2.2. Structural, Morphological and Chemical Characterization
FTIR spectra of NIF, HP-β-CD, NIF-HP-β-CD physical mixture and NIF-HP-β-CD inclusion complexes obtained using the kneading, coprecipitation and lyophilization methods are represented in
Figure 2. The FTIR technique was employed to evaluate the possible interaction of nifedipine with the two used β-cyclodextrin derivatives: HP-β-CD and Me-β-CD, respectively, in their physical mixture and inclusion complexes obtained using kneading, coprecipitation and lyophilization methods.
The main characteristic peaks from the FTIR spectra related to functional groups are shown in
Table 1.
The main FTIR absorption peaks of pure nifedipine (
Figure 2a) are at 3334 cm
−1 due to NH stretching, at 1689 cm
−1 assigned to C=O ester bond, at 1624 cm
−1 characteristic to C=C aromatic vibration, at 1529 cm
−1 due to NO
2 and at 1122 cm
−1 due to -C-O ester [
23,
24]. The FTIR spectrum of HP-β-CD (
Figure 2b) showed the main characteristics of IR absorption peaks belonging to saccharides: the wide and strong absorption bond at 3409 cm
−1 due to O-H stretching vibration caused by the intramolecular hydrogen bond, at 2925 cm
−1 due the anti-symmetric vibration of methyl groups (C-H stretching vibration), at 1640 cm
−1 due to O-H bending vibration and 1157 cm
−1 due to C-O vibration [
25,
26]. The characteristic absorption peak of α-type glycosidic bond found at 846 cm
−1 indicates the formation of HP-β-CD by glucopyranose units through α-1,4-glycosidic bond [
27]. In the FTIR spectra of NIF-HP-β-CD physical mixture (
Figure 2c) and NIF-HP-β-CD obtained using coprecipitation methods (
Figure 2e), no new peaks were observed. The main peaks correlated to the functional groups of the two compounds were kept. In the case of NIF-HP-β-CD inclusion compounds prepared by the kneading (
Figure 2d) and lyophilization (
Figure 2f) methods, a shift in main absorption bands of NIF and HP-β-CD was observed. These findings indicate the involvement of these functional groups in some interactions related to the formation of the inclusion complexes.
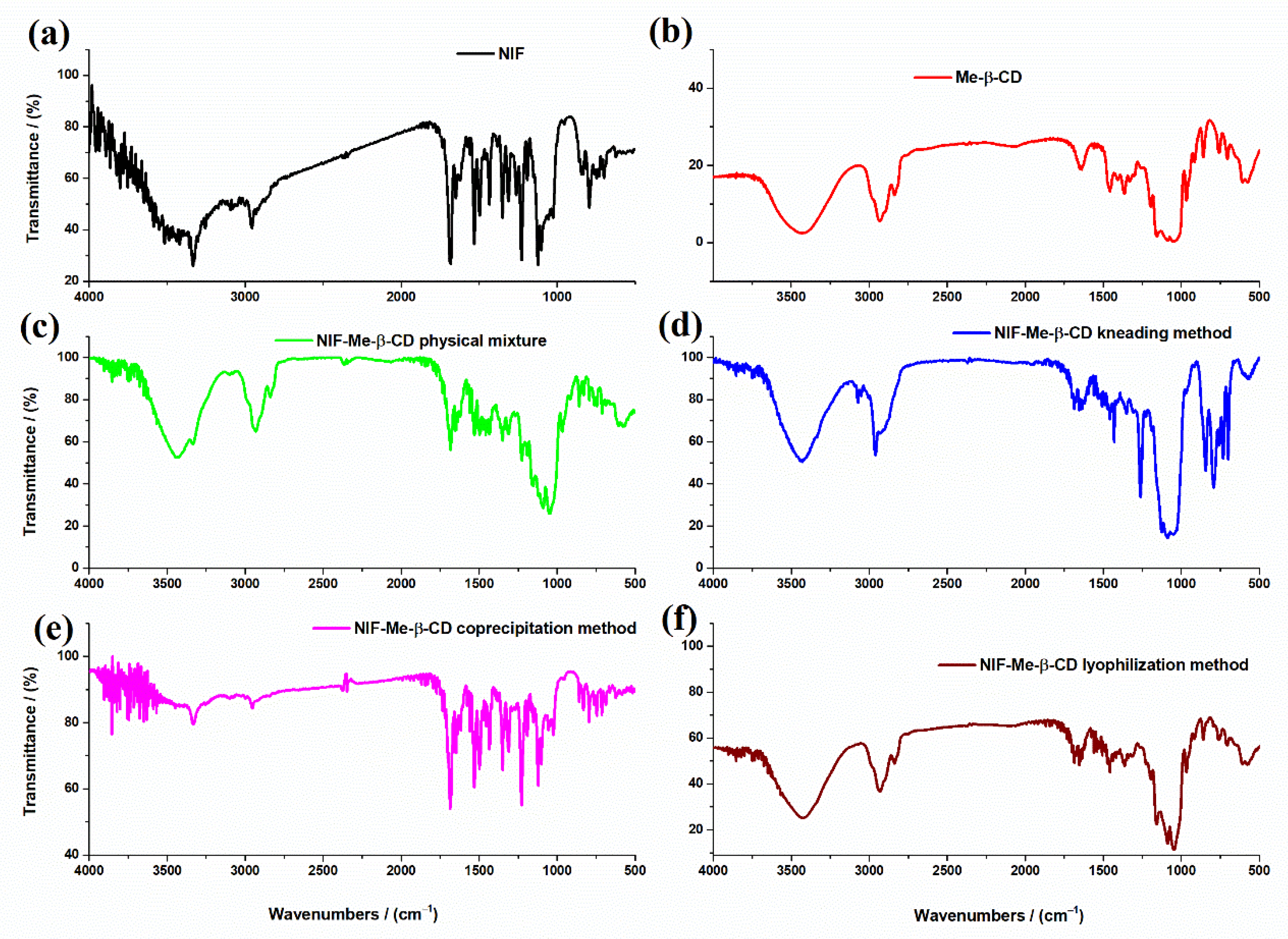
FTIR spectra of NIF, Me-β-CD, their physical mixture and NIF-Me-β-CD inclusion complexes obtained using the kneading, coprecipitation and lyophilization methods are represented in
Figure 3.
The main characteristic peaks from the FTIR spectra related to functional groups are shown in
Table 2.
The FTIR spectrum of Me-β-CD (
Figure 3b) shows the main characteristic bands belonging to saccharides. The FTIR peaks at 2933 cm
−1 and 2840 cm
−1 are due to the aliphatic C-H region of Me-β-CD. The stretching frequency of the C-OH primary and secondary groups from Me-β-CD molecule appears at approximately 1029 cm
−1 [
28,
29,
30]. There are no new peaks found in the spectra of NIF-Me-β-CD physical mixture (
Figure 3c) and the inclusion complexes obtained using different methods of complexation: kneading (
Figure 3d), coprecipitation (
Figure 3e) and lyophilization (
Figure 3f). Some band characteristics of NIF do not appear in any inclusion complexes, even in the physical mixture of NIF-Me-β-CD (
Figure 3c) where no complexation is expected. It is possible that those bands are masked by the very intense and broad bands of the Me-β-CD within the same wavelength region (2960 cm
−1, 1682 cm
−1 and 1122 cm
−1). These bands are visible only in the FTIR spectrum of the compound obtained using the coprecipitation method (
Figure 3e). The disappearance or the strong reduction in the characteristic bands of NIF from the FTIR spectra of compounds NIF-Me-β-CD obtained by kneading (
Figure 3d) and lyophilization (
Figure 3f) methods is an indication of strong interactions between NIF and cyclodextrin compounds and the possible complexation by the inclusion of the drug into the Me-β-CD cavity. All these changes observed in the FTIR spectra of NIF-HP-β-CD and NIF-Me-β-CD compounds obtained using physical and chemical methods of complexation, such as the shift in the absorption peaks or their decrease in intensity, even the whole disappearance is evidence of the various degrees of interaction or amorphization in different products [
28].
SEM analysis of the pure and inclusion complexes is usually used to characterize the structural aspects of the raw compounds and the obtained products but is an inadequate technique for confirming the complexation process. SEM analysis will help us to prove the existence of a single component in the final obtained product [
31].
The morphologies of NIF, HP-β-CD, the physical mixture of the two compounds and the NIF-HP-β-CD inclusion complexes obtained using kneading, coprecipitation and lyophilization methods are represented in
Figure 4. The SEM images of the compounds under study were obtained by scanning the entire surface of the studied samples and magnification factors of 500 until ×3500. The most representative SEM images, which provide significant information regarding the morphologies of the studied samples, are considered. NIF particles (
Figure 4a) show variable shape with a rough surface and showing loose aggregates of irregular shape [
12]. SEM images of HP-β-Cd show spherical forms of particles (
Figure 4b). The SEM image of NIF-HP-β-Cd physical mixture (
Figure 4c) obviously shows the NIF characteristics mixed with HP-β-Cd particles, which clearly displays the presence of the drug in the physical mixture. A drastic modification in the morphologies was observed in the products obtained using kneading, coprecipitation and lyophilization methods. In these compounds, it is not possible to distinguish between the two compounds, revealing a possible interaction between the NIF and HP-β-CD in those systems. In the lyophilization process (
Figure 4f), the formation of a new solid phase with an evident loss of crystallinity can be detected, with respect to the initial compounds.
The morphologies of Me-β-CD, the physical mixture and the NIF-Me-β-CD inclusion complexes obtained using kneading, coprecipitation and lyophilization methods are represented in
Figure 5. Me-β-CD (
Figure 5a) shows an amorphous morphology, composed of spherical particles, some of them being fragments of spherical shells [
32,
33].
The NIF-Me-β-CD physical mixture (
Figure 5a) shows the presence of characteristic NIF crystals in a mixture with Me-β-CD particles or some of them adhered to the Me-β-CD particle surface. In the NIF-Me-β-CD kneading product (
Figure 5c), even if one can detect the development of a new phase, it is still possible to detect some isolated methyl-β-cyclodextrin particles. The coprecipitation technique (
Figure 5d) produced some amorphous aggregates. The SEM image of the NIF-Me-β-CD inclusion product obtained using the lyophilization method shows an amorphous aspect of a new solid phase, demonstrating the possible interaction between the NIF and Me-β-CD by the inclusion of the drug in the cyclodextrin cavity.
X-ray powder diffraction is a suitable technique that clearly shows the cyclodextrin complexation. A true inclusion complex is formed when a new XRD pattern is obtained and is different from that of the superimposition of each pure component [
34]. The X-ray diffraction patterns of NIF, HP-β-CD, their physical mixture and NIF-HP-β-CD obtained using kneading, coprecipitation and lyophilization methods are shown in
Figure 6. The X-ray diffraction pattern of pure nifedipine (
Figure 6a) exhibited characteristic diffraction peaks at 2θ = 8.1, 11.8, 16.6, 19.5 and 24.5°, which are attributed to the crystal planes of Miller indices of (100), (002), (200), (211) and (300) [
35]. This nifedipine diffractogram corresponds to crystalline polymorph A [
18]. The XRD pattern of HP-β-CD (
Figure 6b) showed its amorphous structure with broad peaks around 2θ = 9.76° and 18.7°. In the binary system, prepared by a simple physical mixture (
Figure 6c) and also by the coprecipitation method (
Figure 6e), the presence of the characteristic peaks of the pure NIF and HP-β-CD compounds are observed. This is a confirmation that the complexation between the two compounds did not occur. A possible interaction between them is still evidenced due to the reduction in the intensities of the diffraction peaks. In contrast, in the NIF-HP-β-CD systems obtained using kneading (
Figure 6d) and lyophilization (
Figure 6f) methods, a reduction in the crystallinity degree of the obtained products was noted, even though some specific XRD peaks corresponding to the NIF compound are still noticeable. The additional sharp peaks of NIF indicate that the inclusion is only partial due to the presence of a non-included drug molecule.
The X-ray diffraction patterns of NIF, Me-β-CD, NIF-Me-β-CD physical mixture and NIF-Me-β-CD obtained using kneading, coprecipitation and lyophilization methods are shown in
Figure 7. The XRD diffractogram of Me-β-CD (
Figure 7b) revealed a hollow pattern, which is an indication of its amorphous character. Comparing the XRD diffraction patterns of pure compounds and their NIF-Me-β-CD physical mixture (
Figure 7c), it is observed that the X-ray diffractogram of the physical mixture is a combination of the two initial components, with a slight decrease in the intensity of the diffraction peaks. The coprecipitation system (
Figure 7e) showed an X-ray diffraction pattern relatively comparable to that achieved by combining the diffraction peaks of pure compounds. However, the observed lower intensity of its diffraction peaks can be described by the reciprocal interactions in the liquid state. The kneading (
Figure 7d) and lyophilization (
Figure 7f) products displayed a complete amorphous character, which confirms the strong ability of the amorphous Me-β-CD compound to induce the NIF amorphization [
36]. Further, the lyophilization process can induce a loss of the crystallinity state and the existence of a new solid phase proved the formation of the inclusion complex in the 1:1 molar ratio between NIF and Me-β-CD [
37]. The XRD pattern of the complex obtained using the lyophilization procedure confirmed the inclusion of the NIF compound into the amorphous lattice of the “host” cyclodextrin, since it retained the characteristic XRD pattern of the “host” molecule.
The thermal properties of the pure compounds and their physical mixtures and the products obtained using kneading, coprecipitation and lyophilization methods of preparation were evaluated using differential scanning calorimetry. The DSC thermal curves of NIF, HP-β-CD, their physical mixture and the inclusion complexes are depicted in
Figure 8.
The DSC curve of pure NIF showed a sharp endothermic peak at
Tmin~172 ± 0.7 °C, ascribed to the melting process of the pure compound, with a melting enthalpy of Δ
H = 99.07 ± 0.32 Jg
−1. The DSC curve of HP-β-CD showed a slight decrease in the slope of the curve from room temperature to 130 °C, which could be related to the slow release from the cyclodextrin cavity of the crystallized water molecules [
38,
39]. The DSC curve of the NIF-HP-β-CD physical mixture (
Figure 8c) shows a slight decrease in the peak temperature at
Tmin = 168 ± 0.9 °C (Δ
H = 7.87 ± 0.6 Jg
−1). These results indicate that the two compounds were physically mixed with some interactions. The products obtained using the kneading, coprecipitation and lyophilization methods exhibited a higher decrease in the peak melting intensity of NIF and a shift of peak temperatures at lower values, even its disappearance in the case of the lyophilization technique (
Figure 8f), which indicates an interaction between the two components. The DSC results indicated that NIF was successfully incorporated into the HP-β-CD cavity in the case of the lyophilization product. Generally, when guest molecules are partially or totally included in the cyclodextrin cavity, their sublimation, boiling or melting temperatures are shifted to different temperatures or disappear [
40,
41,
42].
The DSC curves of NIF, Me-β-CD, the physical mixture between NIF and Me-β-CD and the inclusion complexes are illustrated in
Figure 9.
The DSC curve of Me-β-CD (
Figure 9b) shows no thermal effects until 250 °C, when the sample is heated using a linear heating rate of 10 °C min
−1. In the coprecipitation system (
Figure 9e), the melting peak characteristic of the NIF is maintained, indicating that no complexation occurs between NIF and Me-β-CD in the 1:1 molar ratio in this system. Regarding the kneading (
Figure 9d) and lyophilization (
Figure 9f) systems, a considerable peak intensity reduction and a shift to a lower temperature of the melting peak temperature of NIF (
Tmin = 168 °C), even disappearance in the case of lyophilization, were observed. Compared with the physical mixture, it can be pointed out that some NIF-Me-β-CD interactions exist. The absence of the NIF melting peak in the lyophilization product implies the formation of the inclusion complex because of the encapsulation of the drug into the cyclodextrin cavity [
28].
2.3. Preformulation Studies for the Orodispersible Tablets, Which Contain NIF-HP-β-CD and NIF-Me-β-CD, Respectively, Inclusion Complexes
When considering the direct compression technology, the particle size of the blend is an important parameter for the powder flowability, die filling and tablets’ uniformity and integrity. It is essential to achieve an optimal particle size distribution, to obtain tablets with satisfactory pharmacotechnical and biopharmaceutical properties [
43,
44]. The width of the particle size distribution must be established in the preformulation studies.
Figure 10 shows a registered histogram by plotting the particle size distribution on granulometric classes in the case of the studied powders.
A narrow particle size distribution is preferable for ensuring good flowability and compressibility characteristics in the blend [
45]. The studied formulations display different granulometric properties, with similar behaviour between F3 and F6 on one side and the other four (F1, F2, F4 and F5) on the other side. The materials based on the silicified microcrystalline cellulose and Disintequik™ ODT mixture have a considerable proportion of the particles with sizes between 160 and 600 µm, above 90% for F3 and almost 87% for F6, showing a narrower range for all proposed formulations. Meanwhile, F2 and F5, which contain F-Melt
® and Disintequik™ ODT excipients, possess the highest amount of particles in a 80–160 µm range of all formulations. The results prove that the type of cyclodextrin used in the complex formation is not influencing the particle size distribution, but the greatest influence is given by the direct compressible excipient types and amounts.
The pharmacotechnical properties in the direct compression powders are shown in
Table 3.
The moisture content is a very important powder property that highly influences both the blend behaviour during the compression process and its consistency and also the quality of the final tablets [
46]. Considering that water was used for the preparation of the active ingredients by the lyophilization method, we expected to find a certain amount of moisture in the final blends. The results show there is no significant difference between the batches containing NIF-HP-β-CD and the ones based on NIF-Me-β-CD, proving that the type of cyclodextrin is not influencing the moisture retention in the material. On the other hand, obviously, the direct compression excipient types and amounts induce notable variations between formulations in terms of humidity content. The lowest amounts of moisture were detected in F3 (2.45%) and F6 (2.7%), the samples containing silicified microcrystalline cellulose and Disintequik™ ODT mixture. Meanwhile, the other four formulations enclose double amounts of water, 4.69% in F1, 5.13% in F2, 4.85% in F4 and the highest content of 5.76% is included in F5.
The flowability considerably varies between the formulations, regardless of the type of cyclodextrin used. F3 presents the highest flow rate of 6.185 g/mL, followed by F6 with a 5.825 g/mL flow rate. F1, F2, F4 and F5 registered a longer flow time, almost double compared to F3 and F6. According to the European Pharmacopoeia specifications on the angle of repose values, F3 and F6 are classified as having an excellent free flow, while F2 and F5 present a good flow and F1 and F4 have just a fair flow. Considering that the analysis could not be performed on lower-diameter nozzles as the samples did not flow and the results were recorded for the 15 mm nozzle, it can be admitted that only F3 and F6 possess a suitable flowability for the direct compression materials [
47].
The volumetric characteristics proved, more precisely, the differences between the batches and the influence of the cyclodextrin type on the flowability and compressibility parameters. A clear difference between the formulations containing NIF-HP-β-CD complex (F1–F3) and that with NIF-Me-β-CD (F4–F6) was registered. F4–F6 series show higher values for CI and HR than F1–F3 batches that include the same excipients, the cyclodextrin being the only variable. This demonstrates that Me-β-CD decreased the compressibility and flowability properties in the blends. Still, F6 presents a 9.3 CI and 1.10 HR, values which, in accordance with European Pharmacopoeia [
48], mean excellent compressibility and flow character. This is due to the chosen excipients and their weight ratio (silicified microcrystalline cellulose and Disintequik™ ODT mixture). F3 recorded the best values for CI (8.6) and HR (1.09) of all formulations, as a result of using the same excipients in the same proportions as F6, plus enclosing the NIF-HP-β-CD complex, which proved to possess better flowing and compressibility properties. In contrast, F2 and F5, the formulations based on F-MELT
® and Disintequik™ ODT, exhibited low mechanical attributes, with high values for CI (16.3-F2 and 18.3–F5) and also for HR (1.19–F2 and 1.22–F5). However, the highest values for CI (19.3) and HR (1.24) were revealed for F4, which contains silicified microcrystalline cellulose mixed with F-MELT
®. Even so, in agreement with the European Pharmacopoeia [
48], F1, F2, F4 and F5 have fair flowability and compressibility.
2.4. Quality Control of the Orodispersible Tablets
The manufactured tablets are round, white yellowish, with homogeneous appearances and smooth surfaces (
Figure 11).
The pharmacotechnical characteristics of the obtained tablets are shown in
Table 4.
The uniformity of tablet dimensions (thickness and diameter) and weight proves that the dies were homogeneously filled due to the great flowability in the materials, and the compression process was successfully realized based on a good adjustment of all parameters and on the suitable compressibility in the blend [
49]. The registered intra- and inter-batch variabilities are low, demonstrating that the type of cyclodextrin is not affecting the tablet dimensions or mass. The thickness of the tablets is around 4 mm, the diameter is 10 mm and the weight is around 300 mg for both formulations. The values met the European Pharmacopoeia requirements [
48], revealing the appropriate selection of the excipients and compression conditions.
The mechanical resistance of the tablets is satisfactory, both in terms of hardness and friability, but a clear difference between the formulations is observed, indicating that the type of cyclodextrin is responsible for the changes in tablets’ compactness. Me-β-CD induces a higher hardness and friability (67 N and 0.11%) compared to HP-β-CD (55 N and 0.05%). However, the excipients proved to exhibit suitable plasticity and elasticity properties in the materials, leading to tablets with appropriate mechanical strength for both formulations.
In accordance with the hardness results, the disintegration time in both media for the two formulations proved to be faster for the NIF-HP-β-CD tablets compared to NIF-Me-β-CD tablets. Further, for both batches, the disintegration time in the water (13 s for F3 and 21 s for F6) was much lower than in the simulated saliva medium (26 s for F3 and 34 s for F6). The disintegration behaviour displayed by both formulations meets the specified requirements of the European Pharmacopoeia [
48], but the influence of the cyclodextrin type is evidenced. The HP-β-CD inclusion complex led to tablets with lower hardness and faster disintegration performance. All tablets show an excellent disintegration ability due to the superdisintegrant properties of the excipients, characteristic of silicified microcrystalline cellulose, Disintequik™ ODT and, especially, sodium starch glycolate.
The dissolution rates registered by the tablets during 30 min on simulated saliva dissolution medium are shown in
Table 5 and
Figure 12.
After 30 min, the tablets released the entire quantity of nifedipine contained (99.11% for F3 and 98.79% for F6), proving that both formulations lead to fast-dissolving tablets with excellent dissolution rate. Still, the differences between the formulations’ performances are obvious. Even after the first 5 min, it is observed that F3 has a higher dissolution rate of 26.15%, compared to 18.57% for F6, and this behaviour is maintained during the first 20 min, then F6 reaches similar release degrees.