Possible Implication of Nrf2, PPAR-γ and MAPKs Signaling in the Protective Role of Mangiferin against Renal Ischemia/Reperfusion in Rats
Abstract
1. Introduction
2. Results
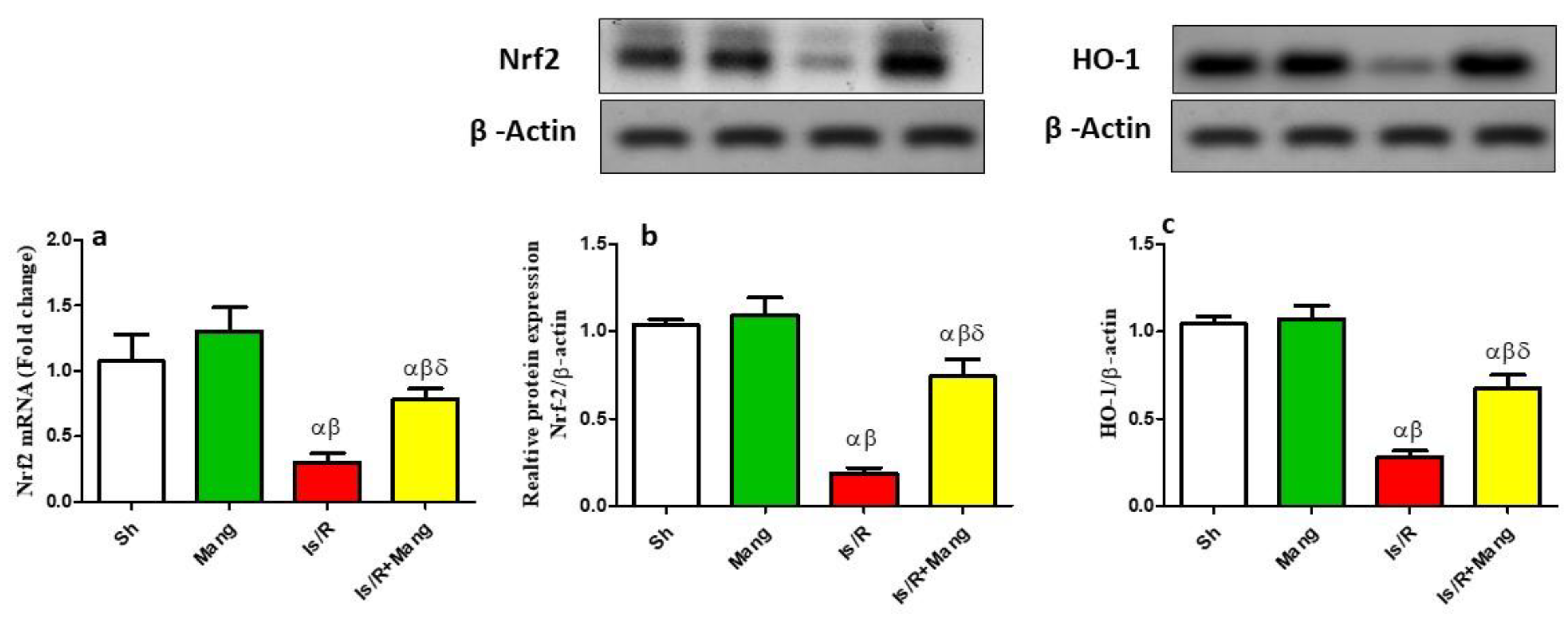
2.1. Impact of Mang on the Expression of Nrf2 and HO-1 Genes
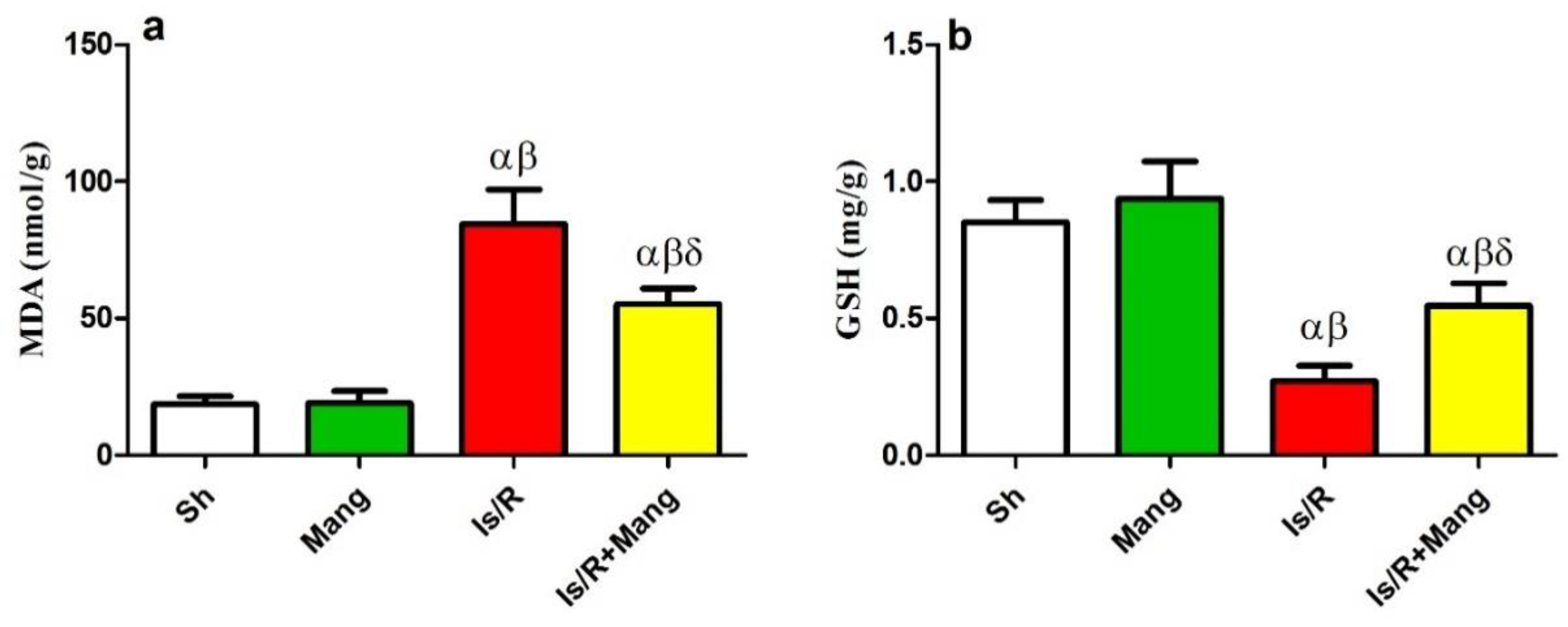
2.2. Impact of Mang on Oxidative Stress Biomarkers
2.3. Impact of Mang on Nitrosative Stress Parameters
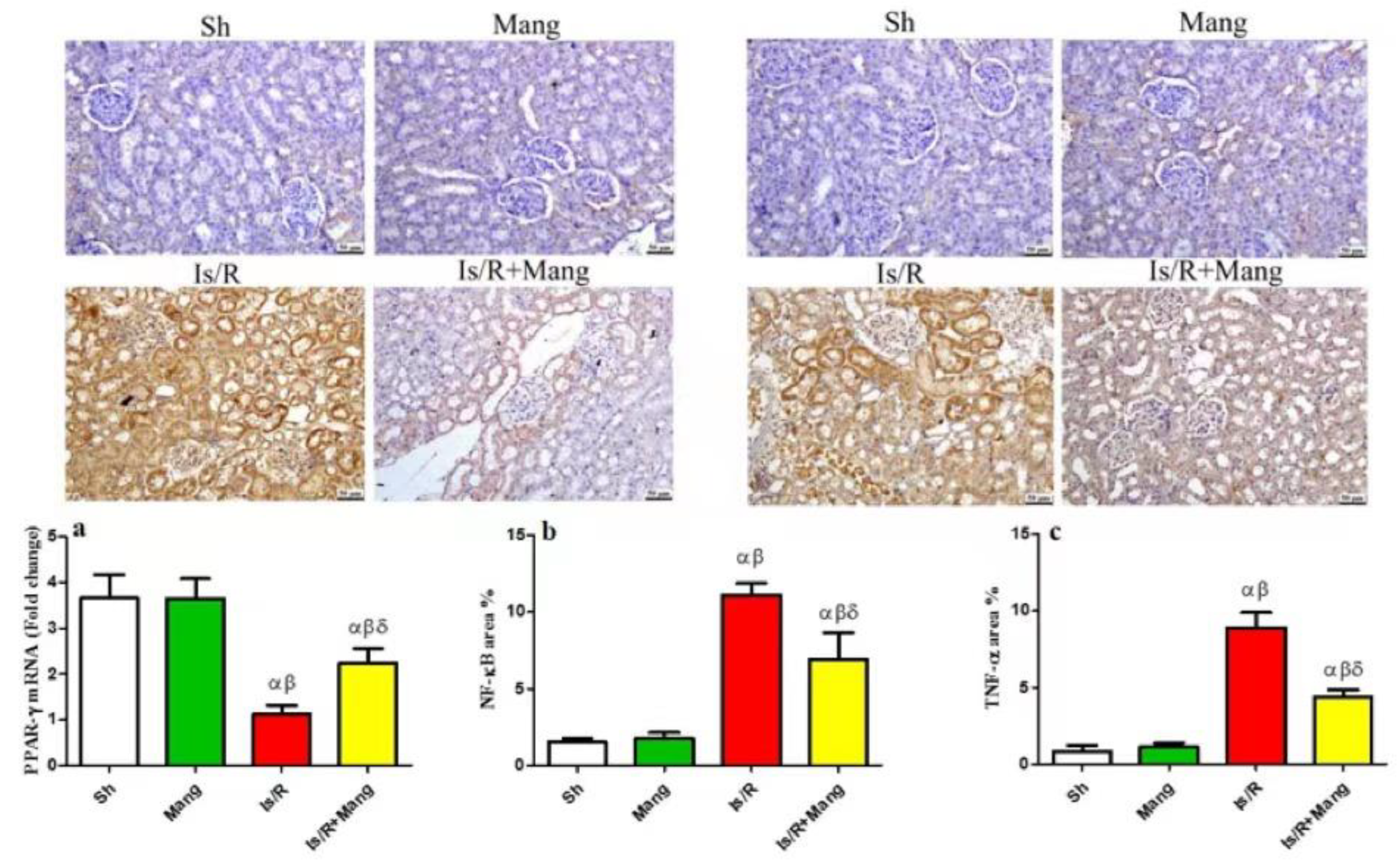
2.4. Impact of Mang on PPAR- γ, NF-κB p65, and TNF-α Expression
2.5. Impact of Mang on p38 MAPK and JNK Proteins Expression
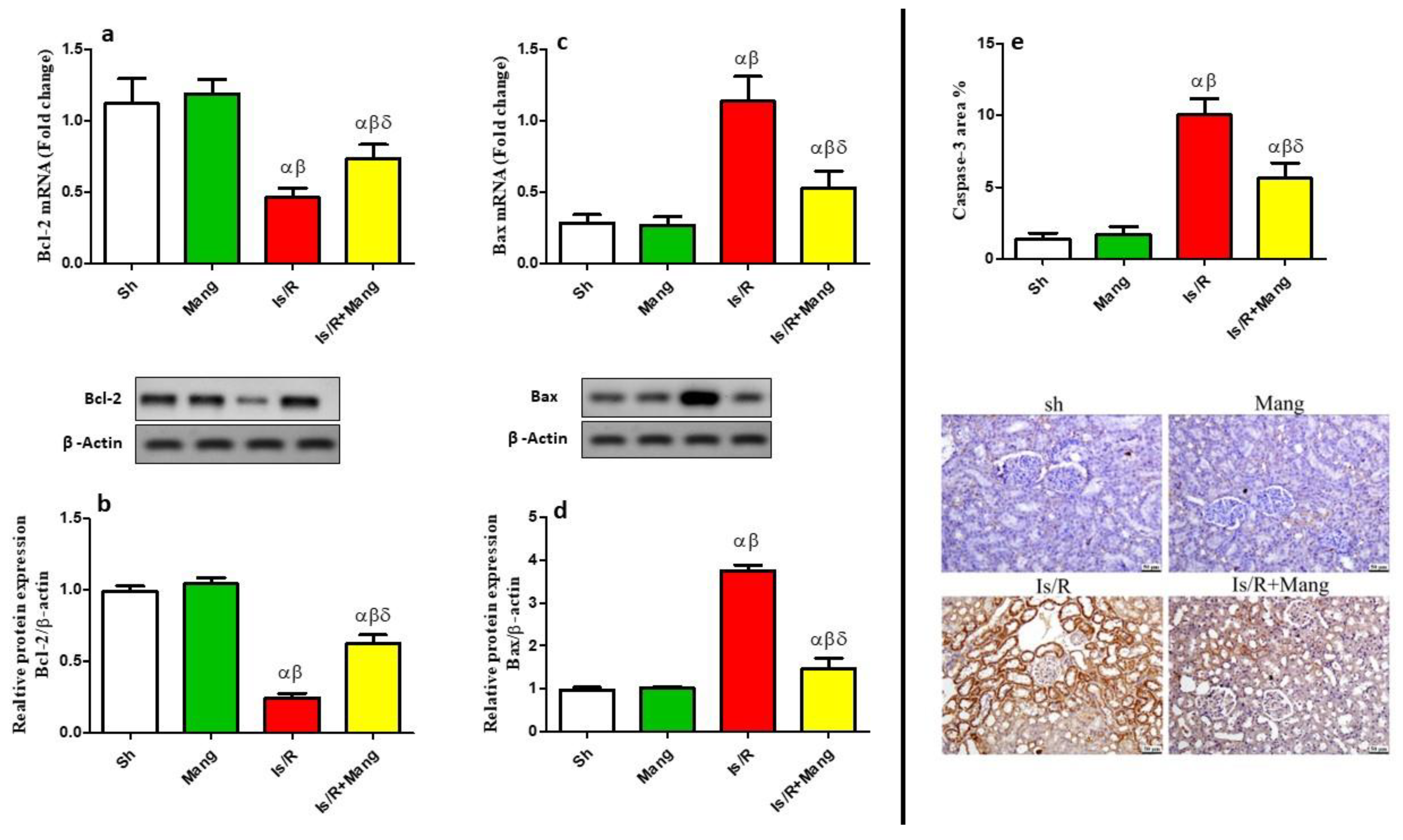
2.6. Impact of Mang on Apoptotic/Anti-Apoptotic Biomarkers
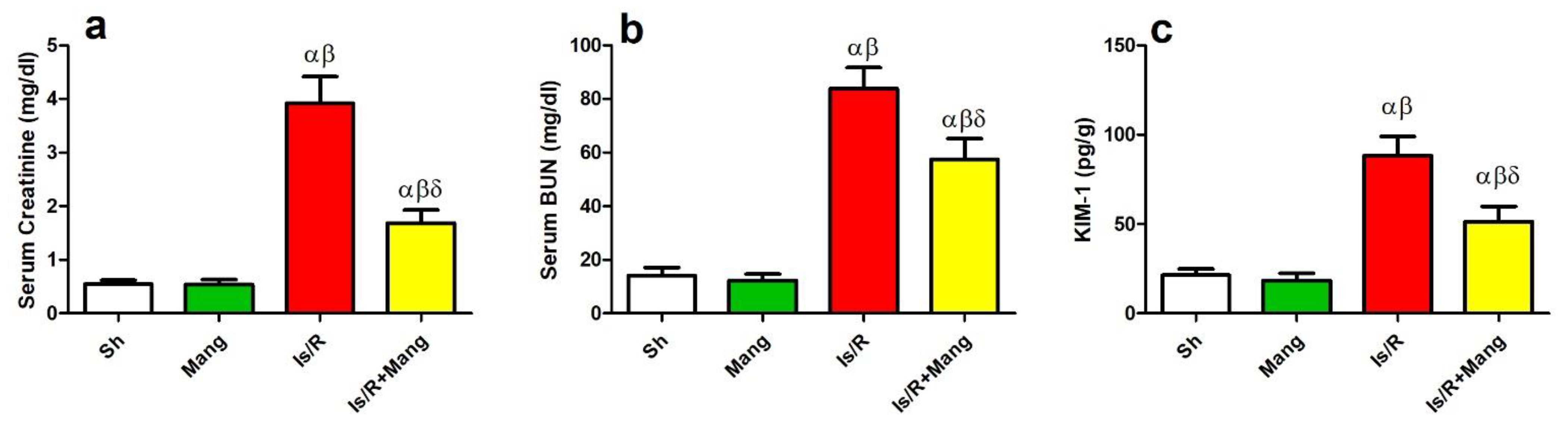
2.7. Impact of Mang on Biomarkers of Renal Function
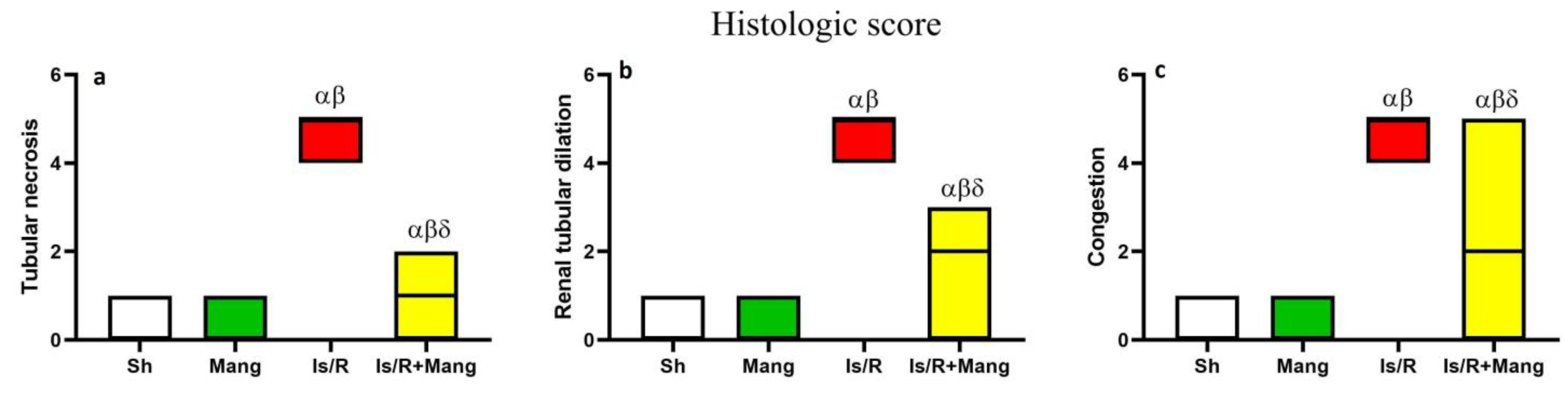
2.8. Impact of Mang on Renal Morphological Changes and Lesion Score
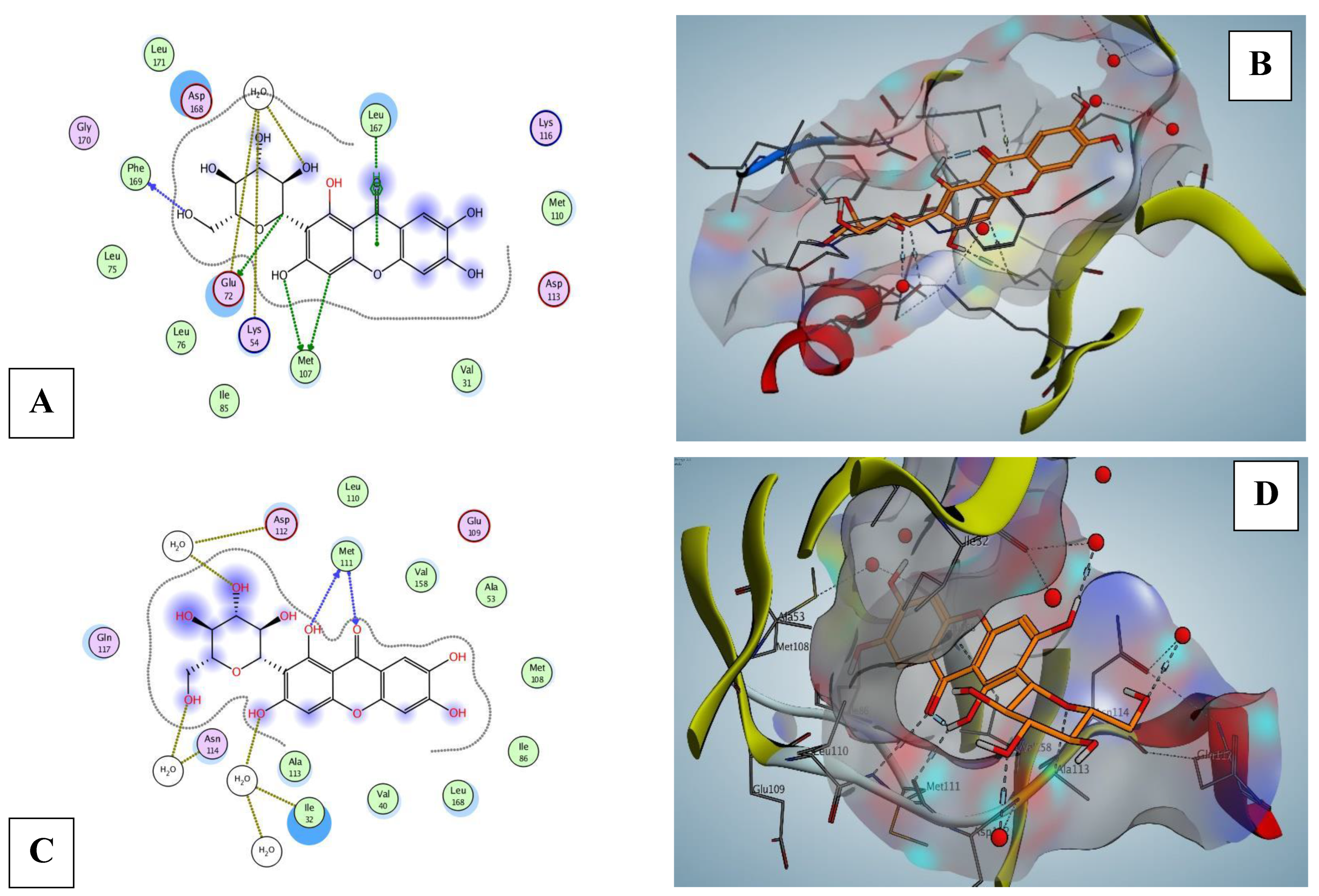
2.9. Mang Molecular Docking of Nrf2, p38 MPAK, and JNK Proteins
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Experimental Design
4.3. Renal Ischemia/Reperfusion Induction
4.4. Sample Preparation
4.5. Biochemical and ELISA Assay
4.6. Western Blot
4.7. qRT-PCR Technique
4.8. Renal Morphology and Lesion Score
4.9. Renal Immunohistochemistry
4.10. Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiva, N.; Sharma, N.; Kulkarni, Y.; Mulay, S.; Gaikwad, A. Renal ischemia/reperfusion injury: An insight on in vitro and in vivo models. Life Sci. 2020, 256, 117860. [Google Scholar] [CrossRef] [PubMed]
- El-Mokadem, B.; El-Abhar, H.; Abdallah, D.; Awad, A.; Soubh, A. Epac-1/Rap-1 signaling pathway orchestrates the reno-therapeutic effect of ticagrelor against renal ischemia/reperfusion model. Biomed. Pharmacother. 2021, 139, 111488. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Abdallah, D.; El-Abhar, H. The dual reno-and neuro-protective effects of dimethyl fumarate against uremic encephalopathy in a renal ischemia/reperfusion model. Pharmacol. Rep. 2020, 72, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.; Pischke, S.; Berger, S.; Sanders, J.; Pol, R.; Struys, M.; Ploeg, R.; Leuvenink, H. Ischemia and reperfusion injury in kidney transplantation: Relevant mechanisms in injury and repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Hue, M.; Rayego-Mateos, S.; Vázquez-Carballo, C.; Palomino-Antolín, A.; García-Caballero, C.; Opazo-Rios, L.; Morgado-Pascual, J.; Herencia, C.; Mas, S.; Ortiz, A. Protective role of Nrf2 in renal disease. Antioxidants 2020, 10, 39. [Google Scholar] [CrossRef]
- Hejazian, S.; Khatibi, S.; Barzegari, A.; Pavon-Djavid, G.; Soofiyani, S.; Hassannejhad, S.; Ahmadian, E.; Ardalan, M.; Vahed, S. Nrf-2 as a therapeutic target in acute kidney injury. Life Sci. 2021, 264, 118581. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, C.; Wang, X.; Hu, H. PPARγ in ischemia-reperfusion injury: Overview of the biology and therapy. Front. Pharmacol. 2021, 12, 600618. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsourouflis, G.; Theocharis, S. Peroxisome proliferator-activated receptor-γ (PPAR-γ) ligands: Novel pharmacological agents in the treatment of ischemia reperfusion injury. Curr. Mol. Med. 2008, 8, 562–579. [Google Scholar] [CrossRef]
- Fawzy, M.; Maher, S.; Bakkar, S.; El-Rehany, M.; Fathy, M. Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)–NF-κB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2021, 22, 10669. [Google Scholar] [CrossRef]
- Sun, W.; Choi, H.; Kim, C.; Bae, E.; Ma, S.; Kim, S. Maslinic Acid Attenuates Ischemia/Reperfusion-Induced Acute Kidney Injury by Suppressing Inflammation and Apoptosis Through Inhibiting NF-κB and MAPK Signaling Pathway. Front. Pharmacol. 2022, 13, 807452. [Google Scholar] [CrossRef]
- Priante, G.; Gianesello, L.; Ceol, M.; Del Prete, D.; Anglani, F. Cell death in the kidney. Int. J. Mol. Sci. 2019, 20, 3598. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Ma, H.; Chen, X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021, 149, 111997. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-T.; Wang, Z.-Z.; Yuan, Y.-H.; Sun, H.-M.; Chen, N.-H.; Zhang, Y. Mangiferin: A multipotent natural product preventing neurodegeneration in Alzheimer’s and Parkinson’s disease models. Pharmacol. Res. 2019, 146, 104336. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wan, J.; Gong, X.; Kuang, G.; Cheng, X.; Min, S. Mangiferin attenuates renal ischemia-reperfusion injury by inhibiting inflammation and inducing adenosine production. Int. Immunopharmacol. 2015, 25, 148–154. [Google Scholar] [CrossRef]
- Aragno, M.; Cutrin, J.; Mastrocola, R.; Perrelli, M.-G.; Restivo, F.; Poli, G.; Danni, O.; Boccuzzi, G. Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: Attenuation by dehydroepiandrosterone. Kidney Int. 2003, 64, 836–843. [Google Scholar] [CrossRef]
- Bayrak, O.; Uz, E.; Bayrak, R.; Turgut, F.; Atmaca, A.; Sahin, S.; Yıldırım, M.; Kaya, A.; Cimentepe, E.; Akcay, A. Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J. Urol. 2008, 26, 285–291. [Google Scholar] [CrossRef]
- Granata, S.; Votrico, V.; Spadaccino, F.; Catalano, V.; Netti, G.; Ranieri, E.; Stallone, G.; Zaza, G. Oxidative Stress and Ischemia/Reperfusion Injury in Kidney Transplantation: Focus on Ferroptosis, Mitophagy and New Antioxidants. Antioxidants 2022, 11, 769. [Google Scholar] [CrossRef]
- Long, C.; Yang, J.; Yang, H.; Li, X.; Wang, G. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti-inflammatory, and anti-apoptotic activities. Mol. Med. Rep. 2016, 13, 4697–4704. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int. J. Mol. Sci. 2020, 21, 2951. [Google Scholar] [CrossRef]
- Mahmoud-Awny, M.; Attia, A.; Abd-Ellah, M.; El-Abhar, H. Mangiferin mitigates gastric ulcer in ischemia/reperfused rats: Involvement of PPAR-γ, NF-κB and Nrf2/HO-1 signaling pathways. PLoS ONE 2015, 10, e0132497. [Google Scholar] [CrossRef]
- Awny, M.; Al-Mokaddem, A.; Ali, B. Mangiferin mitigates di-(2-ethylhexyl) phthalate-induced testicular injury in rats by modulating oxidative stress-mediated signals, inflammatory cascades, apoptotic pathways, and steroidogenesis. Arch. Biochem. Biophys. 2021, 711, 108982. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Elshazly, S.; Abdelaal, M.; Soliman, E. Reno-protective effect of mangiferin against methotrexate-induced kidney damage in male rats: PPARγ-mediated antioxidant activity. Saudi Pharm. J. 2022, 30, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhou, Z.; Xi, X.; Huang, R.; Hu, H. Pioglitazone ameliorates renal ischemia-reperfusion injury via inhibition of NF-κB Activation and Inflammation in Rats. Front. Physiol. 2021, 12, 707344. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zou, C.; Zhang, C.; Wang, X.; Zou, X.; Xiang, Z.; Wang, Z.; Gui, B.; Lin, T.; Hu, H. Protective Effects of PPARγ on Renal Ischemia-Reperfusion Injury by Regulating miR-21. Oxidative Med. Cell Longev. 2022, 2022, 7142314. [Google Scholar] [CrossRef]
- El-Sayyad, S.; Soubh, A.; Awad, A.; El-Abhar, H. Mangiferin protects against intestinal ischemia/reperfusion-induced liver injury: Involvement of PPAR-γ, GSK-3β and Wnt/β-catenin pathway. Eur. J. Pharmacol. 2017, 809, 80–86. [Google Scholar] [CrossRef]
- Yang, K.; Li, W.-F.; Yu, J.-F.; Yi, C.; Huang, W.-F. Diosmetin protects against ischemia/reperfusion-induced acute kidney injury in mice. J. Surg. Res. 2017, 214, 69–78. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Zhang, Q.; Li, F.; Lei, T.; Luo, D.; Zhou, H.; Yang, B. Low molecular weight fucoidan against renal ischemia–reperfusion injury via inhibition of the MAPK signaling pathway. PLoS ONE 2013, 8, e56224. [Google Scholar] [CrossRef]
- Wang, C.; Hao, Z.; Zhou, J.; Zhang, L.; Sun, Y.; Liang, C. Rutaecarpine alleviates renal ischemia reperfusion injury in rats by suppressing the JNK/p38 MAPK signaling pathway and interfering with the oxidative stress response. Mol. Med. Rep. 2017, 16, 922–928. [Google Scholar] [CrossRef]
- Dhanasekaran, D.; Reddy, E. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Sahu, A.; Verma, V.; Mutneja, E.; Malik, S.; Nag, T.; Dinda, A.; Arya, D.; Bhatia, J. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Mol. Cell Biochem. 2019, 452, 141–152. [Google Scholar] [CrossRef]
- Pal, P.; Sinha, K.; Sil, P. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS ONE 2014, 9, e107220. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Lu, J.; Zhang, R.; Nabila, J.; Gao, H.; Wan, Z.; Temitope, I.; Yin, X.; Sun, Y. Mangiferin ameliorates acetaminophen-induced hepatotoxicity through APAP-Cys and JNK modulation. Biomed. Pharmacother. 2019, 117, 109097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rong, S.; Feng, Y.; Zhao, L.; Hong, J.; Wang, R.; Yuan, W. Simvastatin attenuates renal ischemia/reperfusion injury from oxidative stress via targeting Nrf2/HO-1 pathway. Exp. Ther. Med. 2017, 14, 4460–4466. [Google Scholar] [CrossRef] [PubMed]
- Aboutaleb, N.; Jamali, H.; Abolhasani, M.; Toroudi, H. Lavender oil (Lavandula angustifolia) attenuates renal ischemia/reperfusion injury in rats through suppression of inflammation, oxidative stress and apoptosis. Biomed. Pharmacother. 2019, 110, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.; El-Aal, S.A.; El-Abhar, H.; Ahmed, K.; Awny, M. Repositioning of Ticagrelor: Renoprotection mediated by modulating renin-angiotensin system, inflammation, autophagy and galectin-3. Eur. J. Pharmacol. 2022, 918, 174793. [Google Scholar] [CrossRef]
- Gendy, A.; Elnagar, M.; Allam, M.; Mousa, M.; Khodir, A.; El-Haddad, A.; Elnahas, O.; Fayez, S.; El-Mancy, S. Berberine-loaded nanostructured lipid carriers mitigate warm hepatic ischemia/reperfusion-induced lesion through modulation of HMGB1/TLR4/NF-κB signaling and autophagy. Biomed. Pharmacother. 2022, 145, 112122. [Google Scholar] [CrossRef]
- Burnette, W. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Yang, S.-J.; Fan, C.-N.; Wang, M.-J.; Fan, S.-Z.; Tsai, J.-C.; Sun, W.-Z.; Chan, W.-S.; Yeh, Y.-C. Effects of dexmedetomidine on renal microcirculation in ischemia/reperfusion-induced acute kidney injury in rats. Sci. Rep. 2021, 11, 2026. [Google Scholar] [CrossRef]
- Lotfy, B.; Mousa, M.; El-Shehry, M.-S.; Ahmed, S.-F.; Ali, S.; Al Shawoush, A.; Mohamed, A. Therapeutic potency of gallium verum extract on ethanol-induced gastric ulcer in rats. Biointerface Res. Appl. Chem. 2022, 12, 6010–6020. [Google Scholar]




| Proteins | Energy Score (kcal/mol) | No. of Interactions | H-Bonding Residues |
|---|---|---|---|
| Nrf2 protein | |||
| 1X2R | −5.56 | 3 | HOH228, HOH57, TYR572 (H-pi) |
| MAPK8 (JNK1) protein | |||
| 3ELJ | −7.08 | 5 | HOH510, MET111, HOH545, HOH475 |
| MAPK (p38) proteins | |||
| MAPK11 (p38-beta) | |||
| 3GC9 | −5.56 | 5 | ASP112, HOH574, ASN115, HOH480 |
| MAPK13 (p38-delta) | |||
| 4EYJ | −3.51 | 7 | GLU72, ARG68, HOH511, LEU167, HOH709 (pi-H) |
| 4EYM | −6.46 | 6 | MET107, GLU72, PHE169, HOH557, LEU167 (pi-H) |
| MAPK14 (p38-alpha) | |||
| 1OUY | −5.87 | 4 | ALA111, SER154, LYS53 |
| 1OVE | −5.93 | 7 | HOH1241, HOH1177, HOH1256, MET109, LYS53 |
| 1WBS | −4.64 | 6 | LYS53, HOH2029, HOH2208, GLU71, HOH2205 (pi-H) |
| Primer | Primer Sequences | Accession Number |
|---|---|---|
| PPAR-γ | F: 5′-CAGGTACCAGGAGCAGAGCAAAGAGCTG-3′ R: 5′-GAGGTACCGCTCTGTGACAATCTGCCTGA-3′ | NM_001145366.1 |
| Nrf2 | F: 5′-ATGGCC ACACTTTTCTGGAC-3′ R: 5′-AGATGTCAAGCGGGTCACTT-3′ | NM_031789.2 |
| iNOS | F: 5′-TGGGTGAAAGCGGTGTTCTT-3′ R: 5′-TAGCGCTTCCGACTTCCTTG-3′ | S71597.1 |
| Bcl-2 | F:- 5′-GGGGATGACTTCTCTCGTCG-3′ R:- 5′-GACATCTCCCTGTTGACGCT-3′ | NM_016993.2 |
| Bax | F:- 5′-TCATGAAGACAGGGGCCTTT-3′ R:- 5′-CTGCAGCTCCATGTTGTTGT-3′ | NM_017059.2 |
| β-actin | F: 5′-CGT TGA CAT CCG TAA AGA CCT C-3′ R: 5′-TAG GAG CCA GGG CAG TAA TCT-3′ | NM_031144.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gendy, A.M.; El-Gazar, A.A.; Ragab, G.M.; Al-Mokaddem, A.K.; El-Haddad, A.E.; Selim, H.M.R.M.; Yousef, E.M.; Hamed, N.O.; Ibrahim, S.S.A. Possible Implication of Nrf2, PPAR-γ and MAPKs Signaling in the Protective Role of Mangiferin against Renal Ischemia/Reperfusion in Rats. Pharmaceuticals 2023, 16, 6. https://doi.org/10.3390/ph16010006
Gendy AM, El-Gazar AA, Ragab GM, Al-Mokaddem AK, El-Haddad AE, Selim HMRM, Yousef EM, Hamed NO, Ibrahim SSA. Possible Implication of Nrf2, PPAR-γ and MAPKs Signaling in the Protective Role of Mangiferin against Renal Ischemia/Reperfusion in Rats. Pharmaceuticals. 2023; 16(1):6. https://doi.org/10.3390/ph16010006
Chicago/Turabian StyleGendy, Abdallah M., Amira A. El-Gazar, Ghada M. Ragab, Asmaa K. Al-Mokaddem, Alaadin E. El-Haddad, Heba Mohammed Refat M. Selim, Einas Mohamed Yousef, Najat O. Hamed, and Sherihan Salaheldin Abdelhamid Ibrahim. 2023. "Possible Implication of Nrf2, PPAR-γ and MAPKs Signaling in the Protective Role of Mangiferin against Renal Ischemia/Reperfusion in Rats" Pharmaceuticals 16, no. 1: 6. https://doi.org/10.3390/ph16010006
APA StyleGendy, A. M., El-Gazar, A. A., Ragab, G. M., Al-Mokaddem, A. K., El-Haddad, A. E., Selim, H. M. R. M., Yousef, E. M., Hamed, N. O., & Ibrahim, S. S. A. (2023). Possible Implication of Nrf2, PPAR-γ and MAPKs Signaling in the Protective Role of Mangiferin against Renal Ischemia/Reperfusion in Rats. Pharmaceuticals, 16(1), 6. https://doi.org/10.3390/ph16010006








