Combination of Talazoparib and Calcitriol Enhanced Anticancer Effect in Triple−Negative Breast Cancer Cell Lines
Abstract
:1. Introduction
2. Results
2.1. Antiproliferative Effect of Talazoparib, Calcitriol and Their Combination in TNBC Cells
2.2. Cell Migration Profile of TNBC Cells Treated by Talazoparib, Calcitriol and Their Combination
2.3. Talazoparib and Calcitriol Induced Apoptosis in BT−20 Cells
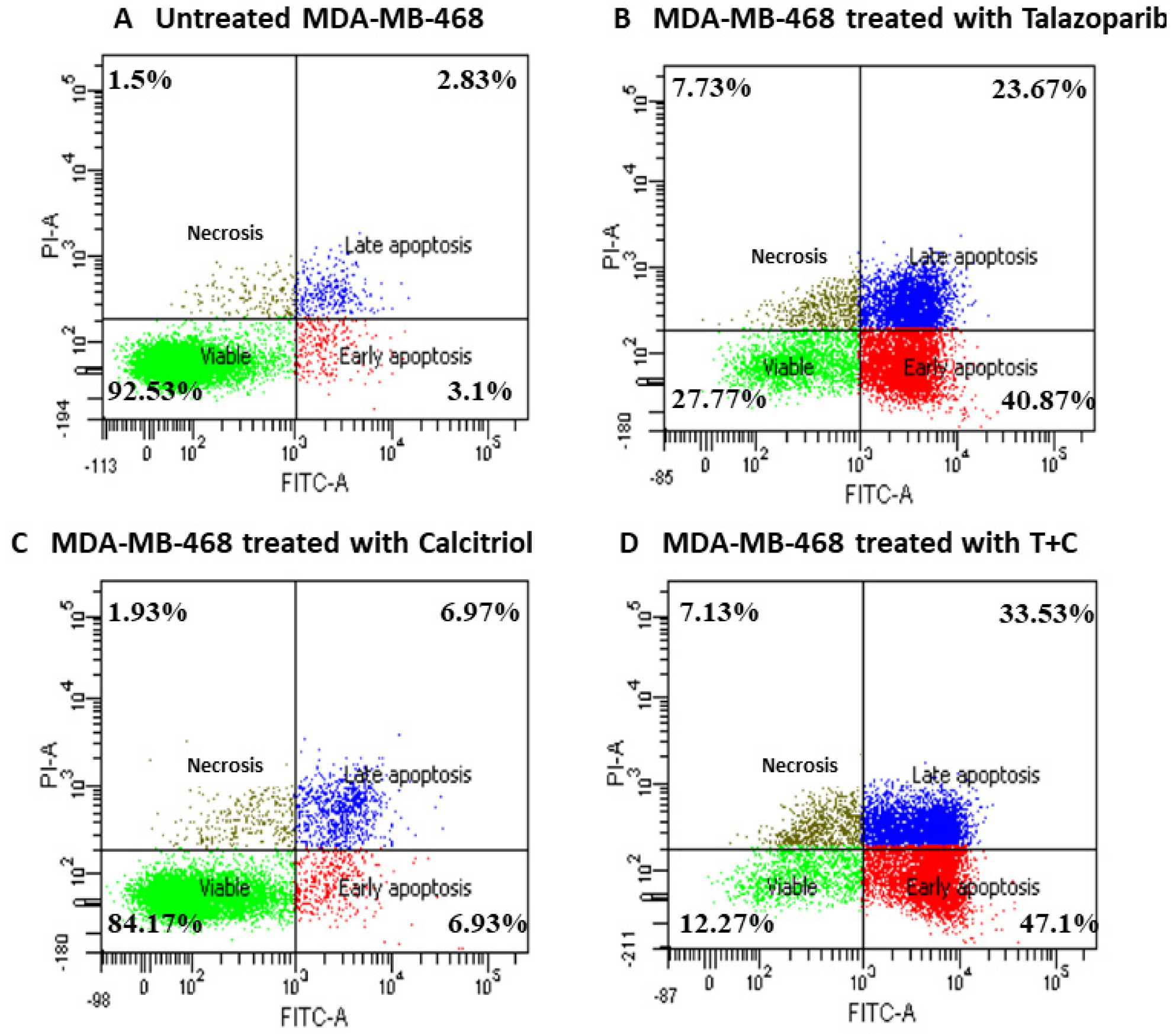
2.4. Talazoparib and Calcitriol Induced Apoptosis in MDA−MB−468 Cells
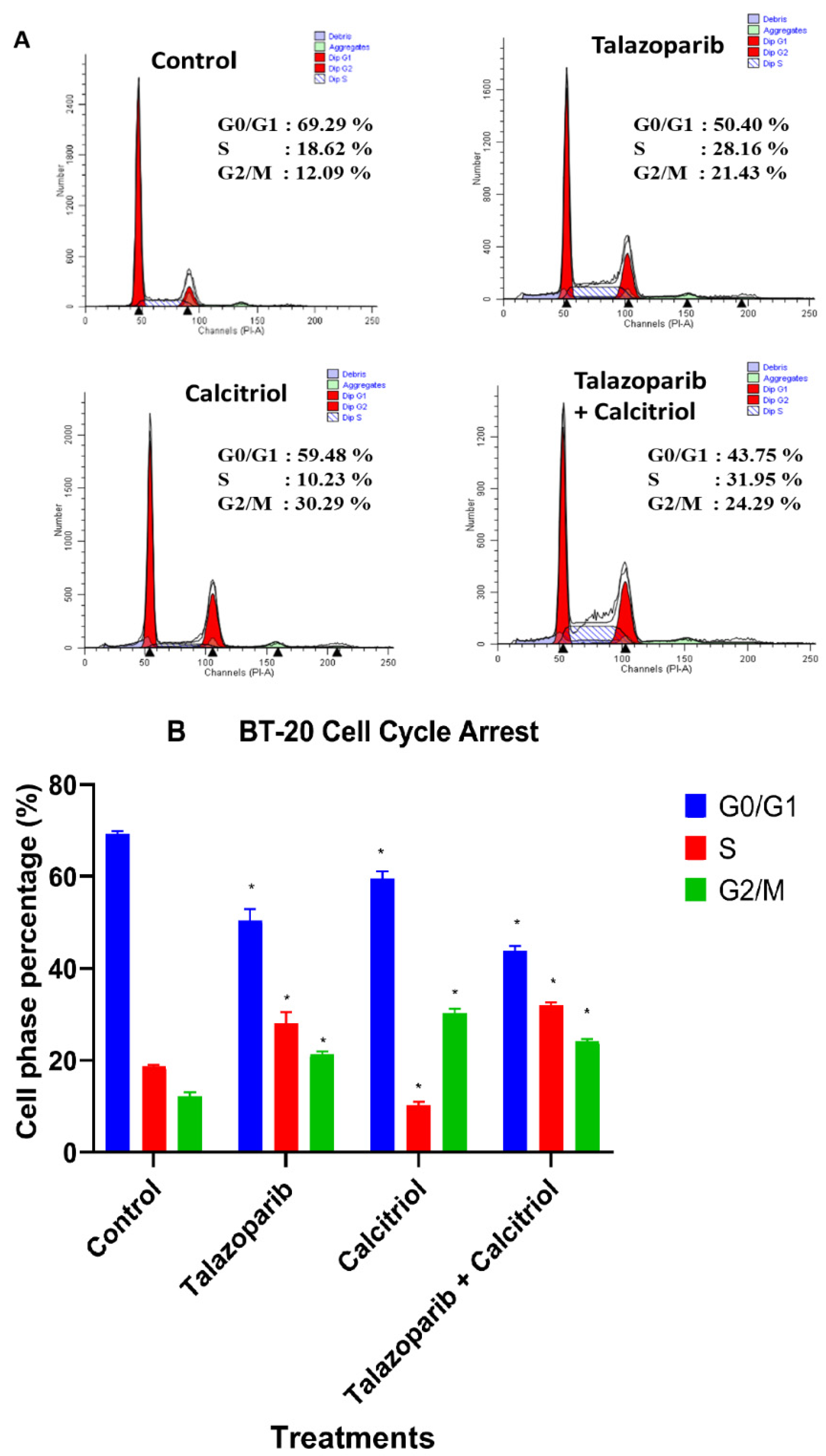
2.5. Cell Cycle Arrest in BT−20 Cells
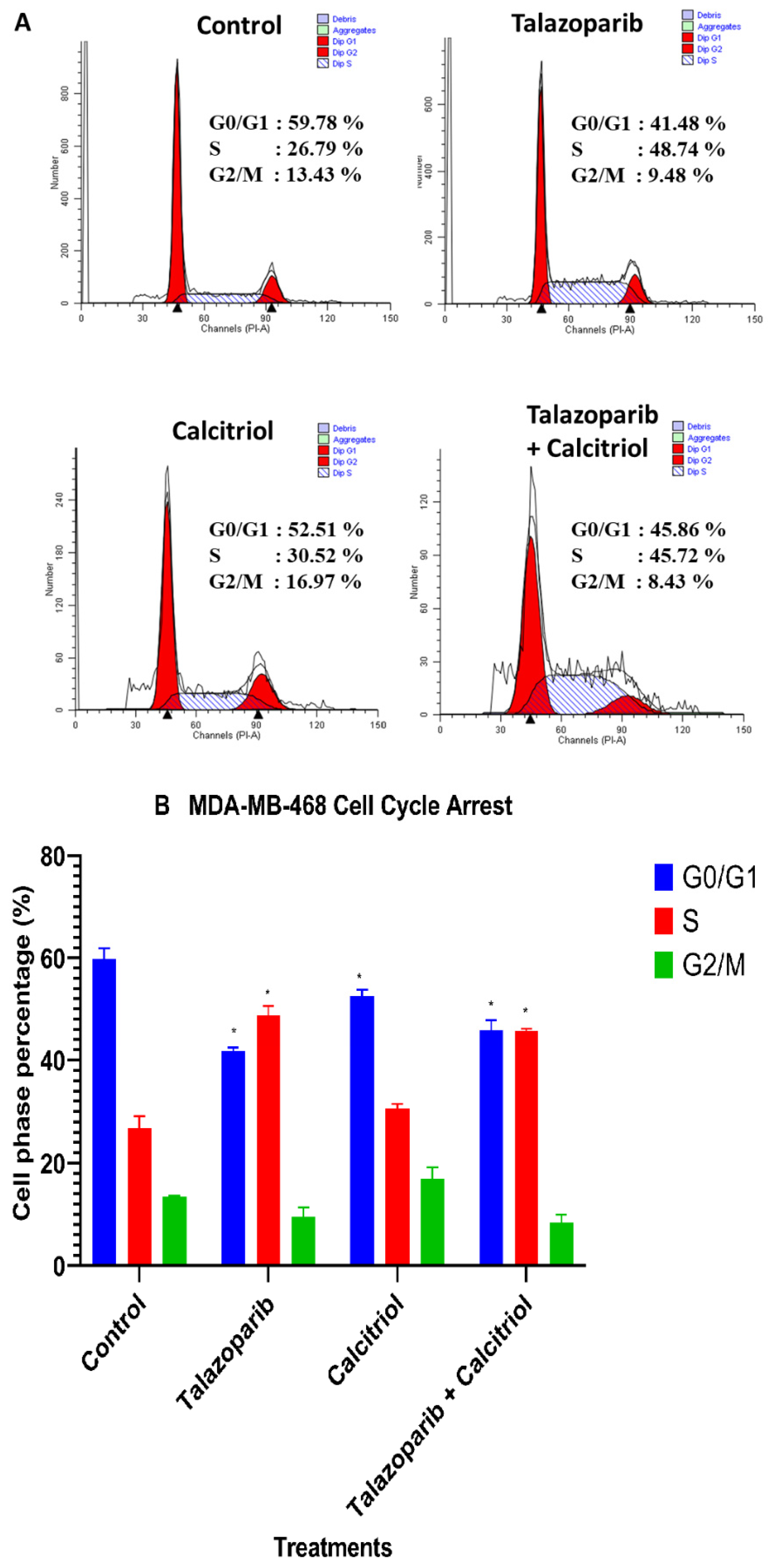
2.6. Cell−Cycle Arrest in MDA−MB−468 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Cell Lines and Cell Culture
4.3. Measuring Antiproliferative Assay using Real−Time Cell Analyzer (RTCA)
4.4. Cell Migration Analysis
4.5. Apoptosis and Cell Cycle Analysis Using Flow Cytometry
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Htay, M.N.N.; Donnelly, M.; Schliemann, D.; Loh, S.Y.; Dahlui, M.; Somasundaram, S.; Ibrahim Tamin, N.S.B.; Su, T.T. Breast Cancer Screening in Malaysia: A Policy Review. Asian Pac. J. Cancer Prev. 2021, 22, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.D.; Caldas, C. Genetic heterogeneity in breast cancer: The road to personalized medicine? BMC Med. 2013, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Amini, F.; Wong, F.H.; Ng, E.S.C.; Ng, C.Y.; Omar, R.; Mohd Rejab, S.; Mohd Noor, I.; Mohd Hussain, B. Mutation Profile of Breast Cancer in Malaysia Patiens. JUMMEC 2021, 24, 37–44. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.3322/caac.21590 (accessed on 31 December 2021).
- Boyle, P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann. Oncol. 2012, 23 (Suppl. S6), 7–12. [Google Scholar] [CrossRef]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. Biofactors 2017, 43, 517–528. [Google Scholar] [CrossRef]
- Lebert, J.M.; Lester, R.; Powell, E.; Seal, M.; McCarthy, J. Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol. 2018, 25, 142–150. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Gene Expression Alterations Associated with Oleuropein-Induced Antiproliferative Effects and S-Phase Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Nutrients 2020, 12, 3755. [Google Scholar] [CrossRef]
- Weiss, A.; Le Roux-Bourdieu, M.; Zoetemelk, M.; Ramzy, G.M.; Rausch, M.; Harry, D.; Miljkovic-Licina, M.; Falamaki, K.; Wehrle-Haller, B.; Meraldi, P.; et al. Identification of a Synergistic Multi-Drug Combination Active in Cancer Cells via the Prevention of Spindle Pole Clustering. Cancers 2019, 11, 1612. [Google Scholar] [CrossRef]
- Sadeghloo, A.Y.; Khorsandi, K.; Kianmehr, Z. Synergistic effect of photodynamic treatment and doxorubicin on triple negative breast cancer cells. Photochem. Photobiol. Sci. 2020, 19, 1580–1589. [Google Scholar] [CrossRef]
- Ren, G.; Wu, C.; Teng, C.; Yao, Y. Synergistic effect of combined protopanaxatiol and ginsenoside Rh2 on antiproliferative activity in MDA-MB-231 human breast cancer cells in vitro. Food Agric. Immunol. 2018, 29, 953–963. [Google Scholar] [CrossRef] [Green Version]
- García-Quiroz, J.; García-Becerra, R.; Santos-Cuevas, C.; Ramírez-Nava, G.J.; Morales-Guadarrama, G.; Cárdenas-Ochoa, N.; Segovia-Mendoza, M.; Prado-Garcia, H.; Ordaz-Rosado, D.; Avila, E.; et al. Synergistic Antitumorigenic Activity of Calcitriol with Curcumin or Resveratrol is Mediated by Angiogenesis Inhibition in Triple Negative Breast Cancer Xenografts. Cancers 2019, 11, 1739. [Google Scholar] [CrossRef] [PubMed]
- Santos-Martínez, N.; Díaz, L.; Ordaz-Rosado, D.; García-Quiroz, J.; Barrera, D.; Avila, E.; Halhali, A.; Medina-Franco, H.; Ibarra-Sánchez, M.J.; Esparza-López, J.; et al. Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: A potential new therapeutic approach. BMC Cancer 2014, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.; Díaz-Muñoz, M.; García-Gaytán, A.C.; Méndez, I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef]
- Exman, P.; Barroso-Sousa, R.; Tolaney, S.M. Evidence to date: Talazoparib in the treatment of breast cancer. Onco Targets Ther. 2019, 12, 5177–5187. [Google Scholar] [CrossRef] [PubMed]
- Keung, M.Y.; Wu, Y.; Badar, F.; Vadgama, J.V. Response of Breast Cancer Cells to PARP Inhibitors Is Independent of BRCA Status. J. Clin. Med. 2020, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, M.; Mountzios, G.; Papadimitriou, C.A. The role of PARP inhibition in triple-negative breast cancer: Unraveling the wide spectrum of synthetic lethality. Cancer Treat. Rev. 2018, 67, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, J.; Wan, F.; Kou, L.; Yin, S.; Sun, Y.; Li, Y.; Zhou, Q.; Wang, T. Calcitriol Alleviates MPP(+)- and MPTP-Induced Parthanatos Through the VDR/PARP1 Pathway in the Model of Parkinson′s Disease. Front. Aging Neurosci. 2021, 13, 657095. [Google Scholar] [CrossRef] [PubMed]
- Mabley, J.G.; Wallace, R.; Pacher, P.; Murphy, K.; Szabó, C. Inhibition of poly(adenosine diphosphate-ribose) polymerase by the active form of vitamin D. Int. J. Mol. Med. 2007, 19, 947–952. [Google Scholar] [CrossRef]
- Qu, H.; Lin, K.; Wang, H.; Wei, H.; Ji, B.; Yang, Z.; Peng, C.; Xiao, X.; Deng, H. 1,25(OH)(2) D(3) improves cardiac dysfunction, hypertrophy, and fibrosis through PARP1/SIRT1/mTOR-related mechanisms in type 1 diabetes. Mol. Nutr. Food Res. 2017, 61, 1600338. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Wang, N.; Luo, G.; Wang, J.; Luo, C.; Yu, W.; Hao, L. 1α,25-Dihydroxyvitamin D(3) prevents renal oxidative damage via the PARP1/SIRT1/NOX4 pathway in Zucker diabetic fatty rats. Am. J. Physiol. Endocrinol. Metab. 2020, 318, 343–356. [Google Scholar] [CrossRef]
- Ajonijebu, D.C.; Olayanju, A.O.; Eduviere, A.T.; Adewumi, F.A.; Atodo, K.U.; Akinsanya, B.T.; Oresile, A.O.; Musa, A.; Abboussi, O.; Sanya, J.O. Effects of Calcitriol Supplementation on the Hematological Parameters of Sleep Deprived Wistar Rats. Available online: http://eprints.abuad.edu.ng/id/eprint/343 (accessed on 31 December 2021).
- Goicoechea, M.; Vazquez, M.I.; Ruiz, M.A.; Gomez-Campdera, F.; Perez-García, R.; Valderrábano, F. Intravenous calcitriol improves anaemia and reduces the need for erythropoietin in haemodialysis patients. Nephron 1998, 78, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Jennifer, K.L.; Joseph, T.B.; Jason, M.J.; Jay, A.; Joanne, L.B.; Lida, A.M.; Raymond, B.; Michael, A.D.; Yuan, Y.; Antonello, A.; et al. Talazoparib (TALA) is a Poly(ADP-Ribose) Polymerase Inhibitor Approved as Monotherapy for Treating Adult Patients (pts) with gBRCA1/2-Mutated HER2-Negative Locally Advanced or Metastatic BC. Available online: https://meetinglibrary.asco.org/record/196833/abstract (accessed on 19 March 2022).
- McCrea, M.A.; Giacino, J.T.; Barber, J.; Temkin, N.R.; Nelson, L.D.; Levin, H.S.; Dikmen, S.; Stein, M.; Bodien, Y.G.; Boase, K.; et al. Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurol. 2021, 78, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Schafer, E.S.; Rau, R.E.; Berg, S.L.; Liu, X.; Minard, C.G.; Bishop, A.J.R.; Romero, J.C.; Hicks, M.J.; Nelson, M.D., Jr.; Voss, S.; et al. Phase 1/2 trial of talazoparib in combination with temozolomide in children and adolescents with refractory/recurrent solid tumors including Ewing sarcoma: A Children′s Oncology Group Phase 1 Consortium study (ADVL1411). Pediatr. Blood Cancer 2020, 67, e28073. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.G.; Granier, C.; Zhao, Y.; Pan, Q.; Tong, Z.; Gschwend, J.E.; Holm, P.S.; Nawroth, R. Combination of Talazoparib and Palbociclib as a Potent Treatment Strategy in Bladder Cancer. J. Pers. Med. 2021, 11, 340. [Google Scholar] [CrossRef]
- Cotte, A.; Beniey, M.; Haque, T.; Béchir, N.; Hubert, A.; Diallo, K.W.; Tran-Thanh, D.; Hassan, S.N. Abstract 1066: Pre-clinical combination of a PARP inhibitor, talazoparib, and carboplatin in triple-negative breast cancer. Cancer Res. 2021, 81, 1066. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wigington, D.P.; Strugnell, S.A.; Knutson, J.C. Growth inhibition of cancer cells by an active metabolite of a novel vitamin D prodrug. Anticancer Res. 2005, 25, 4333–4339. [Google Scholar] [PubMed]
- Yu, W.D.; Ma, Y.; Flynn, G.; Muindi, J.R.; Kong, R.X.; Trump, D.L.; Johnson, C.S. Calcitriol enhances gemcitabine antitumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle 2010, 9, 3022–3029. [Google Scholar] [CrossRef]
- Thill, M.; Reichert, K.; Woeste, A.; Polack, S.; Fischer, D.; Hoellen, F.; Rody, A.; Friedrich, M.; Köster, F. Combined Treatment of Breast Cancer Cell Lines with Vitamin D and COX-2 Inhibitors. Available online: https://ar.iiarjournals.org/content/35/2/1189.long (accessed on 31 December 2021).
- Martínez-Reza, I.; Díaz, L.; Barrera, D.; Segovia-Mendoza, M.; Pedraza-Sánchez, S.; Soca-Chafre, G.; Larrea, F.; García-Becerra, R. Calcitriol Inhibits the Proliferation of Triple-Negative Breast Cancer Cells through a Mechanism Involving the Proinflammatory Cytokines IL-1β and TNF-α. J. Immunol. Res. 2019, 2019, 6384278. [Google Scholar] [CrossRef]
- Latimer, N.R.; Pollard, D.; Towse, A.; Henshall, C.; Sansom, L.; Ward, R.L.; Bruce, A.; Deakin, C. Challenges in valuing and paying for combination regimens in oncology: Reporting the perspectives of a multi-stakeholder, international workshop. BMC Health Serv. Res. 2021, 21, 412. [Google Scholar] [CrossRef]
- Bohl, L.; Guizzardi, S.; Rodríguez, V.; Hinrichsen, L.; Rozados, V.; Cremonezzi, D.; Tolosa de Talamoni, N.; Picotto, G. Combined calcitriol and menadione reduces experimental murine triple negative breast tumor. Biomed. Pharmacother. 2017, 94, 21–26. [Google Scholar] [CrossRef]
- Thakkar, A.; Wang, B.; Picon-Ruiz, M.; Buchwald, P.; Ince, T.A. Vitamin D and androgen receptor-targeted therapy for triple-negative breast cancer. Breast Cancer Res. Treat. 2016, 157, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Rehman, F.L.; Feng, Y.; Boshuizen, J.; Bajrami, I.; Elliott, R.; Wang, B.; Lord, C.J.; Post, L.E.; Ashworth, A. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin. Cancer Res. 2013, 19, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, T.; Lin, J. Synergistic Effect of Bazedoxifene and PARP Inhibitor in the Treatment of Ovarian Cancer Regardless of BRCA Mutation. Available online: https://ar.iiarjournals.org/content/anticanres/41/5/2277.full.pdf (accessed on 31 December 2021).
- Xing, W.Y.; Zhang, Z.H.; Xu, S.; Hong, Q.; Tian, Q.X.; Ye, Q.L.; Wang, H.; Yu, D.X.; Xu, D.X.; Xie, D.D. Calcitriol inhibits lipopolysaccharide-induced proliferation, migration and invasion of prostate cancer cells through suppressing STAT3 signal activation. Int. Immunopharmacol. 2020, 82, 106346. [Google Scholar] [CrossRef] [PubMed]
- Findlay, V.J.; Moretz, R.E.; Wang, C.; Vaena, S.G.; Bandurraga, S.G.; Ashenafi, M.; Marshall, D.T.; Watson, D.K.; Camp, E.R. Slug expression inhibits calcitriol-mediated sensitivity to radiation in colorectal cancer. Mol. Carcinog. 2014, 53 (Suppl. S1), 130–139. [Google Scholar] [CrossRef] [PubMed]
- Gesmundo, I.; Silvagno, F.; Banfi, D.; Monica, V.; Fanciulli, A.; Gamba, G.; Congiusta, N.; Libener, R.; Riganti, C.; Ghigo, E.; et al. Calcitriol Inhibits Viability and Proliferation in Human Malignant Pleural Mesothelioma Cells. Front. Endocrinol. 2020, 11, 559586. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Trump, D.L. Vitamin D Receptor Signaling and Cancer. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1009–1038. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Mehra, N.K.; Tekmal, R.R.; Palakurthi, S. Development and Evaluation of Talazoparib Nanoemulsion for Systemic Therapy of BRCA1-mutant Cancer. Anticancer Res. 2018, 38, 4493–4503. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Cecener, G.; Egeli, U.; Tunca, B. BMN 673 (talazoparib): A potent PARP inhibitor for triple negative breast cancer with different genetic profile. J. Biochem. Mol. Toxicol. 2019, 33, e22286. [Google Scholar] [CrossRef]
- Min, A.; Im, S.-A.; Kim, D.K.; Song, S.-H.; Kim, H.-J.; Lee, K.-H.; Kim, T.-Y.; Han, S.-W.; Oh, D.-Y.; Kim, T.-Y.; et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances antitumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2015, 17, 33. [Google Scholar] [CrossRef] [Green Version]
- Murai, J.; Feng, Y.; Yu, G.K.; Ru, Y.; Tang, S.W.; Shen, Y.; Pommier, Y. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget 2016, 7, 76534–76550. [Google Scholar] [CrossRef] [PubMed]
- Weigert, V.; Jost, T.; Hecht, M.; Knippertz, I.; Heinzerling, L.; Fietkau, R.; Distel, L.V. PARP inhibitors combined with ionizing radiation induce different effects in melanoma cells and healthy fibroblasts. BMC Cancer 2020, 20, 775. [Google Scholar] [CrossRef] [PubMed]
- Michelena, J.; Lezaja, A.; Teloni, F.; Schmid, T.; Imhof, R.; Altmeyer, M. Analysis of PARP inhibitor toxicity by multidimensional fluorescence microscopy reveals mechanisms of sensitivity and resistance. Nat. Commun. 2018, 9, 2678. [Google Scholar] [CrossRef]
- Phan, V.; Protter, A.; Peterson, A.; Uppal, H. Abstract P5-06-01: Talazoparib antitumor effects in BRCA-deficient breast cancer models. Cancer Res. 2017, 77, P5-06-01. [Google Scholar] [CrossRef]
- Gant, D.M.A.; Parris, A.B.; Yang, X. Metformin-induced downregulation of c-Met is a determinant of sensitivity in MDA-MB-468 breast cancer cells. Biochem. Biophys. Res. Commun. 2022, 613, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.E.; Hamzah, J.; Ilett, K.F.; Karunajeewa, H.A.; Reeder, J.C.; Batty, K.T.; Hackett, S.; Barrett, P.H.R. In vitro interactions between piperaquine, dihydroartemisinin, and other conventional and novel antimalarial drugs. Antimicrob. Agents Chemother. 2006, 50, 2883–2885. [Google Scholar] [CrossRef]
- Maki, M.A.A.; Cheah, S.-C.; Bayazeid, O.; Kumar, P.V. Cyclodextrin inclusion complex inhibits circulating galectin-3 and FGF-7 and affects the reproductive integrity and mobility of Caco-2 cells. Sci. Rep. 2020, 10, 17468. [Google Scholar] [CrossRef]
- Maki, M.A.A.; Kumar, P.V.; Cheah, S.-C.; Siew Wei, Y.; Al-Nema, M.; Bayazeid, O.; Majeed, A.B.B.A. Molecular Modeling-Based Delivery System Enhances Everolimus-Induced Apoptosis in Caco-2 Cells. ACS Omega 2019, 4, 8767–8777. [Google Scholar] [CrossRef] [Green Version]
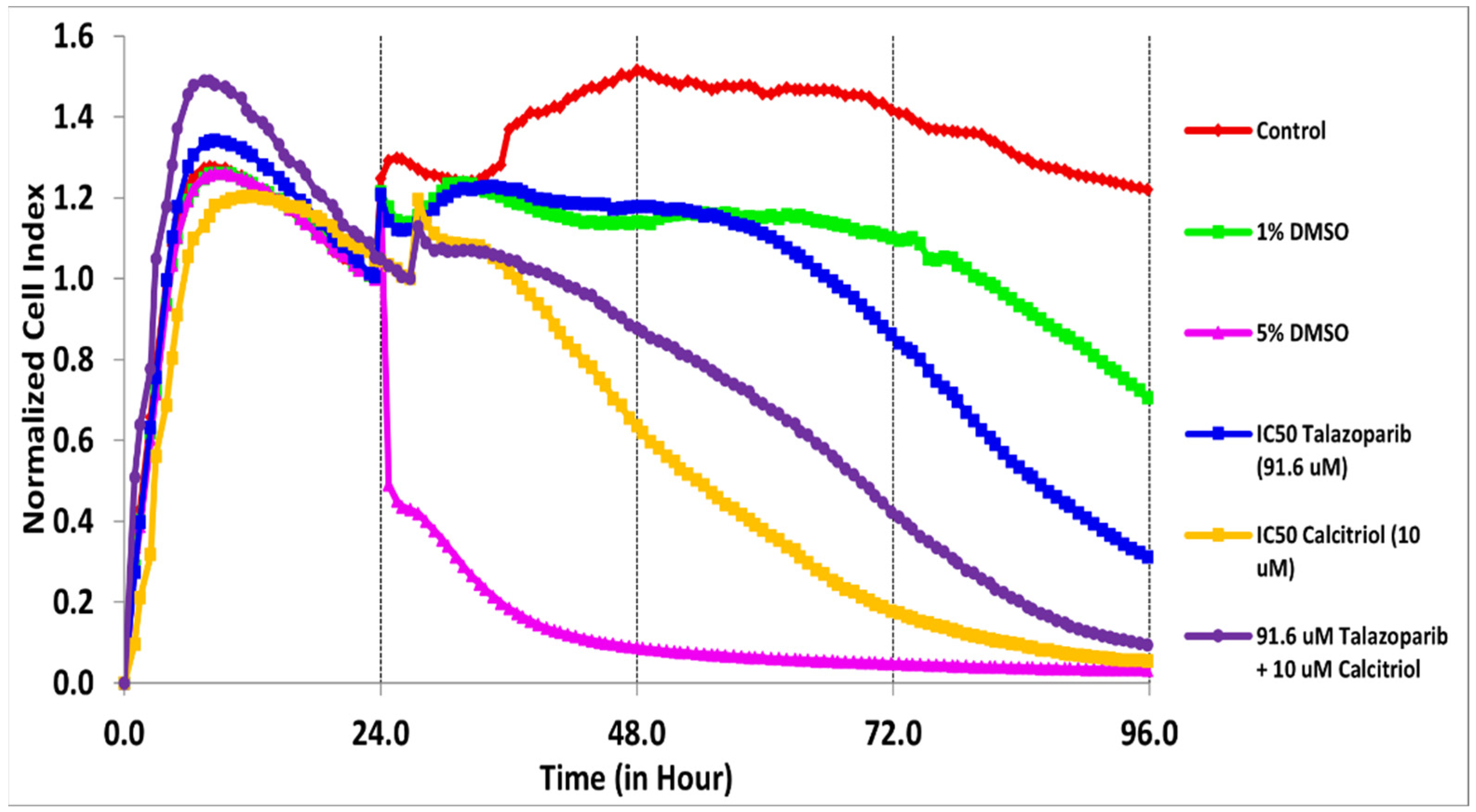
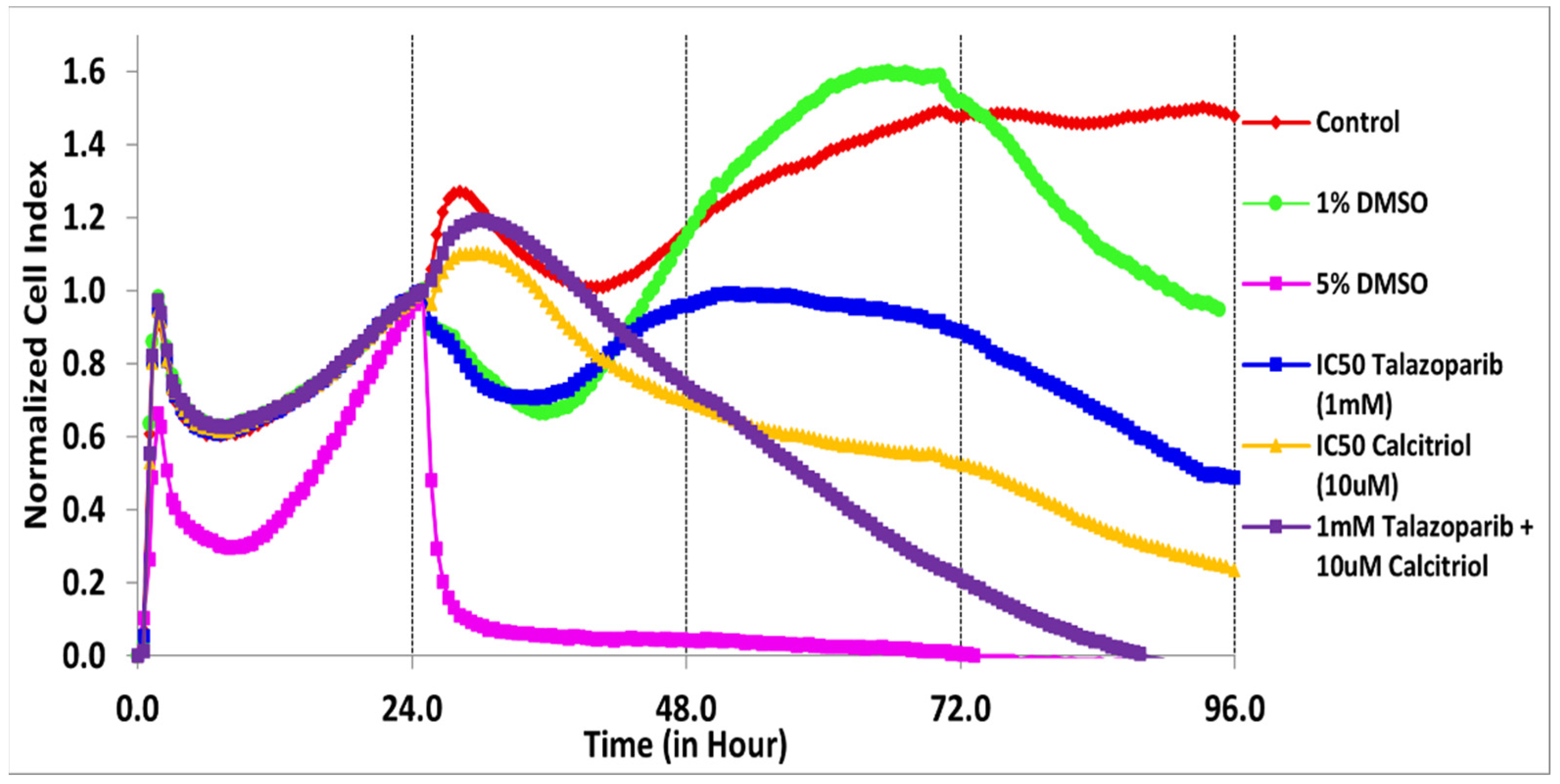
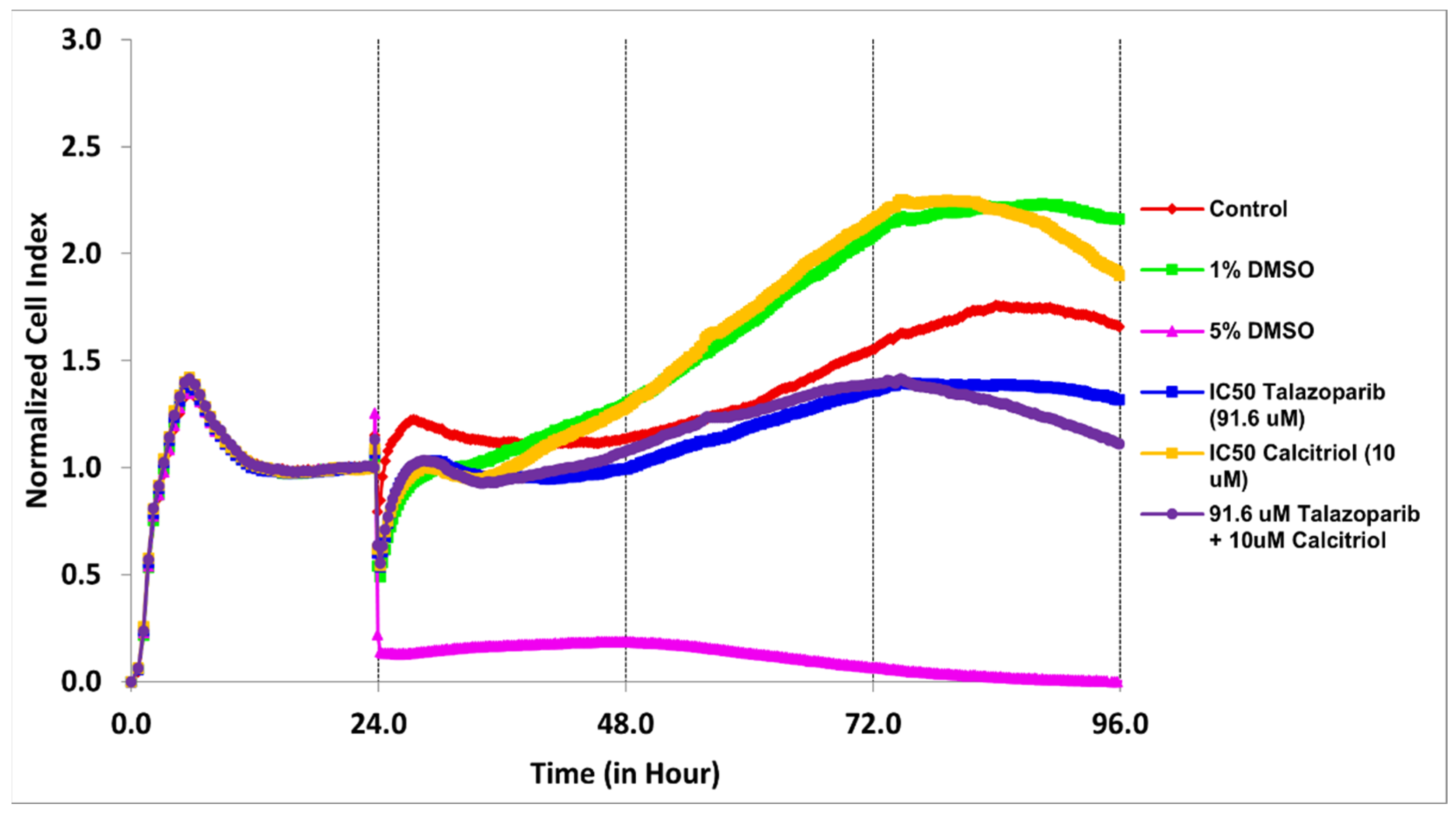
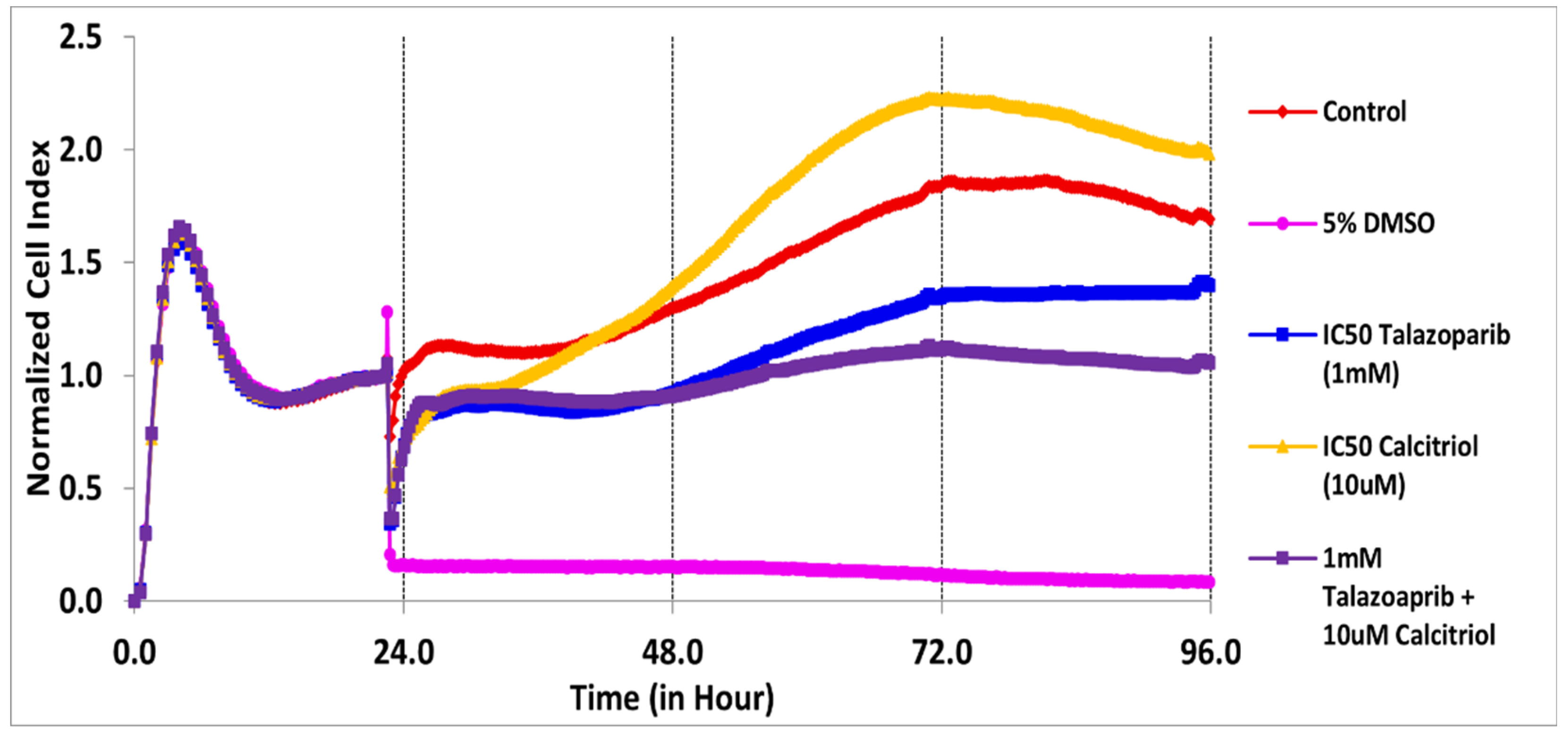
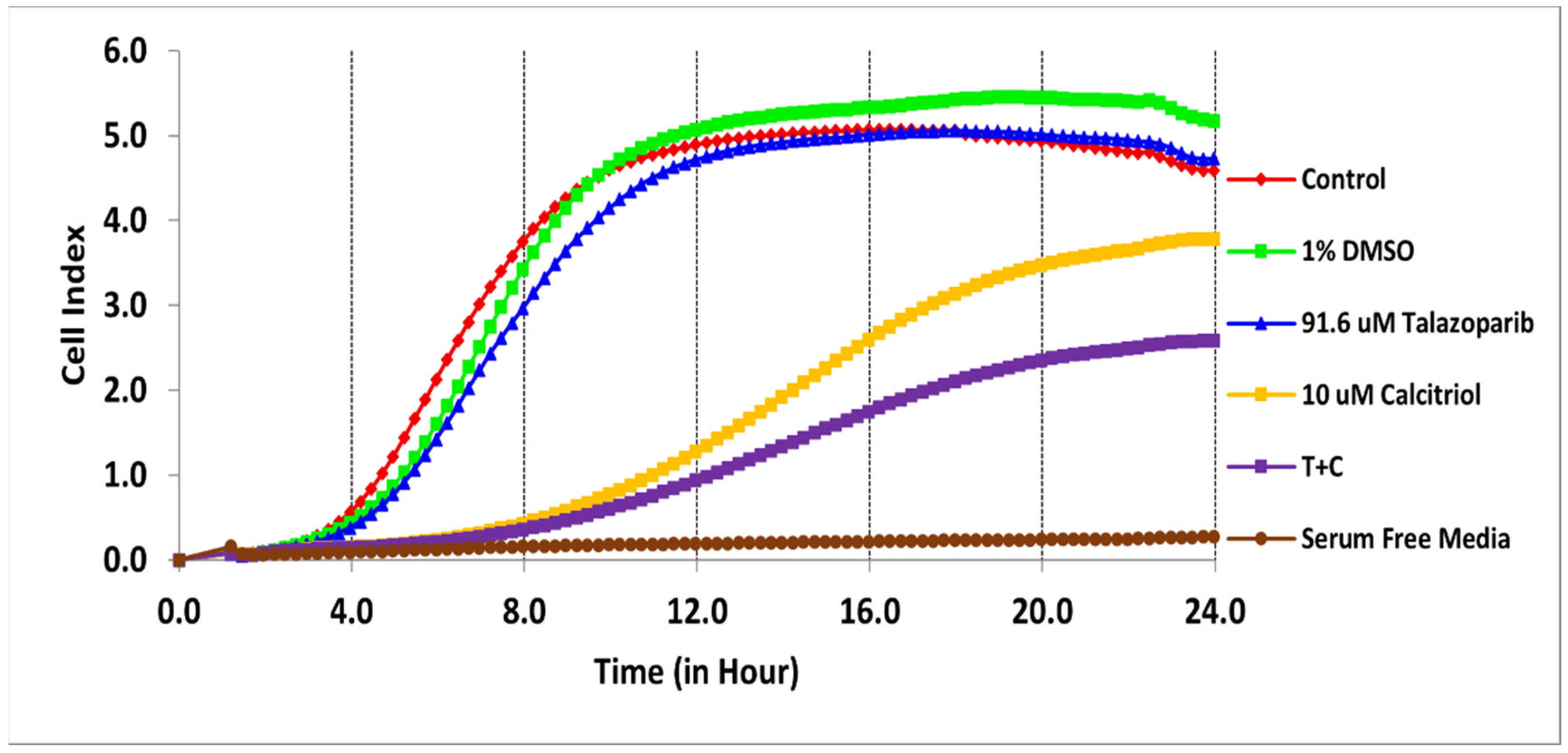
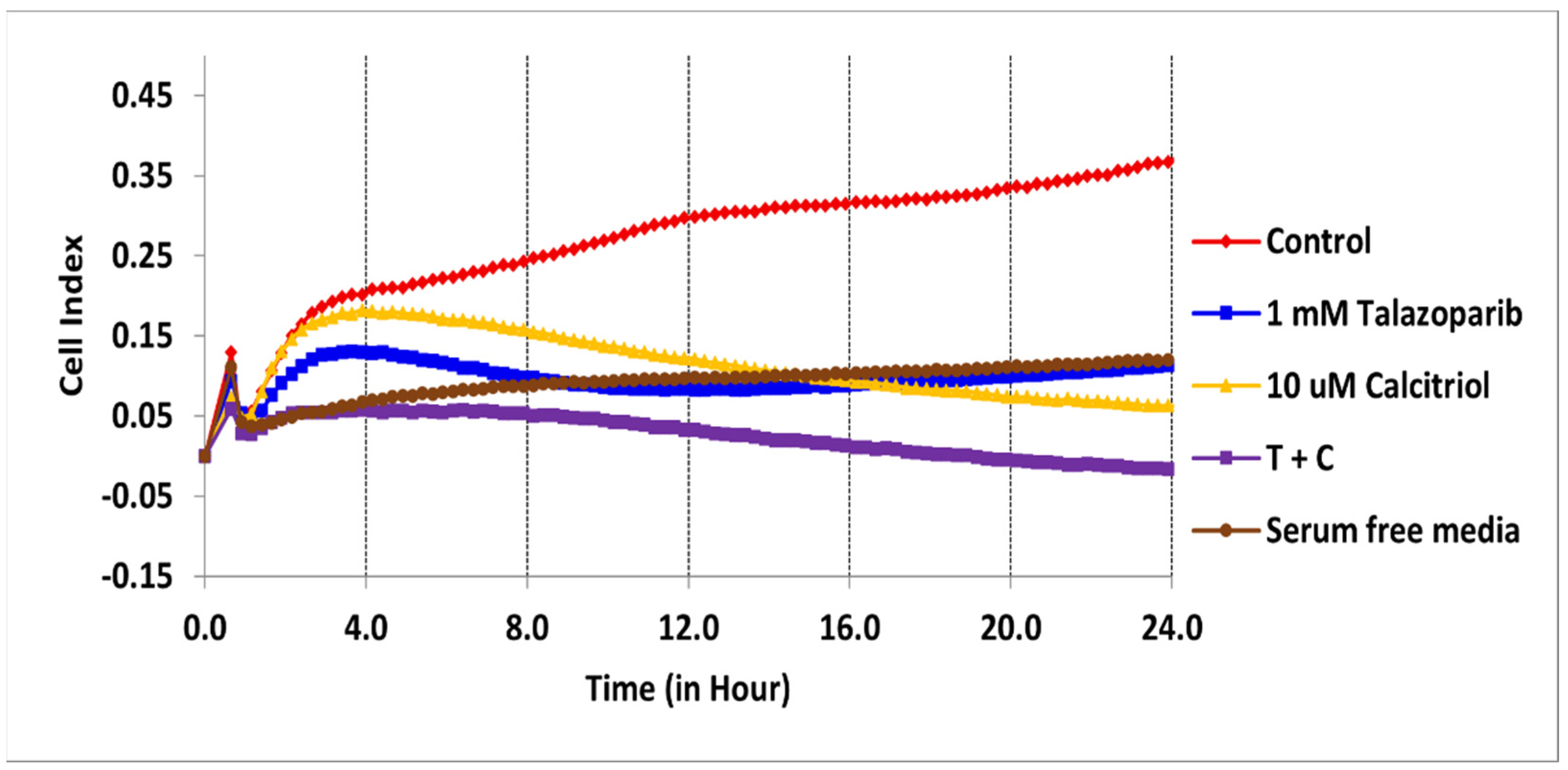
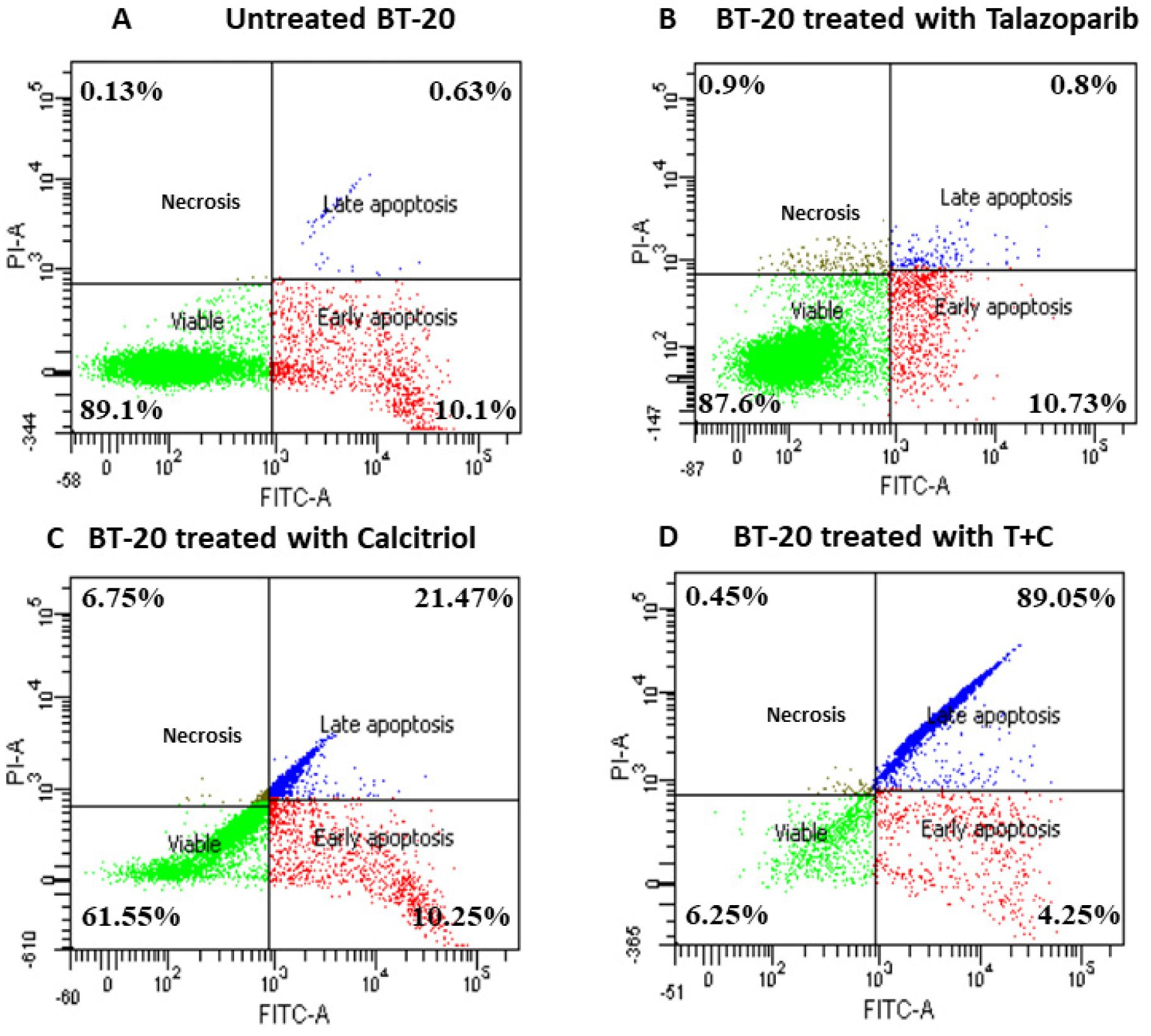
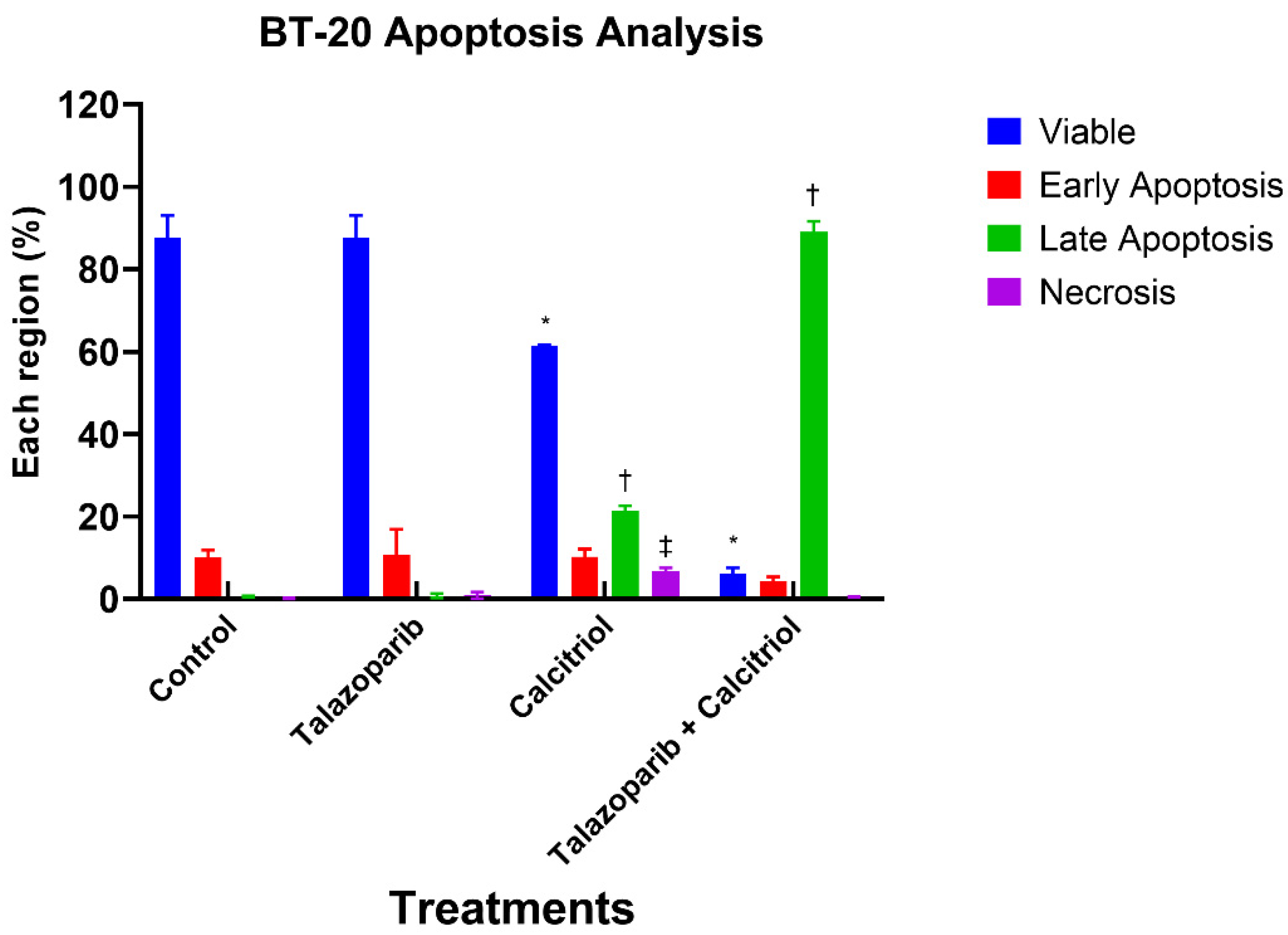

| Treatments | IC50 (µM) | Time (h) |
|---|---|---|
| Talazoparib | 91.6 | 61 |
| Calcitriol Combination | 10 both IC50 | 28 30 |
| Treatments | IC50 (µM) | Time (h) |
|---|---|---|
| Talazoparib | 1000 | 69 |
| Calcitriol Combination | 10 both IC50 | 50 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, F.H.; Palanirajan, V.K.; Ng, E.S.C.; Tan, C.K.; Tan, E.S.S.; Amini, F. Combination of Talazoparib and Calcitriol Enhanced Anticancer Effect in Triple−Negative Breast Cancer Cell Lines. Pharmaceuticals 2022, 15, 1075. https://doi.org/10.3390/ph15091075
Wong FH, Palanirajan VK, Ng ESC, Tan CK, Tan ESS, Amini F. Combination of Talazoparib and Calcitriol Enhanced Anticancer Effect in Triple−Negative Breast Cancer Cell Lines. Pharmaceuticals. 2022; 15(9):1075. https://doi.org/10.3390/ph15091075
Chicago/Turabian StyleWong, Fu Hou, Vijayaraj Kumar Palanirajan, Edmond Siah Chye Ng, Chung Keat Tan, Eugenie Sin Sing Tan, and Farahnaz Amini. 2022. "Combination of Talazoparib and Calcitriol Enhanced Anticancer Effect in Triple−Negative Breast Cancer Cell Lines" Pharmaceuticals 15, no. 9: 1075. https://doi.org/10.3390/ph15091075
APA StyleWong, F. H., Palanirajan, V. K., Ng, E. S. C., Tan, C. K., Tan, E. S. S., & Amini, F. (2022). Combination of Talazoparib and Calcitriol Enhanced Anticancer Effect in Triple−Negative Breast Cancer Cell Lines. Pharmaceuticals, 15(9), 1075. https://doi.org/10.3390/ph15091075








