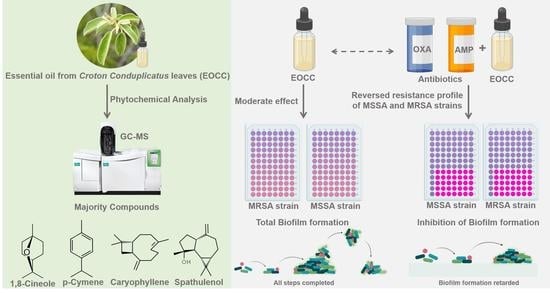
Synergistic and Antibiofilm Effects of the Essential Oil from Croton conduplicatus (Euphorbiaceae) against Methicillin-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterisation of C. conduplicatus Essential Oil
2.2. Antimicrobial Activity of C. conduplicatus Essential Oil
2.3. Synergistic Activity of C. conduplicatus Essential Oil with Oxacillin and Ampicillin against S. aureus
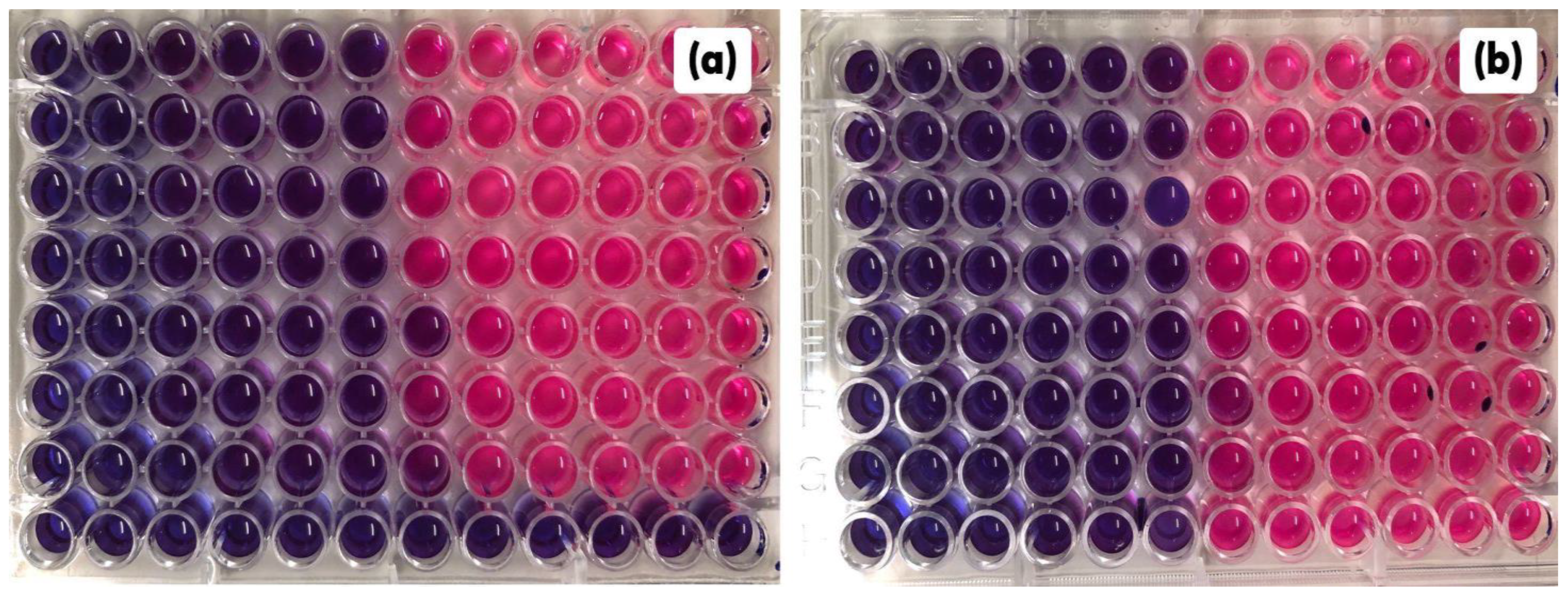
2.4. Antibiofilm Activities of C. conduplicatus Essential Oil
3. Discussion
4. Materials and Methods
4.1. Identification and Harvesting of Plant Material
4.2. Obtaining Plant Drug
4.3. Essential Oil Extraction
4.4. Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
4.5. Antimicrobial Activity of C. conduplicatus Essential Oil
4.5.1. Microbial Strains and Inoculum Standardisation
4.5.2. Antimicrobial Agents
4.5.3. Antimicrobial Screening
4.5.4. Checkerboard Assay against S. aureus
4.5.5. Activity against S. aureus Biofilm Formation
4.5.6. Activity against S. aureus Formed Biofilm
4.5.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 88. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. List of Bacteria for Which New Antibiotics Are Urgently Needed; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Aminov, R. History of antimicrobial drug discovery: Major classes and health impact. Biochem. Pharmacol. 2017, 133, 4–19. [Google Scholar] [CrossRef]
- Shin, J.; Prabhakaran, V.-S.; Kim, K.-s. The multi-faceted potential of plant-derived metabolites as antimicrobial agents against multidrug-resistant pathogens. Microb. Pathog. 2018, 116, 209–214. [Google Scholar] [CrossRef]
- Mérillon, J.-M.; Riviere, C. Natural Antimicrobial Agents; Springer: Berlin/Heidelberg, Germany, 2018; Volume 19. [Google Scholar]
- Sreepian, A.; Popruk, S.; Nutalai, D.; Phutthanu, C.; Sreepian, P.M. Antibacterial Activities and Synergistic Interaction of Citrus Essential Oils and Limonene with Gentamicin against Clinically Isolated Methicillin-Resistant Staphylococcus aureus. Sci. World J. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics 2022, 11, 146. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef] [Green Version]
- Cartaxo, S.L.; de Almeida Souza, M.M.; de Albuquerque, U.P. Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J. Ethnopharmacol. 2010, 131, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Júnior, R.G.; Ferraz, C.A.A.; Silva, J.C.; de Andrade Teles, R.B.; Silva, M.G.; Diniz, T.C.; Dos Santos, U.S.; de Souza, A.V.V.; Nunes, C.E.P.; Salvador, M.J.; et al. Neuropharmacological effects of essential oil from the leaves of Croton conduplicatus Kunth and possible mechanisms of action involved. J. Ethnopharmacol. 2018, 221, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Wei, C.; Zhang, C.; Han, C.; Kuchkarova, N.; Shao, H. Chemical Composition, Phytotoxic, Antimicrobial and Insecticidal Activity of the Essential Oils of Dracocephalum integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Custódio, L.; Vitalini, S.; Iriti, M.; Hassani, L. A Comparative Study of the in Vitro Antimicrobial and Synergistic Effect of Essential Oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with Antimicrobial Drugs: New Approach for Health Promoting Products. Antibiotics 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa, C.; Liu, J.; Dong, Y.; Jiang, L.; Gentana, G.; Wurita, A. Quantification of eucalyptol (1,8-cineole) in rat serum by gas chromatography-mass/mass spectrometry and its application to a rat pharmacokinetic study. Biomed. Chromatogr. 2021, 35, 1–10. [Google Scholar] [CrossRef]
- Agreles, M.A.A.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. The Role of Essential Oils in the Inhibition of Efflux Pumps and Reversion of Bacterial Resistance to Antimicrobials. Curr. Microbiol. 2021, 78, 3609–3619. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured publishing corporation Carol Stream: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- De Oliveira Júnior, R.G.; Ferraz, C.A.A.; Silva, J.C.; De Oliveira, A.P.; Diniz, T.C.; E Silva, M.G.; Quintans Júnior, L.J.; De Souza, A.V.V.; Dos Santos, U.S.; Turatti, I.C.C.; et al. Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae). Molecules 2017, 22, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, J.; Souza, A.V.; Oliveira, A.P.; Santos, U.; Souza, M.; Bispo, L.; Turatti, Z.C.; Lopes, N. Chemical Composition of Essential Oils from Croton conduplicatus (Euphorbiaceae) in Two Different Seasons. J. Essent. Oil-Bear. Plants 2014, 17, 1137–1145. [Google Scholar] [CrossRef]
- Castro, K.N.d.C.; Chagas, A.C.d.S.; Costa-Júnior, L.M.; Canuto, K.M.; Brito, E.S.d.; Rodrigues, T.H.S.; de Andrade, I.M. Acaricidal potential of volatile oils from Croton species on Rhipicephalus microplus. Rev. Bras. Farmacogn. 2020, 29, 811–815. [Google Scholar] [CrossRef]
- Munusamy, K.; Vadivelu, J.; Tay, S.T. A study on Candida biofilm growth characteristics and its susceptibility to aureobasidin A. Rev. Iberoam. Micol. 2018, 35, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Costa-Lima, T.C.; Souza, A.V.V.; Gonçalves-Gervásio, R.d.C.R. Essential oils activity from plants of the Brazilian Caatinga on the vegetable leafminer. Pesqui. Agropecu. Trop. 2020, 50, 1–8. [Google Scholar] [CrossRef]
- de Araújo, L.G.; Veras Neto, J.G.; de Oliveira Alves, J.V.; de Veras, B.O.; Vanusa da Silva, M.; Bacalhau Rodrigues, J.F.; Fook, M.V.L.; Amoah, S.K.S.; Menezes Torres, M.d.C. Chemodiversity and antibacterial activity of the essential oil of leaves of Croton argyrophyllus Kunth. Chem. Biodivers. 2020, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. M100-Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, IL, USA, 2017; p. 249. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.J.C. Composição química e atividade antibacteriana dos óleos essenciais de Croton urticifolius LAM. e Croton adamantinus MÜLL. ARG. (EUPHORBIACEAE). Dissertation, State University of Paraiba, 2021. Available online: https://repositorio.ufpe.br/handle/123456789/25510 (accessed on 13 December 2022).
- Rocha, R.R. Estudo comparativo sobre a composição química, atividade antibacteriana e efeito sinérgico dos óleos essenciais de Croton tetradenius Baill. e c. pulegiodorus Baill. Contra isolados de Staphylococcus aureus. Dissertation, Federal University of Ceará, 2020. Available online: https://repositorio.ufpb.br/jspui/handle/tede/4016 (accessed on 13 December 2022).
- Valarezo, E.; Gaona-Granda, G.; Morocho, V.; Cartuche, L.; Calva, J.; Meneses, M.A. Chemical Constituents of the Essential Oil from Ecuadorian Endemic Species Croton ferrugineus and Its Antimicrobial, Antioxidant and α-Glucosidase Inhibitory Activity. Molecules 2021, 26, 4608. [Google Scholar] [CrossRef] [PubMed]
- Cucho-Medrano, J.L.L.; Mendoza-Beingolea, S.W.; Fuertes-Ruitón, C.M.; Salazar-Salvatierra, M.E.; Herrera-Calderon, O. Chemical Profile of the Volatile Constituents and Antimicrobial Activity of the Essential Oils from Croton adipatus, Croton thurifer, and Croton collinus. Antibiotics 2021, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.M.B.; Chaves, F.C.M.; Almeida, C.A.; Bizzo, H.R.; Duarte, R.S.; Campos-Takaki, G.M.; Alviano, C.S.; Alviano, D.S. Antioxidant and Antimicrobial Activities of 7-Hydroxy-calamenene-Rich Essential Oils from Croton cajucara Benth. Molecules 2013, 18, 1128–1137. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.-H.; Zhao, J.; Zhang, W.-D.; Ma, B.-L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef] [Green Version]
- Nafis, A.; Elhidar, N.; Oubaha, B.; Samri, S.E.; Niedermeyer, T.; Ouhdouch, Y.; Hassani, L.; Barakate, M. Screening for Non-polyenic Antifungal Produced by Actinobacteria from Moroccan Habitats: Assessment of Antimycin A19 Production by Streptomyces albidoflavus AS25. Int. J. Mol. Cell. Med. 2018, 7, 133–145. [Google Scholar]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.; Sanglard, D.; Soković, M. Camphor and Eucalyptol-Anticandidal Spectrum, Antivirulence Effect, Efflux Pumps Interference and Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef]
- Moo, C.-L.; Osman, M.A.; Yang, S.-K.; Yap, W.-S.; Ismail, S.; Lim, S.-H.-E.; Chong, C.-M.; Lai, K.-S. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.Y.; Fu, L.Y.; Long, Q.D.; Shen, X.C. 1,8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Pruss, A.; Wojciuk, B.; Dołęgowska, B.; Wajs-Bonikowska, A.; Sienkiewicz, M.; Mężyńska, M.; Łopusiewicz, Ł. The Influence of Essential Oil Compounds on Antibacterial Activity of Mupirocin-Susceptible and Induced Low-Level Mupirocin-Resistant MRSA Strains. Molecules 2019, 24, 3105. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, K.; Manigandan, V.; Jeyapragash, D.; Bharathidasan, V.; Anandharaj, B.; Sathya, M. Eucalyptol inhibits biofilm formation of Streptococcus pyogenes and its mediated virulence factors. J. Med. Microbiol. 2020, 69, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Keymaram, M.; Falahati, M.; Farahyar, S.; Lotfali, E.; Abolghasemi, S.; Mahmoudi, S.; Sadeghi, F.; Khalandi, H.; Ghasemi, R.; Shamsaei, S.; et al. Anti-biofilm properties of eucalyptol in combination with antifungals against Candida albicans isolates in patients with hematological malignancy. Arch. Microbiol. 2022, 204, 1–8. [Google Scholar]
- Vazquez, N.M.; Mariani, F.; Torres, P.S.; Moreno, S.; Galvan, E.M. Cell death and biomass reduction in biofilms of multidrug resistant extended spectrum β-lactamase-producing uropathogenic Escherichia coli isolates by 1, 8-cineole. PLoS ONE 2020, 15, e0241978. [Google Scholar] [CrossRef]
- Karuppiah, V.; Thirunanasambandham, R.; Thangaraj, G. Anti-quorum sensing and antibiofilm potential of 1,8-cineole derived from Musa paradisiaca against Pseudomonas aeruginosa strain PAO1. World J. Microbiol. Biotechnol. 2021, 37, 1–12. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Gagliano Candela, R.; Maggi, F. Chemical composition of the essential oil of Elaeoselinum asclepium (L.) Bertol subsp. meoides (Desf.) Fiori (Umbelliferae) collected wild in Central Sicily and its antimicrobial activity. Nat. Prod. Res. 2022, 36, 789–797. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Veras, I.N.d.S.; Vasconcelos, M.A.d.; Andrade, A.L.; dos Santos, H.S.; Bandeira, P.N.; Souza, E.B.d.; Albuquerque, M.R.J.R.; Teixeira, E.H. Chemical composition determination and evaluation of the antibacterial activity of essential oils from Ruellia asperula (Mart. Ex Ness) Lindau and Ruellia paniculata L. against oral Streptococci. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can Eucalyptol Replace Antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef]
- Li, H.; Yang, T.; Li, F.Y.; Yao, Y.; Sun, Z.M. Antibacterial activity and mechanism of action of Monarda punctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int J. Clin. Exp. Pathol. 2014, 7, 7389–7398. [Google Scholar]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Griensven, L.J.L.D.v. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-G.; Peng, W.; Yi, J.; Wu, Y.-B.; Chen, T.-Q.; Wong, K.-H.; Wu, J.-Z. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J. Ethnopharmacol. 2014, 154, 198–205. [Google Scholar] [CrossRef]
- Salinas, C.; Florentín, G.; Rodríguez, F.; Alvarenga, N.; Guillén, R. Terpenes Combinations Inhibit Biofilm Formation in Staphyloccocus aureus by Interfering with Initial Adhesion. Microorganisms 2022, 10, 1527. [Google Scholar] [CrossRef]
- Tadić, V.; Oliva, A.; Božović, M.; Cipolla, A.; De Angelis, M.; Vullo, V.; Garzoli, S.; Ragno, R. Chemical and Antimicrobial Analyses of Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, an Endemic of the Western Balkan. Molecules 2017, 22, 1395. [Google Scholar] [CrossRef] [Green Version]
- Aziz, P.; Muhammad, N.; Intisar, A.; Abid, M.A.; Din, M.I.; Yaseen, M.; Kousar, R.; Aamir, A.; Quratulain; Ejaz, R. Constituents and antibacterial activity of leaf essential oil of Plectranthus scutellarioides. Plant Biosyst. 2021, 155, 1247–1252. [Google Scholar] [CrossRef]
- Reynolds, P.E.; Brown, D.F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985, 192, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [PubMed]
- (CLSI), C.L.S.I. M07-Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 2018, p. 91. Available online: https://clsi.org/media/1928/m07ed11_sample.pdf (accessed on 13 December 2022).
- (CLSI), C.L.S.I. M27-Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 2017, 4th Edition, 34. Available online: https://clsi.org/media/1897/m27ed4_sample.pdf (accessed on 13 December 2022).
- Lorian, V. Antibiotics in Laboratory Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Kim, S.-I.; Lee, J. Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling 2017, 33, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Pierce, C.G.; López-Ribot, J.L. Candida albicans biofilm formation and its clinical consequences. Future Microbiol. 2009, 4, 1235–1237. [Google Scholar] [CrossRef] [PubMed]







| No | Compounds a | RILit b | RICalc c | RT (min) | Area (%) | No | Compounds a | RILit b | RICalc c | RT (min) | Area (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-Tricyclene | 926 | 952 | 7.02 | 0.09 | 37 | α-Cubebene | 1348 | 1340 | 17.33 | 1.19 |
| 2 | α-Thujene | 930 | 955 | 7.08 | 0.57 | 38 | β-Bourbonene | 1388 | 1350 | 17.61 | 0.20 |
| 3 | α-Pinene | 939 | 960 | 7.22 | 4.93 | 39 | β–Elemene | 1390 | 1355 | 17.74 | 1.92 |
| 4 | 1-Ethylbutyl Hydroperoxide d | - | 966 | 7.38 | 0.21 | 40 | Caryophyllene | 1419 | 1397 | 18.85 | 9.73 |
| 5 | Camphene | 954 | 970 | 7.50 | 0.73 | 41 | β-Copaene | 1432 | 1409 | 19.16 | 0.31 |
| 6 | Sabinene | 975 | 986 | 7.92 | 1.25 | 42 | cis-Eudesma-6,11-diene | 1477 | 1424 | 19.56 | 0.62 |
| 7 | β-Pinene | 979 | 990 | 8.02 | 2.77 | 43 | α-Caryophyllene | 1454 | 1442 | 20.05 | 1.77 |
| 8 | β-Myrcene | 990 | 997 | 8.21 | 0.33 | 44 | Alloaromadedrene | 1460 | 1448 | 20.20 | 1.99 |
| 9 | 2,3-dihydro-1,8-cineole | 991 | 999 | 8.27 | 0.05 | 45 | γ-Gurjunene | 1477 | 1466 | 20.68 | 0.27 |
| 10 | α-Phellandrene | 1002 | 1012 | 8.60 | 3.08 | 46 | Germacrene D | 1485 | 1474 | 20.90 | 2.64 |
| 11 | α-Terpinene | 1017 | 1021 | 8.85 | 0.20 | 47 | β-Selinene | 1490 | 1484 | 21.17 | 0.87 |
| 12 | p-Cymene | 1024 | 1028 | 9.04 | 10.68 | 48 | Bicyclogermacrene | 1500 | 1493 | 21.40 | 3.40 |
| 13 | D-Limonene | 1029 | 1033 | 9.16 | 1.51 | 49 | α-Muurolene | 1500 | 1496 | 21.48 | 0.72 |
| 14 | β-Thujene d | - | 1034 | 9.20 | 0.69 | 50 | Eremophila-1(10),8,11-triene d | - | 1502 | 21.65 | 0.16 |
| 15 | 1,8-Cineole | 1031 | 1037 | 9.26 | 13.15 | 51 | Germacrene A | 1509 | 1507 | 21.77 | 0.42 |
| 16 | β-cis-Ocimene | 1037 | 1048 | 9.55 | 0.18 | 52 | γ-Cadiene | 1513 | 1513 | 21.95 | 0.43 |
| 17 | γ-Terpinene | 1059 | 1060 | 9.89 | 0.40 | 53 | δ-Cadinene d | - | 1520 | 22.13 | 1.04 |
| 18 | Cis-Sabinene hydrate | 1070 | 1073 | 10.22 | 0.18 | 54 | β-Calacorene | 1545 | 1548 | 22.86 | 0.48 |
| 19 | Isoterpinolene | 1088 | 1087 | 10.59 | 0.16 | 55 | Ciclohexane,1,3-diisopropenyl-6-methyl d | - | 1554 | 23.04 | 1.34 |
| 20 | Linalool | 1096 | 1099 | 10.91 | 2.39 | 56 | Germacrene B | 1561 | 1569 | 23.43 | 0.63 |
| 21 | cis-4-Thujanol | 1098 | 1101 | 10.98 | 0.18 | 57 | Cis-α-Copaene-8-ol d | - | 1574 | 23.56 | 0.51 |
| 22 | Cis-p-Menth-2-en-1-ol | 1121 | 1122 | 11.54 | 0.17 | 58 | Spathulenol | 1578 | 1591 | 24.02 | 6.36 |
| 23 | Trans-pinocarveol | 1139 | 1139 | 11.98 | 0.33 | 59 | Ledol | 1602 | 1622 | 24.85 | 0.50 |
| 24 | (+)-Camphor | 1146 | 1142 | 12.07 | 0.75 | 60 | Humulene epoxide II | 1608 | 1629 | 25.02 | 0.43 |
| 25 | Pinocarvone | 1164 | 1155 | 12.41 | 0.45 | 61 | β-Guayene d | - | 1635 | 25.19 | 0.44 |
| 26 | Terpineol <cis-dihydro-a-> | 1164 | 1161 | 12.57 | 0.23 | 62 | γ-Maaliene d | - | 1643 | 25.39 | 0.12 |
| 27 | Borneol | 1165 | 1163 | 12.63 | 0.67 | 63 | Epicubebol d | - | 1648 | 25.53 | 0.26 |
| 28 | Terpinen-4-ol | 1177 | 1170 | 12.81 | 1.44 | 64 | β-Spathulenol d | 1578 | 1655 | 25.73 | 0.60 |
| 29 | p-Cymen-8-ol | 1182 | 1174 | 12.92 | 0.19 | 65 | Bicyclo[7.2.0]undecan-3-ol, 11,11-dimethyl-4,8-bis(methylene)- d | - | 1660 | 25.85 | 0.34 |
| 30 | α-Terpineol | 1188 | 1181 | 13.11 | 1.96 | 66 | 10-epi-α –Cadinol | 1640 | 1665 | 25.99 | 0.88 |
| 31 | Cis-sabinol d | - | 1188 | 13.29 | 0.40 | 67 | α-Muurolol | 1646 | 1670 | 26.13 | 0.15 |
| 32 | Cis-piperitol | 1196 | 1192 | 13.40 | 0.09 | 68 | Epi-α-Muurolol | 1642 | 1681 | 26.40 | 0.72 |
| 33 | β-Sabinyl Acetate d | - | 1216 | 14.04 | 0.22 | 69 | Xantoxyline | 1668 | 1690 | 26.65 | 0.65 |
| 34 | Bornyl acetate | 1288 | 1250 | 14.94 | 0.36 | 70 | (1R,7S,E)-7-isopropyl-4,10-dimethylene-cyclodec-5-enol | 1686 | 1713 | 27.26 | 0.14 |
| 35 | Thymol | 1290 | 1260 | 15.21 | 0.53 | 71 | NI e | - | 1762 | 28.58 | 0.14 |
| 36 | α-Longipinene | 1352 | 1333 | 17.15 | 0.34 | Total identified | 95.94 |
| Peak | Compounds | RT (min) | % GC-MS |
|---|---|---|---|
| 1 | Tricyclene | 8.447 | 0.08 |
| 2 | α-Thujene | 8.726 | 0.50 |
| 3 | α-Pinene | 8.927 | 2.30 |
| 4 | Camphene | 9.465 | 0.49 |
| 5 | Sabinene | 10.521 | 1.46 |
| 6 | α-Phellandrene | 11.731 | 1.44 |
| 7 | p-Cymene | 12.574 | 12.41 |
| 8 | 1,8-Cineole | 12.792 | 21.42 |
| 9 | NI | 13.694 | 0.07 |
| 10 | γ-Terpinene | 13.942 | 0.14 |
| 11 | Terpinolene | 15.087 | 0.05 |
| 12 | (E)-Sabinene | 15.569 | 0.03 |
| 13 | NI | 15.716 | 0.13 |
| 14 | (Z)-p-Menth-2-en-1-ol | 16.402 | 0.16 |
| 15 | α-Campholenal | 16.535 | 0.01 |
| 16 | (E)-Pinocarveol | 16.977 | 0.18 |
| 17 | Camphor | 17.117 | 0.32 |
| 18 | Pinocarvone | 17.842 | 0.09 |
| 19 | Borneol | 17.989 | 0.52 |
| 20 | NI | 18.130 | 0.05 |
| 21 | Terpinen-4-ol | 18.417 | 2.28 |
| 22 | α-Terpineol | 19.001 | 0.60 |
| 23 | Isobornyl acetate | 22.236 | 0.32 |
| 24 | α-Copaene | 25.167 | 0.20 |
| 25 | β-Bourbonene | 25.450 | 0.21 |
| 26 | β-Elemene | 25.713 | 0.34 |
| 27 | (E)-Caryophylene | 26.560 | 7.52 |
| 28 | α-Humulene | 27.613 | 1.55 |
| 29 | Alloaromadendrene | 27.841 | 1.69 |
| 30 | Germacrene D | 28.473 | 0.31 |
| 31 | β-Selinene | 28.628 | 0.32 |
| 32 | Bicyclogermacrene | 28.955 | 1.61 |
| 33 | δ-Amorphene | 29.488 | 0.58 |
| 34 | δ-Cadinene | 29.776 | 0.53 |
| 35 | α-Calacorene | 30.355 | 0.14 |
| 36 | NI | 30.611 | 0.32 |
| 37 | Spathulenol | 34.413 | 15.47 |
| 38 | Caryophyllene oxide | 31.541 | 12.15 |
| 39 | Ledol | 32.105 | 1.50 |
| 40 | Humulene epoxide | 32.265 | 1.42 |
| 41 | Cubenol | 32.450 | 0.20 |
| 42 | Acorenol | 32.826 | 0.25 |
| 43 | NI | 32.966 | 0.44 |
| 44 | NI | 33.069 | 1.19 |
| 45 | Epi-α-Cadinol | 33.185 | 4.34 |
| 46 | α-Muurolol | 33.352 | 0.50 |
| 47 | β-Eudesmol | 33.449 | 0.51 |
| 48 | α-Cadinol | 33.578 | 1.02 |
| 49 | NI | 33.881 | 0.45 |
| 50 | NI | 35.751 | 0.15 |
| Total identified | 97.2 | ||
| Microrganisms | MIC/MBC or MFC (µg mL−1) | ||||
|---|---|---|---|---|---|
| EOCC | AMP | OXA | POL | ANF | |
| S. aureus ATCC 25923 (MSSA) | 256/512 | 2/4 | 2/4 | - | - |
| S. aureus ATCC 33591 (MRSA) | 512/1024 | 16/32 | 32/64 | - | - |
| E. coli ATCC 25922 | na | nd | - | - | - |
| P. aeruginosa ATCC 27853 | na | - | - | 1/1 | - |
| C. albicans ATCC 10231 | na | - | - | - | 0.5/1 |
| S. aureus | Combination | Individual MIC (µg mL−1) | Combined MIC (µg mL−1) | Individual FIC | FIC Index (FICi) | MIC Reduction (%) | Combination Effect |
|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 (MSSA) | OXA/EOCC | 2/256 | 0.5/16 | 0.25/0.0625 | 0.3125 | 75.0/93.75 | Synergistic |
| AMP/EOCC | 2/256 | 0.25/16 | 0.125/0.0625 | 0.1875 | 87.5/93.75 | Synergistic | |
| S. aureus ATCC 33591 (MRSA) | OXA/EOCC | 32/512 | 1/32 | 0.0313/0.0625 | 0.0938 | 96.9/93.75 | Synergistic |
| AMP/EOCC | 16/512 | 0.5/32 | 0.0313/0.0625 | 0.0938 | 96.9/93.75 | Synergistic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.D.d.; Rocha, W.R.V.d.; Rodrigues, J.F.B.; Alves, H.d.S. Synergistic and Antibiofilm Effects of the Essential Oil from Croton conduplicatus (Euphorbiaceae) against Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 55. https://doi.org/10.3390/ph16010055
Oliveira GDd, Rocha WRVd, Rodrigues JFB, Alves HdS. Synergistic and Antibiofilm Effects of the Essential Oil from Croton conduplicatus (Euphorbiaceae) against Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals. 2023; 16(1):55. https://doi.org/10.3390/ph16010055
Chicago/Turabian StyleOliveira, Genil Dantas de, Wilma Raianny Vieira da Rocha, José Filipe Bacalhau Rodrigues, and Harley da Silva Alves. 2023. "Synergistic and Antibiofilm Effects of the Essential Oil from Croton conduplicatus (Euphorbiaceae) against Methicillin-Resistant Staphylococcus aureus" Pharmaceuticals 16, no. 1: 55. https://doi.org/10.3390/ph16010055
APA StyleOliveira, G. D. d., Rocha, W. R. V. d., Rodrigues, J. F. B., & Alves, H. d. S. (2023). Synergistic and Antibiofilm Effects of the Essential Oil from Croton conduplicatus (Euphorbiaceae) against Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals, 16(1), 55. https://doi.org/10.3390/ph16010055