The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections
Abstract
:1. Introduction
| Detection Method | Nanomaterial | Limit of Detection | Type of Virus | Ref. | |
|---|---|---|---|---|---|
| Electrochemical nanobiosensors | SWCNTs | Nanotubes | 102 CFU/mL | Bacillus subtilis | [17] |
| Change in output voltage | - | 6.9 copies/µL of viral RNA | SARS-CoV-2 | [18] | |
| Amperometric readings | - | - | SARS-CoV-2 | [19] | |
| FET sensor, transfer curve shift | - | 2.29 fM–3.99 fM | SARS-CoV-2 RNA | [20] | |
| Amperometry | Silver graphene QDs (Ag/GQDs) | 1ZM | Legionella | [21] | |
| glip-T | 1,6-Hexanedithiol and chitosan stabilized gold nanoparticle | 0.32 ± 0.01 × 10-[14] | Invasive Aspergillosis (IA) | [22] | |
| Optical nanobiosensors | Fluorescence | CdTe QDs | 0.13 µg mL−1 | Citrus tristeza virus (CTV) | [23] |
| LSPR, plasmonic photothermal heating (dual sensor) | - | 0.22 ± 0.08 pM (2.26 × 104 copies of viral RNA) | Coronavirus 2 | [24] | |
| Fluorescence | - | 12.6 nM of spike RBD | COVID-19 | [25] | |
| Terahertz plasmonic sensor | - | 4.2 fM | SARS-CoV-2 | [26] | |
| SPRi | AuNPs induced with QDs | 0.03 pg/mL and 0.4 pg/mL, 10 PFU/mL | Influenza | [27] | |
| Fluorescence | Nanobeads | 102–103 CFU/mL | E. coli | [28] |
2. The Chemistry of Semiconductor QDs, Carbon QDs, and Graphene QDs and Their Functionalization Strategies
The Comparative Merits and Demerits of Semiconductor QDs, Carbon QDs, and Graphene QDs
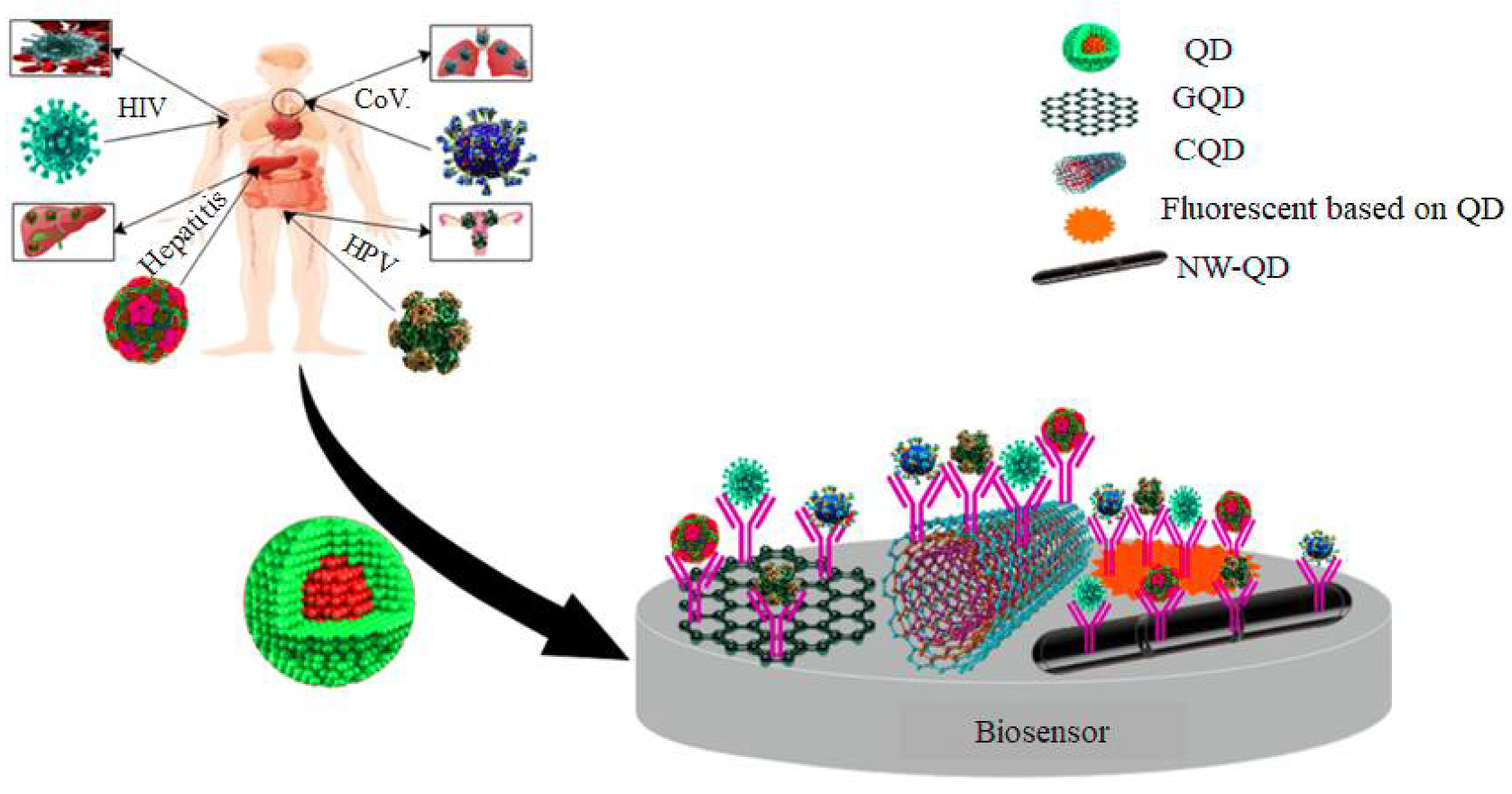
3. QD-Based Nanobiosensors
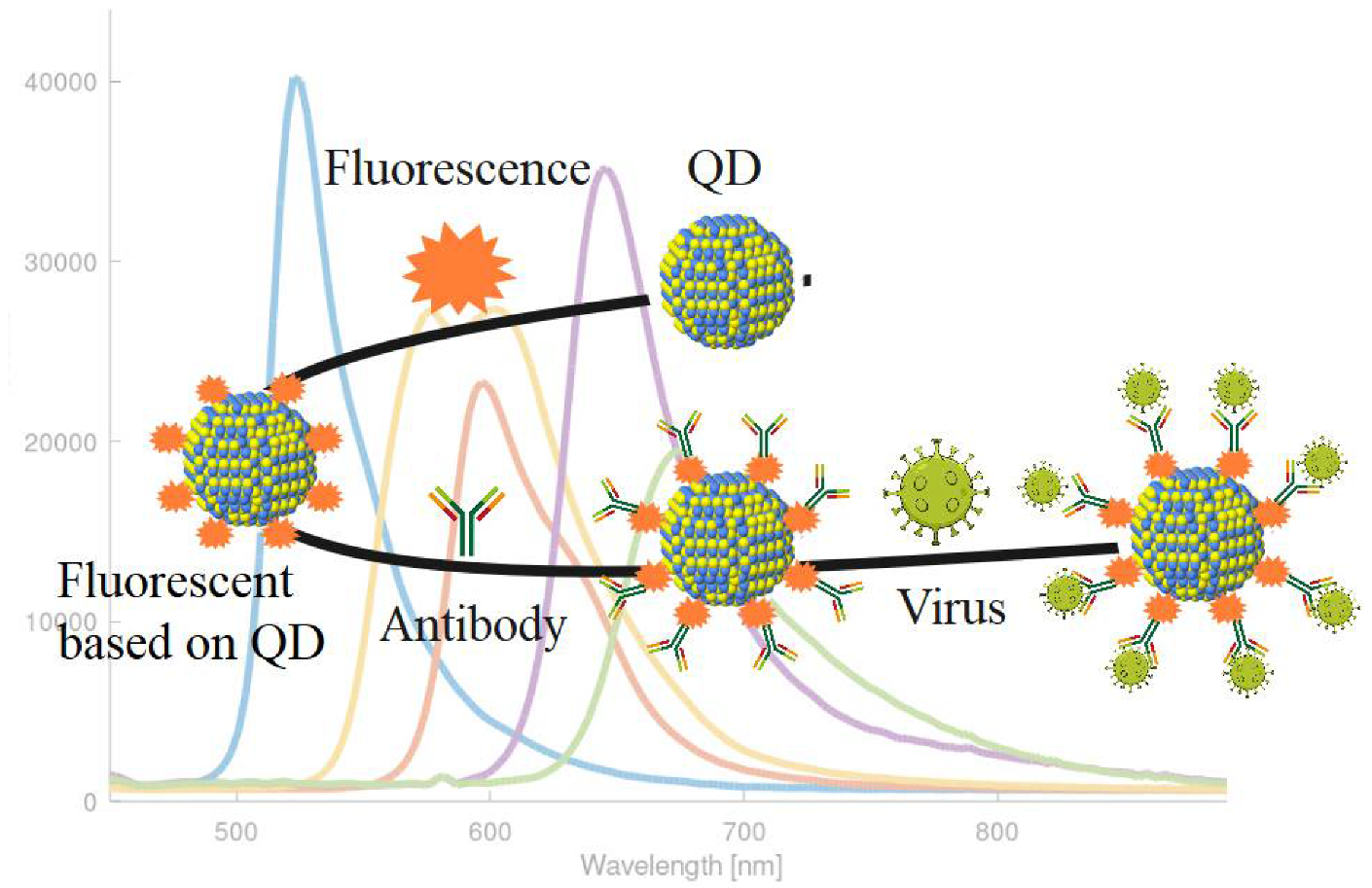
3.1. Fluorescence
3.2. Nanowire
3.3. Graphene
3.4. Carbon Nanotubes
4. QD-Based Biomarkers
5. Viral Infections Detected by QD-Based Biomarkers
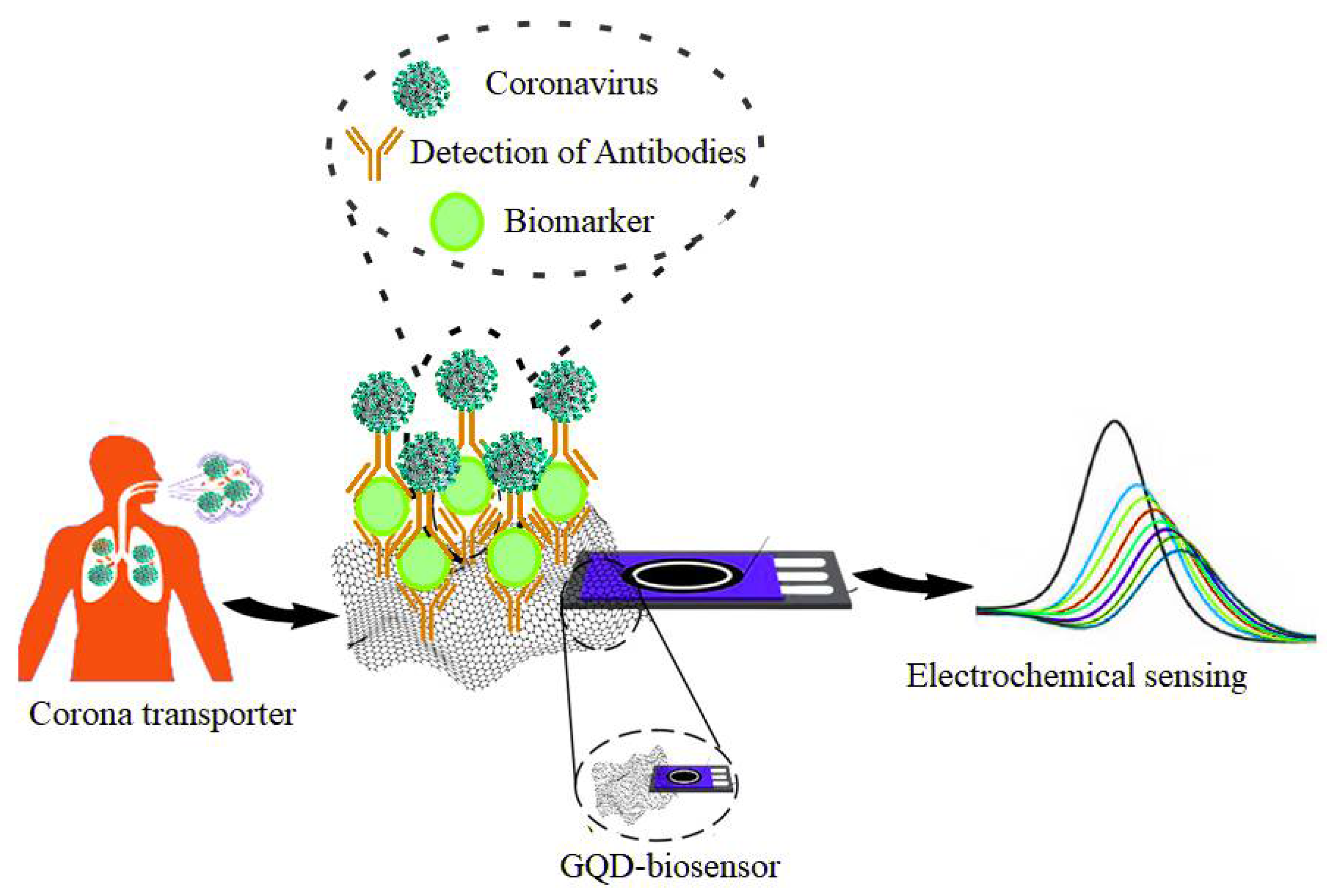
5.1. Coronavirus Disease—2019
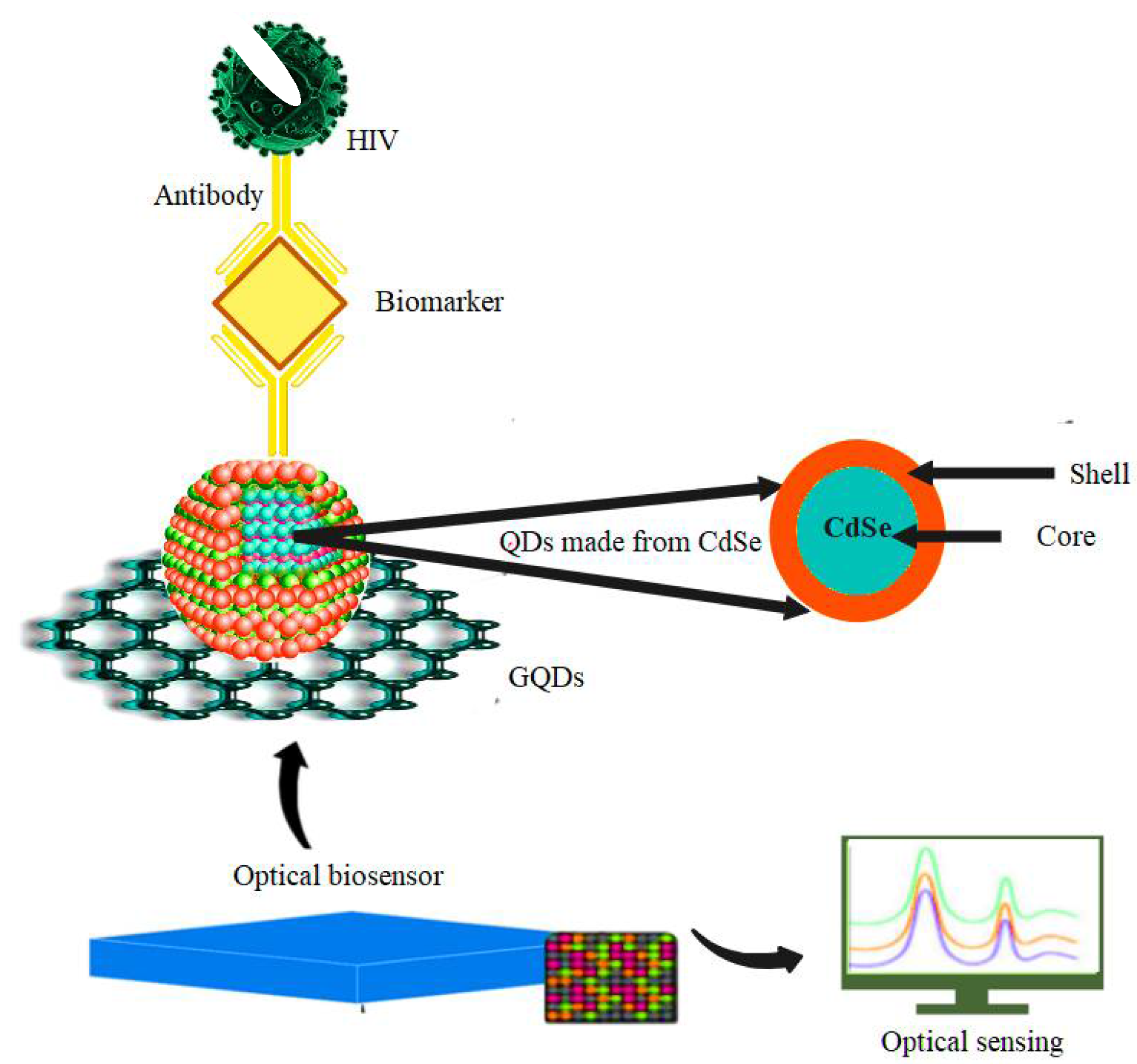
5.2. HIV
5.3. HPV
5.4. Hepatitis
5.5. Dengue Virus, Influenza Virus, Zika Virus, and Norovirus
6. Multiplex Detection of Viral Infection
7. Conclusions and Perspective
Abbreviations
| QDs | Quantum Dots |
| NWs | Nanowires |
| GO | Graphene Oxide |
| GQD | Graphene Quantum Dots |
| CNT | Carbon nanotube |
| CQDs | Carbon Quantum Dot |
| SWCNTs | Single-Walled Carbon Nanotubes |
| CoV | Coronavirus |
| FET | Field-Effect Transistor |
| SARS-CoV | Severe Acute Respiratory Syndrome Coronavirus |
| MERS | Middle East Respiratory Syndrome |
| HIV | Human Immunodeficiency Virus |
| AIDS | Acquired Immune Deficiency Syndrome |
| HPV | Human papillomavirus |
| PCR | Polymerase chain reaction |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fajardo, C.; Martinez-Rodriguez, G.; Blasco, J.; Mancera, J.M.; Thomas, B.; De Donato, M. Nanotechnology in aquaculture: Applications, perspectives and regulatory challenges. Aquac. Fish. 2022, 7, 185–200. [Google Scholar] [CrossRef]
- Belza, J.; Opletalová, A.; Poláková, K. Carbon dots for virus detection and therapy. Mikrochim. Acta 2021, 188, 430. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A.; Omidifar, N.; Shokripour, M. Asymmetric membranes: A potential scaffold for wound healing applications. Symmetry 2020, 12, 1100. [Google Scholar] [CrossRef]
- Khosravi Ardakani, H.; Gerami, M.; Chashmpoosh, M.; Omidifar, N.; Gholami, A. Recent progress in nanobiosensors for precise detection of blood glucose level. Biochem. Res. Int. 2022, 2022, 2964705. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Mazraedoost, S.; Chiang, W.-H.; Arjmand, O.; Omidifar, N.; Babapoor, A. Precise Blood glucose sensing by nitrogen-doped graphene quantum dots for tight control of diabetes. J. Sens. 2021, 2021, 5580203. [Google Scholar] [CrossRef]
- Gholami, A.; Farjami, F.; Ghasemi, Y. The development of an amperometric enzyme biosensor based on a polyaniline-multiwalled carbon nanocomposite for the detection of a chemotherapeutic agent in serum samples from patients. J. Sens. 2021, 2021, 5515728. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable biosensors: An alternative and practical approach in healthcare and disease monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, N.; Mishra, P.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2021, 754, 142363. [Google Scholar] [CrossRef]
- Byrnes, S.A.; Weigl, B.H. Selecting analytical biomarkers for diagnostic applications: A first principles approach. Expert Rev. Mol. Diagn. 2018, 18, 19–26. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Babapoor, A.; Rao, N.V.; Chiang, W.-H. Recent advances in plasma-engineered polymers for biomarker-based viral detection and highly multiplexed analysis. Biosensors 2022, 12, 286. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Menetski, J.P.; Hoffmann, S.C.; Cush, S.S.; Kamphaus, T.N.; Austin, C.P.; Herrling, P.L.; Wagner, J.A. The foundation for the national institutes of health biomarkers consortium: Past accomplishments and new strategic direction. Clin. Pharmacol. Ther. 2019, 105, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Tiwari, S.; Jain, R. Protein based biomarkers for non-invasive Covid-19 detection. Sens. Bio-Sens. Res. 2020, 29, 100362. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Rahmanian, V.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Chiang, W.-H. Highly sensitive flexible SERS-based sensing platform for detection of COVID-19. Biosensors 2022, 12, 466. [Google Scholar] [CrossRef]
- Boissiere, M. Quantum dots as biomarker. In Nanomaterials: A Danger or a Promise? Springer: Berlin, Germany, 2013; pp. 75–97. [Google Scholar]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef] [Green Version]
- Yoo, M.-S.; Shin, M.; Kim, Y.; Jang, Y.-E.; Choi, M.; Park, S.J.; Choi, J.; Lee, J.; Park, C. Development of electrochemical biosensor for detection of pathogenic microorganism in Asian dust events. Chemosphere 2017, 175, 269–274. [Google Scholar] [CrossRef]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano 2020, 14, 17028–17045. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.B.; Gao, W. SARS-CoV-2 RapidPlex: A graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.; Yu, Y.; Li, T.; Li, K.; Xiao, M.-M.; Li, Y.; Zhang, Z.-Y.; Zhang, G.-J. Rapid and unamplified identification of COVID-19 with morpholino-modified graphene field-effect transistor nanosensor. Biosens. Bioelectron. 2021, 183, 113206. [Google Scholar] [CrossRef]
- Mobed, A.; Hasanzadeh, M.; Shadjou, N.; Hassanpour, S.; Saadati, A.; Agazadeh, M. Immobilization of ssDNA on the surface of silver nanoparticles-graphene quantum dots modified by gold nanoparticles towards biosensing of microorganism. Microchem. J. 2020, 152, 104286. [Google Scholar] [CrossRef]
- Bhatnagar, I.; Mahato, K.; Ealla, K.K.R.; Asthana, A.; Chandra, P. Chitosan stabilized gold nanoparticle mediated self-assembled gliP nanobiosensor for diagnosis of Invasive Aspergillosis. Int. J. Biol. Macromol. 2018, 110, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, T.R.; Salleh, M.A.M.; Sijam, K.; Rahim, R.A.; Mohsenifar, A.; Safarnejad, R.; Tabatabaei, M. Detection of Citrus tristeza virus by using fluorescence resonance energy transfer-based biosensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 169, 216–222. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reina, G.; Iglesias, D.; Samorì, P.; Bianco, A. Graphene: A disruptive opportunity for COVID-19 and future pandemics? Adv. Mater. 2021, 33, 2007847. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, A.; Gerislioglu, B.; Ramezani, Z.; Kaushik, A.; Manickam, P.; Ghoreishi, S.A. Functionalized terahertz plasmonic metasensors: Femtomolar-level detection of SARS-CoV-2 spike proteins. Biosens. Bioelectron. 2021, 177, 112971. [Google Scholar] [CrossRef]
- Takemura, K.; Adegoke, O.; Takahashi, N.; Kato, T.; Li, T.-C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Versatility of a localized surface plasmon resonance-based gold nanoparticle-alloyed quantum dot nanobiosensor for immunofluorescence detection of viruses. Biosens. Bioelectron. 2017, 89, 998–1005. [Google Scholar] [CrossRef]
- Xu, L.; Lu, Z.; Cao, L.; Pang, H.; Zhang, Q.; Fu, Y.; Xiong, Y.; Li, Y.; Wang, X.; Wang, J. In-field detection of multiple pathogenic bacteria in food products using a portable fluorescent biosensing system. Food Control 2017, 75, 21–28. [Google Scholar] [CrossRef]
- Zhou, W.; Coleman, J.J. Semiconductor quantum dots. Curr. Opin. Solid State Mater. Sci. 2016, 20, 352–360. [Google Scholar] [CrossRef]
- Adegoke, O.; Montaseri, H.; Nsibande, S.A.; Forbes, P.B. Passivating effect of ternary alloyed AgZnSe shell layer on the structural and luminescent properties of CdS quantum dots. Mater. Sci. Semicond. Process. 2019, 90, 162–170. [Google Scholar] [CrossRef]
- Harrison, P.; Valavanis, A. Quantum Wells, Wires and Dots: Theoretical and Computational Physics of Semiconductor Nanostructures; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac billiard in graphene quantum dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, properties, synthesis, characterization, and applications in health care—An updated review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Reshma, V.; Mohanan, P. Quantum dots: Applications and safety consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon quantum dots: Synthesis, characterization and biomedical applications. Turk. J. Pharm. Sci. 2018, 15, 219. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of mono-disperse carbon quantum dots from fennel seeds: Photoluminescence analysis using machine learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Gao, J.; Chen, X.; Duo, Y.; Zhang, H. Semiconducting quantum dots: Modification and applications in biomedical science. Sci. China Mater. 2020, 63, 1631–1650. [Google Scholar] [CrossRef]
- Pleskova, S.; Mikheeva, E.; Gornostaeva, E. Using of quantum dots in biology and medicine. Cell. Mol. Toxicol. Nanopart. 2018, 1048, 323–334. [Google Scholar]
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1331–1348. [Google Scholar] [CrossRef]
- Desmond, L.J.; Phan, A.N.; Gentile, P. Critical overview on the green synthesis of carbon quantum dots and their application for cancer therapy. Environ. Sci. Nano 2021, 8, 848–862. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, S.; Pillai, V.K. Synthesis and characterization of graphene quantum dots. Phys. Sci. Rev. 2020, 5. [Google Scholar] [CrossRef]
- Shen, J.; Chen, W.; Yang, Z.; Lv, G.; Cao, J.; Li, D.; Liu, X. A critical review of graphene quantum dots: Synthesis and application in biosensors. Nano 2021, 16, 2130001. [Google Scholar] [CrossRef]
- Kairdolf, B.A.; Smith, A.M.; Stokes, T.H.; Wang, M.D.; Young, A.N.; Nie, S. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu. Rev. Anal. Chem. 2013, 6, 143–162. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, X.; Tang, B.; Xu, W.; Li, J.; Hu, J.; Gu, Z. Quantum-dot-tagged bioresponsive hydrogel suspension array for multiplex label-free DNA detection. Adv. Funct. Mater. 2010, 20, 976–982. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Chen, L.; Sheng, Z.; Zhang, A.; Guo, X.; Li, J.; Han, H.; Jin, M. uantum-dots-based fluoroimmunoassay for the rapid and sensitive detection of avian influenza virus subtype H5N1. Luminescence 2010, 25, 419–423. [Google Scholar] [CrossRef]
- Klestova, Z.; Voronina, A.; Yushchenko, A.Y.; Vatlitsova, O.; Dorozinsky, G.; Ushenin, Y.V.; Maslov, V.; Doroshenko, T.; Kravchenko, S. Aspects of “antigen–antibody” interaction of chicken infectious bronchitis virus determined by surface plasmon resonance. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120236. [Google Scholar] [CrossRef]
- Song, M.; Yang, M.; Hao, J. Pathogenic virus detection by optical nanobiosensors. Cell Rep. Phys. Sci. 2021, 2, 100288. [Google Scholar] [CrossRef]
- Lichtman, J.W.; Conchello, J.-A. Fluorescence microscopy. Nat. Methods 2005, 2, 910–919. [Google Scholar] [CrossRef]
- Brus, L.E. Chemistry and Physics of Semiconductor Nanocrystals. In The 37th Middle Atlantic Regional Meeting; Columbia University: New York, NY, USA, 2007. [Google Scholar]
- Sharma, A.; Khan, R.; Catanante, G.; Sherazi, T.A.; Bhand, S.; Hayat, A.; Marty, J.L. Designed strategies for fluorescence-based biosensors for the detection of mycotoxins. Toxins 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddali, H.; Miles, C.E.; Kohn, J.; O’Carroll, D.M. Optical biosensors for virus detection: Prospects for SARS-CoV-2/COVID-19. ChemBioChem 2021, 22, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Álvarez, M.; Martín-Esteban, A. Molecularly imprinted polymer-quantum dot materials in optical sensors: An overview of their synthesis and applications. Biosensors 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.C. Application of nanomaterials-based optical sensors for virus detections. J. Phys. Conf. Ser. 2021, 1906, 012028. [Google Scholar] [CrossRef]
- Raee, M.J.; Ebrahiminezhad, A.; Gholami, A.; Ghoshoon, M.B.; Ghasemi, Y. Magnetic immobilization of recombinant E. coli producing extracellular asparaginase: An effective way to intensify downstream process. Sep. Sci. Technol. 2018, 53, 1397–1404. [Google Scholar] [CrossRef]
- Markwalter, C.F.; Kantor, A.G.; Moore, C.P.; Richardson, K.A.; Wright, D.W. Inorganic complexes and metal-based nanomaterials for infectious disease diagnostics. Chem. Rev. 2018, 119, 1456–1518. [Google Scholar] [CrossRef] [Green Version]
- Altintas, Z.; Fakanya, W.M.; Tothill, I.E. Cardiovascular disease detection using bio-sensing techniques. Talanta 2014, 128, 177–186. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanotechnology for biosensors: A Review. arXiv 2021, arXiv:2101.02430. [Google Scholar]
- Pan, H.; Zhang, P.; Gao, D.; Zhang, Y.; Li, P.; Liu, L.; Wang, C.; Wang, H.; Ma, Y.; Cai, L. Noninvasive visualization of respiratory viral infection using bioorthogonal conjugated near-infrared-emitting quantum dots. ACS Nano 2014, 8, 5468–5477. [Google Scholar] [CrossRef] [PubMed]
- Tedsana, W.; Tuntulani, T.; Ngeontae, W. A highly selective turn-on ATP fluorescence sensor based on unmodified cysteamine capped CdS quantum dots. Anal. Chim. Acta 2013, 783, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Munsch, M.; Malik, N.S.; Dupuy, E.; Delga, A.; Bleuse, J.; Gérard, J.-M.; Claudon, J.; Gregersen, N.; Mørk, J. Dielectric GaAs antenna ensuring an efficient broadband coupling between an InAs quantum dot and a gaussian optical beam. Phys. Rev. Lett. 2013, 110, 177402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Nanowire sensors for medicine and the life sciences. Nanomedicine 2006, 1, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Touhami, A. Biosensors and nanobiosensors: Design and applications. Nanomedicine 2014, 15, 374–403. [Google Scholar]
- Hashemi, S.A.; Mousavi, S.M.; Naderi, H.R.; Bahrani, S.; Arjmand, M.; Hagfeldt, A.; Chiang, W.-H.; Ramakrishna, S. Reinforced polypyrrole with 2D graphene flakes decorated with interconnected nickel-tungsten metal oxide complex toward superiorly stable supercapacitor. Chem. Eng. J. 2021, 418, 129396. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Kalashgrani, M.Y.; Omidifar, N.; Bahrani, S.; Vijayakameswara Rao, N.; Babapoor, A.; Gholami, A.; Chiang, W.-H. Bioactive graphene quantum dots based polymer composite for biomedical applications. Polymers 2022, 14, 617. [Google Scholar] [CrossRef]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, S.M.; Gutha, R.R. Coherent Networks of Si nanocrystals: Tunable collective resonances with narrow spectral widths. Adv. Photonics Res. 2021, 2, 2000129. [Google Scholar] [CrossRef]
- Sedaghat, B.; Taherrian, R.; Hosseini, S.A.; Mousavi, S.M. Rheological properties of bitumen containing nanoclay and organic warm-mix asphalt additives. Constr. Build. Mater. 2020, 243, 118092. [Google Scholar] [CrossRef]
- Smirnov, V.A.; Denisov, N.N.; Alfimov, M.V. Photochemical reduction of graphite oxide. Nanotechnol. Russ. 2013, 8, 1–22. [Google Scholar] [CrossRef]
- Navakul, K.; Warakulwit, C.; Yenchitsomanus, P.-t.; Panya, A.; Lieberzeit, P.A.; Sangma, C. A novel method for dengue virus detection and antibody screening using a graphene-polymer based electrochemical biosensor. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 549–557. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Gholami, A.; Lai, C.W.; Chiang, W.H.; Omidifar, N.; Bahrani, S.; Mazraedoost, S. Recent progress in chemical composition, production, and pharmaceutical effects of kombucha beverage: A complementary and alternative medicine. Evid.-Based Complement. Altern. Med. 2020, 2020, 4397543. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, X.; Li, M.; Gao, H.; Sun, J.; Zhu, S.; Han, S.; Jia, L.-N.; Zhao, X.-E.; Wang, H. A “turn-on” fluorescence sensor for ascorbic acid based on graphene quantum dots via fluorescence resonance energy transfer. Anal. Methods 2018, 10, 611–616. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, D.; Li, R.; Wang, Y.; Zhang, G.; Li, S.; Zheng, J.; Huang, N.; Gu, Y.; Wang, C. Eco-friendly synthesis of size-controllable amine-functionalized graphene quantum dots with antimycoplasma properties. Nanoscale 2013, 5, 1137–1142. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Mousavi, S.; Arjmand, O.; Hashemi, S.; Banaei, N. Modification of the epoxy resin mechanical and thermal properties with silicon acrylate and montmorillonite nanoparticles. Polym. Renew. Resour. 2016, 7, 101–113. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Ferro, S.; De Luca, L.; Monforte, A.M.; Romeo, R.; Buemi, M.R.; Pannecouque, C. Graphene Quantum Dots Based Systems As HIV Inhibitors. Bioconjug. Chem. 2018, 29, 3084–3093. [Google Scholar] [CrossRef]
- Fahmi, M.; Sukmayani, W.; Khairunisa, S.Q.; Witaningrum, A.; Indriati, D.; Matondang, M.; Chang, J.-Y.; Kotaki, T.; Kameoka, M. Design of boronic acid-attributed carbon dots on inhibits HIV-1 entry. RSC Adv. 2016, 6, 92996–93002. [Google Scholar] [CrossRef]
- Xiang, Q.; Huang, J.; Huang, H.; Mao, W.; Ye, Z. A label-free electrochemical platform for the highly sensitive detection of hepatitis B virus DNA using graphene quantum dots. RSC Adv. 2018, 8, 1820–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Raicopol, M.; Prună, A.; Damian, C.; Pilan, L. Functionalized single-walled carbon nanotubes/polypyrrole composites for amperometric glucose biosensors. Nanoscale Res. Lett. 2013, 8, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgakilas, V.; Gournis, D.; Tzitzios, V.; Pasquato, L.; Guldi, D.M.; Prato, M. Decorating carbon nanotubes with metal or semiconductor nanoparticles. J. Mater. Chem. 2007, 17, 2679–2694. [Google Scholar] [CrossRef]
- Aguilar, Z. Nanomaterials for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Kalashgrani, M.Y.; Vijayakameswara Rao, N.; Omidifar, N.; Hsiao, W.W.-W.; Lai, C.W.; Chiang, W.-H. Plasma-Enabled Smart Nanoexosome Platform as Emerging Immunopathogenesis for Clinical Viral Infection. Pharmaceutics 2022, 14, 1054. [Google Scholar] [CrossRef]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef]
- Mohkam, M.; Rasoul-Amini, S.; Shokri, D.; Berenjian, A.; Rahimi, F.; Sadraeian, M.; Khalvati, B.; Gholami, A.; Ghasemi, Y. Characterization and in vitro probiotic assessment of potential indigenous Bacillus strains isolated from soil rhizosphere. Minerva Biotecnol. 2016, 28, 19–28. [Google Scholar]
- Wiriyachaiporn, N.; Sirikett, H.; Maneeprakorn, W.; Dharakul, T. Carbon nanotag based visual detection of influenza A virus by a lateral flow immunoassay. Mikrochim. Acta 2017, 184, 1827–1835. [Google Scholar] [CrossRef]
- Li, X.-M.; Zhan, Z.-M.; Ju, H.-Q.; Zhang, S.-S. Label-Free Electrochemical Detection of Short Sequences Related to the Hepatitis B Virus Using 4,4′-Diaminoazobenzene Based on Multiwalled Carbon Nanotube-Modified GCE. Oligonucleotides 2008, 18, 321–328. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Yari Kalashgrani, M.; Kurniawan, D.; Gholami, A.; Rahmanian, V.; Omidifar, N.; Chiang, W.-H. Recent Advances in Inflammatory Diagnosis with Graphene Quantum Dots Enhanced SERS Detection. Biosensors 2022, 12, 461. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Kalashgrani, M.Y.; Javanmardi, N. Multifunctional Gold nanoparticle: As novel agents for cancer treatment. Adv. Appl. NanoBio-Technol. 2022, 3, 43–48. [Google Scholar]
- Lu, Y.-C.; Chen, J.; Wang, A.-J.; Bao, N.; Feng, J.-J.; Wang, W.; Shao, L. Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury(ii) detection and bioimaging. J. Mater. Chem. C 2015, 3, 73–78. [Google Scholar] [CrossRef]
- Kalashgrani, M.Y.; Nejad, F.F.; Rahmanian, V. Carbon Quantum Dots Platforms: As nano therapeutic for Biomedical Applications. Adv. Appl. NanoBio-Technol. 2022, 3, 38–42. [Google Scholar]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Kalashgrani, M.Y.; Harzand, F.V.; Javanmardi, N.; Nejad, F.F.; Rahmanian, V. Recent Advances in Multifunctional magnetic nano platform for Biomedical Applications: A mini review. Adv. Appl. NanoBio-Technol. 2022, 3, 31–37. [Google Scholar]
- Barras, A.; Pagneux, Q.; Sane, F.; Wang, Q.; Boukherroub, R.; Hober, D.; Szunerits, S. High Efficiency of Functional Carbon Nanodots as Entry Inhibitors of Herpes Simplex Virus Type 1. ACS Appl. Mater. Interfaces 2016, 8, 9004–9013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Xia, L.; Xie, H.-Y.; Zhang, Z.-L.; Pang, D.-W. Quantum Dot Based Biotracking and Biodetection. Anal. Chem. 2018, 91, 532–547. [Google Scholar] [CrossRef]
- Kalashgarani, M.Y.; Babapoor, A. Application of nano-antibiotics in the diagnosis and treatment of infectious diseases. Adv. Appl. NanoBio-Technol. 2022, 3, 22–35. [Google Scholar]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, Endpoints, and Other Tools) Resource; Food and Drug Administration: Silver Spring, MD, USA; National Institutes of Health (US): Bethesda, MD, USA, 2016. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Parvin, N.; Gholami, A.; Ramakrishna, S.; Omidifar, N.; Moghadami, M.; Chiang, W.-H.; Mazraedoost, S. Recent biotechnological approaches for treatment of novel COVID-19: From bench to clinical trial. Drug Metab. Rev. 2021, 53, 141–170. [Google Scholar] [CrossRef] [PubMed]
- Tech, J.E.T. Investigating the activity of antioxidants activities content in Apiaceae and to study antimicrobial and insecticidal activity of antioxidant by using SPME Fiber assembly carboxen/polydimethylsiloxane (CAR/PDMS). J. Environ. Treat. Tech. 2020, 8, 214–224. [Google Scholar]
- Memar, M.Y.; Baghi, H.B. Presepsin: A promising biomarker for the detection of bacterial infections. Biomed. Pharmacother. 2019, 111, 649–656. [Google Scholar] [CrossRef]
- Alipour, A.; Kalashgarani, M.Y. Nano Protein and Peptides for Drug Delivery and Anticancer Agents. Adv. Appl. NanoBio-Technol. 2022, 3, 60–64. [Google Scholar]
- Rabiee, N.; Khatami, M.; Jamalipour Soufi, G.; Fatahi, Y.; Iravani, S.; Varma, R.S. Diatoms with Invaluable Applications in Nanotechnology, Biotechnology, and Biomedicine: Recent Advances. ACS Biomater. Sci. Eng. 2021, 7, 3053–3068. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, N.; Díez, P.; Matarraz, S.; González-González, M.; Paradinas, S.; Orfao, A.; Fuentes, M. Biomarker Discovery by Novel Sensors Based on Nanoproteomics Approaches. Sensors 2012, 12, 2284–2308. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Miralles, N.; Feliu, J.X.; Vandevuer, S.; Müller, A.; Cabrera-Crespo, J.; Ortmans, I.; Hoffmann, F.; Cazorla, D.; Rinas, U.; Prévost, M. Engineering Regulable Escherichia coliβ-Galactosidases as Biosensors for Anti-HIV Antibody Detection in Human Sera. J. Biol. Chem. 2001, 276, 40087–40095. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.R.; Moraes, M.L.; Nigoghossian, K.; Peres, M.F.; Ribeiro, S.J. Silk fibroin-antigenic peptides-YVO 4 :Eu 3+ nanostructured thin films as sensors for hepatitis C. J. Lumin. 2016, 170, 375–379. [Google Scholar] [CrossRef] [Green Version]
- An, J. Expression and Significance of Th17 Cells and Related Factors in Patients with Autoimmune Hepatitis. Comb. Chem. High Throughput Screen. 2019, 22, 232–237. [Google Scholar] [CrossRef]
- Lee, H.J.; Namkoong, K.; Cho, E.C.; Ko, C.; Park, J.C.; Lee, S.S. Surface acoustic wave immunosensor for real-time detection of hepatitis B surface antibodies in whole blood samples. Biosens. Bioelectron. 2009, 24, 3120–3125. [Google Scholar] [CrossRef]
- Omidifar, N.; Lankarani, K.B.; Moghadami, M.; Shokripour, M.; Chashmpoosh, M.; Mousavi, S.M.; Hashemi, S.A.; Gholami, A. Different Laboratory Diagnosis Methods of COVID-19: A Systematic Review. Arch. Clin. Infect. Dis. 2021, 16, e110667. [Google Scholar] [CrossRef]
- Manivannan, S.; Ponnuchamy, K. Quantum dots as a promising agent to combat COVID-19. Appl. Organomet. Chem. 2020, 34, e5887. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C 2020, 112, 110924. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Liang, J.; Dong, N.; Liu, L.; Fang, L.; Xiao, S.; Han, H. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon 2016, 110, 278–285. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Ahn, S.N.; Choudhry, H. Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, S.M. Bacterial cellulose/polyaniline nanocomposite aerogels as novel bioadsorbents for removal of hexavalent chromium: Experimental and simulation study. J. Clean. Prod. 2021, 278, 123817. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Behbahan, N.G.G.; Bahrani, S.; Mousavi, S.M.; Gholami, A.; Ramakrishna, S.; Firoozsani, M.; Moghadami, M.; Lankarani, K.B.; Omidifar, N. Ultra-sensitive viral glycoprotein detection NanoSystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosens. Bioelectron. 2021, 171, 112731. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Samandari, L.; Sheibani, S. Advances in the design of nanomaterial-based electrochemical affinity and enzymatic biosensors for metabolic biomarkers: A review. Mikrochim. Acta 2018, 185, 276. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Mousavi, S.M.; Nazem, H. Anti-bacterial/fungal and anti-cancer performance of green synthesized Ag nanoparticles using summer savory extract. J. Exp. Nanosci. 2020, 15, 363–380. [Google Scholar] [CrossRef]
- Joo, K.-I.; Lei, Y.; Lee, C.-L.; Lo, J.; Xie, J.; Hamm-Alvarez, S.F.; Wang, P. Site-Specific Labeling of Enveloped Viruses with Quantum Dots for Single Virus Tracking. ACS Nano 2008, 2, 1553–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szunerits, S.; Barras, A.; Khanal, M.; Pagneux, Q.; Boukherroub, R. Nanostructures for the Inhibition of Viral Infections. Molecules 2015, 20, 14051–14081. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-S.; Qi, L.; Zhang, R.-L.; Jin, M.; Zhang, Z.-Q. Ratiometric fluorescence biosensor based on CdTe quantum and carbon dots for double strand DNA detection. Sens. Actuators B Chem. 2017, 244, 585–590. [Google Scholar] [CrossRef]
- Jimenez, A.M.J.; Moulick, A.; Richtera, L.; Krejcova, L.; Kalina, L.; Datta, R.; Svobodova, M.; Hynek, D.; Masarik, M.; Heger, Z. Dual-color quantum dots-based simultaneous detection of HPV-HIV co-infection. Sens. Actuators B Chem. 2018, 258, 295–303. [Google Scholar] [CrossRef]
- Xue, J.; Chen, H.; Fan, M.; Zhu, F.; Diao, L.; Chen, X.; Fan, L.; Li, P.; Xia, D. Use of quantum dots to detect human papillomavirus in oral squamous cell carcinoma. J. Oral Pathol. Med. 2009, 38, 668–671. [Google Scholar] [CrossRef]
- Piao, J.Y.; Park, E.H.; Choi, K.; Quan, B.; Kang, D.H.; Park, P.Y.; Chung, D.S. Direct visual detection of DNA based on the light scattering of silica nanoparticles on a human papillomavirus DNA chip. Talanta 2009, 80, 967–973. [Google Scholar] [CrossRef]
- Nejdl, L.; Skalickova, S.; Kudr, J.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Rodrigo, M.A.M.; Kopel, P.; Konecna, M.; Adam, V.; Kizek, R. Interaction of E6 Gene from Human Papilloma Virus 16 (HPV-16) with CdS Quantum Dots. Chromatographia 2014, 77, 1433–1439. [Google Scholar] [CrossRef]
- Hassanpour, S.; Baradaran, B.; de la Guardia, M.; Baghbanzadeh, A.; Mosafer, J.; Hejazi, M.; Mokhtarzadeh, A.; Hasanzadeh, M. Diagnosis of hepatitis via nanomaterial-based electrochemical, optical or piezoelectrical biosensors: A review on recent advancements. Mikrochim. Acta 2018, 185, 568. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Wang, Q.; Wu, Z.; Zhang, X.; Li, B.; Lin, L. Recent advances in quantum dots-based biosensors for antibiotics detection. J. Pharm. Anal. 2021, 12, 355–364. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Ma, Q.; Lin, Z.; Chen, S.; Li, Y.; Lu, L.; Qu, H.; Su, X. Multiplex electrochemiluminescence DNA sensor for determination of hepatitis B virus and hepatitis C virus based on multicolor quantum dots and Au nanoparticles. Anal. Chim. Acta 2016, 916, 92–101. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Liang, X.; Zhang, G.; Ma, H.; Nie, L.; Wang, Y. Detection of Hepatitis B Virus M204I Mutation by Quantum Dot-Labeled DNA Probe. Sensors 2017, 17, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, A.D.; Takemura, K.; Li, T.-C.; Suzuki, T.; Park, E.Y. Electrical pulse-induced electrochemical biosensor for hepatitis E virus detection. Nat. Commun. 2019, 10, 3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, G.-X. A protein array based on quantum dots (QDs) encoded microbeads for detection of hepatitis C virus. Chin. J. Exp. Clin. Virol. 2013, 27, 67–69. [Google Scholar]
- Wang, G.; Leng, Y.; Dou, H.; Wang, L.; Li, W.; Wang, X.; Sun, K.; Shen, L.; Yuan, X.; Li, J. Highly Efficient Preparation of Multiscaled Quantum Dot Barcodes for Multiplexed Hepatitis B Detection. ACS Nano 2013, 7, 471–481. [Google Scholar] [CrossRef]
- Modani, S.; Kharwade, M.; Nijhawan, M. Quantum dots: A novelty of medical field with multiple applications. Int. J. Curr. Pharm. Res. 2013, 5, 55–59. [Google Scholar]
- Tope, S.; Saudagar, S.; Kale, N.; Bhise, K. Therapeutic application of quantum dots (QD). Pharma Innov. 2014, 2, 86. [Google Scholar]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Zou, Z.; Yang, H.; Yan, Q.; Qi, P.; Qing, Z.; Zheng, J.; Xu, X.; Zhang, L.; Feng, F.; Yang, R. Synchronous screening of multiplexed biomarkers of Alzheimer’s disease by a length-encoded aerolysin nanopore-integrated triple-helix molecular switch. Chem. Commun. 2019, 55, 6433–6436. [Google Scholar] [CrossRef]
- Fabri-Faja, N.; Calvo-Lozano, O.; Dey, P.; Terborg, R.A.; Estevez, M.-C.; Belushkin, A.; Yesilköy, F.; Duempelmann, L.; Altug, H.; Pruneri, V. Early sepsis diagnosis via protein and miRNA biomarkers using a novel point-of-care photonic biosensor. Anal. Chim. Acta 2019, 1077, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Ngo, H.T.; Wang, H.-N.; Burke, T.; Ginsburg, G.S.; Vo-Dinh, T. Multiplex detection of disease biomarkers using SERS molecular sentinel-on-chip. Anal. Bioanal. Chem. 2014, 406, 3335–3344. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Ma, X. Clinical identification of diabetic ketosis/diabetic ketoacidosis acid by electrochemical dual channel test strip with medical smartphone. Sens. Actuators B Chem. 2018, 275, 446–450. [Google Scholar] [CrossRef]
- Prigodich, A.E.; Randeria, P.S.; Briley, W.E.; Kim, N.J.; Daniel, W.L.; Giljohann, D.A.; Mirkin, C.A. Multiplexed Nanoflares: mRNA Detection in Live Cells. Anal. Chem. 2012, 84, 2062–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meade, S.O.; Chen, M.Y.; Sailor, M.J.; Miskelly, G.M. Multiplexed DNA Detection Using Spectrally Encoded Porous SiO2 Photonic Crystal Particles. Anal. Chem. 2009, 81, 2618–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirasoli, M.; Bonvicini, F.; Dolci, L.S.; Zangheri, M.; Gallinella, G.; Roda, A. Portable chemiluminescence multiplex biosensor for quantitative detection of three B19 DNA genotypes. Anal. Bioanal. Chem. 2013, 405, 1139–1143. [Google Scholar] [CrossRef]
- Su, S.; Wei, X.; Zhong, Y.; Guo, Y.; Su, Y.; Huang, Q.; Lee, S.-T.; Fan, C.; He, Y. Silicon Nanowire-Based Molecular Beacons for High-Sensitivity and Sequence-Specific DNA Multiplexed Analysis. ACS Nano 2012, 6, 2582–2590. [Google Scholar] [CrossRef]
- Dudak, F.C.; Boyaci, I.H. Multiplex detection of escherichia coli and salmonella enteritidis by using quantum dot-labeled antibodieS. J. Rapid Methods Autom. Microbiol. 2009, 17, 315–327. [Google Scholar] [CrossRef]
- Li, Y.; Cu, Y.T.H.; Luo, D. Multiplexed detection of pathogen DNA with DNA-based fluorescence nanobarcodes. Nat. Biotechnol. 2005, 23, 885–889. [Google Scholar] [CrossRef]
- Bilan, R.; Ametzazurra, A.; Brazhnik, K.; Escorza, S.; Fernández, D.; Uríbarri, M.; Nabiev, I.; Sukhanova, A. Quantum-dot-based suspension microarray for multiplex detection of lung cancer markers: Preclinical validation and comparison with the Luminex xMAP® system. Sci. Rep. 2017, 7, srep44668. [Google Scholar] [CrossRef] [Green Version]
- Xiao, K.; Wang, K.; Qin, W.; Hou, Y.; Lu, W.; Xu, H.; Wo, Y.; Cui, D. Use of quantum dot beads-labeled monoclonal antibody to improve the sensitivity of a quantitative and simultaneous immunochromatographic assay for neuron specific enolase and carcinoembryonic antigen. Talanta 2017, 164, 463–469. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Su, E.; Chen, H.-Y.; Gu, Z.; Zhao, X. Quantitative detection of multiplex cardiac biomarkers with encoded SERS nanotags on a single T line in lateral flow assay. Sens. Actuators B Chem. 2018, 277, 502–509. [Google Scholar] [CrossRef]
- He, H.; Liu, B.; Wen, S.; Liao, J.; Lin, G.; Zhou, J.; Jin, D. Quantitative Lateral Flow Strip Sensor Using Highly Doped Upconversion Nanoparticles. Anal. Chem. 2018, 90, 12356–12360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlov, A.V.; Znoyko, S.L.; Cherkasov, V.R.; Nikitin, M.P.; Nikitin, P.I. Multiplex Biosensing Based on Highly Sensitive Magnetic Nanolabel Quantification: Rapid Detection of Botulinum Neurotoxins A, B, and E in Liquids. Anal. Chem. 2016, 88, 10419–10426. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhou, T.; Yin, B.; He, P. A sensitive and semi-quantitative method for determination of multi-drug residues in animal body fluids using multiplex dipstick immunoassay. Anal. Chim. Acta 2016, 927, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Purrà, M.; Carré-Camps, M.; de Puig, H.; Bosch, I.; Gehrke, L.; Hamad-Schifferli, K. Surface-Enhanced Raman Spectroscopy-Based Sandwich Immunoassays for Multiplexed Detection of Zika and Dengue Viral Biomarkers. ACS Infect. Dis. 2017, 3, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Xianyu, Y.; Yin, B.; Niu, Y.; Wang, S.; Cao, F.; Zhang, X.; Wang, Y.; Jiang, X. A dual-readout chemiluminescent-gold lateral flow test for multiplex and ultrasensitive detection of disease biomarkers in real samples. Nanoscale 2016, 8, 15205–15212. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Ni, H.; Sun, L.; Su, E.; Chen, H.; Gu, Z.; Zhao, X. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens. Bioelectron. 2018, 106, 204–211. [Google Scholar] [CrossRef]
- Guteneva, N.V.; Znoyko, S.L.; Orlov, A.V.; Nikitin, M.P.; Nikitin, P.I. Rapid lateral flow assays based on the quantification of magnetic nanoparticle labels for multiplexed immunodetection of small molecules: Application to the determination of drugs of abuse. Mikrochim. Acta 2019, 186, 621. [Google Scholar] [CrossRef]





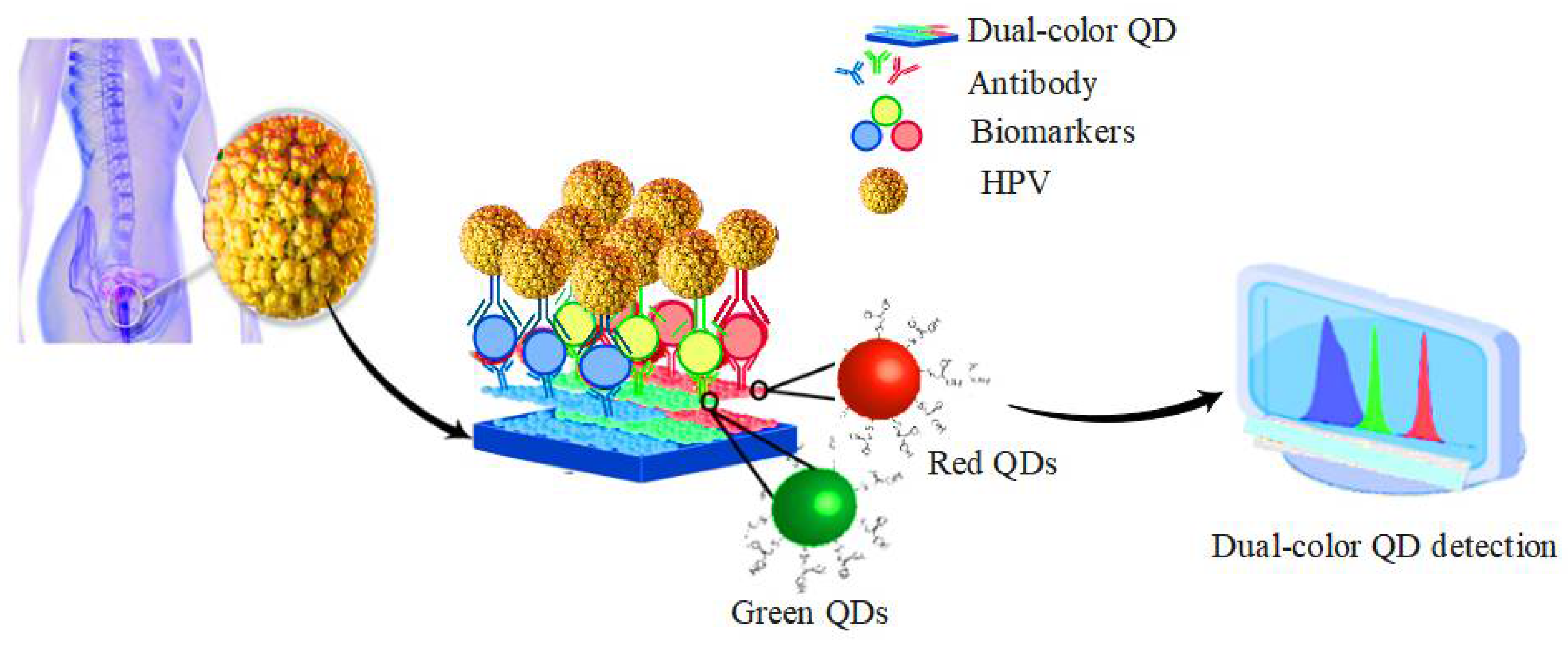
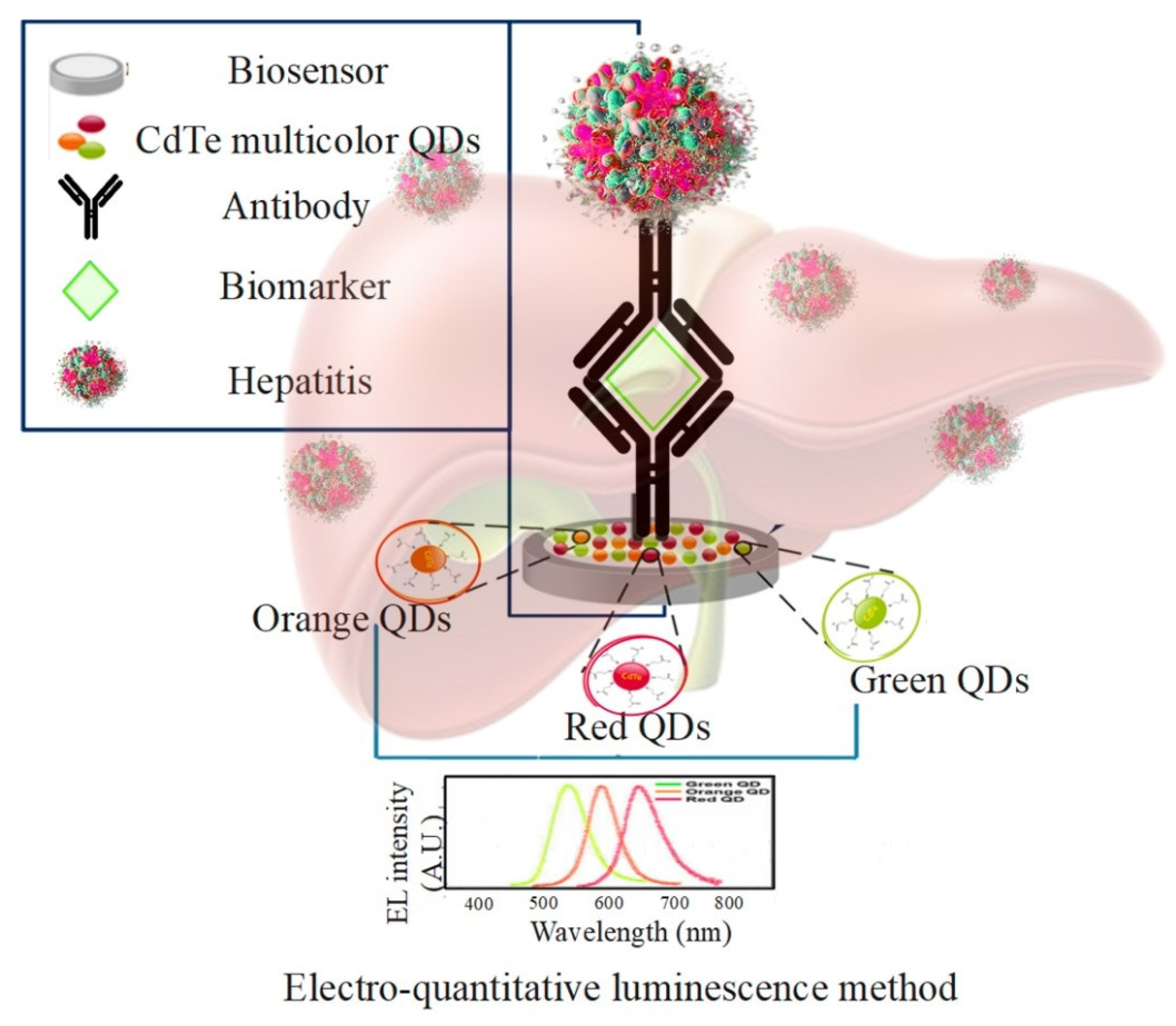
| Advantages | Disadvantages | Ref. | |
|---|---|---|---|
| Semiconductor quantum dots |
|
| [38,39] |
| Carbon quantum dots |
|
| [40,41] |
| Graphene quantum dots |
|
| [42,43,44,45] |
| Infectious Disease | Infectious Biomarker | Detection Techniques | Ref. |
|---|---|---|---|
| Hepatitis B | HBV virus | Microfluidic device with microbead array and QD | [111] |
| HIV | Anti-HIV antibody | Biosensors | [112] |
| Hepatitis C | Anti-HCV antibodies | Optical immunosensors | [113] |
| Autoimmune hepatitis | Serum levels: IL-6, IL-8, IL-17, IL-21, tumor necrosis factor (TNF)-α | enzyme-linked immunosorbent assay | [114] |
| Hepatitis B | Hepatitis B surface antibodies | Surface acoustic wave immunosensor | [115] |
| Biomarkers | Detection Time | Analysis Mode | Detection Method | Ref. |
|---|---|---|---|---|
| CEA and NSE | <15 min | Quantitative | Fluorescent detection | [154] |
| Myo, cTnI, and CKMB | 17 min | Quantitative | SERS detection | [155] |
| PSA and EphA2 | Not mentioned | Quantitative | Fluorescent detection | [156] |
| BoNT-A, BoNT-B, and BoNT-E | 25 min | Quantitative | Magnetic detection | [157] |
| SD, TC, and CT | 10 min | Semiquantitative | Colorimetric detection | [158] |
| DENV NS1 and ZIKV NS1 | Not mentioned | Quantitative | SERS detection | [159] |
| AFP and CEA | 30 min | Semiquantitative | Colorimetric detection | [160] |
| Myo, cTnI, and CKMB | 45 min | Quantitative | SERS detection | [161] |
| MOP, fentanyl, and MET | <20 min | Quantitative | Magnetic detection | [162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, S.M.; Hashemi, S.A.; Yari Kalashgrani, M.; Omidifar, N.; Lai, C.W.; Vijayakameswara Rao, N.; Gholami, A.; Chiang, W.-H. The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections. Pharmaceuticals 2022, 15, 880. https://doi.org/10.3390/ph15070880
Mousavi SM, Hashemi SA, Yari Kalashgrani M, Omidifar N, Lai CW, Vijayakameswara Rao N, Gholami A, Chiang W-H. The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections. Pharmaceuticals. 2022; 15(7):880. https://doi.org/10.3390/ph15070880
Chicago/Turabian StyleMousavi, Seyyed Mojtaba, Seyyed Alireza Hashemi, Masoomeh Yari Kalashgrani, Navid Omidifar, Chin Wei Lai, Neralla Vijayakameswara Rao, Ahmad Gholami, and Wei-Hung Chiang. 2022. "The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections" Pharmaceuticals 15, no. 7: 880. https://doi.org/10.3390/ph15070880
APA StyleMousavi, S. M., Hashemi, S. A., Yari Kalashgrani, M., Omidifar, N., Lai, C. W., Vijayakameswara Rao, N., Gholami, A., & Chiang, W.-H. (2022). The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections. Pharmaceuticals, 15(7), 880. https://doi.org/10.3390/ph15070880










