hepatic Ischemia/Reperfusion Injury in Rats. Exp. Ther. Med. 2018, 15, 3582–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, T.; Zheng, M.; Cao, C.; Chen, C.; Tang, J.; Zhang, W.; Cheng, H.; Chen, K.-H.; Xiao, R.-P. Mitofusin-2 Is a Major Determinant of Oxidative Stress-Mediated Heart Muscle Cell Apoptosis. J. Biol. Chem. 2007, 282, 23354–23361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ke, W.; Zhou, Q.; Wu, Y.; Luo, H.; Zhou, H.; Yang, B.; Guo, Y.; Zheng, Q.; Zhang, Y. Tumour Necrosis Factor-α Promotes Liver Ischaemia-Reperfusion Injury through the PGC-1α/Mfn2 Pathway. J. Cell. Mol. Med. 2014, 18, 1863–1873. [Google Scholar] [CrossRef]
- Vadnais, M.L.; Lin, A.M.; Gerton, G.L. Mitochondrial Fusion Protein MFN2 Interacts with the Mitostatin-Related Protein MNS1 Required for Mouse Sperm Flagellar Structure and Function. Cilia 2014, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Xue, R.-Q.; Sun, L.; Yu, X.-J.; Li, D.-L.; Zang, W.-J. Vagal Nerve Stimulation Improves Mitochondrial Dynamics via an M3 Receptor/CaMKKβ/AMPK Pathway in Isoproterenol-Induced Myocardial Ischaemia. J. Cell. Mol. Med. 2017, 21, 58–71. [Google Scholar] [CrossRef]
- Wang, W.; Dong, R.; Guo, Y.; He, J.; Shao, C.; Yi, P.; Yu, F.; Gu, D.; Zheng, J. CircMTO1 Inhibits Liver Fibrosis via Regulation of MiR-17-5p and Smad7. J. Cell. Mol. Med. 2019, 23, 5486–5496. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Li, K.; Li, Y.; Ding, G.-Q.; Lu, X.-L.; Wang, H.; Liang, X.-Y.; Wang, J.; Meng, Y.-Y.; Zhang, L.; et al. Serum MiR-17 Levels in Patients with Hepatitis B Virus Induced Liver Fibrosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6245–6251. [Google Scholar] [CrossRef]
- Zaafan, M.A.; Abdelhamid, A.M. Dasatinib Ameliorates Thioacetamide-Induced Liver Fibrosis: Modulation of MiR-378 and MiR-17 and Their Linked Wnt/β-Catenin and TGF-β/Smads Pathways. J. Enzyme Inhib. Med. Chem. 2022, 37, 118–124. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, S.; Zhou, H. LncRNA H19 Regulates Proliferation, Apoptosis and ECM Degradation of Aortic Smooth Muscle Cells Via MiR-1-3p/ADAM10 Axis in Thoracic Aortic Aneurysm. Biochem. Genet. 2021, 60, 790–806. [Google Scholar] [CrossRef]
- Chen, H.; Xue, J.; Zhang, Y.; Zhu, X.; Gao, J.; Yu, B. Comparison of Quantum Dots Immunofluorescence Histochemistry and Conventional Immunohistochemistry for the Detection of Caveolin-1 and PCNA in the Lung Cancer Tissue Microarray. J. Mol. Histol. 2009, 40, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Boudonas, G.E. β-Blockers in Coronary Artery Disease Management. Hippokratia 2010, 14, 231–235. [Google Scholar] [PubMed]
- Book, W.M. Carvedilol: A Nonselective β Blocking Agent With Antioxidant Properties. Congest. Heart Fail. 2002, 8, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Serna-Salas, S.A.; Navarro-González, Y.D.; Martínez-Hernández, S.L.; Barba-Gallardo, L.F.; Sánchez-Alemán, E.; Aldaba-Muruato, L.R.; Macías-Pérez, J.R.; Ventura-Juárez, J.; Muñoz-Ortega, M.H. Doxazosin and Carvedilol Treatment Improves Hepatic Regeneration in a Hamster Model of Cirrhosis. BioMed Res. Int. 2018, 2018, e4706976. [Google Scholar] [CrossRef]
- Singh, S.; Preuss, C.V. Carvedilol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Li, T.; Yuan, G.; Ma, C.; Jin, P.; Zhou, C.; Li, W. Clinical Efficacy of Carvedilol Treatment for Dilated Cardiomyopathy. Medicine 2019, 98, e15403. [Google Scholar] [CrossRef]
- Keating, G.M.; Jarvis, B. Carvedilol: A Review of Its Use in Chronic Heart Failure. Drugs 2003, 63, 1697–1741. [Google Scholar] [CrossRef]
- Baraka, S.A.; Tolba, M.F.; Elsherbini, D.A.; El-Naga, R.N.; Awad, A.S.; El-Demerdash, E. Rosuvastatin and Low-Dose Carvedilol Combination Protects against Isoprenaline-Induced Myocardial Infarction in Rats: Role of PI3K/Akt/Nrf2/HO-1 Signalling. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1358–1370. [Google Scholar] [CrossRef]
- Abreu, R.M.; Santos, D.J.; Moreno, A.J. Effects of Carvedilol and Its Analog BM-910228 on Mitochondrial Function and Oxidative Stress. J. Pharmacol. Exp. Ther. 2000, 295, 1022–1030. [Google Scholar]
- Wang, Z.; Niu, Q.; Peng, X.; Li, M.; Liu, Y.; Liu, J.; Wen, S.; Wei, Y. Mitofusin 2 Ameliorates Aortic Remodeling by Suppressing Ras/Raf/ERK Pathway and Regulating Mitochondrial Function in Vascular Smooth Muscle Cells. Int. J. Cardiol. 2015, 178, 165–167. [Google Scholar] [CrossRef]
- Yao, K.; Zhang, W.W.; Yao, L.; Yang, S.; Nie, W.; Huang, F. Carvedilol Promotes Mitochondrial Biogenesis by Regulating the PGC-1/TFAM Pathway in Human Umbilical Vein Endothelial Cells (HUVECs). Biochem. Biophys. Res. Commun. 2016, 470, 961–966. [Google Scholar] [CrossRef]
- Meng, D.; Li, Z.; Wang, G.; Ling, L.; Wu, Y.; Zhang, C. Carvedilol Attenuates Liver Fibrosis by Suppressing Autophagy and Promoting Apoptosis in Hepatic Stellate Cells. Biomed. Pharmacother. Biomedecine Pharmacother. 2018, 108, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhao, C.; Guo, J.; Xie, S.; Yin, F.; Huo, X.; Zhang, X. Carvedilol Attenuates the Progression of Hepatic Fibrosis Induced by Bile Duct Ligation. BioMed Res. Int. 2017, 2017, e4612769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Li, Z.; Xiu, A.-Y.; Meng, D.-X.; Wang, S.-N.; Zhang, C.-Q. Carvedilol Attenuates Carbon Tetrachloride-Induced Liver Fibrosis and Hepatic Sinusoidal Capillarization in Mice. Drug Des. Devel. Ther. 2019, 13, 2667–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, I.; Youle, R.J. Mitochondrial Fission and Fusion. Essays Biochem. 2010, 47, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, E.; Shurland, D.L.; Ryazantsev, S.N.; van der Bliek, A.M. A Human Dynamin-Related Protein Controls the Distribution of Mitochondria. J. Cell Biol. 1998, 143, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Sheffer, R.; Douiev, L.; Edvardson, S.; Shaag, A.; Tamimi, K.; Soiferman, D.; Meiner, V.; Saada, A. Postnatal Microcephaly and Pain Insensitivity Due to a de Novo Heterozygous DNM1L Mutation Causing Impaired Mitochondrial Fission and Function. Am. J. Med. Genet. A. 2016, 170, 1603–1607. [Google Scholar] [CrossRef]
- Fahrner, J.A.; Liu, R.; Perry, M.S.; Klein, J.; Chan, D.C. A Novel de Novo Dominant Negative Mutation in DNM1L Impairs Mitochondrial Fission and Presents as Childhood Epileptic Encephalopathy. Am. J. Med. Genet. A. 2016, 170, 2002–2011. [Google Scholar] [CrossRef] [Green Version]
- Waterham, H.R.; Koster, J.; van Roermund, C.W.T.; Mooyer, P.A.W.; Wanders, R.J.A.; Leonard, J.V. A Lethal Defect of Mitochondrial and Peroxisomal Fission. N. Engl. J. Med. 2007, 356, 1736–1741. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, H.; Chen, S.; Du, F.; Wang, X. The Mitochondrial Phosphatase PGAM5 Functions at the Convergence Point of Multiple Necrotic Death Pathways. Cell 2012, 148, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The Role of Dynamin-Related Protein 1, a Mediator of Mitochondrial Fission, in Apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Palmer, C.S.; Osellame, L.D.; Laine, D.; Koutsopoulos, O.S.; Frazier, A.E.; Ryan, M.T. MiD49 and MiD51, New Components of the Mitochondrial Fission Machinery. EMBO Rep. 2011, 12, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, T.; Jin, S.; Wang, X.; Qu, M.; Uhlén, P.; Tomilin, N.; Shupliakov, O.; Lendahl, U.; Nistér, M. Human MIEF1 Recruits Drp1 to Mitochondrial Outer Membranes and Promotes Mitochondrial Fusion Rather than Fission. EMBO J. 2011, 30, 2762–2778. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Elgass, K.D.; Parton, R.G.; Osellame, L.D.; Stojanovski, D.; Ryan, M.T. Adaptor Proteins MiD49 and MiD51 Can Act Independently of Mff and Fis1 in Drp1 Recruitment and Are Specific for Mitochondrial Fission. J. Biol. Chem. 2013, 288, 27584–27593. [Google Scholar] [CrossRef] [Green Version]
- Losón, O.C.; Song, Z.; Chen, H.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 Mediate Drp1 Recruitment in Mitochondrial Fission. Mol. Biol. Cell 2013, 24, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Koirala, S.; Guo, Q.; Kalia, R.; Bui, H.T.; Eckert, D.M.; Frost, A.; Shaw, J.M. Interchangeable Adaptors Regulate Mitochondrial Dynamin Assembly for Membrane Scission. Proc. Natl. Acad. Sci. USA. 2013, 110, E1342–E1351. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Elsner, D.; Schunkert, H.; Pfeifer, M.; Griese, D.; Bruckschlegel, G.; Muders, F.; Riegger, G.A.; Kromer, E.P. Development of Heart Failure Following Isoproterenol Administration in the Rat: Role of the Renin-Angiotensin System. Cardiovasc. Res. 1998, 37, 91–100. [Google Scholar] [CrossRef]
- Del Mauro, J.S.; Prince, P.D.; Donato, M.; Fernandez Machulsky, N.; Morettón, M.A.; González, G.E.; Bertera, F.M.; Carranza, A.; Gorzalczany, S.B.; Chiappetta, D.A.; et al. Effects of Carvedilol or Amlodipine on Target Organ Damage in L-NAME Hypertensive Rats: Their Relationship with Blood Pressure Variability. J. Am. Soc. Hypertens. JASH 2017, 11, 227–240. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Suvarna, K.S. , Layton, C., Bancroft, J.D. (Eds.) Theory and Practice of Histological Techniques, 7th ed.; Elsevier Churchill Livingston: Edinburgh, UK, 2013; ISBN 978-0-7020-4226-3. [Google Scholar]
- El Azab, I.H.; Saied, E.M.; Osman, A.A.; Mehana, A.E.; Saad, H.A.; Elkanzi, N.A. Novel N-Bridged Pyrazole-1-Carbothioamides with Potential Antiproliferative Activity: Design, Synthesis, In Vitro and In Silico Studies. Future Med. Chem. 2021, 13, 1743–1766. [Google Scholar] [CrossRef]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef]
- Healey, R.D.; Saied, E.M.; Cong, X.; Karsai, G.; Gabellier, L.; Saint-Paul, J.; Del Nero, E.; Jeannot, S.; Drapeau, M.; Fontanel, S.; et al. Discovery and Mechanism of Action of Small Molecule Inhibitors of Ceramidases**. Angew. Chem. Int. Ed. 2022, 61, e202109967. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.I.; Abou-Bakr, D.A.; Ezzat, S.F.; El-Kareem, H.F.A.; Nahas, H.H.A.; Saad, H.A.; Mehana, A.E.; Saied, E.M. Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting MiRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals 2021, 14, 1222. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.I.; Alaa El-Din Aly El-Waseef, D.; Nabih, E.S.; El-Kharashi, O.A.; Abd El-Kareem, H.F.; Abo Nahas, H.H.; Abdel-Wahab, B.A.; Helmy, Y.A.; Alshawwa, S.Z.; Saied, E.M. Acetylsalicylic Acid Suppresses Alcoholism-Induced Cognitive Impairment Associated with Atorvastatin Intake by Targeting Cerebral MiRNA155 and NLRP3: In Vivo, and In Silico Study. Pharmaceutics 2022, 14, 529. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.M.; El-Maradny, Y.A.; Osman, A.A.; Darwish, A.M.G.; Abo Nahas, H.H.; Niedbała, G.; Piekutowska, M.; Abdel-Rahman, M.A.; Balbool, B.A.; Abdel-Azeem, A.M. A Comprehensive Review about the Molecular Structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insights into Natural Products against COVID-19. Pharmaceutics 2021, 13, 1759. [Google Scholar] [CrossRef]
- Samaha, D.; Hamdo, H.H.; Cong, X.; Schumacher, F.; Banhart, S.; Aglar, Ö.; Möller, H.M.; Heuer, D.; Kleuser, B.; Saied, E.M.; et al. Liposomal FRET Assay Identifies Potent Drug-Like Inhibitors of the Ceramide Transport Protein (CERT). Chem.—Eur. J. 2020, 26, 16616–16621. [Google Scholar] [CrossRef]


| Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Serum AST(IU/L) | 32 ± 1.27 | 123.75 ± 8.65 a | 47.75 ± 2.62 ab | 73 ± 5.29 abc |
| Serum ALT(IU/L) | 30.5 ± 2.4 | 92 ± 3.9 a | 45.25 ± 3.90 ab | 55.25 ± 4.03 abc |
| AST/ALT | 1.07 ± 0.22 | 1.35 ± 0.19 a | 1.05 ± 0.11 ab | 1.32 ± 0.09 abc |
| ALP (IU/L) | 111.3 ± 3.88 | 322 ± 7.82 a | 123 ± 14.58 ab | 151.3 ± 5.5 abc |
| Total bilirubin (mg/dL) | 0.55 ± 0.12 | 10.4 ± 0.32 a | 1.62 ± 0.15 ab | 2.77 ± 0.15 abc |
| Direct bilirubin (mg/dL) | 0.12 ± 0.23 | 8.9 ± 0.01 a | 1.7 ± 0.32 ab | 2.6 ± 0.38 abc |
| Albumin (g/dL) | 5.18 ± 0.25 | 2.43 ± 0.16 a | 4.1 ± 0.08 ab | 3.8 ± 0.7 abc |
| Groups | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Congestion 0: None | 18 (90%) | 3 (15%) | 15(83%) | 10 (50%) |
| 1: Minimum | 2 (10%) | 10 (50%) | 3 (17%) | 7 (35%) |
| 2: Slight | 0 | 6 (30%) | 0 | 3 (15%) |
| 3: Moderate 4: Extreme | 0 0 | 1 (5%) 0 | 0 0 | 0 0 |
| Vacuolization 0: None | 19 (95%) | 12 (67%) | 18 (90%) | 16 (80%) |
| 1: Minimum | 1 (5%) | 6(33%) | 2(10%) | 4 (20%) |
| 2: Slight | 0 | 0 | 0 | 0 |
| 3: Moderate 4: Extreme | 0 0 | 0 0 | 0 0 | 0 0 |
| Necrosis 0: None | 20 (100%) | 2 (10%) | 19 (95%) | 13 (65%) |
| 1: Necrosis of individual cell | 0 | 5 (25%) | 1 (5%) | 5 (25%) |
| 2: <30% | 0 | 13 (65%) | 0 | 2 (10%) |
| 3: 30–60% 4: >60% | 0 0 | 0 0 | 0 0 | 0 0 |
| Groups | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Area % of collagen | 1.63 ± 0.26 | 6.41 ± 1.11 a | 2.33 ± 0.60 ab | 5.16 ± 0.93 abc |
| Mean Area Percentage of PCNA | Mean Number of PCNA Positive Nuclei | |
|---|---|---|
| Group I | 2.19 ± 0.35 | 51.85 ± 1.06 |
| Group II | 1.50 ± 0.57 a | 49.28 ± 0.95 a |
| Group III | 11.95 ± 0.82 ab | 130.83 ± 1.47 ab |
| Group IV | 13.43 ± 0.42 abc | 133.42 ± 1.51 abc |
| Protein | PDB Code | Docking Score (kcal/mol) | Interactive Residues | |
|---|---|---|---|---|
| Hydrophilic Interactions | Hydrophobic Interactions | |||
| Dynamin-1-like protein (DNM1L) | 3w6p | −14.83 | Val58, Ser40, Gly37, Lys216, Asp218 | Ile57, Val58, Leu147, Pro148, Leu219, Ile252, Val245 |
| Mitochondrial dynamics protein (MID51) | 4nxx | −12.69 | Gln203, Ser189, Glu345, Val324, Ser340 | Leu194, Leu313, Val324, Leu339, Leu341 |
| Score | Congestion | Vacuolization | Necrosis |
|---|---|---|---|
| 0 | None | None | None |
| 1 | Minimum | Minimum | Necrosis of individual cell |
| 2 | Slight | Slight | < 30% |
| 3 | Moderate | Moderate | 30–60% |
| 4 | Extreme | Extreme | >60% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, D.I.; Ezzat, S.F.; Elayat, W.M.; El-Kharashi, O.A.; El-Kareem, H.F.A.; Nahas, H.H.A.; Abdel-Wahab, B.A.; Alshawwa, S.Z.; Saleh, A.; Helmy, Y.A.; et al. Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting miRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study. Pharmaceuticals 2022, 15, 832. https://doi.org/10.3390/ph15070832
Mohamed DI, Ezzat SF, Elayat WM, El-Kharashi OA, El-Kareem HFA, Nahas HHA, Abdel-Wahab BA, Alshawwa SZ, Saleh A, Helmy YA, et al. Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting miRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study. Pharmaceuticals. 2022; 15(7):832. https://doi.org/10.3390/ph15070832
Chicago/Turabian StyleMohamed, Doaa I., Samar F. Ezzat, Wael M. Elayat, Omnyah A. El-Kharashi, Hanaa F. Abd El-Kareem, Hebatallah H. Abo Nahas, Basel A. Abdel-Wahab, Samar Zuhair Alshawwa, Asmaa Saleh, Yosra A. Helmy, and et al. 2022. "Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting miRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study" Pharmaceuticals 15, no. 7: 832. https://doi.org/10.3390/ph15070832
APA StyleMohamed, D. I., Ezzat, S. F., Elayat, W. M., El-Kharashi, O. A., El-Kareem, H. F. A., Nahas, H. H. A., Abdel-Wahab, B. A., Alshawwa, S. Z., Saleh, A., Helmy, Y. A., Khairy, E., & Saied, E. M. (2022). Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting miRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study. Pharmaceuticals, 15(7), 832. https://doi.org/10.3390/ph15070832