Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights
Abstract
:1. Introduction
2. Applicative and Biomedical Relevance of Fmoc-FF Hydrogel
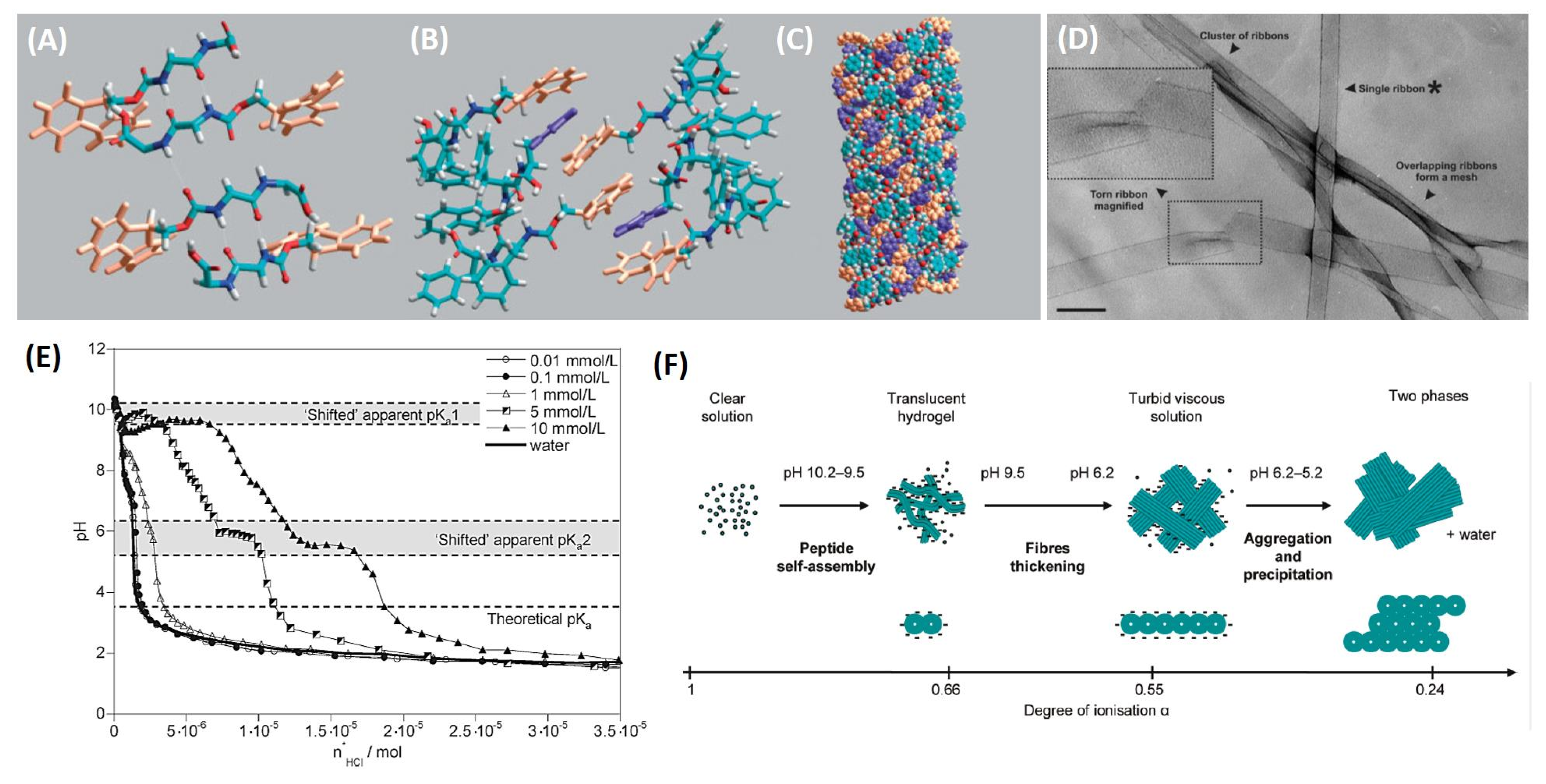
3. Structural Organization and Proposed Model
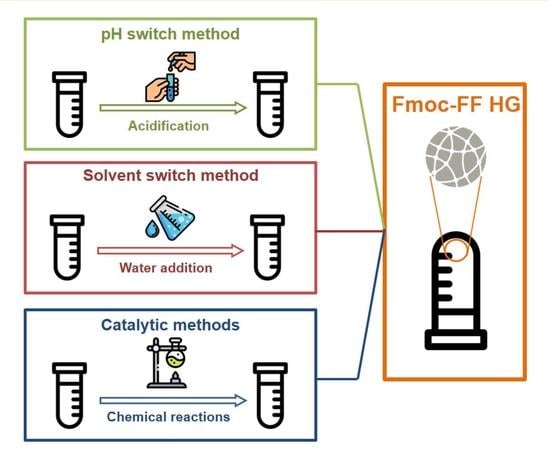
4. Preparation Methods
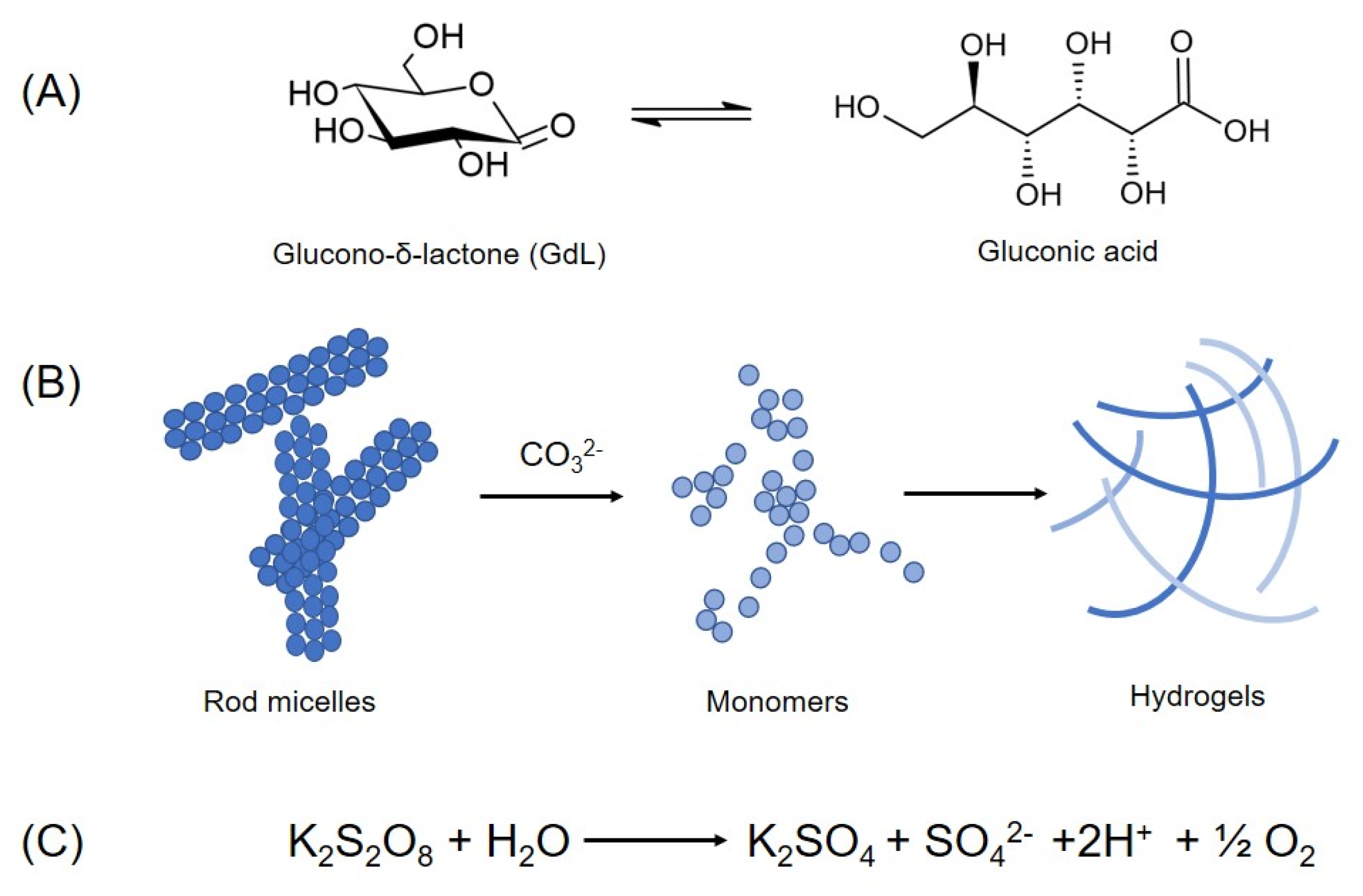
4.1. pH-Switch Method
4.2. Solvent-Switch Method
4.3. Catalytic Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acar, H.; Srivastava, S.; Chung, E.J.; Schnorenberg, M.R.; Barrett, J.C.; LaBelle, J.L.; Tirrell, M. Self-assembling peptide-based building blocks in medical applications. Adv. Drug Deliv. Rev. 2016, 110–111, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Nainytė, M.; Müller, F.; Ganazzoli, G.; Chan, C.Y.; Crisp, A.; Globisch, D.; Carell, T. Amino Acid Modified RNA Bases as Building Blocks of an Early Earth RNA-Peptide World. Chem. A Eur. J. 2020, 26, 14856–14860. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Gianolio, E.; Accardo, A. Peptide-based building blocks as structural elements for supramolecular Gd-containing MRI contrast agents. J. Pept. Sci. 2019, 25, e3157. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, U.; Corra, S.; Zajaczkowski, W.; Ochs, N.A.K.; Shoshan, M.S.; Tanabe, J.; Stappert, S.; Li, C.; Yashima, E.; Pisula, W.; et al. Positional isomers of chromophore–peptide conjugates self-assemble into different morphologies. Chem. Eur. J. 2018, 24, 12623–12629. [Google Scholar] [CrossRef]
- Eisenberg, D.; Jucker, M. The Amyloid State of Proteins in Human Diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef]
- Otzen, D.; Rie, R. Functional amyloids. Cold Spring Harb. Perspect. Biol. 2019, 11, a033860. [Google Scholar] [CrossRef]
- Balasco, N.; Diaferia, C.; Morelli, G.; Vitagliano, L.; Accardo, A. Amyloid-like aggregation in diseases and biomaterials: Osmosis of structural information. Front. Bioeng. Biotechnol. 2021, 9, 641372. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting Metal Nanowires Within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef]
- Görbitz, C.H. Nanotube Formation by Hydrophobic Dipeptides. Chem. A Eur. J. 2001, 7, 5153–5159. [Google Scholar] [CrossRef]
- Fuentes-Caparrós, A.M.; McAulay, K.; Rogers, S.E.; Dalgliesh, R.M.; Adams, D.J. On the Mechanical Properties of N-Functionalised Dipeptide Gels. Molecules 2019, 24, 3855. [Google Scholar] [CrossRef] [Green Version]
- Amdursky, N.; Molotskii, M.; Gazit, E.; Rosenman, G. Elementary Building Blocks of Self-Assembled Peptide Nanotubes. J. Am. Chem. Soc. 2010, 132, 15632–15636. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Avitabile, C.; Leone, M.; Gallo, E.; Saviano, M.; Accardo, A.; Romanelli, A. Diphenylalanine Motif Drives Self-Assembling in Hybrid PNA-Peptide Conjugates. Chem. A Eur. J. 2021, 27, 14307–14316. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Designed aromatic homo-dipeptides: Formation of ordered nanostructures and potential nanotechnological applications. Phys. Biol. 2006, 3, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fei, J.; Li, Q.; Li, J. Covalently assembled dipeptide nanospheres as intrinsic photosensitizers for efficient photodynamic therapy in vitro. Chem. Eur. J. 2016, 22, 1–6. [Google Scholar] [CrossRef]
- Kumara, V.; Vijay, K.; Shruti, K.; Khashti, K.; Joshi, B. Aggregation propensity of amyloidogenic and elastomeric dipeptides constituents. Tetrahedron 2016, 72, 5369–5376. [Google Scholar] [CrossRef]
- Mayans, E.; Casanovas, J.; Gil, A.M.; Jimenez, A.I.; Cativiela, C.; Puiggalí, J.; Aleman, C. Diversity and hierarchy in supramolecular assemblies of triphenylalanine: From laminated helical ribbons to toroids. Langmuir 2017, 33, 4036–4048. [Google Scholar] [CrossRef]
- Diaferia, C.; Roviello, V.; Morelli, G.; Accardo, A. Self-assembly of PEGylated diphenylalanines into photoluminescent fibrillary aggregates. ChemPhysChem 2019, 20, 2774–2782. [Google Scholar] [CrossRef]
- Creasey, R.C.G.; Louzao, I.; Arnon, Z.A.; Marco, P.; Adler-Abramovich, L.; Roberts, C.J.; Gazit, E.; Tendler, S.J.B. Disruption of diphenylalanine assembly by a Boc-modified variant. Soft Matter 2016, 12, 9451–9457. [Google Scholar] [CrossRef]
- Yan, X.; He, Q.; Wang, K.; Duan, L.; Cui, Y.; Li, J. Transition of Cationic Dipeptide Nanotubes into Vesicles and Oligonucleotide Delivery. Angew. Chem. Int. Ed. 2007, 46, 2431–2434. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Formation of Closed-Cage Nanostructures by Self-Assembly of Aromatic Dipeptides. Nano Lett. 2004, 4, 581–585. [Google Scholar] [CrossRef]
- Pellach, M.; Mondal, S.; Shimon, L.J.W.; Adler-Abramovich, L.; Buzhansky, L.; Gazit, E. Molecular Engineering of Self-Assembling Diphenylalanine Analogues Results in the Formation of Distinctive Microstructures. Chem. Mater. 2016, 28, 4341–4348. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Self-Assembly of peptide nanotubes and amyloid-like structures by charged-termini-capped diphenylalanine peptide analogues. Isr. J. Chem. 2005, 45, 363–371. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Gazit, E. Controlled patterning of peptide nanotubes and nanospheres using inkjet printing technology. J. Pep. Sci. 2007, 14, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Amdursky, N.; Molotskii, M.; Gazit, E.; Rosenman, G. Self-assembled bioinspired quantum dots: Optical properties. Appl. Phys. Lett. 2009, 94, 261907. [Google Scholar] [CrossRef]
- Jayawarna, V.; Ali, M.; Jowitt, T.; Miller, A.F.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, self-assembled hydrogel composed of a modified aromatic dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Diaferia, C.; Morelli, G.; Accardo, A. Fmoc-diphenylalanine as a suitable building block for the preparation of hybrid materials and their potential applications. J. Mater. Chem. B 2019, 7, 5142–5155. [Google Scholar] [CrossRef]
- Ghosh, M.; Bera, S.; Schiffmann, S.; Shimon, L.J.W.; Adler-Abramovich, L. Collagen-Inspired Helical Peptide Coassembly Forms a Rigid Hydrogel with Twisted Polyproline II Architecture. ACS Nano 2020, 14, 9990–10000. [Google Scholar] [CrossRef]
- Rosa, E.; Diaferia, C.; Gallo, E.; Morelli, G.; Accardo, A. Stable Formulations of Peptide-Based Nanogels. Molecules 2020, 25, 3455. [Google Scholar] [CrossRef]
- Helen, W.; de Leonardis, P.; Ulijn, R.V.; Gough, J.; Tirelli, N. Mechanosensitive peptide gelation: Mode of agitation controls mechanical properties and nano-scale morphology. Soft Matter 2011, 7, 1732–1740. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Y.; Wang, Y.; Qi, W.; Huang, R.; Su, R.; He, Z. Self-Assembled Microporous Peptide-Polysaccharide Aerogels for Oil–Water Separation. Langmuir 2018, 34, 10732–10738. [Google Scholar] [CrossRef]
- Arakawa, H.; Takeda, K.; Higashi, S.L.; Shibata, A.; Kitamura, Y.; Ikeda, M. Self-assembly and hydrogel formation ability of Fmoc-dipeptides comprising α-methyl-L-phenylalanine. Polym. J. 2020, 52, 923–930. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Ding, B.; Ma, X. Archieving room temperature phosphorescence from organic small molecules on amino acid skeleton. Chi. Chem. Lett. 2020, 31, 2929–2932. [Google Scholar] [CrossRef]
- Sankar, S.; O’Neill, K.; Bagot D’Arc, M.; Rebeca, F.; Buffier, M.; Aleksi, E.; Fan, M.; Matsuda, N.; Gil, E.S.; Spirio, L. Clinical use of the self-assembling peptide RADA16: A review of current and future trends in biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 679525. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Smith, K.; Moore, E.; Schneider, J.; Tycko, R. Molecular structure of monomorphic peptide fibrils within a kinetically trapped hydrogel network. Proc. Natl. Acad. Sci. USA 2015, 112, 9816–9821. [Google Scholar] [CrossRef]
- Yaguchi, A.; Hiramatsu, H.; Ishida, A.; Oshikawa, M.; Ajioka, I.; Muraoka, T. Hydrogel-Stiffening and Non-Cell Adhesive Properties of Amphiphilic Peptides with Central Alkylene Chains. Chem. A Eur. J. 2021, 27, 9295–9301. [Google Scholar] [CrossRef]
- Jadhav, S.P.; Amabili, P.; Stammler, H.-G.; Sewald, N. Remarkable modulation of self-assembly in short γ-Peptides by neighboring ions and orthogonal H-bonding. Chem. Eur. J. 2017, 23, 10352–10357. [Google Scholar] [CrossRef]
- Santagada, V.; Caliendo, G. Peptide and Peptidomimetics; Piccin: Padova, Italy, 2012. [Google Scholar]
- Diaferia, C.; Ghosh, M.; Sibillano, T.; Gallo, E.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Adler-Abramovich, L.; Accardo, A. Fmoc-FF and hexapeptide-based multicomponent hydrogels as scaffold materials. Soft Matter 2019, 15, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, W.; Huang, R.; Su, R.; He, Z. Jet flow directed supramolecular self-assembly at aqueous liquid–liquid interface. RSC Adv. 2014, 4, 15340–15347. [Google Scholar] [CrossRef]
- Choe, R.; Yun, S.I. Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery. e-Polymers 2020, 20, 458–468. [Google Scholar] [CrossRef]
- Diaferia, C.; Balasco, N.; Sibillano, T.; Giannini, C.; Vitagliano, L.; Morelli, G.; Accardo, A. Structural Characterization of Self-Assembled Tetra-Tryptophan Based Nanostructures: Variations on a Common Theme. ChemPhysChem 2018, 19, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Pachahara, S.K.; Adicherla, H.; Nagaraj, R. Self-assembly of Aβ40, Aβ42 and Aβ43 peptides in aqueous mixtures of fluorinated alcohols. PLoS ONE 2015, 10, e0136567. [Google Scholar]
- Schiattarella, C.; Diaferia, C.; Gallo, E.; Della Ventura, B.; Morelli, G.; Vitagliano, L.; Velotta, R.; Accardo, A. Solid-state optical properties of self-assembling amyloid-like peptides with different charged states at the terminal ends. Sci. Rep. 2022, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, H.; Yang, A.Z.; Xu, B. Supramolecular Hydrogels Respond to Ligand−Receptor Interaction. J. Am. Chem. Soc. 2003, 125, 13680–13681. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gu, H.; Zhang, Y.; Wang, L.; Xu, B. Small molecule hydrogels based on a class of anti-inflammatory agents. Chem. Commun. 2004, 208–209. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Fu, D.; Gao, P.; Lam, J.K.; Xu, B. Enzymatic formation of supramolecular hydrogels. Adv. Mater. 2004, 16, 1440–1444. [Google Scholar] [CrossRef]
- Amdursky, N.; Gazit, E.; Rosenman, G. Quantum Confinement in Self-Assembled Bioinspired Peptide Hydrogels. Adv. Mater. 2010, 22, 2311–2315. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, X.; Jing, X.; Chen, X.; Cheng, H.; Zheng, X.; Ren, Z.; Zhuo, Z. Surface self-assembly of N-fluorenyl-9-methoxycarbonyl diphenylalanine on silica wafer. Colloids Surf. B Biointerfaces 2011, 87, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.; Beirne, J.; Redmond, G.; Kilpatrick, J.I.; Guyonnet, J.; Buchete, N.-V.; Kholkin, A.L.; Rodriguez, B.J. Nanoscale piezoelectric properties of self-assembled Fmoc-FF peptide fibrous networks. ACS Appl. Mat. Interfaces 2015, 7, 12702–12707. [Google Scholar] [CrossRef]
- McCloskey, A.P.; Draper, E.R.; Gilmore, B.F.; Laverty, G. Ultrashort self-assembling Fmoc-peptide gelators for anti-infective biomaterial applications. J. Pept. Sci. 2017, 23, 131–140. [Google Scholar] [CrossRef]
- Ben-Zvi, O.; Grinberg, I.; Orr, A.A.; Noy, D.; Tamamis, P.; Yacoby, I.; Adler-Abramovich, L. Protection of Oxygen-Sensitive Enzymes by Peptide Hydrogel. ACS Nano 2021, 15, 6530–6539. [Google Scholar] [CrossRef]
- Smith, A.M.; Williams, R.J.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.L.; Saiani, A.; Ulijn, R.V. Fmoc-diphenylalanine self-assembles to a hydrogel via a novel architecture based on π-π interlocked β-sheets. Adv. Mater. 2008, 20, 37–41. [Google Scholar] [CrossRef]
- Tang, C.; Smith, A.M.; Collins, R.F.; Ulijn, R.V.; Saiani, A. Fmoc-diphenylalanine self-assembly mechanism induces apparent pKa shifts. Langmuir 2009, 25, 9447–9453. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Yuan, C.; Li, S.; Song, J.; Li, J.; Yan, X. Charge-induced secondary structure transformation of amyloid-derived dipeptide assemblies from β-sheet to α-Helix. Angew. Chem. Int. Ed. 2018, 57, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Yuan, C.; Zilberzwige-Tal, S.; Xing, R.; Chakraborty, P.; Tao, K.; Gilead, S.; Yan, X.; Gazit, E. Metal-Ion Modulated Structural Transformation of Amyloid-Like Dipeptide Supramolecular Self-Assembly. ACS Nano 2019, 13, 7300–7309. [Google Scholar] [CrossRef] [PubMed]
- Amdursky, N.; Orbach, R.; Gazit, E.; Huppert, D. Probing the Inner Cavities of Hydrogels by Proton Diffusion. J. Phys. Chem. C 2009, 113, 19500–19505. [Google Scholar] [CrossRef]
- Fuentes, E.; Boháčová, K.; Fuentes-Caparrós, A.M.; Schweins, R.; Draper, E.R.; Adams, D.J.; Silvia Pujals, S.; Albertazzi, L. PAINT-ing Fluorenylmethoxycarbonyl (Fmoc)-diphenylalanine hydrogels. Chem. Eur. J. 2020, 26, 9869–9873. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; IntechOpen: London, UK, 2011. [Google Scholar]
- Taylor, J.; Rahimeh, B.; Alpesh, P.; Kibret, M. Fabrication of highly porous tissue-engineering scaffolds using selective spherical porogens. BioMed. Mater. Eng. 2010, 20, 107–118. [Google Scholar]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tiss. Eng. B 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Raeburn, J.; Pont, G.; Chen, L.; Cesbron, Y.; Levy, R.; Adams, D.J. Fmoc-diphenylalanine hydrogels: Understanding the variability in reported mechanical properties. Soft Matter 2012, 8, 1168–1174. [Google Scholar] [CrossRef]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A new method for maintaining homogeneity during liquid–hydrogel transitions using low molecular weight hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- Berillo, D.; Mattiasson, B.; Yu, I.; Kirsebom, G.H. Formation of macroporous self-assembled hydrogels through cryogelation of Fmoc-Phe-Phe. J. Coll. Interface Sci. 2012, 368, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Li, Y.; Qin, M.; Ding, Y.; Cao, Y.; Wang, W. Two approaches for the engineering of homogeneous small-molecule hydrogels. Soft Matter 2013, 9, 4672–4680. [Google Scholar] [CrossRef]
- Orbach, R.; Adler-Abramovich, L.; Zigerson, S.; Mironi-Harpaz, I.; Seliktar, D.; Gazit, E. Self-Assembled Fmoc-Peptides as a Platform for the Formation of Nanostructures and Hydrogels. Biomacromolecules 2009, 10, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Dudukovic, N.A.; Zukoski, C.F. Mechanical Properties of Self-Assembled Fmoc-Diphenylalanine Molecular Gels. Langmuir 2014, 30, 4493–4500. [Google Scholar] [CrossRef]
- Dudukovic, N.A.; Zukoski, C.F. Evidence for equilibrium gels of valence-limited particles. Soft Matter 2014, 10, 7849–7856. [Google Scholar] [CrossRef]
- Levine, M.S.; Ghosh, M.; Hesser, M.; Hennessy, N.; DiGuiseppi, D.M.; Adler-Abramovich, L.; Schweitzer-Stenner, R. Formation of peptide-based oligomers in dimethylsulfoxide: Identifying the precursor of fibril formation. Soft Matter 2020, 16, 7860–7868. [Google Scholar] [CrossRef]
- Dudukovic, N.A.; Zukoski, C.F. Gelation of Fmoc-diphenylalanine is a first order phase transition. Soft Matter 2015, 11, 7663–7673. [Google Scholar] [CrossRef]
- Raeburn, J.; Mendoza-Cuenca, C.; Cattoz, B.N.; Little, M.A.; Terry, A.E.; Cardoso, A.Z.; Griffiths, P.C.; Adams, D.J. The effect of solvent choice on the gelation and final hydrogel properties of Fmoc–diphenylalanine. Soft Matter 2014, 11, 927–935. [Google Scholar] [CrossRef]
- Dudukovic, N.A.; Hudson, B.C.; Paravastu, A.K.; Zukoski, C.F. Self-assembly pathways and polymorphism in peptide-based nanostructures. Nanoscale 2017, 10, 1508–1516. [Google Scholar] [CrossRef]
- Williams, R.J.; Smith, A.M.; Collins, R.; Hodson, N.; Das, A.K.; Ulijn, R.V. Enzyme-assisted self-assembly under thermodynamic control. Nat. Nanotech. 2009, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Roy, S.; Arora, M.; Das, A.K.; Hodson, N.; Murray, P.; Marshall, S.; Javid, N.; Sefcik, J.; Boekhoven, J.; et al. Biocatalytic induction of supramolecular order. Nat. Chem. 2010, 2, 1089–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Wang, Y.; Qi, W.; Su, R.; He, Z. Chemical catalysis triggered self-assembly for the bottom-up fabrication of peptide nanofibers and hydrogels. Mater. Lett. 2014, 128, 216–219. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Sennato, S.; Bordi, F.; Giannella, D.; Di Nitto, A.; Barbetta, A.; Dentini, M.; Togna, A.R.; Togna, G.I.; Moschini, S.; et al. Designing unconventional Fmoc-peptide-based biomaterials: Structure and related properties. Soft Matter 2013, 10, 1944–1952. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaferia, C.; Rosa, E.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights. Pharmaceuticals 2022, 15, 1048. https://doi.org/10.3390/ph15091048
Diaferia C, Rosa E, Morelli G, Accardo A. Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights. Pharmaceuticals. 2022; 15(9):1048. https://doi.org/10.3390/ph15091048
Chicago/Turabian StyleDiaferia, Carlo, Elisabetta Rosa, Giancarlo Morelli, and Antonella Accardo. 2022. "Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights" Pharmaceuticals 15, no. 9: 1048. https://doi.org/10.3390/ph15091048
APA StyleDiaferia, C., Rosa, E., Morelli, G., & Accardo, A. (2022). Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights. Pharmaceuticals, 15(9), 1048. https://doi.org/10.3390/ph15091048