Pumpkin Seed Oil-Loaded Niosomes for Topical Application: 5α-Reductase Inhibitory, Anti-Inflammatory, and In Vivo Anti-Hair Loss Effects
Abstract
:1. Introduction
2. Results and Discussions
2.1. Skin Permeation and Hair Follicle Accumulation
2.2. Evaluation of the Anti-5α-Reductase Activity in DU-145 Cells
2.2.1. Cell Viability
2.2.2. Anti-5α-Reductase Activity
2.3. Evaluation of Interleukin-6 Inhibition Activity
2.3.1. Cell Viability
2.3.2. Anti-Inflammatory Assay (IL-6 Inhibition)
2.4. Preparation and Characterization of the Hair Scalp Serum Containing PSO-Loaded Niosomes
2.5. Evaluation of Skin Irritation in Reconstructed Human Skin Model
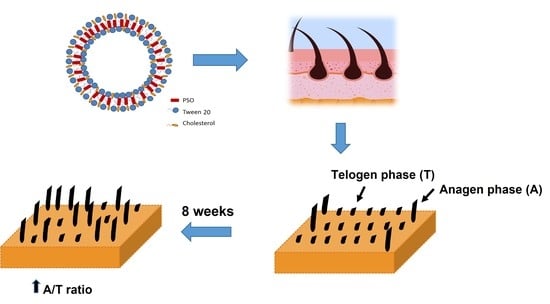
2.6. In Vivo Study of the Anti-Hair Loss Activity of Hair Scalp Serum Containing PSO-Loaded Niosomes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Pumpkin Seed Oil (PSO)-Loaded Niosomes
3.3. Skin Permeation and Hair Follicle Accumulation Study
3.4. Evaluation of Anti-5α-Reductase Activity in DU-145 Cells
3.4.1. Cell Culture
3.4.2. Cell Cytotoxicity Test
3.4.3. Measurement of the mRNA Expression of Hair Loss Genes by Real-Time Polymerase Chain Reaction (qPCR)
3.5. Evaluation of Interleukin-6 (IL-6) Inhibition
3.5.1. Cell Culture
3.5.2. Cell Viability Test
3.5.3. Pro-Inflammatory Activation of RAW 264.7 Cells
3.5.4. IL-6 Assay
3.6. Preparation and Characterization of Hair Scalp Serum Containing PSO-Loaded Niosomes
3.7. Skin Irritation of Hair Scalp Serum Containing PSO-Loaded Niosomes Using Reconstructed Human Skin Model
3.8. Anti-Hair Loss Activity of the Hair Scalp Serum-Containing PSO-Loaded Niosomes
3.8.1. Hair Loss Evaluation by 60-Second Hair Count Test
3.8.2. Hair Loss Evaluation by Phototrichogram Using TrichoScan®
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, S.; Goyal, I.; Mahendra, A. Quality of life assessment in patients with androgenetic alopecia. Int. J. Trichology 2019, 11, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.; Torkamani, N.; Jones, L. Androgenetic alopecia: New insights into the pathogenesis and mechanism of hair loss. F1000Research 2015, 4, 585. [Google Scholar] [CrossRef] [PubMed]
- Kelly, Y.; Blanco, A.; Tosti, A. Androgenetic alopecia: An update of treatment options. Drugs 2016, 76, 1349–1364. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, X.; Fu, S.; Luo, S.; Li, Y. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm.-Venereol. 2022, 102, adv00645. [Google Scholar] [CrossRef]
- Miranda, B.H.; Charlesworth, M.R.; Tobin, D.J.; Sharpe, D.T.; Randall, V.A. Androgens trigger different growth responses in genetically identical human hair follicles in organ culture that reflect their epigenetic diversity in life. FASEB J. 2018, 32, 795–806. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Hair loss and herbs for treatment. J. Cosmet. Dermatol. 2013, 12, 210–222. [Google Scholar] [CrossRef]
- Marchetti, P.M.; Barth, J.H. Clinical biochemistry of dihydrotestosterone. Ann. Clin. Biochem. 2013, 50, 95–107. [Google Scholar] [CrossRef]
- Jacobo, L.M.; Villarreal, C.D.V.; López, R.O.; Candiani, J.O.; Martínez, A.R. Genetic and molecular aspects of androgenetic alopecia. Indian. J. Dermatol. Venereol. Leprol. 2018, 84, 263–268. [Google Scholar] [CrossRef]
- Lai, J.J.; Chang, P.; Lai, K.P.; Chen, L.; Chang, C. The role of androgen and androgen receptor in skin-related disorders. Arch. Dermatol. Res. 2012, 304, 499–510. [Google Scholar] [CrossRef]
- Salisbury, B.H.; Tadi, P. 5 Alpha reductase inhibitors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lee, S.; Lee, Y.B.; Choe, S.J.; Lee, W.S. Adverse sexual effects of treatment with finasteride or dutasteride for male androgenetic alopecia: A systematic review and meta-analysis. Acta Derm. Venereol. 2019, 99, 12–17. [Google Scholar] [CrossRef]
- Fertig, R.M.; Gamret, A.C.; Darwin, E.; Gaudi, S. Sexual side effects of 5-α-reductase inhibitors finasteride and dutasteride: A comprehensive review. Dermatol. Online J. 2017, 23, 3. [Google Scholar] [CrossRef]
- Diviccaro, S.; Melcangi, R.C.; Giatti, S. Post-finasteride syndrome: An emerging clinical problem. Neurobiology 2020, 12, 100209. [Google Scholar] [CrossRef]
- Fertig, R.; Shapiro, J.; Bergfeld, W.; Tosti, A. Investigation of the plausibility of 5-alpha-reductase inhibitor syndrome. Skin Appendage Disord. 2016, 2, 120–129. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P. Review of Cucurbita pepo (pumpkin) its phytochemistry and pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Nawirska-Olszanska, A.; Kita, A.; Biesiada, A.; Sokol-Letowska, A.; Kucharska, A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef]
- Boujemaa, I.; Bernoussi, S.E.; Harhar, H.; Tabyaoui, M. The influence of the species on the quality, chemical composition and antioxidant activity of pumpkin seed oil. OCL 2020, 27, 40. [Google Scholar] [CrossRef]
- Majid, A.K.; Ahmed, Z.; Khan, R. Effect of pumpkin seed oil on cholesterol fractions and systolic/diastolic blood pressure. Food Sci. Technol. 2020, 40, 769–777. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Mohamed, D.A.; Kandil, E.; Ahmed, E.K.; Mohammed, S.E. Antioxidant and anti-cancer effect of Egyptian and European pumpkin seed oil. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 574–580. [Google Scholar]
- Abd-Elnoor, A.V. Hypoglycemic and hypolipidemic effects of pumpkin seeds powder and oil on alloxan-induced diabetic in rats. Egypt. J. Food. Sci. 2019, 47, 255–269. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, A.; Calhelha, R.C.; Rouphael, Y.; Petrovic, J.; Sokovic, M.; Ferreira, I.C.F.R.; Barros, L. Antimicrobial properties, cytotoxic effects, and fatty acids composition of vegetable oils from purslane, linseed, luffa, and pumpkin Seeds. Appl. Sci. 2021, 11, 5738. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [CrossRef]
- Damiano, R.; Cai, T.; Fornara, P.; Franzese, C.A.; Leonardi, R.; Mirone, V. The role of Cucurbita pepo in the management of patients affected by lower urinary tract symptoms due to benign prostatic hyperplasia: A narrative review. Arch. Ital. Urol. Androl. 2016, 88, 136–143. [Google Scholar] [CrossRef]
- Csikos, E.; Horvath, A.; Acs, K.; Papp, N.; Balazs, V.L.; Dolenc, M.S.; Kenda, M.; Glavac, N.K.; Nagy, M.; Protti, M.; et al. Treatment of benign prostatic yyperplasia by natural drugs. Molecules 2021, 26, 7141. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, M.; Bratoeff, E.; Heuze, I.; Ramirez, E.; Sanchez, M.; Flores, E. Effect of beta-sitosterol as inhibitor of 5 alpha-reductase in hamster prostate. Proc. West. Pharmacol. Soc. 2003, 46, 153–155. [Google Scholar] [PubMed]
- Sudeep, H.V.; Thomas, J.V.; Shyamprasad, K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol. 2020, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Bian, Q.; Zhou, Y.; Huang, Q.; Gao, J. Hair follicle-targeting drug delivery strategies for the management of hair follicle-associated disorders. Asian J. Pharm. Sci. 2022, 17, 333–352. [Google Scholar] [CrossRef]
- Tampucci, S.; Paganini, V.; Burgalassi, S.; Chetoni, P.; Monti, D. Nanostructured drug delivery systems for targeting 5-alpha-reductase inhibitors to the hair follicle. Pharmaceutics 2022, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Khoee, S.; Yaghoobian, M. Niosomes: A novel approach in modern drug delivery systems. In Nanostructures for Drug Delivery, 1st ed.; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Walter, J.; Stahl, F.; Scheper, T. Niosomes as nanoparticular drug carriers: Fundamentals and recent applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef]
- Durga, B.G.; Veera, L.P. Recent advances of non-ionic surfactant-based nano-vesicles (niosomes and proniosomes): A brief review of these in enhancing transdermal delivery of drug. Future J. Pharm. Sci. 2020, 6, 100. [Google Scholar] [CrossRef]
- Morakul, B.; Teeranachaideekul, V.; Junyaprasert, V.B. Niosomal delivery of pumpkin seed oil: Development, characterisation, and physical stability. J. Microencapsul. 2019, 36, 120–129. [Google Scholar] [CrossRef]
- Brancato, S.; Cartigliani, C.; Bonfigli, A.; Rigano, L. Quantitative analysis using the phototrichogram technique of an Italian population suffering from androgenic alopecia. Cosmetics 2018, 5, 28. [Google Scholar] [CrossRef]
- Reygagne, P. Phototrichogram. In Agache’s Measuring the Skin, 2nd ed.; Humbert, P., Fanian, F., Maibach, H.I., Agache, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 813–825. [Google Scholar] [CrossRef]
- Oaku, Y.; Abe, A.; Sasano, Y.; Sasaki, F.; Kubota, C.; Yamamoto, N.; Nagahama, T.; Nagai, N. Minoxidil nanoparticles targeting hair follicles enhance hair growth in C57BL/6 mice. Pharmaceutics 2022, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Wosicka-Frackowiak, H.; Cal, K.; Stefanowska, J.; Glowka, E.; Nowacka, M.; Struck-Lewicka, W.; Govedarica, B.; Pasikowska, M.; Debowska, R.; Jesionowski, T.; et al. Roxithromycin-loaded lipid nanoparticles for follicular targeting. Int. J. Pharm. 2015, 495, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Magnani, C.; Guillot, A.; Melero, A.; Corrêa, M. Caffeic acid skin absorption: Delivery of microparticles to hair follicles. Saudi Pharm. J. 2019, 27, 791–797. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The inflammatory aspect of male and female pattern hair loss. J. Inflamm. Res. 2020, 13, 879–881. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Khantham, C.; Yooin, W.; Sringarm, K.; Sommano, S.R.; Jiranusornkul, S.; Carmona, F.D.; Nimlamool, W.; Jantrawut, P.; Rachtanapun, P.; Ruksiriwanich, W. Effects on steroid 5-alpha reductase gene expression of Thai rice bran extracts and molecular dynamics study on SRD5A2. Biology 2021, 10, 319. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M.; Chaikul, P. Para rubber seed oil: The safe and efficient bio-material for hair loss treatment. J. Cosmet. Dermatol. 2021, 20, 2160–2167. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yang, L.; Li, Y.J.; Zhang, D.; Chen, Y.; Kostecka, P.; Kmonickova, E.; Zidek, Z. Effects of sesquiterpene, flavonoid and coumarin types of compounds from Artemisia annua L. on production of mediators of angiogenesis. Pharmacol. Rep. 2013, 65, 410–420. [Google Scholar] [CrossRef]
- Sandhiutami, N.M.; Moordiani, M.; Laksmitawati, D.R.; Fauziah, N.; Maesaroh, M.; Widowati, W. In vitro assesment of anti-inflammatory activities of coumarin and Indonesian cassia extract in RAW264.7 murine macrophage cell line. Iran. J. Basic Med. Sci. 2017, 20, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wasko, C.A.; Mackley, C.L.; Sperling, L.C.; Mauger, D.; Miller, J.J. Standardizing the 60-second hair count. Arch. Dermatol. 2008, 144, 759–762. [Google Scholar] [CrossRef] [PubMed]






| Sample | IL-6 Detection | |
|---|---|---|
| IL-6 Level (pg/mL) | IL-6 Inhibition Activity over Positive Control (%) | |
| Negative control | 2.82 ± 0.27 | - |
| Positive control | 627.94 ± 24.35 | 0.00 |
| PSO-niosomes (100 μg/mL) | 36.50 ± 5.38 | 94.19 ± 0.86 |
| PSO solution (100 μg/mL) | 2.80 ± 0.11 | 99.55 ± 0.02 |
| Diclofenac sodium (1000 µM) | 72.46 ± 13.46 | 88.46 ± 2.14 |
| Time | Average Number of Fallen Hairs | % Reduction of Fallen Hairs | p-Value |
|---|---|---|---|
| Initial | 9.60 ± 5.08 | - | - |
| 4 weeks | 7.33 ± 4.64 | 23.57 ± 1.29 | 0.024 * |
| 8 weeks | 5.33 ± 3.11 | 44.42 ± 1.22 | 0.000 * |
| Time | % Anagen | % Telogen | A/T Ratio | p-Value |
|---|---|---|---|---|
| Initial | 74.53 ± 9.38 | 25.81 ± 9.85 | 3.48 ± 2.06 | |
| 4 weeks | 78.45 ± 5.74 | 21.55 ± 5.74 | 3.92 ± 1.22 | 1.000 |
| 8 weeks | 80.98 ± 6.04 | 19.03 ± 6.04 | 4.72 ± 1.76 | 0.036 * |
| Gene Specific Primers | Sequences | |
|---|---|---|
| Human GAPDH | sense antisense | 5′-CAAATTCCATGGCACCGTCA-3′ 5′-ATCGCCCCACTTGATTTTGG-3′ |
| Human SRD5A1 | sense antisense | 5′-TGGGGATAGAGGAGGAAGCT-3′ 5′-GTATGAACCACCACCAGCAC-3′ |
| Human SRD5A2 | sense antisense | 5′-CAGAGCCCACATTTCCACAC-3′ 5′-GCCCCTTCCTTAGAGAGTCC-3′ |
| Ingredient | Master Formula (% w/w) |
|---|---|
| Deionized water | 88.05 |
| Acrylates/C10-30 alkyl acrylate crosspolymer | 0.30 |
| Glycerin | 1.00 |
| Propylene glycol | 1.00 |
| Disodium EDTA | 0.20 |
| Phenostat | 1.00 |
| Tocopheryl acetate | 0.20 |
| PEG-40 hydrogenated castor oil | 1.00 |
| Fragrance | 0.05 |
| PSO-loaded niosomes | 5.00 |
| 10% w/v triethanolamine solution | q.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teeranachaideekul, V.; Parichatikanond, W.; Junyaprasert, V.B.; Morakul, B. Pumpkin Seed Oil-Loaded Niosomes for Topical Application: 5α-Reductase Inhibitory, Anti-Inflammatory, and In Vivo Anti-Hair Loss Effects. Pharmaceuticals 2022, 15, 930. https://doi.org/10.3390/ph15080930
Teeranachaideekul V, Parichatikanond W, Junyaprasert VB, Morakul B. Pumpkin Seed Oil-Loaded Niosomes for Topical Application: 5α-Reductase Inhibitory, Anti-Inflammatory, and In Vivo Anti-Hair Loss Effects. Pharmaceuticals. 2022; 15(8):930. https://doi.org/10.3390/ph15080930
Chicago/Turabian StyleTeeranachaideekul, Veerawat, Warisara Parichatikanond, Varaporn Buraphacheep Junyaprasert, and Boontida Morakul. 2022. "Pumpkin Seed Oil-Loaded Niosomes for Topical Application: 5α-Reductase Inhibitory, Anti-Inflammatory, and In Vivo Anti-Hair Loss Effects" Pharmaceuticals 15, no. 8: 930. https://doi.org/10.3390/ph15080930
APA StyleTeeranachaideekul, V., Parichatikanond, W., Junyaprasert, V. B., & Morakul, B. (2022). Pumpkin Seed Oil-Loaded Niosomes for Topical Application: 5α-Reductase Inhibitory, Anti-Inflammatory, and In Vivo Anti-Hair Loss Effects. Pharmaceuticals, 15(8), 930. https://doi.org/10.3390/ph15080930