Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing
Abstract
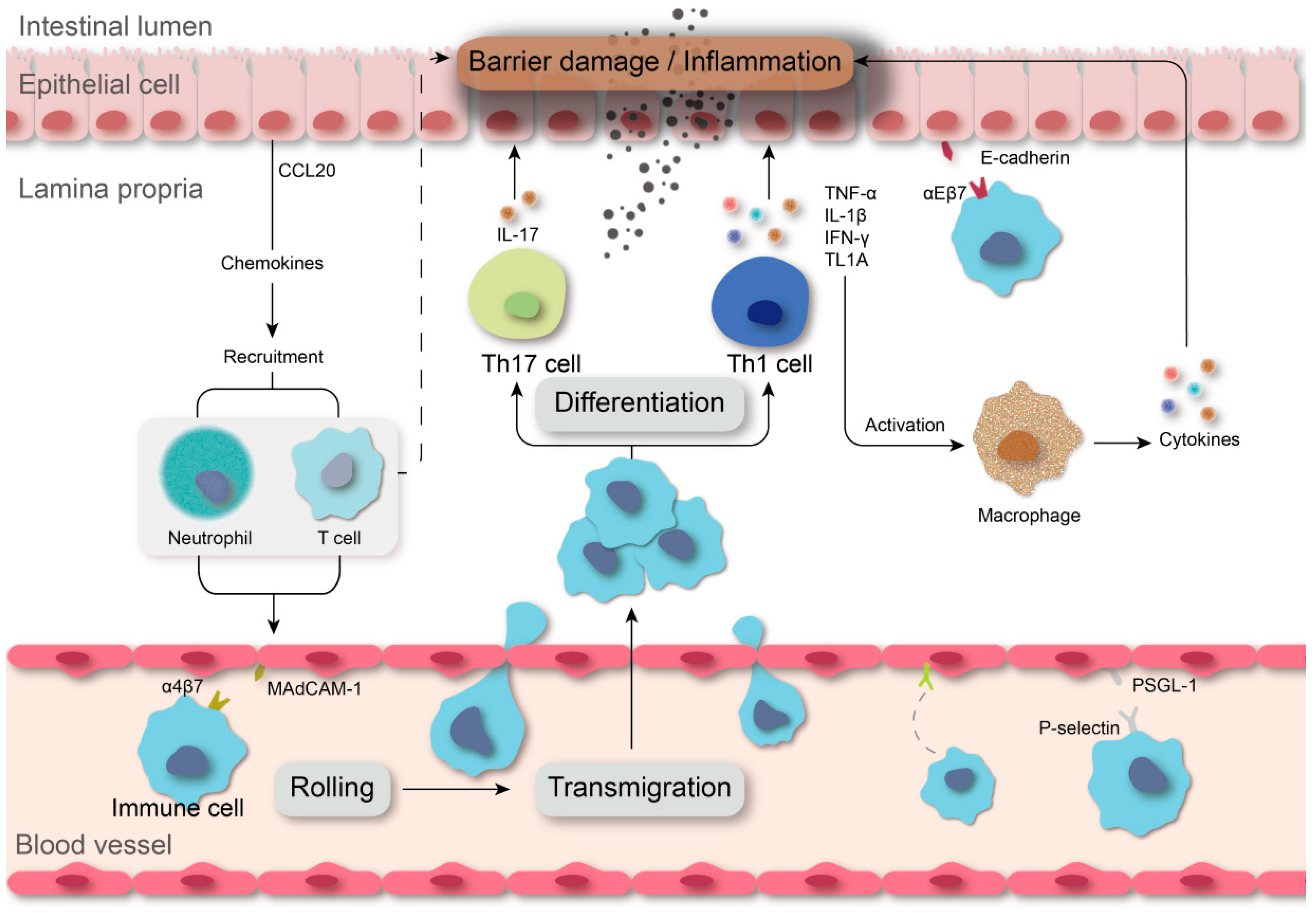
:1. Introduction
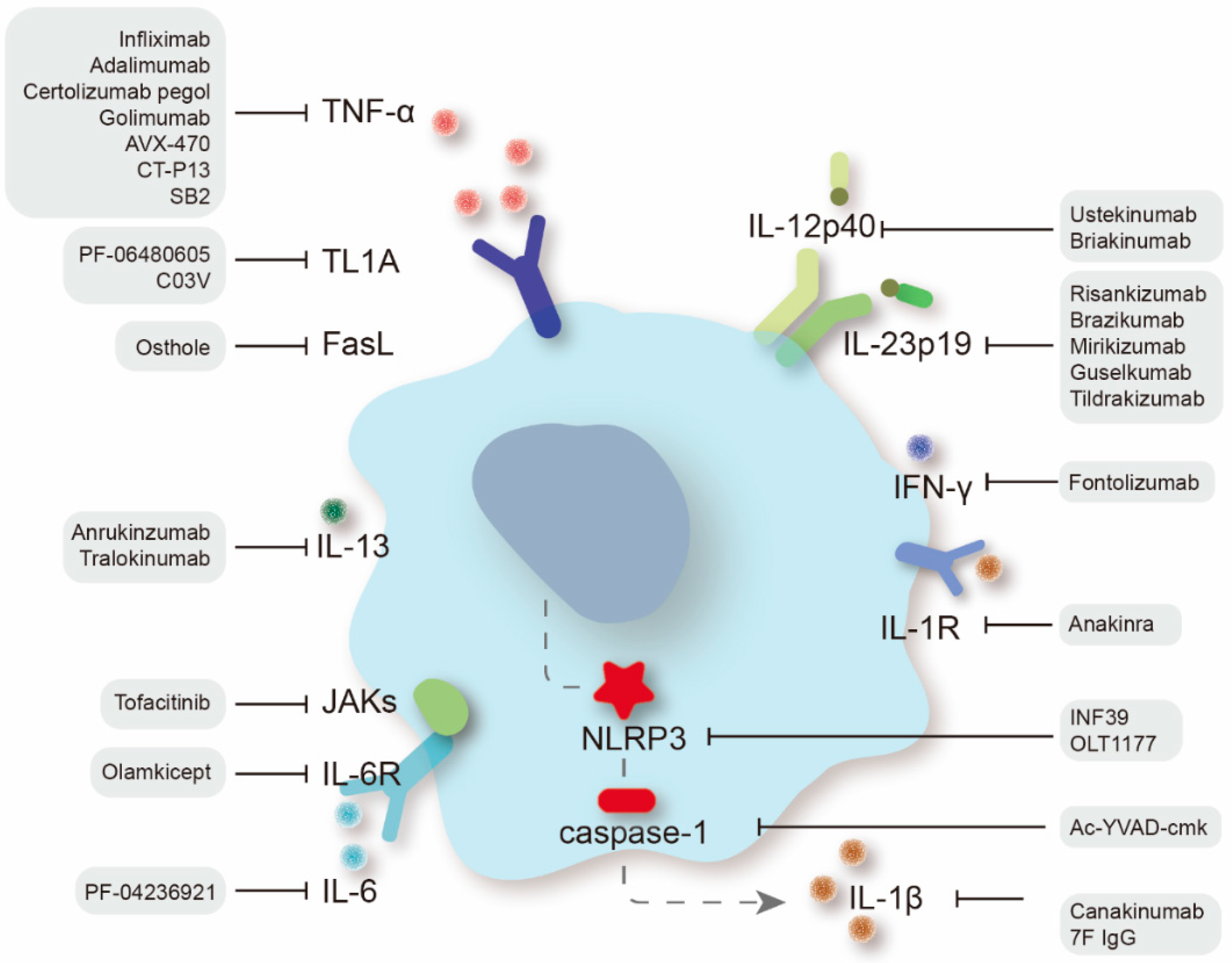
2. Targeting Proinflammatory Cytokines
2.1. Tumor Necrosis Factor Superfamily
2.1.1. Anti-TNF-α Agents
2.1.2. Anti-TL1A Therapy
2.1.3. Targeting the FasL/FAS System
2.1.4. Other TNFSF Members with Potential Efficacy
2.2. Interleukins
2.2.1. Anti-IL-12/IL-23 Agents
2.2.2. IL-6/IL-6R Inhibitors
2.2.3. IL-1β/IL-1R Antagonists
2.2.4. Other Interleukin Neutralizers with Potential Efficacy
2.3. Anti-Interferon Agents
2.4. Janus Kinase Inhibitors
3. Targeting Leukocyte Trafficking
3.1. Integrins
3.1.1. Anti-α4 Antibody
3.1.2. Anti-α4β7 Antibody
3.1.3. Anti-β7 Antibody
3.2. Inhibitors of Cell Adhesion Molecules
3.3. Regulation of Chemokines
3.4. Selectins
3.5. Modulation of Sphingosine 1-Phosphate Signaling
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [CrossRef]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Chen, M.L.; Sundrud, M.S. Cytokine Networks and T-Cell Subsets in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 1157–1167. [Google Scholar] [CrossRef]
- Verstockt, B.; Ferrante, M.; Vermeire, S.; Van Assche, G. New treatment options for inflammatory bowel diseases. J. Gastroenterol. 2018, 53, 585–590. [Google Scholar] [CrossRef]
- Croft, M.; Siegel, R.M. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 217–233. [Google Scholar] [CrossRef]
- Lebioda, T.J.; Kmieć, Z. Tumour necrosis factor superfamily members in the pathogenesis of inflammatory bowel disease. Mediat. Inflamm. 2014, 2014, 325129. [Google Scholar] [CrossRef]
- Koelink, P.J.; Bloemendaal, F.M.; Li, B.; Westera, L.; Vogels, E.W.M.; Van Roest, M.; Gloudemans, A.K.; Wout, A.V.; Korf, H.; Vermeire, S.; et al. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut 2019, 69, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Knight, D.M.; Trinh, H.A.N.; Le, J.; Siegel, S.; Shealy, D.; McDonough, M.; Scallon, B.; Moore, M.A.; Vilcek, J.A.N.; Daddona, P.; et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol. Immunol. 1993, 30, 1443–1453. [Google Scholar] [CrossRef]
- Jongsma, M.M.; Aardoom, M.A.; Cozijnsen, M.A.; van Pieterson, M.; de Meij, T.; Groeneweg, M.; Norbruis, O.F.; Wolters, V.M.; van Wering, H.M.; Hojsak, I.; et al. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn’s disease: An open-label multicentre randomised controlled trial. Gut 2022, 71, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Bouhnik, Y.; Carbonnel, F.; Laharie, D.; Stefanescu, C.; Hébuterne, X.; Abitbol, V.; Nachury, M.; Brixi, H.; Bourreille, A.; Picon, L.; et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bhol, K.C.; Tracey, D.E.; Lemos, B.R.; Lyng, G.D.; Erlich, E.C.; Keane, D.M.; Quesenberry, M.S.; Holdorf, A.D.; Schlehuber, L.D.; Clark, S.A.; et al. AVX-470: A novel oral anti-TNF antibody with therapeutic potential in inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.S.; Hartman, D.; Lemos, B.R.; Erlich, E.C.; Spence, S.; Kennedy, S.; Ptak, T.; Pruitt, R.; Vermeire, S.; Fox, B.S. AVX-470, an Orally Delivered Anti-Tumour Necrosis Factor Antibody for Treatment of Active Ulcerative Colitis: Results of a First-in-Human Trial. J. Crohn’s Colitis 2016, 10, 631–640. [Google Scholar] [CrossRef]
- Zheng, M.K.; Shih, D.Q.; Chen, G.C. Insights on the use of biosimilars in the treatment of inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 1932–1943. [Google Scholar] [CrossRef]
- Danese, S.; Bonovas, S.D.S.; Peyrin-Biroulet, L. Biosimilars in IBD: From theory to practice. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 22–31. [Google Scholar] [CrossRef]
- Gonsky, R.; Fleshner, P.; Deem, R.L.; Biener-Ramanujan, E.; Li, D.; Potdar, A.A.; Bilsborough, J.; Yang, S.; McGovern, D.P.; Targan, S.R. Association of Ribonuclease T2 Gene Polymorphisms With Decreased Expression and Clinical Characteristics of Severity in Crohn’s Disease. Gastroenterology 2017, 153, 219–232. [Google Scholar] [CrossRef]
- Furfaro, F.; Alfarone, L.; Gilardi, D.; Correale, C.; Allocca, M.; Fiorino, G.; Argollo, M.; Zilli, A.; Zacharopoulou, E.; Loy, L.; et al. TL1A: A New Potential Target in the Treatment of Inflammatory Bowel Disease. Curr. Drug Targets 2021, 22, 760–769. [Google Scholar] [CrossRef]
- Jacob, N.; Kumagai, K.; Abraham, J.P.; Shimodaira, Y.; Ye, Y.; Luu, J.; Blackwood, A.Y.; Castanon, S.L.; Stamps, D.T.; Thomas, L.S.; et al. Direct signaling of TL1A-DR3 on fibroblasts induces intestinal fibrosis in vivo. Sci. Rep. 2020, 10, 18189. [Google Scholar] [CrossRef]
- Danese, S.; Klopocka, M.; Scherl, E.J.; Romatowski, J.; Allegretti, J.R.; Peeva, E.; Vincent, M.S.; Schoenbeck, U.; Ye, Z.; Hassan-Zahraee, M.; et al. Anti-TL1A Antibody PF-06480605 Safety and Efficacy for Ulcerative Colitis: A Phase 2a Single-Arm Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2324–2332.e6. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Zahraee, M.; Ye, Z.; Xi, L.; Baniecki, M.L.; Li, X.; Hyde, C.L.; Zhang, J.; Raha, N.; Karlsson, F.; Quan, J.; et al. Antitumor Necrosis Factor-like Ligand 1A Therapy Targets Tissue Inflammation and Fibrosis Pathways and Reduces Gut Pathobionts in Ulcerative Colitis. Inflamm. Bowel Dis. 2022, 28, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.W.; Poulton, L.; Shim, D.; Mabon, D.; Butt, D.; Pollard, M.; Pande, V.; Husten, J.; Lyons, J.; Tian, C.; et al. An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease. MAbs 2018, 10, 664–677. [Google Scholar] [CrossRef]
- Gitlin, A.D.; Heger, K.; Schubert, A.F.; Reja, R.; Yan, D.; Pham, V.C.; Suto, E.; Zhang, J.; Kwon, Y.C.; Freund, E.C.; et al. Integration of innate immune signalling by caspase-8 cleavage of N4BP1. Nature 2020, 587, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, M.; Iizuka, M.; Horie, Y.; Yoneyama, K.; Shirasaka, T.; Itou, H.; Komatsu, M.; Fukushima, T.; Watanabe, S. Systemic and local evidence of increased Fas-mediated apoptosis in ulcerative colitis. Int. J. Color. Dis. 2002, 17, 70–76. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, E.R.; Isidro, R.A.; Cruz, M.L.; Marty, H.; Appleyard, C.B. Adoptive Transfer of Dendritic Cells Expressing Fas Ligand Modulates Intestinal Inflammation in a Model of Inflammatory Bowel Disease. J. Clin. Cell. Immunol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, M.; Yang, S.; Shao, B.; Chen, L.; Dou, L.; Gao, J.; Yang, D. Osthole enhances the immunosuppressive effects of bone marrow—Derived mesenchymal stem cells by promoting the Fas/FasL system. J. Cell. Mol. Med. 2021, 25, 4835–4845. [Google Scholar] [CrossRef]
- Shaikh, R.B.; Santee, S.; Granger, S.W.; Butrovich, K.; Cheung, T.; Kronenberg, M.; Cheroutre, H.; Ware, C.F. Constitutive Expression of LIGHT on T Cells Leads to Lymphocyte Activation, Inflammation, and Tissue Destruction. J. Immunol. 2001, 167, 6330–6337. [Google Scholar] [CrossRef]
- Krause, P.; Zahner, S.P.; Kim, G.; Shaikh, R.B.; Steinberg, M.W.; Kronenberg, M. The Tumor Necrosis Factor Family Member TNFSF14 (LIGHT) Is Required for Resolution of Intestinal Inflammation in Mice. Gastroenterology 2014, 146, 1752–1762.e4. [Google Scholar] [CrossRef]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386.e17. [Google Scholar] [CrossRef] [Green Version]
- Chyuan, I.T.; Tsai, H.F.; Wu, C.S.; Hsu, P.N. TRAIL suppresses gut inflammation and inhibits colitogeic T-cell activation in experimental colitis via an apoptosis-independent pathway. Mucosal Immunol. 2019, 12, 980–989. [Google Scholar] [CrossRef]
- Roblin, X.; Williet, N.; Boschetti, G.; Phelip, J.-M.; Del Tedesco, E.; Berger, A.-E.; Vedrines, P.; Duru, G.; Peyrin-Biroulet, L.; Nancey, S.; et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: A prospective randomised trial. Gut 2020, 69, 1206–1212. [Google Scholar] [CrossRef]
- Guo, B.-J.; Bian, Z.-X.; Qiu, H.-C.; Wang, Y.-T.; Wang, Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1401, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Goulart, R.A.; Barbalho, S.M.; Lima, V.M.; Souza, G.A.; Matias, J.N.; Araújo, A.C.; Rubira, C.J.; Buchaim, R.L.; Buchaim, D.V.; Carvalho, A.; et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn’s Disease: A Systematic Review. J. Med. Food 2021, 24, 675–685. [Google Scholar] [CrossRef]
- Szandruk, M.; Merwid-Ląd, A.; Szelag, A. The impact of mangiferin from Belamcanda chinensis on experimental colitis in rats. Inflammopharmacology 2018, 26, 571–581. [Google Scholar] [CrossRef]
- Du, Y.; Ding, H.; Vanarsa, K.; Soomro, S.; Baig, S.; Hicks, J.; Mohan, C. Low Dose Epigallocatechin Gallate Alleviates Experimental Colitis by Subduing Inflammatory Cells and Cytokines, and Improving Intestinal Permeability. Nutrients 2019, 11, 1743. [Google Scholar] [CrossRef]
- Liso, M.; Sila, A.; Verna, G.; Scarano, A.; Donghia, R.; Castellana, F.; Cavalcanti, E.; Pesole, P.L.; Sommella, E.M.; Lippolis, A.; et al. Nutritional Regimes Enriched with Antioxidants as an Efficient Adjuvant for IBD Patients under Infliximab Administration, a Pilot Study. Antioxidants 2022, 11, 138. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Bowman, E.P.; McElwee, J.J.; Smyth, M.; Casanova, J.-L.; Cooper, A.; Cua, D.J. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015, 21, 719–729. [Google Scholar] [CrossRef]
- Eftychi, C.; Schwarzer, R.; Vlantis, K.; Wachsmuth, L.; Basic, M.; Wagle, P.; Neurath, M.F.; Becker, C.; Bleich, A.; Pasparakis, M. Temporally Distinct Functions of the Cytokines IL-12 and IL-23 Drive Chronic Colon Inflammation in Response to Intestinal Barrier Impairment. Immunity 2019, 51, 367–380.e4. [Google Scholar] [CrossRef]
- Sands, B.E.; Irving, P.M.; Hoops, T.; Izanec, J.L.; Gao, L.L.; Gasink, C.; Greenspan, A.; Allez, M.; Danese, S.; Hanauer, S.B.; et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: A multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 2022, 399, 2200–2211. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Biancone, L.; Ardizzone, S.; Armuzzi, A.; Castiglione, F.; D’Incà, R.; Danese, S.; Daperno, M.; Gionchetti, P.; Rizzello, F.; Scribano, M.L.; et al. Ustekinumab for treating ulcerative colitis: An expert opinion. Expert Opin. Biol. Ther. 2020, 20, 1321–1329. [Google Scholar] [CrossRef]
- Davies, S.C.; Nguyen, T.M.; Parker, C.E.; MacDonald, J.K.; Jairath, V.; Khanna, R. Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2019, 12, Cd012804. [Google Scholar] [CrossRef]
- Sewell, G.W.; Kaser, A. Interleukin-23 in the Pathogenesis of Inflammatory Bowel Disease and Implications for Therapeutic Intervention. J. Crohn’s Colitis 2022, 16, ii3–ii19. [Google Scholar] [CrossRef]
- Ma, C.; Panaccione, R.; Khanna, R.; Feagan, B.G.; Jairath, V. IL12/23 or selective IL23 inhibition for the management of moderate-to-severe Crohn’s disease? Best Pract. Res. Clin. Gastroenterol. 2019, 38–39, 101604. [Google Scholar] [CrossRef]
- Gottlieb, Z.S.; Sands, B.E. Personalised Medicine with IL-23 Blockers: Myth or Reality? J. Crohn’s Colitis 2022, 16 (Suppl. S2), ii73–ii94. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; D’Haens, G.; Panés, J.; Kaser, A.; Ferrante, M.; Louis, E.; Franchimont, D.; Dewit, O.; Seidler, U.; et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: A randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017, 389, 1699–1709. [Google Scholar] [CrossRef]
- Ferrante, M.; Feagan, B.G.; Panés, J.; Baert, F.; Louis, E.; Dewit, O.; Kaser, A.; Duan, W.R.; Pang, Y.; Lee, W.-J.; et al. Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J. Crohn’s Colitis 2021, 15, 2001–2010. [Google Scholar] [CrossRef]
- Visvanathan, S.; Baum, P.; Salas, A.; Vinisko, R.; Schmid, R.; Grebe, K.M.; Davis, J.W.; Wallace, K.; Böcher, W.O.; Padula, S.J.; et al. Selective IL-23 Inhibition by Risankizumab Modulates the Molecular Profile in the Colon and Ileum of Patients With Active Crohn’s Disease: Results From a Randomised Phase II Biopsy Sub-study. J. Crohn’s Colitis 2018, 12, 1170–1179. [Google Scholar] [CrossRef]
- Sands, B.E.; Chen, J.; Feagan, B.G.; Penney, M.; Rees, W.A.; Danese, S.; Higgins, P.; Newbold, P.; Faggioni, R.; Patra, K.; et al. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn’s Disease: A Phase 2a Study. Gastroenterology 2017, 153, 77–86.e6. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ferrante, M.; Bhandari, B.R.; Berliba, E.; Feagan, B.G.; Hibi, T.; Tuttle, J.L.; Klekotka, P.; Friedrich, S.; Durante, M.; et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 537–549.e10. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ferrante, M.; Bhandari, B.R.; Berliba, E.; Hibi, T.; D’Haens, G.R.; Tuttle, J.L.; Krueger, K.; Friedrich, S.; Durante, M.; et al. Efficacy and Safety of Continued Treatment With Mirikizumab in a Phase 2 Trial of Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2022, 20, 105–115.e14. [Google Scholar] [CrossRef]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Tyring, S.K.; Sinclair, R.; Thaçi, D.; Nograles, K.; Mehta, A.; Cichanowitz, N.; Li, Q.; et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): Results from two randomised controlled, phase 3 trials. Lancet 2017, 390, 276–288. [Google Scholar] [CrossRef]
- Valenti, M.; Narcisi, A.; Pavia, G.; Gargiulo, L.; Costanzo, A. What Can IBD Specialists Learn from IL-23 Trials in Dermatology? J. Crohn’s Colitis 2022, 16 (Suppl. S2), ii20–ii29. [Google Scholar]
- Atreya, R.; Mudter, J.; Finotto, S.; Müllberg, J.; Jostock, T.; Wirtz, S.; Schütz, M.; Bartsch, B.; Holtmann, M.; Becker, C.; et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 2000, 6, 583–588. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Harbour, S.N.; DiToro, D.F.; Witte, S.J.; Zindl, C.L.; Gao, M.; Schoeb, T.R.; Jones, G.W.; Jones, S.A.; Hatton, R.D.; Weaver, C.T. T H 17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci. Immunol. 2020, 5, eaaw2262. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; Hellstern, P.; Panaccione, R.; Rogler, G.; Fraser, G.; Kohn, A.; Desreumaux, P.; Leong, R.W.; Comer, G.M.; et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut 2019, 68, 40–48. [Google Scholar] [CrossRef]
- Xiao, Y.-T.; Yan, W.-H.; Cao, Y.; Yan, J.-K.; Cai, W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine 2016, 83, 189–192. [Google Scholar] [CrossRef]
- Sido, A.; Radhakrishnan, S.; Kim, S.W.; Eriksson, E.; Shen, F.; Li, Q.; Bhat, V.; Reddivari, L.; Vanamala, J.K. A food-based approach that targets interleukin-6, a key regulator of chronic intestinal inflammation and colon carcinogenesis. J. Nutr. Biochem. 2017, 43, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, Y.; Zhou, W.; Miao, X.; Zhou, H. Utilization of physiologically-based pharmacokinetic model to assess disease-mediated therapeutic protein-disease-drug interaction in immune-mediated inflammatory diseases. Clin. Transl. Sci. 2022, 15, 464–476. [Google Scholar] [CrossRef]
- Jena, A.; Mishra, S.; Deepak, P.; Kumar-M, P.; Sharma, A.; Patel, Y.I.; Kennedy, N.A.; Kim, A.; Sharma, V.; Sebastian, S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun. Rev. 2022, 21, 102927. [Google Scholar] [CrossRef]
- Schreiber, S.; Aden, K.; Bernardes, J.P.; Conrad, C.; Tran, F.; Höper, H.; Volk, V.; Mishra, N.; Blase, J.I.; Nikolaus, S.; et al. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients With Active Inflammatory Bowel Disease. Gastroenterology 2021, 160, 2354–2366.e11. [Google Scholar] [CrossRef]
- Liso, M.; Verna, G.; Cavalcanti, E.; De Santis, S.; Armentano, R.; Tafaro, A.; Lippolis, A.; Campiglia, P.; Gasbarrini, A.; Mastronardi, M.; et al. Interleukin 1β Blockade Reduces Intestinal Inflammation in a Murine Model of Tumor Necrosis Factor–Independent Ulcerative Colitis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 151–171. [Google Scholar] [CrossRef]
- De Benedetti, F.; Gattorno, M.; Anton, J.; Ben-Chetrit, E.; Frenkel, J.; Hoffman, H.M.; Koné-Paut, I.; Lachmann, H.J.; Ozen, S.; Simon, A.; et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N. Engl. J. Med. 2018, 378, 1908–1919. [Google Scholar] [CrossRef]
- Oh, Y.S.; Kwak, M.-K.; Kim, K.; Cho, E.-H.; Jang, S.-E. Development and application of an antibody that binds to interleukin-1β of various mammalian species for the treatment of inflammatory diseases. Biochem. Biophys. Res. Commun. 2020, 527, 751–756. [Google Scholar] [CrossRef]
- Arend, W.P.; Malyak, M.; Guthridge, C.J.; Gabay, C. Interleukin-1 Receptor Antagonist: Role in Biology. Annu. Rev. Immunol. 1998, 16, 27–55. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Mancuso, G.; Midiri, A.; Di Paola, R.; Cuzzocrea, S. Therapeutic potential of dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice by targeting IL-1β and IL-18. Biochem. Pharmacol. 2018, 155, 150–161. [Google Scholar] [CrossRef]
- Shouval, D.S.; Biswas, A.; Kang, Y.H.; Griffith, A.E.; Konnikova, L.; Mascanfroni, I.D.; Redhu, N.S.; Frei, S.M.; Field, M.; Doty, A.L.; et al. Interleukin 1β Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 2016, 151, 1100–1104. [Google Scholar] [CrossRef]
- Thomas, M.G.; Bayliss, C.; Bond, S.; Dowling, F.; Galea, J.; Jairath, V.; Lamb, C.; Probert, C.; Timperley-Preece, E.; Watson, A.; et al. Trial summary and protocol for a phase II randomised placebo-controlled double-blinded trial of Interleukin 1 blockade in Acute Severe Colitis: The IASO trial. BMJ Open 2019, 9, e023765. [Google Scholar] [CrossRef] [PubMed]
- Gressler, M.; Heddergott, C.; N’Go, I.C.; Renga, G.; Oikonomou, V.; Moretti, S.; Coddeville, B.; Gaifem, J.; Silvestre, R.; Romani, L.; et al. Definition of the Anti-inflammatory Oligosaccharides Derived From the Galactosaminogalactan (GAG) From Aspergillus fumigatus. Front. Cell. Infect. Microbiol. 2019, 9, 365. [Google Scholar] [CrossRef]
- Corcoran, S.E.; Halai, R.; Cooper, M.A. Pharmacological Inhibition of the Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome with MCC950. Pharmacol. Rev. 2021, 73, 968–1000. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Fornai, M.; Colucci, R.; Benvenuti, L.; D’Antongiovanni, V.; Natale, G.; Fulceri, F.; Giorgis, M.; Marini, E.; Gastaldi, S.; et al. A Comparative Study on the Efficacy of NLRP3 Inflammasome Signaling Inhibitors in a Pre-clinical Model of Bowel Inflammation. Front. Pharmacol. 2018, 9, 1405. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhao, S.; Zhou, J.; Yan, J.; Wang, L.; Du, X.; Li, H.; Chen, Y.; Cai, W.; Wu, J. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Gagliani, N.; De Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.D.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef]
- Mokry, L.E.; Zhou, S.; Guo, C.; Scott, R.A.; Devey, L.; Langenberg, C.; Wareham, N.; Waterworth, D.; Cardon, L.; Sanseau, P.; et al. Interleukin-18 as a drug repositioning opportunity for inflammatory bowel disease: A Mendelian randomization study. Sci. Rep. 2019, 9, 9386. [Google Scholar] [CrossRef]
- Wlodek, E.; Kirkpatrick, R.B.; Andrews, S.; Noble, R.; Schroyer, R.; Scott, J.; Watson, C.J.E.; Clatworthy, M.; Harrison, E.M.; Wigmore, S.J.; et al. A pilot study evaluating GSK1070806 inhibition of interleukin-18 in renal transplant delayed graft function. PLoS ONE 2021, 16, e0247972. [Google Scholar] [CrossRef]
- Reinisch, W.; Panes, J.; Khurana, S.; Toth, G.; Hua, F.; Comer, G.M.; Hinz, M.; Page, K.; O’Toole, M.; Moorehead, T.M.; et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: Efficacy and safety from a phase IIa randomised multicentre study. Gut 2015, 64, 894–900. [Google Scholar] [CrossRef]
- Danese, S.; Rudziński, J.; Brandt, W.; Dupas, J.-L.; Peyrin-Biroulet, L.; Bouhnik, Y.; Kleczkowski, D.; Uebel, P.; Lukas, M.; Knutsson, M.; et al. Tralokinumab for moderate-to-severe UC: A randomised, double-blind, placebo-controlled, phase IIa study. Gut 2015, 64, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Fauny, M.; Moulin, D.; D’Amico, F.; Netter, P.; Petitpain, N.; Arnone, D.; Jouzeau, J.-Y.; Loeuille, D.; Peyrin-Biroulet, L. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann. Rheum. Dis. 2020, 79, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, A.-R.; Ahn, S.B.; Eun, C.S.; Han, D.S. Role of innate lymphoid cells in chronic colitis during anti-IL-17A therapy. Sci. Rep. 2020, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Abo, H.; Flannigan, K.L.; Geem, D.; Ngo, V.L.; Harusato, A.; Denning, T.L. Combined IL-2 Immunocomplex and Anti-IL-5 mAb Treatment Expands Foxp3+ Treg Cells in the Absence of Eosinophilia and Ameliorates Experimental Colitis. Front. Immunol. 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, D.; Izzo, R.; Figliuzzi, M.; Pallone, F.; Monteleone, G. IL-21 as a therapeutic target in inflammatory disorders. Expert Opin. Ther. Targets 2014, 18, 1329–1338. [Google Scholar] [CrossRef]
- Phuong, N.N.T.; Palmieri, V.; Adamczyk, A.; Klopfleisch, R.; Langhorst, J.; Hansen, W.; Westendorf, A.M.; Pastille, E. IL-33 Drives Expansion of Type 2 Innate Lymphoid Cells and Regulatory T Cells and Protects Mice From Severe, Acute Colitis. Front. Immunol. 2021, 12, 2764. [Google Scholar] [CrossRef]
- Tong, X.; Zheng, Y.; Li, Y.; Xiong, Y.; Chen, D. Soluble ligands as drug targets for treatment of inflammatory bowel disease. Pharmacol. Ther. 2021, 226, 107859. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Tindemans, I.; Joosse, M.E.; Samsom, J.N. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells 2020, 9, 110. [Google Scholar] [CrossRef]
- Bergemalm, D.; Andersson, E.; Hultdin, J.; Eriksson, C.; Rush, S.T.; Kalla, R.; Adams, A.T.; Keita, A.V.; D’Amato, M.; Gomollon, F.; et al. Systemic Inflammation in Preclinical Ulcerative Colitis. Gastroenterology 2021, 161, 1526–1539.e9. [Google Scholar] [CrossRef]
- Langer, V.; Vivi, E.; Regensburger, D.; Winkler, T.H.; Waldner, M.J.; Rath, T.; Schmid, B.; Skottke, L.; Lee, S.; Jeon, N.L.; et al. IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin–directed vascular barrier disruption. J. Clin. Investig. 2019, 129, 4691–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavragani, C.P.; Nezos, A.; Dovrolis, N.; Andreou, N.-P.; Legaki, E.; Sechi, L.A.; Bamias, G.; Gazouli, M. Type I and II Interferon Signatures Can Predict the Response to Anti-TNF Agents in Inflammatory Bowel Disease Patients: Involvement of the Microbiota. Inflamm. Bowel Dis. 2020, 26, 1543–1553. [Google Scholar] [CrossRef]
- Liu, T.-C.; Kern, J.T.; Jain, U.; Sonnek, N.M.; Xiong, S.; Simpson, K.F.; VanDussen, K.L.; Winkler, E.S.; Haritunians, T.; Malique, A.; et al. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe 2021, 29, 988–1001.e6. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.W.; Constant, D.A.; Nice, T.J. Interferon Lambda in the Pathogenesis of Inflammatory Bowel Diseases. Front. Immunol. 2021, 12, 4234. [Google Scholar] [CrossRef] [PubMed]
- Hommes, D.W.; Mikhajlova, T.L.; Stoinov, S.; Stimac, D.; Vucelic, B.; Lonovics, J.; Zákuciová, M.; D’Haens, G.; Van Assche, G.; Ba, S. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease. Gut 2006, 55, 1131–1137. [Google Scholar] [CrossRef]
- Baker, K.; Isaacs, J. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 2018, 77, 175–187. [Google Scholar] [CrossRef]
- Takahara, M.; Takaki, A.; Hiraoka, S.; Adachi, T.; Shimomura, Y.; Matsushita, H.; Nguyen, T.T.T.; Koike, K.; Ikeda, A.; Takashima, S.; et al. Berberine improved experimental chronic colitis by regulating interferon-γ- and IL-17A-producing lamina propria CD4+ T cells through AMPK activation. Sci. Rep. 2019, 9, 11934. [Google Scholar] [CrossRef]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Casteele, N.V. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Sharara, A.I.; Su, C.; Modesto, I.; Mundayat, R.; Gunay, L.M.; Salese, L.; Sands, B.E. Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status. Clin. Gastroenterol. Hepatol. 2022, 20, 591–601.e8. [Google Scholar] [CrossRef]
- Verstockt, B.; Volk, V.; Jaeckel, C.; Alsoud, D.; Sabino, J.; Nikolaus, S.; Outtier, A.; Krönke, N.; Feuerhake, F.; De Hertogh, G.; et al. Longitudinal monitoring of STAT3 phosphorylation and histologic outcome of tofacitinib therapy in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2022, 56, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Panés, J.; Sandborn, W.J.; Schreiber, S.; Sands, B.E.; Vermeire, S.; D’Haens, G.; Panaccione, R.; Higgins, P.; Colombel, J.F.; Feagan, B.G. Tofacitinib for induction and maintenance therapy of Crohn’s disease: Results of two phase IIb randomised placebo-controlled trials. Gut 2017, 66, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Fenster, M.; Alayo, Q.A.; Khatiwada, A.; Wang, W.; Dimopoulos, C.; Gutierrez, A.; Ciorba, M.A.; Christophi, G.P.; Hirten, R.P.; Ha, C.; et al. Real-World Effectiveness and Safety of Tofacitinib in Crohn’s Disease and IBD-U: A Multicenter Study From the TROPIC Consortium. Clin. Gastroenterol. Hepatol. 2020, 19, 2207–2209.e3. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Nguyen, L.P.; Hadeiba, H.; Butcher, E.C. Leukocyte Trafficking to the Small Intestine and Colon. Gastroenterology 2016, 150, 340–354. [Google Scholar] [CrossRef]
- Wiendl, M.; Becker, E.; Müller, T.M.; Voskens, C.J.; Neurath, M.F.; Zundler, S. Targeting Immune Cell Trafficking—Insights from Research Models and Implications for Future IBD Therapy. Front. Immunol. 2021, 12, 1546. [Google Scholar] [CrossRef]
- Dotan, I.; Allez, M.; Danese, S.; Keir, M.; Tole, S.; McBride, J. The role of integrins in the pathogenesis of inflammatory bowel disease: Approved and investigational anti—Integrin therapies. Med. Res. Rev. 2020, 40, 245–262. [Google Scholar] [CrossRef]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288–300. [Google Scholar] [CrossRef]
- Fischer, A.; Zundler, S.; Atreya, R.; Rath, T.; Bosch-Voskens, C.; Hirschmann, S.; López-Posadas, R.; Watson, A.; Becker, C.; Schuler, G.; et al. Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut 2016, 65, 1642–1664. [Google Scholar] [CrossRef]
- Podolsky, D.K.; Lobb, R.; King, N.; Benjamin, C.D.; Pepinsky, B.; Sehgal, P.; Debeaumont, M. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J. Clin. Investig. 1993, 92, 372–380. [Google Scholar] [CrossRef]
- Lamb, C.; O’Byrne, S.; Keir, M.E.; Butcher, E.C. Gut-Selective Integrin-Targeted Therapies for Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, S653–S668. [Google Scholar] [CrossRef]
- Yoshimura, N.; Watanabe, M.; Motoya, S.; Tominaga, K.; Matsuoka, K.; Iwakiri, R.; Watanabe, K.; Hibi, T. Safety and Efficacy of AJM300, an Oral Antagonist of α4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology 2015, 149, 1775–1783.e2. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Watanabe, M.; Ohmori, T.; Nakajima, K.; Ishida, T.; Ishiguro, Y.; Kanke, K.; Kobayashi, K.; Hirai, F.; Watanabe, K.; et al. AJM300 (carotegrast methyl), an oral antagonist of α4-integrin, as induction therapy for patients with moderately active ulcerative colitis: A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol. Hepatol. 2022, 7, 648–657. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznarić, Ž.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 562–572.e12. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Danese, S.; Colombel, J.-F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Lasa, J.S.; Olivera, P.A.; Danese, S.; Peyrin-Biroulet, L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 161–170. [Google Scholar] [CrossRef]
- Vermeire, S.; D’Haens, G.; Baert, F.; Danese, S.; Kobayashi, T.; Loftus, E.V.; Bhatia, S.; Agboton, C.; Rosario, M.; Chen, C.; et al. Efficacy and Safety of Subcutaneous Vedolizumab in Patients With Moderately to Severely Active Crohn’s Disease: Results From the VISIBLE 2 Randomised Trial. J. Crohn’s Colitis 2022, 16, 27–38. [Google Scholar] [CrossRef]
- Hibi, T.; Motoya, S.; Ashida, T.; Sai, S.; Sameshima, Y.; Nakamura, S.; Maemoto, A.; Nii, M.; Sullivan, B.A.; Gasser, R.A., Jr.; et al. Efficacy and safety of abrilumab, an α4β7 integrin inhibitor, in Japanese patients with moderate-to-severe ulcerative colitis: A phase II study. Intest. Res. 2019, 17, 375–386. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Mattheakis, L.C.; Modi, N.B.; Pugatch, D.; Bressler, B.; Lee, S.; Bhandari, R.; Kanwar, B.; Shames, R.; D’Haens, G.; et al. PTG-100, an Oral α4β7 Antagonist Peptide: Preclinical Development and Phase 1 and 2a Studies in Ulcerative Colitis. Gastroenterology 2021, 161, 1853–1864.e10. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Hart, A.; Bossuyt, P.; Long, M.; Allez, M.; Juillerat, P.; Armuzzi, A.; Loftus, E.V.; Ostad-Saffari, E.; Scalori, A.; et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): A phase 3, randomised, controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 128–140. [Google Scholar] [CrossRef]
- Danese, S.; Colombel, J.-F.; Lukas, M.; Gisbert, J.P.; D’Haens, G.; Hayee, B.; Panaccione, R.; Kim, H.-S.; Reinisch, W.; Tyrrell, H.; et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): A randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol. Hepatol. 2022, 7, 118–127. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Vermeire, S.; Tyrrell, H.; Hassanali, A.; Lacey, S.; Tole, S.; Tatro, A.R. The Etrolizumab Global Steering Committee Etrolizumab for the Treatment of Ulcerative Colitis and Crohn’s Disease: An Overview of the Phase 3 Clinical Program. Adv. Ther. 2020, 37, 3417–3431. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, P.; Neurath, M.F.; Ng, S.C.; El-Omar, E.M.; Sharara, A.I.; Kobayashi, T.; Hisamatsu, T.; Hibi, T.; Rogler, G. Mechanism-Based Treatment Strategies for IBD: Cytokines, Cell Adhesion Molecules, JAK Inhibitors, Gut Flora, and More. Inflamm. Intest. Dis. 2019, 4, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.-T.; Becker, E.; Wiendl, M.; Schleier, L.; Fuchs, F.; Leppkes, M.; Atreya, R.; Neufert, C.; Atreya, I.; Neurath, M.F.; et al. Similar Inhibition of Dynamic Adhesion of Lymphocytes From IBD Patients to MAdCAM-1 by Vedolizumab and Etrolizumab-s. Inflamm. Bowel Dis. 2018, 24, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Roosenboom, B.; van Lochem, E.G.; Meijer, J.; Smids, C.; Nierkens, S.; Brand, E.C.; van Erp, L.W.; Kemperman, L.G.; Groenen, M.J.; Horje, C.S.H.T.; et al. Development of Mucosal PNAd+ and MAdCAM-1+ Venules during Disease Course in Ulcerative Colitis. Cells 2020, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Sandborn, W.J.; Danese, S.; Hébuterne, X.; Kłopocka, M.; Tarabar, D.; Vaňásek, T.; Greguš, M.; Hellstern, P.A.; Kim, J.S.; et al. Long-term Safety and Efficacy of the Anti-MAdCAM-1 Monoclonal Antibody Ontamalimab [SHP647] for the Treatment of Ulcerative Colitis: The Open-label Study TURANDOT II. J. Crohn’s Colitis 2021, 15, 938–949. [Google Scholar] [CrossRef]
- Taftaf, R.; Liu, X.; Singh, S.; Jia, Y.; Dashzeveg, N.K.; Hoffmann, A.D.; El-Shennawy, L.; Ramos, E.K.; Adorno-Cruz, V.; Schuster, E.J.; et al. ICAM1 initiates CTC cluster formation and trans-endothelial migration in lung metastasis of breast cancer. Nat. Commun. 2021, 12, 4867. [Google Scholar] [CrossRef]
- Scarozza, P.; Schmitt, H.; Monteleone, G.; Neurath, M.F.; Atreya, R. Oligonucleotides—A Novel Promising Therapeutic Option for IBD. Front. Pharmacol. 2019, 10, 314. [Google Scholar] [CrossRef]
- Greuter, T.; Vavricka, S.R.; Biedermann, L.; Pilz, J.; Borovicka, J.; Seibold, F.; Sauter, B.; Rogler, G. Alicaforsen, an Antisense Inhibitor of Intercellular Adhesion Molecule-1, in the Treatment for Left-Sided Ulcerative Colitis and Ulcerative Proctitis. Dig. Dis. 2018, 36, 123–129. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Murphy, E.A.; Price, R.L.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine 2016, 77, 44–49. [Google Scholar] [CrossRef]
- Marafini, I.; Monteleone, I.; Dinallo, V.; Di Fusco, D.; De Simone, V.; Laudisi, F.; Fantini, M.C.; Di Sabatino, A.; Pallone, F.; Monteleone, G. CCL20 Is Negatively Regulated by TGF-β1 in Intestinal Epithelial Cells and Reduced in Crohn’s Disease Patients With a Successful Response to Mongersen, a Smad7 Antisense Oligonucleotide. J. Crohn’s Colitis 2017, 11, 603–609. [Google Scholar] [CrossRef]
- Kulkarni, N.; Meitei, H.T.; Sonar, S.A.; Sharma, P.K.; Mujeeb, V.R.; Srivastava, S.; Boppana, R.; Lal, G. CCR6 signaling inhibits suppressor function of induced-Treg during gut inflammation. J. Autoimmun. 2018, 88, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Meitei, H.T.; Jadhav, N.; Lal, G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun. Rev. 2021, 20, 102846. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, H.; Liang, R.; Zhang, R.; Zhang, J.; Zhu, J. Taraxacum officinale extract ameliorates dextran sodium sulphate—Induced colitis by regulating fatty acid degradation and microbial dysbiosis. J. Cell. Mol. Med. 2019, 23, 8161–8172. [Google Scholar] [CrossRef]
- Cho, H.-S.; Shin, H.M.; Haberstock-Debic, H.; Xing, Y.; Owens, T.D.; Funk, J.O.; Hill, R.J.; Bradshaw, J.M.; Berg, L.J. A Small Molecule Inhibitor of ITK and RLK Impairs Th1 Differentiation and Prevents Colitis Disease Progression. J. Immunol. 2015, 195, 4822–4831. [Google Scholar] [CrossRef]
- Bassolas-Molina, H.; Raymond, E.; Labadia, M.; Wahle, J.; Ferrer-Picón, E.; Panzenbeck, M.; Zheng, J.; Harcken, C.; Hughes, R.; Turner, M.; et al. An RORγt Oral Inhibitor Modulates IL-17 Responses in Peripheral Blood and Intestinal Mucosa of Crohn’s Disease Patients. Front. Immunol. 2018, 9, 2307. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Colombel, J.-F.; Ghosh, S.; Sands, B.E.; Dryden, G.; Hébuterne, X.; Leong, R.W.; Bressler, B.; Ullman, T.; Lakatos, P.L.; et al. Eldelumab [Anti-IP-10] Induction Therapy for Ulcerative Colitis: A Randomised, Placebo-Controlled, Phase 2b Study. J. Crohn’s Colitis 2016, 10, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Rutgeerts, P.; Colombel, J.F.; Ghosh, S.; Petryka, R.; Sands, B.E.; Mitra, P.; Luo, A. Eldelumab [anti-interferon-γ-inducible protein-10 antibody] Induction Therapy for Active Crohn’s Disease: A Randomised, Double-blind, Placebo-controlled Phase IIa Study. J. Crohn’s Colitis 2017, 11, 811–819. [Google Scholar] [CrossRef]
- Polosukhina, D.; Singh, K.; Asim, M.; Barry, D.P.; Allaman, M.M.; Hardbower, D.M.; Piazuelo, M.B.; Washington, M.K.; Gobert, A.P.; Wilson, K.T.; et al. CCL11 exacerbates colitis and inflammation-associated colon tumorigenesis. Oncogene 2021, 40, 6540–6546. [Google Scholar] [CrossRef]
- Sands, B.E. Leukocyte Anti-Trafficking Strategies: Current Status and Future Directions. Dig. Dis. 2017, 35, 13–20. [Google Scholar] [CrossRef]
- Cappenberg, A.; Kardell, M.; Zarbock, A. Selectin-Mediated Signaling—Shedding Light on the Regulation of Integrin Activity in Neutrophils. Cells 2022, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Ajdukovic, J.; Salamunic, I.; Hozo, I.; Despalatovic, B.R.; Simunic, M.; Bonacin, D.; Puljiz, Z.; Trgo, G.; Sundov, Z.; Tonkic, A. Soluble P-selectin glycoprotein ligand—A possible new target in ulcerative colitis. Bratisl. Lek. List. 2015, 116, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Bravo, F.; Macpherson, J.A.; Slack, E.; Despalatovic, B.R.; Simunic, M.; Bonacin, D.; Puljiz, Z.; Trgo, G.; Sundov, Z.; Tonkic, A. Prospective Validation of CD-62L (L-Selectin) as Marker of Durable Response to Infliximab Treatment in Patients with Inflammatory Bowel Disease: A 5-Year Clinical Follow-up. Clin. Transl. Gastroenterol. 2021, 12, e00298. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Li, Y.; Johansson-Lindbom, B.; Coskun, M. Sphingosine-1-Phosphate Signaling in Inflammatory Bowel Disease. Trends Mol. Med. 2017, 23, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; D’Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.; Vermeire, S.; Ghosh, S.; Liu, W.J.; Petersen, A.; Charles, L.; Huang, V.; Usiskin, K.; et al. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results From the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohn’s Colitis 2021, 15, 1120–1129. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Danese, S.; Wolf, D.C.; Liu, W.J.; Hua, S.Y.; Minton, N.; Olson, A.; D’Haens, G. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: A single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol. Hepatol. 2020, 5, 819–828. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Zhang, J.; Chiorean, M.; Vermeire, S.; Lee, S.D.; Kühbacher, T.; Yacyshyn, B.; Cabell, C.H.; Naik, S.U.; et al. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 550–561. [Google Scholar] [CrossRef]
- Vermeire, S.; Chiorean, M.; Panés, J.; Peyrin-Biroulet, L.; Zhang, J.; Sands, B.E.; Lazin, K.; Klassen, P.; Naik, S.U.; Cabell, C.H.; et al. Long-term Safety and Efficacy of Etrasimod for Ulcerative Colitis: Results from the Open-label Extension of the OASIS Study. J. Crohn’s Colitis 2021, 15, 950–959. [Google Scholar] [CrossRef]
- Shimano, K.; Maeda, Y.; Kataoka, H.; Murase, M.; Mochizuki, S.; Utsumi, H.; Oshita, K.; Sugahara, K. Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 functional antagonist, inhibits progress of chronic colitis induced by transfer of CD4+CD45RBhigh T cells. PLoS ONE 2019, 14, e0226154. [Google Scholar] [CrossRef]
- D’Haens, G.; Danese, S.; Davies, M.; Watanabe, M.; Hibi, T. A phase II, Multicentre, Randomised, Double-Blind, Placebo-controlled Study to Evaluate Safety, Tolerability, and Efficacy of Amiselimod in Patients with Moderate to Severe Active Crohn’s Disease. J. Crohn’s Colitis 2022, 16, 746–756. [Google Scholar] [CrossRef]
- Abdalla, M.I.; Levesque, B.G. Progress in Corticosteroid Use in the Era of Biologics With Room for Improvement. Am. J. Gastroenterol. 2021, 116, 1187–1188. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Bernstein, C.N.; Benchimol, E.I.; Kaplan, G.G.; Singh, H.; Tennakoon, A.; Nugent, Z.; Coward, S.B.; Kuenzig, M.E.; Murthy, S.K. Trends in Corticosteroid Use During the Era of Biologic Therapy: A Population-Based Analysis. Am. J. Gastroenterol. 2021, 116, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.R. Top-down therapy for IBD: Rationale and requisite evidence. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F. IL-23 Blockade in Anti-TNF Refractory IBD: From Mechanisms to Clinical Reality. J. Crohn’s Colitis 2022, 16 (Suppl. S2), ii54–ii63. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Siegel, C.A. Refocusing IBD Patient Management: Personalized, Proactive, and Patient-Centered Care. Am. J. Gastroenterol. 2018, 113, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Wu, T. The Importance of Adopting Leadership Concepts in Communicating Medicinal Culture of Chinese Medicine in the Western World. Chin. Med. Cult. 2021, 4, 58–65. [Google Scholar] [CrossRef]
- Li, H.; Wei, W.; Xu, H. Drug discovery is an eternal challenge for the biomedical sciences. Acta Mater. Med. 2022, 1, 1–3. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Yuan, M.; Xu, Y.; Xu, H. Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing. Pharmaceuticals 2022, 15, 1080. https://doi.org/10.3390/ph15091080
Song Y, Yuan M, Xu Y, Xu H. Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing. Pharmaceuticals. 2022; 15(9):1080. https://doi.org/10.3390/ph15091080
Chicago/Turabian StyleSong, Yijie, Man Yuan, Yu Xu, and Hongxi Xu. 2022. "Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing" Pharmaceuticals 15, no. 9: 1080. https://doi.org/10.3390/ph15091080
APA StyleSong, Y., Yuan, M., Xu, Y., & Xu, H. (2022). Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing. Pharmaceuticals, 15(9), 1080. https://doi.org/10.3390/ph15091080





