Combining 3D Printing and Microfluidic Techniques: A Powerful Synergy for Nanomedicine
Abstract
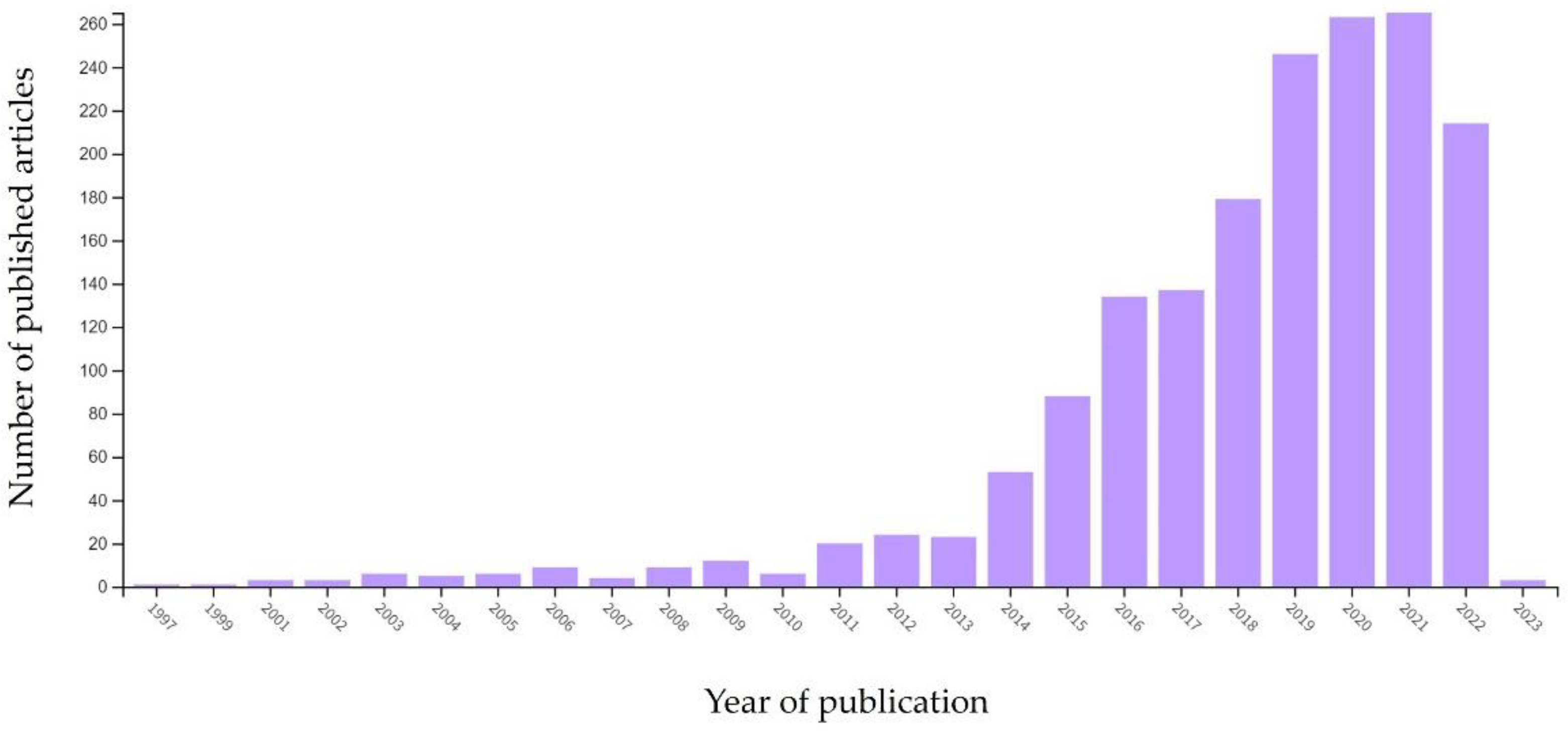
:1. Introduction
2. Method
3. Microfluidic Technique
3.1. Materials for MF Devices
3.1.1. Glass
3.1.2. Silicon
3.1.3. Metals
3.1.4. Polymeric Materials
3.1.5. Paper
3.1.6. Hydrogels
4. Three-Dimensional Printing
4.1. FDM
4.2. Light-Triggered Printing
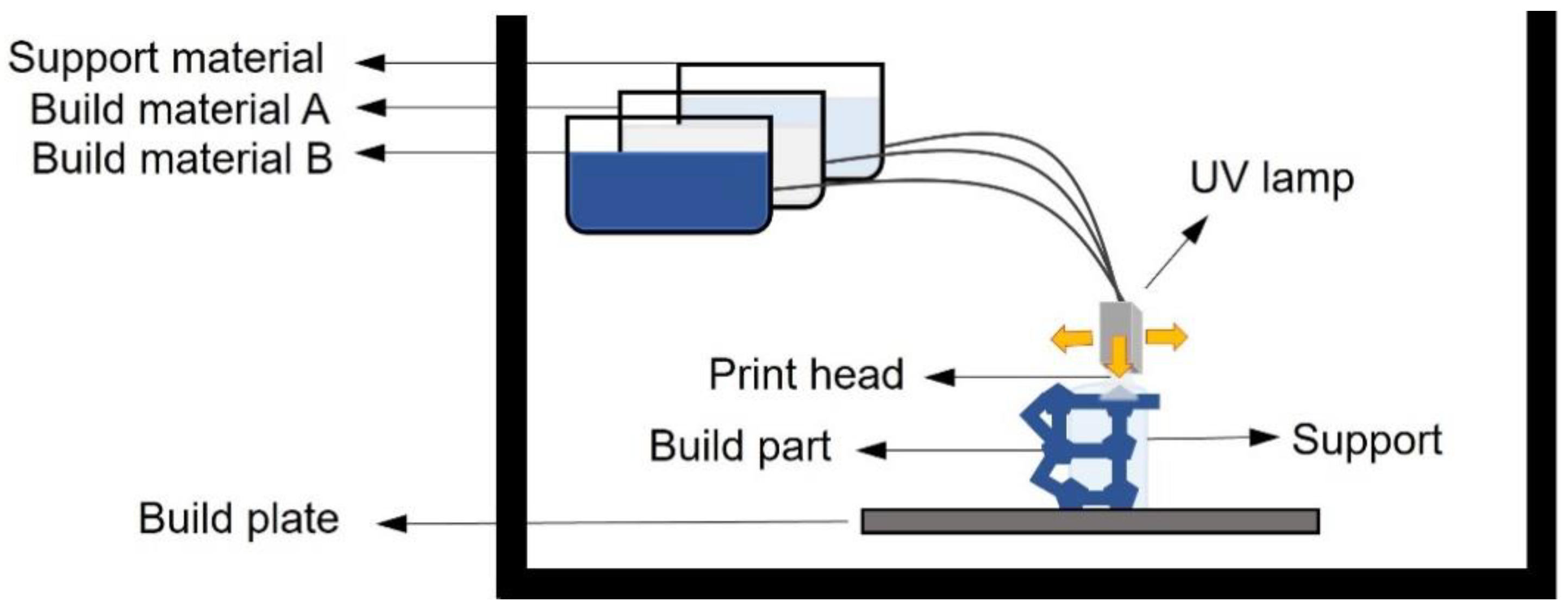
4.3. Inkjet 3D Printing
5. The Synergy of MF and 3DP
- The ability to select the material to be printed, given the wide range of options available, depending on the printer’s specifications and the characteristics desired, such as transparency, resistance to organic solvents, thermostability, and compatibility of surface interactions with substances to be encapsulated within nanoformulations [22];
- The full customization of designs about 3D printed MF devices. With the addition of previously unexplored components, new geometries may be examined. The devices are low-cost, reusable, and more sustainable than those made industrially and subjected to post-processing methods once optimized in the production phase and with reduced energy and material waste [43,112];
- The developed devices may be evaluated for all the formulations that are predicted to be generated in a relatively short time, since they are ready to use once manufactured, and this technique therefore provides for the rapid completion of experimental and comparative research [117].
6. Expert Opinion and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced Nanomedicine and Cancer: Challenges and Opportunities in Clinical Translation. Int. J. Pharm. 2021, 599, 120438. [Google Scholar] [CrossRef] [PubMed]
- Ayub, A.; Wettig, S. An Overview of Nanotechnologies for Drug Delivery to the Brain. Pharmaceutics 2022, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, V.K.; Jindal, S.; Packirisamy, G.; Ojha, S.; Lian, S.; Kaushik, A.; Alzarooni, A.I.M.A.; Metwally, Y.A.F.; Thyagarajan, S.P.; do Jung, Y.; et al. Nanomedicine-Based Cancer Immunotherapy: Recent Trends and Future Perspectives. Cancer Gene Ther. 2021, 28, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef]
- Rahman, M.; Kamal, M.A. Special Issue: Cancer Nanotherapeutics: Targeted Medicine, Therapeutic Vaccination and Challenges with Cancer Nanomedicines. Semin. Cancer Biol. 2021, 69, 1–4. [Google Scholar] [CrossRef]
- Lachowicz, D.; Kaczyńska, A.; Wirecka, R.; Kmita, A.; Szczerba, W.; Bodzoń-Kulakowska, A.; Sikora, M.; Karewicz, A.; Zapotoczny, S. A Hybrid System for Magnetic Hyperthermia and Drug Delivery: SPION Functionalized by Curcumin Conjugate. Materials 2018, 11, 2388. [Google Scholar] [CrossRef] [Green Version]
- Ward, D.M.; Shodeinde, A.B.; Peppas, N.A. Innovations in Biomaterial Design toward Successful RNA Interference Therapy for Cancer Treatment. Adv. Healthc. Mater. 2021, 10, 2100350. [Google Scholar] [CrossRef]
- Beg, S.; Almalki, W.H.; Khatoon, F.; Alharbi, K.S.; Alghamdi, S.; Akhter, M.H.; Khalilullah, H.; Baothman, A.A.; Hafeez, A.; Rahman, M.; et al. Lipid/Polymer-Based Nanocomplexes in Nucleic Acid Delivery as Cancer Vaccines. Drug Discov. Today 2021, 26, 1891–1903. [Google Scholar] [CrossRef]
- Beg, S.; Alharbi, K.S.; Alruwaili, N.K.; Alotaibi, N.H.; Almalki, W.H.; Alenezi, S.K.; Altowayan, W.M.; Alshammari, M.S.; Rahman, M. Nanotherapeutic Systems for Delivering Cancer Vaccines: Recent Advances. Nanomedicine 2020, 15, 1527–1537. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Cabral, H.; Uchida, S.; Perche, F.; Pichon, C. Nanomedicine-Based Approaches for MRNA Delivery. Mol. Pharm. 2020, 17, 3654–3684. [Google Scholar]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines-a New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, K.E.; Bhosle, S.M.; Zurla, C.; Beyersdorf, J.; Rogers, K.A.; Vanover, D.; Xiao, P.; Araínga, M.; Shirreff, L.M.; Pitard, B.; et al. Visualization of Early Events in MRNA Vaccine Delivery in Non-Human Primates via PET–CT and near-Infrared Imaging. Nat. Biomed. Eng. 2019, 3, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Alfagih, I.M.; Aldosari, B.; Alquadeib, B.; Almurshedi, A.; Alfagih, M.M. Nanoparticles as Adjuvants and Nanodelivery Systems for MRNA-Based Vaccines. Pharmaceutics 2021, 13, 45. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of Active Targeting Nanoparticle Delivery System for Chemotherapeutic Drugs and Traditional/Herbal Medicines in Cancer Therapy: A Systematic Review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Sommonte, F.; Arduino, I.; Racaniello, G.F.; Lopalco, A.; Lopedota, A.A.; Denora, N. The Complexity of the Blood-Brain Barrier and the Concept of Age-Related Brain Targeting: Challenges and Potential of Novel Solid Lipid-Based Formulations. J. Pharm. Sci. 2021, 111, 577–592. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic Formulation of Nanoparticles for Biomedical Applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic Nanoparticles for Drug Delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef]
- Weaver, E.; O’Connor, E.; Cole, D.K.; Hooker, A.; Uddin, S.; Lamprou, D.A. Microfluidic-Mediated Self-Assembly of Phospholipids for the Delivery of Biologic Molecules. Int. J. Pharm. 2022, 611, 121347. [Google Scholar] [CrossRef] [PubMed]
- Ballacchino, G.; Weaver, E.; Mathew, E.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Manufacturing of 3d-Printed Microfluidic Devices for the Synthesis of Drug-Loaded Liposomal Formulations. Int. J. Mol. Sci. 2021, 22, 8064. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Increasing the Functionalities of 3D Printed Microchemical Devices by Single Material, Multimaterial, and Print-Pause-Print 3D Printing. Lab Chip 2019, 19, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, G.; Roppolo, I.; Pirri, C.F.; Chiappone, A. Current and Emerging Trends in Polymeric 3D Printed Microfluidic Devices. Addit. Manuf. 2022, 55, 102867. [Google Scholar] [CrossRef]
- Pistone, M.; Racaniello, G.F.; Arduino, I.; Laquinta, V.; Lopalco, A.; Cutrignelli, A.; Rizzi, R.; Franco, M.; Lopedota, A.A.; Denora, N. Direct cyclodextrin-based powder extrusion 3D printing for one-step production of the BCS class II model drug niclosamide. Drug Deliv. Transl. Res. 2022, 12, 1895–1910. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Dwivedi, N.; Khan, R.; Solanki, P.R.; Srivastava, A.K.; Dhand, C. 3D-Printed Microfluidics and Potential Biomedical Applications. Front. Nanotechnol. 2021, 3, 609355. [Google Scholar] [CrossRef]
- Scopus. Available online: www.scopus.com (accessed on 13 December 2022).
- Web of Science. Available online: www.webofscience.com (accessed on 13 December 2022).
- Bohr, A.; Colombo, S.; Jensen, H. Future of Microfluidics in Research and in the Market. In Microfluidics for Pharmaceutical Applications: From Nano/Micro Systems Fabrication to Controlled Drug Delivery; Santos, H.A., Liu, D., Zhang, H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 425–465. [Google Scholar]
- Ahn, J.; Ko, J.; Lee, S.; Yu, J.; Kim, Y.T.; Jeon, N.L. Microfluidics in Nanoparticle Drug Delivery; From Synthesis to Pre-Clinical Screening. Adv. Drug Deliv. Rev. 2018, 128, 29–53. [Google Scholar] [CrossRef]
- Hamdallah, S.I.; Zoqlam, R.; Erfle, P.; Blyth, M.; Alkilany, A.M.; Dietzel, A.; Qi, S. Microfluidics for Pharmaceutical Nanoparticle Fabrication: The Truth and the Myth. Int. J. Pharm. 2020, 584, 119408. [Google Scholar] [CrossRef]
- Martins, J.P.; Torrieri, G.; Santos, H.A. The Importance of Microfluidics for the Preparation of Nanoparticles as Advanced Drug Delivery Systems. Expert Opin. Drug Deliv. 2018, 15, 469–479. [Google Scholar] [CrossRef]
- Preetam, S.; Nahak, B.K.; Patra, S.; Toncu, D.C.; Park, S.; Syväjärvi, M.; Orive, G.; Tiwari, A. Emergence of Microfluidics for next Generation Biomedical Devices. Biosens. Bioelectron. X 2022, 10, 100106. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Zhang, H.; Santos, H.A. Microfluidic Mixing and Devices for Preparing Nanoparticulate Drug Delivery Systems. In Microfluidics for Pharmaceutical Applications: From Nano/Micro Systems Fabrication to Controlled Drug Delivery; Santos, H.A., Liu, D., Zhang, H., Eds.; Elsevier: San Diego, CA, USA, 2018; pp. 155–177. [Google Scholar]
- Jaradat, E.; Weaver, E.; Meziane, A.; Lamprou, D.A. Microfluidics Technology for the Design and Formulation of Nanomedicines. Nanomaterials 2021, 11, 3440. [Google Scholar] [CrossRef] [PubMed]
- Arduino, I.; Liu, Z.; Iacobazzi, R.M.; Lopedota, A.A.; Lopalco, A.; Cutrignelli, A.; Laquintana, V.; Porcelli, L.; Azzariti, A.; Franco, M.; et al. Microfluidic Preparation and in Vitro Evaluation of IRGD-Functionalized Solid Lipid Nanoparticles for Targeted Delivery of Paclitaxel to Tumor Cells. Int. J. Pharm. 2021, 610, 121246. [Google Scholar] [CrossRef] [PubMed]
- Arduino, I.; Liu, Z.; Rahikkala, A.; Figueiredo, P.; Correia, A.; Cutrignelli, A.; Denora, N.; Santos, H.A. Preparation of Cetyl Palmitate-Based PEGylated Solid Lipid Nanoparticles by Microfluidic Technique. Acta Biomater. 2021, 121, 566–578. [Google Scholar] [CrossRef]
- Iacobazzi, R.M.; Arduino, I.; di Fonte, R.; Lopedota, A.A.; Serratì, S.; Racaniello, G.; Bruno, V.; Laquintana, V.; Lee, B.C.; Silvestris, N.; et al. Microfluidic-Assisted Preparation of Targeted Ph-Responsive Polymeric Micelles Improves Gemcitabine Effectiveness in Pdac: In Vitro Insights. Cancers 2022, 14, 5. [Google Scholar] [CrossRef]
- Ailuno, G.; Iacobazzi, R.M.; Lopalco, A.; Baldassari, S.; Arduino, I.; Azzariti, A.; Pastorino, S.; Caviglioli, G.; Denora, N. The Pharmaceutical Technology Approach on Imaging Innovations from Italian Research. Pharmaceutics 2021, 13, 1214. [Google Scholar] [CrossRef]
- Trantidou, T.; Friddin, M.S.; Salehi-Reyhani, A.; Ces, O.; Elani, Y. Droplet Microfluidics for the Construction of Compartmentalised Model Membranes. Lab Chip 2018, 18, 2488–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, A.V.; Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. 3D Printed Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Patel, V.B.; Singh, B.; Varade, D. Microfluidic Device Based Molecular Self-Assembly Structures. J. Mol. Liq. 2022, 362, 119760. [Google Scholar] [CrossRef]
- Weaver, E.; O’Hagan, C.; Lamprou, D.A. The Sustainability of Emerging Technologies for Use in Pharmaceutical Manufacturing. Expert Opin. Drug Deliv. 2022, 19, 861–872. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary Microfluidics in Microchannels: From Microfluidic Networks to Capillaric Circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Helmer, D.; Rapp, B.E. Emerging Technologies and Materials for High-Resolution 3D Printing of Microfluidic Chips. Adv. Biochem. Eng. Biotechnol. 2022, 179, 37–66. [Google Scholar] [PubMed]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel Fabrication on Glass Materials for Microfluidic Devices. IJPEM 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Tian, J.; Wu, T.; Li, Z.; Xing, F.; Fu, S. Polymer-Based Microfluidic Devices: A Comprehensive Review on Preparation and Applications. Polym. Eng. Sci. 2022, 62, 3–24. [Google Scholar] [CrossRef]
- James, M.; Revia, R.A.; Stephen, Z.; Zhang, M. Microfluidic Synthesis of Iron Oxide Nanoparticles. Nanomaterials 2020, 10, 2113. [Google Scholar] [CrossRef]
- Persson, H.; Park, S.; Mohan, M.; Cheung, K.K.; Simmons, C.A.; Young, E.W.K. Rapid Assembly of PMMA Microfluidic Devices with PETE Membranes for Studying the Endothelium. Sens. Actuators B Chem. 2022, 356, 131342. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Schaumburg, F.; Berli, C.L.A. Assessing the Rapid Flow in Multilayer Paper-Based Microfluidic Devices. Microfluid. Nanofluidics 2019, 23, 98. [Google Scholar] [CrossRef]
- Zargaryan, A.; Farhoudi, N.; Haworth, G.; Ashby, J.F.; Au, S.H. Hybrid 3D Printed-Paper Microfluidics. Sci. Rep. 2020, 10, 18379. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Fu, J.; He, Y. Hydrogels: The Next Generation Body Materials for Microfluidic Chips? Small 2020, 16, 2003797. [Google Scholar] [CrossRef] [PubMed]
- Nash, A.T.; Foster, D.A.N.; Thompson, S.I.; Han, S.; Fernandez, M.K.; Hwang, D.K. A New Rapid Microfluidic Detection Platform Utilizing Hydrogel-Membrane under Cross-Flow. Adv. Mater. Technol. 2022, 7, 2101396. [Google Scholar] [CrossRef]
- Nasello, G.; Cóndor, M.; Vaughan, T.; Schiavi, J. Designing Hydrogel-Based Bone-on-Chips for Personalized Medicine. Appl. Sci. 2021, 11, 4495. [Google Scholar] [CrossRef]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef] [Green Version]
- Moroni, S.; Casettari, L.; Lamprou, D.A. 3D and 4D Printing in the Fight against Breast Cancer. Biosensors 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Kumar, A.; Dev, A.; Gupta, V.K.; Han, S.S. Advances in Hydrogel-Based Microfluidic Blood–Brain-Barrier Models in Oncology Research. Pharmaceutics 2022, 14, 993. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Abdoli, A.; Rahmanian, M.; Bardania, H.; Bayandori, M.; Moosavi Basri, S.M.; Kalbasi, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders. Mol. Neurobiol. 2019, 56, 8489–8512. [Google Scholar] [CrossRef]
- Bhusal, A.; Dogan, E.; Nguyen, H.A.; Labutina, O.; Nieto, D.; Khademhosseini, A.; Miri, A.K. Multi-Material Digital Light Processing Bioprinting of Hydrogel-Based Microfluidic Chips. Biofabrication 2022, 14, 014103. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Gagandeep; Bhatia, R. Paper-based microfluidic devices: Fabrication, detection, and significant applications in various fields. Rev. Anal. Chem. 2022, 41, 112–136. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, H.; Shi, X.; Tan, W.; Zhu, G. Fabrication for paper-based microfluidic analytical devices and saliva analysis application. Microfluid. Nanofluidics 2021, 25, 80. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Balakrishnan, H.K.; Badar, F.; Doeven, E.H.; Novak, J.I.; Merenda, A.; Dumée, L.F.; Loy, J.; Guijt, R.M. 3D Printing: An Alternative Microfabrication Approach with Unprecedented Opportunities in Design. Anal. Chem. 2021, 93, 350–366. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. Three-Dimensional Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulhameed, O.; Al-Ahmari, A.; Ameen, W.; Mian, S.H. Additive Manufacturing: Challenges, Trends, and Applications. Adv. Mech. Eng. 2019, 11, 1687814018822880. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Solomon, I.J.; Sevvel, P.; Gunasekaran, J. A Review on the Various Processing Parameters in FDM. Mater. Today Proc. 2020, 37, 509–514. [Google Scholar] [CrossRef]
- Gal-Or, E.; Gershoni, Y.; Scotti, G.; Nillson, S.M.E.; Saarinen, J.; Jokinen, V.; Strachan, C.J.; af Gennäs, G.B.; Yli-Kauhaluoma, Y.; Kotiaho, T. Chemical analysis using 3D printed glass microfluidics. Anal. Methods 2019, 11, 1802. [Google Scholar] [CrossRef] [Green Version]
- Weisgrab, G.; Ovsianikov, A.; Costa, P.F. Functional 3D Printing for Microfluidic Chips. Adv. Mater. Technol. 2019, 4, 1900275. [Google Scholar] [CrossRef]
- Mehta, V.; Rath, S.N. 3D Printed Microfluidic Devices: A Review Focused on Four Fundamental Manufacturing Approaches and Implications on the Field of Healthcare. Bio-Des. Manuf. 2021, 4, 311–343. [Google Scholar] [CrossRef]
- Grösche, M.; Zoheir, A.E.; Stegmaier, J.; Mikut, R.; Mager, D.; Korvink, J.G.; Rabe, K.S.; Niemeyer, C.M. Microfluidic Chips for Life Sciences—A Comparison of Low Entry Manufacturing Technologies. Small 2019, 15, 1901956. [Google Scholar] [CrossRef] [PubMed]
- Santoliquido, O.; Colombo, P.; Ortona, A. Additive Manufacturing of Ceramic Components by Digital Light Processing: A Comparison between the “Bottom-up” and the “Top-down” Approaches. J. Eur. Ceram. Soc. 2019, 39, 2140–2148. [Google Scholar] [CrossRef]
- Chaudhary, R.; Fabbri, P.; Leoni, E.; Mazzanti, F.; Akbari, R.; Antonini, C. Additive Manufacturing by Digital Light Processing: A Review. Prog. Addit. Manuf. 2022. [Google Scholar] [CrossRef]
- Hayes, B.; Hainsworth, T.; MacCurdy, R. Liquid–Solid Co-Printing of Multi-Material 3D Fluidic Devices via Material Jetting. Addit. Manuf. 2022, 55, 102785. [Google Scholar] [CrossRef]
- Pranzo, D.; Larizza, P.; Filippini, D.; Percoco, G. Extrusion-Based 3D Printing of Microfluidic Devices for Chemical and Biomedical Applications: A Topical Review. Micromachines 2018, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Jaisingh Sheoran, A.; Kumar, H. Fused Deposition Modeling Process Parameters Optimization and Effect on Mechanical Properties and Part Quality: Review and Reflection on Present Research. Mater. Today Proc. 2020, 21, 1659–1672. [Google Scholar] [CrossRef]
- Quero, R.F.; Da Silveira, G.D.; Da Silva, J.A.F.; de Jesus, D.P. Understanding and Improving FDM 3D Printing to Fabricate High-Resolution and Optically Transparent Microfluidic Devices. Lab Chip 2021, 21, 3715. [Google Scholar] [CrossRef]
- Quero, R.F.; de Castro Costa, B.M.; da Silva, J.A.F.; de Jesus, D.P. Using Multi-Material Fused Deposition Modeling (FDM) for One-Step 3D Printing of Microfluidic Capillary Electrophoresis with Integrated Electrodes for Capacitively Coupled Contactless Conductivity Detection. Sens. Actuators B Chem. 2022, 365, 131959. [Google Scholar] [CrossRef]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible, Transparent, Sub-100 Μm Microfluidic Channels with Fused Deposition Modeling 3D-Printed Thermoplastic Polyurethane. J. Micromech. Microeng. 2019, 29, 095010. [Google Scholar] [CrossRef]
- Bressan, L.P.; Lima, T.M.; da Silveira, G.D.; da Silva, J.A.F. Low-Cost and Simple FDM-Based 3D-Printed Microfluidic Device for the Synthesis of Metallic Core–Shell Nanoparticles. SN Appl. Sci. 2020, 2, 984. [Google Scholar] [CrossRef]
- Klusák, J.; Mucha, J.; Večeř, M. 3D-Printed Microfluidic Device for Monodisperse Emulsions Preparation. Chem. Pap. 2021, 75, 6101–6113. [Google Scholar] [CrossRef]
- Mader, M.; Rein, C.; Konrat, E.; Meermeyer, S.L.; Lee-Thedieck, C.; Kotz-Helmer, F.; Rapp, B.E. Fused Deposition Modeling of Microfluidic Chips in Transparent Polystyrene. Micromachines 2021, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Musgrove, H.B.; Catterton, M.A.; Pompano, R.R. Applied Tutorial for the Design and Fabrication of Biomicrofluidic Devices by Resin 3D Printing. Anal. Chim. Acta 2022, 1209, 339842. [Google Scholar] [CrossRef]
- Krkobabić, M.; Medarević, D.; Cvijić, S.; Grujić, B.; Ibrić, S. Hydrophilic Excipients in Digital Light Processing (DLP) Printing of Sustained Release Tablets: Impact on Internal Structure and Drug Dissolution Rate. Int. J. Pharm. 2019, 572, 118790. [Google Scholar] [CrossRef]
- Lee, B.J.; Hsiao, K.; Lipkowitz, G.; Samuelsen, T.; Tate, L.; DeSimone, J.M. Characterization of a 30 Μm Pixel Size CLIP-Based 3D Printer and Its Enhancement through Dynamic Printing Optimization. Addit. Manuf. 2022, 55, 102800. [Google Scholar]
- Zhao, Z.; Tian, X.; Song, X. Engineering Materials with Light: Recent Progress in Digital Light Processing Based 3D Printing. J. Mater. Chem. C Mater. 2020, 8, 13896–13917. [Google Scholar] [CrossRef]
- Mele, M.; Campana, G. An Experimental Approach to Manufacturability Assessment of Microfluidic Devices Produced by Stereolithography. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2020, 234, 4905–4916. [Google Scholar] [CrossRef]
- Ahmed, I.; Sullivan, K.; Priye, A. Multi-Resin Masked Stereolithography (MSLA) 3D Printing for Rapid and Inexpensive Prototyping of Microfluidic Chips with Integrated Functional Components. Biosensors 2022, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Valentinčič, J.; Prijatelj, M.; Jerman, M.; Lebar, A.; Sabotin, I. Characterization of a Custom-Made Digital Light Processing Stereolithographic Printer Based on a Slanted Groove Micromixer Geometry. J. Micro. Nanomanuf. 2020, 8, 010911. [Google Scholar] [CrossRef]
- Goralczyk, A.; Mayoussi, F.; Sanjaya, M.; Corredor, S.F.; Bhagwat, S.; Song, Q.; Schwenteck, S.; Warmbold, A.; Pezeshkpour, P.; Rapp, B.E. On-Chip Chemical Synthesis Using One-Step 3D Printed Polyperfluoropolyether. Chem. Ing. Tech. 2022, 94, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rivas, O.; Hernández-Velázquez, D.; Piazza, V.; Marquez, S. Rapid Prototyping of Microfluidic Devices by SL 3D Printing and Their Biocompatibility Study for Cell Culturing. Mater. Today Proc. 2019, 13, 436–445. [Google Scholar] [CrossRef]
- Subirada, F.; Paoli, R.; Sierra-Agudelo, J.; Lagunas, A.; Rodriguez-Trujillo, R.; Samitier, J. Development of a Custom-Made 3D Printing Protocol with Commercial Resins for Manufacturing Microfluidic Devices. Polymers 2022, 14, 2955. [Google Scholar] [CrossRef]
- Brenda, B.M.; Griveau, S.; Bedioui, F.; d’ Orlye, F.; da Silva, J.A.F.; Varenne, A. Stereolithography Based 3D-Printed Microfluidic Device with Integrated Electrochemical Detection. Electrochim. Acta 2022, 407, 139888. [Google Scholar]
- Chen, Z.; Han, J.Y.; Shumate, L.; Fedak, R.; DeVoe, D.L. High Throughput Nanoliposome Formation Using 3D Printed Microfluidic Flow Focusing Chips. Adv. Mater. Technol. 2019, 4, 1800511. [Google Scholar] [CrossRef]
- Shan, H.; Lin, Q.; Wang, D.; Sun, X.; Quan, B.; Chen, X.; Chen, Z. 3D Printed Integrated Multi-Layer Microfluidic Chips for Ultra-High Volumetric Throughput Nanoliposome Preparation. Front. Bioeng. Biotechnol. 2021, 9, 773705. [Google Scholar] [CrossRef]
- Tzivelekis, C.; Sgardelis, P.; Waldron, K.; Whalley, R.; Huo, D.; Dalgarno, K. Fabrication Routes via Projection Stereolithography for 3D-Printing of Microfluidic Geometries for Nucleic Acid Amplification. PLoS ONE 2020, 15, e0240237. [Google Scholar] [CrossRef]
- Xenikakis, I.; Tsongas, K.; Tzimtzimis, E.K.; Zacharis, C.K.; Theodoroula, N.; Kalogianni, E.P.; Demiri, E.; Vizirianakis, I.S.; Tzetzis, D.; Fatouros, D.G. Fabrication of hollow microneedles using liquid crystal display (LCD) vat polymerization 3D printing technology for transdermal macromolecular delivery. Int. J. Pharm. 2021, 597, 120303. [Google Scholar] [CrossRef]
- Weaver, E.; Mathew, E.; Caldwell, J.; Hooker, A.; Uddin, S.; Lamprou, A.D. The manufacturing of 3D-printed microfluidic chips to analyse the effect upon particle size during the synthesis of lipid nanoparticles. J. Pharm. Pharmacol. 2022, rgac085. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, J.M.; Liu, Y.; Li, X.; Lv, P.; Jin, D.; Duan, H. A Modular Microfluidic Device via Multimaterial 3D Printing for Emulsion Generation. Sci. Rep. 2018, 8, 4791. [Google Scholar] [CrossRef] [PubMed]
- Castiaux, A.D.; Pinger, C.W.; Hayter, E.A.; Bunn, M.E.; Martin, R.S.; Spence, D.M. PolyJet 3D-Printed Enclosed Microfluidic Channels without Photocurable Supports. Anal. Chem. 2019, 91, 6910–6917. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.; Siller, I.G.; Urmann, K.; Hoffmann, M.R.; Bahnemann, J. 3D Printed Microfluidic Mixers—A Comparative Study on Mixing Unit Performances. Small 2019, 15, 1804326. [Google Scholar] [CrossRef] [Green Version]
- Barbaresco, F.; Cocuzza, M.; Pirri, C.F.; Marasso, S.L. Application of a Micro Free-Flow Electrophoresis 3d Printed Lab-on-a-Chip for Micro-Nanoparticles Analysis. Nanomaterials 2020, 10, 1277. [Google Scholar] [CrossRef]
- Chang, Y.; Jiang, J.; Chen, W.; Yang, W.; Chen, L.; Chen, P.; Shen, J.; Quian, S.; Zhou, T.; Wu, L.; et al. Biomimetic Metal-Organic Nanoparticles Prepared with a 3D-Printed Microfluidicdevice as a Novel Formulation for Disulfiram-Based Therapy against Breast Cancer. Appl. Mater. Today 2020, 18, 100492. [Google Scholar] [CrossRef]
- Kara, A.; Vassiliadou, A.; Ongoren, B.; Keeble, W.; Hinng, R.; Lalatsa, A.; Serrano, D.R.; Parhizkar, M.; Tsaoulidis, D. Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines. Pharmaceutics 2021, 13, 2134. [Google Scholar] [CrossRef]
- Tiboni, M.; Tiboni, M.; Pierro, A.; del Papa, M.; Sparaventi, S.; Cespi, M.; Casettari, L. Microfluidics for Nanomedicines Manufacturing: An Affordable and Low-Cost 3D Printing Approach. Int. J. Pharm. 2021, 599, 120464. [Google Scholar] [CrossRef]
- Sommonte, F.; Arduino, I.; Iacobazzi, R.M.; Tiboni, M.; Catalano, F.; Marotta, R.; Di Francesco, M.; Casettari, L.; Decuzzi, P.; Lopedota, A.A.; et al. Microfluidic assembly of “turtle-like” shaped solid lipid nanoparticles for lysozyme delivery. Int. J. Pharm. 2022, in press. [Google Scholar] [CrossRef]
- Drishya, C.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Preparation of Emulsion for Nutrient Delivery Using 3D Printed Microfluidic Chips. Pharma Innov. J. 2021, 10, 490–494. [Google Scholar] [CrossRef]
- Vasilescu, S.A.; Bazaz, S.R.; Jin, D.; Shimoni, O.; Warkiani, M.E. 3D Printing Enables the Rapid Prototyping of Modular Microfluidic Devices for Particle Conjugation. Appl. Mater. Today 2020, 20, 100726. [Google Scholar] [CrossRef]
- Aşık, M.D.; Kaplan, M.; Çetin, B.; Sağlam, N. Synthesis of Iron Oxide Core Chitosan Nanoparticles in a 3D Printed Microfluidic Device. J. Nanopart. Res. 2021, 23, 62. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Wu, D.; Chen, Q.; Zhou, Z.; Zhang, R.; Peng, X.; Su, Y.-C.; Sun, D. 3D Printed Microfluidic Chip for Multiple Anticancer Drug Combinations. Sens. Actuators B Chem. 2018, 276, 507–516. [Google Scholar] [CrossRef]
- Sommonte, F.; Weaver, E.; Mathew, E.; Denora, N.; Lamprou, A.D. In-House Innovative “Diamond Shaped” 3D Printed Microfluidic Devices for Lysozyme-Loaded Liposomes. Pharmaceutics 2022, 14, 2484. [Google Scholar] [CrossRef]
- Khashayar, P.; Al-Madhagi, S.; Azimzadeh, M.; Scognamiglio, V.; Arduini, F. New Frontiers in Microfluidics Devices for MiRNA Analysis. Trends Analyt. Chem. 2022, 156, 116706. [Google Scholar] [CrossRef]
- Rebelo, R.; Barbosa, A.I.; Caballero, D.; Kwon, I.K.; Oliveira, J.M.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. 3D Biosensors in Advanced Medical Diagnostics of High Mortality Diseases. Biosens. Bioelectron. 2019, 130, 20–39. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Puliga, S.; Serrano, D.R.; Bucchi, A.; Halbert, G.; Lalatsa, A. Microfluidic Manufacture of Lipid-Based Nanomedicines. Pharmaceutics 2022, 14, 1940. [Google Scholar] [CrossRef]
- Weaver, E.; Uddin, S.; Cole, D.K.; Hooker, A.; Lamprou, D.A. The Present and Future Role of Microfluidics for Protein and Peptide-Based Therapeutics and Diagnostics. Appl. Sci. 2021, 11, 4109. [Google Scholar] [CrossRef]
- Niranjan, Y.C.; Channabasavanna, S.G.; Krishnapillai, S.; Velmurugan, R.; Kannan, A.R.; Mohan, D.G.; Karganroudi, S.S. The Unprecedented Role of 3D Printing Technology in Fighting the COVID-19 Pandemic: A Comprehensive Review. Materials 2022, 15, 6827. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Gomes Dos Santos, A.L.; Lamprou, D.A. Optimization of Printing Parameters for Digital Light Processing 3d Printing of Hollow Microneedle Arrays. Pharmaceutics 2021, 13, 1837. [Google Scholar] [CrossRef]








| Material | Fabrication Method | Refs. |
|---|---|---|
| Glass | Laminate manufacturing Wet etching/Dry etching Micromilling Microgrinding | [46,48,64] |
| Silicon and polymeric materials | Lithography Laser ablation Hot embossing Inject moulding Soft lithography | [44,46,49,65] |
| Metals | Laser ablation Lithography | [65] |
| Paper | Paper cutting Ink plotting Wax/inkjet/laser printing Plasma etching Photolithography | [66,67,68] |
| Hydrogels | Micromoulding Sacrificial template replication Photo-patterning | [56] |
| Material | Production Method | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Glass | Laminate manufacturing | Easy to use, scalable | Misalignment of layers, air bubble, possibility of leakage, time-consuming | [64] |
| Wet/Dry etching | Fast, precise | Expensive instrumentation, low etching rate | [48] | |
| Mechanical processes | Crack-free surfaces | Low precision, need of controlled environment | [48,74] | |
| 3D printing | Time-effective, crack-free processes | Need of heated chamber to prevent thermal shock | [74] | |
| Polymeric materials | Soft lithography | Suitable for most materials, scalable, easy to use | Easy to deform, Low repeatability | [49] |
| Hot embossing and imprinting | Rapid, high resolution and precision | High cost | [49] | |
| Laser ablation | Fast | High cost, elevate roughness of surfaces | [49] | |
| Lithography | High resolution, good reliability | High cost, no scalable procedures | [49] | |
| 3D printing | Customizable features, low-cost, time-effective, printing on-demand | Not suitable for all materials, resolution issues | [46,49] | |
| Hydrogel | Micromoulding | Controlled microstructures | Stress damages during demoulding | [56,62] |
| Photo-patterning | Fast, high resolution and repeatability | Restricted to photo-sensitive hydrogels | [56] | |
| 3D printing | Time-effective, controlled microstructures, lower cost | Restrictions related to mechanical properties | [62,63] |
| 3D Printing Technique | Material Type | Pros | Cons | Refs. |
|---|---|---|---|---|
| FDM | Thermoplastic filament | Rapid prototyping, low-cost, no post-production processes, easy to use | Mechanical drawbacks (air gap, layers misalignment), poor surface properties, low optical transparency | [71,72,77] |
| Light- triggered printing | UV curable resin | Highest accuracy, visual clarity, high mechanical properties, smooth surface | Post-production requirement to remove uncured resin, less cost-effectiveness material, delamination process | [71,72,78,79] |
| Inkjet printing | UV curable acrylic | High speed, possibility to use different material in the same print | Lack of adhesion between layers, coarse resolution, post-production removal of support, difficulty to fabricate “voids” | [72,75,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommonte, F.; Denora, N.; Lamprou, D.A. Combining 3D Printing and Microfluidic Techniques: A Powerful Synergy for Nanomedicine. Pharmaceuticals 2023, 16, 69. https://doi.org/10.3390/ph16010069
Sommonte F, Denora N, Lamprou DA. Combining 3D Printing and Microfluidic Techniques: A Powerful Synergy for Nanomedicine. Pharmaceuticals. 2023; 16(1):69. https://doi.org/10.3390/ph16010069
Chicago/Turabian StyleSommonte, Federica, Nunzio Denora, and Dimitrios A. Lamprou. 2023. "Combining 3D Printing and Microfluidic Techniques: A Powerful Synergy for Nanomedicine" Pharmaceuticals 16, no. 1: 69. https://doi.org/10.3390/ph16010069
APA StyleSommonte, F., Denora, N., & Lamprou, D. A. (2023). Combining 3D Printing and Microfluidic Techniques: A Powerful Synergy for Nanomedicine. Pharmaceuticals, 16(1), 69. https://doi.org/10.3390/ph16010069










