Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity
Abstract
1. Introduction
2. Trends in Network Pharmacology Research
2.1. On the Road to Network-Based Precision Medicine
2.2. Methods of Network Pharmacology
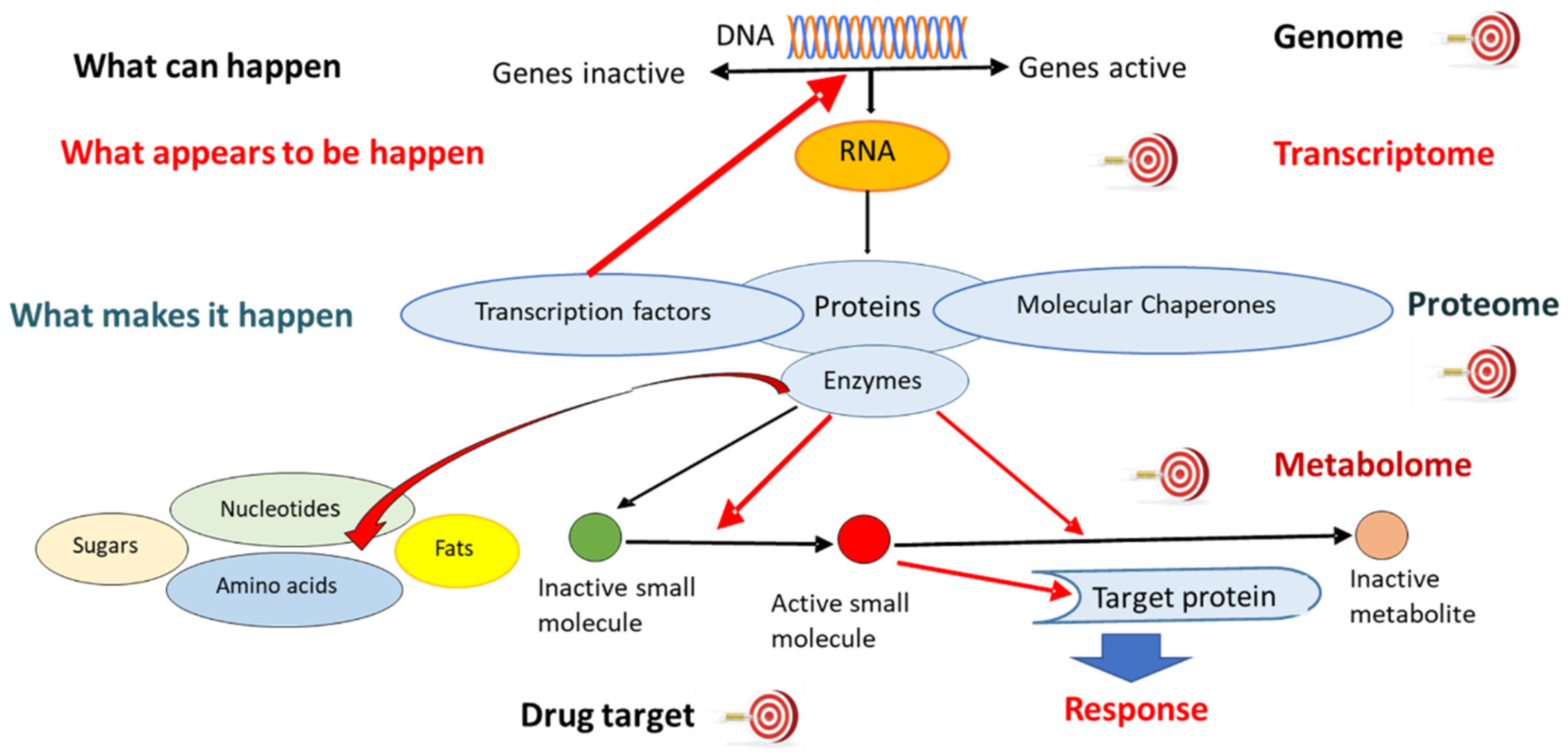
2.2.1. Genomics
2.2.2. Transcriptomics
2.2.3. Proteomics
2.2.4. Metabolomics
2.2.5. Other “Omics” Technologies
2.3. “Multi-Omics” Technologies
2.4. Single-Cell “-Omics” Technologies
2.5. Network Modeling
2.6. “Omics”-Based Biomarker Development
2.7. “Omics”-Based Therapy Monitoring
2.8. Network Pharmacology with a Natural Product and Complex Herbal Mixtures
2.9. Toxicology
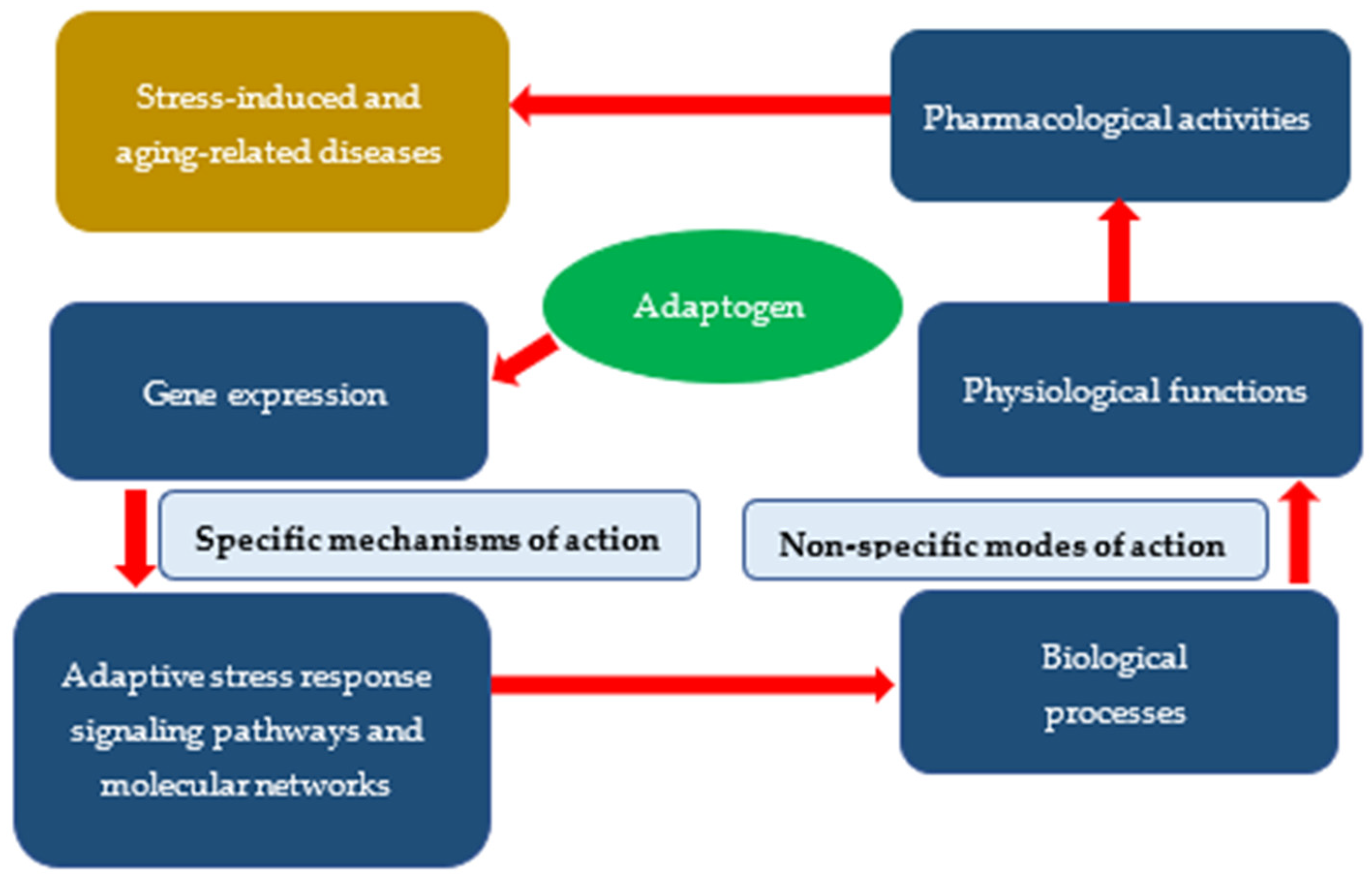
3. Specific and Nonspecific Actions of Adaptogens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H. Reductionism and complexity in molecular biology. Scientists now have the tools to unravel biological and overcome the limitations of reductionism. EMBO Rep. 2004, 5, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Fliri, A.F.; Loging, W.T.; Volkmann, R.A. Cause-effect relationships in medicine: A protein network perspective. Trends Pharmacol. Sci. 2010, 31, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Klipp, E.; Wade, R.C.; Kummer, U. Biochemical network-based drug-target prediction. Curr. Opin. Biotechnol. 2010, 21, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network pharmacology of red ginseng (part I): Effects of ginsenoside Rg5 at physiological and sub-physiological concentrations. Pharmaceuticals 2021, 14, 999. [Google Scholar] [CrossRef]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network pharmacology of ginseng (part II): The differential effects of red ginseng and ginsenoside Rg5 in cancer and heart diseases as determined by transcriptomics. Pharmaceuticals 2021, 14, 1010. [Google Scholar] [CrossRef]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.J.; Efferth, T. Effects of anti-inflammatory and adaptogenic herbal extracts on gene expression of eicosanoids signaling pathways in isolated brain cells. Phytomedicine 2019, 60, 152881. [Google Scholar] [CrossRef]
- Seo, E.J.; Klauck, S.M.; Efferth, T.; Panossian, A. Adaptogens in chemobrain (Part III): Antitoxic effects of plant extracts towards cancer chemotherapy-induced toxicity—Transcriptome-wide microarray analysis of neuroglia cells. Phytomedicine 2019, 56, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Klauck, S.M.; Efferth, T.; Panossian, A. Adaptogens in chemobrain (part I): Plant extracts attenuate cancer chemotherapy-induced cognitive impairment—Transcriptome-wide microarray profiles of neuroglia cells. Phytomedicine 2019, 55, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.J.; Wikman, G.; Efferth, T. Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling. Phytomedicine 2015, 22, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Hamm, R.; Wikman, G.; Efferth, T. Mechanism of action of Rhodiola, salidroside, tyrosol, and triandrin in isolated neuroglial cells: An interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine 2014, 21, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Hamm, R.; Kadioglu, O.; Wikman, G.; Efferth, T. Synergy and antagonism of active constituents of ADAPT-232 on transcriptional level of metabolic regulation of isolated neuroglial cells. Front. Neurosci. 2013, 7, 16. [Google Scholar] [CrossRef]
- Robin, X.; Creixell, P.; Radetskaya, O.; Santini, C.C.; Longden, J.; Linding, R. Personalized network-based treatments in oncology. Clin. Pharmacol. Ther. 2013, 94, 646–650. [Google Scholar] [CrossRef]
- Lay, J.O.; Borgmann, S.; Liyanage, R.; Wilkins, C.L. Problems with the “omics”. Trends Anal. Chem. 2006, 25, 1046–1056. [Google Scholar] [CrossRef]
- Ouedraogo, M.; Baudoux, T.; Stévigny, C.; Nortier, J.; Colet, J.M.; Efferth, T.; Qu, F.; Zhou, J.; Chan, K.; Shaw, D.; et al. Review of current and “omics” methods for assessing the toxicity (genotoxicity, teratogenicity and nephrotoxicity) of herbal medicines and mushrooms. J. Ethnopharmacol. 2012, 140, 492–512. [Google Scholar] [CrossRef]
- Kinross, J.M.; Darzi, A.W.; Nicholson, J.K. Gut microbiome-host interactions in health and disease. Genome Med. 2011, 3, 14. [Google Scholar] [CrossRef]
- Sun, Y.V.; Hu, Y.J. Integrative analysis of multi-omics data for discovery and functional studies of complex human diseases. Adv. Genet. 2016, 93, 147–190. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Dong, X.; Liu, C.; Dozmorov, M. Review of multi-omics data resources and integrative analysis for human brain disorders. Brief Funct. Genom. 2021, 20, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Hack, C.J. Integrated transcriptome and proteome data: The challenges ahead. Brief Funct. Genom. Proteom. 2004, 3, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wiench, B.; Chen, Y.R.; Paulsen, M.; Hamm, R.; Schröder, S.; Yang, N.S.; Efferth, T. Integration of different “-omics” technologies identifies inhibition of the IGF1R-Akt-mTOR signaling cascade involved in the cytotoxic effect of shikonin against leukemia cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 818709. [Google Scholar] [CrossRef] [PubMed]
- Cifani, P.; Kentsis, A. Towards comprehensive and quantitative proteomics for diagnosis and therapy of human disease. Proteomics 2017, 17, 1600079. [Google Scholar] [CrossRef]
- Monti, C.; Zilocchi, M.; Colugnat, I.; Alberio, T. Proteomics turns functional. J. Proteom. 2019, 198, 36–44. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Korcsmaros, T.; Schneider, M.V.; Superti-Furga, G. Next generation of network medicine: Interdisciplinary signaling approaches. Integr. Biol. (Camb.) 2017, 9, 97–108. [Google Scholar] [CrossRef]
- Addepalli, R.V.; Mullangi, R. A concise review on lipidomics analysis in biological samples. ADMET DMPK 2020, 9, 1–22. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics data integration, interpretation, and its application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Varol, A.; Efferth, T. Multi-omics approaches to improve malaria therapy. Pharmacol. Res. 2021, 167, 105570. [Google Scholar] [CrossRef]
- Nam, A.S.; Chaligne, R.; Landau, D.A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet. 2021, 22, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vergallo, A.; Aguilar, L.F.; Benda, N.; Broich, K.; Cuello, A.C.; Cummings, J.; Dubois, B.; Federoff, H.J.; Fiandaca, M.; et al. Precision pharmacology for Alzheimer’s disease. Pharmacol. Res. 2018, 130, 331–365. [Google Scholar] [CrossRef]
- Lederer, A.R.; La Manno, G. The emergence and promise of single-cell temporal-omics approaches. Curr. Opin. Biotechnol. 2020, 63, 70–78. [Google Scholar] [CrossRef]
- Silverman, E.K.; Schmidt, H.H.H.W.; Anastasiadou, E.; Altucci, L.; Angelini, M.; Badimon, L.; Balligand, J.L.; Benincasa, G.; Capasso, G.; Conte, F.; et al. Molecular networks in Network Medicine: Development and applications. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1489. [Google Scholar] [CrossRef]
- Collins, F.S.; Green, E.D.; Guttmacher, A.E.; Guyer, M.S.; US National Human Genome Research Institute. A vision for the future of genomics research. Nature 2003, 422, 835–847. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol 2017, 18, 83. [Google Scholar] [CrossRef]
- Emilsson, V.; Thorleifsson, G.; Zhang, B.; Leonardson, A.S.; Zink, F.; Zhu, J.; Carlson, S.; Helgason, A.; Walters, G.B.; Gunnarsdottir, S.; et al. Genetics of gene expression and its effect on disease. Nature 2008, 452, 423–428. [Google Scholar] [CrossRef]
- Khan, S.R.; Manialawy, Y.; Wheeler, M.B.; Cox, B.J. Unbiased data analytic strategies to improve biomarker discovery in precision medicine. Drug Discov. Today 2019, 24, 1735–1748. [Google Scholar] [CrossRef]
- Danhof, M. Systems pharmacology—Towards the modeling of network interactions. Eur. J. Pharm. Sci. 2016, 94, 4–14. [Google Scholar] [CrossRef]
- Clifton, D.A.; Niehaus, K.E.; Charlton, P.; Colopy, G.W. Health informatics via machine learning for the clinical management of patients. Yearb. Med. Inform. 2015, 10, 38–43. [Google Scholar] [CrossRef][Green Version]
- Hung, A.J. Can machine-learning algorithms replace conventional statistics? BJU Int. 2019, 123, 1. [Google Scholar] [CrossRef]
- Reel, P.S.; Reel, S.; Pearson, E.; Trucco, E.; Jefferson, E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol. Adv. 2021, 49, 107739. [Google Scholar] [CrossRef]
- Liao, J.G.; Chin, K.V. Logistic regression for disease classification using microarray data: Model selection in a large p and small n case. Bioinformatics 2007, 23, 1945–1951. [Google Scholar] [CrossRef]
- Vidal, M.; Cusick, M.E.; Barabási, A.L. Interactome networks and human disease. Cell 2011, 144, 986–998. [Google Scholar] [CrossRef]
- Barabási, A.L. Network medicine—From obesity to the “diseasome”. N. Engl. J. Med. 2007, 357, 404–407. [Google Scholar] [CrossRef]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Breiteneder, H.; Peng, Y.Q.; Agache, I.; Diamant, Z.; Eiwegger, T.; Fokkens, W.J.; Traidl-Hoffmann, C.; Nadeau, K.; O’Hehir, R.E.; O’Mahony, L.; et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy 2020, 75, 3039–3068. [Google Scholar] [CrossRef] [PubMed]
- Koen, N.; Du Preez, I.; Loots, D.T. Metabolomics and personalized medicine. Adv. Protein Chem. Struct. Biol. 2016, 102, 53–78. [Google Scholar] [CrossRef]
- Brandão, M.; Pondé, N.; Piccart-Gebhart, M. Mammaprint™: A comprehensive review. Future Oncol. 2019, 15, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Post, T.M.; Peletier, L.A.; Boroujerdi, M.A.; Danhof, M. Coping with time scales in disease systems analysis: Application to bone remodeling. J. Pharmacokinet. Pharmacodyn. 2011, 38, 873–900. [Google Scholar] [CrossRef][Green Version]
- Post, T.M.; Schmidt, S.; Peletier, L.A.; de Greef, R.; Kerbusch, T.; Danhof, M. Application of a mechanism-based disease systems model for osteoporosis to clinical data. J. Pharmacokinet. Pharmacodyn. 2013, 40, 143–156. [Google Scholar] [CrossRef]
- Klaeger, S.; Heinzlmeir, S.; Wilhelm, M.; Polzer, H.; Vick, B.; Koenig, P.A.; Reinecke, M.; Ruprecht, B.; Petzoldt, S.; Meng, C.; et al. The target landscape of clinical kinase drugs. Science 2017, 358, eaan4368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, X.; He, S.; Jiang, H.; Feng, F.; Liu, W.; Qu, W.; Sun, H. Rational design of multitarget-directed ligands: Strategies and emerging paradigms. J. Med. Chem. 2019, 62, 8881–8914. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Identification of target associations for polypharmacology from analysis of crystallographic ligands of the Protein Data Bank. J. Chem. Inf. Model. 2020, 60, 372–390. [Google Scholar] [CrossRef]
- Garuti, L.; Roberti, M.; Bottegoni, G. Multi-kinase inhibitors. Curr. Med. Chem. 2015, 22, 695–712. [Google Scholar] [CrossRef]
- Lim, H.; He, D.; Qiu, Y.; Krawczuk, P.; Sun, X.; Xie, L. Rational discovery of dual-indication multitarget PDE/Kinase inhibitor for precision anti-cancer therapy using structural systems pharmacology. PLoS Comput. Biol. 2019, 15, e1006619. [Google Scholar] [CrossRef]
- Kuenzi, B.M.; Remsing Rix, L.L.; Stewart, P.A.; Fang, B.; Kinose, F.; Bryant, A.T.; Boyle, T.A.; Koomen, J.M.; Haura, E.B.; Rix, U. Polypharmacology-based ceritinib repurposing using integrated functional proteomics. Nat. Chem. Biol. 2017, 13, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Sugimoto, Y.; Greten, H.J.; Efferth, T. Repurposing of bromocriptine for cancer therapy. Front. Pharmacol. 2018, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Dinić, J.; Efferth, T.; García-Sosa, A.T.; Grahovac, J.; Padrón, J.M.; Pajeva, I.; Rizzolio, F.; Saponara, S.; Spengler, G.; Tsakovska, I. Repurposing old drugs to fight multidrug resistant cancers. Drug Resist. Updates 2020, 52, 100713. [Google Scholar] [CrossRef]
- Boulos, J.C.; Saeed, M.E.M.; Chatterjee, M.; Bülbül, Y.; Crudo, F.; Marko, D.; Munder, M.; Klauck, S.M.; Efferth, T. Repurposing of the ALK inhibitor crizotinib for acute leukemia and multiple myeloma cells. Pharmaceuticals 2021, 14, 1126. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Efferth, T. Repurposing of artemisinin-type drugs for the treatment of acute leukemia. Semin. Cancer Biol. 2021, 68, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Desai, R.J.; Handy, D.E.; Wang, R.; Schneeweiss, S.; Barabási, A.L.; Loscalzo, J. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat. Commun. 2018, 9, 2691. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Chaudhari, R.; Fong, L.W.; Tan, Z.; Huang, B.; Zhang, S. An up-to-date overview of computational polypharmacology in modern drug discovery. Expert Opin. Drug Discov. 2020, 15, 1025–1044. [Google Scholar] [CrossRef]
- Schneider, P.; Röthlisberger, M.; Reker, D.; Schneider, G. Spotting and designing promiscuous ligands for drug discovery. Chem. Commun. (Camb.) 2016, 52, 1135–1138. [Google Scholar] [CrossRef]
- Da, C.; Zhang, D.; Stashko, M.; Vasileiadi, E.; Parker, R.E.; Minson, K.A.; Huey, M.G.; Huelse, J.M.; Hunter, D.; Gilbert, T.S.K.; et al. Data-driven construction of antitumor agents with controlled polypharmacology. J. Am. Chem. Soc. 2019, 141, 15700–15709. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Wu, X.; Xiong, Z.; Yang, T.; Fu, Z.; Liu, X.; Tan, X.; Zhong, F.; Wan, X.; et al. Deep learning enhancing kinome-wide polypharmacology profiling: Model construction and experiment validation. J. Med. Chem. 2020, 63, 8723–8737. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Liu, C.; Wang, Q.; Lin, P.; Cheng, F. In silico polypharmacology of natural products. Brief. Bioinform. 2018, 19, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multitarget therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Casey, S.C.; Amedei, A.; Aquilano, K.; Azmi, A.S.; Benencia, F.; Bhakta, D.; Bilsland, A.E.; Boosani, C.S.; Chen, S.; Ciriolo, M.R.; et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef] [PubMed]
- Meerson, A.; Khatib, S.; Mahajna, J. Natural products targeting cancer stem cells for augmenting cancer therapeutics. Int. J. Mol. Sci. 2021, 22, 13044. [Google Scholar] [CrossRef]
- Schmidt, F.; Efferth, T. Tumor heterogeneity, single-cell sequencing, and drug resistance. Pharmaceuticals 2016, 9, 33. [Google Scholar] [CrossRef]
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, X.X.; Liu, J.P.; Luo, H.; Ma, L.X.; Alraek, T. Traditional Chinese medicine for chronic fatigue syndrome: A systematic review of randomized clinical trials. Complement. Ther. Med. 2014, 22, 826–833. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Shergis, J.; Zhang, L.; Zhang, A.L.; Guo, X.; Qin, X.; Johnson, D.; Liu, X.; Lu, C.; et al. Chinese herbal medicine for diabetic kidney disease: A systematic review and meta-analysis of randomised placebo-controlled trials. BMJ. Open 2019, 9, e025653. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, X.; Yuan, P.; Liu, J.; Wang, B.; Wang, G. Efficacy of traditional Chinese Medicine combined with chemotherapy in patients with non-small cell lung cancer (NSCLC): A meta-analysis of randomized clinical trials. Support. Care Cancer 2020, 28, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.B.; Fang, M.; Liang, C.H.; Lan, H.D.; Shen, C.; Yan, L.J.; Hu, X.Y.; Han, M.; Robinson, N.; Liu, J.P. Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: A rapid systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2021, 60, 102744. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Jin, X.; Ma, Y.; Yang, Y.; Li, J.; Liang, L.; Liu, R.; Li, Z. A comprehensive application: Molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine. Comput. Biol. Chem. 2021, 90, 107402. [Google Scholar] [CrossRef]
- Han, S.; Lv, A.P.; Li, J. Application review of network pharmacology in the study of properties theory of traditional Chinese medicine. J. Basic Chin. Med. 2019, 25, 127–130. [Google Scholar]
- Zhou, Z.; Chen, B.; Chen, S.; Lin, M.; Chen, Y.; Jin, S.; Chen, W.; Zhang, Y. Applications of Network Pharmacology in Traditional Chinese Medicine Research. Evid. Based Complement. Alternat. Med. 2020, 2020, 1646905. [Google Scholar] [CrossRef]
- Lee, D.Y.W.; Li, Q.Y.; Liu, J.; Efferth, T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: Clinical experience and scientific basis. Phytomedicine 2021, 80, 153337. [Google Scholar] [CrossRef]
- Chen, K.X.; Jiang, H.L.; Luo, X.M.; Shen, J.H. Drug discovery in postgenome era: Trend and practice. Chin. J. Nat. Med. 2004, 2, 257–260. [Google Scholar]
- Li, S.; Zhang, Z.; Wu, L.; Zhang, X.; Li, Y.; Wang, Y. Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst. Biol. 2007, 1, 51–60. [Google Scholar] [CrossRef]
- de Villiers, L. Loots, DT Using metabolomics for elucidating the mechanisms related to tuberculosis treatment failure. Curr. Metab. 2013, 1, 306–317. [Google Scholar] [CrossRef]
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based approaches in pharmacology. Mol. Inform. 2017, 36, 1700048. [Google Scholar] [CrossRef]
- Young, M.; Hoheisel, J.D.; Efferth, T. Toxicogenomics for the prediction of toxicity related to herbs from traditional Chinese medicine. Planta Med. 2010, 76, 2019–2025. [Google Scholar] [CrossRef]
- Börner, F.U.; Schütz, H.; Wiedemann, P. The fragility of omics risk and benefit perceptions. Toxicol. Lett. 2011, 201, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Aardema, M.J.; MacGregor, J.T. Toxicology and genetic toxicology in the new era of “toxicogenomics”: Impact of “-omics” technologies. Mutat. Res. 2002, 499, 13–25. [Google Scholar] [CrossRef]
- Lewis, W.H.; Elwin-Lewis, M.P.F. Panaceas, Adaptogens, and Tonics. In Medical Botany: Plants Affecting Human Health, 2nd ed.; Lewis, W.H., Elwin-Lewis, M.P.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; Part II, Chapter 18; pp. 608–628. [Google Scholar]
- de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; Miola, V.; Guissoni Campos, L.M.; Spilla, C.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component, ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, B.; Bajpai, V. Andrographis aniculate (Burm.f.) Nees: Traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J. Ethnopharmacol. 2021, 275, 114054. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Wei, A.; Zhou, Q.; Yuan, M.; Lei, K.; Liu, Y.; Song, J.; Guo, L.; Ye, Q. Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytother. Res. 2022, 36, 336–364. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Khan, M.I.; Maqsood, M.; Saeed, R.A.; Alam, A.; Sahar, A.; Kieliszek, M.; Miecznikowski, A.; Muzammil, H.S.; Aadil, R.M. Phytochemistry, food application, and therapeutic potential of the medicinal plant (Withania coagulans): A review. Molecules 2021, 26, 6881. [Google Scholar] [CrossRef]
- Ahsan, R.; Arshad, M.; Khushtar, M.; Ahmad, M.A.; Muazzam, M.; Akhter, M.S.; Gupta, G.; Muzahid, M. A Comprehensive review on physiological effects of curcumin. Drug Res. 2020, 70, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Bahroudi, Z.; Hussen, B.M.; Talebi, S.F.; Taheri, M.; Ayatollahi, S.A. Nrf2-Related therapeutic effects of curcumin in different disorders. Biomolecules 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Montecucco, F.; Carbone, F.; Sahebkar, A. Effects of curcumin on aging: Molecular mechanisms and experimental evidence. Biomed. Res. Int. 2021, 2021, 8972074. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Gabrielian, E.; Wagner, H. Plant adaptogens. II. Bryonia as an adaptogen. Phytomedicine 1997, 4, 85–99. [Google Scholar] [CrossRef]
- Forsdike, K.; Pirotta, M. St John’s wort for depression: Scoping review about perceptions and use by general practitioners in clinical practice. J. Pharm. Pharmacol. 2019, 71, 117–128. [Google Scholar] [CrossRef]
- Xiao, C.Y.; Mu, Q.; Gibbons, S. The phytochemistry and pharmacology of Hypericum. Prog. Chem. Org. Nat. Prod. 2020, 112, 85–182. [Google Scholar] [CrossRef]
- Tanaka, N.; Kashiwada, Y. Characteristic metabolites of Hypericum plants: Their chemical structures and biological activities. J. Nat. Med. 2021, 75, 423–433. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Spagnolo, E.V.; Musolino, C.; Gangemi, S. Antiproliferative effects of St. John’s Wort, its derivatives, and other Hypericum species in hematologic malignancies. Int. J. Mol. Sci. 2020, 22, 146. [Google Scholar] [CrossRef]
- Brekhman, I.I.; Dardymov, I.V. New substances of plant origin which increase nonspecific resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef]
- Leitão, S.G.; Leitão, G.G.; de Oliveira, D.R. Saracura-Mirá, a Proposed Brazilian Amazonian Adaptogen from Ampelozizyphus amazonicus. Plants 2022, 11, 191. [Google Scholar] [CrossRef]
- Allen, K.; Bennett, J.W. Tour of truffles: Aromas, aphrodisiacs, adaptogens, and more. Mycobiology 2021, 49, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Brendler, T. The role of adaptogens in prophylaxis and treatment of viral respiratory infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.Y.; He, Y.F.; Li, L.; Meng, H.; Dong, Y.M.; Yi, F.; Xiao, P.G. A preliminary review of studies on adaptogens: Comparison of their bioactivity in TCM with that of ginseng-like herbs used worldwide. Chin. Med. 2018, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, E.; Amato, M.; Izzo, A.A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000, 71 (Suppl. S1), S1–S5. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, L.H.; Zhang, J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol. Sin. 2005, 26, 143–149. [Google Scholar] [CrossRef]
- Yoon, S.J.; Kim, S.K.; Lee, N.Y.; Choi, Y.R.; Kim, H.S.; Gupta, H.; Youn, G.S.; Sung, H.; Shin, M.J.; Suk, K.T. Effect of Korean Red Ginseng on metabolic syndrome. J. Ginseng Res. 2021, 45, 380–389. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, L.; Wang, L. A narrative review of the pharmacology of ginsenoside compound K. Ann. Transl. Med. 2022, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Hiai, S.; Yokoyama, H.; Oura, H.; Yano, S. Stimulation of pituitary-adrenocortical system by ginseng saponin. Endocrinol. Jpn. 1979, 26, 661–665. [Google Scholar] [CrossRef]
- Filaretov, A.A.; Bogdanova, T.S.; Podvigina, T.T.; Bodganov, A.I. Role of pituitary-adrenocortical system in body adaptation abilities. Exp. Clin. Endocrinol. 1988, 92, 129–136. [Google Scholar] [CrossRef]
- Zhang, J.T.; Qu, Z.W.; Liu, Y.; Deng, H.L. Preliminary study on antiamnestic mechanism of ginsenoside Rg1 and Rb1. Chin. Med. J. 1990, 103, 932–938. [Google Scholar] [CrossRef]
- Jin, W.; Ma, R.; Zhai, L.; Xu, X.; Lou, T.; Huang, Q.; Wang, J.; Zhao, D.; Li, X.; Sun, L. Ginsenoside Rd attenuates ACTH-induced corticosterone secretion by blocking the MC2R-cAMP/PKA/CREB pathway in Y1 mouse adrenocortical cells. Life Sci. 2020, 245, 117337. [Google Scholar] [CrossRef]
- Zarneshan, S.N.; Fakhri, S.; Khan, H. Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacol. Res. 2022, 177, 106099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Kwak, Y.S.; Han, C.K.; Hyun, S.H.; Rhee, M.H. Adaptogenic effects of Panax ginseng on modulation of cardiovascular functions. J. Ginseng Res. 2020, 44, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Kim, M.; Rhee, M.H. Anti-platelet role of Korean ginseng and ginsenosides in cardiovascular diseases. J. Ginseng Res. 2020, 44, 24–32. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, J.; Xu, J.F.; Tang, F.; Chen, L.; Tan, Y.Z.; Rao, C.L.; Ao, H.; Peng, C. Panax ginseng and its ginsenosides: Potential candidates for the prevention and treatment of chemotherapy-induced side effects. J. Ginseng Res. 2021, 45, 617–630. [Google Scholar] [CrossRef]
- “Difference between Mode of Action and Mechanism of Action”. Difference Between.Com. Available online: http://www.differencebetween.com/difference-between-mode-of-action-and-vsmechanism-of-action/ (accessed on 22 February 2022).
- Todorova, V.; Ivanov, K.; Ivanova, S. Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants 2021, 11, 64. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens-History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef]
- Lee, T.X.Y.; Wu, J.; Jean, W.H.; Condello, G.; Alkhatib, A.; Hsieh, C.C.; Hsieh, Y.W.; Huang, C.Y.; Kuo, C.H. Reduced stem cell aging in exercised human skeletal muscle is enhanced by ginsenoside Rg1. Aging (Albany New York) 2021, 13, 16567–16576. [Google Scholar] [CrossRef]
- Wu, J.; Saovieng, S.; Cheng, I.S.; Liu, T.; Hong, S.; Lin, C.Y.; Su, I.C.; Huang, C.Y.; Kuo, C.H. Ginsenoside Rg1 supplementation clears senescence-associated β-galactosidase in exercising human skeletal muscle. J. Ginseng Res. 2019, 43, 580–588. [Google Scholar] [CrossRef]
- Hou, C.W.; Lee, S.D.; Kao, C.L.; Cheng, I.S.; Lin, Y.N.; Chuang, S.J.; Chen, C.Y.; Ivy, J.L.; Huang, C.Y.; Kuo, C.H. Improved inflammatory balance of human skeletal muscle during exercise after supplementations of the ginseng-based steroid Rg1. PLoS ONE 2015, 10, e0116387. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, W.-S.; Gao, C.H.; Deng, L.C.; Shen, D. Protective effects of salidroside on epirubicin-induced early left ventricular regional systolic dysfunction in patients with breast cancer. Drugs RD 2012, 12, 101–106. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, R.A.; Zotova, M.I.; Nekhoda, M.F.; Cherdintsev, S.G. Comparative characteristics of the stimulating and adaptogenic effects of Rhodiola rosea preparations. In Stimulants of the Central Nervous System; Saratikov, A.S., Ed.; Tomsk University Press: Tomsk, Russia, 1968; Volume 2, pp. 3–12. [Google Scholar]
- Ciampi, E.; Uribe-San-Martin, R.; Cárcamo, C.; Cruz, J.P.; Reyes, A.; Reyes, D.; Pinto, C.; Vásquez, M.; Burgos, R.A.; Hancke, J. Efficacy of andrographolide in not active progressive multiple sclerosis: A prospective exploratory double-blind, parallel-group, randomized, placebo-controlled trial. BMC Neurol. 2020, 20, 173. [Google Scholar] [CrossRef] [PubMed]



| Stimulating and tonic effects on the CNS system |
| Modulation of the stress response system, including the hypothalamus–hypophysis–adrenal (HPA) axis |
| Modulation of the endocrine system and metabolic regulation |
| Regulation of cellular homeostasis and metabolism |
| Modulation of the immune response |
|
| Anti-inflammatory activity |
|
| Detoxification and reparation of oxidative stress-induced damages in compromised cells |
|
| Direct antiviral activity |
|
| Type(s) | Gene Symbol | Gene and Regulated Protein Name | Signaling Pathways | Biological Processes | Physiol. Functions | Diseases |
|---|---|---|---|---|---|---|
| Hormones | CRH | Corticotropin-releasing hormone | 2 | 54 | 7 | 18 |
| ACTH | Adrenocorticotrophic hormone; ACTH | 2 | ||||
| UCN | Urocortin (corticotropin-releasing factor family) | 1 | 53 | 7 | 4 | |
| GNRH1 | Gonadotropin-releasing hormone 1 | 3 | 25 | 10 | 12 | |
| Transmembrane receptors | TLR9 | Toll-like receptor 9, member of PI3K (complex) | 152 | 65 | 7 | 66 |
| CHRNE | Cholinergic receptor nicotinic epsilon subunit | 3 | 12 | 8 | 22 | |
| PRLR | Prolactin receptor | 2 | 17 | 7 | 11 | |
| G-protein coupled receptor | CHRM4 | Cholinergic receptor muscarinic 4 | 5 | 11 | 5 | 173 |
| Nuclear receptor | RORA | RAR-related orphan receptor A (RZR) | Melatonin signaling | - | 16 | 17 |
| Transcription regulators | STAT5A | Signal transducer and activator of transcription 5A | 19 | 57 | 13 | 10 |
| FOS | Fos proto-oncogene, AP-1 transcription factor subunit | 21 | 37 | 15 | 52 | |
| FOXO6 | Forkhead box O6 | 3 | 7 | 5 | 10 | |
| Kinases | FLT1 | Fms-related tyrosine kinase 1 | 9 | |||
| MAPK10 JNK, SAPK1 | Mitogen-activated protein kinase 10, c-Jun N-terminal kinase | 77 | 12 | 11 | 8 | |
| MAPK13 p38, SAPK2 | Mitogen-activated protein kinase 13, p-38 MAP kinase | 59 | 14 | 10 | 15 | |
| PRKCH | Protein kinase C eta | 72 | 15 | 11 | 20 | |
| PKA | protein kinase A ACTH induced | cAMP/PKA/CREB signaling | ||||
| PKB | Protein kinase B - AKT | |||||
| Metabolic enzymes | GUCY1A2 | Guanylate cyclase 1 soluble subunit alpha 2 | 19 | 4 | 6 | 32 |
| HSPA6 | Heat shock protein family A (Hsp70) member 6 | 6 | 3 | 5 | 3 | |
| PDE3B | Phosphodiesterase 3B | 16 | ||||
| PDE9A | Phosphodiesterase 9A | 6 |
| Canonical Pathways |
|---|
| AMPK signaling Axonal guidance signaling Calcium signaling cAMP-mediated signaling Cardiac β-adrenergic signaling Chronic obstructive pulmonary disease signaling Colorectal cancer metastasis signaling Corticotropin-releasing hormone signaling CREB signaling in neurons CXCR4 signaling Dendritic cell maturation signaling Dopamine-DARPP32 feedback in cAMP signaling eNOS signaling Glutamate receptor signaling GP6 signaling pathway G-protein-coupled receptor signaling Inositol pyrophosphate biosynthesis Leptin signaling in obesity LPS-stimulated MAPK signaling Melatonin signaling and degradation Neuroinflammation signaling pathway Neuropathic pain signaling in dorsal horn neurons Nitric oxide signaling in the cardiovascular system NRF2-mediated oxidative stress response signaling Opioid signaling pathway Protein kinase A signaling Relaxin signaling Renin–angiotensin signaling Osteoblasts, osteoclasts, and chondrocytes in rheumatoid arthritis signaling Salvage pathways of pyrimidine nucleotide signaling Sperm motility signaling Super pathway of inositol phosphate compounds signaling Synaptic long-term depression signaling Telomere extension by telomerase signaling tRNA splicing signaling |
| Cellular Function | Genes |
|---|---|
Cellular compromise:
| AIPL1, ALOX12, CDHR1, NGB3, GNLY, HLA-B, NCAM1, SERPINA1, ULBP3, XRCC5 |
| Cell signaling | PDE3A, MUC20, PDE4D, PDE11A, ESR1, CCKBR |
| DNA replication, recombination, and repair | PARPBP, PDE3A, APLF, PDE4D, PDE11A, XRCC5, AICDA |
| Nucleic acid metabolism | PFKFB1, MTNR1A, PDE3A, APOBEC2, TAAR1, PDE4D, PDE11A, AIPL1, ESR1, AICDA |
| Lipid metabolism | NR4A3, RGS3, SLC27A2, AKR1D1, TNXB, SERPINA1, ALOX12, ESR1, CCKBR, CETP, NCAM1 |
| Category | Diseases | Genes Affected by Adaptogens |
|---|---|---|
| Organismal injury and abnormalities | Physical disability Degeneration of retinal cone cells Atrophy of gastric mucosa Hypoestrogenism Postmenopausal vulvar atrophy Nociception Cone dystrophy Pelvic organ prolapse | PDE11A,PDE3A,PDE4D AIPL1,CNGB3 CCKBR ESR1 ESR1б MTNR1A KCNK10, PDE11A,PDE3A,PDE4D,SCN2B CDHR1,CNGB3 ESR1, SERPINA1 |
| Inflammatory and pulmonary diseases | Pulmonary emphysema Bronchiectasis Chronic bronchitis Chronic obstructive pulmonary disease | PDE11A,PDE3A,PDE4D,SERPINA1 PDE11A,PDE3A,PDE4D MMP8,MTNR1A PDE11A,PDE3A,PDE4D,SERPINA1 |
| Neurological and psychological diseases | Non-24 h sleep–wake disorder Sleep–wake schedule disorder | MTNR1A PDE3A |
| Cardiovascular diseases | Ischemic cardiomyopathy Cholesteryl ester transfer protein deficiency Angina pectoris Cerebral small vessel disease | PDE11A,PDE3A,PDE4D,PPP1R1A CETP PDE11A,PDE3A,PDE4D–all upregulated PDE3A-unregulated |
| Skeletal and connective tissues | Osteochondrodysplasia | COL9A1, PDE4D |
| Metabolic disease | Estrogen resistance | ESR1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panossian, A.; Efferth, T. Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity. Pharmaceuticals 2022, 15, 1051. https://doi.org/10.3390/ph15091051
Panossian A, Efferth T. Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity. Pharmaceuticals. 2022; 15(9):1051. https://doi.org/10.3390/ph15091051
Chicago/Turabian StylePanossian, Alexander, and Thomas Efferth. 2022. "Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity" Pharmaceuticals 15, no. 9: 1051. https://doi.org/10.3390/ph15091051
APA StylePanossian, A., & Efferth, T. (2022). Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity. Pharmaceuticals, 15(9), 1051. https://doi.org/10.3390/ph15091051







