The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy
Abstract
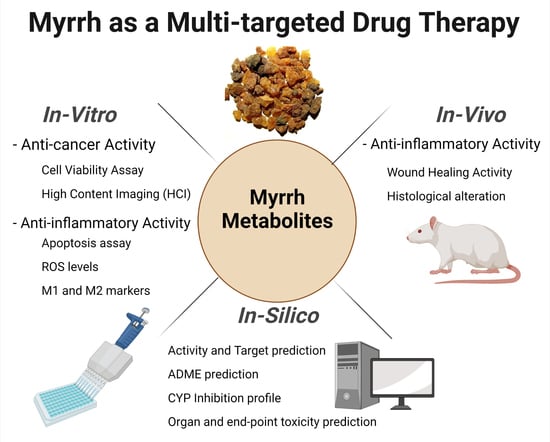
:1. Introduction
2. Results and Discussion
2.1. Metabolite Profiling Using HPLC and QTOF-LCMS
2.1.1. Metabolite A: 4,5-Dihydrofuranodiene
2.1.2. Metabolite B: Furanodienone
2.1.3. Metabolite C: Mansumbinone
2.1.4. Metabolite D: Cembrene-A
2.1.5. Metabolite E: Campes-tan-3b-ol
2.1.6. Metabolite F: Pregnadienes
2.1.7. Metabolite G: Z,4Z-Furanodien-6-one
2.1.8. Metabolite H: Guggulsterone
2.1.9. Metabolite I: 3,4-Seco-mansumbinoic Acid
2.2. Anti-Cancer Activity of Myrrh Resin Extract
2.3. In Vitro Anti-Inflammatory Activity Investigation
2.4. In Vivo Anti-Inflammatory and Wound-Healing Activity Investigation
2.5. In Silico Computational Investigation
2.5.1. Activity Prediction
2.5.2. Target Prediction
Molinspiration
GPCR Ligand
Ion Channel Modulator
Kinase Inhibitor
Nuclear Receptor Ligand
Protease Inhibitor
Enzyme Inhibitor
SEA Search and Swiss Target Predictions
2.5.3. ADME Prediction
2.5.4. CYP Inhibition Profile
2.5.5. Organ and End-Point Toxicity Prediction
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Metabolites Profiling Using HPLC and QTOF-LCMS
3.3. In Vitro Anticancer Activity of Myrrh Resins Extract
3.3.1. Cell Viability Assay
3.3.2. High Content Imaging (HCI) Assay
3.4. In Vitro Anti-Inflammatory Activity Investigation
3.4.1. Cell Lines
3.4.2. Apoptosis Assay
3.4.3. Measurement of ROS Levels
3.5. In Vivo Anti-Inflammatory Activity Investigation
3.6. In Silico Computational Investigation
3.6.1. Activity Prediction
3.6.2. Target Prediction
Molinspiration
SEA Search
Swiss Target Prediction
3.6.3. ADME Prediction
3.6.4. CYP Inhibition Profile
3.6.5. Organ and End-Point Toxicity Prediction
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rapper, S.; Van Vuuren, S.F.; Kamatou, G.P.P.; Viljoen, A.M.; Dagne, E. The additive and synergistic antimicrobial effects of select frankincense and myrrh oils—A combination from the pharaonic pharmacopoeia. Lett. Appl. Microbiol. 2012, 54, 352–358. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, B.; Dekebo, A.; Dagne, E. Essential oils of some Boswellia spp., Myrrh and Opopanax. Undefined 2003, 18, 153–156. [Google Scholar]
- US Food and Drug Administration. CFR—Code of Federal Regulations Title 21. New Hampshire Ave. 2015. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (accessed on 25 May 2021).
- Schrankel, K.R. Safety evaluation of food flavorings. Toxicology 2004, 198, 203–211. [Google Scholar] [CrossRef]
- Ahamad, S.R.; Al-Ghadeer, A.R.; Ali, R.; Qamar, W.; Aljarboa, S. Analysis of inorganic and organic constituents of myrrh resin by GC-MS and ICP-MS: An emphasis on medicinal assets. Saudi Pharm. J. 2017, 25, 788–794. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Rashed, N.; Salama, O.M.; Saleh, A. Components, therapeutic value and uses of myrrh. Die Pharm. Int. J. Pharm. Sci. 2003, 58, 163–168. [Google Scholar]
- NCI Dictionary of Cancer Terms-National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/ (accessed on 16 January 2022).
- Fatani, A.J.; Alrojayee, F.S.; Parmar, M.Y.; Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S. Myrrh attenuates oxidative and inflammatory processes in acetic acid-induced ulcerative colitis. Exp. Ther. Med. 2016, 12, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Germano, A.; Occhipinti, A.; Barbero, F.; Maffei, M.E. A pilot study on bioactive constituents and analgesic effects of MyrLiq®, a Commiphora myrrha extract with a high furanodiene content. BioMed Res. Int. 2017, 2017, 3804356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, M. Medicinal Plants: Chemistry and Properties; Science Publishers: Hamburg, Germany, 2006. [Google Scholar]
- Mohamed, A.; Shahid SM, A.; Alkhamaiseh, S.I.; Ahmed, M.Q.; Albalwi, F.O.; Al-gholaigah, M.H.; Alshammari, M.G. Antibacterial activity of Commiphora molmol in wound infections. J. Diagn. Pathol. Oncol. 2017, 2, 32–36. [Google Scholar]
- Hassan, A.B.; Shahid, S.M.A.; Alkhamaiseh, S.I.; Ahmed, M.Q.; Albalwi, F.O.; Al-Gholaigah, M.H.; Alqahtani, M.M.; Alshammari, M.G.; Kausar, M.A. Antibacterial activity of Commiphora molmol in wound infections. Biochem. Cell. Arch. 2017, 17, 639–644. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. Available online: https://europepmc.org/articles/PMC7147972 (accessed on 16 January 2022).
- Corley, D.A.; Kerlikowse, K.; Verma, R.; Buffler, P. Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysis. Gastroenterology 2003, 124, 47–56. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Hua, J.; Liu, G.; Huang, P.; Liu, N.; He, X. Myrrh induces the apoptosis and inhibits the proliferation and migration of gastric cancer cells through down-regulating cyclooxygenase-2 expression. Biosci. Rep. 2020, 40, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alhawassi, T.; Qamar, W.; El-Gamal, A. Cytotoxic evaluation and anti-angiogenic effects of two furano-sesquiterpenoids from Commiphora myrrh resin. Molecules 2020, 25, 1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corticosteroid Adverse Effects-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30285357/ (accessed on 16 January 2022).
- Stucker, F.; Ackermann, D. Immunsuppressiva-Wirkungen, nebenwirkungen und interaktionen. Ther. Umsch. 2011, 68, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef]
- Gao, W.; Su, X.; Dong, X.; Chen, Y.; Zhou, C.; Xin, P.; Yu, C.; Wei, T. Cycloartan-24-ene-1α,2α,3β-triol, a cycloartane-type triterpenoid from the resinous exudates of Commiphora myrrha, induces apoptosis in human prostatic cancer PC-3 cells. Oncol. Rep. 2015, 33, 1107–1114. Available online: https://pubmed.ncbi.nlm.nih.gov/25591732/ (accessed on 16 January 2022).
- Shen, T.; Yuan, H.-Q.; Wan, W.-Z.; Wang, X.-L.; Ji, M.; Lou, H.-X. Cycloartane-type triterpenoids from the resinous exudates of Commiphora opobalsamum. J. Nat. Prod. 2008, 71, 81–86. Available online: https://pubmed.ncbi.nlm.nih.gov/18177010/ (accessed on 16 January 2022).
- Mohammed, H.A.; Al-Omar, M.S.; Aly, M.S.; Hegazy, M.M. Essential oil constituents and biological activities of the halophytic plants, Suaeda vermiculata Forssk and Salsola cyclophylla Bakera growing in Saudi Arabia. J. Essent. Oil Bear. Plants 2019, 22, 82–93. [Google Scholar] [CrossRef]
- Ali, R.; Samman, N.; Al Zahrani, H.; Nehdi, A.; Rahman, S.; Khan, A.L.; Al Balwi, M.; Alriyees, L.A.; Alzaid, M.; Al Askar, A.; et al. Isolation and characterization of a new naturally immortalized human breast carcinoma cell line, KAIMRC1. BMC Cancer 2017, 17, 803. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3812-5 (accessed on 19 January 2022).
- Way2Drug-Main. Available online: http://way2drug.com/PassOnline/ (accessed on 25 May 2021).
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. Available online: https://pubmed.ncbi.nlm.nih.gov/11099264/ (accessed on 9 July 2021).
- Molinspiration Cheminformatics. Available online: https://www.molinspiration.com/ (accessed on 26 December 2021).
- Wang, Z.; Liang, L.; Yin, Z.; Lin, J. Improving chemical similarity ensemble approach in target prediction. J. Cheminform 2016, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W3664. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 26 December 2021).
- ProTox-II-Prediction of TOXicity of Chemicals. Available online: https://tox-new.charite.de/protox_II/ (accessed on 21 October 2021).
- Mohammed, A.E.; Sonbol, H.; Alwakeel, S.S.; Alotaibi, M.O.; Alotaibi, S.; Alothman, N.; Suliman, R.S.; Ahmedah, H.T.; Ali, R. Investigation of biological activity of soil fungal extracts and LC/MS-QTOF based metabolite profiling. Sci. Rep. 2021, 11, 4760. Available online: https://www.nature.com/articles/s41598-021-83556-8 (accessed on 19 January 2022).
- Arepalli, S.K.; Sridhar, V.; Venkateswara Rao, J.; Kavin Kennady, P.; Venkateswarlu, Y. Furano-sesquiterpene from soft coral, Sinularia kavarittiensis: Induces apoptosis via the mitochondrial-mediated caspase-dependent pathway in THP-1, leukemia cell line. Apoptosis 2009, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Gigliarelli, G.; Palmier, A.; Marcotullio, M.C. Furanodienone: An emerging bioactive furanosesquiterpenoid. Curr. Org. Chem. 2017, 21, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Morikawa, T.; Ando, S.; Oominami, H.; Murakami, T.; Kimura, I.; Yoshikawa, M. Absolute Stereostructures of Polypodane-Type Triterpenes, Myrrhanol A and Myrrhanone A, from Guggul-Gum Resin (the Resin of Balsamodendron mukul). ChemInform 2005, 36, 1200–1209. Available online: https://www.researchgate.net/publication/8248888_Absolute_Stereostructures_of_Polypodane-Type_Triterpenes_Myrrhanol_A_and_Myrrhanone_A_from_Guggul-Gum_Resin_the_Resin_of_Balsamodendron_mukul (accessed on 30 January 2022).
- Yao, Y.Q.; Ding, X.; Jia, Y.C.; Huang, C.X.; Wang, Y.Z.; Xu, Y.H. Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008, 264, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lemenith, M.; Teketay, D. Frankincense and myrrh resources of Ethiopia: II. Medicinal and industrial uses. SINET Ethiop. J. Sci. 2003, 26, 161–172. [Google Scholar] [CrossRef]
- Bai, Z.; Yao, C.; Zhu, J.; Xie, Y.; Ye, X.Y.; Bai, R.; Xie, T. Anti-tumor drug discovery based on natural product β-elemene: Anti-tumor mechanisms and structural modification. Molecules 2021, 26, 1499. [Google Scholar] [CrossRef]
- Zhai, B.; Zhang, N.; Han, X.; Li, Q.; Zhang, M.; Chen, X.; Li, G.; Zhang, R.; Chen, P.; Wang, W.; et al. Molecular targets of β-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: A review. Biomed. Pharmacother. 2019, 114, 108812. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, X.; Hu, D. Furanodienone induces G0/G1 arrest and causes apoptosis via the ROS/MAPKs-mediated caspase-dependent pathway in human colorectal cancer cells: A study in vitro and in vivo. Cell Death Dis. 2017, 8, e2815. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhou, S.; Gustafsson, J.Å. Nuclear Receptors: Recent Drug Discovery for Cancer Therapies. Endocr. Rev. 2019, 40, 1207–1249. [Google Scholar] [CrossRef] [PubMed]
- Duwiejua, M.; Zeitlin, I.J.; Waterman, P.G.; Chapman, J.; Mhango, G.J.; Provan, G.J. Anti-inflammatory activity of resins from some species of the plant family Burseraceae. Planta Med. 1993, 59, 12–16. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, X.; Xu, J.; Li, C.; Yu, Y.; Wang, W.; Zhu, L. The protective effect of dietary phytosterols on cancer risk: A systematic meta-analysis. J. Oncol. 2019, 2019, 7479518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essa, A.F.; El-Hawary, S.S.; Abd-El Gawad, A.M.; Kubacy, T.M.; El-Khrisy, E.E.-D.A.M.; Elshamy, A.I.; Younis, I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar] [CrossRef] [PubMed]
- Vanderah, D.J.; Rutledge, N.; Schmitz, F.J.; Ciereszko, L.S. Marine natural products: Cembrene-A and cembrene-C from a soft coral, Nephthea species. J. Org. Chem. 1978, 43, 1614–1616. [Google Scholar] [CrossRef]
- You, Z.; Heiman, A.S.; Hudson, C.E.; Lee, H.J. Effect of chirality at C-20 of methyl 11β, 17α, 20-trihydroxy-3-oxo-1, 4-pregnadien-21-oate derivatives on antiinflammatory activity. Steroids 2002, 67, 353–359. [Google Scholar] [CrossRef]
- Bratoeff, E.; Cabeza, M.; Pérez-Ornelas, V.; Recillas, S.; Heuze, I. In vivo and in vitro effect of novel 4, 16-pregnadiene-6, 20-dione derivatives, as 5α-reductase inhibitors. J. Steroid Biochem. Mol. Biol. 2008, 111, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Long, B.J.; Grigoryev, D.N.; Nnane, I.P.; Liu, Y.; Ling, Y.Z.; Brodie, A.M. Antiandrogenic effects of novel androgen synthesis inhibitors on hormone-dependent prostate cancer. Cancer Res. 2000, 60, 6630–6640. [Google Scholar]
- Li, C.L.; Chang, L.; Guo, L.; Zhao, D.; Liu, H.B.; Wang, Q.S.; Zhang, P.; Du, W.Z.; Liu, X.; Zhang, H.T. β-elemene induces caspase-dependent apoptosis in human glioma cells in vitro through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Asian Pac. J. Cancer Prev. 2015, 15, 10407–10412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eatemadi, A.; Aiyelabegan, H.T.; Negahdari, B.; Mazlomi, M.A.; Daraee, H.; Daraee, N.; Eatemadi, R.; Sadroddiny, E. Role of protease and protease in-hibitors in cancer pathogenesis and treatment. Biomed. Pharm. 2017, 86, 221–231. [Google Scholar] [CrossRef]
- Bhore, S.J.; Preveena, J.; Kandasamy, K.I. Isolation and identification of bacterial endophytes from pharmaceutical agarwood-producing Aquilaria species. Pharmacogn. Res. 2013, 5, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greve, H.L.; Kaiser, M.; Schmidt, T.J. Investigation of antiplasmodial effects of terpenoid compounds isolated from myrrh. Planta Med. 2020, 86, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Y.; Li, Y.; Zhao, P.; Liu, C.; Ma, Y.; Zhang, T. Sesquiterpenoids from the resinous exudates of Commiphora myrrha and their neuroprotective effects. Planta Med. 2011, 77, 2023–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Gao, Y. β-elemene against Burkitt’s lymphoma via activation of PUMA mediated apoptotic pathway. Biomed. Pharmacother. 2018, 106, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sugimoto, K. Guggulsterone and its role in chronic diseases. Drug Discov. Mother Nat. 2016, 929, 329–361. [Google Scholar]
- Rahman, M.M.; Garvey, M.; Piddock, L.J.; Gibbons, S. Antibacterial terpenes from the oleo-resin of Commiphora molmol (Engl.). Phytother. Res. 2008, 22, 1356–1360. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Wang, C.; Wu, C.; Guo, P.; Wei, J. Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishodia, S.; Azu, N.; ARosenzweig, J.; AJackson, D. Guggulsterone for Chemoprevention of Cancer. Curr. Pharm. Des. 2016, 22, 294–306. [Google Scholar] [CrossRef]
- Shi, J.-J.; Jia, X.-L.; Li, M.; Yang, N.; Li, Y.-P.; Zhang, X.; Gao, N.; Dang, S.-S. Guggulsterone induces apoptosis of human hepatocellular carcinoma cells through intrinsic mitochondrial pathway. World J. Gastroenterol. 2015, 21, 13277. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhao, J.H.; Liu, Y.; Wang, Z.; Zhang, Y.T.; Feng, N.P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033. [Google Scholar]
- Hamad, G.M.; Taha, T.H.; Alshehri, A.; El-Deeb, N.M. Myrrh as a Functional Food with Therapeutic Properties Against Colon Cancer in Traditional Meals. J. Food Process. Preserv. 2017, 41, e12963. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-L.; Kong, F.; Shen, T.; Young, C.Y.F.; Lou, H.-X.; Yuan, H.-Q. Sesquiterpenoids from myrrh inhibit androgen receptor expression and function in human prostate cancer cells. Acta Pharmacol. Sin. 2011, 32, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Miao, X.; Sun, C.; Su, S.; Zhu, Y.; Qian, D.; Ouyang, Z.; Duan, J. Frankincense and myrrh and their bioactive compounds ameliorate the multiple myeloma through regulation of metabolome profiling and JAK/STAT signaling pathway based on U266 cells. BMC Complement. Med. Ther. 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Y.Z.H.Y.; Phirdaous, A.; Azura, A. Screening of anticancer activity from agarwood essential oil. Pharmacogn. Res. 2014, 6, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Wang, T.; Chen, T.; Duan, J.-A.; Li, Y.; Tang, Y. Cytotoxicity activity of extracts and compounds from Commiphora myrrha resin against human gynecologic cancer cells. J. Med. Plants Res. 2011, 5, 1382–1389. Available online: http://www.academicjournals.org/JMPR (accessed on 19 January 2022).
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 5, 195727. [Google Scholar] [CrossRef] [Green Version]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 86–97. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Principles of innate and adaptive immunity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: Hamburg, Germany, 2001. [Google Scholar]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [Green Version]
- Kongara, S.; Karantza, V. The interplay between autophagy and ROS in tumorigenesis. Front. Oncol. 2012, 2, 171. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, I.; Carito, V.; Russo, E.; Tripepi, S.; Aquila, S.; Donato, G. Macrophage autophagy and oxidative stress: An ultrastructural and immunoelectron microscopical study. Oxid. Med. Cell. Longev. 2011, 2011, 282739. [Google Scholar] [CrossRef]
- Wolf, A.; Herb, M.; Schramm, M.; Langmann, T. The TSPO-NOX1 axis controls phagocyte-triggered pathological angiogenesis in the eye. Nat. Commun. 2020, 11, 2709. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, J.M.; Kim, S.J.; Seo, W.; Kim, M.H.; Choi, W.M.; Jeong, W.I. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat. Commun. 2017, 8, 2247. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.B.; Greten, F.R. Immune cell-produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 32–48. [Google Scholar] [CrossRef]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Ramulu, U.; Ramesh, D.; Srikanth, D.; Bhattacharya, P.; Prabhakar, P.; Kalivendi, S.V.; Babu, K.S.; Venkateswarlu, Y.; Navath, S. Anti-cancer evaluation of carboxamides of furano-sesquiterpene carboxylic acids from the soft coral Sinularia kavarattiensis. Bioorg. Med. Chem. Lett. 2013, 23, 6234–6238. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.A.; Hashim, Y.Z.H.; El-Kersh, D.M.; Abd-Azziz, S.S.; Salleh, H.M.; Isa, M.L.M.; Abd Warif, N.M. Metabolite Profiling of Aquilaria malaccensis leaf extract using Liquid Chromatography-Q-TOF-Mass spectrometry and investigation of its potential antilipoxygenase activity in-vitro. Processes 2020, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Brieskorn, C.H.; Noble, P. Two furanoeudesmanes from the essential oil of myrrh. Phytochemistry 1983, 22, 187–189. [Google Scholar] [CrossRef]
- Kerns, E.H.; Di, L. Drug-Like Properties: Concepts, Structure Design and Methods: From ADME to Toxicity Optimization; Elsevier: Burlington, MA, USA, 2008; p. 526. [Google Scholar]
- Zulkifle, N.L.B. Antidiabetic Activity of Aquilaria Malaccensis (Agarwood) Leaves Extracts. Ph.D. Thesis, Universiti Malaysia Pahang, Gambang, Malaysia, 2018. [Google Scholar]
- Tao, L.; Zhou, L.; Zheng, L.; Yao, M. Elemene displays anti-cancer ability on laryngeal cancer cells in vitro and in vivo. Cancer Chemother. Pharm. 2006, 58, 24–34. [Google Scholar] [CrossRef]
- [Beta-Elemene Induces Apoptosis of K562 Leukemia Cells]-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11783084/ (accessed on 19 January 2022).
- Cao, B.; Wei, X.-C.; Xu, X.-R.; Zhang, H.-Z.; Luo, C.-H.; Feng, B.; Xu, R.-C.; Zhao, S.-Y.; Du, X.-J.; Han, L.; et al. Seeing the Unseen of the Combination of Two Natural Resins, Frankincense and Myrrh: Changes in Chemical Constituents and Pharmacological Activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef] [Green Version]
- Wanwimolruk, S.; Prachayasittikul, V. Cytochrome P450 enzyme mediated herbal drug interactions (Part 1). EXCLI J. 2014, 13, 347. [Google Scholar] [PubMed]
- Lynch, T.; Neff, A.P. The Effect of Cytochrome P450 Metabolism on Drug Response, Interactions, and Adverse Effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Shankar, K.; Mehendale, H.M. Cytochrome P450. In Encyclopedia of Toxicology; Elsevier: Burlington, MA, USA, 2014; pp. 1125–1127. [Google Scholar]
- Ismail, F.; Wahab, A.Y.A.; Isa, M.L.M.; Muhammad, H.; Ismail, R.A.S.R.; Razak, R.N.H.A. The effects of Aquilaria malaccensis leaves aqueous extract on sperm of Sprague Dawley Rats towards early embryogenesis. IIUM Med. J. Malays. 2019, 18. [Google Scholar] [CrossRef]
- Mishra, S.; Dahima, R. In vitro ADME studies of TUG-891, a GPR-120 inhibitor using SWISS ADME predictor. J. Drug Deliv. Ther. 2019, 9, 366–369. [Google Scholar]
- Lohidakshan, K.; Rajan, M.; Ganesh, A.; Paul, M.; Jerin, J. Pass and Swiss ADME collaborated in silico docking approach to the synthesis of certain pyrazoline spacer compounds for dihydrofolate reductase inhibition and antimalarial activity. Bangladesh J. Pharmacol. 2018, 13, 23–29. [Google Scholar] [CrossRef]
- Al Azzam, K.M.; Negim, E.S.; Aboul-Enein, H.Y. ADME studies of TUG-770 (a GPR-40 inhibitor agonist) for the treatment of type 2 diabetes using SwissADME predictor: In silico study. J. Appl. Pharm. Sci. 2022, 12, 159–169. [Google Scholar]
- Roman, M.; Roman, D.L.; Ostafe, V.; Ciorsac, A.; Isvoran, A. Computational assessment of pharmacokinetics and biological effects of some anabolic and androgen steroids. Pharm. Res. 2018, 35, 41. [Google Scholar] [CrossRef]
- Mazumder, K.; Hossain, M.; Aktar, A.; Mohiuddin, M.; Sarkar, K.K.; Biswas, B.; Fukase, K. In Silico Analysis and Experimental Evaluation of Ester Prodrugs of Ketoprofen for Oral Delivery: With a View to Reduce Toxicity. Processes 2021, 9, 2221. [Google Scholar] [CrossRef]
- Ranjith, D.; Ravikumar, C. SwissADME predictions of pharmacokinetics and drug-likeness properties of small molecules present in Ipomoea mauritiana Jacq. J. Pharmacogn. Phytochem. 2019, 8, 2063–2073. [Google Scholar]
- Sympli, H.D. Estimation of drug-likeness properties of GC-MS separated bioactive compounds in rare medicinal Pleione maculata using molecular docking technique and SwissADME in silico tools. Netw. Modeling Anal. Health Inform. Bioinform. 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Strong, J.M.; Zhang, L.; Reynolds, K.S.; Nallani, S.; Temple, R.; Lesko, L.J. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J. Clin. Pharmacol. 2008, 48, 662–670. [Google Scholar] [CrossRef]








| Peak No. | ESI-MS Rt (min) | Observed Mass (m/z) | Calculated Mass (m/z) | Error (ppm) | Molecular Formula | Key MSE Fragment Ions (m/z) | Identification |
|---|---|---|---|---|---|---|---|
| A | 6.13 | 288.148 | 288.136 | −0.5 | C17H20O4 | [M-CH3]+ 273.113, [M-2CH3]+ 259.089, [M-3CH3]+ 243.118, and [M-CO2CH3]+ 246.118 | 4,5-Dihydrofuranodienone |
| B | 6.35 | 231.155 | 231.131 | −1.8 | C15H18O2 | [M-CH3]+ 215.107, [M-2CH3]+ 200.084, and [M-3CH3]+ 185.023 | Furanodienone |
| C | 6.78 | 313.157 | 313.149 | −0.5 | C22H32O2 | [M-CH3]+ 297.222, [M-2CH3]+ 282.198, [M-3CH3]+ 267.175, [M-4CH3]+ 252.151, and [M-5CH3]+ 237.128, | Mansumbinone |
| D | 7.25 | 269.133 | 269.129 | −0.7 | C20H30 | [M-CH3]+ 258.235, [M-2CH3]+ 243.211, [M-3CH3]+ 228.188, [M-4CH3]+ 213.164, and [M-C3H5]+ 231.211, 41.039 | Cembrene-A |
| E | 6.93 | 404.421 | 404.417 | −0.6 | C28H48O | [M-CH3]+ 385.347, [M-2CH3]+ 370.324, [M-3CH3]+ 355.300, [M-4CH3]+ 340.277, [M-5CH3]+ 325.253, and [M-C9H19]+ 273.222, 127.149. | Campestan-3b-ol |
| F | 7.70 | 301.158 | 301.147 | −0.9 | C21H28O2 | [M-CH3]+ 301.217, [M-2CH3]+ 286.193, and [M-3CH3]+ 271.170. | Pregnadienes |
| G | 8.08 | 253.133 | 253.125 | −0.8 | C15H18O2 | [M-CH3]+ 238.131, [M-2CH3]+ 223.107, and [M-3CH3]+ 208.084. | Z,4Z-Furanodien-6-one |
| H | 8.64 | 418.7695 | 416 | −1.2 | C27H44O3 | [M-CH3]+ 374.266, [M-2CH3]+ 359.243, [M-3CH3]+ 355.300, [M-4CH3]+ 344.243, [M-5CH3]+ 329.219, and [M-C8H17]+ 273.222, 113.133. | Guggulsterols |
| I | 9.66 | 331.2109 | 331.2097 | −1.4 | C22H34O2 | [M-CH3]+ 315.232, [M-2CH3]+ 300.209, [M-3CH3]+ 285.185, and [M-C2H3O2]+ 271.243 | 3,4-Seco-mansumbinoic acid |
| Cancer Cell Line | IC50 ± SE | R2 |
|---|---|---|
| Leukemia (HL60) | 26.29 ± 7.09 | 0.957 |
| Leukemia (K562) | 88.27± 12.58 | 0.805 |
| Breast Cancer (KAIMRC1) | 95.73 ± 6.48 | 0.979 |
| Breast Cancer (MDA-MB-231) | 272.6 ± 6.561 | 0.976 |
| Colorectal Cancer (HCT8) | 150 ± 11.35 | 0.958 |
| Colorectal Cancer (HCT116) | 132.9 ± 4.90 | 0.988 |
| Normal Blood Sample (N1) | 27.59 ± 11.14 | 0.911 |
| Normal Primary Fibroblasts (P1) | 295.4 ± 15.98 | 0.927 |
| Compound Number (Name) | Predicted Activity | Pa | Pi |
|---|---|---|---|
| 1 (4,5-Dihydrofuranodienone) | Anti-inflammatory | 0.390 | 0.101 |
| Anti-oxidant | 0.324 | 0.019 | |
| Wound-healing agent | - | - | |
| Free radical scavenger | 0.284 | 0.034 | |
| Anti-neoplastic | 0.879 | 0.005 | |
| 2 (Furanodienone) | Anti-inflammatory | 0.449 | 0.073 |
| Anti-oxidant | 0.319 | 0.020 | |
| Wound-healing agent | - | - | |
| Free radical scavenger | 0.255 | 0.044 | |
| Anti-neoplastic | 0.788 | 0.013 | |
| 3 (Mansumbinone) | Anti-inflammatory | 0.506 | 0.055 |
| Anti-oxidant | 0.155 | 0.097 | |
| Wound-healing agent | 0.168 | 0.161 | |
| Free radical scavenger | - | - | |
| Anti-neoplastic | 0.814 | 0.010 | |
| 4 (Campestan-3b-ol) | Anti-inflammatory | 0.602 | 0.031 |
| Anti-oxidant | 0.157 | 0.095 | |
| Wound-healing agent | 0.197 | 0.121 | |
| Free radical scavenger | - | - | |
| Anti-neoplastic | 0.824 | 0.009 | |
| 5 (Cembrene-A) | Anti-inflammatory | 0.497 | 0.058 |
| Anti-oxidant | 0.181 | 0.068 | |
| Wound-healing agent | 0.416 | 0.020 | |
| Free radical scavenger | - | - | |
| Anti-neoplastic | 0.340 | 0.130 | |
| 6 (Pregnadienes) | Anti-inflammatory | 0.564 | 0.040 |
| Anti-oxidant | 0.171 | 0.078 | |
| Wound-healing agent | - | - | |
| Free radical scavenger | - | - | |
| Anti-neoplastic | 0.888 | 0.005 | |
| 7 (Z,4Z-Furanodien-6-one) | Anti-inflammatory | 0.449 | 0.073 |
| Anti-oxidant | 0.319 | 0.020 | |
| Wound-healing agent | - | - | |
| Free radical scavenger | 0.255 | 0.044 | |
| Anti-neoplastic | 0.788 | 0.013 | |
| 8 (Guggulsterols) | Anti-inflammatory | 0.559 | 0.041 |
| Anti-oxidant | 0.192 | 0.060 | |
| Wound-healing agent | 0.192 | 0.127 | |
| Free radical scavenger | - | - | |
| Anti-neoplastic | 0.593 | 0.047 | |
| 9 (3,4-Seco-mansumbinoic acid) | Anti-inflammatory | 0.510 | 0.054 |
| Anti-oxidant | 0.192 | 0.060 | |
| Wound-healing agent | - | - | |
| Free radical scavenger | - | - | |
| Anti-neoplastic | 0.735 | 0.020 |
| Compounds Number (Name) | GPCR Ligand | Ion Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor |
|---|---|---|---|---|---|---|
| Crude myrrh resin | ||||||
| 1 (4,5-Dihydrofuranodienone) | −0.20 | −0.23 | −0.77 | 0.04 | −0.48 | 0.30 |
| 2 (Furanodienone) | −0.45 | −0.42 | −0.96 | −0.27 | −0.85 | 0.05 |
| 3 (Mansumbinone) | 0.08 | −0.22 | −0.72 | 0.60 | −0.20 | 0.46 |
| 4 (Campestan-3b-ol) | 0.02 | 0.07 | −0.51 | 0.38 | −0.23 | 0.24 |
| 5 (Cembrene-A) | 0.13 | 0.13 | −0.46 | 0.78 | 0.08 | 0.56 |
| 6 (Pregnadienes) | −0.06 | −0.09 | −0.91 | 0.99 | −0.23 | 0.50 |
| 7 (Z,4Z-Furanodien-6-one) | −0.45 | −0.42 | −0.96 | −0.27 | −0.85 | 0.05 |
| 8 (Guggulsterols) | 0.20 | 0.08 | −0.60 | 0.99 | 0.12 | 0.71 |
| 9 (3,4-Seco-mansumbinoic acid) | 0.17 | 0.05 | −0.58 | 0.73 | −0.10 | 0.43 |
| Compound Number (Name) | SEA Search | Swiss Target | |||
|---|---|---|---|---|---|
| Predicted Targets | p Value | MaxTC | Predicted Targets | Probability | |
| 1 (4,5-Dihydrofuranodienone) | Glucocorticoid receptor Androgen receptor | Inactive Inactive | Inactive Inactive | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen receptor DNA topoisomerase I | NA NA NA 0.098 NA |
| 2 (Furanodienone) | Glucocorticoid receptor Androgen receptor | Inactive Inactive | Inactive Inactive | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen receptor DNA topoisomerase I | 0.049 NA 0.049 NA NA |
| 3 (Mansumbinone) | Glucocorticoid receptor Androgen Receptor | Inactive Inactive | Inactive Inactive | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen Receptor DNA topoisomerase I | NA 0.102 NA 0.102 NA |
| 4 (Campestan-3b-ol) | Glucocorticoid receptor Androgen receptor | Inactive Inactive | Inactive Inactive | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen receptor DNA topoisomerase I | NA NA NA NA NA |
| 5 (Cembrene-A) | Glucocorticoid receptor Androgen receptor | Inactive 4.441 × 10−16 | Inactive 0.53 | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen receptor DNA topoisomerase I | NA NA NA 0.631 NA |
| 6 (Pregnadienes) | Glucocorticoid receptor Androgen receptor | 1.121 × 10−12 1.121 × 10−12 | 1.00 1.00 | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen receptor DNA topoisomerase I | NA 1.0 NA 1.0 NA |
| 7 (Z,4Z-Furanodien-6-one) | Glucocorticoid receptor Androgen receptor | Inactive Inactive | Inactive Inactive | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen receptor DNA topoisomerase I | 0.049 NA 0.049 NA NA |
| 8 (Guggulsterols) | Glucocorticoid receptor Androgen receptor | 1.868 × 10−23 1.218 × 10−19 | 0.40 0.53 | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen Receptor DNA topoisomerase I | 0.119 0.473 NA 0.376 NA |
| 9 (3,4-Seco-mansumbinoic acid) | Glucocorticoid receptor Androgen Receptor | Inactive Inactive | Inactive Inactive | Cyclooxygenase-2 Glucocorticoid receptor Beta amyloid A4 protein Androgen receptor DNA topoisomerase I | 0.105 0.105 NA 0.113 0.105 |
| Compounds Number (Name) | Molecular Weight (g/mol | HB Donor | HB Acceptor | Log Po/w (WLOGP) | Log S (SILICO S-IT) | BBB Permeant | GI Absorption | Rule of Five (ROF) |
|---|---|---|---|---|---|---|---|---|
| Crude Myrrh Resin | ||||||||
| 1 (4,5-Dihydrofuranodienone) | 288.34 | 0 | 4 | 3.54 | −4.23 Moderately soluble | Yes | High | Yes; 0 violation |
| 2 (Furanodienone) | 230.30 | 0 | 2 | 4.00 | −4.40 Moderately soluble | Yes | High | Yes; 0 violation |
| 3 (Mansumbinone) | 312.49 | 0 | 1 | 5.71 | −5.18 Moderately soluble | No | High | Yes; 0 violation |
| 4 (Campestan-3b-ol) | 272.47 | 0 | 0 | 6.76 | −5.18 Moderately soluble | No | High | Yes; 0 violation |
| 5 (Cembrene-A) | 400.68 | 1 | 1 | 7.78 | −6.17 Poorly soluble | No | Low | Yes; 0 violation |
| 6 (Pregnadienes) | 312.45 | 0 | 2 | 4.64 | −4.58 Moderately soluble | Yes | High | Yes; 0 violation |
| 7 (Z,4Z-Furanodien-6-one) | 230.30 | 0 | 2 | 4.00 | −4.40 Moderately soluble | Yes | High | Yes; 0 violation |
| 8 (Guggulsterols) | 432.64 | 3 | 4 | 4.65 | −4.12 Moderately soluble | No | High | Yes; 0 violation |
| 9 (3,4-Seco-mansumbinoic acid) | 230.50 | 1 | 2 | 5.84 | −4.44 Moderately soluble | Yes | High | Yes; 0 violation |
| Compound Number (Name) | CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 |
|---|---|---|---|---|---|
| 1 (4,5-Dihydrofuranodienone) | No | Yes | No | No | No |
| 2 (Furanodienone) | Yes | No | No | No | No |
| 3 (Mansumbinone) | No | Yes | Yes | No | No |
| 4 (Campestan-3b-ol) | No | Yes | Yes | No | No |
| 5 (Cembrene-A) | No | No | No | No | No |
| 6 (Pregnadienes) | No | Yes | Yes | No | No |
| 7 (Z,4Z-Furanodien-6-one) | Yes | No | No | No | No |
| 8 (Guggulsterols) | No | No | No | No | No |
| 9 (3,4-Seco-mansumbinoic acid) | Yes | No | No | No | No |
| Compound Number (Name) | Oral Toxicity of Compounds | Prediction of Active Organ Toxicity/Toxicity Endpoints | Probability | |
|---|---|---|---|---|
| Predicted LD50 (mg/kg) | Predicted Toxicity Class | |||
| 1 (4,5-Dihydrofuranodienone) | 590 | 4 | Hepatotoxicity | 0.61 (Inactive) |
| Immunotoxicity | 0.73 (Inactive) | |||
| Carcinogenicity | 0.52 (Inactive) | |||
| Mutagenicity | 0.65 (Inactive) | |||
| 2 (Furanodienone) | 116 | 3 | Hepatotoxicity | 0.71 (Inactive) |
| Immunotoxicity | 0.96 (Inactive) | |||
| Carcinogenicity | 0.58 (Inactive) | |||
| Mutagenicity | 0.84 (Inactive) | |||
| 3 (Mansumbinone) | 15,000 | 6 | Hepatotoxicity | 0.70 (Inactive) |
| Immunotoxicity | 0.73 (Inactive) | |||
| Carcinogenicity | 0.57 (Inactive) | |||
| Mutagenicity | 0.97 (Inactive) | |||
| 4 (Campestan-3b-ol) | 4400 | 5 | Hepatotoxicity | 0.77 (Inactive) |
| Immunotoxicity | 0.93 (Inactive) | |||
| Carcinogenicity | 0.66 (Inactive) | |||
| Mutagenicity | 0.87 (Inactive) | |||
| 5 (Cembrene-A) | 890 | 4 | Hepatotoxicity | 0.87 (Inactive) |
| Immunotoxicity | 0.99 (Active) | |||
| Carcinogenicity | 0.60 (Inactive) | |||
| Mutagenicity | 0.98 (Inactive) | |||
| 6 (Pregnadienes) | 2300 | 5 | Hepatotoxicity | 0.67 (Inactive) |
| Immunotoxicity | 0.98 (Active) | |||
| Carcinogenicity | 0.56 (Active) | |||
| Mutagenicity | 0.99 (Inactive) | |||
| 7 (Z,4Z-Furanodien-6-one) | 116 | 3 | Hepatotoxicity | 0.71 (Inactive) |
| Immunotoxicity | 0.96 (Inactive) | |||
| Carcinogenicity | 0.58 (Inactive) | |||
| Mutagenicity | 0.84 (Inactive) | |||
| 8 (Guggulsterols) | 5010 | 6 | Hepatotoxicity | 0.82 (Inactive) |
| Immunotoxicity | 0.98 (Active) | |||
| Carcinogenicity | 0.66 (Active) | |||
| Mutagenicity | 0.67 (Inactive) | |||
| 9 (3,4-Seco-mansumbinoic acid) | 11,800 | 2 | Hepatotoxicity | 0.51 (Active) |
| Immunotoxicity | 0.93 (Inactive) | |||
| Carcinogenicity | 0.67 (Inactive) | |||
| Mutagenicity | 0.88 (Inactive) | |||
| Class 1: | Fatal if swallowed (LD50 ≤ 5) |
| Class 2: | Fatal if swallowed (5 < LD50 ≤ 50) |
| Class 3: | Toxic if swallowed (50 < LD50 ≤ 300) |
| Class 4: | Harmful if swallowed (300 < LD50 ≤ 2000) |
| Class 5: | It may be harmful if swallowed (2000 < LD50 ≤ 5000) |
| Class 6: | Non-toxic (LD50 > 5000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Aljatli, D.; Aljammaz, N.A.; Huwaizi, S.; Suliman, R.; Kahtani, K.M.; Albadrani, G.M.; Barhoumi, T.; et al. The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy. Pharmaceuticals 2022, 15, 944. https://doi.org/10.3390/ph15080944
Suliman RS, Alghamdi SS, Ali R, Aljatli D, Aljammaz NA, Huwaizi S, Suliman R, Kahtani KM, Albadrani GM, Barhoumi T, et al. The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy. Pharmaceuticals. 2022; 15(8):944. https://doi.org/10.3390/ph15080944
Chicago/Turabian StyleSuliman, Rasha Saad, Sahar Saleh Alghamdi, Rizwan Ali, Dimah Aljatli, Norah Abdulaziz Aljammaz, Sarah Huwaizi, Rania Suliman, Khawla Mohammed Kahtani, Ghadeer M. Albadrani, Tlili Barhoumi, and et al. 2022. "The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy" Pharmaceuticals 15, no. 8: 944. https://doi.org/10.3390/ph15080944
APA StyleSuliman, R. S., Alghamdi, S. S., Ali, R., Aljatli, D., Aljammaz, N. A., Huwaizi, S., Suliman, R., Kahtani, K. M., Albadrani, G. M., Barhoumi, T., Altolayyan, A., & Rahman, I. (2022). The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy. Pharmaceuticals, 15(8), 944. https://doi.org/10.3390/ph15080944