Clinical Efficacy and Safety of Antimicrobial Photodynamic Therapy in Residual Periodontal Pockets during the Maintenance Phase
Abstract
:1. Introduction
2. Results
2.1. Effect of a-PDT on PPD
2.2. Effect of a-PDT on BOP
2.3. Effects of a-PDT on Plaque Retention and Tooth Mobility
2.4. Adverse Events after a-PDT Treatment
3. Discussion
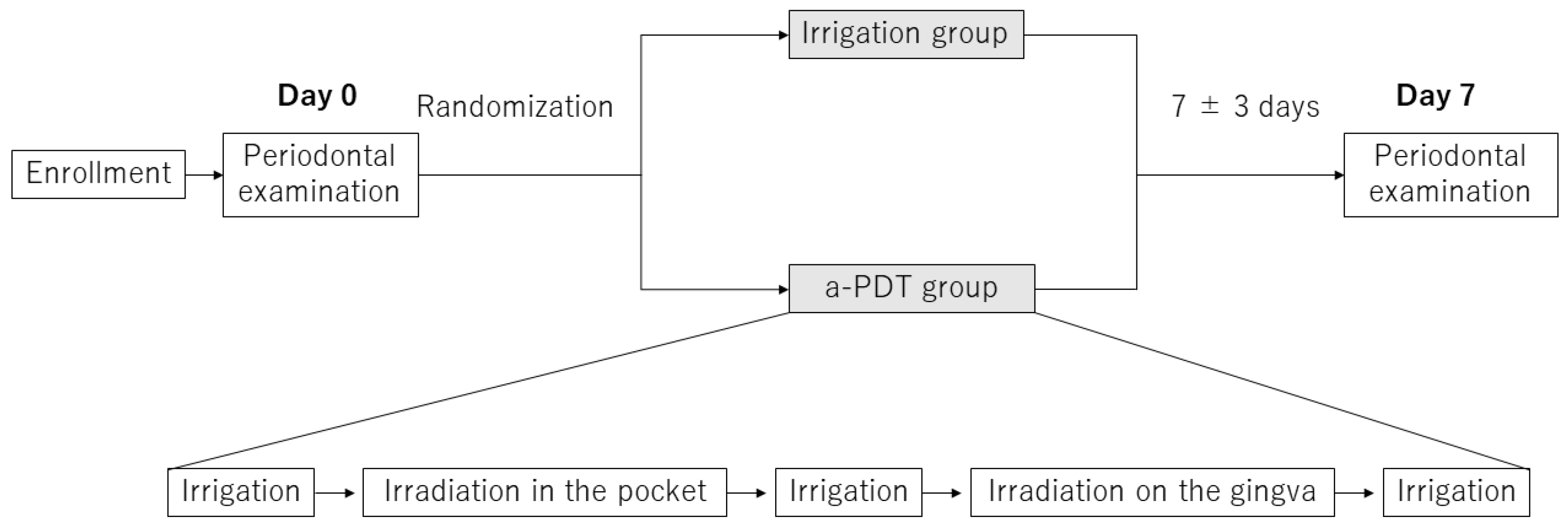
4. Materials and Methods
4.1. Study Population
- (1)
- Patients whose periodontal examination had revealed multiple BOP-positive sites with PPD of 4–6 mm.
- (2)
- Patients aged 20 years or older and less than 90 years old.
- (3)
- Patients who provided written informed consent to participate in this study of their own accord.
- (1)
- Patients lacking the ability to make decisions.
- (2)
- Photosensitive patients.
- (3)
- Patients hypersensitive to drugs, such as toluidine blue.
- (4)
- Patients taking antimicrobial or anti-inflammatory drugs.
- (5)
- Pregnant or lactating women.
- (6)
- Patients with severe diabetes, hepatic disease, renal disease, or other conditions leaving them particularly susceptible to infections.
- (7)
- Patients who were otherwise considered unsuitable for this study.
4.2. Ultrasonic Irrigation and a-PDT Treatment
4.3. Evaluation of Periodontal Status
4.4. General and Local Complications
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, R.; Botelho, J.; Machado, V.; Mascarenhas, P.; Alcoforado, G.; Mendes, J.J.; Chambrone, L. Predictors of tooth loss during long-term periodontal maintenance: An updated systematic review. J. Clin. Periodontol. 2021, 48, 1019–1036. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Academy of Periodontology. Comprehensive periodontal therapy: A statement by the American Academy of Periodontology. J. Periodontol. 2011, 82, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Periodontol. 2018, 89, S9–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol. 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ragas, X.; He, X.; Agut, M.; Roxo-Rosa, M.; Gonsalves, A.R.; Serra, A.C.; Nonell, S. Singlet oxygen in antimicrobial photodynamic therapy: Photosensitizer-dependent production and decay in E. coli. Molecules 2013, 18, 2712–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couto, A.C.F.; de Carvalho, R.V.H.; Brancini, G.T.P.; Martins, F.G.; Sorgi, C.A.; da Silva, R.A.B.; Nelson-Filho, P.; Paula-Silva, F.W.G.; Borsatto, M.C.; Braga, G.U.L.; et al. Photosensitizers attenuate LPS-induced inflammation: Implications in dentistry and general health. Lasers Med. Sci. 2021, 36, 913–926. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Nesi-Reis, V.; Lera-Nonose, D.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of photodynamic therapy in wound healing: A systematic review. Photodiagnosis Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.; Loebel, N.; Hammond, D.; Wilson, M. Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J. Clin. Dent. 2007, 18, 34–38. [Google Scholar] [PubMed]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Graziani, F.; Gatto, R.; Monaco, A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40, 514–526. [Google Scholar] [CrossRef]
- Xue, D.; Tang, L.; Bai, Y.; Ding, Q.; Wang, P.; Zhao, Y. Clinical efficacy of photodynamic therapy adjunctive to scaling and root planing in the treatment of chronic periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 18, 119–127. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Gatto, R.; Marzo, G.; Monaco, A. Photodynamic therapy in the treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2013, 28, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Mongardini, C.; Di Tanna, G.L.; Pilloni, A. Light-activated disinfection using a light-emitting diode lamp in the red spectrum: Clinical and microbiological short-term findings on periodontitis patients in maintenance. A randomized controlled split-mouth clinical trial. Lasers Med. Sci. 2014, 29, 1–8. [Google Scholar] [CrossRef]
- Joseph, B.; Janam, P.; Narayanan, S.; Anil, S. Is antimicrobial photodynamic therapy effective as an adjunct to scaling and root planing in patients with chronic periodontitis? A systematic review. Biomolecules 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller Campanile, V.S.; Giannopoulou, C.; Campanile, G.; Cancela, J.A.; Mombelli, A. Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: Clinical, microbiological, and local biological effects. Lasers Med. Sci. 2015, 30, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Feres, M.; Cortelli, S.C.; Figueiredo, L.C.; Haffajee, A.D.; Socransky, S.S. Microbiological basis for periodontal therapy. J. Appl. Oral Sci. 2004, 12, 256–266. [Google Scholar] [CrossRef] [Green Version]
- Zolfaghari, P.S.; Packer, S.; Singer, M.; Nair, S.P.; Bennett, J.; Street, C.; Wilson, M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Akram, Z.; Al-Shareef, S.A.; Daood, U.; Asiri, F.Y.; Shah, A.H.; AlQahtani, M.A.; Vohra, F.; Javed, F. Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: A systematic review. Photomed. Laser Surg. 2016, 34, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Khan, A.U. Antimicrobial photodynamic therapy (aPDT) against vancomycin resistant Staphylococcus aureus (VRSA) biofilm disruption: A putative role of phagocytosis in infection control. Photodiagnosis Photodyn. Ther. 2021, 36, 102552. [Google Scholar] [CrossRef] [PubMed]
- Bourre, L.; Giuntini, F.; Eggleston, I.M.; Mosse, C.A.; Macrobert, A.J.; Wilson, M. Effective photoinactivation of Gram-positive and Gram-negative bacterial strains using an HIV-1 Tat peptide-porphyrin conjugate. Photochem. Photobiol. Sci. 2010, 9, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Misba, L.; Zaidi, S.; Khan, A.U. A comparison of antibacterial and antibiofilm efficacy of phenothiazinium dyes between Gram positive and Gram negative bacterial biofilm. Photodiagnosis Photodyn. Ther. 2017, 18, 24–33. [Google Scholar] [CrossRef]
- Bhatti, M.; Nair, S.P.; Macrobert, A.J.; Henderson, B.; Shepherd, P.; Cridland, J.; Wilson, M. Identification of photolabile outer membrane proteins of Porphyromonas gingivalis. Curr. Microbiol. 2001, 43, 96–99. [Google Scholar] [CrossRef]
- Beytollahi, L.; Pourhajibagher, M.; Chiniforush, N.; Ghorbanzadeh, R.; Raoofian, R.; Pourakbari, B.; Bahador, A. The efficacy of photodynamic and photothermal therapy on biofilm formation of Streptococcus mutans: An in vitro study. Photodiagnosis Photodyn. Ther. 2017, 17, 56–60. [Google Scholar] [CrossRef]
- Wood, S.; Metcalf, D.; Devine, D.; Robinson, C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J. Antimicrob. Chemother. 2006, 57, 680–684. [Google Scholar] [CrossRef]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: In vitro study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef]
- Braun, A.; Dehn, C.; Krause, F.; Jepsen, S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2008, 35, 877–884. [Google Scholar] [CrossRef]
- Alwaeli, H.A.; Al-Khateeb, S.N.; Al-Sadi, A. Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. Lasers Med. Sci. 2015, 30, 801–807. [Google Scholar] [CrossRef]
- Dilsiz, A.; Canakci, V.; Aydin, T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2013, 84, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Bassir, S.H.; Moslemi, N.; Jamali, R.; Mashmouly, S.; Fekrazad, R.; Chiniforush, N.; Shamshiri, A.R.; Nowzari, H. Photoactivated disinfection using light-emitting diode as an adjunct in the management of chronic periodontitis: A pilot double-blind split-mouth randomized clinical trial. J. Clin. Periodontol. 2013, 40, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, Q.; Wu, Q.; Yao, M.; Chen, Y.; Zhou, H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: A systematic review and Bayesian network meta-analysis. BMC Oral Health 2019, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamoudi, N. Is antimicrobial photodynamic therapy an effective treatment for chronic periodontitis in diabetes mellitus and cigarette smokers: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 19, 375–382. [Google Scholar] [CrossRef] [PubMed]

| Irrigation | a-PDT | |||||
|---|---|---|---|---|---|---|
| Whole | 4 mm | 5–6 mm | Whole | 4 mm | 5–6 mm | |
| n | 30 | 16 | 14 | 30 | 17 | 13 |
| Gender (M/F) | 13/17 | 6/10 | 7/7 | 13/17 | 6/11 | 7/6 |
| Age (years) | 62.0 ± 14.2 | 59.5 ± 15.4 | 66.0 ± 11.1 | 62.0 ± 14.2 | 57.5 ± 15.5 | 67.8 ± 9.1 |
| PPD (mm) | 4.53 ± 0.62 | 4.00 ± 0.00 | 5.14 ± 0.36 | 4.50 ± 0.63 | 4.00 ± 0.00 | 5.07 ± 0.47 |
| BOP (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Plaque (%) | 100 | 100 | 100 | 77 | 100 | 46 |
| Mobility | 0.57 ± 0.50 | 0.44 ±0.51 | 0.71 ±0.47 | 0.60 ± 0.50 | 0.47 ± 0.51 | 0.77 ± 0.43 |
| Smoking (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Irrigation | a-PDT | Odds Ratio | p Value * | ||
|---|---|---|---|---|---|
| Whole group | Pre | 30 (100%) | 30 (100%) | 0.35 | 0.011 |
| Post | 29 (97%) | 10 (33%) | |||
| 4 mm PPD | Pre | 16 (100%) | 17 (100%) | 0.57 | 0.42 |
| Post | 15 (94%) | 9 (53%) | |||
| 5–6 mm PPD | Pre | 14 (100%) | 13 (100%) | 0.077 | 0.006 |
| Post | 14 (100%) | 1 (8%) | |||
| Irrigation | a-PDT | Odds Ratio | p Value * | ||
|---|---|---|---|---|---|
| Whole group | Pre | 30 (100%) | 23 (77%) | 1.30 | 0.65 |
| Post | 15 (50%) | 15 (50%) | |||
| 4 mm PPD | Pre | 16 (100%) | 17 (100%) | 0.65 | 0.58 |
| Post | 13 (81%) | 9 (53%) | |||
| 5–6 mm PPD | Pre | 14 (100%) | 6 (46%) | 7.00 | 0.044 |
| Post | 2 (14%) | 6 (46%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, Y.; Mae, M.; Oohira, M.; Ozaki, Y.; Ohba, S.; Asahina, I.; Yoshimura, A. Clinical Efficacy and Safety of Antimicrobial Photodynamic Therapy in Residual Periodontal Pockets during the Maintenance Phase. Pharmaceuticals 2022, 15, 924. https://doi.org/10.3390/ph15080924
Yamashita Y, Mae M, Oohira M, Ozaki Y, Ohba S, Asahina I, Yoshimura A. Clinical Efficacy and Safety of Antimicrobial Photodynamic Therapy in Residual Periodontal Pockets during the Maintenance Phase. Pharmaceuticals. 2022; 15(8):924. https://doi.org/10.3390/ph15080924
Chicago/Turabian StyleYamashita, Yasunori, Megumi Mae, Masayuki Oohira, Yukio Ozaki, Seigo Ohba, Izumi Asahina, and Atsutoshi Yoshimura. 2022. "Clinical Efficacy and Safety of Antimicrobial Photodynamic Therapy in Residual Periodontal Pockets during the Maintenance Phase" Pharmaceuticals 15, no. 8: 924. https://doi.org/10.3390/ph15080924
APA StyleYamashita, Y., Mae, M., Oohira, M., Ozaki, Y., Ohba, S., Asahina, I., & Yoshimura, A. (2022). Clinical Efficacy and Safety of Antimicrobial Photodynamic Therapy in Residual Periodontal Pockets during the Maintenance Phase. Pharmaceuticals, 15(8), 924. https://doi.org/10.3390/ph15080924







