Strategies and Tools for Supporting the Appropriateness of Drug Use in Older People
Abstract
:1. Introduction
2. Diagnosis and Medical Prescription in Older Adults
3. Medication Adherence
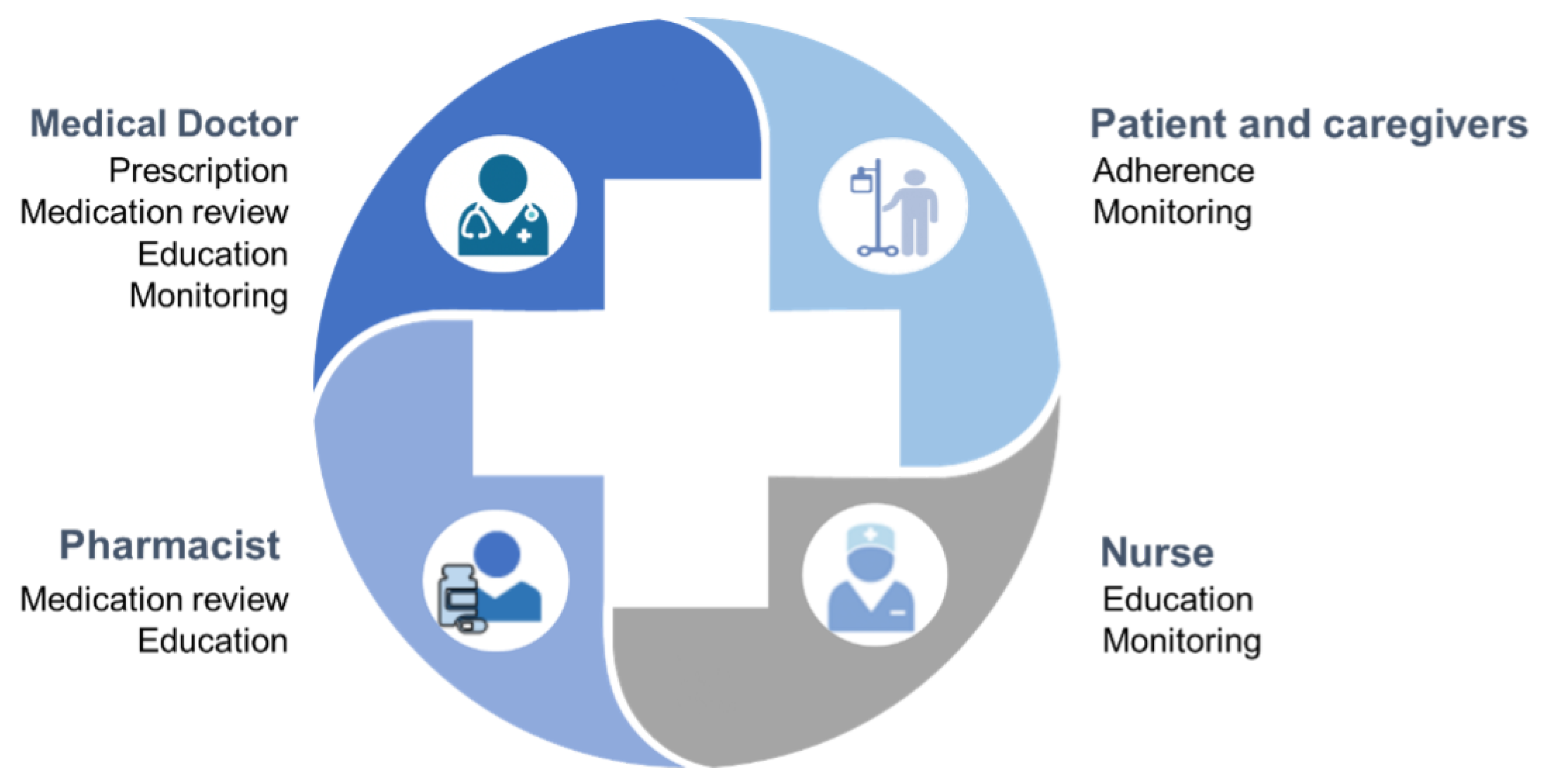
4. Strategies Supporting the Appropriateness of Medication Use
4.1. Prescriber’S Tools
4.1.1. Lists and Indexes
4.1.2. Medication Review in Team
4.1.3. Electronic Tools Supporting Appropriate Prescription
4.1.4. Web Resources on Adverse Reaction Prevention
4.2. Patient’s Tools
4.2.1. Digital Tools for the Patient
4.2.2. Dose-Dispensing Tools
5. Communication between Physician and Patient
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenzi, J.; Avaldi, V.M.; Rucci, P.; Pieri, G.; Fantini, M.P. Burden of multimorbidity in relation to age, gender and immigrant status: A cross-sectional study based on administrative data. BMJ Open 2016, 6, e012812. [Google Scholar] [CrossRef] [Green Version]
- Sirois, C.; Domingues, N.S.; Laroche, M.L.; Zongo, A.; Lunghi, C.; Guénette, L.; Kröger, E.; Émond, V. Polypharmacy Definitions for Multimorbid Older Adults Need Stronger Foundations to Guide Research, Clinical Practice and Public Health. Pharmacy 2019, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Midão, L.; Giardini, A.; Menditto, E.; Kardas, P.; Costa, E. Polypharmacy prevalence among older adults based on the survey of health, ageing and retirement in Europe. Arch. Gerontol. Geriatr. 2018, 78, 213–220. [Google Scholar] [CrossRef]
- Molino, C.d.G.R.C.; Chocano-Bedoya, P.O.; Sadlon, A.; Theiler, R.; Orav, J.E.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Kanis, J.A.; Guyonnet, S.; et al. Prevalence of polypharmacy in community-dwelling older adults from seven centres in five European countries: A cross-sectional study of DO-HEALTH. BMJ Open 2022, 12, e051881. [Google Scholar] [CrossRef]
- Onder, G.; Bonassi, S.; Abbatecola, A.M.; Folino-Gallo, P.; Lapi, F.; Marchionni, N.; Pani, L.; Pecorelli, S.; Sancarlo, D.; Scuteri, A.; et al. High Prevalence of Poor Quality Drug Prescribing in Older Individuals: A Nationwide Report From the Italian Medicines Agency (AIFA). J. Gerontol. Ser. Biomed. Sci. Med. Sci. 2014, 69, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, F.; Orlando, V.; Tari, D.U.; Di Giorgio, A.; Cittadini, A.; Trifirò, G.; Menditto, E. How healthy is community-dwelling elderly population? Results from Southern Italy. Transl. Med. UniSa 2015, 13, 59–64. [Google Scholar]
- Valent, F. Polypharmacy in the general population of a Northern Italian area: Analysis of administrative data. Ann. Dellístituto Super. Sanitá 2019, 55, 233–239. [Google Scholar] [CrossRef]
- Fickweiler, F.; Fickweiler, W.; Urbach, E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: A systematic review. BMJ Open 2017, 7, e016408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, S.E.; Figert, A.E. Medicalization and pharmaceuticalization at the intersections: Looking backward, sideways and forward. Soc. Sci. Med. 2012, 75, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J. Pharmaceuticalization of Society in Context: Theoretical, Empirical and Health Dimensions. Sociology 2010, 44, 603–622. [Google Scholar] [CrossRef]
- Ailabouni, N.J.; Hilmer, S.N.; Kalisch, L.; Braund, R.; Reeve, E. COVID-19 Pandemic: Considerations for Safe Medication Use in Older Adults with Multimorbidity and Polypharmacy. J. Gerontol. Ser. A 2021, 76, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Elbeddini, A.; Prabaharan, T.; Almasalkhi, S.; Tran, C.; Zhou, Y. Barriers to conducting deprescribing in the elderly population amid the COVID-19 pandemic. Res. Soc. Adm. Pharm. 2021, 17, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Campitelli, M.A.; Bronskill, S.E.; Maclagan, L.C.; Harris, D.A.; Cotton, C.A.; Tadrous, M.; Gruneir, A.; Hogan, D.B.; Maxwell, C.J. Comparison of Medication Prescribing Before and After the COVID-19 Pandemic Among Nursing Home Residents in Ontario, Canada. JAMA Netw. Open 2021, 4, e2118441. [Google Scholar] [CrossRef] [PubMed]
- Enners, S.; Gradl, G.; Kieble, M.; Böhm, M.; Laufs, U.; Schulz, M. Utilization of drugs with reports on potential efficacy or harm on COVID-19 before, during, and after the first pandemic wave. Pharmacoepidemiol. Drug Saf. 2021, 30, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Fried, T.R.; O’Leary, J.; Towle, V.; Goldstein, M.K.; Trentalange, M.; Martin, D.K. Health Outcomes Associated with Polypharmacy in Community-Dwelling Older Adults: A Systematic Review. J. Am. Geriatr. Soc. 2014, 62, 2261–2272. [Google Scholar] [CrossRef] [Green Version]
- Steinman, M.A.; Seth Landefeld, C.; Rosenthal, G.E.; Berthenthal, D.; Sen, S.; Kaboli, P.J. Polypharmacy and Prescribing Quality in Older People: Polypharmacy and prescribing quality. J. Am. Geriatr. Soc. 2006, 54, 1516–1523. [Google Scholar] [CrossRef]
- Onder, G.; Vetrano, D.L.; Palmer, K.; Trevisan, C.; Amato, L.; Berti, F.; Campomori, A.; Catalano, L.; Corsonello, A.; Kruger, P.; et al. Italian guidelines on management of persons with multimorbidity and polypharmacy. Aging Clin. Exp. Res. 2022, 34, 989–996. [Google Scholar] [CrossRef]
- Chisaki, Y.; Aoji, S.; Yano, Y. Analysis of Adverse Drug Reaction Risk in Elderly Patients Using the Japanese Adverse Drug Event Report (JADER) Database. Biol. Pharm. Bull. 2017, 40, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Oscanoa, T.J.; Lizaraso, F.; Carvajal, A. Hospital admissions due to adverse drug reactions in the elderly: A meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 759–770. [Google Scholar] [CrossRef]
- Bloomfield, H.E.; Greer, N.; Linsky, A.M.; Bolduc, J.; Naidl, T.; Vardeny, O.; MacDonald, R.; McKenzie, L.; Wilt, T.J. Deprescribing for Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2020, 35, 3323–3332. [Google Scholar] [CrossRef]
- Kua, C.H.; Yeo, C.Y.Y.; Tan, P.C.; Char, C.W.T.; Tan, C.W.Y.; Mak, V.; Leong, I.Y.O.; Lee, S.W.H. Association of Deprescribing With Reduction in Mortality and Hospitalization: A Pragmatic Stepped-Wedge Cluster-Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2021, 22, 82–89.e3. [Google Scholar] [CrossRef] [PubMed]
- Muth, C.; Blom, J.W.; Smith, S.M.; Johnell, K.; Gonzalez-Gonzalez, A.I.; Nguyen, T.S.; Brueckle, M.S.; Cesari, M.; Tinetti, M.E.; Valderas, J.M. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: A systematic guideline review and expert consensus. J. Intern. Med. 2019, 285, 272–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucalo, I.; Hadžiabdić, M.O.; Brajković, A.; Lukić, S.; Marić, P.; Marinović, I.; Bačić-Vrca, V. Potentially inappropriate medicines in elderly hospitalised patients according to the EU(7)-PIM list, STOPP version 2 criteria and comprehensive protocol. Eur. J. Clin. Pharmacol. 2017, 73, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Debacq, C.; Bourgueil, J.; Aidoud, A.; Bleuet, J.; Mennecart, M.; Dardaine-Giraud, V.; Fougère, B. Persistence of Effect of Medication Review on Potentially Inappropriate Prescriptions in Older Patients Following Hospital Discharge. Drugs Aging 2021, 38, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, E.K.; Drey, M.; Pudritz, Y.M. Influence of a ward-based pharmacist on the medication quality of geriatric inpatients: A before–after study. Int. J. Clin. Pharm. 2022, 44, 480–488. [Google Scholar] [CrossRef]
- Shiber, S.; Zuker-Herman, R.; Drescher, M.J.; Glezerman, M. Gender differences in the comprehension of care plans in an emergency department setting. Isr. J. Health Policy Res. 2018, 7, 50. [Google Scholar] [CrossRef]
- Kerse, N.; Buetow, S.; Mainous, A.G.; Young, G.; Coster, G.; Arroll, B. Physician-patient relationship and medication compliance: A primary care investigation. Ann. Fam. Med. 2004, 2, 455–461. [Google Scholar] [CrossRef]
- Neri, L.; Peris, K.; Longo, C.; Calvieri, S.; Frascione, P.; Parodi, A.; Eibenschuz, L.; Bottoni, U.; Pellacani, G. Physician-patient communication and patient-reported outcomes in the actinic keratosis treatment adherence initiative (AK-TRAIN): A multicenter, prospective, real-life study of treatment satisfaction, quality of life and adherence to topical field-directed therapy for the treatment of actinic keratosis in Italy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.H. Potential for physician communication to build favorable medication beliefs among older adults with hypertension: A cross-sectional survey. PLoS ONE 2019, 14, e0210169. [Google Scholar] [CrossRef] [Green Version]
- Calkins, D.R.; Davis, R.B.; Reiley, P.; Phillips, R.S.; Pineo, K.L.; Delbanco, T.L.; Iezzoni, L.I. Patient-physician communication at hospital discharge and patients’ understanding of the postdischarge treatment plan. Arch. Intern. Med. 1997, 157, 1026–1030. [Google Scholar] [CrossRef]
- Makaryus, A.N.; Friedman, E.A. Patients’ understanding of their treatment plans and diagnosis at discharge. Mayo Clin. Proc. 2005, 80, 991–994. [Google Scholar] [CrossRef] [Green Version]
- Samuels-Kalow, M.E.; Stack, A.M.; Porter, S.C. Effective Discharge Communication in the Emergency Department. Ann. Emerg. Med. 2012, 60, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Counselman, F.L.; Caggiano, C.D. Emergency department discharge instructions and patient literacy: A problem of disparity. Am. J. Emerg. Med. 1996, 14, 19–22. [Google Scholar] [CrossRef]
- McInnes, G.T. Integrated approaches to management of hypertension: Promoting treatment acceptance. Am. Heart J. 1999, 138, S252–S255. [Google Scholar] [CrossRef]
- Shariff, Z.B.; Dahmash, D.T.; Kirby, D.J.; Missaghi, S.; Rajabi-Siahboomi, A.; Maidment, I.D. Does the Formulation of Oral Solid Dosage Forms Affect Acceptance and Adherence in Older Patients? A Mixed Methods Systematic Review. J. Am. Med. Dir. Assoc. 2020, 21, 1015–1023.e8. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; Sabaté, E., Ed.; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Lindquist, L.A.; Go, L.; Fleisher, J.; Jain, N.; Friesema, E.; Baker, D.W. Relationship of Health Literacy to Intentional and Unintentional Non-Adherence of Hospital Discharge Medications. J. Gen. Intern. Med. 2012, 27, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.; Mainous Iii, A.G.; Gray, D.P.; Love, M.M. Exploration of the relationship between continuity, trust in regular doctors and patient satisfaction with consultations with family doctors. Scand. J. Prim. Health Care 2003, 21, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Ridd, M.; Shaw, A.; Lewis, G.; Salisbury, C. The patient–doctor relationship: A synthesis of the qualitative literature on patients’ perspectives. Br. J. Gen. Pract. 2009, 59, e116–e133. [Google Scholar] [CrossRef]
- Thom, D.H.; Kravitz, R.L.; Bell, R.A.; Krupat, E.; Azari, R. Patient trust in the physician: Relationship to patient requests. Fam. Pract. 2002, 19, 476–483. [Google Scholar] [CrossRef] [Green Version]
- Clyne, B.; Cooper, J.A.; Boland, F.; Hughes, C.M.; Fahey, T.; Smith, S.M. Beliefs about prescribed medication among older patients with polypharmacy: A mixed methods study in primary care. Br. J. Gen. Pract. 2017, 67, e507–e518. [Google Scholar] [CrossRef] [Green Version]
- Leonard, M.B.; Pursley, D.M.; Robinson, L.A.; Abman, S.H.; Davis, J.M. The importance of trustworthiness: Lessons from the COVID-19 pandemic. Pediatr. Res. 2022, 91, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Mielke, N.; Huscher, D.; Douros, A.; Ebert, N.; Gaedeke, J.; van der Giet, M.; Kuhlmann, M.K.; Martus, P.; Schaeffner, E. Self-reported medication in community-dwelling older adults in Germany: Results from the Berlin Initiative Study. BMC Geriatr. 2020, 20, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiMatteo, M.R.; Giordani, P.J.; Lepper, H.S.; Croghan, T.W. Patient adherence and medical treatment outcomes: A meta-analysis. Med. Care 2002, 40, 794–811. [Google Scholar] [CrossRef]
- Marcum, Z.A.; Hanlon, J.T.; Murray, M.D. Improving Medication Adherence and Health Outcomes in Older Adults: An Evidence-Based Review of Randomized Controlled Trials. Drugs Aging 2017, 34, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcum, Z.A.; Gellad, W.F. Medication Adherence to Multidrug Regimens. Clin. Geriatr. Med. 2012, 28, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Cooper, V.; Metcalf, L.; Versnel, J.; Upton, J.; Walker, S.; Horne, R. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: A UK-wide, cross-sectional study. Npj Prim. Care Respir. Med. 2015, 25, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Koya, T.; Hasegawa, T.; Ueno, H.; Yoshizawa, K.; Kimura, Y.; Hayashi, M.; Watanabe, S.; Kikuchi, T. Characterization of low adherence population in asthma patients from Japan using Adherence Starts with Knowledge-12. Allergol. Int. 2020, 69, 61–65. [Google Scholar] [CrossRef]
- Pazan, F.; Kather, J.; Wehling, M. A systematic review and novel classification of listing tools to improve medication in older people. Eur. J. Clin. Pharmacol. 2019, 75, 619–625. [Google Scholar] [CrossRef]
- American Geriatrics Society. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2014, 44, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.M.; Chen, M.J.; Tsai, C.Y.; Ho, L.H.; Hsieh, H.L.; Chau, Y.L.; Liu, C.Y. Medical conditions and medications as risk factors of falls in the inpatient older people: A case–control study. Int. J. Geriatr. Psychiatry 2011, 26, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.; Schmiedl, S.; Thürmann, P.A. Potentially Inappropriate Medications in the Elderly: The PRISCUS List. Dtsch. Arztebl. Int. 2010, 107, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.L.; Charmes, J.P.; Merle, L. Potentially inappropriate medications in the elderly: A French consensus panel list. Eur. J. Clin. Pharmacol. 2007, 63, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Lavan, A.H.; Gallagher, P.; Parsons, C.; O’Mahony, D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): Consensus validation. Age Ageing 2017, 46, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Rancourt, C.; Moisan, J.; Baillargeon, L.; Verreault, R.; Laurin, D.; Grégoire, J.P. Potentially inappropriate prescriptions for older patients in long-term care. BMC Geriatr. 2004, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Renom-Guiteras, A.; Meyer, G.; Thürmann, P.A. The EU(7)-PIM list: A list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur. J. Clin. Pharmacol. 2015, 71, 861–875. [Google Scholar] [CrossRef] [Green Version]
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Kuhn-Thiel, A.M.; Weiß, C.; Wehling, M.; FORTA Authors/Expert Panel Members. Consensus validation of the FORTA (Fit fOR The Aged) List: A clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging 2014, 31, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Perpétuo, C.; Plácido, A.I.; Rodrigues, D.; Aperta, J.; Piñeiro-Lamas, M.; Figueiras, A.; Herdeiro, M.T.; Roque, F. Prescription of Potentially Inappropriate Medication in Older Inpatients of an Internal Medicine Ward: Concordance and Overlap Among the EU(7)-PIM List and Beers and STOPP Criteria. Front. Pharmacol. 2021, 12, 676020. [Google Scholar] [CrossRef]
- Roux, B.; Berthou-Contreras, J.; Beuscart, J.B.; Charenton-Blavignac, M.; Doucet, J.; Fournier, J.P.; de la Gastine, B.; Gautier, S.; Gonthier, R.; Gras, V.; et al. REview of potentially inappropriate MEDIcation pr[e]scribing in Seniors (REMEDI[e]S): French implicit and explicit criteria. Eur. J. Clin. Pharmacol. 2021, 77, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, G.; Straand, J.; Klovning, A.; Brekke, M. The Norwegian General Practice–Nursing Home criteria (NORGEP-NH) for potentially inappropriate medication use: A web-based Delphi study. Scand. J. Prim. Health Care 2015, 33, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fialová, D.; Brkić, J.; Laffon, B.; Reissigová, J.; Grešáková, S.; Dogan, S.; Doro, P.; Tasić, L.; Marinković, V.; Valdiglesias, V.; et al. Applicability of EU(7)-PIM criteria in cross-national studies in European countries. Ther. Adv. Drug Saf. 2019, 10, 2042098619854014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshehri, A.A.; Jalal, Z.; Cheema, E.; Haque, M.S.; Jenkins, D.; Yahyouche, A. Impact of the pharmacist-led intervention on the control of medical cardiovascular risk factors for the primary prevention of cardiovascular disease in general practice: A systematic review and meta-analysis of randomised controlled trials. Br. J. Clin. Pharmacol. 2020, 86, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loots, E.; Goossens, E.; Vanwesemael, T.; Morrens, M.; Van Rompaey, B.; Dilles, T. Interventions to Improve Medication Adherence in Patients with Schizophrenia or Bipolar Disorders: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10213. [Google Scholar] [CrossRef]
- Tho, P.C.; Ang, E. The effectiveness of patient navigation programs for adult cancer patients undergoing treatment: A systematic review. JBI Evid. Synth. 2016, 14, 295–321. [Google Scholar] [CrossRef]
- Laberge, M.; Sirois, C.; Lunghi, C.; Gaudreault, M.; Nakamura, Y.; Bolduc, C.; Laroche, M.L. Economic Evaluations of Interventions to Optimize Medication Use in Older Adults with Polypharmacy and Multimorbidity: A Systematic Review. Clin. Interv. Aging 2021, 16, 767–779. [Google Scholar] [CrossRef]
- Eysenbach, G. What is e-health? J. Med. Internet Res. 2001, 3, e20. [Google Scholar] [CrossRef]
- Kampmeijer, R.; Pavlova, M.; Tambor, M.; Golinowska, S.; Groot, W. The use of e-health and m-health tools in health promotion and primary prevention among older adults: A systematic literature review. BMC Health Serv. Res. 2016, 16, 290. [Google Scholar] [CrossRef] [Green Version]
- Linn, N.; Goetzinger, C.; Regnaux, J.P.; Schmitz, S.; Dessenne, C.; Fagherazzi, G.; Aguayo, G.A. Digital Health Interventions among People Living with Frailty: A Scoping Review. J. Am. Med. Dir. Assoc. 2021, 22, 1802–1812.e21. [Google Scholar] [CrossRef]
- Laverty, H.; Benson, C.; Cartwright, E.; Cross, M.; Garland, C.; Hammond, T.; Holloway, C.; McMahon, N.; Milligan, J.; Park, B.; et al. How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines?: Cardiovascular toxicity of medicines. Br. J. Pharmacol. 2011, 163, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016, 14, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, P.J.; Woosley, R.L. Predicting the Unpredictable. J. Am. Coll. Cardiol. 2006, 67, 1639–1650. [Google Scholar] [CrossRef]
- Poluzzi, E.; Raschi, E.; Diemberger, I.; De Ponti, F. Drug-Induced Arrhythmia: Bridging the Gap Between Pathophysiological Knowledge and Clinical Practice. Drug Saf. 2017, 40, 461–464. [Google Scholar] [CrossRef]
- Gatti, M.; Raschi, E.; Poluzzi, E.; Martignani, C.; Salvagni, S.; Ardizzoni, A.; Diemberger, I. The Complex Management of Atrial Fibrillation and Cancer in the COVID-19 Era: Drug Interactions, Thromboembolic Risk, and Proarrhythmia. Curr. Heart Fail. Rep. 2020, 17, 365–383. [Google Scholar] [CrossRef]
- Skullbacka, S.; Airaksinen, M.; Puustinen, J.; Toivo, T. Risk assessment tools for QT prolonging pharmacotherapy in older adults: A systematic review. Eur. J. Clin. Pharmacol. 2022, 78, 765–779. [Google Scholar] [CrossRef]
- Hayashi, P.H.; Lucena, M.I.; Fontana, R.J.; Bjornsson, E.S.; Aithal, G.P.; Barnhart, H.; Gonzalez-Jimenez, A.; Yang, Q.; Gu, J.; Andrade, R.J.; et al. A revised electronic version of RUCAM for the diagnosis of DILI. Hepatology 2022, 76, 18–31. [Google Scholar] [CrossRef]
- Backes, C.; Moyano, C.; Rimaud, C.; Bienvenu, C.; Schneider, M.P. Digital Medication Adherence Support: Could Healthcare Providers Recommend Mobile Health Apps? Front. Med. Technol. 2021, 2, 616242. [Google Scholar] [CrossRef]
- Sourbati, M.; Loos, E.F. Interfacing age: Diversity and (in)visibility in digital public service. J. Digit. Media Policy 2019, 10, 275–293. [Google Scholar] [CrossRef]
- Matthew-Maich, N.; Harris, L.; Ploeg, J.; Markle-Reid, M.; Valaitis, R.; Ibrahim, S.; Gafni, A.; Isaacs, S. Designing, Implementing, and Evaluating Mobile Health Technologies for Managing Chronic Conditions in Older Adults: A Scoping Review. JMIR mHealth uHealth 2016, 4, e29. [Google Scholar] [CrossRef] [PubMed]
- Gulliford, M.; Alageel, S. Digital health intervention at older ages. Lancet Digit. Health 2019, 1, e382–e383. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, O.; Weinman, J.; Jackson, S.H.D. Intentional Non-Adherence to Medications by Older Adults. Drugs Aging 2014, 31, 149–157. [Google Scholar] [CrossRef]
- Amorim, W.W.; Passos, L.C.; Gama, R.S.; Souza, R.M.; Oliveira, M.G. Factors associated with older patients’ misunderstandings of medication dosage regimen instructions after consultation in primary care in Brazil. J. Eval. Clin. Pract. 2021, 27, 817–825. [Google Scholar] [CrossRef]
- Mertens, B.J.; Kwint, H.F.; van Marum, R.J.; Bouvy, M.L. Are multidose drug dispensing systems initiated for the appropriate patients? Eur. J. Clin. Pharmacol. 2018, 74, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Hersberger, K.E.; Boeni, F.; Arnet, I. Dose-dispensing service as an intervention to improve adherence to polymedication. Expert Rev. Clin. Pharmacol. 2013, 6, 413–421. [Google Scholar] [CrossRef]
- Levings, B.; Szep, S.; Helps, S. Towards the safer use of dosettes. J. Qual. Clin. Pract. 1999, 19, 69–72. [Google Scholar] [CrossRef]
- Smith, D.L. Compliance Packaging: A Patient Education Tool. Am. Pharm. 1989, 29, 42–53. [Google Scholar] [CrossRef]
- Schwartz, J.K. Pillbox use, satisfaction, and effectiveness among persons with chronic health conditions. Assist. Technol. 2017, 29, 181–187. [Google Scholar] [CrossRef]
- Barton, E.; Twining, L.; Walters, L. Understanding the decision to commence a dose administration aid. Aust. Fam. Physician 2017, 46, 943–947. [Google Scholar]
- Straka, I.; Minar, M.; Grofik, M.; Skorvanek, M.; Bolekova, V.; Gazova, A.; Kyselovic, J.; Valkovic, P. Effect of Pillbox Organizers with Alarms on Adherence to Pharmacotherapy in Parkinson Disease Patients Taking Three and More Daily Doses of Dopaminergic Medications. J. Pers. Med. 2022, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Boeni, F.; Spinatsch, E.; Suter, K.; Hersberger, K.E.; Arnet, I. Effect of drug reminder packaging on medication adherence: A systematic review revealing research gaps. Syst. Rev. 2014, 3, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stip, E.; Vincent, P.D.; Sablier, J.; Guevremont, C.; Zhornitsky, S.; Tranulis, C. A randomized controlled trial with a Canadian electronic pill dispenser used to measure and improve medication adherence in patients with schizophrenia. Front. Pharmacol. 2013, 4, 100. [Google Scholar] [CrossRef] [Green Version]
- Zedler, B.K.; Kakad, P.; Colilla, S.; Murrelle, L.; Shah, N.R. Does Packaging with a Calendar Feature Improve Adherence to Self-Administered Medication for Long-Term Use? A Systematic Review. Clin. Ther. 2011, 33, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Beckman, H.B. The Effect of Physician Behavior on the Collection of Data. Ann. Intern. Med. 1984, 101, 692. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, J.A.; Herrera, J.L. The Role of Effective Clinician-Patient Communication in the Management of Irritable Bowel Syndrome and Chronic Constipation. J. Clin. Gastroenterol. 2012, 46, 748–751. [Google Scholar] [CrossRef]
- Singh Ospina, N.; Phillips, K.A.; Rodriguez-Gutierrez, R.; Castaneda-Guarderas, A.; Gionfriddo, M.R.; Branda, M.E.; Montori, V.M. Eliciting the Patient’s Agenda- Secondary Analysis of Recorded Clinical Encounters. J. Gen. Intern. Med. 2019, 34, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Roter, D.L.; Hall, J.A.; Aoki, Y. Physician Gender Effects in Medical Communication: A Meta-analytic Review. JAMA 2002, 288, 756. [Google Scholar] [CrossRef]
- Vogliotti, E.; Pintore, G.; Zoccarato, F.; Biasin, M.; Sergi, G.; Inelmen, E.M.; Trevisan, C. Communicating Bad News to Older Patients from the Physician’s Point of View: Focus on the Influence of Gender and Length of Work Experience. Gerontology 2021, 1–7. [Google Scholar] [CrossRef]
- Cooper, L.A.; Roter, D.L.; Johnson, R.L.; Ford, D.E.; Steinwachs, D.M.; Powe, N.R. Patient-Centered Communication, Ratings of Care, and Concordance of Patient and Physician Race. Ann. Intern. Med. 2003, 139, 907. [Google Scholar] [CrossRef]
- Mira, J.J.; Orozco-Beltran, D.; Perez-Jover, V.; Martinez-Jimeno, L.; Gil-Guillen, V.F.; Carratala-Munuera, C.; Sanchez-Molla, M.; Pertusa-Martinez, S.; Asencio-Aznar, A. Physician patient communication failure facilitates medication errors in older polymedicated patients with multiple comorbidities. Fam. Pract. 2013, 30, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Advinha, A.M.; Lopes, M.J.; de Oliveira-Martins, S. Assessment of the elderly’s functional ability to manage their medication: A systematic literature review. Int. J. Clin. Pharm. 2017, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Noh, Y.; Kang, H.; Hong, S.H. The mediatory role of medication adherence in improving patients’ medication experience through patient-physician communication among older hypertensive patients. Patient Prefer. Adherence 2017, 11, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Marcum, Z.A.; Pugh, M.J.V.; Amuan, M.E.; Aspinall, S.L.; Handler, S.M.; Ruby, C.M.; Hanlon, J.T. Prevalence of Potentially Preventable Unplanned Hospitalizations Caused by Therapeutic Failures and Adverse Drug Withdrawal Events among Older Veterans. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Ozavci, G.; Bucknall, T.; Woodward-Kron, R.; Hughes, C.; Jorm, C.; Joseph, K.; Manias, E. A systematic review of older patients’ experiences and perceptions of communication about managing medication across transitions of care. Res. Soc. Adm. Pharm. 2021, 17, 273–291. [Google Scholar] [CrossRef]
| Tools | Description | Examples |
|---|---|---|
| Prescriber’s tools | ||
| Lists and indexes | Lists of medications to be or not to be used based on efficacy, safety, and appropriateness | Beers criteria; START/STOPP * criteria; FORTA * list; REMEDI[e]S *; PRISCUS list; NORGEP-NH criteria; Eu(7)-PIM list |
| General appropriateness indexes | MAI * | |
| Electronic tools | DHIs * providing recommendations, videos, and apps for specific therapeutic areas | www.deprescribing.org, accessed on 27 July 2022 |
| Websites and bookshelves supporting patients and prescribers in therapy optimization | www.medstopper.com, accessed on 27 July 2022; www.drugs.com, accessed on 27 July 2022 [folder: interaction checker]; www.intercheckweb.marionegri.it, accessed on 27 July 2022 | |
| Web resources on adverse drug reactions | Websites and bookshelves supporting patients and prescribers in therapy optimization | www.crediblemeds.org, accessed on 27 July 2022; www.ncbi.nlm.nih.gov/books/NBK547852/, accessed on 27 July 2022 |
| RATs * and diagnostic algorithms to identify patients at risk for adverse reactions | Risk scores; computerized physician order entry systems; clinical decision support systems; RECAM * | |
| Patient’s tools | ||
| Digital tools for the patients | Mobile applications facilitating communications between patients and physicians | Apps reporting suspect adverse reactions, adherence information, and vital signs parameters directly to the electronic healthcare record |
| DHIs * helping patients and caregivers adhering to treatment | Apps reminding the patient that a pill should be taken at a specific time; apps making more accessible information included in the package insert | |
| DHIs * for information sharing among different stakeholders | Apps that remind the patient to take pills, alert the caregiver in case of omission, and show the adherence interpolated with biomarker data in the electronic healthcare record for the physician | |
| Dose-dispensing tools | Dose-dispensing services for patients experiencing unintentional non-adherence | Pillboxes with seven compartments for each day of the week; pillboxes with visual and sound alarms |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunghi, C.; Trevisan, C.; Fusaroli, M.; Giunchi, V.; Raschi, E.; Sangiorgi, E.; Domenicali, M.; Volpato, S.; De Ponti, F.; Poluzzi, E. Strategies and Tools for Supporting the Appropriateness of Drug Use in Older People. Pharmaceuticals 2022, 15, 977. https://doi.org/10.3390/ph15080977
Lunghi C, Trevisan C, Fusaroli M, Giunchi V, Raschi E, Sangiorgi E, Domenicali M, Volpato S, De Ponti F, Poluzzi E. Strategies and Tools for Supporting the Appropriateness of Drug Use in Older People. Pharmaceuticals. 2022; 15(8):977. https://doi.org/10.3390/ph15080977
Chicago/Turabian StyleLunghi, Carlotta, Caterina Trevisan, Michele Fusaroli, Valentina Giunchi, Emanuel Raschi, Elisa Sangiorgi, Marco Domenicali, Stefano Volpato, Fabrizio De Ponti, and Elisabetta Poluzzi. 2022. "Strategies and Tools for Supporting the Appropriateness of Drug Use in Older People" Pharmaceuticals 15, no. 8: 977. https://doi.org/10.3390/ph15080977
APA StyleLunghi, C., Trevisan, C., Fusaroli, M., Giunchi, V., Raschi, E., Sangiorgi, E., Domenicali, M., Volpato, S., De Ponti, F., & Poluzzi, E. (2022). Strategies and Tools for Supporting the Appropriateness of Drug Use in Older People. Pharmaceuticals, 15(8), 977. https://doi.org/10.3390/ph15080977










