Tumor-Derived Membrane Vesicles: A Promising Tool for Personalized Immunotherapy
Abstract
:1. Introduction
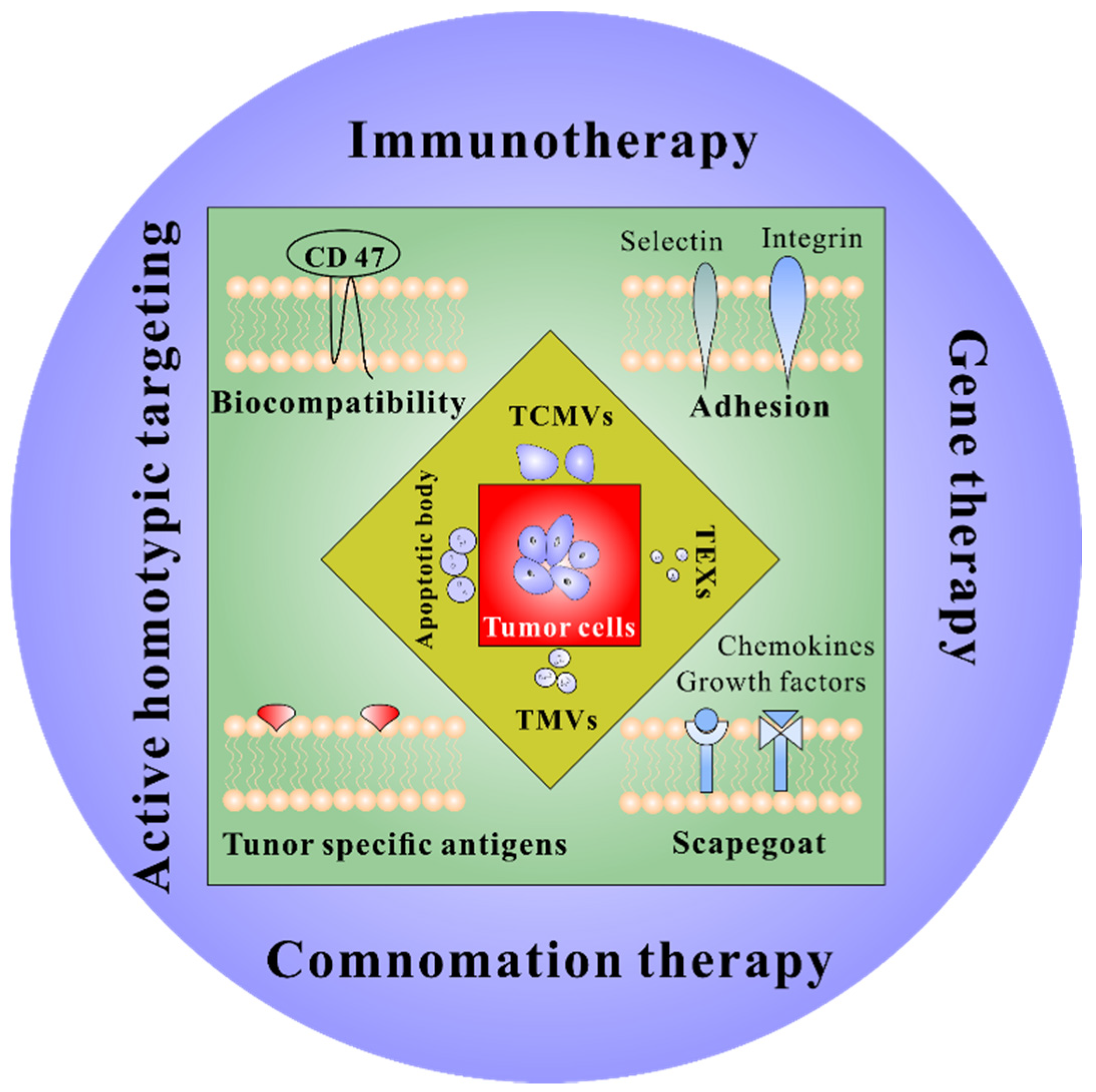
2. Tumor Cell-Derived Membrane Vesicles
2.1. Tumor Cell Membrane Vesicles
2.2. Tumor Extracellular Vesicles
2.2.1. Exosomes
2.2.2. Microvesicles
2.2.3. Apoptotic Bodies
3. Engineering Tumor Cell-Derived Membrane Vesicles in Cancer Treatment
3.1. Encapsulation
3.2. Surface Modification
3.3. Membrane Fusion
3.4. Genetic Engineering
4. The Application of Tumor Cell-Derived Membrane Vesicles to Personalized Immunotherapy
4.1. Cancer Vaccines
4.2. Immune Checkpoint Therapy
4.3. Combination Therapy
5. Concluding Remarks and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.-F.; Chen, W.-H.; Zeng, X.; Zhang, X.-Z. Cell primitive-based biomimetic functional materials for enhanced cancer therapy. Chem. Soc. Rev. 2020, 50, 945–985. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 17, 147–167. [Google Scholar] [CrossRef]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef] [Green Version]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Jensen, C.; Madsen, D.H.; Hansen, M.; Schmidt, H.; Svane, I.M.; Karsdal, M.A.; Willumsen, N. Non-invasive biomarkers derived from the extracellular matrix associate with response to immune checkpoint blockade (anti-CTLA-4) in metastatic melanoma patients. J. Immunother. Cancer 2018, 6, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linette, G.P.; Becker-Hapak, M.; Skidmore, Z.L.; Baroja, M.L.; Xu, C.; Hundal, J.; Spencer, D.H.; Fu, W.; Cummins, C.; Robnett, M.; et al. Immunological ignorance is an enabling feature of the oligo-clonal T cell response to melanoma neoantigens. Proc. Natl. Acad. Sci. USA 2019, 116, 23662–23670. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Huang, K.; Gu, Z.; Wu, J. Tumor immune microenvironment modulation-based drug delivery strategies for cancer immunotherapy. Nanoscale 2019, 12, 413–436. [Google Scholar] [CrossRef]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices and immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Wang, Z.; Wang, W.; Yang, M.; Dong, X.-C. Organic/inorganic nanohybrids rejuvenate photodynamic cancer therapy. J. Mater. Chem. B 2020, 8, 4748–4763. [Google Scholar] [CrossRef]
- Karmali, P.P.; Simberg, D. Interactions of nanoparticles with plasma proteins: Implication on clearance and toxicity of drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 343–357. [Google Scholar] [CrossRef]
- Emam, S.E.; Elsadek, N.E.; Abu Lila, A.S.; Takata, H.; Kawaguchi, Y.; Shimizu, T.; Ando, H.; Ishima, Y.; Ishida, T. Anti-PEG IgM production and accelerated blood clearance phenomenon after the administration of PEGylated exosomes in mice. J. Control. Release 2021, 334, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Che, J.; Ji, X.; Li, Y.; Xie, J.; Chen, X. Recent advances in biomaterial-boosted adoptive cell therapy. Chem. Soc. Rev. 2022, 51, 1766–1794. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Gu, Z. Bioinspired and Biomimetic Nanomedicines. Accounts Chem. Res. 2019, 52, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Gao, W.W.; Zhang, L.F. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef] [PubMed]
- Thanuja, M.Y.; Anupama, C.; Ranganath, S.H. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: So near and yet so far. Adv. Drug Deliv. Rev. 2018, 132, 57–80. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, G.; Chen, X. Nanobiotechnology: Cell membrane-based delivery systems. Nano Today 2016, 13, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, N.; Yoshioka, Y.; Ochiya, T. A novel platform for cancer therapy using extracellular vesicles. Adv. Drug Deliv. Rev. 2015, 95, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Srivatsav, A.T.; Kapoor, S. The Emerging World of Membrane Vesicles: Functional Relevance, Theranostic Avenues and Tools for Investigating Membrane Function. Front. Mol. Biosci. 2021, 8, 640355. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Zheng, Z.; Chen, S.; Pang, X.; Xiang, X.; Tang, J.; Ren, E.; Chen, Y.; You, M.; et al. Vesicular Antibodies: A Bioactive Multifunctional Combination Platform for Targeted Therapeutic Delivery and Cancer Immunotherapy. Adv. Mater. 2019, 31, e1808294. [Google Scholar] [CrossRef]
- Noh, Y.-W.; Kim, S.-Y.; Kim, J.-E.; Kim, S.; Ryu, J.; Kim, I.; Lee, E.; Um, S.H.; Lim, Y.T. Multifaceted Immunomodulatory Nanoliposomes: Reshaping Tumors into Vaccines for Enhanced Cancer Immunotherapy. Adv. Funct. Mater. 2017, 27, 1605398. [Google Scholar] [CrossRef]
- Möller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef]
- Park, W.; Seong, K.Y.; Han, H.H.; Yang, S.Y.; Hahn, S.K. Dissolving microneedles delivering cancer cell membrane coated nanoparticles for cancer immunotherapy. RSC Adv. 2021, 11, 10393–10399. [Google Scholar] [CrossRef]
- Wang, D.; Dong, H.; Li, M.; Cao, Y.; Yang, F.; Zhang, K.; Dai, W.; Wang, C.; Zhang, X. Erythrocyte–Cancer Hybrid Membrane Camouflaged Hollow Copper Sulfide Nanoparticles for Prolonged Circulation Life and Homotypic-Targeting Photothermal/Chemotherapy of Melanoma. ACS Nano 2018, 12, 5241–5252. [Google Scholar] [CrossRef]
- Durymanov, M.; Rosenkranz, A.; Sobolev, A.S. Current Approaches for Improving Intratumoral Accumulation and Distribution of Nanomedicines. Theranostics 2015, 5, 1007–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, J.; Li, C.; Xia, Y.; Wang, Z.; Feng, J.; Zhang, X. Cytomembrane-Mediated Transport of Metal Ions with Biological Specificity. Adv. Sci. 2019, 6, 1900835. [Google Scholar] [CrossRef]
- Xia, J.; Cheng, Y.; Zhang, H.; Li, R.; Hu, Y.; Liu, B. The role of adhesions between homologous cancer cells in tumor progression and targeted therapy. Expert Rev. Anticancer Ther. 2017, 17, 517–526. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane–Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef] [PubMed]
- Khaldoyanidi, S.K.; Glinsky, V.; Sikora, L.; Glinskii, A.B.; Mossine, V.V.; Quinn, T.P.; Glinsky, G.V.; Sriramarao, P. MDA-MB-435 Human Breast Carcinoma Cell Homo- and Heterotypic Adhesion under Flow Conditions Is Mediated in Part by Thomsen-Friedenreich Antigen-Galectin-3 Interactions. J. Biol. Chem. 2003, 278, 4127–4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, W.-X.; Zhang, M.-K.; Liu, L.-H.; Gao, F.; Zhang, L.; Li, S.; Xie, B.-R.; Zhang, C.; Feng, J.; Zhang, X.-Z. A self-delivery membrane system for enhanced anti-tumor therapy. Biomaterials 2018, 161, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Sharma, S.C.; Das, S.N. Galectin-1 and galectin-3: Plausible tumour markers for oral squamous cell carcinoma and suitable targets for screening high-risk population. Clin. Chim. Acta 2015, 442, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bombardelli, L.; Cavallaro, U. Immunoglobulin-like cell adhesion molecules: Novel signaling players in epithelial ovarian cancer. Int. J. Biochem. Cell Biol. 2010, 42, 590–594. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, Q.; Xin, N.; Wang, W.; Zhao, C. CD 155, an onco-immunologic molecule in human tumors. Cancer Sci. 2017, 108, 1934–1938. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Su, J.; Meng, Q.; Yin, Q.; Chen, L.; Gu, W.; Zhang, P.; Zhang, Z.; Yu, H.; Wang, S.; et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv. Mater. 2016, 28, 9581–9588. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; Hu, Y.; Li, W.; Di, W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics 2020, 10, 3325–3339. [Google Scholar] [CrossRef]
- Wan, J.; Wang, J.; Zhou, M.; Rao, Z.; Ling, X. A cell membrane vehicle co-delivering sorafenib and doxorubicin remodel the tumor microenvironment and enhance immunotherapy by inducing immunogenic cell death in lung cancer cells. J. Mater. Chem. B 2020, 8, 7755–7765. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; He, R.; Xu, D.; Zang, J.; Weeranoppanant, N.; Dong, H.; Li, Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2019, 8, 552–568. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.-L.; Cai, B.; Xu, J.-H.; Li, A.; Zhang, W.-F.; Sun, Z.-J.; Guo, S.-S.; Liu, W.; Wang, T.-H.; et al. Cancer Cell Membrane-Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging. Adv. Mater. 2016, 28, 3460–3466. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Guo, K.; Cao, Y.; Yang, F.; Wang, D.; Dou, L.; Liu, Y.; Dong, H. Cancer Cell Membrane Vesicle for Multiplex MicroRNA Imaging in Living Cells. Anal. Chem. 2019, 92, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Wang, J.; Hu, Q.; Langworthy, B.; Ye, Y.; Sun, W.; Lin, J.; Wang, T.; Fine, J.; et al. PD-1 Blockade Cellular Vesicles for Cancer Immunotherapy. Adv. Mater. 2018, 30, e1707112. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Zhou, J.; Chekuri, S.; Wei, X.; Kroll, A.V.; Yu, C.L.; Duan, Y.; Gao, W.; Fang, R.H.; et al. Engineered Cell-Membrane-Coated Nanoparticles Directly Present Tumor Antigens to Promote Anticancer Immunity. Adv. Mater. 2020, 32, e2001808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; Zhang, M.-K.; Ding, X.-G.; Qiu, W.-X.; Yu, W.-Y.; Feng, J.; Zhang, X.-Z. Virus-Inspired Nanogenes Free from Man-Made Materials for Host-Specific Transfection and Bio-Aided MR Imaging. Adv. Mater. 2018, 30, e1707459. [Google Scholar] [CrossRef]
- Wang, P.; Kankala, R.K.; Chen, B.; Zhang, Y.; Zhu, M.; Li, X.; Long, R.; Yang, D.; Krastev, R.; Wang, S.; et al. Cancer Cytomembrane-Cloaked Prussian Blue Nanoparticles Enhance the Efficacy of Mild-Temperature Photothermal Therapy by Disrupting Mitochondrial Functions of Cancer Cells. ACS Appl. Mater. Interfaces 2021, 13, 37563–37577. [Google Scholar] [CrossRef]
- Browning, M.J. Antigen presenting cell/tumor cell fusion vaccines for cancer immunotherapy. Hum. Vaccines Immunother. 2013, 9, 1545–1548. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Peng, K.; Qiu, L.-Y.; Li, M.; Ruan, J.-H.; He, L.-L.; Yuan, Z.-X. Hitchhiking on Controlled-Release Drug Delivery Systems: Opportunities and Challenges for Cancer Vaccines. Front. Pharmacol. 2021, 12, 679602. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, T.; Zhang, B.; Yan, C.; Lu, C.; Liu, L.; Chen, Z.; Ran, H.; Shi, Q.; Pan, H.; et al. Cancer-macrophage hybrid membrane-camouflaged photochlor for enhanced sonodynamic therapy against triple-negative breast cancer. Nano Res. 2022, 15, 4224–4232. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, W.; Huang, J.; Li, F.; Sheng, J.; Song, H.; Chen, Y. Development of a Dendritic Cell/Tumor Cell Fusion Cell Membrane Nano-Vaccine for the Treatment of Ovarian Cancer. Front. Immunol. 2022, 13, 828263. [Google Scholar] [CrossRef]
- Liu, W.; Zou, M.; Liu, T.; Zeng, J.; Li, X.; Yu, W.; Li, C.; Ye, J.; Song, W.; Feng, J.; et al. Expandable Immunotherapeutic Nano-platforms Engineered from Cytomembranes of Hybrid Cells Derived from Cancer and Dendritic Cells. Adv. Mater. 2019, 31, e1900499. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-L.; Zou, M.-Z.; Liu, T.; Zeng, J.-Y.; Li, X.; Yu, W.-Y.; Li, C.-X.; Ye, J.-J.; Song, W.; Feng, J.; et al. Cytomembrane nano-vaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat. Commun. 2019, 10, 3199. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, H.; Qiu, W.; Han, Y.; Liu, H.; Li, Z. Biomimetic nanoparticles directly remodel immunosuppressive mi-croenvironment for boosting glioblastoma immunotherapy. Bioact. Mater. 2022, 16, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-X.; Liu, J.-H.; Wu, J.-Y.; Li, Y.-J.; Qiu, X.-H.; Xu, W.-J.; Xu, P.; Xiang, D.-X. Hybrid Cell Membrane-Functionalized Bio-mimetic Nanoparticles for Targeted Therapy of Osteosarcoma. Int. J. Nanomed. 2022, 17, 837–854. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, G.; Wu, W.; Wang, J.; Hu, J.; Mao, J.; Chu, P.; Bai, H.; Tang, G. A Hybrid Eukaryotic-Prokaryotic Nano-platform with Photothermal Modality for Enhanced Antitumor Vaccination. Adv. Mater. 2020, 32, e1908185. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, Y.; Yang, Y.; Miao, Z.; Zhu, J.; Zhong, M.; Feng, C.; Tang, W.; Zhou, J.; Wang, L.; et al. Biomimetic cy-tomembrane nanovaccines prevent breast cancer development in the long term. Nanoscale 2021, 13, 3594–3601. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, J.; Pan, J.; Liu, C.; Guo, X.; Zhou, S. Personalized Nanovaccine Coated with Calcinetin-Expressed Cancer Cell Membrane Antigen for Cancer Immunotherapy. Nano Lett. 2021, 21, 8418–8425. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Wang, Y.; Luan, Z.; Zhao, C.; Bai, C.; Yang, K. Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy. Eur. J. Pharm. Sci. 2019, 142, 105136. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, C.; Feng, J.; Huangfu, Y.; Wang, K.; Zhang, Z.-L. Personalized gel-droplet monocyte vaccines for cancer immu-notherapy. Lab Chip 2021, 21, 4414–4426. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Albero, M.; Encinas-Giménez, M.; Sebastián, V.; Pérez, E.; Luján, L.; Santamaría, J.; Martin-Duque, P. Transfer of photothermal nanoparticles using stem cell derived small extracellular vesicles for in vivo treatment of primary and mul-tinodular tumours. J. Extracell. Vesicles 2022, 11, e12193. [Google Scholar] [CrossRef] [PubMed]
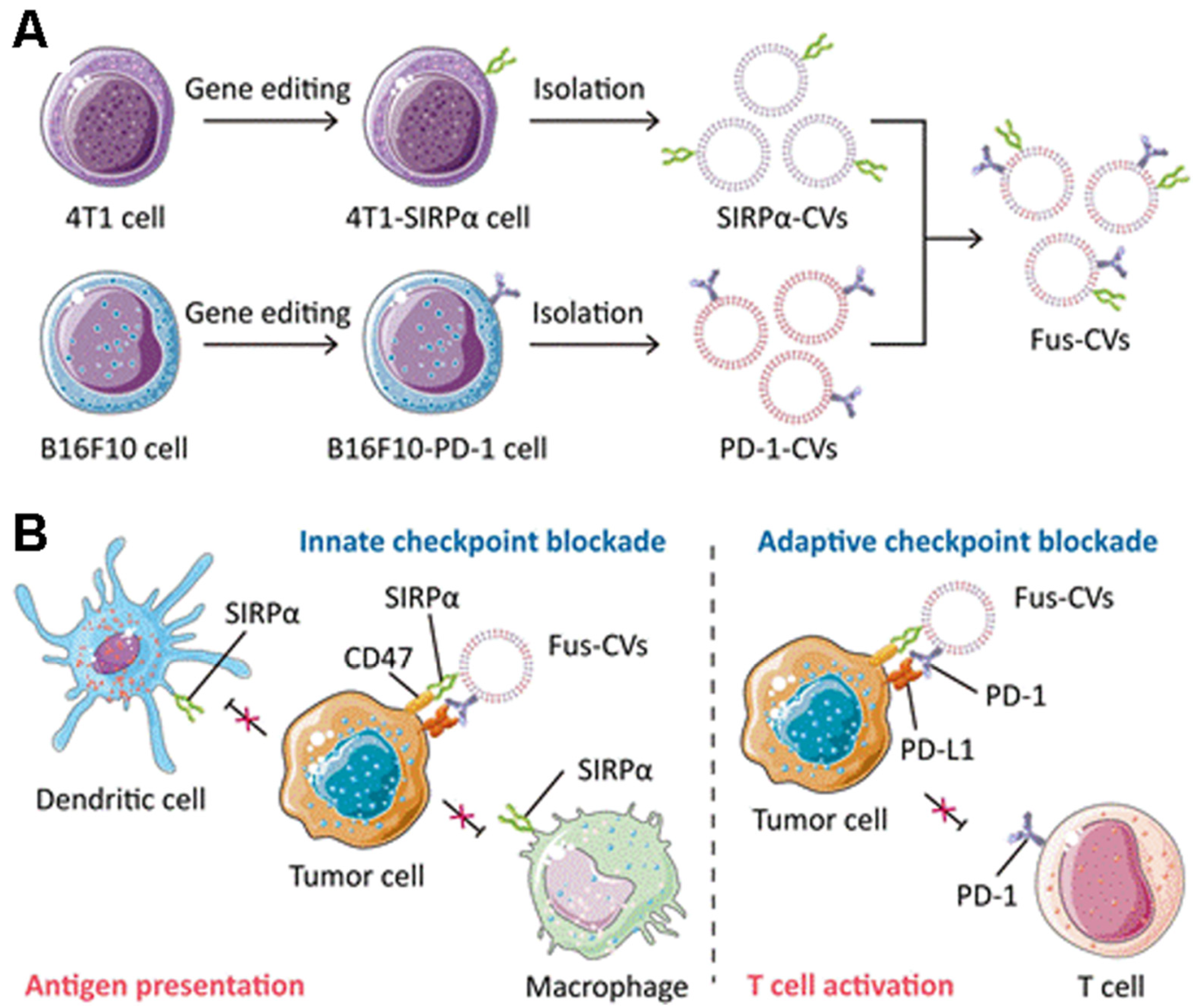
- Meng, Q.; Zhao, Y.; Dong, C.; Liu, L.; Pan, Y.; Lai, J.; Liu, Z.; Yu, G.; Chen, X.; Rao, L. Genetically Programmable Fusion Cel-lular Vesicles for Cancer Immunotherapy. Angew. Chem. Int. Ed. 2021, 60, 26320–26326. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Z.; Qin, X.; Zhang, M.; Du, Q.; Li, Z.; Luan, Y. A Checkpoint-Regulatable Immune Niche Created by Injectable Hydrogel for Tumor Therapy. Adv. Funct. Mater. 2021, 31, 2104630. [Google Scholar] [CrossRef]
- Wang, H.; Wang, K.; He, L.; Liu, Y.; Dong, H.; Li, Y. Engineering antigen as photosensitiser nanocarrier to facilitate ROS triggered immune cascade for photodynamic immunotherapy. Biomaterials 2020, 244, 119964. [Google Scholar] [CrossRef]
- Pack, C.D.; Bommireddy, R.; Munoz, L.E.; Patel, J.M.; Bozeman, E.N.; Dey, P.; Radhakrishnan, V.; Vartabedian, V.F.; Venkat, K.; Ramachandiran, S.; et al. Tumor membrane-based vaccine immunotherapy in combination with anti-CTLA-4 antibody confers protection against immune checkpoint resistant murine triple-negative breast cancer. Hum. Vaccines Immunother. 2020, 16, 3184–3193. [Google Scholar] [CrossRef]
- Ye, X.; Liang, X.; Chen, Q.; Miao, Q.; Chen, X.; Zhang, X.; Mei, L. Surgical Tumor-Derived Personalized Photothermal Vac-cine Formulation for Cancer Immunotherapy. ACS Nano 2019, 13, 2956–2968. [Google Scholar] [CrossRef]
- Wu, M.; Liu, X.; Bai, H.; Lai, L.; Chen, Q.; Huang, G.; Liu, B.; Tang, G. Surface-Layer Protein-Enhanced Immunotherapy Based on Cell Membrane-Coated Nanoparticles for the Effective Inhibition of Tumor Growth and Metastasis. ACS Appl. Mater. In-terfaces 2019, 11, 9850–9859. [Google Scholar] [CrossRef]
- Li, F.; Nie, W.; Zhang, F.; Lu, G.; Lv, C.; Lv, Y.; Bao, W.; Zhang, L.; Wang, S.; Gao, X.; et al. Engineering Magnetosomes for High-Performance Cancer Vaccination. ACS Central Sci. 2019, 5, 796–807. [Google Scholar] [CrossRef] [Green Version]
- Kooijmans, S.A.A.; Gitz-Francois, J.J.J.M.; Schiffelers, R.M.; Vader, P. Recombinant phosphatidylserine-binding nanobodies for targeting of extracellular vesicles to tumor cells: A plug-and-play approach. Nanoscale 2018, 10, 2413–2426. [Google Scholar] [CrossRef] [Green Version]
- Kroll, A.V.; Fang, R.H.; Jiang, Y.; Zhou, J.; Wei, X.; Yu, C.L.; Gao, J.; Luk, B.T.; Dehaini, D.; Gao, W.; et al. Nanoparticulate Delivery of Cancer Cell Membrane Elicits Multiantigenic Antitumor Immunity. Adv. Mater. 2017, 29, 1703969. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; De Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesi-cles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Veerman, R.E.; Akpinar, G.G.; Eldh, M.; Gabrielsson, S. Immune Cell-Derived Extracellular Vesicles-Functions and Thera-peutic Applications. Trends Mol. Med. 2019, 25, 382–394. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2020, 269, 120467. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Jiang, L.; Gu, Y.; Liu, J. Cell-derived biomimetic nanocarriers for targeted cancer therapy: Cell membranes and extracellular vesicles. Drug Deliv. 2021, 28, 1237–1255. [Google Scholar] [CrossRef]
- Lima Correa, B.; El Harane, N.; Gomez, I.; Rachid Hocine, H.; Vilar, J.; Desgres, M.; Bellamy, V.; Keirththana, K.; Guillas, C.; Perotto, M.; et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc. Res. 2020, 117, 292–307. [Google Scholar] [CrossRef]
- Barenholz-Cohen, T.; Merkher, Y.; Haj, J.; Shechter, D.; Kirchmeier, D.; Shaked, Y.; Weihs, D. Lung mechanics modifications facilitating metastasis are mediated in part by breast cancer-derived extracellular vesicles. Int. J. Cancer 2020, 147, 2924–2933. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J. Potential of Cancer Cell-Derived Exosomes in Clinical Application: A Review of Recent Research Advances. Clin. Ther. 2014, 36, 863–872. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-derived microvesicles: Shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revenfeld, A.L.S.; Bæk, R.; Nielsen, M.H.; Stensballe, A.; Varming, K.; Jørgensen, M. Diagnostic and Prognostic Potential of Extracellular Vesicles in Peripheral Blood. Clin. Ther. 2014, 36, 830–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Li, Q.; Zhao, B.; Wang, Y. Stem Cell-Derived Extracellular Vesicles as a Novel Potential Therapeutic Tool for Tis-sue Repair. STEM CELLS Transl. Med. 2017, 6, 1753–1758. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Telford, W.; Skommer, J.; Darzynkiewicz, Z. Apoptosis and Beyond: Cytometry in Studies of Programmed Cell Death. Methods Cell Biol. 2011, 103, 55–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015, 6, 7164. [Google Scholar] [CrossRef] [Green Version]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dommelen, S.M.; Vader, P.; Lakhal, S.; Kooijmans, S.; van Solinge, W.W.; Wood, M.J.; Schiffelers, R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release 2012, 161, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Wu, X.; Jiang, Z.; Kasman, I.; Yao, J.; Guan, Y.; Oeh, J.; Modrusan, Z.; Bais, C.; Sampath, D.; et al. Tu-mour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012, 31, 3513–3523. [Google Scholar] [CrossRef]
- Antonyak, M.A.; Li, B.; Boroughs, L.K.; Johnson, J.L.; Druso, J.E.; Bryant, K.L.; Holowka, D.A.; Cerione, R.A. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4852–4857. [Google Scholar] [CrossRef] [Green Version]
- Reich, C.F.; Pisetsky, D.S. The content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosis. Exp. Cell Res. 2009, 315, 760–768. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 con-tributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Lee, E.J.; Nam, G.-H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.-S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, C.; Wangmo, D.; Subramanian, S. Tumor-Secreted Extracellular Vesicles Regulate T-Cell Costimulation and Can Be Manipulated To Induce Tumor-Specific T-Cell Responses. Gastroenterology 2021, 161, 560–574.e11. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, X.; Tang, J.; Lv, Q.; Liu, J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials 2021, 275, 120964. [Google Scholar] [CrossRef]
- Zuo, B.; Qi, H.; Lu, Z.; Chen, L.; Sun, B.; Yang, R.; Zhang, Y.; Liu, Z.; Gao, X.; You, A.; et al. Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat. Commun. 2020, 11, 1790. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.-M.; Duo, Y.; Suo, M.; Zhao, Y.; Xia, L.; Zheng, Z.; Li, Y.; Tang, B.Z. Tumor-Exocytosed Exosome/Aggregation-Induced Emission Luminogen Hybrid Nanovesicles Facilitate Efficient Tumor Penetration and Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 13836–13843. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, X.; Wu, H.; Mo, L.; Xie, S.; Li, J.; Peng, C.; Xu, S.; Qiu, L.; Tan, W. In Vivo Monocyte/Macrophage-Hitchhiked Intratumoral Accumulation of Nanomedicines for Enhanced Tumor Therapy. J. Am. Chem. Soc. 2019, 142, 382–391. [Google Scholar] [CrossRef]
- He, L.; Nie, T.; Xia, X.; Liu, T.; Huang, Y.; Wang, X.; Chen, T. Designing Bioinspired 2D MoSe 2 Nanosheet for Efficient Pho-tothermal-Triggered Cancer Immunotherapy with Reprogramming Tumor-Associated Macrophages. Adv. Funct. Mater. 2019, 29, 1901240. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, T.; Zheng, Y.; Yang, S.-L.; Yu, Z.; Duo, Y.; Zhang, Y.; Adah, D.; Shi, L.; Sun, Z.; et al. Immunogenic exo-some-encapsulated black phosphorus nanoparticles as an effective anticancer photo-nanovaccine. Nanoscale 2020, 12, 19939–19952. [Google Scholar] [CrossRef]
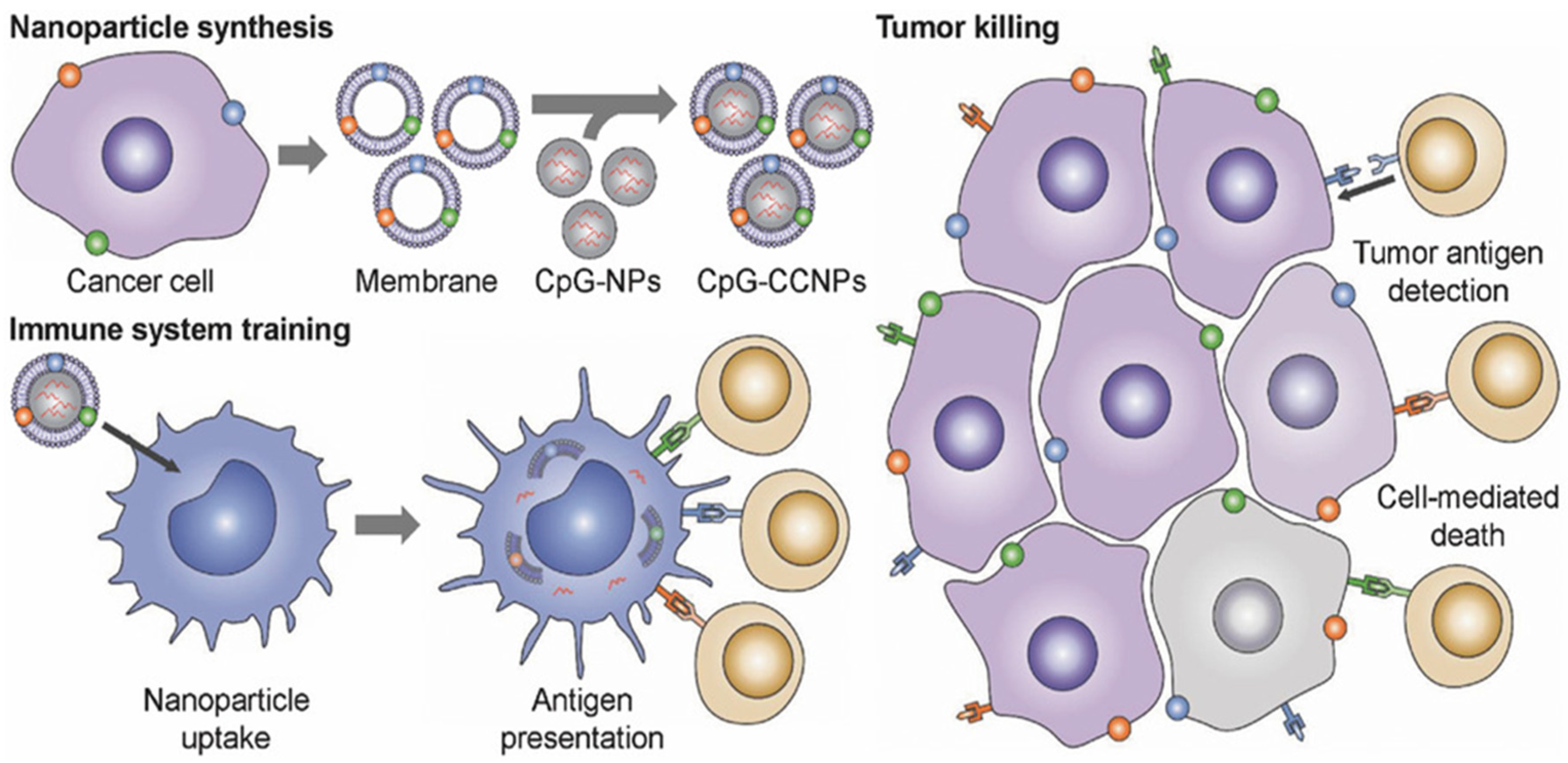
- Morishita, M.; Takahashi, Y.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 2016, 111, 55–65. [Google Scholar] [CrossRef]
- Davidson, S.M. Benefit of Extracellular Vesicles at the Blood-Brain Barrier. Arter. Thromb. Vasc. Biol. 2021, 41, 1146–1148. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [Green Version]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nano-platforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Das, C.K.; Jena, B.C.; Banerjee, I.; Das, S.; Parekh, A.; Bhutia, S.K.; Mandal, M. Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers. Mol. Pharm. 2018, 16, 24–40. [Google Scholar] [CrossRef]
- Chopra, N.; Arya, B.D.; Jain, N.; Yadav, P.; Wajid, S.; Singh, S.P.; Choudhury, S. Biophysical Characterization and Drug De-livery Potential of Exosomes from Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. ACS Omega 2019, 4, 13143–13152. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, W.; Lu, X.; Fu, Y.; Li, L.; Luo, Y. High expression of small GTPase Rab3D promotes cancer progression and me-tastasis. Oncotarget 2015, 6, 11125–11138. [Google Scholar] [CrossRef] [Green Version]
- López-Cobo, S.; Campos-Silva, C.; Moyano, A.; Oliveira-Rodríguez, M.; Paschen, A.; Yáñez-Mó, M.; Blanco-López, M.C.; Valés-Gómez, M. Immunoassays for scarce tumour-antigens in exosomes: Detection of the human NKG2D-Ligand, MICA, in tetraspanin-containing nanovesicles from melanoma. J. Nanobiotechnol. 2018, 16, 47. [Google Scholar] [CrossRef]
- Subramanian, A.; Gupta, V.; Sarkar, S.; Maity, G.; Banerjee, S.; Ghosh, A.; Harris, L.; Christenson, L.K.; Hung, W.; Bansal, A.; et al. Exosomes in carcinogenesis: Molecular palkis carry signals for the regulation of cancer progression and metastasis. J. Cell Commun. Signal. 2016, 10, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Mörgelin, M.; Belting, M. Exosome Uptake Depends on ERK1/2-Heat Shock Protein 27 Signaling and Lipid Raft-mediated Endocytosis Negatively Regulated by Caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724. [Google Scholar] [CrossRef] [Green Version]
- Plebanek, M.P.; Mutharasan, R.K.; Volpert, O.; Matov, A.; Gatlin, J.C.; Thaxton, C.S. Nanoparticle Targeting and Cholesterol Flux Through Scavenger Receptor Type B-1 Inhibits Cellular Exosome Uptake. Sci. Rep. 2015, 5, 15724. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar] [CrossRef] [Green Version]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.-P.; Goh, B.-C. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transi-tion to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenviron-ment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef]
- Clayton, A.; Mitchell, J.P.; Court, J.; Linnane, S.; Mason, M.D.; Tabi, Z. Human Tumor-Derived Exosomes Down-Modulate NKG2D Expression. J. Immunol. 2008, 180, 7249–7258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, Y.; Hao, L.; Hou, A.; Chen, X.; Li, Y.; Wang, R.; Luo, P.; Ruan, Z.; Ou, J.; et al. Macrophages induce resistance to 5-fluorouracil chemotherapy in colorectal cancer through the release of putrescine. Cancer Lett. 2016, 381, 305–313. [Google Scholar] [CrossRef]
- Dong, N.; Shi, X.; Wang, S.; Gao, Y.; Kuang, Z.; Xie, Q.; Li, Y.; Deng, H.; Wu, Y.; Li, M.; et al. M2 macrophages mediate soraf-enib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma. Br. J. Cancer 2019, 121, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.W.; Sceneay, J.; Lima, L.G.; Wong, C.S.; Becker, M.; Krumeich, S.; Lobb, R.J.; Castillo, V.; Ni Wong, K.; Ellis, S.; et al. The Biodistribution and Immune Suppressive Effects of Breast Cancer-Derived Exosomes. Cancer Res. 2016, 76, 6816–6827. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Guo, S.; Li, F.; Sun, Q.; Liang, G. Exosomal PD-L1: New Insights Into Tumor Immune Escape Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 569219. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef] [Green Version]
- Pi, Y.-N.; Xia, B.-R.; Jin, M.-Z.; Jin, W.-L.; Lou, G. Exosomes: Powerful weapon for cancer nano-immunoengineering. Biochem. Pharmacol. 2021, 186, 114487. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A Novel Nanoparti-cle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Victor, C.T.-S.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef] [Green Version]
- Cesselli, D.; Parisse, P.; Aleksova, A.; Veneziano, C.; Cervellin, C.; Zanello, A.; Beltrami, A.P. Extracellular Vesicles: How Drug and Pathology Interfere with Their Biogenesis and Function. Front. Physiol. 2018, 9, 1394. [Google Scholar] [CrossRef]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived from Exocytosis of Multivesicular Bodies and -Granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, J.G. Multiple Roles for Arf6: Sorting, Structuring, and Signaling at the Plasma Membrane. J. Biol. Chem. 2003, 278, 41573–41576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidharan-Chari, V.; Clancy, J.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communi-cation during cancer progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonomidis, J.S.; Nadeau, E.K.; Akerman, A.W.; Stroud, R.E.; Mukherjee, R.; Jones, J.A. Regulation of membrane type-1 ma-trix metalloproteinase activity and intracellular localization in clinical thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2016, 153, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.; Zhang, Y.; Sheehan, C.; D’Souza-Schorey, C. An ARF6-Exportin-5 axis delivers pre-miRNA cargo to tumour mi-crovesicles. Nat. Cell Biol. 2019, 21, 856–866. [Google Scholar] [CrossRef]
- Bian, X.; Xiao, Y.-T.; Wu, T.; Yao, M.; Du, L.; Ren, S.; Wang, J. Microvesicles and chemokines in tumor microenvironment: Mediators of intercellular communications in tumor progression. Mol. Cancer 2019, 18, 50. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Baj-Krzyworzeka, M.; Szatanek, R.; Weglarczyk, K.; Baran, J.; Urbanowicz, B.; Brański, P.; Ratajczak, M.Z.; Zembala, M. Tu-mour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these de-terminants to monocytes. Cancer Immunol. Immunother. 2005, 55, 808–818. [Google Scholar] [CrossRef]
- Wieckowski, E.; Whiteside, T.L. Human Tumor-Derived vs. Dendritic Cell-Derived Exosomes Have Distinct Biologic Roles and Molecular Profiles. Immunol. Res. 2006, 36, 247–254. [Google Scholar] [CrossRef]
- Yi, M.; Xu, L.; Jiao, Y.; Luo, S.; Li, A.; Wu, K. The role of cancer-derived microRNAs in cancer immune escape. J. Hematol. On-col. 2020, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Gilkes, D.M.; Takano, N.; Xiang, L.; Luo, W.; Bishop, C.J.; Chaturvedi, P.; Green, J.J.; Semenza, G.L. Hypox-ia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, E3234–E3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finley, S.D.; Popel, A.S. Effect of Tumor Microenvironment on Tumor VEGF during Anti-VEGF Treatment: Systems Biolo-gy Predictions. JNCI J. Natl. Cancer Inst. 2013, 105, 802–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szubert, S.; Szpurek, D.; Moszynski, R.; Nowicki, M.; Frankowski, A.; Sajdak, S.; Michalak, S. Extracellular matrix metallo-proteinase inducer (EMMPRIN) expression correlates positively with active angiogenesis and negatively with basic fibro-blast growth factor expression in epithelial ovarian cancer. J. Cancer Res. Clin. Oncol. 2013, 140, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Bai, M.; Deng, T.; Liu, R.; Wang, X.; Qu, Y.; Duan, J.; Zhang, L.; Ning, T.; Ge, S.; et al. Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett. 2016, 375, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, S.; Inal, J.M. The role of microvesicles in cancer progression and drug resistance. Biochem. Soc. Trans. 2013, 41, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Cai, Y.; He, D.; Zou, C.; Zhang, P.; Lo, C.Y.; Xu, Z.; Chan, F.L.; Yu, S.; Chen, Y.; et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc. Natl. Acad. Sci. USA 2012, 109, 16282–16287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Pan, Q.; Jiang, L.; Chen, Z.; Zhang, F.; Liu, Y.; Xing, H.; Shi, M.; Li, J.; Li, X.; et al. Tumor endothelial expression of P-glycoprotein upon microvesicular transfer of TrpC5 derived from adriamycin-resistant breast cancer cells. Biochem. Biophys. Res. Commun. 2014, 446, 85–90. [Google Scholar] [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.-J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotrans-poson elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Wickman, G.; Julian, L.; Olson, M.F. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012, 19, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [Green Version]
- Takizawa, F.; Tsuji, S.; Nagasawa, S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 1996, 397, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Erwig, L.-P.; Henson, P.M. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2007, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Vandivier, R.W.; Ogden, C.A.; Fadok, V.A.; Hoffmann, P.R.; Brown, K.K.; Botto, M.; Walport, M.; Fisher, J.H.; Henson, P.M.; Greene, K.E. Role of Surfactant Proteins A, D, and C1q in the Clearance of Apoptotic Cells In Vivo and In Vitro: Calreticulin and CD91 as a Common Collectin Receptor Complex. J. Immunol. 2002, 169, 3978–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergsmedh, A.; Szeles, A.; Henriksson, M.; Bratt, A.; Folkman, M.J.; Spetz, A.-L.; Holmgren, L. Horizontal transfer of onco-genes by uptake of apoptotic bodies. Proc. Natl. Acad. Sci. USA 2001, 98, 6407–6411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmgren, L.; Szeles, A.; Rajnavölgyi, E.; Folkman, J.; Klein, G.; Ernberg, I.; Falk, K.I. Horizontal Transfer of DNA by the Uptake of Apoptotic Bodies. Blood 1999, 93, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Turnier, J.L.; Kahlenberg, J.M. Using autoantibody signatures to define cancer risk in dermatomyositis. J. Clin. Investig. 2022, 132, e156025. [Google Scholar] [CrossRef]
- Gregory, C.D.; Dransfield, I. Apoptotic Tumor Cell-Derived Extracellular Vesicles as Important Regulators of the On-co-Regenerative Niche. Front. Immunol. 2018, 9, 1111. [Google Scholar] [CrossRef] [Green Version]
- McGaha, T.L.; Karlsson, M.C.I. Apoptotic cell responses in the splenic marginal zone: A paradigm for immunologic reac-tions to apoptotic antigens with implications for autoimmunity. Immunol. Rev. 2015, 269, 26–43. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.B.; Ohlsson, M.; Beroukas, D.; Hiscock, J.; Bradley, J.; Buyon, J.P.; Gordon, T.P. Subcelluar Redistribution of La/SSB Autoantigen During Rhysiologic Apoptosis in the Fetal Mouse Heart and Conduction System. Arthr. Rheum. 2002, 46, 202–208. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nano-particles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Yang, B.; Peng, Y.; Huang, J.; Wong, S.H.D.; Bian, L.; Zhu, K.; Shuai, X.; Han, S. Nanomedicine-Boosting Tumor Immunogenicity for Enhanced Immunotherapy. Adv. Funct. Mater. 2021, 31, 2011171. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Kolosnjaj-Tabi, J.; Bonneau, S.; Marangon, I.; Boggetto, N.; Aubertin, K.; Clément, O.; Bureau, M.F.; Luciani, N.; Gazeau, F.; et al. Magnetic and Photoresponsive Theranosomes: Translating Cell-Released Vesicles into Smart Nanovectors for Cancer Therapy. ACS Nano 2013, 7, 4954–4966. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Huang, J.; Li, Y.; Li, S.; Luo, M.; Luo, J.; Lee, A.W.; Fu, L.; Hu, F.; Guan, X. Near-Infrared Responsive Membrane Nanovesicles Amplify Homologous Targeting Delivery of Anti-PD Immunotherapy against Metastatic Tumors. Adv. Health Mater. 2021, 11, 2101496. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef]
- Taherkhani, S.; Mohammadi, M.; Daoud, J.; Martel, S.; Tabrizian, M. Covalent Binding of Nanoliposomes to the Surface of Magnetotactic Bacteria for the Synthesis of Self-Propelled Therapeutic Agents. ACS Nano 2014, 8, 5049–5060. [Google Scholar] [CrossRef]
- Liu, L.; He, H.; Luo, Z.; Zhou, H.; Liang, R.; Pan, H.; Ma, Y.; Cai, L. In Situ Photocatalyzed Oxygen Generation with Photo-synthetic Bacteria to Enable Robust Immunogenic Photodynamic Therapy in Triple-Negative Breast Cancer. Adv. Funct. Mater. 2020, 30, 1910176. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultane-ous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef]
- Nie, W.; Wu, G.; Zhang, J.; Huang, L.; Ding, J.; Jiang, A.; Zhang, Y.; Liu, Y.; Li, J.; Pu, K.; et al. Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 59, 2018–2022. [Google Scholar] [CrossRef]
- Higashi, K.; Mibu, F.; Saito, K.; Limwikrant, W.; Yamamoto, K.; Moribe, K. Composition-dependent structural changes and antitumor activity of ASC-DP/DSPE-PEG nanoparticles. Eur. J. Pharm. Sci. 2017, 99, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, N.; Moghadam, N.S.; Pazuki, G.; Parvin, P.; Shahi, F. In Vitro Evaluation of DSPE-PEG (5000) Amine SWCNT Tox-icity and Efficacy as a Novel Nanovector Candidate in Photothermal Therapy by Response Surface Methodology (RSM). Cells 2021, 10, 2874. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Takeuchi, Y.; Takada, M.; Namba, Y.; Oku, N. Synthesis of angiogenesis-targeted peptide and hydrophobized polyethylene glycol conjugate. Bioorganic Med. Chem. Lett. 2004, 14, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cy-tosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef] [PubMed]
- Banz, A.; Cremel, M.; Rembert, A.; Godfrin, Y. In situ targeting of dendritic cells by antigen-loaded red blood cells: A novel approach to cancer immunotherapy. Vaccine 2010, 28, 2965–2972. [Google Scholar] [CrossRef]
- Han, X.; Shen, S.; Fan, Q.; Chen, G.; Archibong, E.; Dotti, G.; Liu, Z.; Gu, Z.; Wang, C. Red blood cell-derived nanoerythro-some for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 2019, 5, eaaw6870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Chen, D.; Kashiwaba, M.; Kufe, D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat. Med. 1997, 3, 558–561. [Google Scholar] [CrossRef]
- He, H.; Guo, C.; Wang, J.; Korzun, W.J.; Wang, X.-Y.; Ghosh, S.; Yang, H. Leutusome: A Biomimetic Nanoplatform Integrat-ing Plasma Membrane Components of Leukocytes and Tumor Cells for Remarkably Enhanced Solid Tumor Homing. Nano Lett. 2018, 18, 6164–6174. [Google Scholar] [CrossRef]
- Rao, L.; Wu, L.; Liu, Z.; Tian, R.; Yu, G.; Zhou, Z.; Yang, K.; Xiong, H.-G.; Zhang, A.; Yu, G.-T.; et al. Hybrid cellular mem-brane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat. Commun. 2020, 11, 4909. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, J.; Rawding, P.; Bu, J.; Hong, S.; Hu, Q. Chemically and Biologically Engineered Bacteria-Based Deliv-ery Systems for Emerging Diagnosis and Advanced Therapy. Adv. Mater. 2021, 33, 2102580. [Google Scholar] [CrossRef]
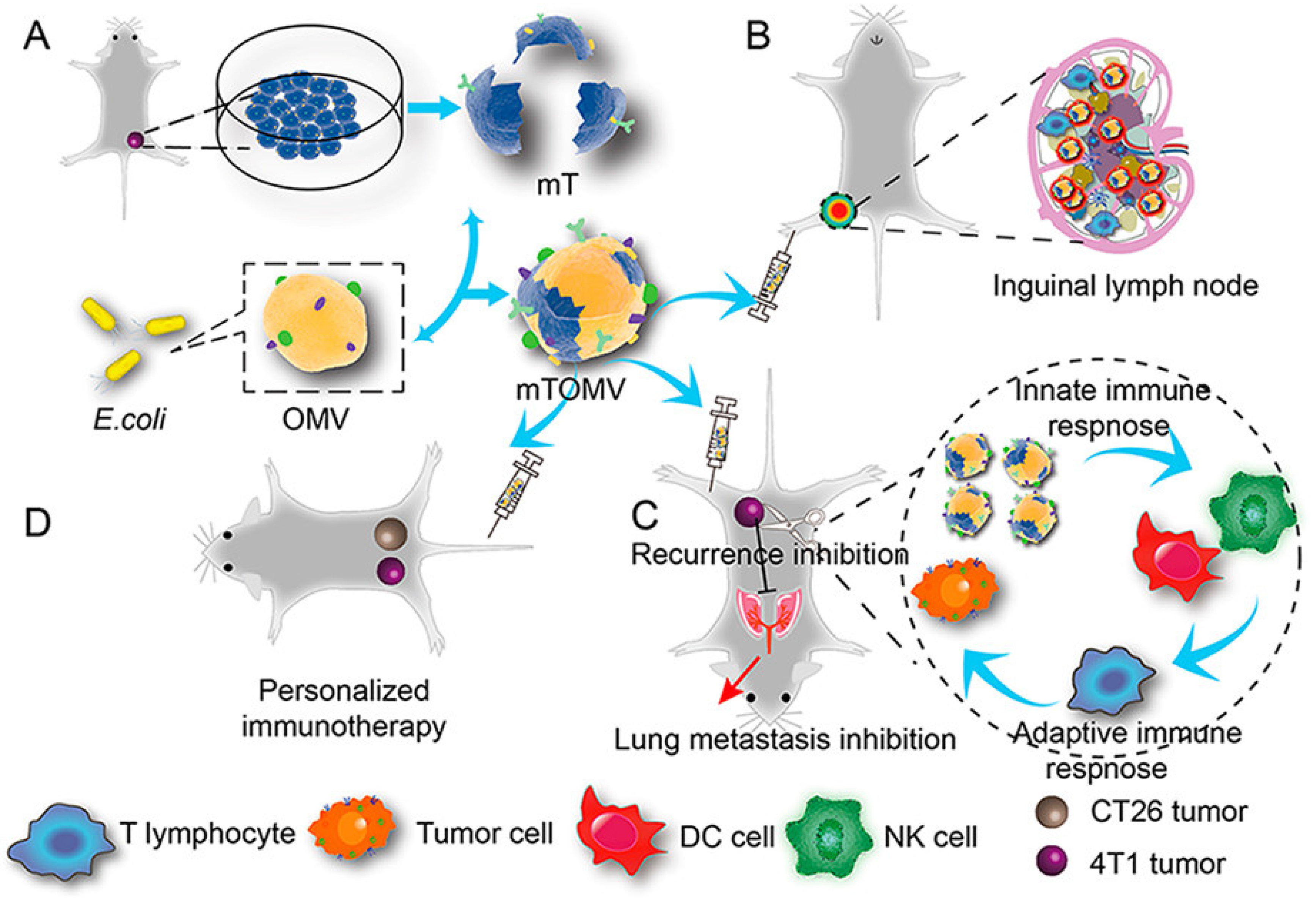
- Zou, M.-Z.; Li, Z.-H.; Bai, X.-F.; Liu, C.-J.; Zhang, X.-Z. Hybrid Vesicles Based on Autologous Tumor Cell Membrane and Bacterial Outer Membrane to Enhance Innate Immune Response and Personalized Tumor Immunotherapy. Nano Lett. 2021, 21, 8609–8618. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Miao, Q.; Meng, F.; Li, B.; Xue, T.; Fang, T.; Zhang, Z.; Zhang, J.; Ye, X.; Kang, Y.; et al. Genetic engineering cellular vesicles expressing CD64 as checkpoint antibody carrier for cancer immunotherapy. Theranostics 2021, 11, 6033–6043. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Q.; Gu, Z. Leveraging Engineering of Cells for Drug Delivery. Acc. Chem. Res. 2018, 51, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Mizrak, A.; Bolukbasi, M.F.; Ozdener, G.B.; Brenner, G.J.; Madlener, S.; Erkan, E.P.; Ströbel, T.; Breakefield, X.O.; Saydam, O. Genetically Engineered Microvesicles Carrying Suicide mRNA/Protein Inhibit Schwannoma Tumor Growth. Mol. Ther. 2013, 21, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Ridder, K.; Sevko, A.; Heide, J.; Dams, M.; Rupp, A.-K.; Macas, J.; Starmann, J.; Tjwa, M.; Plate, K.H.; Sultmann, H.; et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology 2015, 4, e1008371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Chen, J.; Su, F.; Yu, B.; Su, F.; Lin, L.; Liu, Y.; Huang, J.-D.; Song, E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer 2011, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; Van Solinge, W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored an-ti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef]
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Postnikov, Y.V.; Li, Y.; Tewary, P.; De La Rosa, G.; Wei, F.; Klinman, D.; Gioannini, T.; Weiss, J.; Furusawa, T.; et al. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced im-mune responses. J. Exp. Med. 2011, 209, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Shi, X.; Han, M.; Smbatyan, G.; Lenz, H.-J.; Zhang, Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018, 140, 16413–16417. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.-J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2019, 28, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, B.; Zheng, P.; Li, D.; Wang, M.; Jiang, F.; Wang, Z.; Ma, P.; Lin, J. Tumor microenvironment-triggered in situ cancer vaccines inducing dual immunogenic cell death for elevated antitumor and antimetastatic therapy. Nanoscale 2021, 13, 10906–10915. [Google Scholar] [CrossRef] [PubMed]
- Ochyl, L.J.; Bazzill, J.D.; Park, C.; Xu, Y.; Kuai, R.; Moon, J.J. PEGylated tumor cell membrane vesicles as a new vaccine plat-form for cancer immunotherapy. Biomaterials 2018, 182, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Badrinath, S.; Dellacherie, M.O.; Li, A.; Zheng, S.; Zhang, X.; Sobral, M.; Pyrdol, J.W.; Smith, K.L.; Lu, Y.; Haag, S.; et al. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nature 2022, 606, 992–998. [Google Scholar] [CrossRef]
- Islam, M.A.; Rice, J.; Reesor, E.; Zope, H.; Tao, W.; Lim, M.; Ding, J.; Chen, Y.; Aduluso, D.; Zetter, B.R.; et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 2020, 266, 120431. [Google Scholar] [CrossRef]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evo-lution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, C.; Zhang, X.; Hu, Q.; Zhang, Y.; Liu, Q.; Wen, D.; Milligan, J.; Bellotti, A.; Huang, L.; et al. A mela-nin-mediated cancer immunotherapy patch. Sci. Immunol. 2017, 2, eaan5692. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Diao, L.; Peng, Z.; Jia, Y.; Xie, H.; Li, B.; Ma, J.; Zhang, M.; Cheng, L.; Ding, D.; et al. Immunotherapy and Prevention of Cancer by Nanovaccines Loaded with Whole-Cell Components of Tumor Tissues or Cells. Adv. Mater. 2021, 33, 2104849. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.; Yang, Y.; Chen, X.; Ge, F.; Wang, J.; Deng, J. Inhibition of tumor recurrence and metastasis via a surgical tumor-derived personalized hydrogel vaccine. Biomater. Sci. 2022, 10, 1352–1363. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.-M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128, 69–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z.; et al. A general strategy towards per-sonalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.; Cao, X. The exosomes in tumor immunity. OncoImmunology 2015, 4, e1027472. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xiu, F.; Cai, Z.; Wang, J.; Wang, Q.; Fu, Y.; Cao, X. Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J. Cancer Res. Clin. Oncol. 2007, 133, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhou, X.; Wang, B.; Wang, Q.; Fu, Y.-X.; Chen, T.; Wan, T.; Yu, Y.; Cao, X. Enhanced induction of dendritic cell matu-ration and HLA-A*0201-restricted CEA-specific CD8+ CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. Klin. Wochenschr. 2006, 84, 1067–1076. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Cao, H.; Li, Q.; Li, Y.; Han, M.; Wang, H.; Li, J. Photodynamic Therapy with Liposomes Encapsulating Photosensitizers with Aggregation-Induced Emission. Nano Lett. 2019, 19, 1821–1826. [Google Scholar] [CrossRef]
- Han, X.; Chen, J.; Chu, J.; Liang, C.; Ma, Q.; Fan, Q.; Liu, Z.; Wang, C. Platelets as platforms for inhibition of tumor recur-rence post-physical therapy by delivery of anti-PD-L1 checkpoint antibody. J. Control. Release 2019, 304, 233–241. [Google Scholar] [CrossRef]
- Cazares-Cortes, E.; Cabana, S.; Boitard, C.; Nehlig, E.; Griffete, N.; Fresnais, J.; Wilhelm, C.; Abou-Hassan, A.; Ménager, C. Recent insights in magnetic hyperthermia: From the “hot-spot” effect for local delivery to combined magne-to-photo-thermia using magneto-plasmonic hybrids. Adv. Drug Deliv. Rev. 2018, 138, 233–246. [Google Scholar] [CrossRef]
- Marangoni, V.S.; Bernardi, J.C.; Reis, I.B.; Fávaro, W.J.; Zucolotto, V. Photothermia and Activated Drug Release of Natural Cell Membrane Coated Plasmonic Gold Nanorods and β-Lapachone. ACS Appl. Bio Mater. 2019, 2, 728–736. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Tang, K.; Zhang, H.; Yin, X.; Li, Y.; Xu, P.; Sun, Y.; Ma, R.; Ji, T.; et al. Reversing drug resistance of soft tu-mor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016, 26, 713–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome mdr in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ye, Z.; Xiang, M.; Chang, B.; Cui, J.; Ji, T.; Zhao, L.; Li, Q.; Deng, Y.; Xu, L.; et al. Functional extracellular vesicles en-gineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance. Biomaterials 2019, 223, 119475. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.; Kumar, S.U.; Zeng, Y.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.J.; Sinclair, R.; et al. Tumor Cell-Derived Extracellular Vesicle-Coated Nanocarriers: An Efficient Theranostic Platform for the Cancer-Specific Delivery of Anti-miR-21 and Imaging Agents. ACS Nano 2018, 12, 10817–10832. [Google Scholar] [CrossRef]

| TCMVs Sourse | Engineering Strategy | Drug | Application | Ref. |
|---|---|---|---|---|
| Ovarian cancer cell | DC/TCMVs Fusion; PLGA core | CpG ODN | Vaccine | [60] |
| 4T1 cell | DC/TCMVs fusion; MOF core | photosensitizer | PDT and vaccine | [61] |
| 4T1 cell | DC/TCMVs fusion; MOF core | - | vaccine | [62] |
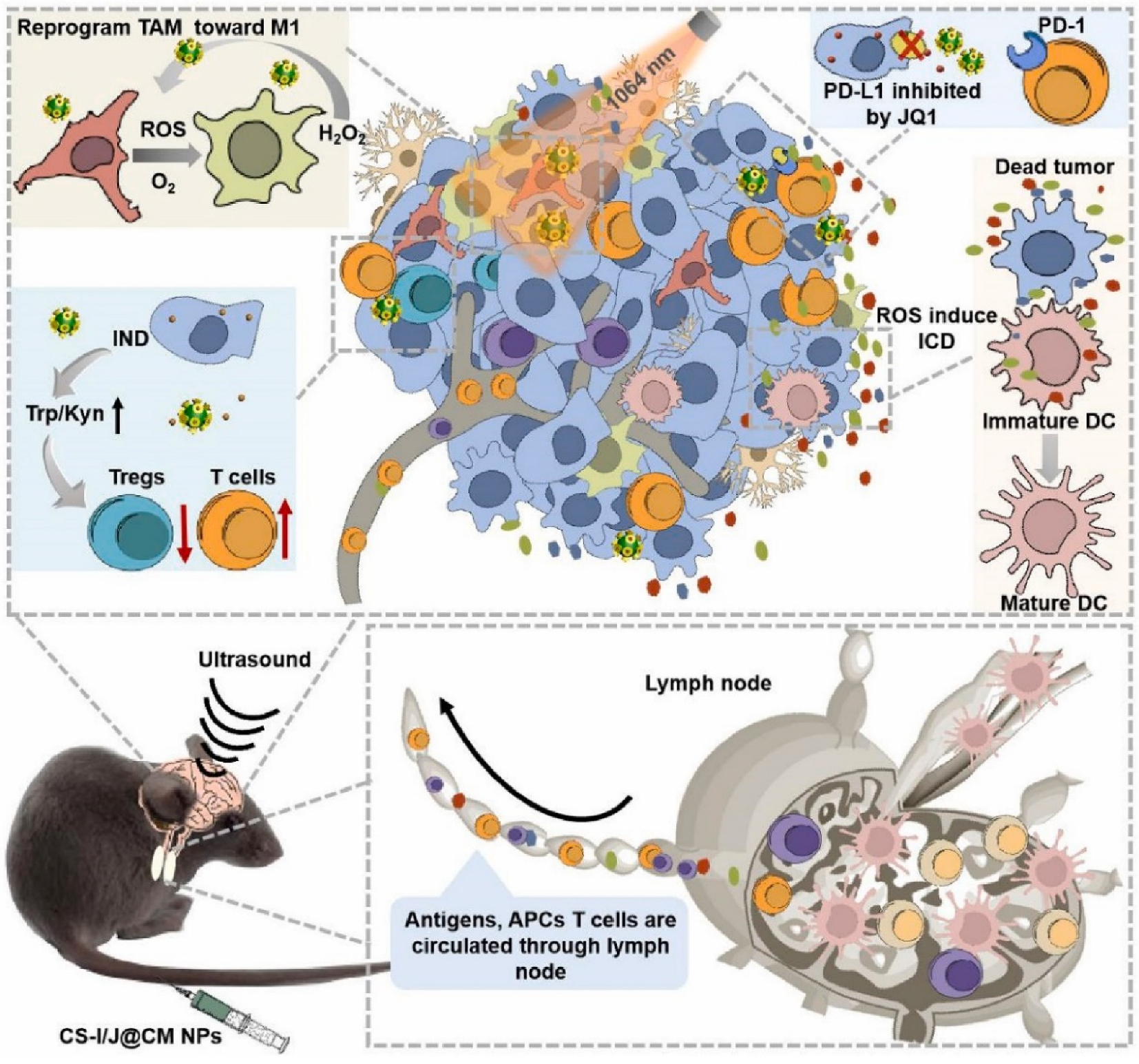
| Glioblastoma cell | Cu2-xSe NPs core | PD-L1 inhibitor and indoximod | ICT, PDT and ICD | [63] |
| Osteosarcoma cell | Macrophage cell membrane/TCMVs fusion; PLGA core | PTX | targeted tumor | [64] |
| solid tumor | Bacterial membrane/TCMVs fusion | - | vaccine | [64,65] |
| B16F10 cell | Bacterial membrane/TCMVs fusion; PLGA core | ICG | Vaccine and PTT | [66] |
| B16F10 | RBC/TCMVs fusion; CuS NPs core | DOX | Chemo-immunotherapy | [34] |
| 4T1 cell | PLGA core | R837 | vaccine | [67,68] |
| Ovarian cancer cell | RBC/TCMVs fusion; Fe3O4 core | ICG | PTT and Immunotherapy | [69] |
| 4T1 cell | RBC/TCMVs fusion; Fe3O4 core | CSF-1R inhibitor: | Immunotherapy | [70] |
| HepG2 cell | Prussian blue Nps core | - | PTT | [56] |
| 4T1 cell | alginate gel encapsulation | anti-PD-1 antibodies | vaccine | [71] |
| 4T1 cell | PAMAM core | DOX | targeting and anti-metastasis treatment | [72] |
| HCT116 | F127 core | R837 | vaccine | [33] |
| 4T1 cell and B16F10 cell | Encoded SIRPα and PD-1 | - | ICT and CD47 blockade | [73] |
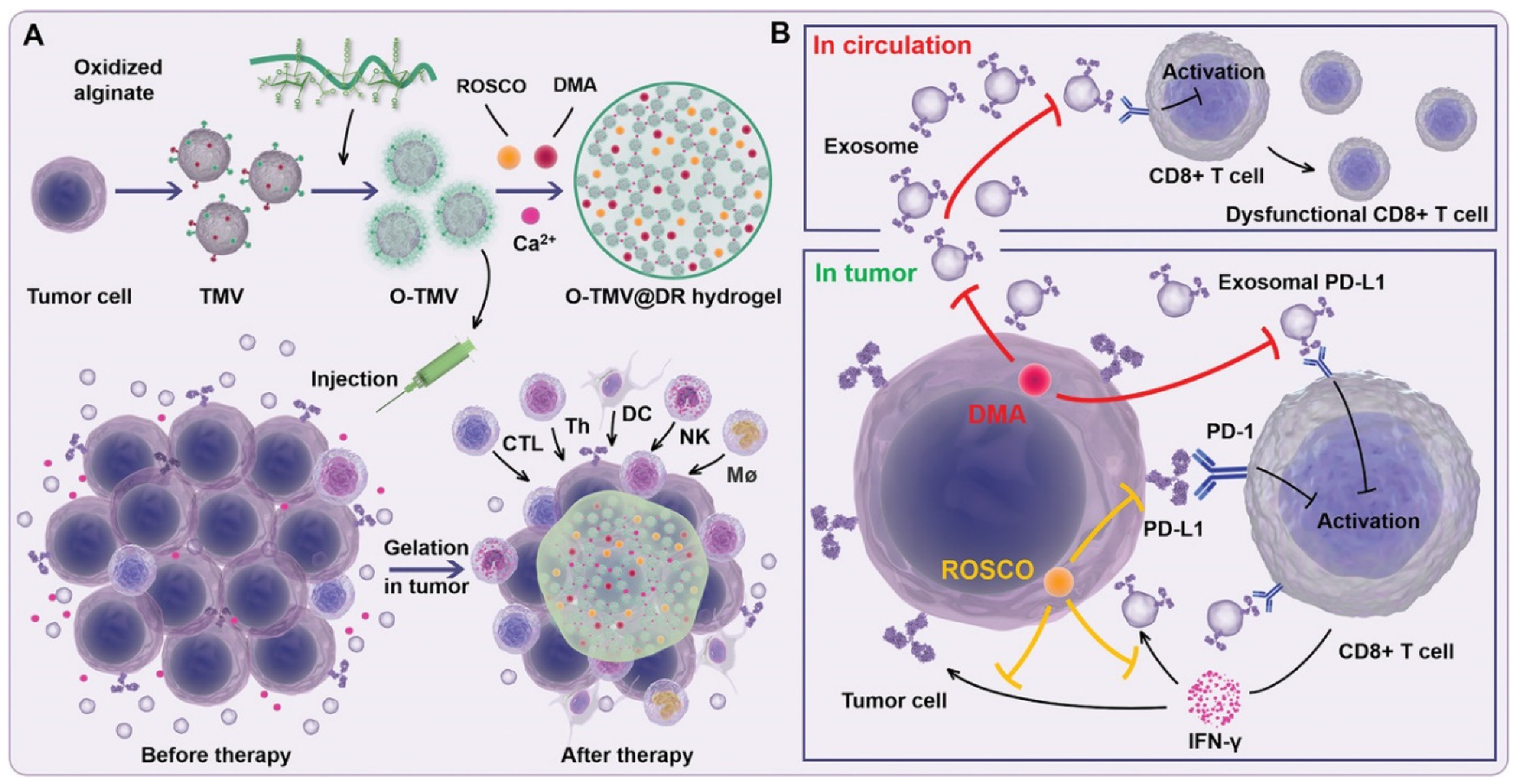
| B16F10 | - | DMA and Cdk5 inhibitor | ICT | [74] |
| Hela cell | PLGA core | PTX and siRNA | Chemo-immunotherapy | [48] |
| B16-OVA cell | OVA assembly core | Ce6 | PDT | [75] |
| lung carcinoma cell | - | Dox and Sorafenib | ICD and ICT | [49] |
| 4T1 cell | Surface-anchored CD80 and IL-12 | - | vaccine | [76] |
| MCF-7 | Au NPs core | MicroRNA | cancer diagnosis | [52] |
| 4T1 cell and B16F10 cell | thermosensitive hydrogel encapsulation | black phosphorus | Vaccine, PTT and ICT | [77] |
| B16F10 cell | cationic polymers core | DOX | Chemo-immunotherapy | [78] |
| Tumor cell | Surface-anchored anti CD205 | - | vaccine | [79] |
| B16-OVA | Surface-anchored mannose; PLGA core | R837 | vaccine | [80] |
| B16F10 | PLGA core | CpG | vaccine | [81] |
| Exosomes | Microvesicles | Apoptotic Bodies | |
|---|---|---|---|
| Size | 20–100 nm | 50–1000 nm | 500–2000 nm |
| Biogenesis | The inner membrane forms multivesicles within the cell that fuses with the plasma membrane to release exosomes into the extracellular compartment | Local changes in plasma membrane stiffness and curvature, cell surface shrinkage and outward blistering | Released by belting of apoptotic cell membrane |
| Contents | mRNA, microRNA, cytoplasmic and membrane protein, MHC | mRNA, miroRNA, noncoding RNAs, cytoplasmic and membrane protein | nuclear fractions, cytoplasmic protein, cell organelles |
| Biomakers | Tetraspanins, ESCRT peoteins, flotillin, TSG101 | Integrins, seltctins, CD40 ligand | Phosphatidylserine, annexin V |
| Ref. | [89,90] | [91,92] | [93,94] |
| TEVs Sourse | Engineering Strategy | Drug | Application | Ref. |
|---|---|---|---|---|
| MDA-MB-231 cells | alpha-lactalbumin-engineered TEVs | ELANE and Hiltonol | Vaccine and ICD | [104] |
| Colorectal cancer cell | - | microRNA 424 | immunotherapy | [105] |
| CT26 cell | thermosensitive liposomes/gene-engineered TEVs Fusion | - | PTT and CD47 blockade | [106] |
| HEPA1–6 | - | HMGN1 | vaccine | [107] |
| 4T1 cell | AIE luminogen/TEVs fusion | Dexamethasone | PDT | [108] |
| EL4 cell | gold-silver nanorods core | CpG | PTT and immunotherapy | [109] |
| HER2 expressing breast cancer | encoded anti-CD3 and anti-HER2 antibodies | - | immunotherapy | [110] |
| Serum exosomes from tumor-bearing mice | - | black phosphorus | PTT and vaccine | [111] |
| B16BL6 cells | encoding streptavidin lactadherin protein | CpG | immunotherapy | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Cao, W.; Wang, P.; Liu, H. Tumor-Derived Membrane Vesicles: A Promising Tool for Personalized Immunotherapy. Pharmaceuticals 2022, 15, 876. https://doi.org/10.3390/ph15070876
Xu J, Cao W, Wang P, Liu H. Tumor-Derived Membrane Vesicles: A Promising Tool for Personalized Immunotherapy. Pharmaceuticals. 2022; 15(7):876. https://doi.org/10.3390/ph15070876
Chicago/Turabian StyleXu, Jiabin, Wenqiang Cao, Penglai Wang, and Hong Liu. 2022. "Tumor-Derived Membrane Vesicles: A Promising Tool for Personalized Immunotherapy" Pharmaceuticals 15, no. 7: 876. https://doi.org/10.3390/ph15070876
APA StyleXu, J., Cao, W., Wang, P., & Liu, H. (2022). Tumor-Derived Membrane Vesicles: A Promising Tool for Personalized Immunotherapy. Pharmaceuticals, 15(7), 876. https://doi.org/10.3390/ph15070876







