Skin Anti-Aging Potential of Ipomoea pes-caprae Ethanolic Extracts on Promoting Cell Proliferation and Collagen Production in Human Fibroblasts (CCD-986sk Cells)
Abstract
1. Introduction
2. Results
2.1. Chemical Profiles, Total Phenolic, Flavonoid, Tannin Contents of Ipomoea pes-caprae Extracts
2.2. Antioxidant and Collagenase Inhibitory Activities of Ipomoea pes-caprae Extracts
2.3. Cytotoxicity of Ipomoea pes-caprae Extracts on Human Fibroblasts (CCD-986sk Cells)
2.4. Ipomoea pes-caprae Extracts Induced Cell Proliferation
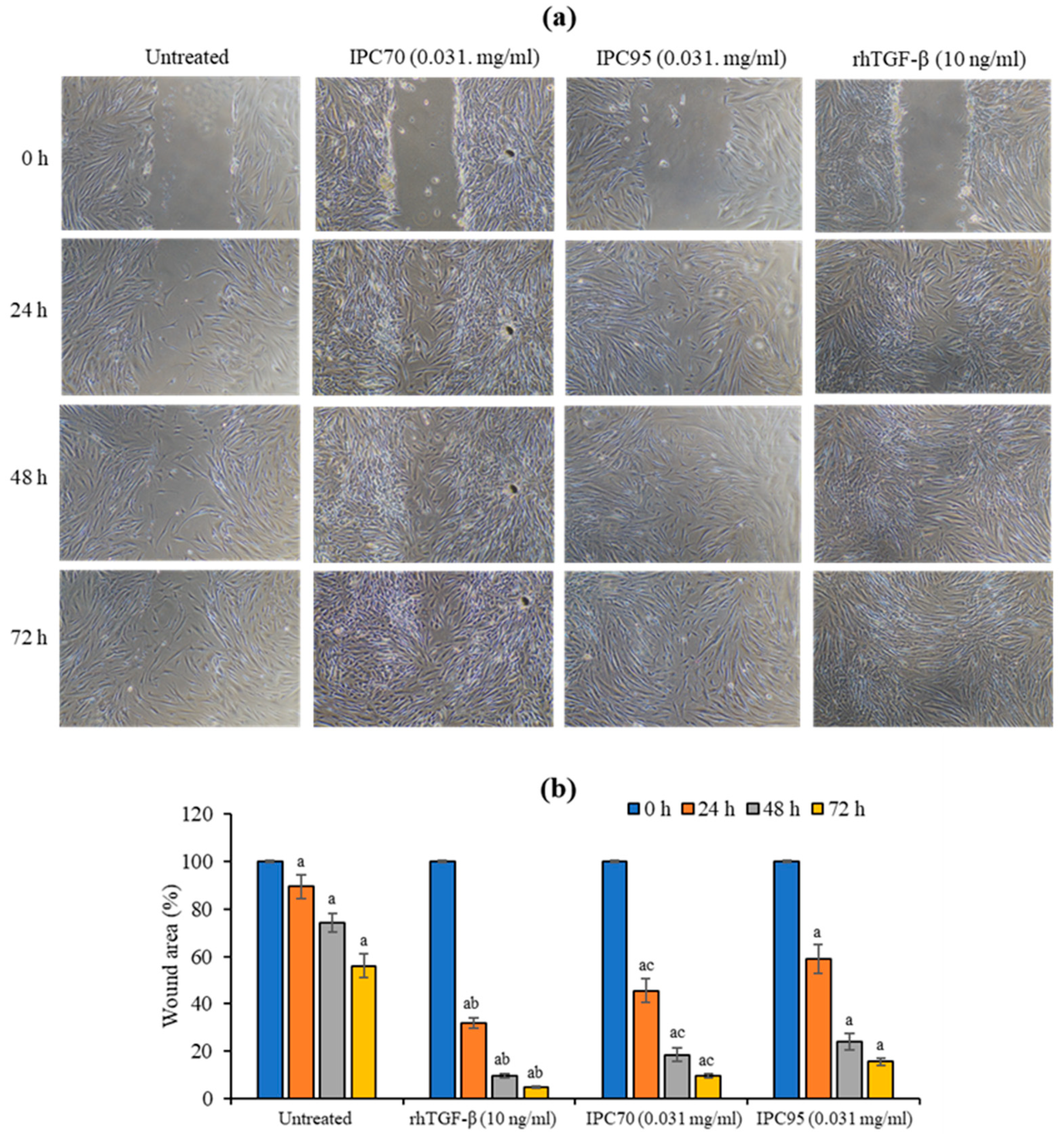
2.5. Ipomoea pes-caprae Extracts Induced In Vitro Wound Healing
2.6. Effect of Ipomoea pes-caprae Extracts on Production of Type I Collagen and Expression of COL1A1, TGFB1, and FGF2 Genes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials and Extraction
4.3. Determination of Total Phenolic Content
4.4. Determination of Flavonoid Content
4.5. Detection of Tannin Content
4.6. Phytochemical-Screening Assay by HPLC
4.7. Determination of Antioxidant Activity
4.7.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical-Scavenging Assay
4.7.2. 2,2′-Azinobis-(3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS) Radical-Scavenging Assay
4.7.3. Ferric-Reducing Antioxidant Power (FRAP)
4.8. Collagenase Activity Assay
4.9. Cell Viability Assay
4.10. Cell Proliferation Assay
4.11. In Vitro Scratch Wound Assay
4.12. Detection of Type I Collagen and Expression of Collagen Type I (COL1A1), Tumor Growth Factor-Beta 1 (TGFB1), and Beta-Fibroblast Growth Factor (FGF2) Genes
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gragnani, A.; Cornick, S.M.; Chominski, V.; de Noronha, S.M.R.; de Noronha, S.A.A.C.; Ferreira, L.M. Review of major theories of skin aging. Adv. Aging Res. 2014, 3, 265–284. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A minireview. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Lee, J.; Huh, S.; Kim, J.; Park, M.; So, J.; Ham, Y.; Jung, K.; Hyun, C.G.; et al. Panax ginseng induces human type I collagen synthesis through activation of Smad signaling. J. Ethnopharmacol. 2007, 109, 29–34. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.A.; Son, D.; Park, D.; Jung, E. Anti-skin-aging activity of a standardized extract from Panax ginseng leaves in vitro and in human volunteer. Cosmetics 2017, 4, 18. [Google Scholar] [CrossRef]
- Bindschadler, M.; McGrath, J.L. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J. Cell Sci. 2007, 120, 876–884. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Wasuwat, S. Extract of lpomoea pes-caprae (Convolvulaceae) antagonistic to histamine and jellyfish poison. Nature 1970, 225, 758. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Bohlin, L.; Soonthornsaratune, P.; Wasuwat, S. Anti-inflammatory activity of Ipomoea pes-caprae (L.) R. Br. Phytother. Res. 1991, 5, 63–66. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Bohlin, L.; Wasuwat, S. Neutralization of toxic effects of different crude jelly fish venoms by an extract of Ipomoea pes-caprae (L.). R. Br. J. Ethnopharmacol. 1991, 35, 65–69. [Google Scholar] [CrossRef]
- De Souza, M.M.; Madeira, A.; Berti, C.; Krogh, R.; Yunes, R.A.; Cechinel-Filho, V. Antinociceptive properties of the methanolic extract obtained from Ipomoea pes-caprae (L.) R.Br. J. Ethnopharmacol. 2000, 69, 85–90. [Google Scholar] [CrossRef]
- Akinniyi, G.; Lee, J.; Kim, H.; Lee, J.G.; Yang, I.A. Medicinal halophyte Ipomoea pes-caprae (Linn.) R. Br.: A review of its botany, traditional uses, phytochemistry, and bioactivity. Mar. Drugs 2022, 20, 329. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Santos, J.B.; Passos, J.G.R.; Gomes, J.A.S.; Cruz, J.V.C.; Alves, J.S.F.; Garcia, V.B.; da Silva, R.M.; Lopes, N.P.; Araujo-Junior, R.F.; Zucolotto, S.M.; et al. Topical gel containing phenolic-rich extract from Ipomoea pes-caprae leaf (Convolvulaceae) has anti-inflammatory, wound healing, and antiophidic properties. Biomed. Pharmacother. 2022, 149, 112921. [Google Scholar] [CrossRef]
- Nilam, R.; Jyoti, P.; Sumitra, C. Pharmacognostic and phytochemical studies of Ipomoea pes-caprae, an halophyte from Gujarat. J. Pharmacogn. Phytochem. 2018, 7, 11–18. [Google Scholar]
- Barth, C.S.; Tolentino de Souza, H.G.; Rocha, L.W.; da Silva, G.F.; dos Anjos, M.F.; Pastor, V.D.; Belle Bresolin, T.M.; Garcia Couto, A.; Roberto Santin, J.; Meira Quintao, N.L. Ipomoea pes-caprae (L.) R. Br (Convolvulaceae) relieved nociception and inflammation in mice—A topical herbal medicine against effects due to cnidarian venom-skin contact. J. Ethnopharmacol. 2017, 200, 156–164. [Google Scholar] [CrossRef]
- Venkataraman, N.D.; Atleeb, W.C.; Prabhuc, T.P.; Kannana, R. Anti-inflammatory potential of ethanolic extracts from aerial parts of Ipomoea pes-caprae (L.) R. Br using cotton pellet induced granuloma model. J. Appl. Pharm. Sci. 2013, 3, 61–63. [Google Scholar] [CrossRef]
- Ameamsri, U.; Tanee, T.; Chaveerach, A.; Peigneur, S.; Tytgat, J.; Sudmoond, R. Anti-inflammatory and detoxification activities of some Ipomoea species determined by ion channel inhibition and their phytochemical constituents. ScienceAsia 2021, 47, 321–329. [Google Scholar] [CrossRef]
- Teramachi, F.; Koyano, T.; Kowithayakor, T.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Collagenase inhibitory quinic acid ester from Ipomoea pes-caprae. J. Nat. Prod. 2005, 68, 794–796. [Google Scholar] [CrossRef]
- Lu, L.; Ying, K.; Wei, S.; Fang, Y.; Liu, Y.; Lin, H.; Ma, L.; Mao, Y. Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int. J. Dermatol. 2004, 43, 801–807. [Google Scholar] [CrossRef]
- Hormozi-Moghaddam, Z.; Mokhtari-Dizaji, M.; Nilforoshzadeh, M.A.; Bakhshandeh, M. Low-intensity ultrasound to induce proliferation and collagen I expression of adipose-derived mesenchymal stem cells and fibroblast cells in co-culture. Measurement 2021, 167, 108280. [Google Scholar] [CrossRef]
- Ignotz, R.A.; Massagué, J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar] [CrossRef]
- Xie, J.; Bian, H.; Qi, S.; Xu, Y.; Tang, J.; Li, T.; Liu, X. Effects of basic fibroblast growth factor on the expression of extracellular matrix and matrix metalloproteinase-1 in wound healing. Clin. Exp. Dermatol. 2008, 33, 176–182. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tanuja, Y.; Mishra, S.; Das, S.; Aggarwal, S.; Rani, V. Antioxidants and natural prevention of environmental toxicants induced accelerated aging of skin. Environ. Toxicol. Pharmacol. 2015, 39, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Meira, M.; Dasilva, E.P.; David, J.M.; David, J.P. Review of genus Ipomoea: Traditional uses, chemistry and biological activities. Braz. J. Pharmacogn. 2012, 22, 682–713. [Google Scholar] [CrossRef]
- Banerjee, D.; Hazra, A.K.; Chakraborti, S.; Ray, J.; Mukherjee, B. Variation of total phenolic content, flavonoid and radical scavenging activity of Ipomoea pes-caprae with respect to harvesting time and location. Int. J. Geo-Mar. Sci. 2013, 42, 106–109. [Google Scholar]
- Kumar, A.; Paul, S.; Pingalkumari, S.; Somasundarum, I.; Kathiresan, K. Antibacterial and phytochemical assessment of various extracts of Ipomoea pes-caprae (L) R Br. through FTIR and GC-MS spectroscopic analysis. Asian J. Pharm. Clin. Res. 2014, 7, 134–138. [Google Scholar]
- Malartna, A.; Etholsha, P. Phytochemical tests, antioxidant potential and TLC analysis of Ipomoea pes-caprae and Cathoranthus roseus. Int. J. Nat. Prod. Res. 2014, 4, 58–64. [Google Scholar]
- Kumar, A.; Paul, S.; Kumari, P.; Thirugnanasambandan, S.S.; Kathiresan, K. Antioxidant and free radical scavenging activities of Ipomoea pes-caprae (L.) R. Br. Extracts. Int. J. Curr. Pharm. Rev. Res. 2015, 5, 91–109. [Google Scholar]
- Sugimoto, K.; Nakagawa, K.; Hayashi, S.; Amakura, Y.; Yoshimura, M.; Yoshida, J.; Yamali, R.; Nakiano, Y.; Inui, H. Hydrolyzable tannins as antioxidants in the leaf extract of Eucalyptus globules possessing tyrosinase and hyaluronidase inhibitory activites. Food Sci. Technol. Res. 2009, 15, 331–336. [Google Scholar] [CrossRef]
- Antognoni, F.; Lianza, M.; Poli, F.; Buccioni, M.; Santinelli, C.; Caprioli, G.; Iannarelli, R.; Lupidi, G.; Damiani, E.; Beghelli, D.; et al. Polar extracts from the berry-like fruits of Hypericum androsaemum L. as a promising ingredient in skin care formulations. J. Ethnopharmacol. 2017, 195, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Girsang, E.; Lister, N.E.; Ginting, C.N.; Bethasari, M.; Amalia, A.; Widowati, W. Comparison of antiaging and antioxidant activities of protocatechuic and ferulic acids. Mol. Cell Biomed. Sci. 2020, 4, 68–75. [Google Scholar] [CrossRef]
- Thakur, R.; Jain, N.; Pathak, R.; Sandhu, S.S. Practices in wound healing studies of plants. Evid. Based Complement. Altern. Med. 2011, 2011, 438056. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, J.; Chen, M.; Guan, S.; Sawcer, D.; Bokoch, G.M.; Woodley, D.T. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet derived growth factor-BB. Mol. Biol. Cell 2004, 15, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Ermertcan, A.T.; Inan, S.; Ozturkcan, S.; Bilac, C.; Cilaker, S. Comparison of the effects of collagenase and extract of Centella asiatica in an experimental model of wound healing: An immunohistochemical and histopathological study. Wound Repair Regen. 2008, 16, 674–681. [Google Scholar] [CrossRef]
- Tsukahara, K.; Nakagawa, H.; Moriwaki, S.; Takema, Y.; Fujimura, T.; Imokawa, G. Inhibition of ultraviolet-B induced wrinkle formation by an elastase-inhibiting herbal extract: Implication for the mechanism underlying elastase-associated wrinkles. Int. J. Dermatol. 2006, 45, 460–468. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Li, T.S.C.; Mazza, G.; Cottrell, A.C.; Gao, L. Ginsenosides in roots and leaves of American ginseng. J. Agric. Food Chem. 1996, 44, 717–720. [Google Scholar] [CrossRef]
- Boraschi-Diaz, I.; Wang, J.; Mort, J.S.; Komarova, S.V. Collagen type I as a ligand for receptor-mediated signaling. Front. Phys. 2017, 5, 12. [Google Scholar] [CrossRef]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; El-Ammawi, T.S.; Medhat, W.; Moawad, O.; Mahoney, M.G.; Uitto, J. Expression of transforming growth factor- β after different non-invasive facial rejuvenation modalities. Int. J. Dermatol. 2015, 54, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Toshniwal, P.; Nguyen, M.; Guedin, A.; Viola, H.; Ho, D.; Kim, Y.; Bhatt, U.; Bond, C.S.; Hool, L.; Hurley, L.H.; et al. TGF-β-induced fibrotic stress increases G-quadruplex formation in human fibroblasts. FEBS Lett. 2019, 593, 3149–3161. [Google Scholar] [CrossRef]
- Panichakul, T.; Rodboon, T.; Suwannalert, P.; Tripetch, C.; Rungruang, R.; Boohuad, N.; Youdee, P. Additive effect of a combination of Artocarpus lakoocha and Glycyrrhiza glabra extracts on tyrosinase inhibition in melanoma B16 cells. Pharmaceuticals 2020, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Naima, S.; Muhammad, R.K.; Maria, S. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar]
- Govindappa, M.; Naga, S.; Poojashri, M.N.; Sadananda, T.S.; Chandrappa, C.P. Antimicrobial, antioxidant and in vitro anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L.) Hitchc. J. Pharmacogn. Phytother. 2011, 3, 43–51. [Google Scholar]
- Dutra, D.M.; Barth, C.d.S.; Block, L.C.; Quintao, N.L.M.; Couto, A.G.; Filho, V.C.; Bresolin, T.M.B. Simultaneous determination of four phenolic compounds in extracts of aerial parts of Ipomoea pes-caprae (L.) R.Br. (Convolvulaceae) by HPLC-UV. Quim. Nova 2014, 37, 1510–1514. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of Teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.W. Anti-aging activities of Pyrus pyrifolia var culta plant callus. Trop. J. Pharm. Res. 2017, 16, 1579–1588. [Google Scholar] [CrossRef][Green Version]
- Strand, D.W.; Liang, Y.Y.; Yang, F.; Barron, D.A.; Ressler, S.J.; Schauer, I.G.; Feng, X.H.; Rowley, D.R. TGF-β induction of FGF-2 expression in stromal cells requires integrated Smad3 and MAPK pathways. Am. J. Clin. Exp. Urol. 2014, 2, 239–248. [Google Scholar] [PubMed]
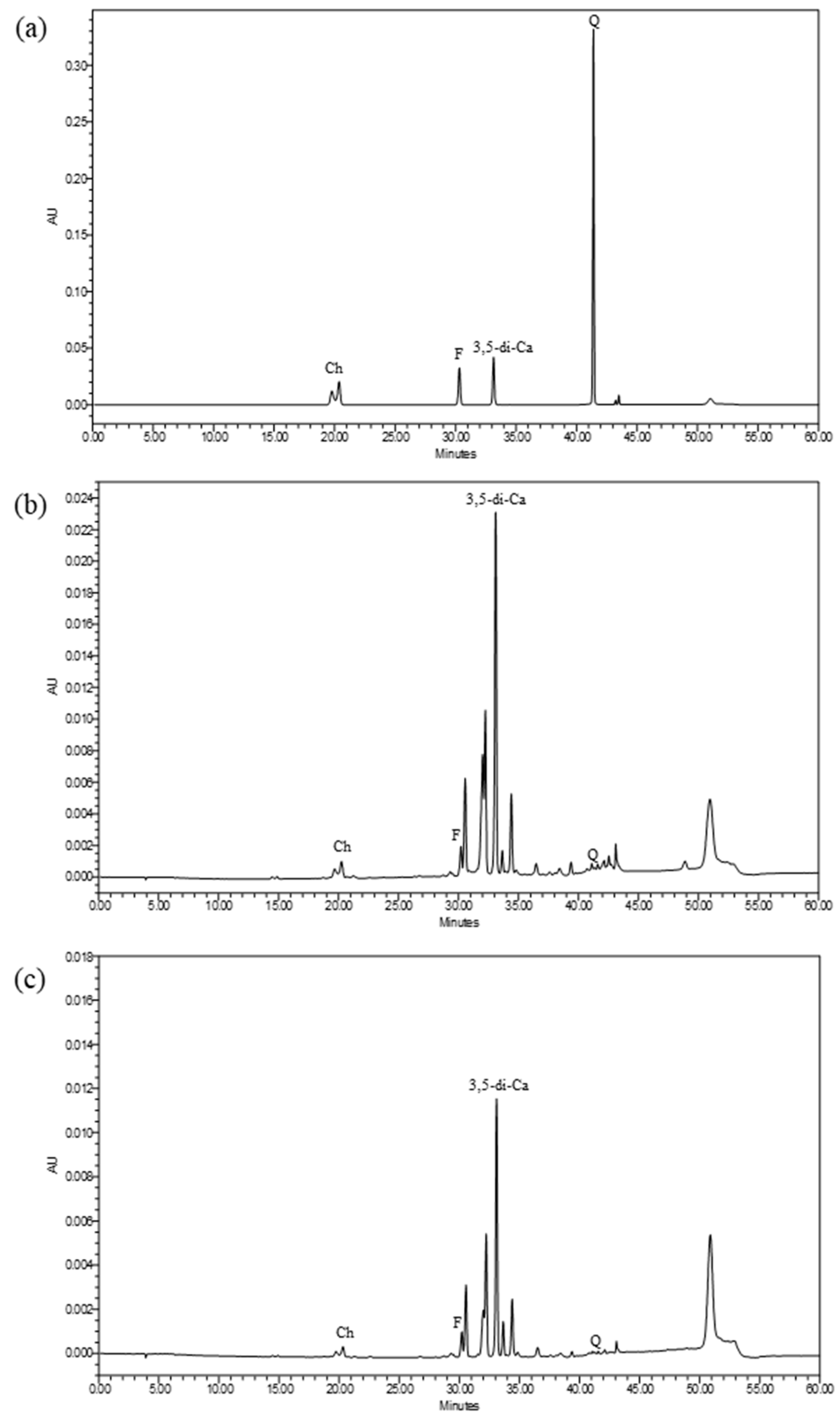



| Extracts | Total Phenolic Contents mg GAE/g Extract | Total Flavonoid Contents mg QE/g Extract | Total Tannin Contents mg TAE/g Extract |
|---|---|---|---|
| IPC70 | 75.315 ± 9.074 | 17.641 ± 0.966 | 18.39 ± 0.77 a |
| IPC95 | 67.095 ± 6.202 | 26.815 ±1.133 a | 6.57 ± 0.68 |
| Extracts | Antioxidant Activity | Collagenase Inhibitory Activity IC50 (mg/mL)/ (% of Inhibition) | ||
|---|---|---|---|---|
| DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | FRAP (µM TE/g Extract) | ||
| IPC70 | 0.342 ± 0.021 | 0.259 ± 0.070 a | 170,427.67 ± 1325.24 a | 5.541 ± 0.044 a |
| IPC95 | 0.403 ± 0.036 | 0.601 ± 0.057 | 53,530.40 ± 4587.89 | 12.179 ± 0.413 |
| L-ascorbic acid | 0.037 ± 0.002 | 0.034 ± 0.002 | - | - |
| 1,10-Phenanthroline (10 mM) | - | - | - | (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panichakul, T.; Ponnikorn, S.; Tupchiangmai, W.; Haritakun, W.; Srisanga, K. Skin Anti-Aging Potential of Ipomoea pes-caprae Ethanolic Extracts on Promoting Cell Proliferation and Collagen Production in Human Fibroblasts (CCD-986sk Cells). Pharmaceuticals 2022, 15, 969. https://doi.org/10.3390/ph15080969
Panichakul T, Ponnikorn S, Tupchiangmai W, Haritakun W, Srisanga K. Skin Anti-Aging Potential of Ipomoea pes-caprae Ethanolic Extracts on Promoting Cell Proliferation and Collagen Production in Human Fibroblasts (CCD-986sk Cells). Pharmaceuticals. 2022; 15(8):969. https://doi.org/10.3390/ph15080969
Chicago/Turabian StylePanichakul, Tasanee, Saranyoo Ponnikorn, Wipa Tupchiangmai, Woraphot Haritakun, and Kitima Srisanga. 2022. "Skin Anti-Aging Potential of Ipomoea pes-caprae Ethanolic Extracts on Promoting Cell Proliferation and Collagen Production in Human Fibroblasts (CCD-986sk Cells)" Pharmaceuticals 15, no. 8: 969. https://doi.org/10.3390/ph15080969
APA StylePanichakul, T., Ponnikorn, S., Tupchiangmai, W., Haritakun, W., & Srisanga, K. (2022). Skin Anti-Aging Potential of Ipomoea pes-caprae Ethanolic Extracts on Promoting Cell Proliferation and Collagen Production in Human Fibroblasts (CCD-986sk Cells). Pharmaceuticals, 15(8), 969. https://doi.org/10.3390/ph15080969






