Looking beyond the Skin: Pathophysiology of Cardiovascular Comorbidity in Psoriasis and the Protective Role of Biologics
Abstract
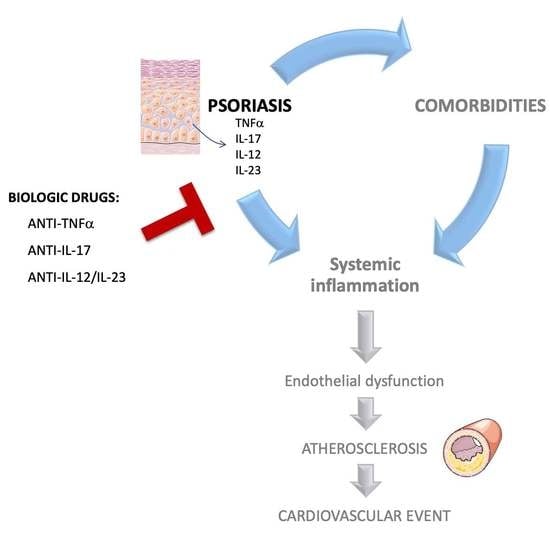
:1. Introduction
2. Link between Atherosclerosis and Psoriasis
3. Biologics in the Treatment of Psoriasis. Do They Also Address the Increased Cardiovascular Risk?
3.1. Anti-TNFα Drugs
3.2. Drugs That Target the IL-23/Th17 Axis
3.2.1. Anti-IL-17 Drugs
3.2.2. Anti-IL12/23 Drugs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.M.; Loring, B.; John, S.M. A Systematic Review of Worldwide Epidemiology of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Korman, N.J. Management of Psoriasis as a Systemic Disease: What Is the Evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum Levels of TNF-Alpha, IFN-Gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in Patients with Active Psoriasis and Correlation with Disease Severity. Mediat. Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Li, K.; Fuentes-Duculan, J.; Hayden, K.; Brodmerkel, C.; Krueger, J.G. Expanding the Psoriasis Disease Profile: Interrogation of the Skin and Serum of Patients with Moderate-to-Severe Psoriasis. J. Investig. Dermatol. 2012, 132, 2552–2564. [Google Scholar] [CrossRef]
- De Oliveira, P.S.S.; Cardoso, P.R.G.; Lima, E.V.D.A.; Pereira, M.C.; Duarte, A.L.B.P.; Pitta, I.D.R.; Rêgo, M.J.B.D.M.; Pitta, M.G.D.R. IL-17A, IL-22, IL-6, and IL-21 Serum Levels in Plaque-Type Psoriasis in Brazilian Patients. Mediat. Inflamm. 2015, 2015, 819149. [Google Scholar] [CrossRef]
- Wang, W.-M.; Jin, H.-Z. Role of Neutrophils in Psoriasis. J. Immunol. Res. 2020, 2020, 3709749. [Google Scholar] [CrossRef]
- Schön, M.P.; Broekaert, S.M.C.; Erpenbeck, L. Sexy Again: The Renaissance of Neutrophils in Psoriasis. Exp. Dermatol. 2017, 26, 305–311. [Google Scholar] [CrossRef]
- Mehta, N.N.; Yu, Y.D.; Saboury, B.; Foroughi, N.; Krishnamoorthy, P.; Raper, A.; Baer, A.; Antigua, J.; Van Voorhees, A.S.; Torigian, D.A.; et al. Systemic and Vascular Inflammation in Patients with Moderate to Severe Psoriasis as Measured by [18F]-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography (FDG-PET/CT): A Pilot Study. Arch. Dermatol. 2011, 147, 1031–1039. [Google Scholar] [CrossRef] [Green Version]
- Hjuler, K.F.; Gormsen, L.C.; Vendelbo, M.H.; Egeberg, A.; Nielsen, J.; Iversen, L. Increased Global Arterial and Subcutaneous Adipose Tissue Inflammation in Patients with Moderate-to-Severe Psoriasis. Br. J. Dermatol. 2017, 176, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Machado-Pinto, J.; Diniz, M.D.S.; Bavoso, N.C. Psoriasis: New Comorbidities. An. Bras. Dermatol. 2016, 91, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Mallbris, L.; Warren, R.B.; Bachelez, H.; Gislason, G.H.; Hansen, P.R.; Skov, L. Association between Psoriasis and Inflammatory Bowel Disease: A Danish Nationwide Cohort Study. Br. J. Dermatol. 2016, 175, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Faustini, F.; Simon, D.; Oliveira, I.; Kleyer, A.; Haschka, J.; Englbrecht, M.; Cavalcante, A.R.; Kraus, S.; Tabosa, T.P.; Figueiredo, C.; et al. Subclinical Joint Inflammation in Patients with Psoriasis without Concomitant Psoriatic Arthritis: A Cross-Sectional and Longitudinal Analysis. Ann. Rheum. Dis. 2016, 75, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Yu, Y.; Pinnelas, R.; Krishnamoorthy, P.; Shin, D.B.; Troxel, A.B.; Gelfand, J.M. Attributable Risk Estimate of Severe Psoriasis on Major Cardiovascular Events. Am. J. Med. 2011, 124, 775.e1–775.e6. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Patients with Severe Psoriasis Are at Increased Risk of Cardiovascular Mortality: Cohort Study Using the General Practice Research Database. Eur. Heart J. 2010, 31, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Patel, R.; Pradhan, D.; Deval, R.; Singh, H.; Thomas, G.; Jain, A.K. Psoriasis and Cardiovascular Disorders: Association or Epiphenomenon? Meta-Analysis of Observational Studies. 3 Biotech 2020, 10, 104. [Google Scholar] [CrossRef]
- Boehncke, W.-H.H.; Boehncke, S. Cardiovascular Mortality in Psoriasis and Psoriatic Arthritis: Epidemiology, Pathomechanisms, Therapeutic Implications, and Perspectives. Curr. Rheumatol. Rep. 2012, 14, 343–348. [Google Scholar] [CrossRef]
- Candia, R.; Ruiz, A.; Torres-Robles, R.; Chávez-Tapia, N.; Méndez-Sánchez, N.; Arrese, M. Risk of Non-Alcoholic Fatty Liver Disease in Patients with Psoriasis: A Systematic Review and Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 656–662. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Gislason, G.H.; Charlot, M.; Jørgensen, C.H.; Lindhardsen, J.; Olesen, J.B.; Abildstrøm, S.Z.; Skov, L.; Torp-Pedersen, C.; Hansen, P.R. Psoriasis Is Associated with Clinically Significant Cardiovascular Risk: A Danish Nationwide Cohort Study. J. Intern. Med. 2011, 270, 147–157. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Skov, L.; Gislason, G.; Lindhardsen, J.; Kristensen, S.L.; Iversen, L.; Lasthein, S.; Gniadecki, R.; Dam, T.N.; Torp-Pedersen, C.; et al. Cardiovascular Disease Event Rates in Patients with Severe Psoriasis Treated with Systemic Anti-Inflammatory Drugs: A Danish Real-World Cohort Study. J. Intern. Med. 2013, 273, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.-S.; Lan, C.-C.E. Psoriasis and Cardiovascular Comorbidities: Focusing on Severe Vascular Events, Cardiovascular Risk Factors and Implications for Treatment. Int. J. Mol. Sci. 2017, 18, 2211. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Thyssen, J.P.; Jensen, P.; Gislason, G.H.; Skov, L. Risk of Myocardial Infarction in Patients with Psoriasis and Psoriatic Arthritis: A Nationwide Cohort Study. Acta Derm. Venereol. 2017, 97, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, E.A.; Kavousi, M.; Nijsten, T.; Ikram, M.A.; Hofman, A.; Franco, O.H.; Wakkee, M. Psoriasis Is Not Associated with Atherosclerosis and Incident Cardiovascular Events: The Rotterdam Study. J. Investig. Dermatol. 2013, 133, 2347–2354. [Google Scholar] [CrossRef]
- Wakkee, M.; Meijer, W.; Neumann, H.A.M.; Herings, R.M.C.; Nijsten, T. Psoriasis May Not Be an Independent Predictor for the Use of Cardiovascular and Anti-Diabetic Drugs: A 5-Year Prevalence Study. Acta Derm. Venereol. 2009, 89, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B. Risk of Myocardial Infarction in Patients With Psoriasis. JAMA 2006, 296, 1735. [Google Scholar] [CrossRef] [PubMed]
- Ahlehoff, O.; Gislason, G.H.; Jorgensen, C.H.; Lindhardsen, J.; Charlot, M.; Olesen, J.B.; Abildstrom, S.Z.; Skov, L.; Torp-Pedersen, C.; Hansen, P.R.; et al. Psoriasis and Risk of Atrial Fibrillation and Ischaemic Stroke: A Danish Nationwide Cohort Study. Eur. Heart J. 2012, 33, 2054–2064. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Dommasch, E.D.; Shin, D.B.; Azfar, R.S.; Kurd, S.K.; Wang, X.; Troxel, A.B. The Risk of Stroke in Patients with Psoriasis. J. Investig. Dermatol. 2009, 129, 2411–2418. [Google Scholar] [CrossRef]
- Fernández-Armenteros, J.M.; Gómez-Arbonés, X.; Buti-Soler, M.; Betriu-Bars, A.; Sanmartin-Novell, V.; Ortega-Bravo, M.; Martínez-Alonso, M.; Garí, E.; Portero-Otín, M.; Santamaria-Babi, L.; et al. Psoriasis, Metabolic Syndrome and Cardiovascular Risk Factors. A Population-Based Study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 128–135. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Prim. 2016, 2, 16082. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and Comorbid Diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Yew, Y.W. Psoriasis as an Independent Risk Factor for Cardiovascular Disease: An Epidemiologic Analysis Using a National Database. J. Cutan. Med. Surg. 2016, 20, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Troxel, A.B.; Mehta, N.N.; Gelfand, J.M. Psoriasis and Cardiovascular Risk: Strength in Numbers Part 3. J. Investig. Dermatol. 2015, 135, 2148–2150. [Google Scholar] [CrossRef] [PubMed]
- Raaby, L.; Ahlehoff, O.; de Thurah, A. Psoriasis and Cardiovascular Events: Updating the Evidence. Arch. Dermatol. Res. 2017, 309, 225–228. [Google Scholar] [CrossRef]
- Martinez-Moreno, A.; Ocampo-Candiani, J.; Garza-Rodriguez, V. Psoriasis and Cardiovascular Disease: A Narrative Review. Korean J. Fam. Med. 2021, 42, 345–355. [Google Scholar] [CrossRef]
- Puig, L. Cardiometabolic Comorbidities in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2018, 19, 58. [Google Scholar] [CrossRef]
- Lockshin, B.; Balagula, Y.; Merola, J.F. Interleukin 17, Inflammation, and Cardiovascular Risk in Patients with Psoriasis. J. Am. Acad. Dermatol. 2018, 79, 345–352. [Google Scholar] [CrossRef]
- Gonzalez-Cantero, A.; Gonzalez-Cantero, J.; Sanchez-Moya, A.I.; Perez-Hortet, C.; Arias-Santiago, S.; Schoendorff-Ortega, C.; Gonzalez-Calvin, J.L. Subclinical Atherosclerosis in Psoriasis. Usefulness of Femoral Artery Ultrasound for the Diagnosis, and Analysis of Its Relationship with Insulin Resistance. PLoS ONE 2019, 14, e0211808. [Google Scholar] [CrossRef]
- Youn, S.W.; Kang, S.Y.; Kim, S.A.; Park, G.Y.; Lee, W.W. Subclinical Systemic and Vascular Inflammation Detected by (18) F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Patients with Mild Psoriasis. J. Dermatol. 2015, 42, 559–566. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the Site of Inflammation: The Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Jackson, S.P. Arterial Thrombosis-Insidious, Unpredictable and Deadly. Nat. Med. 2011, 17, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and Platelets: An Update. Eur. Heart J. 2016, 38, ehw550. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, G.; Fabbrocini, G.; Di Caprio, R.; Raimondo, A.; Scala, E.; Balato, N.; Balato, A. Psoriasis, Cardiovascular Events, and Biologics: Lights and Shadows. Front. Immunol. 2018, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation and Atherosclerosis: The End of a Controversy. Circulation 2017, 136, 1875–1877. [Google Scholar] [CrossRef]
- Späh, F. Inflammation in Atherosclerosis and Psoriasis: Common Pathogenic Mechanisms and the Potential for an Integrated Treatment Approach. Br. J. Dermatol. 2008, 159, 10–17. [Google Scholar] [CrossRef]
- Boehncke, W.-H.H. Systemic Inflammation and Cardiovascular Comorbidity in Psoriasis Patients: Causes and Consequences. Front. Immunol. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.H.; Boehncke, S.; Tobin, A.-M.M.; Kirby, B. The “Psoriatic March”: A Concept of How Severe Psoriasis May Drive Cardiovascular Comorbidity. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, S.; Thaci, D.; Beschmann, H.; Ludwig, R.J.; Ackermann, H.; Badenhoop, K.; Boehncke, W.-H. Psoriasis Patients Show Signs of Insulin Resistance. Br. J. Dermatol. 2007, 157, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Ucak, S.; Ekmekci, T.R.; Basat, O.; Koslu, A.; Altuntas, Y. Comparison of Various Insulin Sensitivity Indices in Psoriatic Patients and Their Relationship with Type of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 517–522. [Google Scholar] [CrossRef]
- Karadag, A.S.; Yavuz, B.; Ertugrul, D.T.; Akin, K.O.; Yalcin, A.A.; Deveci, O.S.; Ata, N.; Kucukazman, M.; Dal, K. Is Psoriasis a Pre-Atherosclerotic Disease? Increased Insulin Resistance and Impaired Endothelial Function in Patients with Psoriasis. Int. J. Dermatol. 2010, 49, 642–646. [Google Scholar] [CrossRef]
- Gisondi, P.; Fantin, F.; Del Giglio, M.; Valbusa, F.; Marino, F.; Zamboni, M.; Girolomoni, G. Chronic Plaque Psoriasis Is Associated with Increased Arterial Stiffness. Dermatology 2009, 218, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Balci, D.D.; Balci, A.; Karazincir, S.; Ucar, E.; Iyigun, U.; Yalcin, F.; Seyfeli, E.; Inandi, T.; Egilmez, E. Increased Carotid Artery Intima-Media Thickness and Impaired Endothelial Function in Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, R.E.; Karabudak, O.; Yokusoglu, M.; Kilicaslan, F.; Kirilmaz, A.; Cebeci, B.S. Noninvasive Assessment of Impaired Endothelial Function in Psoriasis. Rheumatol. Int. 2010, 30, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Cerman, A.A.; Bozkurt, S.; Sav, A.; Tulunay, A.; Elbaşi, M.O.; Ergun, T. Serum Leptin Levels, Skin Leptin and Leptin Receptor Expression in Psoriasis. Br. J. Dermatol. 2008, 159, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, Z. Vascular Function, Insulin Action, and Exercise: An Intricate Interplay. Trends Endocrinol. Metab. 2015, 26, 297–304. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Breslin, J.W.; Perrin, R.; Gaudreault, N.; Guo, M.; Kargozaran, H.; Wu, M.H. Microvascular Permeability in Diabetes and Insulin Resistance. Microcirculation 2007, 14, 363–373. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Bastard, J.-P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent Advances in the Relationship between Obesity, Inflammation, and Insulin Resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Lago, R.; Gómez, R.; Lago, F.; Gómez-Reino, J.; Gualillo, O. Leptin beyond Body Weight Regulation—Current Concepts Concerning Its Role in Immune Function and Inflammation. Cell. Immunol. 2008, 252, 139–145. [Google Scholar] [CrossRef]
- Sajja, A.P.; Joshi, A.A.; Teague, H.L.; Dey, A.K.; Mehta, N.N. Potential Immunological Links between Psoriasis and Cardiovascular Disease. Front. Immunol. 2018, 9, 1234. [Google Scholar] [CrossRef]
- Ford, M.L.; Adams, A.B.; Pearson, T.C. Targeting Co-Stimulatory Pathways: Transplantation and Autoimmunity. Nat. Rev. Nephrol. 2014, 10, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Akkara Veetil, B.M.; Matteson, E.L.; Maradit-Kremers, H.; McEvoy, M.T.; Crowson, C.S. Trends in Lipid Profiles in Patients with Psoriasis: A Population-Based Analysis. BMC Dermatol. 2012, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Li, R.; Krishnamoorthy, P.; Yu, Y.; Farver, W.; Rodrigues, A.; Raper, A.; Wilcox, M.; Baer, A.; DerOhannesian, S.; et al. Abnormal Lipoprotein Particles and Cholesterol Efflux Capacity in Patients with Psoriasis. Atherosclerosis 2012, 224, 218–221. [Google Scholar] [CrossRef]
- Asha, K.; Singal, A.; Sharma, S.B.; Arora, V.K.; Aggarwal, A. Dyslipidaemia & Oxidative Stress in Patients of Psoriasis: Emerging Cardiovascular Risk Factors. Indian J. Med. Res. 2017, 146, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Purushothaman, K.R.; Fuster, V.; Echeverri, D.; Truszczynska, H.; Sharma, S.K.; Badimon, J.J.; O’Connor, W.N. Plaque Neovascularization Is Increased in Ruptured Atherosclerotic Lesions of Human Aorta: Implications for Plaque Vulnerability. Circulation 2004, 110, 2032–2038. [Google Scholar] [CrossRef]
- Nilsson, J.; Wigren, M.; Shah, P.K. Vaccines against Atherosclerosis. Expert Rev. Vaccines 2013, 12, 311–321. [Google Scholar] [CrossRef]
- Karunakaran, D.; Geoffrion, M.; Wei, L.; Gan, W.; Richards, L.; Shangari, P.; DeKemp, E.M.; Beanlands, R.A.; Perisic, L.; Maegdefessel, L.; et al. Targeting Macrophage Necroptosis for Therapeutic and Diagnostic Interventions in Atherosclerosis. Sci. Adv. 2016, 2, e1600224. [Google Scholar] [CrossRef] [PubMed]
- Tuenter, A.; Selwaness, M.; Arias Lorza, A.; Schuurbiers, J.C.H.; Speelman, L.; Cibis, M.; van der Lugt, A.; de Bruijne, M.; van der Steen, A.F.W.; Franco, O.H.; et al. High Shear Stress Relates to Intraplaque Haemorrhage in Asymptomatic Carotid Plaques. Atherosclerosis 2016, 251, 348–354. [Google Scholar] [CrossRef]
- Flammer, A.J.; Ruschitzka, F. Psoriasis and Atherosclerosis: Two Plaques, One Syndrome? Eur. Heart J. 2012, 33, 1989–1991. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Voyles, S.V.; Armstrong, E.J.; Fuller, E.N.; Rutledge, J.C. A Tale of Two Plaques: Convergent Mechanisms of T-Cell-Mediated Inflammation in Psoriasis and Atherosclerosis. Exp. Dermatol. 2011, 20, 544–549. [Google Scholar] [CrossRef]
- Stemme, S.; Faber, B.; Holm, J.; Wiklund, O.; Witztum, J.L.; Hansson, G.K. T Lymphocytes from Human Atherosclerotic Plaques Recognize Oxidized Low Density Lipoprotein. Proc. Natl. Acad. Sci. USA 1995, 92, 3893–3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frostegård, J.; Ulfgren, A.K.; Nyberg, P.; Hedin, U.; Swedenborg, J.; Andersson, U.; Hansson, G.K. Cytokine Expression in Advanced Human Atherosclerotic Plaques: Dominance of pro-Inflammatory (Th1) and Macrophage-Stimulating Cytokines. Atherosclerosis 1999, 145, 33–43. [Google Scholar] [CrossRef]
- Methe, H.; Brunner, S.; Wiegand, D.; Nabauer, M.; Koglin, J.; Edelman, E.R. Enhanced T-Helper-1 Lymphocyte Activation Patterns in Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2005, 45, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The Role of IL-23 and the IL-23/TH 17 Immune Axis in the Pathogenesis and Treatment of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef]
- Amin, M.; Darji, K.; No, D.J.; Bhutani, T.; Wu, J.J. Review of IL-17 Inhibitors for Psoriasis. J. Dermatol. Treat. 2018, 29, 347–352. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Toussirot, E. The IL23/Th17 Pathway as a Therapeutic Target in Chronic Inflammatory Diseases. Inflamm. Allergy Drug Targets 2012, 11, 159–168. [Google Scholar] [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Lorthois, I.; Asselineau, D.; Seyler, N.; Pouliot, R. Contribution of In Vivo and Organotypic 3D Models to Understanding the Role of Macrophages and Neutrophils in the Pathogenesis of Psoriasis. Mediat. Inflamm. 2017, 2017, 7215072. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Papp, K.A.; Matheson, R.T.; Tu, J.H.; Bissonnette, R.; Bourcier, M.; Gratton, D.; Kunynetz, R.A.; Poulin, Y.; Rosoph, L.A.; et al. Evidence That a Neutrophil-Keratinocyte Crosstalk Is an Early Target of IL-17A Inhibition in Psoriasis. Exp. Dermatol. 2015, 24, 529–535. [Google Scholar] [CrossRef]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Leeuwen, M.; Gijbels, M.J.J.; Duijvestijn, A.; Smook, M.; van de Gaar, M.J.; Heeringa, P.; de Winther, M.P.J.; Tervaert, J.W.C. Accumulation of Myeloperoxidase-Positive Neutrophils in Atherosclerotic Lesions in LDLR−/− Mice. Arter. Thromb. Vasc. Biol. 2008, 28, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Rotzius, P.; Thams, S.; Soehnlein, O.; Kenne, E.; Tseng, C.-N.; Björkström, N.K.; Malmberg, K.-J.; Lindbom, L.; Eriksson, E.E. Distinct Infiltration of Neutrophils in Lesion Shoulders in ApoE−/− Mice. Am. J. Pathol. 2010, 177, 493–500. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kumon, Y.; Kobayashi, T.; Enzan, H.; Nishioka, Y.; Yuri, K.; Wakiguchi, H.; Sugiura, T. Neutrophil Infiltration and Oxidant-Production in Human Atherosclerotic Carotid Plaques. Histol. Histopathol. 2011, 26, 1–11. [Google Scholar] [CrossRef]
- Kramer, M.C.A.; Rittersma, S.Z.H.; de Winter, R.J.; Ladich, E.R.; Fowler, D.R.; Liang, Y.-H.; Kutys, R.; Carter-Monroe, N.; Kolodgie, F.D.; van der Wal, A.C.; et al. Relationship of Thrombus Healing to Underlying Plaque Morphology in Sudden Coronary Death. J. Am. Coll. Cardiol. 2010, 55, 122–132. [Google Scholar] [CrossRef]
- Deng, Y.; Chang, C.; Lu, Q. The Inflammatory Response in Psoriasis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 377–389. [Google Scholar] [CrossRef]
- Costa, S.; Marini, O.; Bevilacqua, D.; DeFranco, A.L.; Hou, B.; Lonardi, S.; Vermi, W.; Rodegher, P.; Panato, A.; Tagliaro, F.; et al. Role of MyD88 Signaling in the Imiquimod-Induced Mouse Model of Psoriasis: Focus on Innate Myeloid Cells. J. Leukoc. Biol. 2017, 102, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Lichtman, A.H. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef]
- Peled, M.; Fisher, E.A. Dynamic Aspects of Macrophage Polarization during Atherosclerosis Progression and Regression. Front. Immunol. 2014, 5, 579. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, L.; Jiang, H.; Lin, Y.; Wang, Z. Platelet Dysfunction and Its Role in the Pathogenesis of Psoriasis. Dermatology 2021, 237, 56–65. [Google Scholar] [CrossRef]
- Weber, B.; Merola, J.F.; Husni, M.E.; Di Carli, M.; Berger, J.S.; Garshick, M.S. Psoriasis and Cardiovascular Disease: Novel Mechanisms and Evolving Therapeutics. Curr. Atheroscler. Rep. 2021, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.J.; Schultz, J.E.; Boehncke, W.-H.; Podda, M.; Tandi, C.; Krombach, F.; Baatz, H.; Kaufmann, R.; von Andrian, U.H.; Zollner, T.M. Activated, Not Resting, Platelets Increase Leukocyte Rolling in Murine Skin Utilizing a Distinct Set of Adhesion Molecules. J. Investig. Dermatol. 2004, 122, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in Inflammation and Atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Gisterå, A.; Hansson, G.K. The Immunology of Atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Jamasbi, J.; Ayabe, K.; Goto, S.; Nieswandt, B.; Peter, K.; Siess, W. Platelet Receptors as Therapeutic Targets: Past, Present and Future. Thromb. Haemost. 2017, 117, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Boehncke, S. More than Skin-Deep: The Many Dimensions of the Psoriatic Disease. Swiss Med. Wkly. 2014, 144, w13968. [Google Scholar] [CrossRef]
- Gisondi, P.; Fostini, A.C.; Fossà, I.; Girolomoni, G.; Targher, G. Psoriasis and the Metabolic Syndrome. Clin. Dermatol. 2017, 36, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Poon, K.-Y.T. Tumor Necrosis Factor Inhibitor Therapy and Myocardial Infarction Risk in Patients with Psoriasis, Psoriatic Arthritis, or Both. J. Drugs Dermatol. 2014, 13, 932–934. [Google Scholar]
- Prodanovich, S.; Prodanowich, S.; Ma, F.; Taylor, J.R.; Pezon, C.; Fasihi, T.; Kirsner, R.S. Methotrexate Reduces Incidence of Vascular Diseases in Veterans with Psoriasis or Rheumatoid Arthritis. J. Am. Acad. Dermatol. 2005, 52, 262–267. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Ko, Y.-C.; Yu, H.-S.; Wu, C.-S.; Li, W.-C.; Lu, Y.-W.; Chen, Y.-C.; Chin, Y.-Y.; Yang, Y.-H.; Chen, G.-S. Methotrexate Reduces the Occurrence of Cerebrovascular Events among Taiwanese Psoriatic Patients: A Nationwide Population-Based Study. Acta Derm. Venereol. 2012, 92, 349–352. [Google Scholar] [CrossRef]
- Bălănescu, A.-R.; Bojincă, V.C.; Bojincă, M.; Donisan, T.; Bălănescu, S.M. Cardiovascular Effects of Methotrexate in Immune-Mediated Inflammatory Diseases. Exp. Ther. Med. 2019, 17, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, F.; Prati, C.; Chouk, M.; Demougeot, C.; Wendling, D. Methotrexate and Cardiovascular Risk in Rheumatic Diseases:A Comprehensive Review. Expert Rev. Clin. Pharmacol. 2021, 14, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.L.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR Recommendations for Cardiovascular Disease Risk Management in Patients with Rheumatoid Arthritis and Other Forms of Inflammatory Joint Disorders: 2015/2016 Update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef]
- Westlake, S.L.; Colebatch, A.N.; Baird, J.; Curzen, N.; Kiely, P.; Quinn, M.; Choy, E.; Ostor, A.J.K.; Edwards, C.J. Tumour Necrosis Factor Antagonists and the Risk of Cardiovascular Disease in Patients with Rheumatoid Arthritis: A Systematic Literature Review. Rheumatology 2011, 50, 518–531. [Google Scholar] [CrossRef]
- Mäki-Petäjä, K.M.; Elkhawad, M.; Cheriyan, J.; Joshi, F.R.; Ostör, A.J.K.; Hall, F.C.; Rudd, J.H.F.; Wilkinson, I.B.; Östör, A.J.K.; Hall, F.C.; et al. Anti-Tumor Necrosis Factor-α Therapy Reduces Aortic Inflammation and Stiffness in Patients with Rheumatoid Arthritis. Circulation 2012, 126, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Tam, L.S.; Kitas, G.D.; Gonźlez-gay, M.A. Can Suppression of Inflammation by Anti-TNF Prevent Progression of Subclinical Atherosclerosis in Inflammatory Arthritis? Rheumatology 2014, 53, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The Effects of Tumour Necrosis Factor Inhibitors, Methotrexate, Non-Steroidal Anti-Inflammatory Drugs and Corticosteroids on Cardiovascular Events in Rheumatoid Arthritis, Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-Analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M. Does Treatment of Psoriasis Reduce Cardiovascular Comorbidities? J. Investig. Dermatol. 2017, 137, 1612–1613. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Sbidian, E.; Chaimani, A.; Afach, S.; Doney, L.; Dressler, C.; Hua, C.; Mazaud, C.; Phan, C.; Hughes, C.; Riddle, D.; et al. Systemic Pharmacological Treatments for Chronic Plaque Psoriasis: A Network Meta-Analysis. Cochrane Database Syst. Rev. 2020, 1, CD011535. [Google Scholar] [CrossRef]
- Daudén, E.; Puig, L.; Ferrándiz, C.; Sánchez-Carazo, J.L.; Hernanz-Hermosa, J.M. Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology Consensus Document on the Evaluation and Treatment of Moderate-to-Severe Psoriasis: Psoriasis Group of the Spanish Academy of Dermatology and Venereology. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. S2), 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, M.; Mabuchi, T. New Treatment Addressing the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 7488. [Google Scholar] [CrossRef] [PubMed]
- Stiff, K.M.; Glines, K.R.; Porter, C.L.; Cline, A.; Feldman, S.R. Current Pharmacological Treatment Guidelines for Psoriasis and Psoriatic Arthritis. Expert Rev. Clin. Pharmacol. 2018, 11, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Hugh, J.; Van Voorhees, A.S.; Nijhawan, R.I.; Bagel, J.; Lebwohl, M.; Blauvelt, A.; Hsu, S.; Weinberg, J.M. From the Medical Board of the National Psoriasis Foundation: The Risk of Cardiovascular Disease in Individuals with Psoriasis and the Potential Impact of Current Therapies. J. Am. Acad. Dermatol. 2014, 70, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the Systemic Treatment of Psoriasis Vulgaris—Part 1: Treatment and Monitoring Recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef]
- Molina-Leyva, A.; Garrido-Pareja, F.; Ruiz-Carrascosa, J.C.; Ruiz-Villaverde, R. TNF-Alpha Inhibition Could Reduce Biomarkers of Endothelial Dysfunction in Patients with Moderate to Severe Psoriasis: A 52-Week Echo-Doppler Based Quasi-Experimental Study. Med. Clin. 2018, 150, 465–468. [Google Scholar] [CrossRef]
- Yang, Z.S.; Lin, N.N.; Li, L.; Li, Y. The Effect of TNF Inhibitors on Cardiovascular Events in Psoriasis and Psoriatic Arthritis: An Updated Meta-Analysis. Clin. Rev. Allergy Immunol. 2016, 51, 240–247. [Google Scholar] [CrossRef]
- Shaaban, D.; Al-Mutairi, N. The Effect of Tumor Necrosis Factor Inhibitor Therapy on the Incidence of Myocardial Infarction in Patients with Psoriasis: A Retrospective Study. J. Dermatol. Treat. 2018, 29, 3–7. [Google Scholar] [CrossRef]
- Wu, J.J.; Sundaram, M.; Cloutier, M.; Gauthier-Loiselle, M.; Guérin, A.; Singh, R.; Ganguli, A. The Risk of Cardiovascular Events in Psoriasis Patients Treated with Tumor Necrosis Factor-α Inhibitors versus Phototherapy: An Observational Cohort Study. J. Am. Acad. Dermatol. 2018, 79, 60–68. [Google Scholar] [CrossRef]
- Wu, J.J.; Guérin, A.; Sundaram, M.; Dea, K.; Cloutier, M.; Mulani, P. Cardiovascular Event Risk Assessment in Psoriasis Patients Treated with Tumor Necrosis Factor-α Inhibitors versus Methotrexate. J. Am. Acad. Dermatol. 2017, 76, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Rungapiromnan, W.; Yiu, Z.Z.N.; Warren, R.B.; Griffiths, C.E.M.; Ashcroft, D.M. Impact of Biologic Therapies on Risk of Major Adverse Cardiovascular Events in Patients with Psoriasis: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Br. J. Dermatol. 2017, 176, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Cui, L.; Wang, Y.; Li, Y.; Zhang, X.; Shi, Y. Cardiometabolic Comorbidities in Patients With Psoriasis: Focusing on Risk, Biological Therapy, and Pathogenesis. Front. Pharmacol. 2021, 12, 774808. [Google Scholar] [CrossRef]
- Bissonnette, R.; Harel, F.; Krueger, J.G.; Guertin, M.-C.; Chabot-Blanchet, M.; González, J.; Maari, C.; Delorme, I.; Lynde, C.; Tardif, J.-C. TNF-α Antagonist and Vascular Inflammation in Patients with Psoriasis Vulgaris: A Randomized Placebo-Controlled Study. J. Investig. Dermatol. 2017, 137, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.B.; Lebwohl, M.G. Psoriasis: Which Therapy for Which Patient: Psoriasis Comorbidities and Preferred Systemic Agents. J. Am. Acad. Dermatol. 2019, 80, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Shin, D.B.; Joshi, A.A.; Dey, A.K.; Armstrong, A.W.; Duffin, K.C.; Fuxench, Z.C.; Harrington, C.L.; Hubbard, R.A.; Kalb, R.E.; et al. Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial. Circ. Cardiovasc. Imaging 2018, 11, e007394. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Hoke, M.; Sabeti-Sandor, S.; Perkmann, T.; Rauscher, A.; Strassegger, B.; Radakovic, S.; Tanew, A. Disparate Effects of Adalimumab and Fumaric Acid Esters on Cardiovascular Risk Factors in Psoriasis Patients: Results from a Prospective, Randomized, Observer-Blinded Head-to-Head Trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 441–449. [Google Scholar] [CrossRef]
- García-Martínez, P.; Collado-Díaz, V.; Mateu-Puchades, A.; Villarroel-Vicente, C.; Rovira-Llopis, S.; Blas-García, A.; Álvarez, Á.; Esplugues, J.V.; Andújar, I. Differential Effects of Biologics on Psoriasis-Related Vascular Inflammation and Risk of Thrombosis. J. Investig. Dermatol. 2020, 140, 2294–2298.e6. [Google Scholar] [CrossRef]
- Knowles, L.; Nadeem, N.; Chowienczyk, P.J. Do Anti-Tumour Necrosis Factor-α Biologics Affect Subclinical Measures of Atherosclerosis and Arteriosclerosis? A Systematic Review. Br. J. Clin. Pharmacol. 2020, 86, 837–851. [Google Scholar] [CrossRef]
- González-Cantero, A.; Ortega-Quijano, D.; Álvarez-Díaz, N.; Ballester, M.A.; Jimenez-Gomez, N.; Jaen, P.; González-Cantero, J.; González-Calvin, J.L.; Barderas, M.G.; Shin, D.B.; et al. Impact of Biological Agents on Imaging and Biomarkers of Cardiovascular Disease in Patients with Psoriasis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. J. Investig. Dermatol. 2021, 141, 2402–2411. [Google Scholar] [CrossRef]
- von Stebut, E.; Boehncke, W.-H.; Ghoreschi, K.; Gori, T.; Kaya, Z.; Thaci, D.; Schäffler, A. IL-17A in Psoriasis and Beyond: Cardiovascular and Metabolic Implications. Front. Immunol. 2019, 10, 3096. [Google Scholar] [CrossRef]
- Erbel, C.; Akhavanpoor, M.; Okuyucu, D.; Wangler, S.; Dietz, A.; Zhao, L.; Stellos, K.; Little, K.M.; Lasitschka, F.; Doesch, A.; et al. IL-17A Influences Essential Functions of the Monocyte/Macrophage Lineage and Is Involved in Advanced Murine and Human Atherosclerosis. J. Immunol. 2014, 193, 4344–4355. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yu, X.; Ding, Y.-J.; Fu, Q.-Q.; Xie, J.-J.; Tang, T.-T.; Yao, R.; Chen, Y.; Liao, Y.-H. The Th17/Treg Imbalance in Patients with Acute Coronary Syndrome. Clin. Immunol. 2008, 127, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Zeng, Q.T. Role of Interleukin-17 and Interleukin-17-Induced Cytokines Interleukin-6 and Interleukin-8 in Unstable Coronary Artery Disease. Coron. Artery Dis. 2006, 17, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.; Romain, M.; Ramkhelawon, B.; Uyttenhove, C.; Pasterkamp, G.; Herbin, O.; Esposito, B.; Perez, N.; Yasukawa, H.; Van Snick, J.; et al. Loss of SOCS3 Expression in T Cells Reveals a Regulatory Role for Interleukin-17 in Atherosclerosis. J. Exp. Med. 2009, 206, 2067–2077. [Google Scholar] [CrossRef]
- Simon, T.; Taleb, S.; Danchin, N.; Laurans, L.; Rousseau, B.; Cattan, S.; Montely, J.-M.; Dubourg, O.; Tedgui, A.; Kotti, S.; et al. Circulating Levels of Interleukin-17 and Cardiovascular Outcomes in Patients with Acute Myocardial Infarction. Eur. Heart J. 2013, 34, 570–577. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Shin, D.B.; Duffin, K.C.; Armstrong, A.W.; Blauvelt, A.; Tyring, S.K.; Menter, A.; Gottlieb, S.; Lockshin, B.N.; Simpson, E.L.; et al. A Randomized Placebo-Controlled Trial of Secukinumab on Aortic Vascular Inflammation in Moderate-to-Severe Plaque Psoriasis (VIP-S). J. Investig. Dermatol. 2020, 140, 1784–1793.e2. [Google Scholar] [CrossRef]
- von Stebut, E.; Reich, K.; Thaçi, D.; Koenig, W.; Pinter, A.; Körber, A.; Rassaf, T.; Waisman, A.; Mani, V.; Yates, D.; et al. Impact of Secukinumab on Endothelial Dysfunction and Other Cardiovascular Disease Parameters in Psoriasis Patients over 52 Weeks. J. Investig. Dermatol. 2019, 139, 1054–1062. [Google Scholar] [CrossRef]
- Makavos, G.; Ikonomidis, I.; Andreadou, I.; Varoudi, M.; Kapniari, I.; Loukeri, E.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Thymis, J.; et al. Effects of Interleukin 17A Inhibition on Myocardial Deformation and Vascular Function in Psoriasis. Can. J. Cardiol. 2020, 36, 100–111. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Deodhar, A.; Mcinnes, I.B.; Baraliakos, X.; Reich, K.; Schreiber, S.; Bao, W.; Marfo, K.; Richards, H.B.; Pricop, L.; et al. Long-Term Safety of Secukinumab Over Five Years in Patients with Moderate-to-Severe Plaque Psoriasis, Psoriatic Arthritis and Ankylosing Spondylitis: Update on Integrated Pooled Clinical Trial and Post-Marketing Surveillance Data. Acta Derm. Venereol. 2022, 102, adv00698. [Google Scholar] [CrossRef]
- Van De Kerkhof, P.C.M.; Griffiths, C.E.M.; Reich, K.; Leonardi, C.L.; Blauvelt, A.; Tsai, T.F.; Gong, Y.; Huang, J.; Papavassilis, C.; Fox, T. Secukinumab Long-Term Safety Experience: A Pooled Analysis of 10 Phase II and III Clinical Studies in Patients with Moderate to Severe Plaque Psoriasis. J. Am. Acad. Dermatol. 2016, 75, 83–98. [Google Scholar] [CrossRef]
- Frieder, J.; Kivelevitch, D.; Menter, A. Secukinumab: A Review of the Anti-IL-17A Biologic for the Treatment of Psoriasis. Ther. Adv. Chronic Dis. 2018, 9, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Wu, J.J.; Korman, N.; Solomon, J.A.; Goldblum, O.; Zhao, F.; Mallbris, L. Ixekizumab Treatment Shows a Neutral Impact on Cardiovascular Parameters in Patients with Moderate-to-Severe Plaque Psoriasis: Results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J. Am. Acad. Dermatol. 2018, 79, 104–109.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munjal, A.; Khandia, R. Atherosclerosis: Orchestrating Cells and Biomolecules Involved in Its Activation and Inhibition. Adv. Protein Chem. Struct. Biol. 2020, 120, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Y.; Wang, Z.; Liu, L.; Yang, Z.; Wang, M.; Xu, Y.; Ye, D.; Zhang, J.; Lin, Y.; et al. Roles and Mechanisms of Interleukin-12 Family Members in Cardiovascular Diseases: Opportunities and Challenges. Front. Pharmacol. 2020, 11, 129. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Libby, P.; Tedgui, A. Anticytokine Immune Therapy and Atherothrombotic Cardiovascular Risk. Arter. Thromb. Vasc. Biol. 2019, 39, 1510–1519. [Google Scholar] [CrossRef]
- Reich, K.; Langley, R.G.; Lebwohl, M.; Szapary, P.; Guzzo, C.; Yeilding, N.; Li, S.; Hsu, M.C.; Griffiths, C.E.M. Cardiovascular Safety of Ustekinumab in Patients with Moderate to Severe Psoriasis: Results of Integrated Analyses of Data from Phase II and III Clinical Studies. Br. J. Dermatol. 2011, 164, 862–872. [Google Scholar] [CrossRef]
- Ryan, C.; Leonardi, C.L.; Krueger, J.G.; Kimball, A.B.; Strober, B.E.; Gordon, K.B.; Langley, R.G.; de Lemos, J.A.; Daoud, Y.; Blankenship, D.; et al. Association between Biologic Therapies for Chronic Plaque Psoriasis and Cardiovascular Events: A Meta-Analysis of Randomized Controlled Trials. JAMA 2011, 306, 864–871. [Google Scholar] [CrossRef]
- Gordon, K.B.; Langley, R.G.; Gottlieb, A.B.; Papp, K.A.; Krueger, G.G.; Strober, B.E.; Williams, D.A.; Gu, Y.; Valdes, J.M. A Phase III, Randomized, Controlled Trial of the Fully Human IL-12/23 MAb Briakinumab in Moderate-to-Severe Psoriasis. J. Investig. Dermatol. 2012, 132, 304–314. [Google Scholar] [CrossRef]
- Tzellos, T.; Kyrgidis, A.; Zouboulis, C.C. Re-Evaluation of the Risk for Major Adverse Cardiovascular Events in Patients Treated with Anti-IL-12/23 Biological Agents for Chronic Plaque Psoriasis: A Meta-Analysis of Randomized Controlled Trials. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 622–627. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Gisondi, P.; Girolomoni, G. The Pharmacological Management of Patients with Comorbid Psoriasis and Obesity. Expert Opin. Pharmacother. 2019, 20, 863–872. [Google Scholar] [CrossRef]
- Poizeau, F.; Nowak, E.; Kerbrat, S.; Le Nautout, B.; Droitcourt, C.; Drici, M.D.; Sbidian, E.; Guillot, B.; Bachelez, H.; Ait-Oufella, H.; et al. Association between Early Severe Cardiovascular Events and the Initiation of Treatment with the Anti-Interleukin 12/23p40 Antibody Ustekinumab. JAMA Dermatol. 2020, 156, 1208. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Griffiths, C.E.M.; Gordon, K.; Lebwohl, M.; Szapary, P.O.; Wasfi, Y.; Chan, D.; Hsu, M.C.; Ho, V.; Ghislain, P.D.; et al. Long-Term Safety of Ustekinumab in Patients with Moderate-to-Severe Psoriasis: Final Results from 5 Years of Follow-Up. Br. J. Dermatol. 2013, 168, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Gottlieb, A.B.; Naldi, L.; Pariser, D.; Ho, V.; Goyal, K.; Fakharzadeh, S.; Chevrier, M.; Calabro, S.; Langholff, W.; et al. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Drugs Dermatol. 2015, 14, 706–714. [Google Scholar] [PubMed]
- Kim, B.-S.; Lee, W.-K.; Pak, K.; Han, J.; Kim, G.-W.; Kim, H.-S.; Ko, H.-C.; Kim, M.-B.; Kim, S.-J. Ustekinumab Treatment Is Associated with Decreased Systemic and Vascular Inflammation in Patients with Moderate-to-Severe Psoriasis: Feasibility Study Using 18F-Fluorodeoxyglucose PET/CT. J. Am. Acad. Dermatol. 2019, 80, 1322–1331. [Google Scholar] [CrossRef]
- Choi, H.; Uceda, D.E.; Dey, A.K.; Abdelrahman, K.M.; Aksentijevich, M.; Rodante, J.A.; Elnabawi, Y.A.; Reddy, A.; Keel, A.; Erb-Alvarez, J.; et al. Treatment of Psoriasis With Biologic Therapy Is Associated With Improvement of Coronary Artery Plaque Lipid-Rich Necrotic Core: Results From a Prospective, Observational Study. Circ. Cardiovasc. Imaging 2020, 13, e011199. [Google Scholar] [CrossRef]
- Menter, M.A.; Mehta, N.N.; Lebwohl, M.G.; Gottlieb, A.B.; Mendelsohn, A.M.; Rozzo, S.J.; Leonardi, C. The Effect of Tildrakizumab on Cardiometabolic Risk Factors in Psoriasis by Metabolic Syndrome Status: Post Hoc Analysis of Two Phase 3 Trials (ReSURFACE 1 and ReSURFACE 2). J. Drugs Dermatol. 2020, 19, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.G.; Mehta, N.N.; Gottlieb, A.B.; Mendelsohn, A.M. Tildrakizumab Efficacy by Metabolic Syndrome Status in Psoriasis: Post Hoc Analysis of 3-Year Data from the Phase 3 Resurface 1 And Resurface 2 Studies. Ski. J. Cutan. Med. 2020, 4, s42. [Google Scholar] [CrossRef]
- Blauvelt, A.; Reich, K.; Papp, K.A.; Kimball, A.B.; Gooderham, M.; Tyring, S.K.; Sinclair, R.; Thaçi, D.; Li, Q.; Cichanowitz, N.; et al. Safety of Tildrakizumab for Moderate-to-Severe Plaque Psoriasis: Pooled Analysis of Three Randomized Controlled Trials. Br. J. Dermatol. 2018, 179, 615–622. [Google Scholar] [CrossRef]
- Blauvelt, A.; Papp, K.A.; Griffiths, C.E.M.; Randazzo, B.; Wasfi, Y.; Shen, Y.K.; Li, S.; Kimball, A.B. Efficacy and Safety of Guselkumab, an Anti-Interleukin-23 Monoclonal Antibody, Compared with Adalimumab for the Continuous Treatment of Patients with Moderate to Severe Psoriasis: Results from the Phase III, Double-Blinded, Placebo- and Active Comparator–Controlled VOYAGE 1 Trial. J. Am. Acad. Dermatol. 2017, 76, 405–417. [Google Scholar] [CrossRef]
- Reich, K.; Armstrong, A.W.; Foley, P.; Song, M.; Wasfi, Y.; Randazzo, B.; Li, S.; Shen, Y.K.; Gordon, K.B. Efficacy and Safety of Guselkumab, an Anti-Interleukin-23 Monoclonal Antibody, Compared with Adalimumab for the Treatment of Patients with Moderate to Severe Psoriasis with Randomized Withdrawal and Retreatment: Results from the Phase III, Double-Blind, Placebo- and Active Comparator–Controlled VOYAGE 2 Trial. J. Am. Acad. Dermatol. 2017, 76, 418–431. [Google Scholar] [CrossRef]
- Gordon, K.B.; Strober, B.; Lebwohl, M.; Augustin, M.; Blauvelt, A.; Poulin, Y.; Papp, K.A.; Sofen, H.; Puig, L.; Foley, P.; et al. Efficacy and Safety of Risankizumab in Moderate-to-Severe Plaque Psoriasis (UltIMMa-1 and UltIMMa-2): Results from Two Double-Blind, Randomised, Placebo-Controlled and Ustekinumab-Controlled Phase 3 Trials. Lancet 2018, 392, 650–661. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Kadoglou, N.; Makavos, G.; Katogiannis, K.; Kountouri, A.; Thymis, J.; Kostelli, G.; Kapniari, I.; Theodoropoulos, K.; et al. Apremilast Improves Endothelial Glycocalyx Integrity, Vascular and Left Ventricular Myocardial Function in Psoriasis. Pharmaceuticals 2022, 15, 172. [Google Scholar] [CrossRef]
| Drug | N of Trials or Patients | MACE Risk |
|---|---|---|
| Anti-TNFα | 5 studies (49795 patients with psoriasis or psoriatic arthritis, mean duration follow-up: 38 months) comparing TNFi (adalimumab, etanercept, golimumab, and infliximab) vs. topical/photo therapy or MTX [117] | vs. topical/photo therapy: RR, 0.58; 95% CI, 0.43 to 0.77; p < 0.001; I2 = 66.2% vs. MTX: RR, 0.67; 95% CI, 0.52 to 0.88; p = 0.003; I2 = 9.3% overall, vs. control group: RR, 0.60; 95% CI, 0.48 to 0.74; p < 0.001; I2 = 57.3% |
| 4762 psoriasis patients (1058 patients treated with TNFi (adalimumab, etanercept, infliximab); 1331 treated with MTX; 2372 treated with topical agents); a median of 3.9 years follow-up [118] | IR per 1000 SY (95% CI): TNFi: 4.88 (2.5–7.2) (significantly lower than topical cohort, p = 0.01) MTX: 5.38 (3.04–8.3) (significantly lower than topical cohort, p = 0.02) Topical: 12.34 (9.6–20.8) No significant difference between TNFi and MTX | |
| 11410 TNFi vs. 12433 phototherapy patients [119] | HR = 0.77; p < 0.05 | |
| 9148 TNFi users vs. 8581 MTX users [120] | HR = 0.55; p < 0.01 | |
| 18 RCTs comparing TNFi (4 adalimumab, 9 etanercept, 5 infliximab) vs. placebo [121] | OR, 0.67 (95% CI, 0.10–4.63, p = 0.69) | |
| 15 RCTs comparing TNFi (8 etanercept, 4 infliximab, 3 adalimumab) vs. placebo [147] | (Mantel–Haenszel risk difference, −0.0005/SY; 95% CI, −0.010 to 0.009; p = 0.94) | |
| Anti-IL17 (Secukinumab) | 28 clinical trials and post-marketing surveillance data, 12,637 patients (15063, 5985 and 3527 patient-years of exposure in psoriasis, psoriatic arthritis and ankylosing spondylitis patients, respectively); 5 years cumulative data [139] | IR < 0.4/100 SY for psoriasis and psoriatic arthritis, with no apparent increases over time |
| 10 phase II/III clinical trials, 52 weeks follow-up [140] | IR: 0.42/100 SY (300 mg dose) IR: 0.35/100 SY (150 mg dose) | |
| Anti-IL12/23 | 7 RCTs (ustekinumab vs. placebo), 30 weeks follow-up [121] | OR, 4.48 (95% CI, 0.24–84.77; p = 0.32) |
| 1582 ustekinumab vs. 732 placebo-treated patients. 20 weeks follow-up [146] | IR, 0.3%; (95% CI, 0.1–0.70) vs. 0.0% (95% CI, 0.0–0.5%) | |
| 9 RCTs comparing anti-IL-12/23 (5 ustekinumab, 4 briakinumab) vs. placebo [147] | (Mantel–Haenszel risk difference, 0.012/SY; 95% CI, −0.001 to 0.026; p = 0.12 | |
| 9 RCTs (ustekinumab vs. placebo), 30 weeks follow-up [149] | OR, 4.23, 95% CI: 1.07–16.75, p = 0.04) | |
| Case-time-control study with 9290 ustekinumab [151] | Patients with high CV risk: OR, 4.17; 95% CI, 1.19–14.59 Patients with low CV risk: OR, 0.30; 95% CI, 0.03–3.13 | |
| 4 phase II and phase III studies, 3177 patients, 5 years follow-up [152] | IR (45 mg) 0.56/100 SY IR (90 mg) 0.36/100 SY | |
| Psoriasis Longitudinal Assessment and Registry (PSOLAR), 12093 patients [153] | IR 0.33/100 SY | |
| 1465 patients (981 briakinumab vs. 484 placebo) [148] | Exposure-adjusted rate: 1.06/100SY, 95% CI 0.43, 2.18. | |
| 9 RCTs (briakinumab vs. placebo), 30 weeks follow-up [149] | OR, 4.47 (95% CI, 0.69–28.89; p = 0.12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andújar, I.; Esplugues, J.V.; García-Martínez, P. Looking beyond the Skin: Pathophysiology of Cardiovascular Comorbidity in Psoriasis and the Protective Role of Biologics. Pharmaceuticals 2022, 15, 1101. https://doi.org/10.3390/ph15091101
Andújar I, Esplugues JV, García-Martínez P. Looking beyond the Skin: Pathophysiology of Cardiovascular Comorbidity in Psoriasis and the Protective Role of Biologics. Pharmaceuticals. 2022; 15(9):1101. https://doi.org/10.3390/ph15091101
Chicago/Turabian StyleAndújar, Isabel, Juan V. Esplugues, and Patricia García-Martínez. 2022. "Looking beyond the Skin: Pathophysiology of Cardiovascular Comorbidity in Psoriasis and the Protective Role of Biologics" Pharmaceuticals 15, no. 9: 1101. https://doi.org/10.3390/ph15091101
APA StyleAndújar, I., Esplugues, J. V., & García-Martínez, P. (2022). Looking beyond the Skin: Pathophysiology of Cardiovascular Comorbidity in Psoriasis and the Protective Role of Biologics. Pharmaceuticals, 15(9), 1101. https://doi.org/10.3390/ph15091101