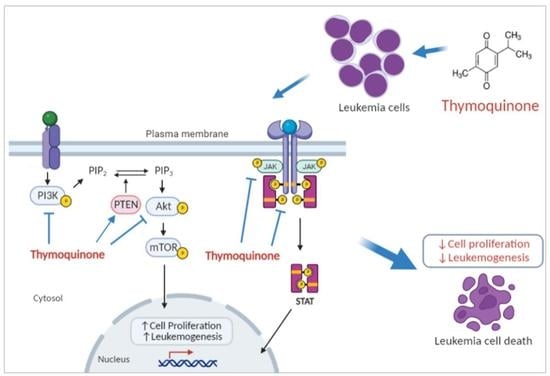
Thymoquinone Inhibits JAK/STAT and PI3K/Akt/ mTOR Signaling Pathways in MV4-11 and K562 Myeloid Leukemia Cells
Abstract
:1. Introduction
2. Results
2.1. Thymoquinone Exhibits a Growth Inhibitory Effect in K562 Cells
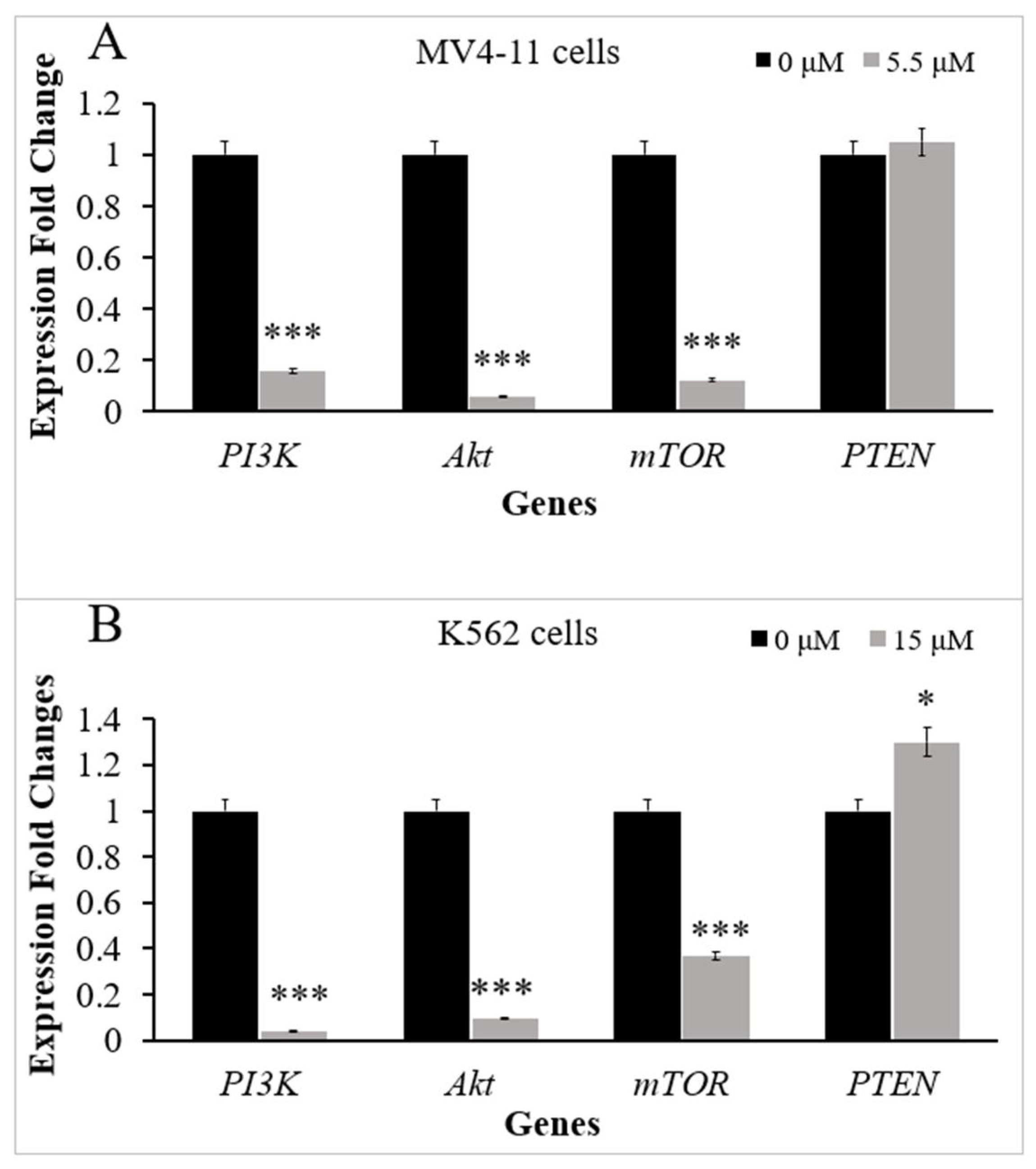
2.2. Thymoquinone Downregulates Several Genes in PI3K/Akt/mTOR Pathway in MV4-11 and K562 Cells
2.3. Thymoquinone Inhibits JAK/STAT Signaling in MV4-11 Cells
2.4. Thymoquinone Inhibits PI3K/Akt/mTOR Signaling in MV4-11 Cells
2.5. Thymoquinone Inhibits PI3K/Akt/mTOR Signaling in K562 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Treatment with Thymoquinone
4.3. Cytotoxicity Analysis by WST-8 Assay
4.4. RNA Extraction
4.5. Reverse Transcriptase Quantitative PCR (RT-qPCR)
4.6. Total Cell Lysates Preparation
4.7. Protein Expression Analysis Using JESS Simple Western Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bispo, J.A.B.; Pinheiro, P.S.; Kobetz, E.K. Epidemiology and etiology of leukemia and lymphoma. Cold Spring Harb. Perspect. Med. 2020, 10, a034819. [Google Scholar] [CrossRef]
- Liu, X.-L.; Liu, H.-Q.; Li, J.; Mao, C.-Y.; He, J.-T.; Zhao, X. Role of epigenetic in leukemia: From mechanism to therapy. Chem.-Biol. Interact. 2020, 317, 108963. [Google Scholar] [CrossRef] [PubMed]
- Vela-Ojeda, J.; Cardenas, P.V.; Esparza, M.A.G.-R.; Cervantes, L.A.M.; Chavez, J.G.; Caballero, A.H.; Majluf-Cruz, A.; Vega-López, A.; Reyes-Maldonado, E. FLT3-ITD and CD135 over-expression are frequent findings of poor survival in adult patients with acute leukemias. Arch. Med. Res. 2021, 52, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Boddu, P.C.; Kadia, T.M.; Garcia-Manero, G.; Cortes, J.; Alfayez, M.; Borthakur, G.; Konopleva, M.; Jabbour, E.J.; Daver, N.G.; DiNardo, C.D. Validation of the 2017 European LeukemiaNet classification for acute myeloid leukemia with NPM1 and FLT3-internal tandem duplication genotypes. Cancer 2019, 125, 1091–1100. [Google Scholar] [CrossRef]
- Niparuck, P.; Limsuwanachot, N.; Pukiat, S.; Chantrathammachart, P.; Rerkamnuaychoke, B.; Magmuang, S.; Phusanti, S.; Boonyawat, K.; Puavilai, T.; Angchaisuksiri, P.; et al. Cytogenetics and FLT3-ITD mutation predict clinical outcomes in non transplant patients with acute myeloid leukemia. Exp. Hematol. Oncol. 2019, 8, 1–14. [Google Scholar] [CrossRef]
- Rowley, J.D. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Gorantla, S.P.; Müller-Rudorf, A.; Müller, T.A.; Kreutmair, S.; Albers, C.; Jakob, L.; Lippert, L.J.; Yue, Z.; Engelhardt, M.; et al. Phosphorylation of BECLIN-1 by BCR-ABL suppresses autophagy in chronic myeloid leukemia. Haematologica 2020, 105, 1285. [Google Scholar] [CrossRef]
- Braun, T.P.; Eide, C.A.; Druker, B.J. Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell 2020, 37, 530–542. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Q.; Wang, X.; Liao, J.; Zhang, W.; Gao, L.; Liu, Y.; Zhang, C.; Zhang, X.; Rao, J.; et al. THBS1 is a novel serum prognostic factors of acute myeloid leukemia. Front. Oncol. 2020, 9, 1567. [Google Scholar] [CrossRef]
- Patel, A.B.; Pomicter, A.D.; Yan, D.; Eiring, A.M.; Antelope, O.; Schumacher, J.A.; Kelley, T.W.; Tantravahi, S.K.; Kovacsovics, T.J.; Shami, P.J. Dasatinib overcomes stroma-based resistance to the FLT3 inhibitor quizartinib using multiple mechanisms. Leukemia 2020, 34, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wu, Z.; Chu, J.; Yang, L.; Wang, N. Promoter methylation and expression of SOCS3 affect the clinical outcome of pediatric acute lymphoblastic leukemia by JAK/STAT pathway. Biomed. Pharmacother. 2019, 115, 108913. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Bar-Natan, M.; Mascarenhas, J.O. JAKs to STATs: A tantalizing therapeutic target in acute myeloid leukemia. Blood Rev. 2020, 40, 100634. [Google Scholar] [CrossRef]
- Zhu, W.-Q.; Wang, J.; Guo, X.-F.; Liu, Z.; Dong, W.-G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149. [Google Scholar] [CrossRef]
- Darici, S.; Alkhaldi, H.; Horne, G.; Jørgensen, H.G.; Marmiroli, S.; Huang, X. Targeting PI3K/Akt/mTOR in AML: Rationale and clinical evidence. J. Clin. Med. 2020, 9, 2934. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, I.; Hatfield, K.J.; Grønningsæter, I.S.; Reikvam, H. The PI3K-Akt-mTOR signaling pathway in human acute myeloid leukemia (AML) cells. Int. J. Mol. Sci. 2020, 21, 2907. [Google Scholar] [CrossRef]
- Zheng, C.; Tang, F.; Min, L.; Hornicek, F.; Duan, Z.; Tu, C. PTEN in osteosarcoma: Recent advances and the therapeutic potential. Biochim. Biophys. Acta-Rev. Cancer 2020, 1874, 188405. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Conciatori, F.; Pallocca, M.; Falcone, I.; Fanciulli, M.; Cognetti, F.; Milella, M.; Ciuffreda, L. PTEN as a prognostic/predictive biomarker in cancer: An unfulfilled promise? Cancers 2019, 11, 435. [Google Scholar] [CrossRef]
- Hackl, H.; Astanina, K.; Wieser, R. Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 1–16. [Google Scholar] [CrossRef]
- Maiti, A.; Franquiz, M.J.; Ravandi, F.; Cortes, J.E.; Jabbour, E.J.; Sasaki, K.; Marx, K.; Daver, N.G.; Kadia, T.M.; Konopleva, M.Y.; et al. Venetoclax and BCR-ABL tyrosine kinase inhibitor combinations: Outcome in patients with philadelphia chromosome-positive advanced myeloid leukemias. Acta Haematol. 2020, 143, 567–573. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Xu, Z.; Yang, B.; Luo, P.; He, Q. Molecular basis for class side effects associated with PI3K/AKT/mTOR pathway inhibitors. Expert Opin. Drug Metab. Toxicol. 2019, 15, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, V.; Gupta, S.K.; Kumari, G.; Verma, M. Combating TKI resistance in CML by inhibiting the PI3K/Akt/mTOR pathway in combination with TKIs: A review. Med. Oncol. 2021, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Wang, L.; Goh, B.C.; Ahn, K.S.; Bishayee, A.; Sethi, G. Modulation of diverse oncogenic transcription factors by thymoquinone, an essential oil compound isolated from the seeds of Nigella sativa Linn. Pharmacol. Res. 2018, 129, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Shen, N.; Yan, F.; Zhao, N.; Dou, L.; Wu, L.-C.; Seiler, C.L.; Yu, L.; Yang, K.; Bachanova, V.; et al. Thymoquinone exerts potent growth-suppressive activity on leukemia through DNA hypermethylation reversal in leukemia cells. Oncotarget 2017, 8, 34453. [Google Scholar] [CrossRef]
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 593–605. [Google Scholar] [CrossRef]
- Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N. Thymoquinone, as a novel therapeutic candidate of cancers. Pharmaceuticals 2021, 14, 369. [Google Scholar] [CrossRef]
- Al-Rawashde, F.A.; Johan, M.F.; Taib, W.R.W.; Ismail, I.; Johari, S.A.T.T.; Almajali, B.; Al-Wajeeh, A.S.; Vishkaei, M.N.; Nazari, V.M.; Al-Jamal, H.A.N. Thymoquinone Inhibits Growth of Acute Myeloid Leukemia Cells through Reversal SHP-1 and SOCS-3 Hypermethylation: In Vitro and In Silico Evaluation. Pharmaceuticals 2021, 14, 1287. [Google Scholar] [CrossRef]
- Al-Jamal, H.A.N.; Johan, M.F.; Jusoh, S.A.M.; Ismail, I.; Taib, W.R.W. Re-expression of bone marrow proteoglycan-2 by 5-azacytidine is associated with STAT3 inactivation and sensitivity response to imatinib in resistant CML cells. Asian Pac. J. Cancer Prev. 2018, 19, 1585. [Google Scholar]
- Roshandel, E.; Noorazar, L.; Farhadihosseinabadi, B.; Mehdizadeh, M.; Kazemi, M.H.; Parkhideh, S. PI3 kinase signaling pathway in hematopoietic cancers: A glance in miRNA’s role. J. Clin. Lab. Anal. 2021, 35, e23725. [Google Scholar] [CrossRef]
- Raut, P.K.; Lee, H.S.; Joo, S.H.; Chun, K.-S. Thymoquinone induces oxidative stress-mediated apoptosis through downregulation of Jak2/STAT3 signaling pathway in human melanoma cells. Food Chem. Toxicol. 2021, 157, 112604. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Y.; Huang, S.; Wang, G.; Han, R.; Lei, F.; Luo, A.; Jing, X.; Zhao, L.; Gu, S.; et al. Thymoquinone inhibits the metastasis of renal cell cancer cells by inducing autophagy via AMPK/mTOR signaling pathway. Cancer Sci. 2018, 109, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawashde, F.A.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Al-Wajeeh, A.S.; Al-Jamal, H.A.N. Thymoquinone Induces Downregulation of BCR-ABL/JAK/STAT Pathway and Apoptosis in K562 Leukemia Cells. Asian Pac. J. Cancer Prev. 2021, 22, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Panah, M.Z.; Nikbakht, M.; Sajjadi, S.M.; Rostami, S.; Norooznezhad, A.H.; Fumani, H.K.; Ghavamzadeh, A.; Mohammadi, S. Anti-apoptotic effects of osteopontin via the up-regulation of AKT/mTOR/β-catenin loop in acute myeloid leukemia cells. Int. J. Hematol. Oncol. Stem Cell. Res. 2017, 11, 148. [Google Scholar]
- Xie, C.; He, Y.; Zhen, M.; Wang, Y.; Xu, Y.; Lou, L. Puquitinib, a novel orally available PI 3Kδ inhibitor, exhibits potent antitumor efficacy against acute myeloid leukemia. Cancer Sci. 2017, 108, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Hu, S.; Chen, Y.; Zhang, Q.; Xia, C.; Deng, H.; Wang, Y.; Hu, Q. Thymoquinone loaded calcium alginate and polyvinyl alcohol carrier inhibits the 7, 12-dimethylbenz [a] anthracene-induced hamster oral cancer via the down-regulation of PI3K/AKT/mTOR signaling pathways. Environ. Toxicol. 2021, 36, 339–351. [Google Scholar] [CrossRef]
- Arafa, E.-S.A.; Zhu, Q.; Shah, Z.I.; Wani, G.; Barakat, B.M.; Racoma, I.; El-Mahdy, M.A.; Wani, A.A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 706, 28–35. [Google Scholar] [CrossRef]
- Khan, A.; Aldebasu, Y.H.; Alsuhaibani, S.A.; Khan, M.A. Thymoquinone augments cyclophosphamide-mediated inhibition of cell proliferation in breast cancer cells. Asian Pac. J. Cancer Prev. 2019, 20, 1153. [Google Scholar] [CrossRef]
- Ren, X.; Luo, W. Exploration of pro-apoptotic effect of Thymoquinone on oral squamous cell carcinoma cells through PI3K/Akt signaling pathway. Cell. Mol. Biol. 2019, 65, 61–64. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Zhu, M.; Lei, P.-P.; Pan, X.-Y.; Ma, W.-N. ND-09 inhibits chronic myeloid leukemia K562 cell growth by regulating BCR-ABL signaling. Oncol. Rep. 2021, 46, 1–10. [Google Scholar] [CrossRef]
- Fasouli, E.S.; Katsantoni, E. JAK-STAT in early hematopoiesis and leukemia. Front. Cell Dev. Biol. 2021, 9, 1219. [Google Scholar] [CrossRef]
- Hammarén, H.M.; Virtanen, A.T.; Raivola, J.; Silvennoinen, O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine 2019, 118, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lindström, H.J.G.; Friedman, R. Combating drug resistance in acute myeloid leukaemia by drug rotations: The effects of quizartinib and pexidartinib. Cancer Cell Int. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhu, X.; Jia, H.; Zhou, L.; Liu, H. Combination Therapies in Chronic Myeloid Leukemia for Potential Treatment-Free Remission: Focus on Leukemia Stem Cells and Immune Modulation. Front. Oncol. 2021, 11, 1657. [Google Scholar] [CrossRef] [PubMed]
- Almajali, B.; Johan, M.F.; Al-Wajeeh, A.S.; Taib, W.R.W.; Ismail, I.; Alhawamdeh, M.; Al-Tawarah, N.M.; Ibrahim, W.N.; Al-Rawashde, F.A.; Al-Jamal, H.A.N. Gene Expression Profiling and Protein Analysis Reveal Suppression of the C-Myc Oncogene and Inhibition JAK/STAT and PI3K/AKT/mTOR Signaling by Thymoquinone in Acute Myeloid Leukemia Cells. Pharmaceuticals 2022, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, M.; Lu, J.; Wei, W.; Lv, Y.; Zhang, X.; Yao, Y.; Wang, L.; Ling, T.; Zou, X. Effects of proton pump inhibitors on reversing multidrug resistance via downregulating V-ATPases/PI3K/Akt/mTOR/HIF-1α signaling pathway through TSC1/2 complex and Rheb in human gastric adenocarcinoma cells in vitro and in vivo. Oncol. Targets Ther. 2018, 11, 6705. [Google Scholar] [CrossRef]
- Yi, W.; Li, D.; Guo, Y.; Zhang, Y.; Huang, B.; Li, X. Sevoflurane inhibits the migration and invasion of glioma cells by upregulating microRNA-637. Int. J. Mol. Med. 2016, 38, 1857–1863. [Google Scholar] [CrossRef]
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Thymoquinone promotes pancreatic cancer cell death and reduction of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation. Adv. Prev. Med. 2016, 2016, 1407840. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford assay for determining protein concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef] [PubMed]

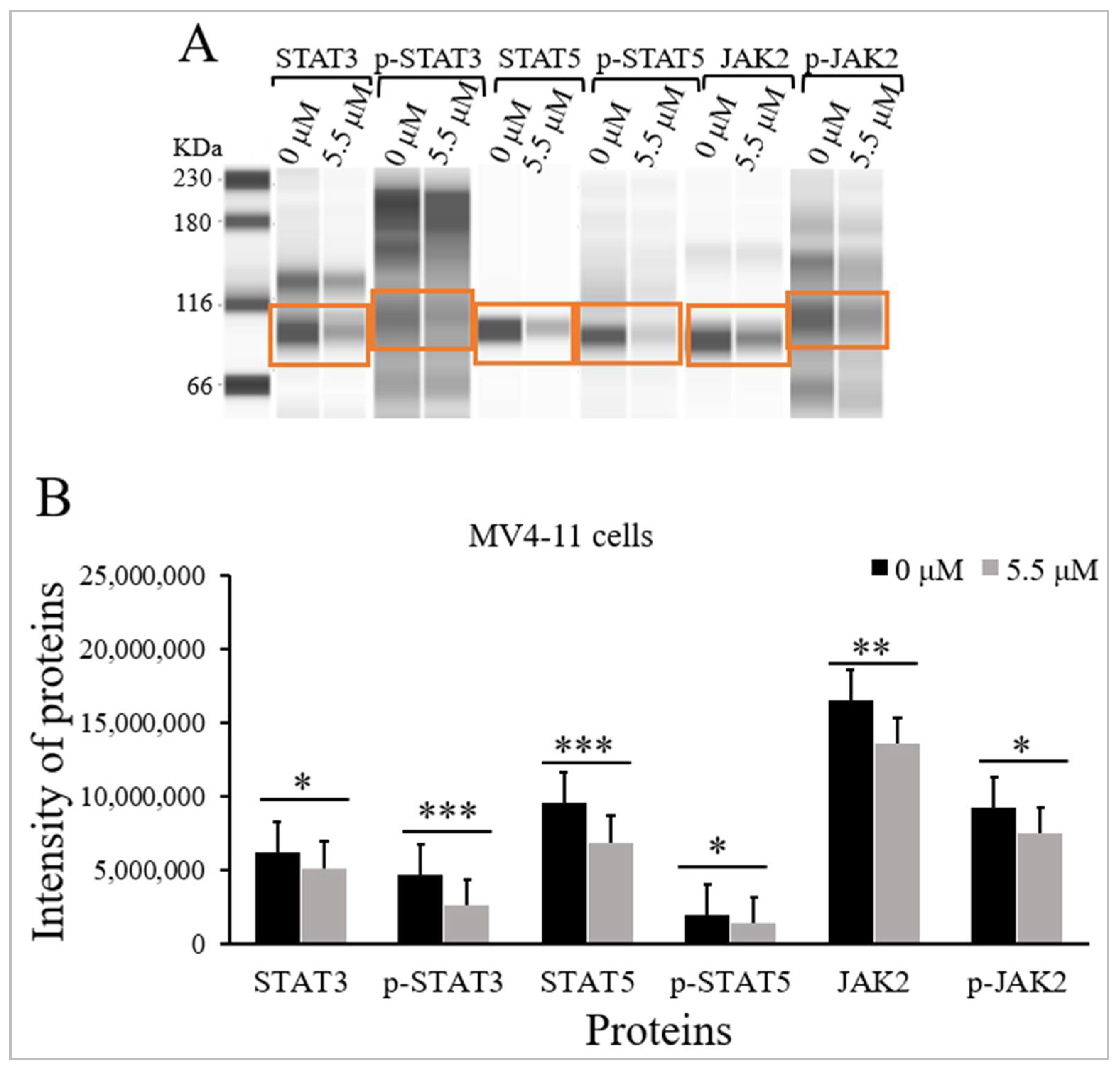
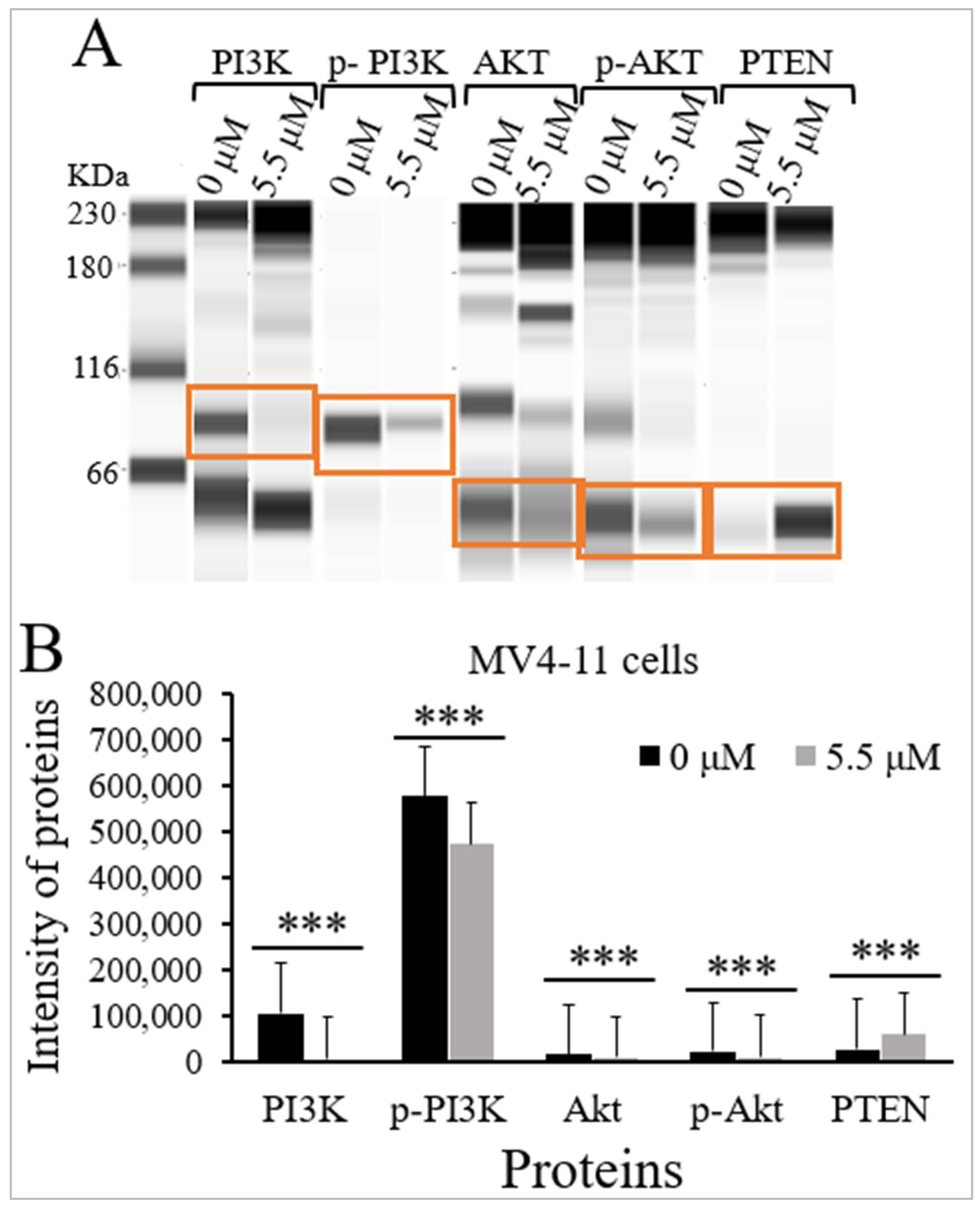

| Proteins | M.W (kDa) | Intensity of Proteins Median (IqR) (n = 3) | p-Value a | |
|---|---|---|---|---|
| Untreated MV4-11 Cells | Treated MV4-11 Cells | |||
| STAT3 | 80–90 | 6,238,528 (382,810) | 5,156,854 (196,459) | <0.05 |
| p-STAT3 | 95 | 4,644,236 (217,641) | 2,588,108 (131,717) | <0.001 |
| STAT5 | 91 | 9,545,158 (541,613) | 6,908,133 (315,229) | <0.001 |
| p-STAT5 | 92 | 1,944,235 (463,715) | 1,376,497 (681,108) | <0.05 |
| JAK2 | 130 | 16,523,707(831,602) | 13,585,639 (719,317) | <0.01 |
| p-JAK2 | 130 | 9,233,971 (691,001) | 7,481,068 (592,419) | <0.05 |
| PI3K | 85 | 107,064 (45,613) | 6627 (8731) | <0.001 |
| p-PI3K | 89 | 575,923 (104,621) | 473,130 (85,372) | <0.001 |
| Akt | 62 | 17,443 (4794) | 9897 (7803) | <0.001 |
| p-Akt | 60 | 22,879 (9117) | 11,646 (2712) | <0.001 |
| PTEN | 60 | 28,705 (9062) | 60,137 (11,823) | <0.001 |
| Proteins | M.W (kDa) | Intensity of Proteins Median (IqR) (n = 3) | p-Value a | |
|---|---|---|---|---|
| Untreated K562 Cells | TQ-Treated K562 Cells | |||
| PI3K | 85 | 520,529 (73,119) | 332,532 (57,649) | <0.001 |
| p-PI3K | 89 | 29,977 (6881) | 9633 (437) | <0.001 |
| Akt | 62 | 43,372 (9653) | 12,165 (1711) | <0.001 |
| p-Akt | 60 | 72,651 (8091) | 16,152 (5793) | <0.001 |
| PTEN | 60 | 194,916 (39,293) | 295,205 (11,719) | <0.001 |
| Gene | Accession Number | Primer Set Sequences (5′–3′) | Reference |
|---|---|---|---|
| PI3K | NG_012113.2 | Forward: TTAGCTATTCCCACGCAGGA Reverse: CACAATAGTGTCTGTGACTC | [46] |
| AKT | NG_012188.1 | Forward: CTGAGATTGTGTCAGCCCTG Reverse: CACAGCCCGAAGTCTGTGATCTTA | [47] |
| mTOR | NG_033239.1 | Forward: ATGCAGCTGTCCTGGTTCTC Reverse: AATCAGACAGGCACGAAG | [46] |
| PTEN | AF_067844.1 | Forward: TGGATTCGACTTAGACT GACCT Reverse: TTTGGCGGTGTCATAATGTCTT | [33] |
| β-actin | NC_000071.7 | Forward: CTGGCACCCAGGACAATG Reverse: GCCGATCCACACGGAGTA | [48] |
| Primary Antibodies | Catalogue Number | COMPANY |
|---|---|---|
| Anti-JAK2 | NBP2-59451 | Novus Biologicals, LLC, Littleton, CO, USA |
| Anti-phospho-JAK2 | NBP2-67429 | Novus Biologicals, LLC, Littleton, CO, USA |
| Anti-STAT3 | MAB1799 | R&D System, Minneapolis, MN, USA |
| Anti-phospho-STAT3 | AF4607 | R&D System, Minneapolis, MN, USA |
| Anti-STAT5 | AF2168 | R&D System, Minneapolis, MN, USA |
| Anti-phospho-STAT5 | MAB41901 | R&D System, Minneapolis, MN, USA |
| Anti-PI3K | MAB2998 | R&D System, Minneapolis, MN, USA |
| Anti-phospho-PI3K | PA5-177387 | Invitrogen, Waltham, MA, USA |
| Anti-Akt | MAB2055 | R&D System, Minneapolis, MN, USA |
| Anti-phospho-Akt | AF887 | R&D System, Minneapolis, MN, USA |
| Anti-PTEN | AF847 | R&D System, Minneapolis, MN, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rawashde, F.A.; Al-wajeeh, A.S.; Vishkaei, M.N.; Saad, H.K.M.; Johan, M.F.; Taib, W.R.W.; Ismail, I.; Al-Jamal, H.A.N. Thymoquinone Inhibits JAK/STAT and PI3K/Akt/ mTOR Signaling Pathways in MV4-11 and K562 Myeloid Leukemia Cells. Pharmaceuticals 2022, 15, 1123. https://doi.org/10.3390/ph15091123
Al-Rawashde FA, Al-wajeeh AS, Vishkaei MN, Saad HKM, Johan MF, Taib WRW, Ismail I, Al-Jamal HAN. Thymoquinone Inhibits JAK/STAT and PI3K/Akt/ mTOR Signaling Pathways in MV4-11 and K562 Myeloid Leukemia Cells. Pharmaceuticals. 2022; 15(9):1123. https://doi.org/10.3390/ph15091123
Chicago/Turabian StyleAl-Rawashde, Futoon Abedrabbu, Abdullah Saleh Al-wajeeh, Mansoureh Nazari Vishkaei, Hanan Kamel M. Saad, Muhammad Farid Johan, Wan Rohani Wan Taib, Imilia Ismail, and Hamid Ali Nagi Al-Jamal. 2022. "Thymoquinone Inhibits JAK/STAT and PI3K/Akt/ mTOR Signaling Pathways in MV4-11 and K562 Myeloid Leukemia Cells" Pharmaceuticals 15, no. 9: 1123. https://doi.org/10.3390/ph15091123
APA StyleAl-Rawashde, F. A., Al-wajeeh, A. S., Vishkaei, M. N., Saad, H. K. M., Johan, M. F., Taib, W. R. W., Ismail, I., & Al-Jamal, H. A. N. (2022). Thymoquinone Inhibits JAK/STAT and PI3K/Akt/ mTOR Signaling Pathways in MV4-11 and K562 Myeloid Leukemia Cells. Pharmaceuticals, 15(9), 1123. https://doi.org/10.3390/ph15091123