Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma
Abstract
1. Introduction
2. Results
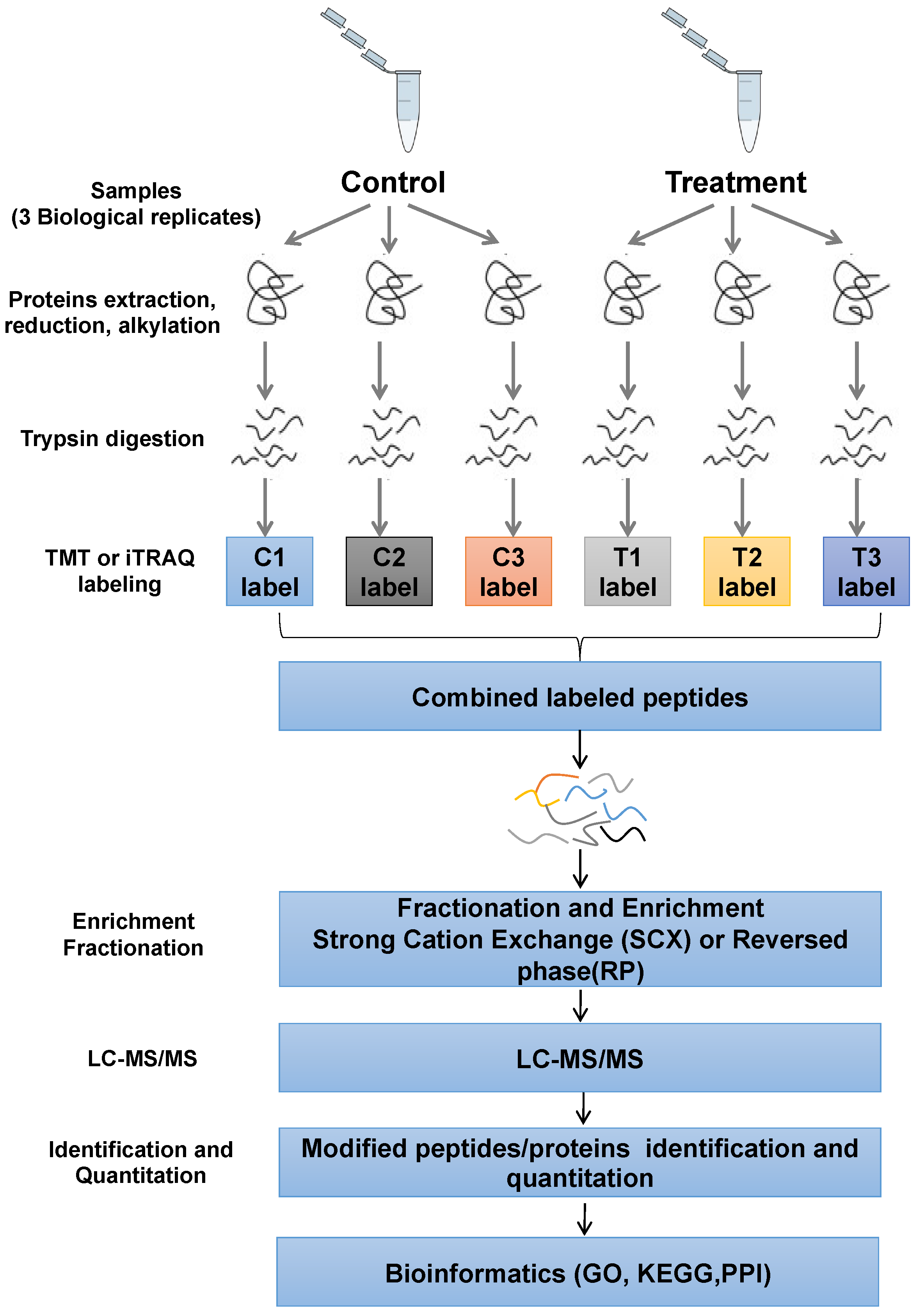
2.1. Profile of Phosphoproteomics Data upon Afatinib and IR Treatment in NPC Cells
2.2. Functional Characteristics and KEGG Pathway Analysis of DEPs
2.3. CD44-STAT3 Axis Contributing to Affect the Radiosensitivity of NPC Cells
2.4. Exploring the Inhibition of EMT Process in Combination with Afatinib and IR Treatment in NPC Cells
2.5. Stat3 Agonist Reversed Sensitivity to IR by Regulating EMT
2.6. Afatinib Enhanced Sensitivity to IR In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and CCK-8 Assay
4.2. Cell Migration and Wound Healing Assay
4.3. Immunoblotting
4.4. Xenograft Mouse Experiment
4.5. Protein Extraction and Digestion
4.6. Tandem Mass Tagging Labeling
4.7. Phosphopeptide Enrichment and LC-MS/MS
4.8. Bioinformatic Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMT | Epithelial-Mesenchymal Transition |
| NPC | Nasopharyngeal carcinoma |
| EBV | Epstein–Barr virus |
| CSCs | Cancer stem cells |
| NSCLC | Non-small cell lung cancer |
| PTMs | Protein posttranslational modifications |
| DEPs | Differentially expressed proteins |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tang, L.-L.; Chen, W.-Q.; Xue, W.-Q.; He, Y.-Q.; Zheng, R.-S.; Zeng, Y.-X.; Jia, W.-H. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016, 374, 22–30. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Blanchard, P.; Lee, A.; Marguet, S.; Leclercq, J.; Ng, W.T.; Ma, J.; Chan, A.T.C.; Huang, P.-Y.; Benhamou, E.; Zhu, G.; et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015, 16, 645–655. [Google Scholar] [CrossRef]
- Luftig, M. Heavy LIFting: Tumor promotion and radioresistance in NPC. J. Clin. Investig. 2013, 123, 4999–5001. [Google Scholar] [CrossRef]
- Tan, Z.; Xiao, L.; Tang, M.; Bai, F.; Li, J.; Li, L.; Shi, F.; Li, N.; Li, Y.; Du, Q.; et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics 2018, 8, 2329–2347. [Google Scholar] [CrossRef]
- Liao, K.; Xia, B.; Zhuang, Q.Y.; Hou, M.J.; Zhang, Y.J.; Luo, B.; Qiu, Y.; Gao, Y.F.; Li, X.J.; Chen, H.F.; et al. Parthenolide inhibits cancer stem-like side population of nasopharyngeal carcinoma cells via suppression of the NF-κB/COX-2 pathway. Theranostics 2015, 5, 302–321. [Google Scholar] [CrossRef]
- Ma, J.; Mai, H.Q.; Hong, M.H.; Min, H.Q.; Mao, Z.D.; Cui, N.J.; Lu, T.X.; Mo, H.Y. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J. Clin. Oncol. 2001, 19, 1350–1357. [Google Scholar] [CrossRef]
- Hareyama, M.; Sakata, K.; Shirato, H.; Nishioka, T.; Nishio, M.; Suzuki, K.; Saitoh, A.; Oouchi, A.; Fukuda, S.; Himi, T. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer 2002, 94, 2217–2223. [Google Scholar] [CrossRef]
- Willers, H.; Pan, X.; Borgeaud, N.; Korovina, I.; Koi, L.; Egan, R.; Greninger, P.; Rosenkranz, A.; Kung, J.; Liss, A.S.; et al. Screening and Validation of Molecular Targeted Radiosensitizers. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e63–e74. [Google Scholar] [CrossRef]
- Huang, F.; Liang, X.; Min, X.; Zhang, Y.; Wang, G.; Peng, Z.; Peng, F.; Li, M.; Chen, L.; Chen, Y. Simultaneous Inhibition of EGFR and HER2 via Afatinib Augments the Radiosensitivity of Nasopharyngeal Carcinoma Cells. J. Cancer 2019, 10, 2063–2073. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Al-Assar, O.; Demiciorglu, F.; Lunardi, S.; Gaspar-Carvalho, M.M.; McKenna, W.G.; Muschel, R.M.; Brunner, T.B. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother. Oncol. 2014, 111, 243–251. [Google Scholar] [CrossRef]
- Cojoc, M.; Peitzsch, C.; Kurth, I.; Trautmann, F.; Kunz-Schughart, L.A.; Telegeev, G.D.; Stakhovsky, E.A.; Walker, J.R.; Simin, K.; Lyle, S.; et al. Aldehyde Dehydrogenase Is Regulated by β-Catenin/TCF and Promotes Radioresistance in Prostate Cancer Progenitor Cells. Cancer Res. 2015, 75, 1482–1494. [Google Scholar] [CrossRef]
- Chang, L.; Graham, P.H.; Hao, J.; Ni, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013, 4, e875. [Google Scholar] [CrossRef]
- Uddin, N.; Kim, R.; Yoo, K.; Kim, Y.; Cui, Y.; Kim, I.; Suh, Y.; Lee, S. Persistent activation of STAT3 by PIM2-driven positive feedback loop for epithelial-mesenchymal transition in breast cancer. Cancer Sci. 2015, 106, 718–725. [Google Scholar] [CrossRef]
- Tahmasebi-Birgani, M.J.; Teimoori, A.; Ghadiri, A.; Mansoury-Asl, H.; Danyaei, A.; Khanbabaei, H. Fractionated radiotherapy might induce epithelial-mesenchymal transition and radioresistance in a cellular context manner. J. Cell. Biochem. 2018, 120, 8601–8610. [Google Scholar] [CrossRef]
- Luo, M.; Wu, C.; Guo, E.; Peng, S.; Zhang, L.; Sun, W.; Liu, D.; Hu, G.; Hu, G. FOXO3a knockdown promotes radioresistance in nasopharyngeal carcinoma by inducing epithelial-mesenchymal transition and the Wnt/β-catenin signaling pathway. Cancer Lett. 2019, 455, 26–35. [Google Scholar] [CrossRef]
- Zeilstra, J.; Joosten, S.P.; van Andel, H.; Tolg, C.; Berns, A.; Snoek, M.; Van De Wetering, M.; Spaargaren, M.; Clevers, H.; Pals, S.T. Stem cell CD44v isoforms promote intestinal cancer formation in Apc(min) mice downstream of Wnt signaling. Oncogene 2014, 33, 665–670. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Brown, R.L.; Wei, Y.; Zhao, P.; Liu, S.; Liu, X.; Deng, Y.; Hu, X.; Zhang, J.; Gao, X.D.; et al. CD44 splice isoform switching determines breast cancer stem cell state. Genes Dev. 2019, 33, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Reinke, L.M.; Damerow, M.S.; Perez, D.; Chodosh, L.A.; Yang, J.; Cheng, C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J. Clin. Investig. 2011, 121, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Pietras, A.; Katz, A.M.; Ekström, E.J.; Wee, B.; Halliday, J.J.; Pitter, K.L.; Werbeck, J.L.; Amankulor, N.M.; Huse, J.T.; Holland, E.C. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell 2014, 14, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Xia, W.; Wong, G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates β-catenin signaling and NFκB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J. Biol. Chem. 2009, 284, 2657–2671. [Google Scholar] [CrossRef]
- Lee, M.; Hirpara, J.L.; Eu, J.Q.; Sethi, G.; Wang, L.; Goh, B.C.; Wong, A.L. Targeting STAT3 and oxidative phosphorylation in oncogene-addicted tumors. Redox Biol. 2019, 25, 101073. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, X.; Yang, K.; Chen, Y.; Wang, L.; Luo, W.; Li, Y.; Liao, J.; Zhou, Y.; Lei, Y.; et al. Protein tyrosine phosphatase receptor type D gene promotes radiosensitivity via STAT3 dephosphorylation in nasopharyngeal carcinoma. Oncogene 2021, 40, 3101–3117. [Google Scholar] [CrossRef]
- Li, H.; Luo, D.; Huttad, L.; Zhang, M.; Wang, Y.; Feng, J.; Ding, Y.; Han, B. RIPK4 Suppresses the Invasion and Metastasis of Hepatocellular Carcinoma by Inhibiting the Phosphorylation of STAT3. Front. Mol. Biosci. 2021, 8, 654766. [Google Scholar] [CrossRef]
- Du, C.; Zhang, C.; Hassan, S.; Biswas, M.H.; Balaji, K.C. Protein kinase D1 suppresses epithelial-to-mesenchymal transition through phosphorylation of snail. Cancer Res. 2010, 70, 7810–7819. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef]
- Shawver, L.K.; Slamon, D.; Ullrich, A. Smart drugs: Tyrosine kinase inhibitors in cancer therapy. Cancer Cell 2002, 1, 117–123. [Google Scholar] [CrossRef]
- Liang, S.; Chen, Z.; Jiang, G.; Zhou, Y.; Liu, Q.; Su, Q.; Wei, W.; Du, J.; Wang, H. Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-κB/IL-6 signals. Cancer Lett. 2017, 386, 12–23. [Google Scholar] [CrossRef]
- Marotta, L.L.; Almendro, V.; Marusyk, A.; Shipitsin, M.; Schemme, J.; Walker, S.R.; Bloushtain-Qimron, N.; Kim, J.J.; Choudhury, S.A.; Maruyama, R.; et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24− stem cell-like breast cancer cells in human tumors. J. Clin. Investig. 2011, 121, 2723–2735. [Google Scholar] [CrossRef]
- Anand, V.; Khandelwal, M.; Appunni, S.; Gupta, N.; Seth, A.; Singh, P.; Mathur, S.; Sharma, A. CD44 splice variant (CD44v3) promotes progression of urothelial carcinoma of bladder through Akt/ERK/STAT3 pathways: Novel therapeutic approach. J. Cancer Res. Clin. Oncol. 2019, 145, 2649–2661. [Google Scholar] [CrossRef]
- Lin, C.H.; Chiang, M.C.; Chen, Y.J. STAT3 mediates resistance to anoikis and promotes invasiveness of nasopharyngeal cancer cells. Int. J. Mol. Med. 2017, 40, 1549–1556. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Q.; Liu, B.; Zhang, N.; Cheng, G. Vitamin D Enhances Radiosensitivity of Colorectal Cancer by Reversing Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2021, 9, 684855. [Google Scholar] [CrossRef]
- Dongre, A.; Rashidian, M.; Reinhardt, F.; Bagnato, A.; Keckesova, Z.; Ploegh, H.L.; Weinberg, R.A. Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas. Cancer Res. 2017, 77, 3982–3989. [Google Scholar] [CrossRef]
- Way, T.D.; Huang, J.T.; Chou, C.H.; Huang, C.H.; Yang, M.H.; Ho, C.T. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the β-catenin and Akt pathways. Eur. J. Cancer 2014, 50, 366–378. [Google Scholar] [CrossRef]






| Upregulated DEPs (Top 20) | Downregulated DEPs (Top 20) | ||||||
|---|---|---|---|---|---|---|---|
| ID | Proteins | FC Value | p Value | ID | Proteins | FC Value | p Value |
| P20020 | ATP2B1 | 1.764 | 0.048 | Q04637 | EIF4G1 | 0.4 | 0.006 |
| Q13459 | MYO9B | 1.517 | 0.009 | Q04637 | EIF4G1 | 0.429 | 0.018 |
| Q9H8Y8 | GORASP2 | 1.49 | 0.014 | Q15648 | MED1 | 0.467 | 0.014 |
| Q5VT25 | CDC42BPA | 1.481 | 0.033 | P16070 | CD44 | 0.475 | 0.011 |
| Q9Y570 | PPME1 | 1.466 | 0.027 | P40763 | STAT3 | 0.476 | 0.029 |
| O94921 | CDK14 | 1.46 | 0.004 | Q86X27 | RALGPS2 | 0.497 | 0.012 |
| Q14669 | TRIP12 | 1.443 | 0.02 | Q7RTP6 | MICAL3 | 0.504 | 0.008 |
| O15355 | PPM1G | 1.432 | 0.007 | Q09666 | AHNAK | 0.509 | 0.043 |
| Q9H3H3 | C11orf68 | 1.429 | <0.001 | Q9H2G2 | SLK | 0.512 | 0.007 |
| P18433 | PTPRA | 1.423 | 0.006 | Q9BRS8 | LARP6 | 0.524 | 0.021 |
| P13796 | LCP1 | 1.401 | 0.047 | Q9H0X4 | FAM234A | 0.526 | 0.02 |
| Q12802 | AKAP13 | 1.386 | 0.005 | Q01196 | RUNX1 | 0.535 | 0.025 |
| Q9NP64 | ZCCHC17 | 1.37 | 0.007 | P80723 | BASP1 | 0.543 | 0.033 |
| P53999 | SUB1 | 1.365 | 0.021 | Q09666 | AHNAK | 0.545 | 0.01 |
| Q9H2H9 | SLC38A1 | 1.364 | 0.004 | Q13555 | CAMK2G | 0.546 | 0.049 |
| Q8WX93 | PALLD | 1.363 | 0.01 | Q5T5U3 | ARHGAP21 | 0.558 | 0.003 |
| Q14160 | SCRIB | 1.362 | 0.016 | Q9Y520 | PRRC2C | 0.565 | 0.001 |
| Q9UQ35 | SRRM2 | 1.355 | 0.016 | Q5T5U3 | ARHGAP21 | 0.594 | 0.022 |
| Q96L91 | EP400 | 1.354 | 0.015 | Q9NUQ3 | TXLNG | 0.596 | 0.001 |
| Q96MU7 | YTHDC1 | 1.349 | 0.034 | Q04637 | EIF4G1 | 0.602 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Huang, F.; Liang, X.; Fu, Y.; Cheng, Z.; Huang, Y.; Chen, Z.; Duan, Y.; Chen, Y. Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma. Pharmaceuticals 2023, 16, 37. https://doi.org/10.3390/ph16010037
Huang H, Huang F, Liang X, Fu Y, Cheng Z, Huang Y, Chen Z, Duan Y, Chen Y. Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma. Pharmaceuticals. 2023; 16(1):37. https://doi.org/10.3390/ph16010037
Chicago/Turabian StyleHuang, Huichao, Fangling Huang, Xujun Liang, Ying Fu, Zhe Cheng, Yan Huang, Zhuchu Chen, Yankun Duan, and Yongheng Chen. 2023. "Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma" Pharmaceuticals 16, no. 1: 37. https://doi.org/10.3390/ph16010037
APA StyleHuang, H., Huang, F., Liang, X., Fu, Y., Cheng, Z., Huang, Y., Chen, Z., Duan, Y., & Chen, Y. (2023). Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma. Pharmaceuticals, 16(1), 37. https://doi.org/10.3390/ph16010037







