The Core-Targeted RRM2 Gene of Berberine Hydrochloride Promotes Breast Cancer Cell Migration and Invasion via the Epithelial–Mesenchymal Transition
Abstract
:1. Introduction
2. Results
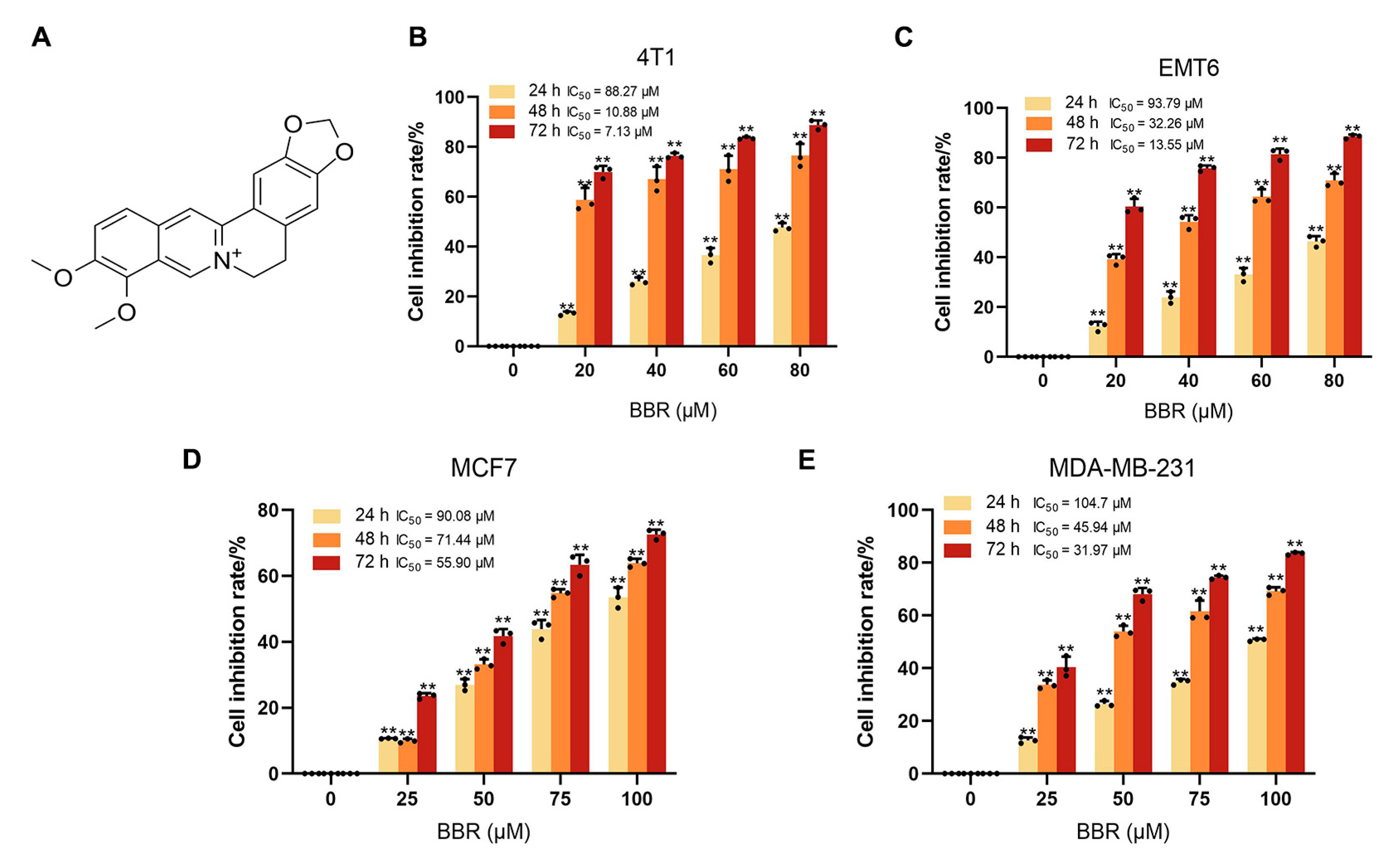
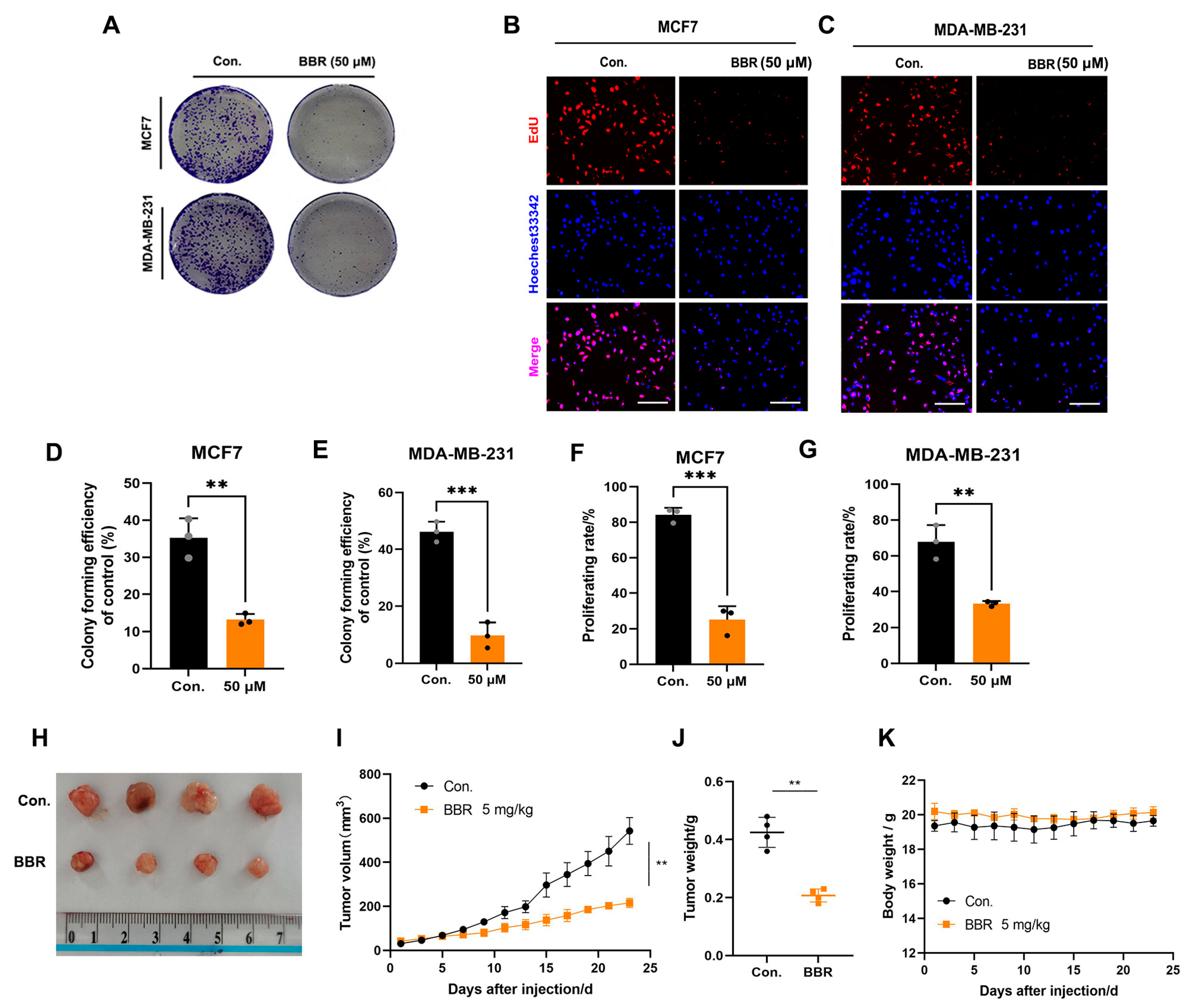
2.1. BBR Inhibits the Proliferation of BRCA Cells In Vitro and In Vivo
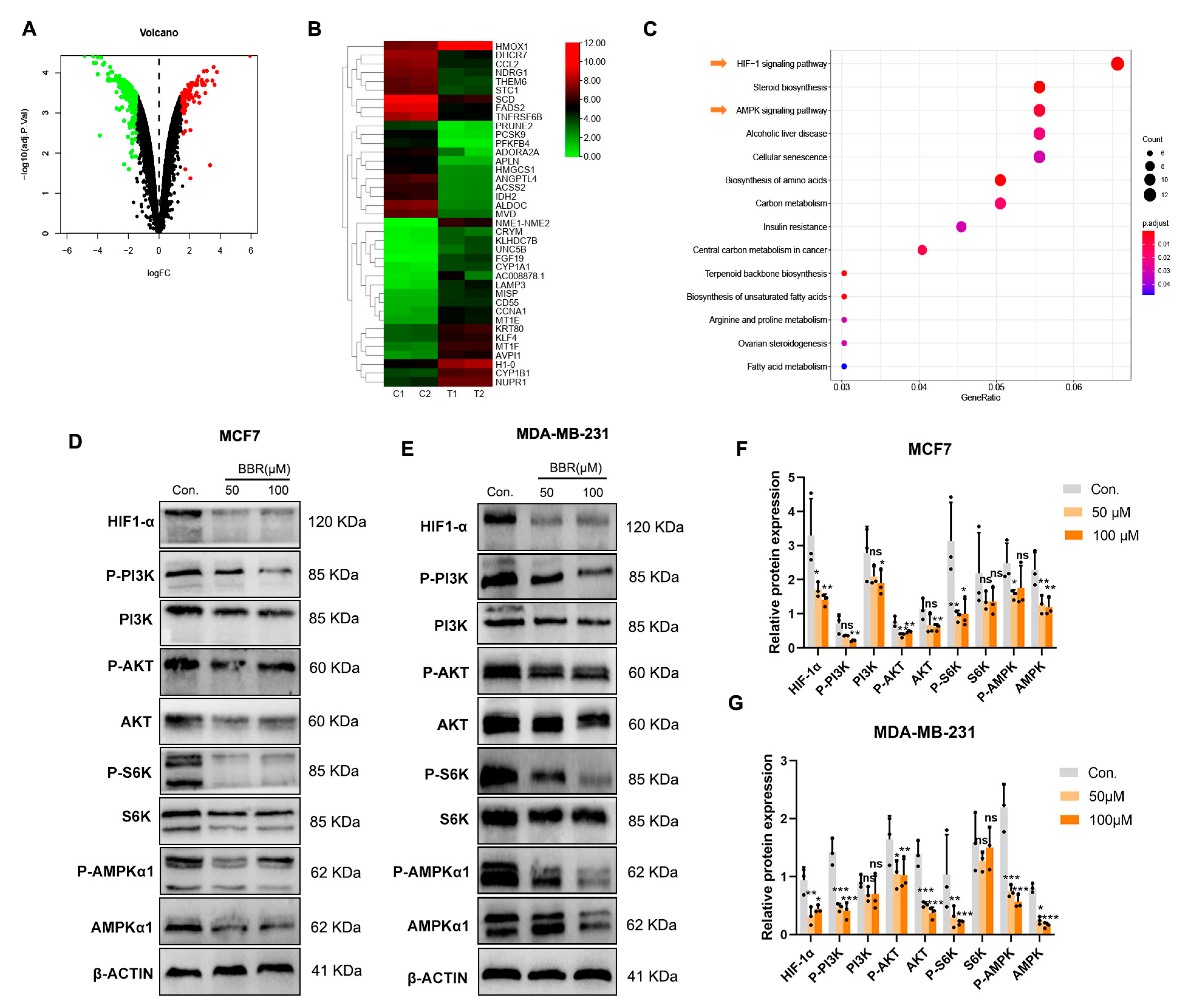
2.2. Signaling Pathway Enrichment of BBR Based on RNA-Seq
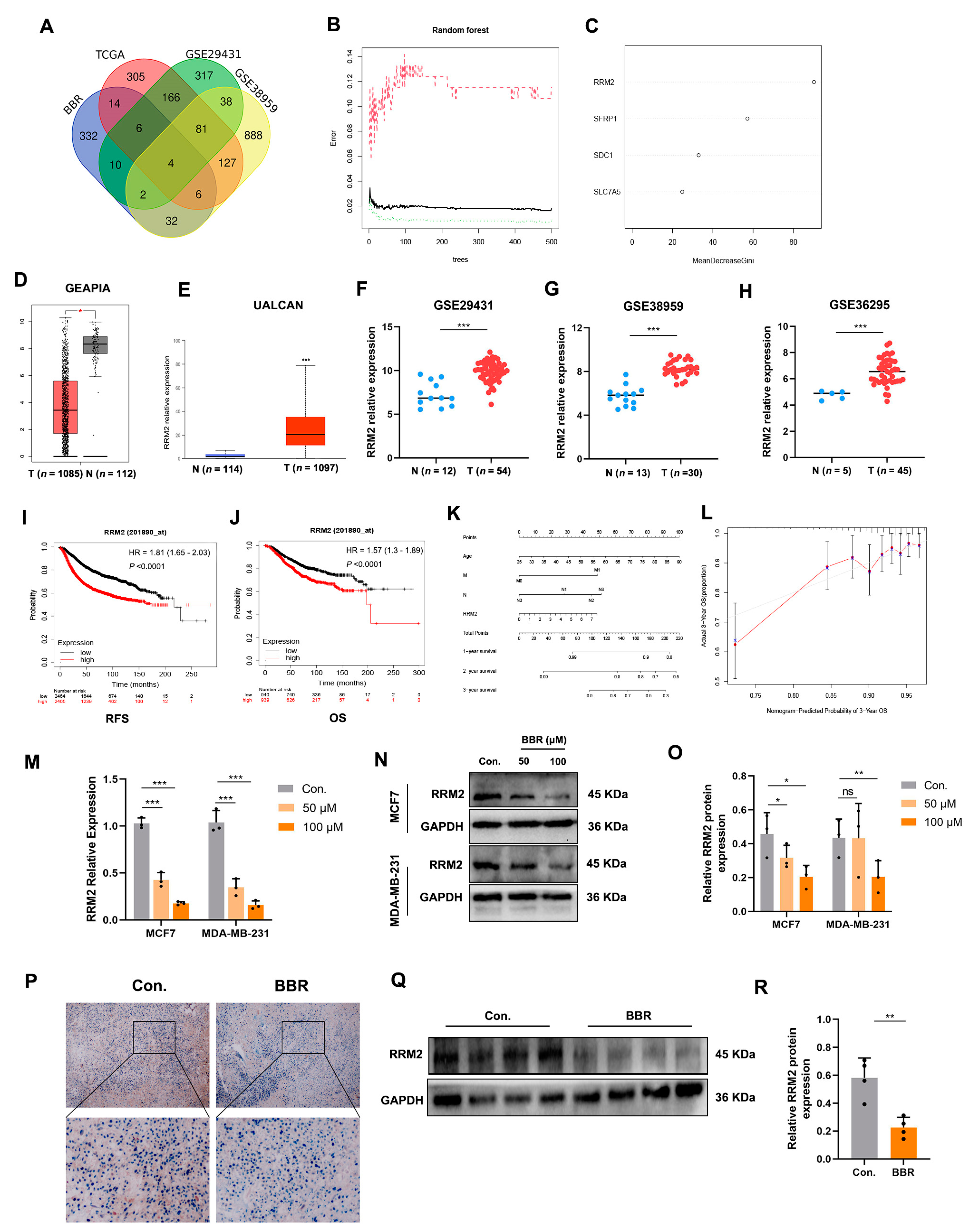
2.3. Random Forest Screening for DEGs
2.4. RRM2 as a Potential Regulator in BBR-Treated BRCA Cells
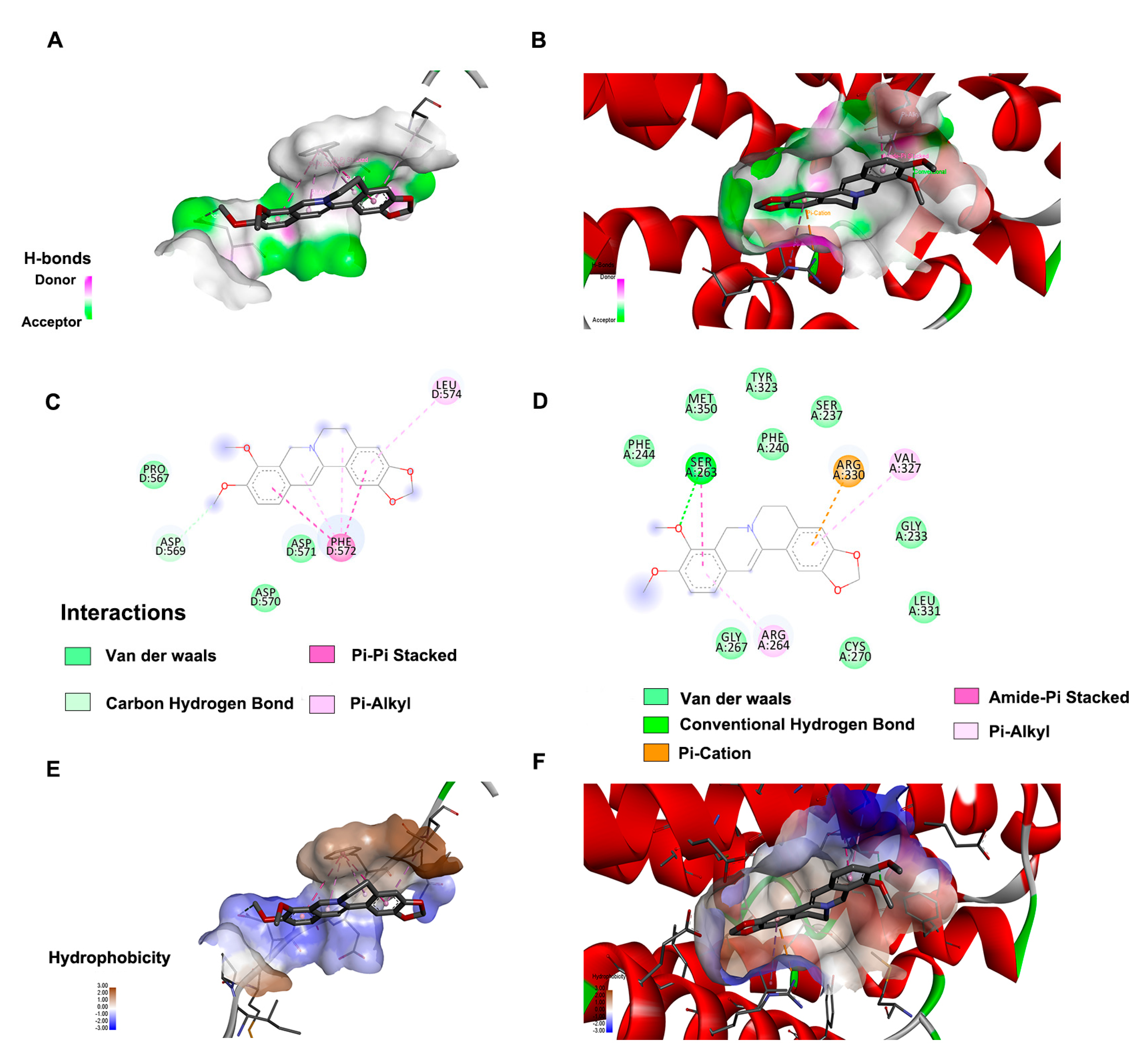
2.5. Molecular Docking
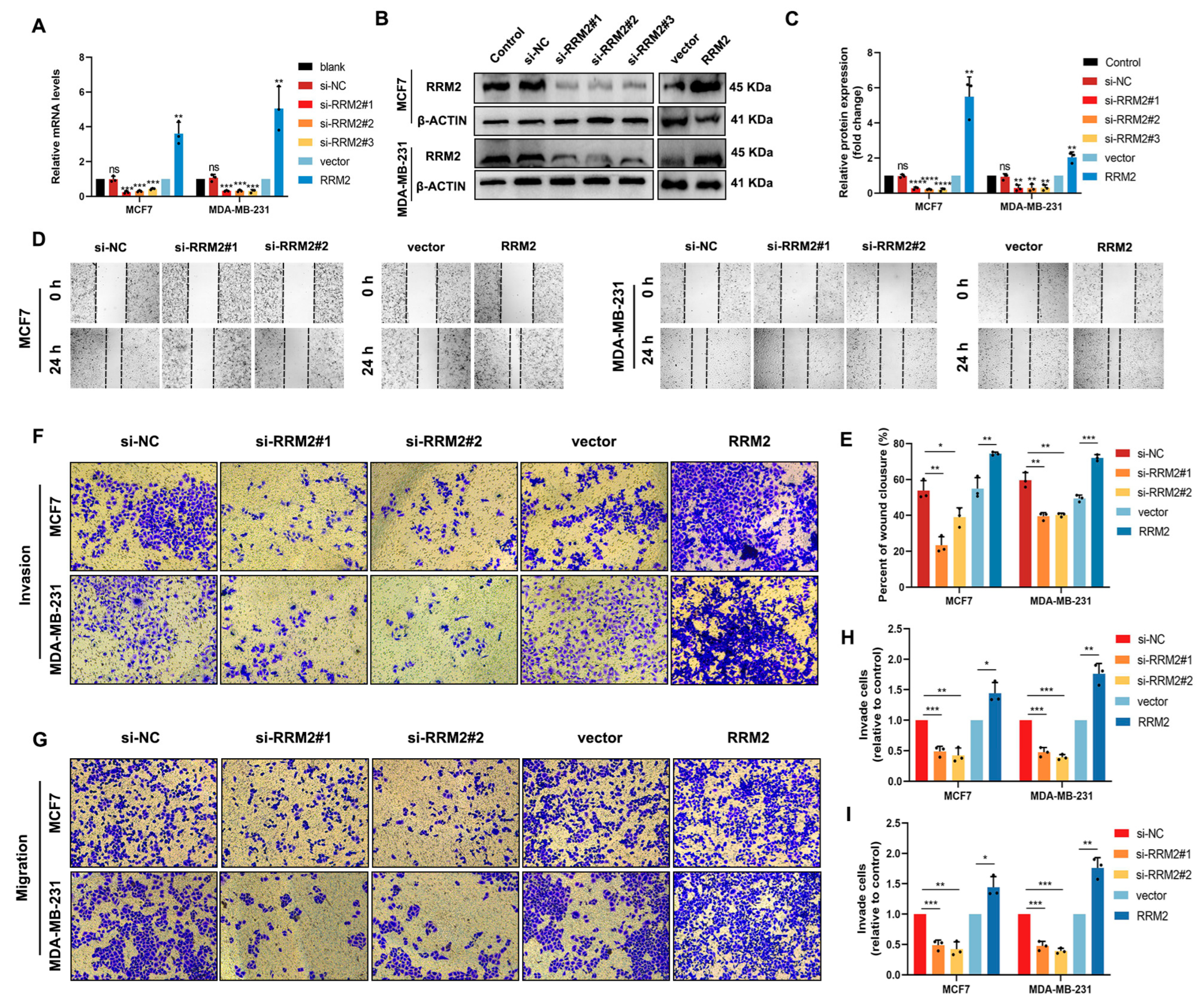
2.6. RRM2 Promotes Migration and Invasion of BRCA Cells In Vitro
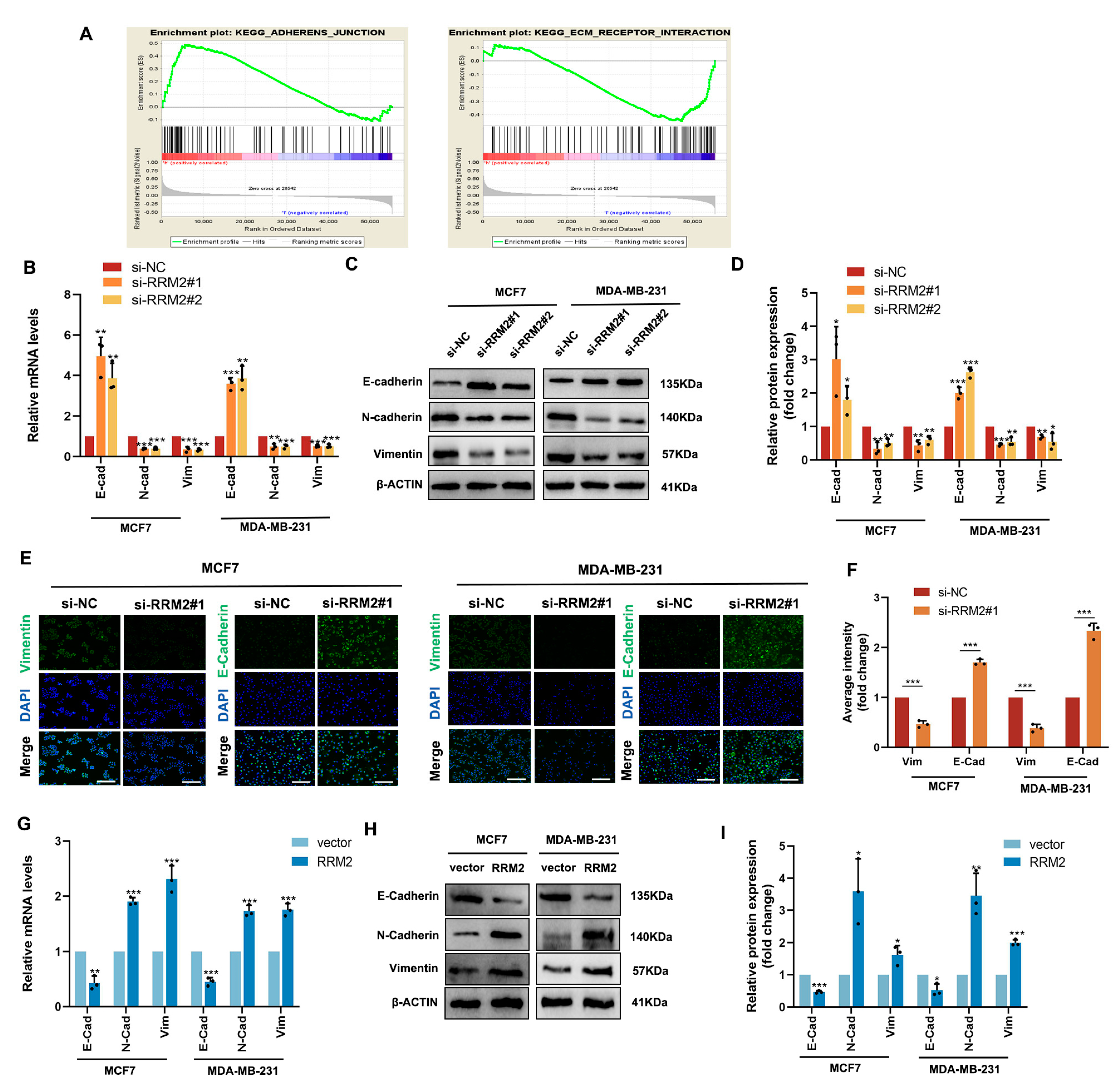
2.7. RRM2 Regulates the EMT Program of BRCA Cells In Vitro
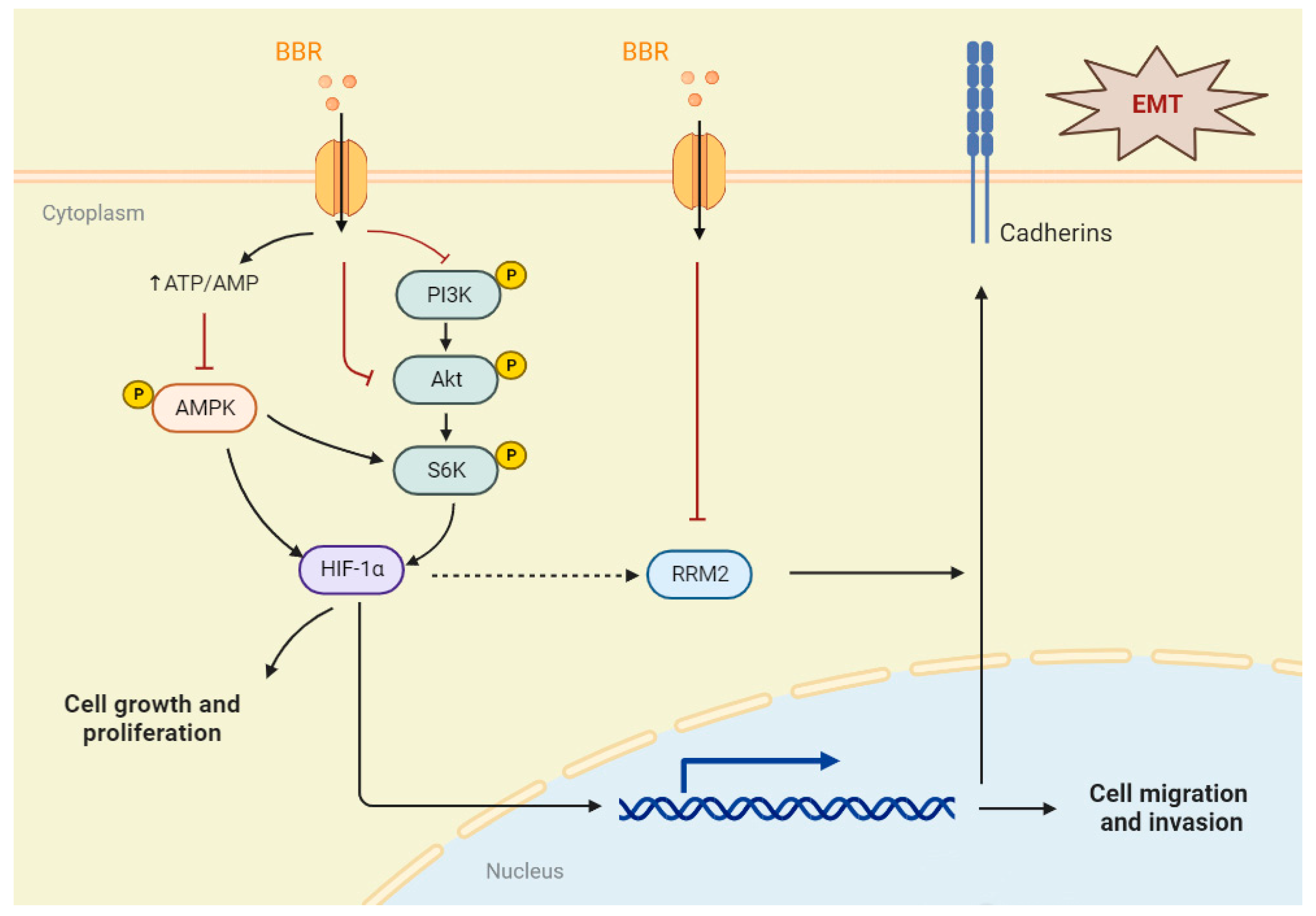
3. Discussion
4. Materials and Methods
4.1. Online Data
4.2. Random Forest Algorithm
4.3. Nomogram Analysis
4.4. GSEA Analysis
4.5. Molecular Docking
4.6. Reagent and Antibodies
4.7. Cell Culture
4.8. Cell Viability Assay
4.9. Transcriptome Sequencing
4.10. Cell Colony Formation and 5-Ethynyl-2′-Deoxyuridine (EdU) Assay
4.11. Wound-Healing Assay
4.12. Transwell Assay
4.13. Western Blot (WB) Assay
4.14. Immunofluorescence (IF) Staining
4.15. Quantitative Real-Time PCR Assay (RT-qPCR)
4.16. siRNA and Plasmids Transfection
4.17. Xenograft Models in Nude Mice
4.18. Immunohistochemistry (IHC) Analysis
4.19. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and Nutritional Supplements on Breast Cancer. Biomed. Res. Int. 2017, 2017, 7207983. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef]
- Kuo, H.P.; Chuang, T.C.; Tsai, S.C.; Tseng, H.H.; Hsu, S.C.; Chen, Y.C.; Kuo, C.L.; Kuo, Y.H.; Liu, J.Y.; Kao, M.C. Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt pathway modulation. J. Agric. Food Chem. 2012, 60, 9649–9658. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, W.; Li, Y.; Xian, X.; Zhao, Z.; Liu, C.; Zou, J.; Li, J.; Fang, X.; Zhu, Y. Berberine suppresses colon cancer cell proliferation by inhibiting the SCAP/SREBP-1 signaling pathway-mediated lipogenesis. Biochem. Pharmacol. 2020, 174, 113776. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Jiang, S.; Liu, J.; Chen, X.; Zhang, S.; Zhao, H. Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/Bax signaling pathways. Oncol. Lett. 2018, 15, 7409–7414. [Google Scholar] [CrossRef]
- Du, H.; Gu, J.; Peng, Q.; Wang, X.; Liu, L.; Shu, X.; He, Q.; Tan, Y. Berberine Suppresses EMT in Liver and Gastric Carcinoma Cells through Combination with TGFβR Regulating TGF-β/Smad Pathway. Oxidative Med. Cell Longev. 2021, 2021, 2337818. [Google Scholar] [CrossRef]
- Li, Y.; Gong, Y.; Zhang, X.; Wang, J.; Cheng, Y.; Liu, F.; Shi, X.; Xu, W.; Dong, L. Exploring the synergistic mechanism of Gegen Qinlian Decoction on the Wnt signaling pathway using an integrated strategy of network pharmacology and RNA-seq. J. Ethnopharmacol. 2021, 278, 114283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Bi, Y.; Zhong, J. Pristimerin suppresses colorectal cancer through inhibiting inflammatory responses and Wnt/β-catenin signaling. Toxicol. Appl. Pharmacol. 2020, 386, 114813. [Google Scholar] [CrossRef]
- Uhlin, U.; Eklund, H. Structure of ribonucleotide reductase protein R1. Nature 1994, 370, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Jiang, L.; Jin, X. Inhibiting RRM2 to enhance the anticancer activity of chemotherapy. Biomed. Pharmacother. 2021, 133, 110996. [Google Scholar] [CrossRef] [PubMed]
- Aye, Y.; Li, M.; Long, M.J.; Weiss, R.S. Ribonucleotide reductase and cancer: Biological mechanisms and targeted therapies. Oncogene 2015, 34, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, B.; Yu, H.; Zhu, L.; Yi, L.; Jin, X. RRM2 Regulates Sensitivity to Sunitinib and PD-1 Blockade in Renal Cancer by Stabilizing ANXA1 and Activating the AKT Pathway. Adv. Sci. 2021, 8, e2100881. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lu, S.; Yang, D.; Zhang, L.; Ye, J.; Li, M.; Hu, W. MiR-20a-5p regulates gemcitabine chemosensitivity by targeting RRM2 in pancreatic cancer cells and serves as a predictor for gemcitabine-based chemotherapy. Biosci. Rep. 2019, 39, BSR20181374. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Li, Y.; Zhang, N.; Gao, Y.; Han, L.; Li, S.; Li, J.; Liu, X.; Gong, Y.; Xie, C. RRM2 silencing suppresses malignant phenotype and enhances radiosensitivity via activating cGAS/STING signaling pathway in lung adenocarcinoma. Cell Biosci. 2021, 11, 74. [Google Scholar] [CrossRef]
- Li, J.; Pang, J.; Liu, Y.; Zhang, J.; Zhang, C.; Shen, G.; Song, L. Suppression of RRM2 inhibits cell proliferation, causes cell cycle arrest and promotes the apoptosis of human neuroblastoma cells and in human neuroblastoma RRM2 is suppressed following chemotherapy. Oncol. Rep. 2018, 40, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Amin, A.R.; Wang, D.; Koenig, L.; Nannapaneni, S.; Chen, Z.; Wang, Z.; Sica, G.; Deng, X.; Chen, Z.G.; et al. RRM2 regulates Bcl-2 in head and neck and lung cancers: A potential target for cancer therapy. Clin. Cancer Res. 2013, 19, 3416–3428. [Google Scholar] [CrossRef]
- Gandhi, M.; Groß, M.; Holler, J.M. The lncRNA lincNMR regulates nucleotide metabolism via a YBX1—RRM2 axis in cancer. Nat. Commun. 2020, 11, 3214. [Google Scholar] [CrossRef] [PubMed]
- Quan, D.; Chen, K.; Zhang, J.; Guan, Y.; Yang, D.; Wu, H.; Wu, S.; Lv, L. Identification of lncRNA NEAT1/miR-21/RRM2 axis as a novel biomarker in breast cancer. J. Cell Physiol. 2020, 235, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhong, T.; Wang, M.; Xiang, X.; Ren, G.; Jia, Z.; Lin, Q.; Liu, Q.; Dong, J.; Li, L.; et al. Integrative Analysis Reveals Across-Cancer Expression Patterns and Clinical Relevance of Ribonucleotide Reductase in Human Cancers. Front. Oncol. 2019, 9, 956. [Google Scholar] [CrossRef]
- Morikawa, T.; Maeda, D.; Kume, H.; Homma, Y.; Fukayama, M. Ribonucleotide reductase M2 subunit is a novel diagnostic marker and a potential therapeutic target in bladder cancer. Histopathology 2010, 57, 885–892. [Google Scholar] [CrossRef]
- Duff, D.; Long, A. Roles for RACK1 in cancer cell migration and invasion. Cell Signal. 2017, 35, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.S.; Chiu, Y.C.; Tsai, Y.H.; Tsai, Y.F.; Wang, J.Y.; Tseng, L.M.; Chiu, J.H. Different mechanisms involved in the berberine-induced antiproliferation effects in triple-negative breast cancer cell lines. J. Cell Biochem. 2019, 120, 13531–13544. [Google Scholar] [CrossRef] [PubMed]
- de Heer, E.C.; Jalving, M.; Harris, A.L. HIFs, angiogenesis, and metabolism: Elusive enemies in breast cancer. J. Clin. Investig. 2020, 130, 5074–5087. [Google Scholar] [CrossRef]
- Laughner, E.; Taghavi, P.; Chiles, K.; Mahon, P.C.; Semenza, G.L. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell Biol. 2001, 21, 3995–4004. [Google Scholar] [CrossRef] [Green Version]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Li, J.; Zhong, L.; Wang, F.; Zhu, H. Dissecting the role of AMP-activated protein kinase in human diseases. Acta Pharm. Sin. B 2017, 7, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Lo, H.L.; Tang, W.C.; Hsiao, H.H.; Yang, P.M. A gene expression signature-based approach reveals the mechanisms of action of the Chinese herbal medicine berberine. Sci. Rep. 2014, 4, 6394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.N.; Yang, W.K.; Kim, J.; Kim, H.S.; Kim, E.J.; Yun, H.; Park, H.; Kim, S.S.; Choe, W.; Kang, I.; et al. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis 2008, 29, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Li, X.; Ma, H.; Yang, Q.; Shang, Q.; Song, L.; Zheng, Z.; Zhang, S.; Pan, Y.; Huang, P.; et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic. Biol. Med. 2020, 161, 339–350. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, J.; Lan, M.; Zou, T. Construction and analysis of a joint diagnosis model of random forest and artificial neural network for heart failure. Aging 2020, 12, 26221–26235. [Google Scholar] [CrossRef]
- Huang, N.; Guo, W.; Ren, K.; Li, W.; Jiang, Y.; Sun, J.; Dai, W.; Zhao, W. LncRNA AFAP1-AS1 Supresses miR-139-5p and Promotes Cell Proliferation and Chemotherapy Resistance of Non-small Cell Lung Cancer by Competitively Upregulating RRM2. Front. Oncol. 2019, 9, 1103. [Google Scholar] [CrossRef] [Green Version]
- Du, S.M. The SNHG16/miR-30a axis promotes breast cancer cell proliferation and invasion by regulating RRM2. Neoplasma 2020, 67, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Mai, H.; Zhu, Y.; Li, G.; Sun, J.; Li, G.; Liang, B.; Chen, S. MicroRNA-4500 Inhibits Migration, Invasion, and Angiogenesis of Breast Cancer Cells via RRM2-Dependent MAPK Signaling Pathway. Mol. Ther. Nucleic Acids 2020, 21, 278–289. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhou, W.; Lu, S.; Wu, C.; Wu, Z.; Liu, R.; Li, X.; Wu, J.; Liu, Y.; et al. Identification of Key Genes Associated With the Process of Hepatitis B Inflammation and Cancer Transformation by Integrated Bioinformatics Analysis. Front. Genet. 2021, 12, 654517. [Google Scholar] [CrossRef]
- Wang, Z.; Embaye, K.S.; Yang, Q.; Qin, L.; Zhang, C.; Liu, L.; Zhan, X.; Zhang, F.; Wang, X.; Qin, S. A Novel Metabolism-Related Signature as a Candidate Prognostic Biomarker for Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.C.; Zhang, Y.; Wang, T. High RRM2 expression has poor prognosis in specific types of breast cancer. PLoS ONE 2022, 17, e0265195. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Mahfouz, M.; Habashy, H.O. RRM2 expression in different molecular subtypes of breast cancer and its prognostic significance. Diagn. Pathol. 2022, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.L.; Wu, Z.Z.; Yang, Q.C.; Xiong, H.G.; Xiao, Y.; Li, H.; Sun, Z.J. Overexpression of RRM2 is related to poor prognosis in oral squamous cell carcinoma. Oral Dis. 2021, 27, 204–214. [Google Scholar] [CrossRef]
- Yang, M.; Yao, P.; Lang, X.; Li, X.; Zhang, D. Ribonucleotide reductase subunit M2 promotes proliferation and epithelial-mesenchymal transition via the JAK2/STAT3 signaling pathway in retinoblastoma. Bioengineered 2021, 12, 12800–12811. [Google Scholar] [CrossRef]
- Yan, T.; Li, H.Y.; Wu, J.S.; Niu, Q.; Duan, W.H.; Han, Q.Z.; Ji, W.M.; Zhang, T.; Lv, W. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol. Lett. 2017, 14, 5400–5408. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Zhan, T.; Ke, T.; Huang, X.; Ke, D.; Wang, Q.; Li, H. Increased expression of RRM2 by human papillomavirus E7 oncoprotein promotes angiogenesis in cervical cancer. Br. J. Cancer 2014, 110, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.J.; Zhao, G.Z.; Jiang, R.; He, S.; Xu, H.; He, J.M.; Sun, Y.; Wu, M.N.; Ran, J.H.; Chen, D.L.; et al. Brusatol Inhibits Proliferation and Metastasis of Colorectal Cancer by Targeting and Reversing the RhoA/ROCK1 Pathway. Biomed. Res. Int. 2022, 2022, 7132159. [Google Scholar] [CrossRef]
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Age | 1.97 (1.39–2.81) | 0.00 * | 2.13 (1.47–3.08) | 0.00 * |
| Gender | 0.85 (0.12–6.13) | 0.88 | 0.58 (0.08–4.21) | 0.59 |
| Tumor stage | 2.70 (1.90–3.85) | 0.00 * | 1.88 (1.09–3.24) | 0.02 * |
| T N M RRM2 | 1.87 (1.26–2.78) 2.21 (1.52–3.21) 6.54 (3.67–11.65) 1.10 (0.94–1.28) | 0.00 * 0.00 * 0.00 * 0.25 | 0.94 (0.57–1.57) 1.64 (1.04–2.58) 2.86 (1.49–5.48) 1.17 (1.00–1.37) | 0.81 0.03 * 0.00 * 0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Wei, Q.; Jiang, R.; Luan, T.; He, S.; Lu, R.; Xu, H.; Ran, J.; Li, J.; Chen, D. The Core-Targeted RRM2 Gene of Berberine Hydrochloride Promotes Breast Cancer Cell Migration and Invasion via the Epithelial–Mesenchymal Transition. Pharmaceuticals 2023, 16, 42. https://doi.org/10.3390/ph16010042
He J, Wei Q, Jiang R, Luan T, He S, Lu R, Xu H, Ran J, Li J, Chen D. The Core-Targeted RRM2 Gene of Berberine Hydrochloride Promotes Breast Cancer Cell Migration and Invasion via the Epithelial–Mesenchymal Transition. Pharmaceuticals. 2023; 16(1):42. https://doi.org/10.3390/ph16010042
Chicago/Turabian StyleHe, Jiaming, Qiang Wei, Rong Jiang, Tiankuo Luan, Shuang He, Ruijin Lu, Hang Xu, Jianhua Ran, Jing Li, and Dilong Chen. 2023. "The Core-Targeted RRM2 Gene of Berberine Hydrochloride Promotes Breast Cancer Cell Migration and Invasion via the Epithelial–Mesenchymal Transition" Pharmaceuticals 16, no. 1: 42. https://doi.org/10.3390/ph16010042
APA StyleHe, J., Wei, Q., Jiang, R., Luan, T., He, S., Lu, R., Xu, H., Ran, J., Li, J., & Chen, D. (2023). The Core-Targeted RRM2 Gene of Berberine Hydrochloride Promotes Breast Cancer Cell Migration and Invasion via the Epithelial–Mesenchymal Transition. Pharmaceuticals, 16(1), 42. https://doi.org/10.3390/ph16010042









