Statin Use in Cancer Patients with Acute Myocardial Infarction and Its Impact on Long-Term Mortality
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Lipid Profile, Dyslipidemia, and Use of Statins in Cancer vs. Non-Cancer Patients
2.3. Insights into Oncological Subgroups
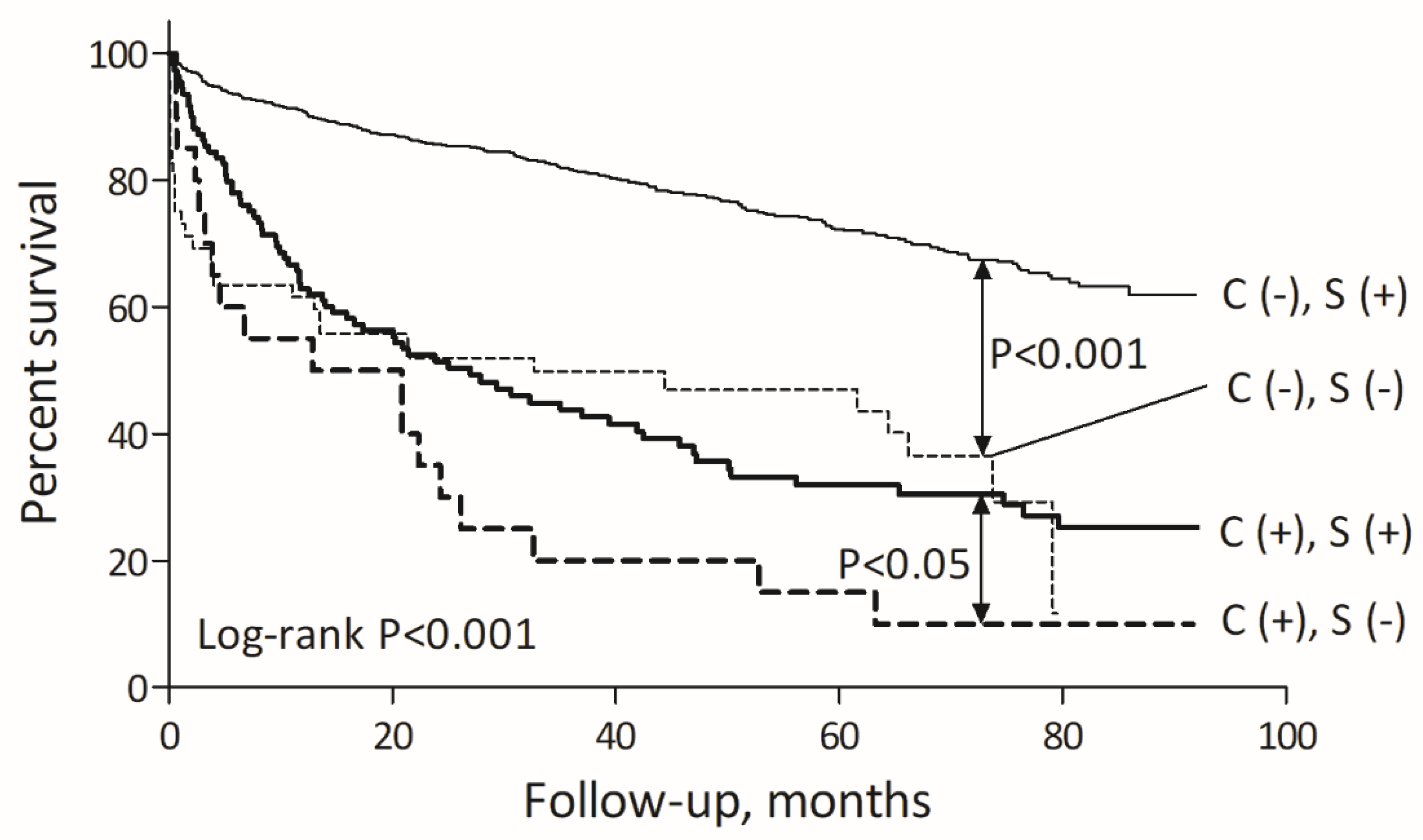
2.4. Long-Term Mortality and Its Determinants
2.5. Statins in MI with Non-Obstructive Coronary Arteries (MINOCA) Subgroup
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics
4.2. Angiography
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, J.P.; Thiele, H.; Barbato, E.; Bauersachs, J.; Dendale, P.; Edvardsen, T.; Gale, C.P.; Jobs, A.; Lambrinou, E.; Mehilli, J.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and Safety of More Intensive Lowering of LDL Cholesterol: A Meta-Analysis of Data from 170,000 Participants in 26 Randomised Trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strongman, H.; Gadd, S.; Matthews, A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Medium and Long-Term Risks of Specific Cardiovascular Diseases in Survivors of 20 Adult Cancers: A Population-Based Cohort Study Using Multiple Linked UK Electronic Health Records Databases. Lancet 2019, 394, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Koo, C.Y.; Zheng, H.; Tan, L.L.; Foo, L.L.; Seet, R.; Chong, J.H.; Hausenloy, D.J.; Chng, W.J.; Richards, A.M.; Lee, C.H.; et al. Lipid Profiles and Outcomes of Patients with Prior Cancer and Subsequent Myocardial Infarction or Stroke. Sci. Rep. 2021, 11, 21167. [Google Scholar] [CrossRef]
- Luo, F.; Zeng, K.-M.; Cao, J.-X.; Zhou, T.; Lin, S.-X.; Ma, W.-J.; Yang, Y.-P.; Zhang, Z.-H.; Lu, F.-T.; Huang, Y.; et al. Predictive Value of a Reduction in the Level of High-Density Lipoprotein-Cholesterol in Patients with Non-Small-Cell Lung Cancer Undergoing Radical Resection and Adjuvant Chemotherapy: A Retrospective Observational Study. Lipids Health Dis. 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Tian, W.; Yao, Y.; Fan, G.; Zhou, Y.; Wu, M.; Xu, D.; Deng, Y. Changes in Lipid Profiles during and after (Neo)Adjuvant Chemotherapy in Women with Early-Stage Breast Cancer: A Retrospective Study. PLoS ONE 2019, 14, e0221866. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.L.; Wu, Y.T.; Wu, H.; Dai, W.; Arshad, B.; Xu, Z.; Li, H.; Wu, K.N.; Kong, L.Q. Status of Lipid and Lipoprotein in Female Breast Cancer Patients at Initial Diagnosis and during Chemotherapy. Lipids Health Dis. 2018, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Cao, R.; Wang, Y.; Qian, G.; Dan, H.C.; Jiang, W.; Ju, L.; Wu, M.; Xiao, Y.; Wang, X. Simvastatin Induces Cell Cycle Arrest and Inhibits Proliferation of Bladder Cancer Cells via PPARγ Signalling Pathway. Sci. Rep. 2016, 6, 35783. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin Use and Reduced Cancer-Related Mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Zhang, X.; Chen, L.; Ma, T.; Tang, J.; Zhao, J. Statin Use and Mortality in Cancer Patients: Systematic Review and Meta-Analysis of Observational Studies. Cancer Treat. Rev. 2015, 41, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.O.; Wun, C.-C.; et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. Cholesterol and Recurrent Events Trial Investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.E.M.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): A Randomised Controlled Trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Dale, K.M.; Coleman, C.I.; Henyan, N.N.; Kluger, J.; White, C.M. Statins and Cancer Risk: A Meta-Analysis. JAMA 2006, 295, 74–80. [Google Scholar] [CrossRef]
- Pun, N.T.; Jeong, C.H. Statin as a Potential Chemotherapeutic Agent: Current Updates as a Monotherapy, Combination Therapy, and Treatment for Anti-Cancer Drug Resistance. Pharmaceuticals 2021, 14, 470. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women. Pharmaceuticals 2020, 13, 422. [Google Scholar] [CrossRef]
- Marcianò, G.; Palleria, C.; Casarella, A.; Rania, V.; Basile, E.; Catarisano, L.; Vocca, C.; Bianco, L.; Pelaia, C.; Cione, E.; et al. Effect of Statins on Lung Cancer Molecular Pathways: A Possible Therapeutic Role. Pharmaceuticals 2022, 15, 589. [Google Scholar] [CrossRef]
- Duarte, J.A.; de Barros, A.L.B.; Leite, E.A. The Potential Use of Simvastatin for Cancer Treatment: A Review. Biomed. Pharm. 2021, 141, 111858. [Google Scholar] [CrossRef]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.V.; Panageas, K.S.; DeAngelis, L.M. Risk of Arterial Thromboembolism in Patients With Cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Zalewski, J.; Pac, A.; Undas, A. Extended Treatment with Non-Vitamin K Antagonist Oral Anticoagulants versus Low-Molecular-Weight Heparins in Cancer Patients Following Venous Thromboembolism. A Pilot Study. Vasc. Pharmacol. 2019, 120, 106567. [Google Scholar] [CrossRef] [PubMed]
- Pastuszczak, M.; Kotlarz, A.; Mostowik, M.; Zalewski, J.; Zmudka, K.; Undas, A. Prior Simvastatin Treatment Is Associated with Reduced Thrombin Generation and Platelet Activation in Patients with Acute ST-Segment Elevation Myocardial Infarction. Thromb. Res. 2010, 125, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Bielecka-Dąbrowa, A.; Hannamm, S.; Rysz, J.; Banach, M. Malignancy-Associated Dyslipidemia. Open Cardiovasc. Med. J. 2011, 5, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, S.W.; Daraban, N.; Abbasi, N.; Lei, X.; Durand, J.B.; Daher, I.N. Treatment and Outcomes of Acute Coronary Syndrome in the Cancer Population. Clin. Cardiol. 2012, 35, 443–450. [Google Scholar] [CrossRef]
- Rose, G.; Shipley, M.J. Plasma Lipids and Mortality: A Source of Error. Lancet 1980, 1, 523–526. [Google Scholar] [CrossRef]
- Kutner, J.S.; Blatchford, P.J.; Taylor, D.H.; Ritchie, C.S.; Bull, J.H.; Fairclough, D.L.; Hanson, L.C.; LeBlanc, T.W.; Samsa, G.P.; Wolf, S.; et al. Safety and Benefit of Discontinuing Statin Therapy in the Setting of Advanced, Life-Limiting Illness: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Frisk, G.; Bergström, H.; Frankling, M.H.; Björkhem-Bergman, L. Sex-Differences in Discontinuation of Statin Treatment in Cancer Patients the Year before Death. Pharmaceuticals 2021, 14, 368. [Google Scholar] [CrossRef]
- Ciliberti, G.; Compagnucci, P.; Urbinati, A.; Bianco, F.; Stronati, G.; Lattanzi, S.; dello Russo, A.; Guerra, F. Myocardial Infarction Without Obstructive Coronary Artery Disease (MINOCA): A Practical Guide for Clinicians. Curr. Probl. Cardiol. 2021, 46, 100761. [Google Scholar] [CrossRef]
- Gasior, P.; Desperak, A.; Gierlotka, M.; Milewski, K.; Wita, K.; Kalarus, Z.; Fluder, J.; Kazmierski, M.; Buszman, P.E.; Gasior, M.; et al. Clinical Characteristics, Treatments, and Outcomes of Patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Results from a Multicenter National Registry. J. Clin. Med. 2020, 9, 2779. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Saturi, G.; D’Angelo, E.C.; Magnani, I.; Toniolo, S.; Stefanizzi, A.; Rinaldi, A.; Bartoli, L.; Angeli, F.; et al. Secondary Prevention Medical Therapy and Outcomes in Patients With Myocardial Infarction With Non-Obstructive Coronary Artery Disease. Front. Pharmacol. 2020, 10, 1606. [Google Scholar] [CrossRef] [Green Version]
- Masson, W.; Lobo, M.; Barbagelata, L.; Lavalle-Cobo, A.; Molinero, G. Prognostic Value of Statin Therapy in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): A Meta-Analysis. Acta Cardiol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, M. Co-Occurrence of Depressive Disorders and Cardiovascular Diseases—Selected Aspects. Psychiatr. Psychol. Klin. 2021, 20, 183–190. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Skorek, P.; Baravik, V.; Kozynacka, A.; Nessler, J.; Zalewski, J. Baseline Indicators of Coronary Artery Disease Burden in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Minerva Cardiol. Angiol. 2019, 67, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of Direct Oral Anticoagulants in the Treatment of Cancer-Associated Venous Thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [Green Version]
- Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available online: https://apps.who.int/iris/handle/10665/85839 (accessed on 16 June 2022).
- ten Berg, M.J.; van den Bemt, P.M.L.A.; Shantakumar, S.; Bennett, D.; Voest, E.E.; Huisman, A.; van Solinge, W.W.; Egberts, T.C.G. Thrombocytopenia in Adult Cancer Patients Receiving Cytotoxic Chemotherapy: Results from a Retrospective Hospital-Based Cohort Study. Drug Saf. 2011, 34, 1151–1160. [Google Scholar] [CrossRef]
- Reiner, Ž.; Catapano, A.L.; de Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [CrossRef] [Green Version]
- Limbruno, U.; de Carlo, M.; Pistolesi, S.; Micheli, A.; Petronio, A.S.; Camacci, T.; Fontanini, G.; Balbarini, A.; Mariani, M.; de Caterina, R. Distal Embolization during Primary Angioplasty: Histopathologic Features and Predictability. Am. Heart J. 2005, 150, 102–108. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; de Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC Working Group Position Paper on Myocardial Infarction with Non-Obstructive Coronary Arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef]
- Stępień, K.; Nowak, K.; Nessler, J.; Zalewski, J. Worse Long-term Prognosis in Myocardial Infarction Occurring at Weekends or Public Holidays with Insight into Myocardial Infarction with Nonobstructive Coronary Arteries. Pol. Arch. Intern. Med. 2020, 130, 15615. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Szlosarczyk, B.; Nessler, J.; Zalewski, J. Clinical Characteristics and Long-Term Outcomes of MINOCA Accompanied by Active Cancer: A Retrospective Insight Into a Cardio-Oncology Center Registry. Front. Cardiovasc. Med. 2022, 9, 785246. [Google Scholar] [CrossRef]


| Cancer MI | Non-Cancer MI | p-Value | |
|---|---|---|---|
| n = 134 | n = 877 | ||
| Male gender | 96 (71.6) | 618 (70.5) | 0.78 |
| Age, years | 73 (66; 79) | 68 (60; 78) | 0.004 |
| Body mass index, kg/m2 | 27.1 (23.5; 30.1) | 27.7 (24.9; 30.9) | 0.012 |
| Diabetes mellitus | 47 (35.1) | 331 (37.9) | 0.53 |
| Hypertension | 112 (83.6) | 755 (86.4) | 0.38 |
| Dyslipidemia | 85 (63.4) | 742 (84.9) | <0.001 |
| Pre-ESRD or ESRD | 7 (5.22) | 22 (2.51) | 0.11 |
| Anemia | 61 (45.5) | 180 (20.5) | <0.001 |
| Thrombocytopenia | 5 (3.7) | 11 (1.3) | 0.030 |
| Prior myocardial infarction | 42 (31.4) | 247 (28.3) | 0.46 |
| Prior stroke | 12 (9.0) | 59 (6.8) | 0.35 |
| Killip class on admission: | 0.10 | ||
| I/II | 117 (87.3) | 804 (91.7) | |
| III/IV | 17 (12.7) | 73 (8.3) | |
| Clinical presentation: | 0.84 | ||
| NSTEMI | 89 (66.4) | 575 (65.6) | |
| STEMI | 45 (33.6) | 302 (34.4) | |
| LVEF, % | 45 (37; 55) | 50 (40; 55) | 0.008 |
| Type of cancer: | |||
| Genitourinary | 44 (32.8) | - | |
| Breast | 12 (9.0) | - | |
| Lung | 31 (23.1) | - | |
| Gastrointestinal | 22 (16.4) | - | |
| Other | 25 (18.7) | - | |
| Metastatic disease: | |||
| Lymph nodes | 16 (11.9) | - | |
| Distant | 28 (20.9) | - | |
| Prior oncological treatment: | |||
| Surgery | 30 (22.4) | - | |
| Surgery with curative intent | 4 (3.0) | - | |
| Radiotherapy | 16 (11.9) | - | |
| Chemotherapy | 32 (23.9) | - | |
| Platinum compounds | 11 (8.2) | - | |
| Taxanes | 4 (3.0) | - | |
| Fluoropyrimidines | 10 (7.5) | - | |
| Anthracyclines | 3 (2.2) | - | |
| Other | 4 (3.0) | - | |
| Hormonotherapy | 19 (14.2) | - | |
| Newly diagnosed cancer | 24 (17.9) | - | |
| Coronary angiography: | |||
| ≥50% stenosis | 113 (84.3) | 826 (94.2) | <0.001 |
| Epicardial thrombus | 14 (10.4) | 117 (13.3) | 0.35 |
| Distal embolization | 9 (6.7) | 20 (2.3) | 0.004 |
| Treatment strategy: | 0.074 | ||
| Percutaneous coronary intervention | 101 (75.4) | 724 (82.6) | |
| Coronary artery bypass graft surgery | 3 (2.2) | 24 (2.7) | |
| Optimal medical treatment | 30 (22.4) | 129 (14.7) | |
| Pharmacotherapy: | |||
| Aspirin | 127 (94.8) | 854 (97.3) | 0.17 |
| P2Y12 inhibitor | 115 (85.8) | 812 (92.6) | 0.008 |
| Proton pump inhibitor | 92 (68.7) | 652 (75.0) | 0.11 |
| ACEI/ARB | 120 (89.6) | 763 (87.0) | 0.41 |
| β-blocker | 117 (87.3) | 780 (89.7) | 0.39 |
| Statin | 107 (80.5) | 801 (92.1) | <0.001 |
| Cancer MI | Non-Cancer MI | p-Value | |
|---|---|---|---|
| n = 134 | n = 877 | ||
| Hemoglobin, g/dL | 12.8 (11.2; 14.0) | 13.8 (12.8; 15.0) | <0.001 |
| Hematocrit, % | 38.5 (33.9; 41.6) | 41.2 (38.3; 44.5) | <0.001 |
| White blood cells, ×103/µL | 9.7 (7.4; 12.9) | 9.3 (7.4; 11.9) | 0.34 |
| Platelet count, ×103/µL | 237.5 (181.5; 290.5) | 221.0 (184.0; 270.0) | 0.26 |
| Creatinine, µmol/L | 92.5 (77.0; 114.5) | 88.0 (76.0; 104.0) | 0.11 |
| Glomerular filtration rate, ml/min | 65.5 (48; 85) | 71 (57; 86) | 0.09 |
| Glucose, mmol/L | 7.5 (5.7; 8.9) | 6.9 (5.8; 9.1) | 0.45 |
| Troponin, ng/mL | 0.19 (0.05; 1.07) | 0.11 (0.03; 0.42) | <0.001 |
| Troponin peak, ng/mL | 0.61 (0.15; 6.27) | 0.45 (0.14; 1.91) | 0.013 |
| Creatine kinase MB isoenzyme, IU/L | 24 (15; 51) | 22 (15; 42) | 0.57 |
| Creatine kinase MB isoenzyme peak, IU/L | 41 (22; 119) | 36 (19; 98) | 0.44 |
| Total cholesterol, mmol/L | 4.1 (3.4; 4.8) | 4.4 (3.6; 5.3) | 0.006 |
| LDL, mmol/L | 2.5 (1.9; 3.1) | 2.6 (1.7; 3.4) | 0.70 |
| HDL, mmol/L | 1.1 (0.9; 1.4) | 1.2 (1.0; 1.6) | <0.001 |
| Triglycerides, mmol/L | 1.1 (0.9; 1.5) | 1.3 (0.9; 1.7) | 0.013 |
| Independent Variables | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| All patients, Chi2 = 40.1, df = 7, p < 0.001 | ||||||
| p-value | OR | 95% CI for OR | p-value | OR | 95% CI for OR | |
| Age, per 1 year | 0.017 | 1.03 | 1.01–1.04 | 0.956 | 1.01 | 0.97–1.03 |
| Cancer, yes vs. no | <0.001 | 2.63 | 1.56–4.35 | 0.038 | 2.13 | 1.04–4.35 |
| Lack of coronary stenosis of >50%, yes vs. no | <0.001 | 3.80 | 2.12–6.81 | <0.001 | 4.47 | 2.13–9.40 |
| Hypertension, yes vs. no | <0.001 | 0.40 | 0.24–0.65 | 0.258 | 0.65 | 0.31–1.37 |
| Anemia, yes vs. no | <0.001 | 2.56 | 1.67–4.00 | 0.045 | 1.89 | 1.01–3.57 |
| Glomerular filtration rate, per 1 mL/min | <0.001 | 0.97 | 0.96–0.98 | 0.006 | 0.98 | 0.96–0.99 |
| LDL cholesterol, per 1 mmol/L | 0.041 | 0.78 | 0.61–0.99 | 0.210 | 0.83 | 0.63–1.11 |
| Cancer group, Chi2 = 10.9, df = 3, p = 0.012 | ||||||
| p-value | OR | 95% CI for OR | p-value | OR | 95% CI for OR | |
| Lack of coronary stenosis of >50%, yes vs. no | 0.059 | 2.74 | 0.96–7.78 | 0.314 | 1.89 | 0.55–6.53 |
| Hypertension, yes vs. no | 0.021 | 0.30 | 0.11–0.83 | 0.029 | 0.28 | 0.09–0.88 |
| Glomerular filtration rate, per 1 mL/min | 0.045 | 1.02 | 1.00–1.04 | 0.091 | 1.02 | 0.99–1.04 |
| Non-cancer group, Chi2 = 26.9, df = 6, p < 0.001 | ||||||
| p-value | OR | 95% CI for OR | p-value | OR | 95% CI for OR | |
| Age, per 1 year | 0.037 | 1.02 | 1.01–1.06 | 0.993 | 1.00 | 0.97–1.03 |
| Lack of coronary stenosis of >50%, yes vs. no | <0.001 | 3.61 | 1.76–7.40 | <0.001 | 5.66 | 2.36–13.57 |
| Hypertension, yes vs. no | 0.006 | 0.44 | 0.25–0.79 | 0.945 | 0.97 | 0.36–2.58 |
| Anemia, yes vs. no | <0.001 | 2.79 | 1.67–4.67 | 0.025 | 2.28 | 1.11–4.68 |
| Glomerular filtration rate, per 1 mL/min | <0.001 | 0.97 | 0.95–0.98 | 0.008 | 0.98 | 0.96–0.99 |
| LDL cholesterol, per 1 mmol/L | 0.031 | 0.74 | 0.56–0.98 | 0.200 | 1.23 | 0.90–1.67 |
| Independent Variables | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| All patients, Chi2 = 393, df = 12, p < 0.001 | ||||||
| p-value | HR | 95% CI for HR | p-value | HR | 95% CI for HR | |
| Age, per 1 year | <0.001 | 1.05 | 1.04–1.06 | <0.001 | 1.04 | 1.03–1.05 |
| Male gender, yes vs. no | 0.790 | 0.97 | 0.78–1.21 | 0.898 | 1.02 | 0.80–1.29 |
| Body mass index, per 1 kg/m2 | <0.001 | 0.95 | 0.93–0.98 | 0.311 | 0.99 | 0.96–1.01 |
| Active cancer, yes vs. no | <0.001 | 3.34 | 2.64–4.22 | <0.001 | 2.42 | 1.89–3.11 |
| Diabetes mellitus, yes vs. no | 0.002 | 1.39 | 1.13–1.70 | 0.111 | 1.20 | 0.96–1.01 |
| Hypertension, yes vs. no | 0.010 | 0.70 | 0.54–0.92 | <0.001 | 0.50 | 0.37–0.65 |
| Coronary stenosis of >50%, yes vs. no | 0.786 | 1.05 | 0.72–1.54 | 0.003 | 1.86 | 1.23–2.80 |
| Left ventricular ejection fraction, per 1% | <0.001 | 0.97 | 0.96–0.98 | <0.001 | 0.97 | 0.96–0.98 |
| Hemoglobin, per 1 g/dL | <0.001 | 0.80 | 0.77–0.83 | <0.001 | 0.88 | 0.83–0.92 |
| LDL cholesterol, per 1 mmol/L | <0.001 | 0.77 | 0.70–0.85 | 0.068 | 0.90 | 0.80–1.01 |
| Creatinine, per 1 µmol/L | <0.001 | 1.003 | 1.002–1.004 | 0.011 | 1.002 | 1.001–1.003 |
| Statin use, yes vs. no | <0.001 | 0.29 | 0.22–0.37 | <0.001 | 0.47 | 0.36–0.62 |
| Cancer group, Chi2 = 22.4, df = 6, p = 0.001 | ||||||
| p-value | HR | 95% CI for HR | p-value | HR | 95% CI for HR | |
| Age, per 1 year | 0.048 | 1.02 | 1.00–1.05 | 0.007 | 1.04 | 1.01–1.06 |
| Statin use, yes vs. no | 0.004 | 0.50 | 0.31–0.81 | 0.034 | 0.56 | 0.32–0.96 |
| Hypertension, yes vs. no | 0.089 | 0.64 | 0.38–1.07 | 0.018 | 0.50 | 0.28–0.89 |
| Hemoglobin, per 1 g/dL | 0.016 | 0.90 | 0.82–0.98 | 0.075 | 0.91 | 0.83–1.01 |
| Coronary stenosis of >50%, yes vs. no | 0.228 | 1.42 | 0.80–2.51 | 0.037 | 1.92 | 1.04–3.53 |
| LDL cholesterol, per 1 mmol/L | 0.090 | 0.78 | 0.59–1.04 | 0.677 | 0.94 | 0.68–1.28 |
| Statin MINOCA | Non-Statin MINOCA | p-Value | |
|---|---|---|---|
| n = 54 | n = 18 | ||
| Male gender | 27 (50.0) | 10 (55.6) | 0.68 |
| Age, years | 72.5 (66; 79) | 72 (54; 78) | 0.17 |
| Body mass index, kg/m2 | 28.1 (25.1; 31.6) | 26.1 (23.0; 29.9) | 0.08 |
| Diabetes mellitus | 18 (33.3) | 2 (11.1) | 0.06 |
| Hypertension | 46 (85.2) | 8 (44.4) | 0.001 |
| Dyslipidemia | 51 (94.4) | 8 (44.4) | <0.001 |
| Pre-ESRD or ESRD | 3 (5.6) | 0 (0.0) | 0.42 |
| Active cancer | 14 (25.9) | 7 (38.9) | 0.45 |
| Killip class on admission: | 0.005 | ||
| I/II | 53 (98.1) | 13 (72.2) | |
| III/IV | 1 (1.9) | 5 (27.8) | |
| Clinical presentation: | 0.27 | ||
| NSTEMI | 47 (87.0) | 13 (72.2) | |
| STEMI | 7 (13.0) | 5 (27.8) | |
| LVEF, % | 55 (45; 60) | 40 (30; 55) | 0.019 |
| Lipid profile: | |||
| Total cholesterol, mmol/L | 4.4 (3.5; 5.4) | 4.0 (3.1; 4.2) | 0.06 |
| LDL, mmol/L | 2.7 (1.9; 3.8) | 1.9 (1.1; 2.6) | 0.008 |
| HDL, mmol/L | 1.1 (1.0; 1.5) | 1.3 (1.1; 2.0) | 0.52 |
| Triglycerides, mmol/L | 1.3 (0.9; 1.6) | 1.1 (0.8; 1.5) | 0.69 |
| Pharmacotherapy: | |||
| Aspirin | 28 (51.9) | 10 (55.6) | 0.50 |
| P2Y12 inhibitor | 26 (48.2) | 4 (22.2) | 0.047 |
| Proton pump inhibitor | 36 (66.7) | 9 (50.0) | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepien, K.; Nowak, K.; Kachnic, N.; Horosin, G.; Walczak, P.; Karcinska, A.; Schwarz, T.; Wojtas, M.; Zalewska, M.; Pastuszak, M.; et al. Statin Use in Cancer Patients with Acute Myocardial Infarction and Its Impact on Long-Term Mortality. Pharmaceuticals 2022, 15, 919. https://doi.org/10.3390/ph15080919
Stepien K, Nowak K, Kachnic N, Horosin G, Walczak P, Karcinska A, Schwarz T, Wojtas M, Zalewska M, Pastuszak M, et al. Statin Use in Cancer Patients with Acute Myocardial Infarction and Its Impact on Long-Term Mortality. Pharmaceuticals. 2022; 15(8):919. https://doi.org/10.3390/ph15080919
Chicago/Turabian StyleStepien, Konrad, Karol Nowak, Natalia Kachnic, Grzegorz Horosin, Piotr Walczak, Aleksandra Karcinska, Tomasz Schwarz, Mariusz Wojtas, Magdalena Zalewska, Maksymilian Pastuszak, and et al. 2022. "Statin Use in Cancer Patients with Acute Myocardial Infarction and Its Impact on Long-Term Mortality" Pharmaceuticals 15, no. 8: 919. https://doi.org/10.3390/ph15080919
APA StyleStepien, K., Nowak, K., Kachnic, N., Horosin, G., Walczak, P., Karcinska, A., Schwarz, T., Wojtas, M., Zalewska, M., Pastuszak, M., Wegrzyn, B., Nessler, J., & Zalewski, J. (2022). Statin Use in Cancer Patients with Acute Myocardial Infarction and Its Impact on Long-Term Mortality. Pharmaceuticals, 15(8), 919. https://doi.org/10.3390/ph15080919





