Evaluation of Antineoplastic Delayed-Type Hypersensitivity Skin Reactions In Vitro
Abstract
:1. Introduction
2. Results
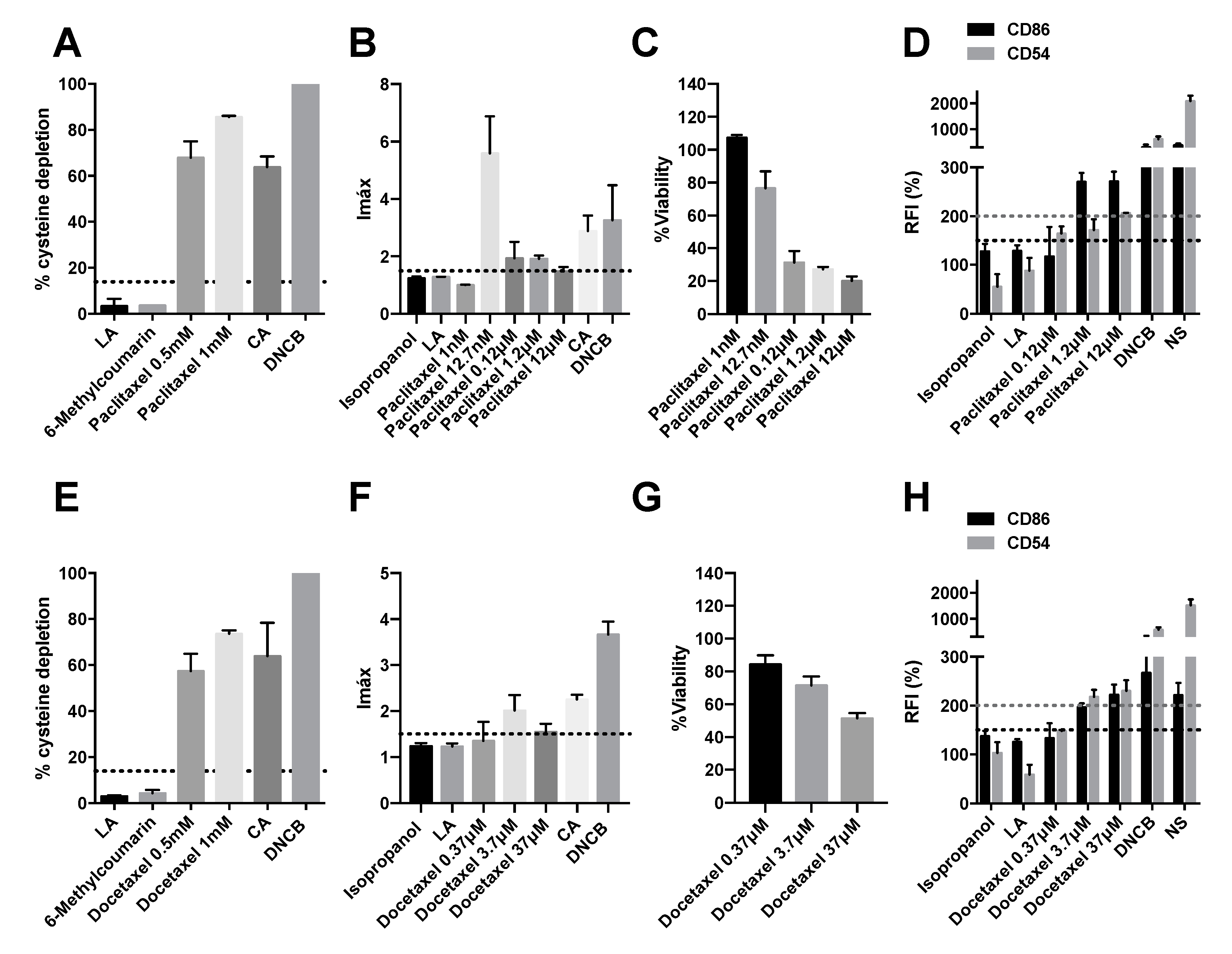
2.1. Taxanes Induce Delayed-Type Hypersensitivity
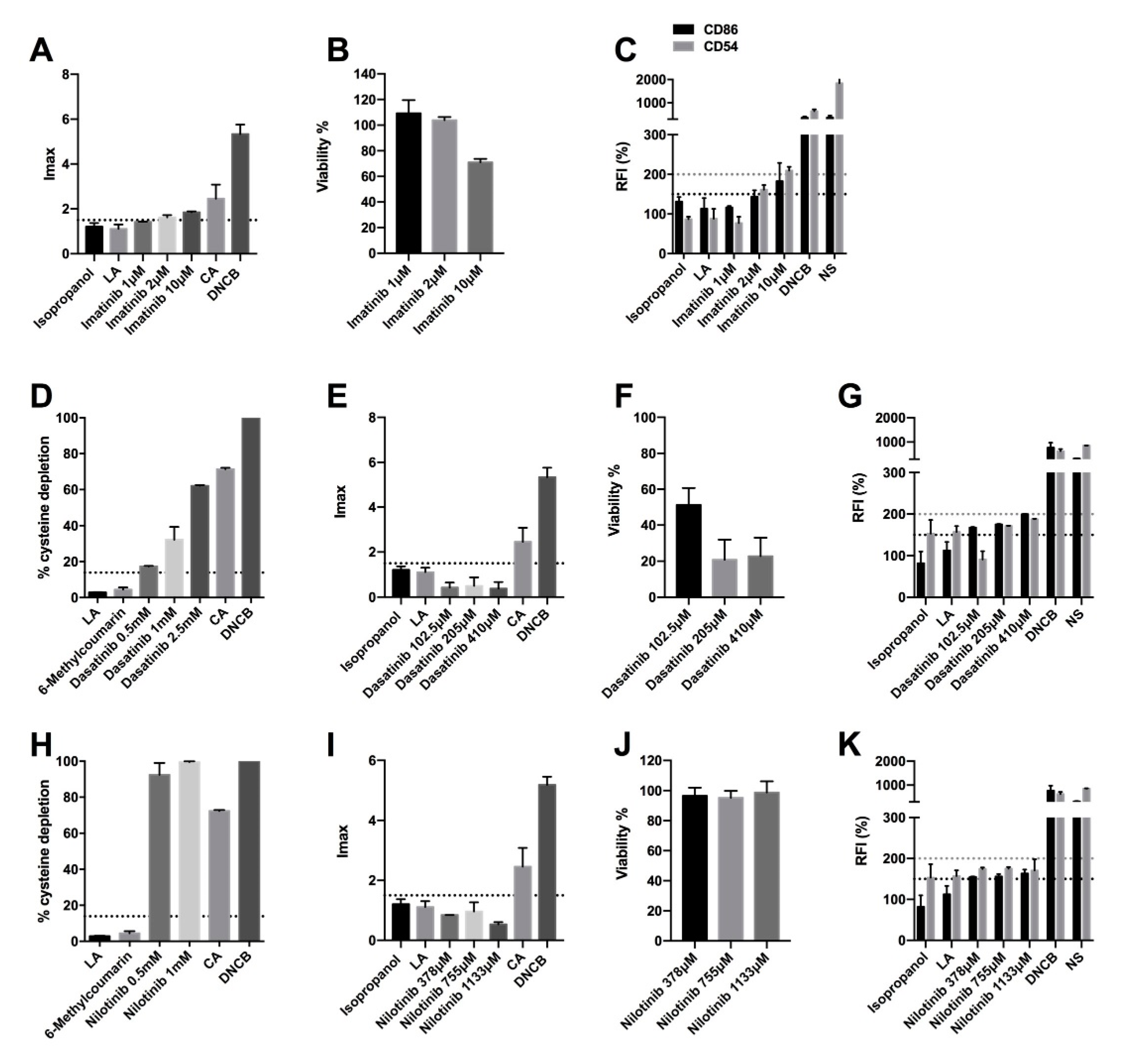
2.2. BCR-ABL, C-KIT, and PDGF Inhibitors Induce Delayed-Type Hypersensitivity
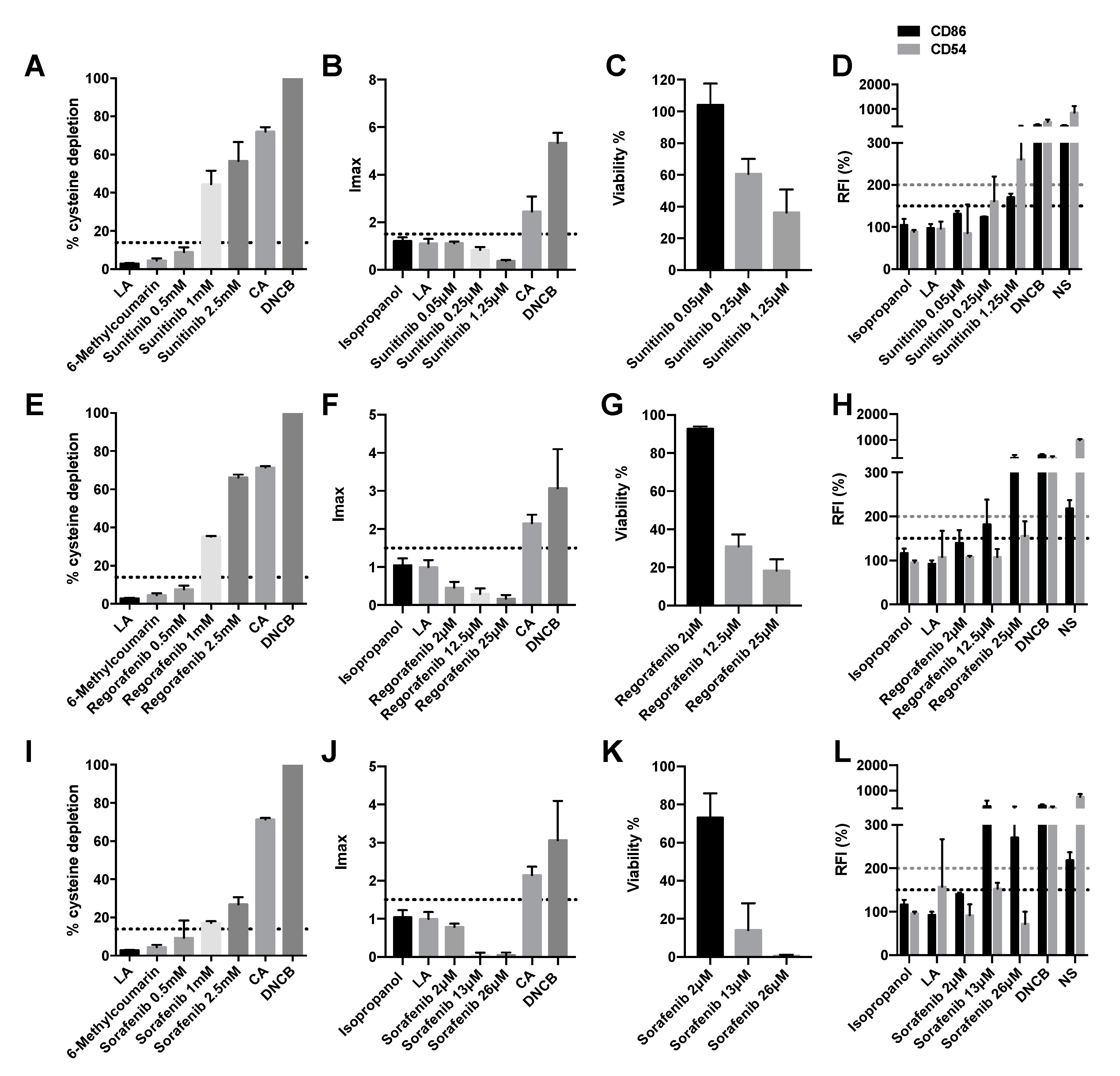
2.3. Olaparib and Palbociclib Do Not Induce Delayed-Type Hypersensitivity
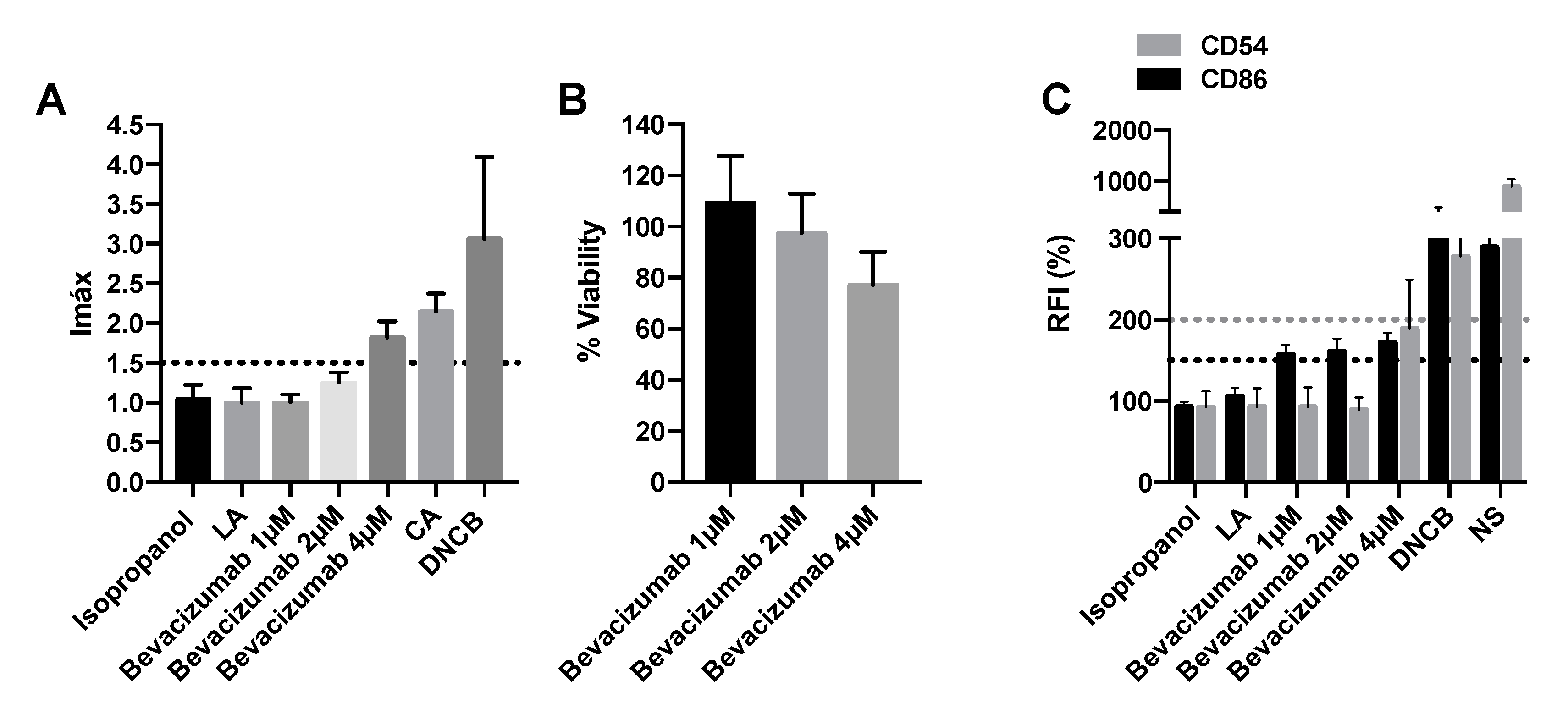
2.4. Vegf Inhibitors Induce Delayed-Type Hypersensitivity
2.5. SiRNA-VEGFR1 Induces Delayed-Type Hypersensitivity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Antineoplastic
4.3. Direct Peptide Reactivity Assay (DPRA)
4.4. KeratinoSensTM Assay
- -
- The Imáx must be equal to or greater than 1.5 and statistically significant compared to the negative control.
- -
- Cell viability must be greater than 70% for the lowest concentration of the compound with an Imáx ≥ 1.5
- -
- There should be a dose–response increase in luminescence.
4.5. Human Cell Line Activation Test (hCLAT)
4.6. The “2 out of 3” Prediction Model
4.7. Real-Time RT-PCR and Sirna Experiments in KeratinoSensTM and THP-1 Cells
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brandt, O.; Bircher, A.J. Delayed-type hypersensitivity to oral and parenteral drugs. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2017, 15, 1111–1132. [Google Scholar] [CrossRef] [PubMed]
- Syrigou, E.; Makrilia, N.; Koti, I.; Saif, M.W.; Syrigos, K.N. Hypersensitivity reactions to antineoplastic agents: An overview. Anticancer. Drugs 2009, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 2007, 12, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.; Ortega-Loayza, A.G. Adverse cutaneous reactions to chemotherapeutic drugs. Clin. Dermatol. 2020, 38, 712–728. [Google Scholar] [CrossRef]
- Brandi, G.; Pantaleo, M.A.; Galli, C.; Falcone, A.; Antonuzzo, A.; Mordenti, P.; Di Marco, M.C.; Biasco, G. Hypersensitivity reactions related to oxaliplatin (OHP). Br. J. Cancer 2003, 89, 477–481. [Google Scholar] [CrossRef]
- Charles, A.J., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology, 5th ed.; Garland Science: New York, NY, USA, 2001; ISBN 978-081-533-642-6. [Google Scholar]
- Strickland, J.; Zang, Q.; Kleinstreuer, N.; Paris, M.; Lehmann, D.M.; Choksi, N.; Matheson, J.; Jacobs, A.; Lowit, A.; Allen, D.; et al. Integrated Decision Strategies for Skin Sensitization Hazard. J. Appl. Toxicol. JAT 2016, 36, 1150–1162. [Google Scholar] [CrossRef]
- Weaver, J.L.; Chapdelaine, J.M.; Descotes, J.; Germolec, D.; Holsapple, M.; House, R.; Lebrec, H.; Meade, J.; Pieters, R.; Hastings, K.L.; et al. Evaluation of a lymph node proliferation assay for its ability to detect pharmaceuticals with potential to cause immune-mediated drug reactions. J. Immunotoxicol. 2005, 2, 11–20. [Google Scholar] [CrossRef]
- Corsini, E.; Casula, M.; Tragni, E.; Galbiati, V.; Pallardy, M. Tools to investigate and avoid drug-hypersensitivity in drug development. Expert Opin. Drug Discov. 2018, 13, 425–433. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins; Test No. 442C; Organisation for Economic Co-Operation and Development: Paris, France, 2021; Available online: https://www.oecd-ilibrary.org/environment/test-no-442c-in-chemico-skin-sensitisation_9789264229709-en (accessed on 1 June 2022).
- Organisation for Economic Co-Operation and Development. In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method; Test No. 442D; Organisation for Economic Co-Operation and Development: Paris, France, 2018; Available online: https://www.oecd-ilibrary.org/environment/test-no-442d-in-vitro-skin-sensitisation_9789264229822-en (accessed on 1 June 2022).
- Organisation for Economic Co-Operation and Development. In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation; Test No. 442E; Organisation for Economic Co-Operation and Development: Paris, France, 2018; Available online: https://www.oecd-ilibrary.org/environment/test-no-442e-in-vitro-skin-sensitisation_9789264264359-en (accessed on 1 June 2022).
- Organisation for Economic Co-Operation and Development. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins. Part 1: Scientific Evidence; Series on Testing and Assessment No. 168; Organisation for Economic Co-Operation and Development: Paris, France, 2012; Available online: https://read.oecd-ilibrary.org/environment/the-adverse-outcome-pathway-for-skin-sensitisation-initiated-by-covalent-binding-to-proteins_9789264221444-en#page1 (accessed on 1 June 2022).
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 7th ed.; United Nations (UN), Ed.; United Nations: Geneva, NY, USA, 2017; ISBN 978-921-117-131-0.
- Sakaguchi, H.; Ryan, C.; Ovigne, J.-M.; Schroeder, K.R.; Ashikaga, T. Predicting skin sensitization potential and inter-laboratory reproducibility of a human Cell Line Activation Test (h-CLAT) in the European Cosmetics Association (COLIPA) ring trials. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2010, 24, 1810–1820. [Google Scholar] [CrossRef]
- Cho, S.-A.; Choi, M.; Park, S.-R.; An, S.; Park, J.-H. Application of Spectro-DPRA, KeratinoSensTM and h-CLAT to estimation of the skin sensitization potential of cosmetics ingredients. J. Appl. Toxicol. JAT 2020, 40, 300–312. [Google Scholar] [CrossRef]
- Bauch, C.; Kolle, S.N.; Fabian, E.; Pachel, C.; Ramirez, T.; Wiench, B.; Wruck, C.J.; van Ravenzwaay, B.; Landsiedel, R. Intralaboratory validation of four in vitro assays for the prediction of the skin sensitizing potential of chemicals. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2011, 25, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Urbisch, D.; Mehling, A.; Guth, K.; Ramirez, T.; Honarvar, N.; Kolle, S.; Landsiedel, R.; Jaworska, J.; Kern, P.S.; Gerberick, F.; et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul. Toxicol. Pharmacol. RTP 2015, 71, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Papale, A.; Kummer, E.; Corsini, E. In vitro Models to Evaluate Drug-Induced Hypersensitivity: Potential Test Based on Activation of Dendritic Cells. Front. Pharmacol. 2016, 7, 204. [Google Scholar] [CrossRef]
- Ng, C.Y.; Chen, C.-B.; Wu, M.-Y.; Wu, J.; Yang, C.-H.; Hui, R.C.-Y.; Chang, Y.-C.; Lu, C.-W. Anticancer Drugs Induced Severe Adverse Cutaneous Drug Reactions: An Updated Review on the Risks Associated with Anticancer Targeted Therapy or Immunotherapies. J. Immunol. Res. 2018, 2018, 5376476. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wang, C.-W.; Hui, C.-Y.R.; Chang, Y.-C.; Yang, C.-H.; Cheng, C.-Y.; Chen, W.-W.; Ke, W.-M.; Chung, W.-H. Delayed-type hypersensitivity reactions induced by proton pump inhibitors: A clinical and in vitro T-cell reactivity study. Allergy 2018, 73, 221–229. [Google Scholar] [CrossRef]
- Dodiuk-Gad, R.P.; Chung, W.-H.; Valeyrie-Allanore, L.; Shear, N.H. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. Am. J. Clin. Dermatol. 2015, 16, 475–493. [Google Scholar] [CrossRef]
- Quah, B.J.C.; O’Neill, H.C. Maturation of function in dendritic cells for tolerance and immunity. J. Cell. Mol. Med. 2005, 9, 643–654. [Google Scholar] [CrossRef]
- Martin, S.F. Allergic contact dermatitis: Xenoinflammation of the skin. Curr. Opin. Immunol. 2012, 24, 720–729. [Google Scholar] [CrossRef]
- Kattan, J.G.; Farhat, F.S.; Chahine, G.Y.; Nasr, F.L.; Moukadem, W.T.; Younes, F.C.; Yazbeck, N.J.; Ghosn, M.G.; Cancer Research Group. Weekly docetaxel, zoledronic acid and estramustine in hormone-refractory prostate cancer (HRPC). Investig. New Drugs 2008, 26, 75–79. [Google Scholar] [CrossRef]
- Kilic, M.O.; Yalaza, M.; Bilgic, C.I.; Dener, C. Docetaxel-induced Scleroderma in A Breast Cancer Patient: A Case Report. J. Breast Health 2015, 11, 95–97. [Google Scholar] [CrossRef]
- Sawada, Y.; Sugita, K.; Kabashima, R.; Nakamura, M.; Tokura, Y. Docetaxel-induced Stevens-Johnson syndrome with regenerating epidermis composed of atypical keratinocytes. J. Eur. Acad. Dermatol. Venereol. JEADV 2009, 23, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Dourakis, S.P.; Sevastianos, V.A.; Alexopoulou, A.; Deutsch, M.; Stavrianeas, N. Treatment side effects. Case 2. Toxic, epidermal, necrolysis-like reaction associated with docetaxel chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 3030–3032. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, A.; Aoe, K.; Murakami, T.; Maeda, T.; Eda, R.; Takeyama, H. Stevens-Johnson syndrome induced by paclitaxel in a patient with squamous cell carcinoma of the lung: A case report. Anticancer Res. 2004, 24, 1135–1137. [Google Scholar] [PubMed]
- Pretel-Irazabal, M.; Tuneu-Valls, A.; Ormaechea-Pérez, N. Adverse skin effects of imatinib, a tyrosine kinase inhibitor. Actas Dermosifiliogr. 2014, 105, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N. Imatinib mesylate and dermatology part 2: A review of the cutaneous side effects of imatinib mesylate. J. Drugs Dermatol. JDD 2006, 5, 228–231. [Google Scholar]
- Saglio, G.; Kim, D.-W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Lobo, C.; Pasquini, R.; Clark, R.E.; Hochhaus, A.; Hughes, T.P.; et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2010, 362, 2251–2259. [Google Scholar] [CrossRef]
- Amitay-Laish, I.; Stemmer, S.M.; Lacouture, M.E. Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol. Ther. 2011, 24, 386–395. [Google Scholar] [CrossRef]
- Namba, M.; Tsunemi, Y.; Kawashima, M. Sorafenib-induced erythema multiforme: Three cases. Eur. J. Dermatol. EJD 2011, 21, 1015–1016. [Google Scholar] [CrossRef]
- Kodaira, M.; Takahashi, S.; Takeuchi, K.; Yuasa, T.; Saotome, T.; Yonese, J.; Fukui, I.; Hatake, K. Sorafenib-induced erythema multiforme for metastatic renal cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 1563–1565. [Google Scholar] [CrossRef]
- Bilaç, C.; Müezzinoğlu, T.; Ermertcan, A.T.; Kayhan, T.C.; Temeltaş, G.; Oztürkcan, S.; Temiz, P. Sorafenib-induced erythema multiforme in metastatic renal cell carcinoma. Cutan. Ocul. Toxicol. 2009, 28, 90–92. [Google Scholar] [CrossRef]
- MacGregor, J.L.; Silvers, D.N.; Grossman, M.E.; Sherman, W.H. Sorafenib-induced erythema multiforme. J. Am. Acad. Dermatol. 2007, 56, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Kollmannsberger, C.; Soulieres, D.; Wong, R.; Scalera, A.; Gaspo, R.; Bjarnason, G. Sunitinib therapy for metastatic renal cell carcinoma: Recommendations for management of side effects. Can. Urol. Assoc. J. 2007, 1, S41–S54. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Mantovani, A.; Álvares-Da-Silva, M.R. Anaphylaxis preceded by erythema multiforme with sorafenib: First case report. Ann. Hepatol. 2019, 18, 777–779. [Google Scholar] [CrossRef]
- Ikeda, M.; Fujita, T.; Mii, S.; Tanabe, K.; Tabata, K.; Matsumoto, K.; Satoh, T.; Iwamura, M. Erythema multiforme induced by sorafenib for metastatic renal cell carcinoma. Jpn. J. Clin. Oncol. 2012, 42, 820–824. [Google Scholar] [CrossRef]
- Akaza, H.; Tsukamoto, T.; Murai, M.; Nakajima, K.; Naito, S. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn. J. Clin. Oncol. 2007, 37, 755–762. [Google Scholar] [CrossRef]
- Zimmerman, E.I.; Gibson, A.A.; Hu, S.; Vasilyeva, A.; Orwick, S.J.; Du, G.; Mascara, G.P.; Ong, S.S.; Chen, T.; Vogel, P.; et al. Multikinase Inhibitors Induce Cutaneous Toxicity through OAT6-Mediated Uptake and MAP3K7-Driven Cell Death. Cancer Res. 2016, 76, 117–126. [Google Scholar] [CrossRef]
- Yamamoto, K.; Mizumoto, A.; Nishimura, K.; Uda, A.; Mukai, A.; Yamashita, K.; Kume, M.; Makimoto, H.; Bito, T.; Nishigori, C.; et al. Association of Toxicity of Sorafenib and Sunitinib for Human Keratinocytes with Inhibition of Signal Transduction and Activator of Transcription 3 (STAT3). PLoS ONE 2014, 9, e102110. [Google Scholar] [CrossRef]
- Fam, A.; Finger, P.T. Cutaneous Cell-Mediated Delayed Hypersensitivity to Intravitreal Bevacizumab. Middle East Afr. J. Ophthalmol. 2020, 27, 182–184. [Google Scholar] [CrossRef]
- Hagura, A.; Asai, J.; Maruyama, K.; Takenaka, H.; Kinoshita, S.; Katoh, N. The VEGF-C/VEGFR3 signaling pathway contributes to resolving chronic skin inflammation by activating lymphatic vessel function. J. Dermatol. Sci. 2014, 73, 135–141. [Google Scholar] [CrossRef]
- Huggenberger, R.; Siddiqui, S.S.; Brander, D.; Ullmann, S.; Zimmermann, K.; Antsiferova, M.; Werner, S.; Alitalo, K.; Detmar, M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 2011, 117, 4667–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, S.; Kleinstreuer, N.; Alépée, N.; Allen, D.; Api, A.M.; Ashikaga, T.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (I): The Cosmetics Europe database. Crit. Rev. Toxicol. 2018, 48, 344–358. [Google Scholar] [CrossRef]
- Potter, T.M.; Neun, B.W.; Dobrovolskaia, M.A. Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2018; pp. 197–210. [Google Scholar]
- Iulini, M.; Maddalon, A.; Galbiati, V.; Corsini, E. The Modified THP-1 Activation Assay for the In Vitro Identification of Drug-Inducing Systemic Hypersensitivity. Front. Toxicol. 2022, 4, 814050. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Galbiati, V.; Gatti, N.; Marinovich, M.; Galli, C.L.; Corsini, E. Optimization of the THP-1 activation assay to detect pharmaceuticals with potential to cause immune mediated drug reactions. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2015, 29, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Iulini, M.; Maddalon, A.; Galbiati, V.; Marinovich, M.; Corsini, E. In vitro identification of drugs inducing systemic hypersensitivity reactions known in vivo to be associated with specific HLA genotypes. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2020, 68, 104953. [Google Scholar] [CrossRef]
- Warbrick, E.V.; Dearman, R.J.; Kimber, I. Prediction of drug allergenicity: Possible use of the local lymph node assay. Curr. Opin. Drug Discov. Devel. 2001, 4, 60–65. [Google Scholar]
- Sohn, K.H.; Oh, S.Y.; Lim, K.W.; Kim, M.Y.; Lee, S.Y.; Kang, H.R. Sorafenib induces delayed-onset cutaneous hypersensitivity: A case series. Allergy Asthma Immunol. Res. 2015, 7, 304–307. [Google Scholar] [CrossRef]
- Vincenzi, B.; Santini, D.; Russo, A.; Addeo, R.; Giuliani, F.; Montella, L.; Rizzo, S.; Venditti, O.; Frezza, A.M.; Caraglia, M.; et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist 2010, 15, 85–92. [Google Scholar] [CrossRef]
- Pieters, R. Detection of autoimmunity by pharmaceuticals. Methods San Diego Calif. 2007, 41, 112–117. [Google Scholar] [CrossRef]
- Höper, T.; Mussotter, F.; Haase, A.; Luch, A.; Tralau, T. Application of proteomics in the elucidation of chemical-mediated allergic contact dermatitis. Toxicol. Res. 2017, 6, 595–610. [Google Scholar] [CrossRef]
- Natsch, A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers--functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 113, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Emter, R. Skin Sensitizers Induce Antioxidant Response Element Dependent Genes: Application to the In Vitro Testing of the Sensitization Potential of Chemicals. Toxicol. Sci. 2008, 102, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emter, R.; Ellis, G.; Natsch, A. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol. Appl. Pharmacol. 2010, 245, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Gerberick, G.F. Integrated skin sensitization assessment based on OECD methods (II): Hazard and potency by combining kinetic peptide reactivity and the “2 out of 3” Defined Approach. ALTEX 2022. [Google Scholar] [CrossRef]


| Drug | DPRA | KeratinoSensTM | hCLAT | Classification |
|---|---|---|---|---|
| Sunitinib | + | − * | + | Positive |
| Regorafenib | + | − * | + | Positive |
| Sorafenib | + | − * | + | Positive |
| Bevacizumab | − | + | Positive | |
| Olaparib | + | − | − | Negative |
| Palbociclib | − | − | + | Negative |
| Paclitaxel | + | + | + | Positive |
| Docetaxel | + | + | + | Positive |
| Imatinib | Inconclusive (Coelution) | + | + | Positive |
| Nilotinib | + | − | + | Positive |
| Dasatinib | + | − * | + | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roger, I.; Montero, P.; García, A.; Milara, J.; Ribera, P.; Pérez-Fidalgo, J.A.; Cortijo, J. Evaluation of Antineoplastic Delayed-Type Hypersensitivity Skin Reactions In Vitro. Pharmaceuticals 2022, 15, 1111. https://doi.org/10.3390/ph15091111
Roger I, Montero P, García A, Milara J, Ribera P, Pérez-Fidalgo JA, Cortijo J. Evaluation of Antineoplastic Delayed-Type Hypersensitivity Skin Reactions In Vitro. Pharmaceuticals. 2022; 15(9):1111. https://doi.org/10.3390/ph15091111
Chicago/Turabian StyleRoger, Inés, Paula Montero, Antonio García, Javier Milara, Pilar Ribera, Jose Alejandro Pérez-Fidalgo, and Julio Cortijo. 2022. "Evaluation of Antineoplastic Delayed-Type Hypersensitivity Skin Reactions In Vitro" Pharmaceuticals 15, no. 9: 1111. https://doi.org/10.3390/ph15091111
APA StyleRoger, I., Montero, P., García, A., Milara, J., Ribera, P., Pérez-Fidalgo, J. A., & Cortijo, J. (2022). Evaluation of Antineoplastic Delayed-Type Hypersensitivity Skin Reactions In Vitro. Pharmaceuticals, 15(9), 1111. https://doi.org/10.3390/ph15091111






