Antibladder Cancer Effects of Excavatolide C by Inducing Oxidative Stress, Apoptosis, and DNA Damage In Vitro
Abstract
:1. Introduction
2. Results
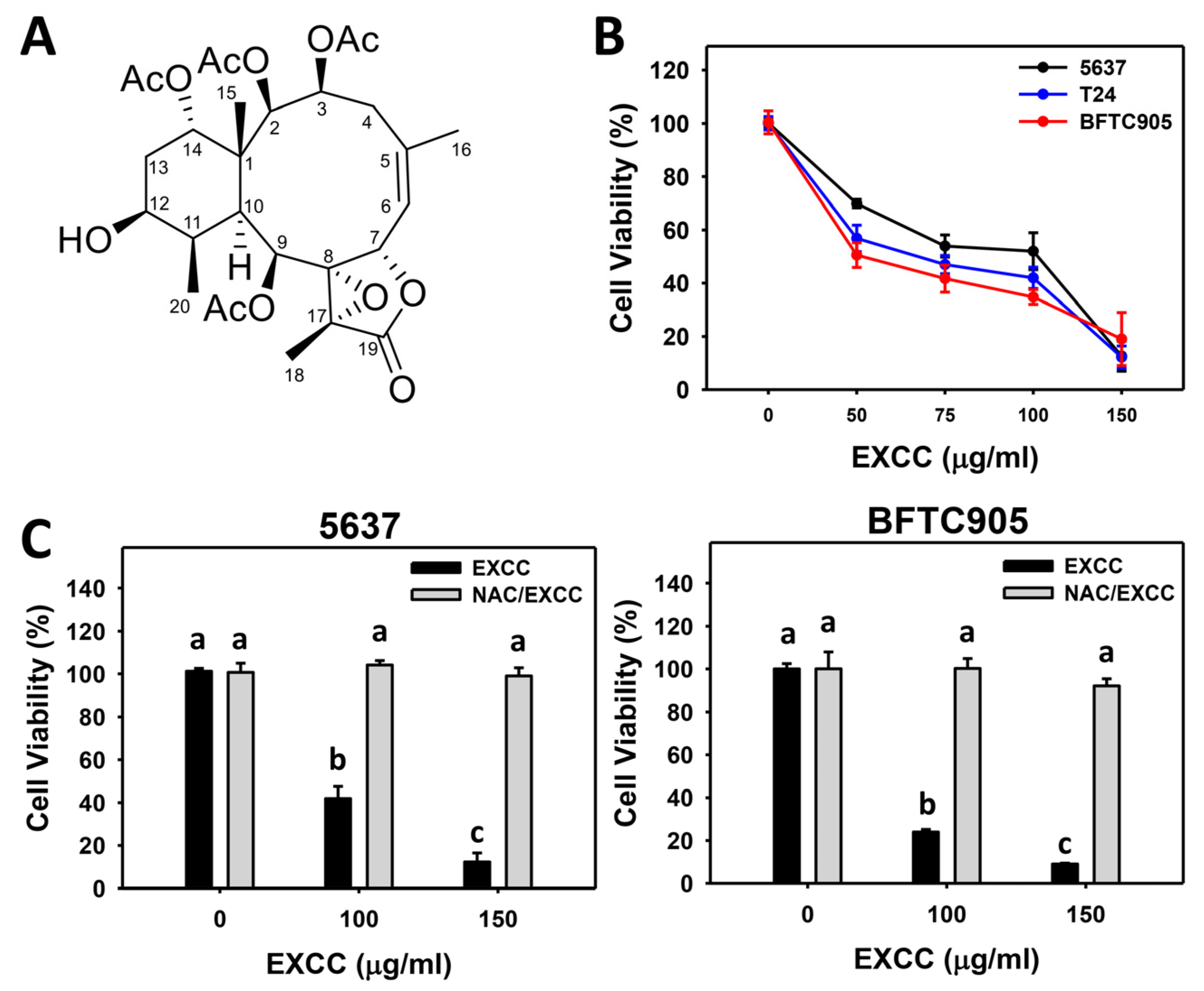
2.1. Proliferation Change by EXCC
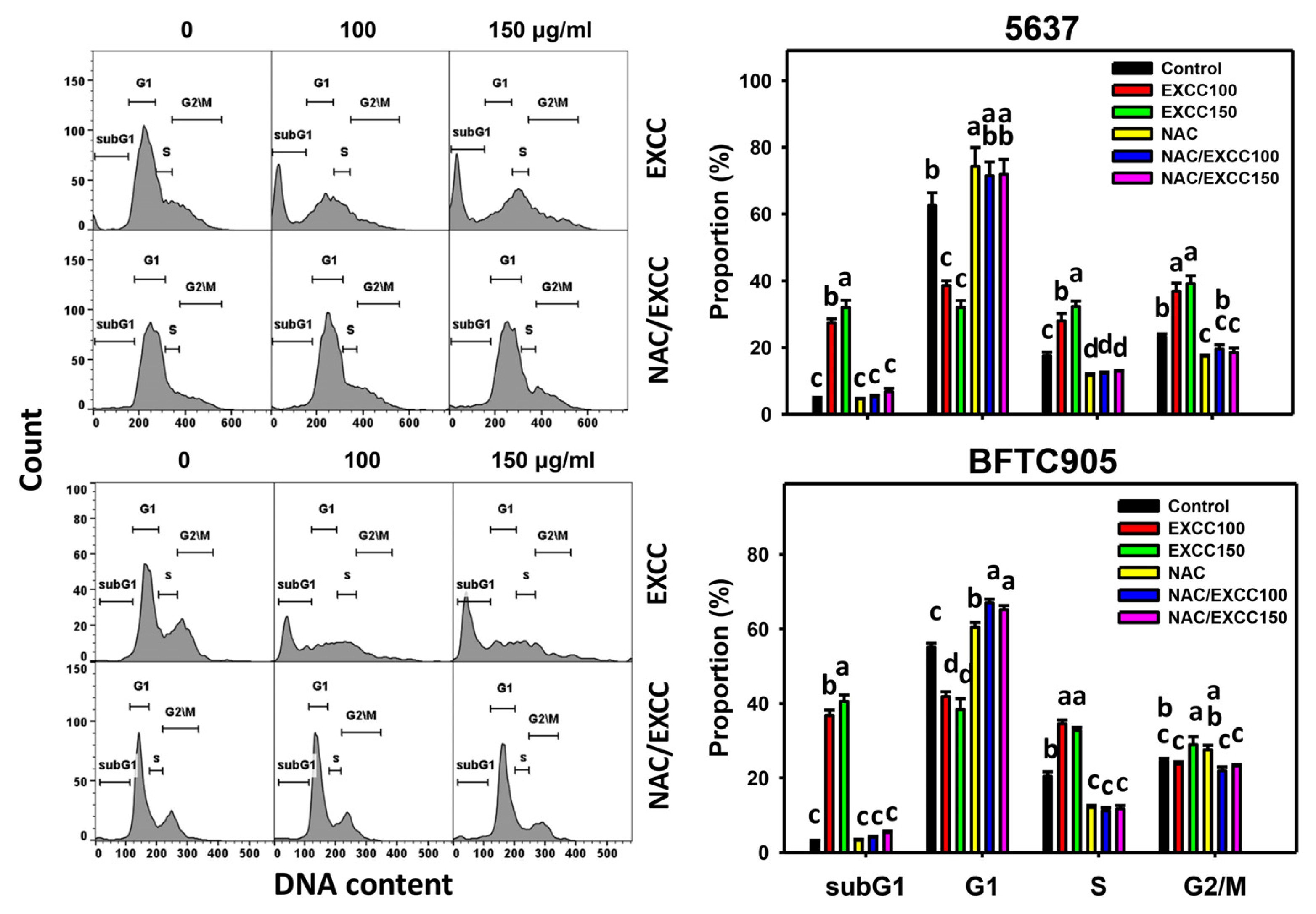
2.2. Cell Cycle Change by EXCC
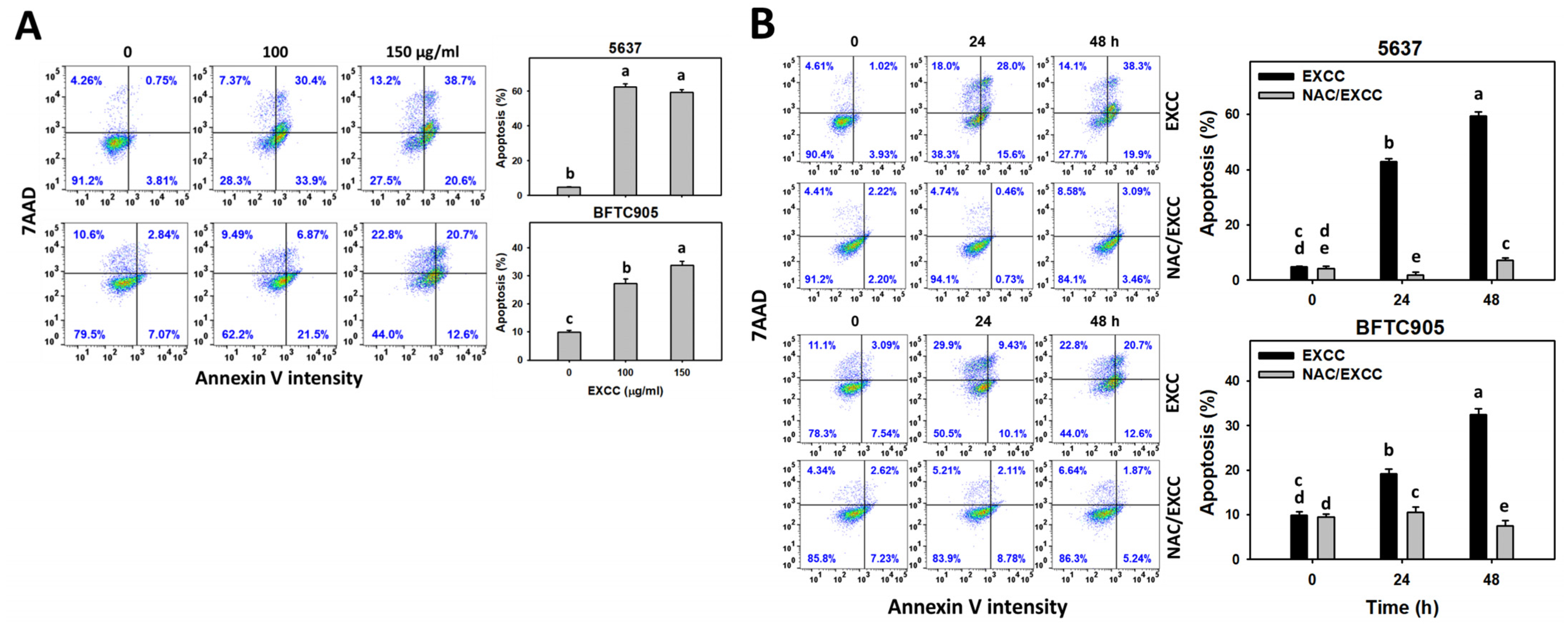
2.3. Apoptosis (Annexin V) Change by EXCC
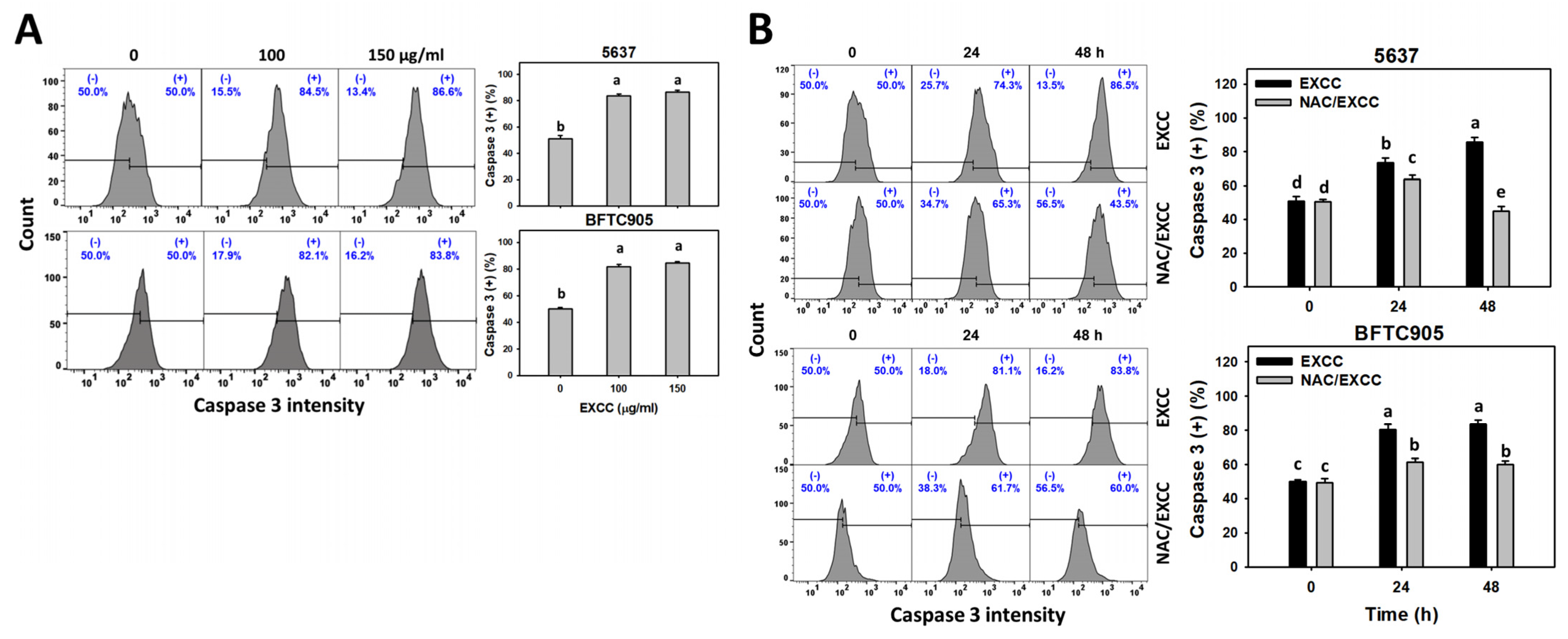
2.4. Caspase 3 (Cas 3) Activation Change by EXCC
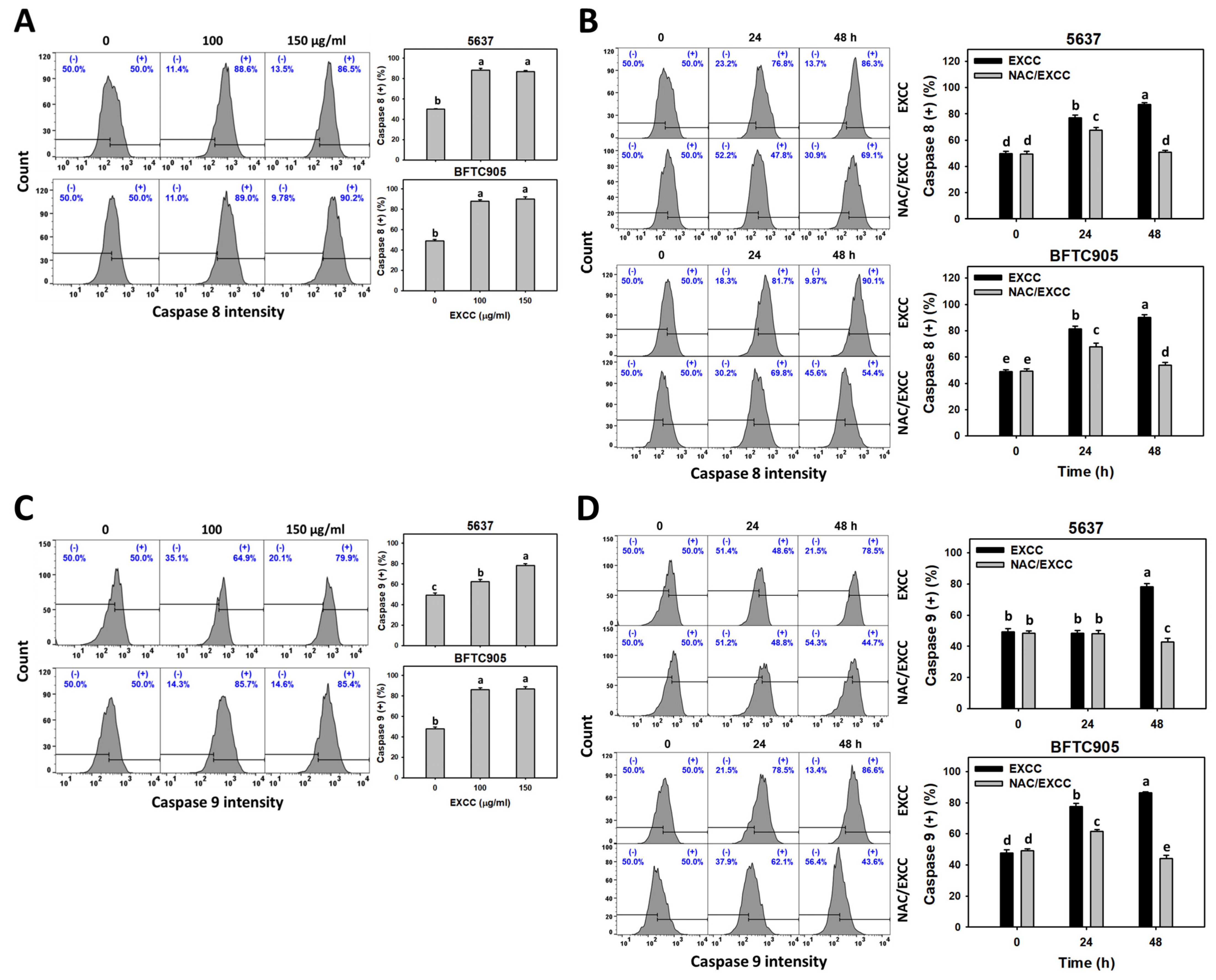
2.5. Caspase 8/9 (Cas 8/9) Changes by EXCC
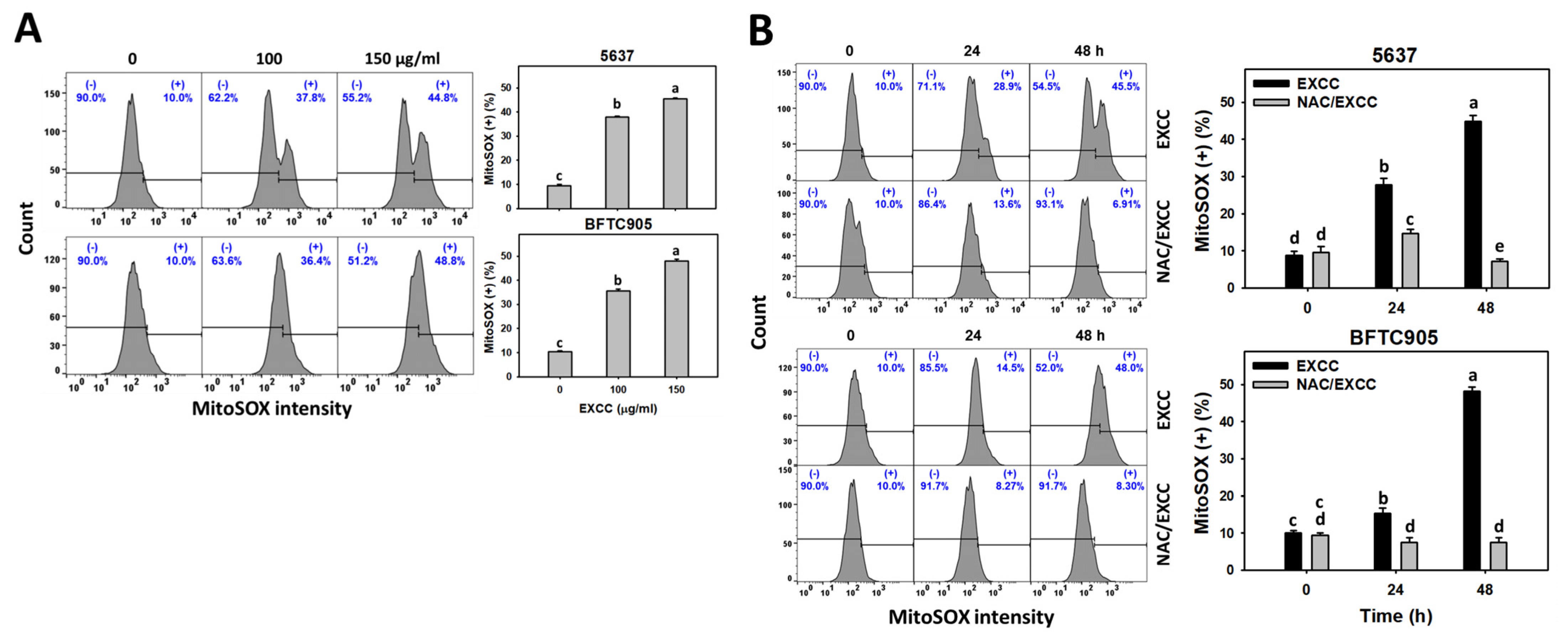
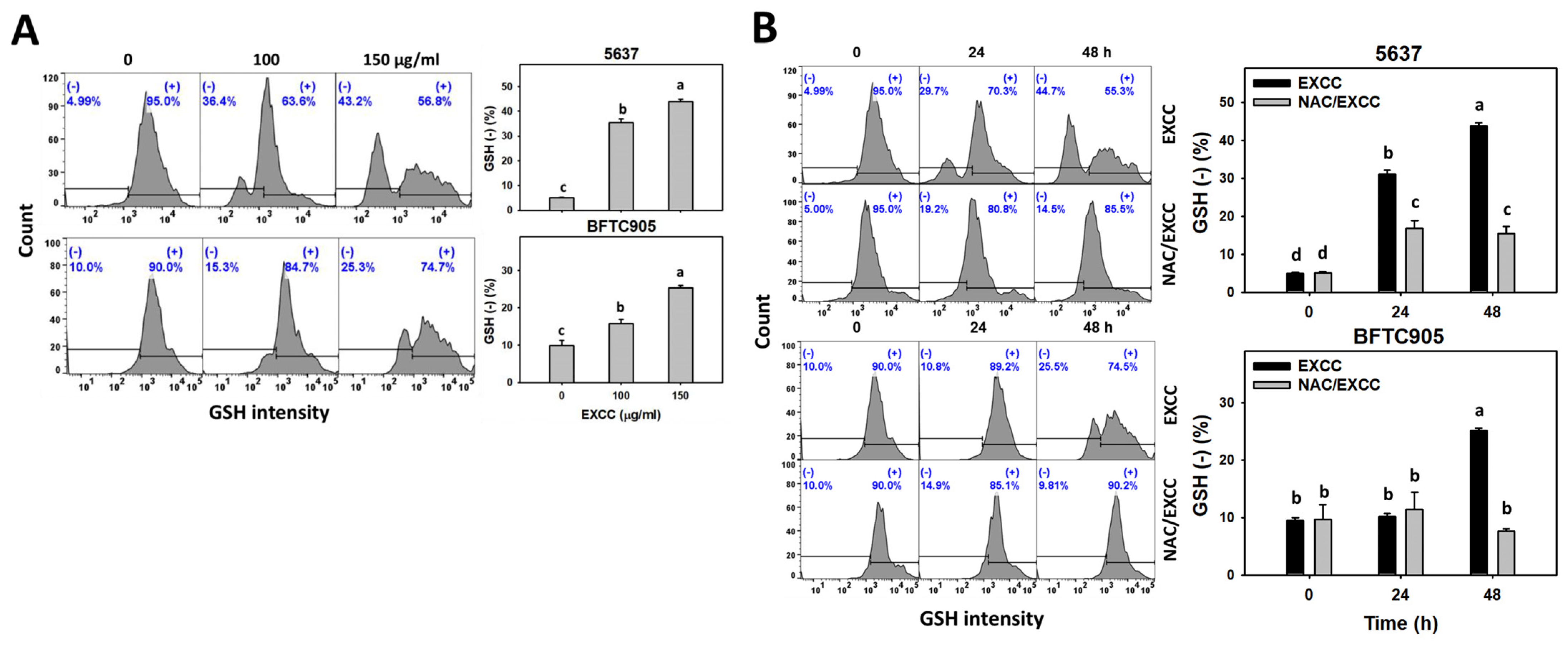
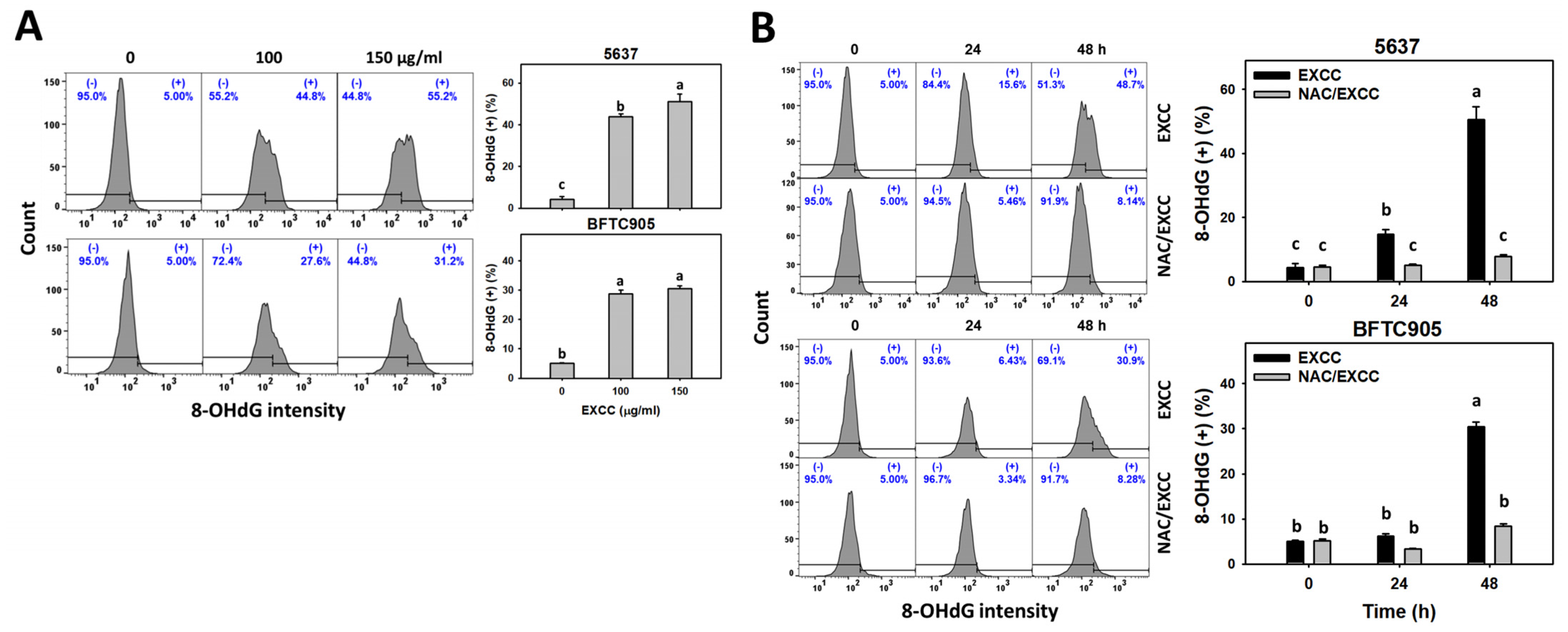
2.6. Oxidative Stress Change by EXCC
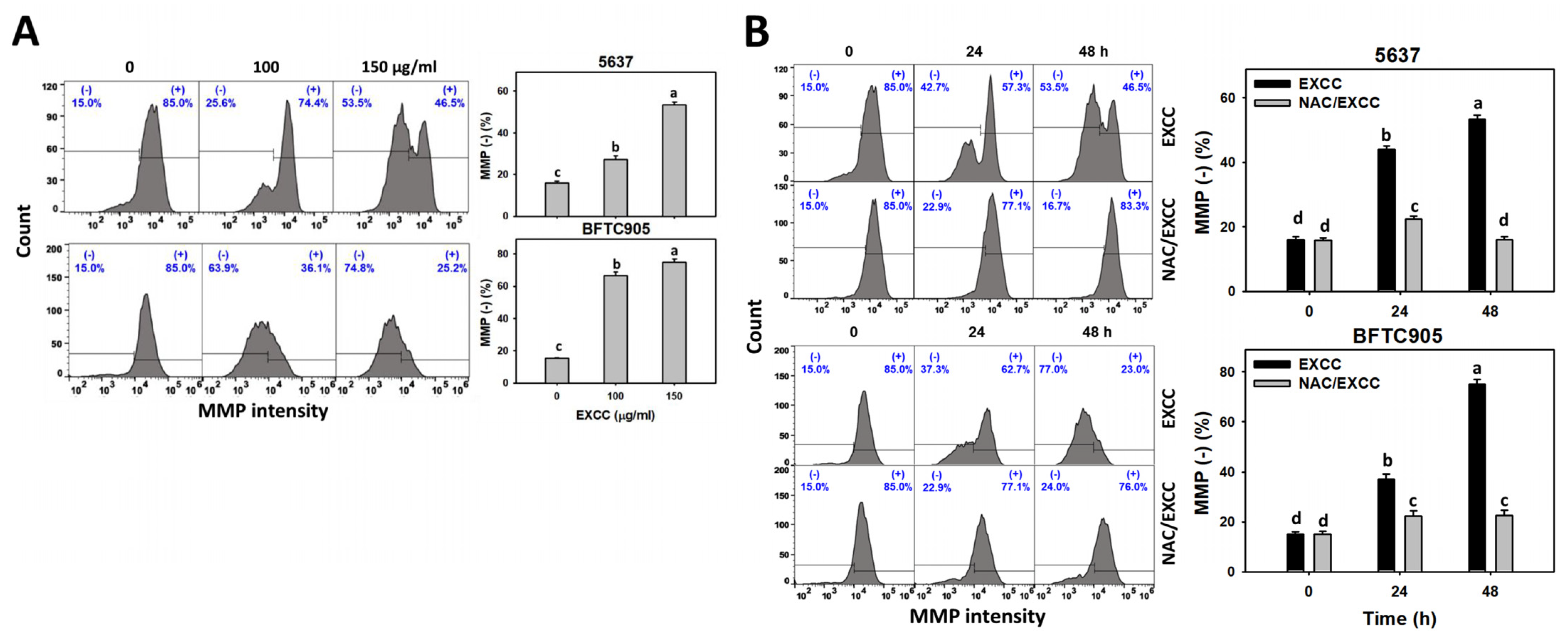
2.7. Mitochondrial Membrane Potential (MMP) Change by EXCC
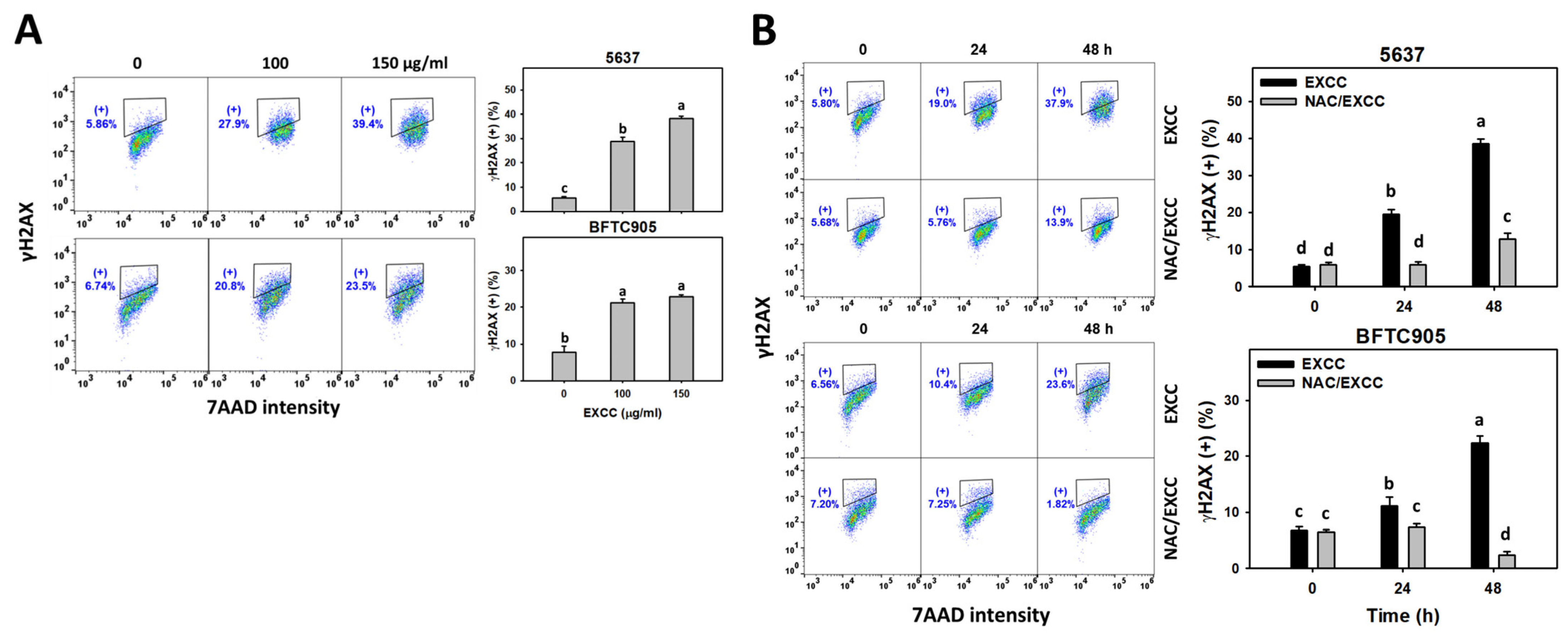
2.8. DNA Damage Change by EXCC
3. Discussion
3.1. Comparison of Antiproliferation of EXCC in Different Cancer Cell Lines
3.2. Role of Oxidative Stress in Antiproliferation of EXCC
3.3. Apoptosis and DNA Damage Effects of EXCC Involving Oxidative Stress
4. Materials and Methods
4.1. Extraction and Separation of EXCC
4.2. Oxidative Stress Inhibitor
4.3. Cell Culture and Viability
4.4. Cell Cycle Assays
4.5. Apoptosis (Annexin V/7AAD)
4.6. Apoptosis (Cas 3, 8, 9)
4.7. ROS, MitoSOX, and GSH
4.8. MMP
4.9. γH2AX
4.10. 8-OHdG
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Bellmunt, J.; Comperat, E.; de Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef]
- Harraz, A.M.; El-Shabrawy, M.; El-Nahas, A.R.; El-Kappany, H.; Osman, Y. Single versus maintenance intravesical chemotherapy for the prevention of bladder recurrence after radical nephroureterectomy for upper tract urothelial carcinoma: A randomized clinical trial. Clin. Genitourin. Cancer 2019, 17, e1108–e1115. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.R.A.H.; Ridwan, F.R.; Parikesit, D.; Widia, F.; Mochtar, C.A.; Umbas, R. Meta-analysis of neoadjuvant chemotherapy compared to radical cystectomy alone in improving overall survival of muscle-invasive bladder cancer patients. BMC Urol. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.M.; Hai, Y.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Chemical and bioactive marine natural products of coral-derived microorganisms (2015–2017). Curr. Med. Chem. 2019, 26, 6930–6941. [Google Scholar] [CrossRef]
- Sang, V.T.; Dat, T.T.H.; Vinh, L.B.; Cuong, L.C.V.; Oanh, P.T.T.; Ha, H.; Kim, Y.H.; Anh, H.L.T.; Yang, S.Y. Coral and coral-associated microorganisms: A prolific source of potential bioactive natural products. Mar. Drugs 2019, 17, 468. [Google Scholar] [CrossRef] [Green Version]
- Alves, C.; Diederich, M. Marine natural products as anticancer agents. Mar. Drugs 2021, 19, 447. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Hossain, R.; Hassan, S.M.H.; Salehi, B.; Martins, N.; Sharifi-Rad, J.; Amarowicz, R. Biological activities of sinularin: A literature-based review. Cell. Mol. Biol. 2020, 66, 33–36. [Google Scholar] [CrossRef]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.H.; Sung, P.J.; Cheng, M.C.; Liu, H.Y.; Fang, L.S.; Duh, C.Y.; Chiang, M.Y. Novel cytotoxic diterpenes, excavatolides A-E, isolated from the Formosan gorgonian Briareum excavatum. J. Nat. Prod. 1998, 61, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-T.; Wang, S.-K.; Dai, C.-F.; Duh, C.-Y. Briacavatolides A–C, new briaranes from the Taiwanese octocoral Briareum excavatum. Mar. Drugs 2012, 10, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.F.; Su, Y.D.; Hwang, T.L.; Liao, Z.J.; Tsui, K.H.; Wen, Z.H.; Wu, Y.C.; Sung, P.J. Briarenols C-E, new polyoxygenated briaranes from the octocoral Briareum excavatum. Molecules 2017, 22, 475. [Google Scholar] [CrossRef] [Green Version]
- Sung, P.J.; Li, G.Y.; Su, Y.D.; Lin, M.R.; Chang, Y.C.; Kung, T.H.; Lin, C.S.; Chen, Y.H.; Su, J.H.; Lu, M.C.; et al. Excavatoids O and P, new 12-hydroxybriaranes from the octocoral Briareum excavatum. Mar. Drugs 2010, 8, 2639–2646. [Google Scholar] [CrossRef]
- Sung, P.J.; Lin, M.R.; Chiang, M.Y.; Hwang, T.L. Briaexcavatins V-Z, discovery of new briaranes from a cultured octocoral Briareum excavatum. Bull. Chem. Soc. Jpn. 2009, 82, 987–996. [Google Scholar] [CrossRef]
- Huynh, T.H.; Chang, Y.M.; Yang, S.N.; Lee, G.H.; Wen, Z.H.; Wu, Y.J.; Su, T.R.; Sung, P.J. Briarenol L, a new chlorine-containing briarane from Briareum excavatum (Briareidae). J. Mol. Struct. 2021, 1223, 128970. [Google Scholar] [CrossRef]
- Chi, W.C.; Kuo, L.M.; Yang, S.N.; Lee, Y.T.; Wen, Z.H.; Tsui, K.H.; Hwang, T.L.; Zhang, Y.L.; Sung, P.J. Briarenols O and P: Novel briaranes from a cultured octocoral Briareum excavatum (Briareidae). Phytochem. Lett. 2021, 41, 134–138. [Google Scholar] [CrossRef]
- Ortega, M.J.; Zubia, E.; Sanchez, M.C.; Carballo, J.L. Cembrane diterpenes from the gorgonian Leptogorgia laxa. J. Nat. Prod. 2008, 71, 1637–1639. [Google Scholar] [CrossRef]
- Marrero, J.; Benitez, J.; Rodriguez, A.D.; Zhao, H.; Raptis, R.G. Bipinnatins K-Q, minor cembrane-type diterpenes from the West Indian Gorgonian Pseudopterogorgia kallos: Isolation, structure assignment, and evaluation of biological activities. J. Nat. Prod. 2008, 71, 381–389. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Yang, H.H.; Sung, P.J.; Weng, C.F. Excavatolide B inhibits nonsmall cell lung cancer proliferation by altering peroxisome proliferator activated receptor gamma expression and PTEN/AKT/NF-Kbeta expression. Environ. Toxicol. 2017, 32, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef]
- Kauffman, M.E.; Kauffman, M.K.; Traore, K.; Zhu, H.; Trush, M.A.; Jia, Z.; Li, Y.R. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maharjan, S.; Oku, M.; Tsuda, M.; Hoseki, J.; Sakai, Y. Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci. Rep. 2014, 4, 5896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction—A mini-review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef] [Green Version]
- Yoon, C.Y.; Lee, J.S.; Kim, B.S.; Jeong, S.J.; Hong, S.K.; Byun, S.S.; Lee, S.E. Sunitinib malate synergistically potentiates anti-tumor effect of gemcitabine in human bladder cancer cells. Korean J. Urol. 2011, 52, 55–63. [Google Scholar] [CrossRef]
- Powles, T.; Perry, J.; Shamash, J.; Liu, W.; Oliver, T.; Joel, S. A comparison of the platinum analogues in bladder cancer cell lines. Urol. Int. 2007, 79, 67–72. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Teppo, H.R.; Soini, Y.; Karihtala, P. Reactive oxygen species-mediated mechanisms of action of targeted cancer therapy. Oxid. Med. Cell. Longev. 2017, 2017, 1485283. [Google Scholar] [CrossRef]
- Tang, J.Y.; Ou-Yang, F.; Hou, M.F.; Huang, H.W.; Wang, H.R.; Li, K.T.; Fayyaz, S.; Shu, C.W.; Chang, H.W. Oxidative stress-modulating drugs have preferential anticancer effects-involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, D.; Huang, L.; Wang, S.; Jin, Y. Targeting reactive oxygen species capacity of tumor cells with repurposed drug as an anticancer therapy. Oxid. Med. Cell. Longev. 2021, 2021, 8532940. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown algae-derived fucoidan exerts oxidative stress-dependent antiproliferation on oral cancer cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yang, C.W.; Chan, T.F.; Farooqi, A.A.; Chang, H.S.; Yen, C.H.; Huang, M.Y.; Chang, H.W. Cryptocaryone promotes ROS-dependent antiproliferation and apoptosis in ovarian cancer cells. Cells 2022, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Eustress and distress in redox homeostasis. In Stress: Physiology, Biochemistry, and Pathology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–163. [Google Scholar]
- Ahmad, T.; Suzuki, Y.J. Juglone in oxidative stress and cell signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Jasek-Gajda, E.; Jurkowska, H.; Jasinska, M.; Lis, G.J. Targeting the MAPK/ERK and PI3K/AKT signaling pathways affects NRF2, Trx and GSH antioxidant systems in leukemia cells. Antioxidants 2020, 9, 633. [Google Scholar] [CrossRef]
- Rostila, A.M.; Anttila, S.L.; Lalowski, M.M.; Vuopala, K.S.; Toljamo, T.I.; Lindstrom, I.; Baumann, M.H.; Puustinen, A.M. Reactive oxygen species-regulating proteins peroxiredoxin 2 and thioredoxin, and glyceraldehyde-3-phosphate dehydrogenase are differentially abundant in induced sputum from smokers with lung cancer or asbestos exposure. Eur. J. Cancer Prev. 2020, 29, 238–247. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Hung, J.H.; Chen, C.Y.; Omar, H.A.; Huang, K.Y.; Tsao, C.C.; Chiu, C.C.; Chen, Y.L.; Chen, P.H.; Teng, Y.N. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ. Toxicol. 2016, 31, 1888–1898. [Google Scholar] [CrossRef]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.C.; Hsieh, Y.C.; Li, L.H.; Chang, C.C.; Janouskova, K.; Ramani, M.V.; Subbaraju, G.V.; Cheng, K.T.; Chang, C.C. Dehydroxyhispolon methyl ether, a hispolon derivative, inhibits WNT/beta-catenin signaling to elicit human colorectal carcinoma cell apoptosis. Int. J. Mol. Sci. 2020, 21, 8839. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shih, Y.L.; Lee, M.H.; Au, M.K.; Chen, Y.L.; Lu, H.F.; Chung, J.G. Bufalin induces apoptosis of human osteosarcoma U-2 OS cells through endoplasmic reticulum stress, caspase- and mitochondria-dependent signaling pathways. Molecules 2017, 22, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, W.H.; Liu, K.L.; Shih, Y.L.; Chuang, Y.Y.; Chou, J.; Lu, H.F.; Jair, H.W.; Lee, M.Z.; Au, M.K.; Chung, J.G. Ouabain induces apoptotic cell death through caspase- and mitochondria-dependent pathways in human osteosarcoma U-2 OS cells. Anticancer Res. 2018, 38, 169–178. [Google Scholar]
- Liu, Y.C.; Peng, B.R.; Hsu, K.C.; El-Shazly, M.; Shih, S.P.; Lin, T.E.; Kuo, F.W.; Chou, Y.C.; Lin, H.Y.; Lu, M.C. 13-Acetoxysarcocrassolide exhibits cytotoxic activity against oral cancer cells through the interruption of the Keap1/Nrf2/p62/SQSTM1 pathway: The need to move beyond classical concepts. Mar. Drugs 2020, 18, 382. [Google Scholar] [CrossRef]
- Sgarbi, G.; Gorini, G.; Liuzzi, F.; Solaini, G.; Baracca, A. Hypoxia and IF(1) expression promote ROS decrease in cancer cells. Cells 2018, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.C.; Tseng, C.N.; Yang, J.I.; Huang, H.W.; Fang, Y.; Tang, J.Y.; Chang, F.R.; Chang, H.W. Antiproliferation and induction of apoptosis in Ca9-22 oral cancer cells by ethanolic extract of Gracilaria tenuistipitata. Molecules 2012, 17, 10916–10927. [Google Scholar] [CrossRef] [Green Version]
- Sung, P.J.; Lin, M.R.; Su, Y.D.; Chiang, M.Y.; Hu, W.P.; Su, J.H.; Cheng, M.C.; Hwang, T.L.; Sheu, J.H. New briaranes from the octocorals Briareum excavatum (Briareidae) and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 2596–2604. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-W.; Chien, T.-M.; Yen, C.-H.; Wu, W.-J.; Sheu, J.-H.; Chang, H.-W. Antibladder Cancer Effects of Excavatolide C by Inducing Oxidative Stress, Apoptosis, and DNA Damage In Vitro. Pharmaceuticals 2022, 15, 917. https://doi.org/10.3390/ph15080917
Yang C-W, Chien T-M, Yen C-H, Wu W-J, Sheu J-H, Chang H-W. Antibladder Cancer Effects of Excavatolide C by Inducing Oxidative Stress, Apoptosis, and DNA Damage In Vitro. Pharmaceuticals. 2022; 15(8):917. https://doi.org/10.3390/ph15080917
Chicago/Turabian StyleYang, Che-Wei, Tsu-Ming Chien, Chia-Hung Yen, Wen-Jeng Wu, Jyh-Horng Sheu, and Hsueh-Wei Chang. 2022. "Antibladder Cancer Effects of Excavatolide C by Inducing Oxidative Stress, Apoptosis, and DNA Damage In Vitro" Pharmaceuticals 15, no. 8: 917. https://doi.org/10.3390/ph15080917
APA StyleYang, C.-W., Chien, T.-M., Yen, C.-H., Wu, W.-J., Sheu, J.-H., & Chang, H.-W. (2022). Antibladder Cancer Effects of Excavatolide C by Inducing Oxidative Stress, Apoptosis, and DNA Damage In Vitro. Pharmaceuticals, 15(8), 917. https://doi.org/10.3390/ph15080917







