Nanoencapsulation of Vaccinium ashei Leaf Extract in Eudragit® RS100-Based Nanoparticles Increases Its In Vitro Antioxidant and In Vivo Antidepressant-like Actions
Abstract
1. Introduction
2. Results and Discussion
2.1. Nanoparticle Preparation and Characterization
2.2. Antioxidant Activities
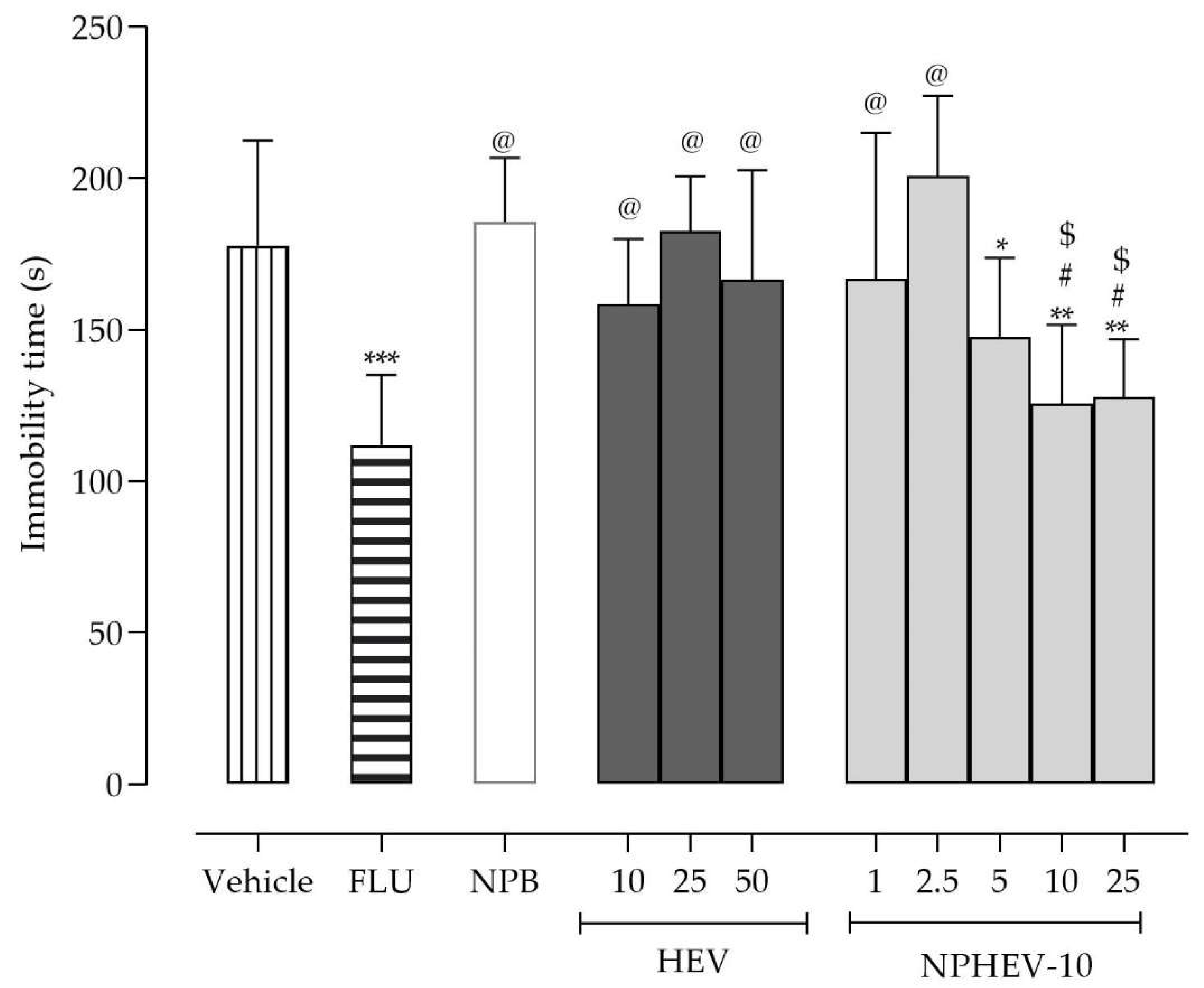
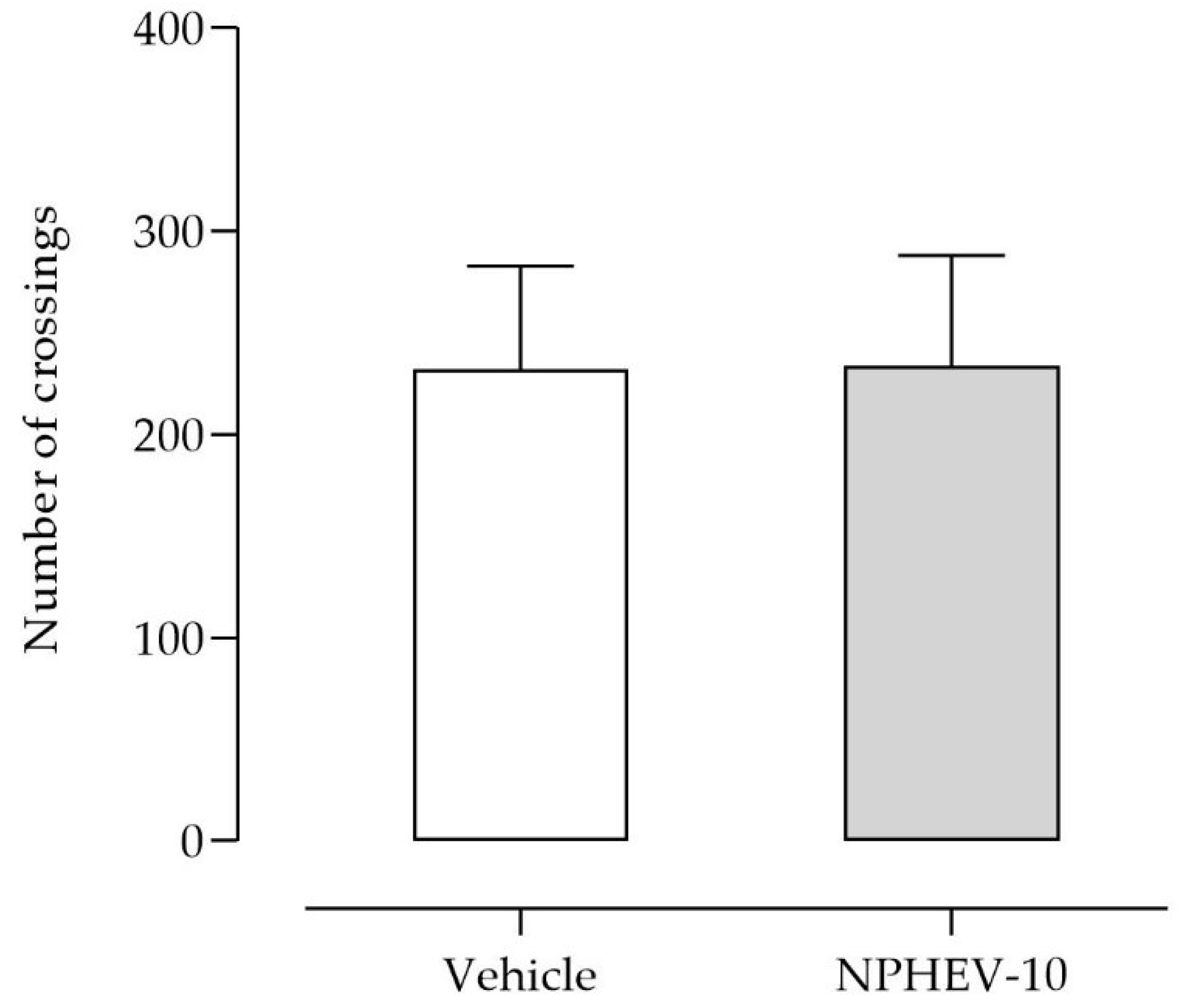
2.3. Acute Antidepressant-like Activity
3. Materials and Methods
3.1. Chemicals
3.2. Planta Material and Extract Preparation
3.3. Analytical Method
3.4. Nanoparticle Suspensions Containing HEV
3.5. Physicochemical Characterization
3.5.1. Particle Size Analysis, Polydispersity Index, pH, and Zeta Potential
3.5.2. Phenolic Content and Encapsulation Efficiency
3.6. Antioxidant Assays
3.6.1. DPPH Assay
3.6.2. Oxygen Radical Absorbance Capacity Test
3.7. Acute Antidepressant-like Action Investigation
3.7.1. Animals
3.7.2. Tail Suspension Test
3.7.3. Forced Swimming Test
3.7.4. Locomotor Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakamura, C.A.; Scazufca, M.; Moretti, F.A.; Didone, T.V.N.; de Sá Martins, M.M.; Pereira, L.A.; de Souza, C.H.Q.; de Oliveira, G.M.; da Costa, M.O.; Machado, M.; et al. Digital Psychosocial Intervention for Depression among Older Adults in Socioeconomically Deprived Areas in Brazil (PRODIGITAL-D): Protocol for an Individually Randomised Controlled Trial. Trials 2022, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- WHO Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 28 December 2022).
- Mrejen, M.; Hone, T.; Rocha, R. Socioeconomic and Racial/Ethnic Inequalities in Depression Prevalence and the Treatment Gap in Brazil: A Decomposition Analysis. SSM-Popul. Health 2022, 20. [Google Scholar] [CrossRef] [PubMed]
- WHO Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017.
- Pelucio, L.; Simões, P.; Dourado, M.C.N.; Quagliato, L.A.; Nardi, A.E. Depression and Anxiety among Online Learning Students during the COVID-19 Pandemic: A Cross-Sectional Survey in Rio de Janeiro, Brazil. BMC Psychol. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Hoefler, R.; Galvão, T.F.; Ribeiro-Vaz, I.; Silva, M.T. Trends in Brazilian Market of Antidepressants: A Five-Year Dataset Analysis. Front. Pharmacol. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Faquih, A.E.; Memon, R.I.; Hafeez, H.; Zeshan, M.; Naveed, S. A Review of Novel Antidepressants: A Guide for Clinicians. Cureus 2019. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J. Herbal Medicines in the Treatment of Psychiatric Disorders: 10-Year Updated Review. Phyther. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zhang, K.; Zhang, R.; Shi, H.; Bi, K.; Chen, X. Antidepressant-like Effect of the Water Extract of the Fixed Combination of Gardenia Jasminoides, Citrus Aurantium and Magnolia Officinalis in a Rat Model of Chronic Unpredictable Mild Stress. Phytomedicine 2015, 22, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.Z.; Lu, C.; Dong, L.M.; Le Zhai, J.; Liao, Y.H.; Aibai, S.; Yang, Y.; Liu, X.M. Antidepressant-like Effects and Cognitive Enhancement of the Total Phenols Extract of Hemerocallis Citrina Baroni in Chronic Unpredictable Mild Stress Rats and Its Related Mechanism. J. Ethnopharmacol. 2016, 194, 819–826. [Google Scholar] [CrossRef]
- Spohr, L.; Luduvico, K.P.; Soares, M.S.P.; Bona, N.P.; Oliveira, P.S.; de Mello, J.E.; Alvez, F.L.; Teixeira, F.C.; de Oliveira Campello Felix, A.; Stefanello, F.M.; et al. Blueberry Extract as a Potential Pharmacological Tool for Preventing Depressive-like Behavior and Neurochemical Dysfunctions in Mice Exposed to Lipopolysaccharide. Nutr. Neurosci. 2020, 25, 857–870. [Google Scholar] [CrossRef]
- Gapski, A.; Gomes, T.M.; Bredun, M.A.; Ferreira-Lima, N.E.; Ludka, F.K.; Bordignon-Luiz, M.T.; Burin, V.M. Digestion Behavior and Antidepressant-like Effect Promoted by Acute Administration of Blueberry Extract on Mice. Food Res. Int. 2019, 125. [Google Scholar] [CrossRef]
- Radünz, A.L.; Herter, F.G.; Radünz, M.; Radünz, L.L.; Scariot, M.A.; Silva, V.N.; Tonin, S.T.; Gilson, I.K.; Bizollo, A.R.; Ducatti, R.D.B. Productive Characterization of the Blueberry Cultivar Bluegem in Brazil. Agron. Colomb. 2021, 39, 89–96. [Google Scholar] [CrossRef]
- Cantuarias-Avilés, T.; Da Silva, S.R.; Medina, R.B.; Moraes, A.F.G.; Alberti, M.F. Cultivo Do Mirtilo: Atualizações e Desempenho Inicial de Variedades de Baixa Exigência Em Frio No Estado de São Paulo. Rev. Bras. Frutic. 2014, 36, 139–147. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Barcia, M.T.; Jacques, A.C.; Vizzotto, M.; Godoy, H.T.; Zambiazi, R.C. Quantification of Several Bioactive Compounds and Antioxidant Activities of Six Cultivars of Brazilian Blueberry. Nat. Prod. J. 2012, 2, 188–195. [Google Scholar] [CrossRef]
- Ferlemi, A.; Lamari, F.N. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cezarotto, V.; Giacomelli, S.; Vendruscolo, M.; Vestena, A.; Cezarotto, C.; da Cruz, R.; Maurer, L.; Ferreira, L.; Emanuelli, T.; Cruz, L. Influence of Harvest Season and Cultivar on the Variation of Phenolic Compounds Composition and Antioxidant Properties in Vaccinium Ashei Leaves. Molecules 2017, 22, 1603. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmiański, J.; Kostrzewa-Nowak, D.; Tarasiuk, J. In Vitro Antileukaemic Activity of Extracts from Berry Plant Leaves against Sensitive and Multidrug Resistant HL60 Cells. Cancer Lett. 2006, 236, 282–291. [Google Scholar] [CrossRef]
- Sakaida, H.; Nagao, K.; Higa, K.; Shirouchi, B.; Inoue, N.; Hidaka, F.; Kai, T.; Yanagita, T. Effect of Vaccinium Ashei Reade Leaves on Angiotensin Converting Enzyme Activity in Vitro and on Systolic Blood Pressure of Spontaneously Hypertensive Rats in Vivo. Biosci. Biotechnol. Biochem. 2007, 71, 2335–2337. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, B.X.; Geng, L.J. Hypolipidemic and Antioxidant Effects of Total Flavonoids from Blueberry Leaves. Eur. Food Res. Technol. 2011, 233, 897–903. [Google Scholar] [CrossRef]
- Yuji, K.; Sakaida, H.; Kai, T.; Fukuda, N.; Yukizaki, C.; Sakai, M.; Tsubouchi, H.; Kataoka, H. Effect of Dietary Blueberry (Vaccinium Ashei Reade) Leaves on Serum and Hepatic Lipid Levels in Rats. J. Oleo Sci. 2013, 62, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries Decrease Cardiovascular Risk Factors in Obese Men and Women with Metabolic Syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef]
- Mechikova, G.Y.; Kuzmich, A.S.; Ponomarenko, L.P.; Kalinovsky, A.I.; Stepanova, T.A.; Fedorov, S.N.; Stonik, V.A. Cancer-Preventive Activities of Secondary Metabolites from Leaves of the Bilberry Vaccinium Smallii A. Gray. Phyther. Res. 2010, 24, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, C.; Kanjilal, S.; Gupta, A.; Katiyar, S. Drug Discovery from Plant Sources: An Integrated Approach. AYU (An. Int. Q. J. Res. Ayurveda) 2012, 33, 10. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sharangi, A.B. Nanotechnology: A Potential Tool in Exploring Herbal Benefits. Nanotechnol. Life Sci. 2020, 27–46. [Google Scholar] [CrossRef]
- Bansal, G.; Suthar, N.; Kaur, J.; Jain, A. Stability Testing of Herbal Drugs: Challenges, Regulatory Compliance and Perspectives. Phyther. Res. 2016, 30, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Teja, P.K.; Mithiya, J.; Kate, A.S.; Bairwa, K.; Chauthe, S.K. Herbal Nanomedicines: Recent Advancements, Challenges, Opportunities and Regulatory Overview. Phytomedicine 2022, 96, 153890. [Google Scholar] [CrossRef]
- Sandhiya, V.; Ubaidulla, U. A Review on Herbal Drug Loaded into Pharmaceutical Carrier Techniques and Its Evaluation Process. Futur. J. Pharm. Sci. 2020, 6, 51. [Google Scholar] [CrossRef]
- Mazdaei, M.; Asare-Addo, K. A Mini-Review of Nanocarriers in Drug Delivery Systems. Br. J. Pharm. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Ansari, S.; Sameem, M.; Islam, F. Influence of Nanotechnology on Herbal Drugs: A Review. J. Adv. Pharm. Technol. Res. 2012, 3, 142. [Google Scholar] [CrossRef]
- Júlia, A.; Dalcin, F.; Ferreira Ourique, A.; Gomes, P. Cationic Nanocapsules Containing Eudragit RS100® and Its Potential for Application in Nanomedicine. Discip. Sci.|Nat. Tecnol. 2017, 18, 545–566. [Google Scholar]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Jana, U.; Mohanty, A.K.; Pal, S.L.A.L.; Manna, P.K.; Mohanta, G.P. Preparation and in Vitro Characterization of Felodipine Loaded Eudragit® RS100 Nanoparticles. Innovare Acad. Sci. 2014, 6, 4–7. [Google Scholar]
- Santos, S.S.; Lorenzoni, A.; Pegoraro, N.S.; Denardi, L.B.; Alves, S.H.; Schaffazick, S.R.; Cruz, L. Formulation and in Vitro Evaluation of Coconut Oil-Core Cationic Nanocapsules Intended for Vaginal Delivery of Clotrimazole. Colloids Surf. B Biointerfaces 2014, 116, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Barola, C.; Blasi, F.; Moretti, S.; Galarini, R.; Cossignani, L. Investigation on Chlorogenic Acid Stability in Aqueous Solution after Microwave Treatment. Food Chem. 2022, 374. [Google Scholar] [CrossRef] [PubMed]
- Frutos, M.J.; Rincón-Frutos, L.; Valero-Cases, E. Rutin. Nonvitam. Nonminer. Nutr. Suppl. 2019, 111–117. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, H.R.; Lee, K.A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC) and Phenolic and Anthocyanin Concentrations in Fruit and Leaf Tissues of Highbush Blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- Piljac-Zegarac, J.; Belscak, A. Piljac, a Antioxidant Capacity and Polyphenolic Content of Blueberry (Vaccinium Corymbosum L.) Leaf Infusions. J. Med. Food 2009, 12, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Mishra, Y.; Amin, H.I.M.; Mishra, V.; Vyas, M.; Prabhakar, P.K.; Gupta, M.; Kanday, R.; Sudhakar, K.; Saini, S.; Hromić-Jahjefendić, A.; et al. Application of Nanotechnology to Herbal Antioxidants as Improved Phytomedicine: An Expanding Horizon. Biomed. Pharmacother. 2022, 153, 113413. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, M.C.L.; Rigon, C.; Copetti, P.M.; Sagrillo, M.R.; Cruz, L. Nanoencapsulation Improves Scavenging Capacity and Decreases Cytotoxicity of Silibinin and Pomegranate Oil Association. AAPS PharmSciTech 2017, 18, 3236–3246. [Google Scholar] [CrossRef] [PubMed]
- Mattiazzi, J.; Sari, M.H.M.; Lautenchleger, R.; Dal Prá, M.; Braganhol, E.; Cruz, L. Incorporation of 3,3′-Diindolylmethane into Nanocapsules Improves Its Photostability, Radical Scavenging Capacity, and Cytotoxicity Against Glioma Cells. AAPS PharmSciTech 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Faizi, M.; Jahani, R.; Khaledyan, D.; Jahani, A.; Jamshidi, E.; Kamalinejad, M.; Khoramjouy, M.; Faizi, M. Evaluation and Comparison of the Antidepressant-like Activity of Artemisia Dracunculus and Stachys Lavandulifolia Ethanolic Extracts: An in Vivo Study. Res. Pharm. Sci. 2019, 14, 544–553. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of Rutin with β-Cyclodextrin as Potential Delivery System. PLoS ONE 2015, 10, e0120858. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Cervi, V.F.; Prado, V.C.; Nadal, J.M.; Azambuja, J.H.; da Silveira, E.F.; Nogueira, C.W.; Farago, P.V.; Braganhol, E.; et al. Design of Pegylated-Nanocapsules to Diphenyl Diselenide Administration: In Vitro Evidence of Hemocompatible and Selective Antiglioma Formulation. AAPS PharmSciTech 2020, 21. [Google Scholar] [CrossRef]
- Castagné, V.; Porsolt, R.D.; Moser, P. Early Behavioral Screening for Antidepressants and Anxiolytics. Drug Dev. Res. 2006, 67, 729–742. [Google Scholar] [CrossRef]
- Castagné, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent Models of Depression: Forced Swim and Tail Suspension Behavioral Despair Tests in Rats and Mice. Curr. Protoc. Neurosci. 2011, 55, 8.10A.1–8.10A.14. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, D.; Sun, H.; Qu, Y.; Su, X. Behavioral Tests for Evaluating the Characteristics of Brain Diseases in Rodent Models: Optimal Choices for Improved Outcomes (Review). Mol. Med. Rep. 2022, 25, 183. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Bitencourt, P.E.R.; Ferreira, L.M.; Cargnelutti, L.O.; Denardi, L.; Boligon, A.; Fleck, M.; Brandão, R.; Athayde, M.L.; Cruz, L.; Zanette, R.A.; et al. A New Biodegradable Polymeric Nanoparticle Formulation Containing Syzygium Cumini: Phytochemical Profile, Antioxidant and Antifungal Activity and in Vivo Toxicity. Ind. Crops Prod. 2016, 83, 400–407. [Google Scholar] [CrossRef]
- Berrocoso, E.; Ikeda, K.; Sora, I.; Uhl, G.R.; Sánchez-Blázquez, P.; Mico, J.A. Active Behaviours Produced by Antidepressants and Opioids in the Mouse Tail Suspension Test. Int. J. Neuropsychopharmacol. 2013, 16, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The Tail Suspension Test: A New Method for Screening Antidepressants in Mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jafre, M. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Stein, A.C.; Viana, A.F.; Müller, L.G.; Nunes, J.M.; Stolz, E.D.; Rego, J.D.; Costentin, J.; Von Poser, G.L.; Rates, S.M.K. Uliginosin B, a Phloroglucinol Derivative from Hypericum Polyanthemum: A Promising New Molecular Pattern for the Development of Antidepressant Drugs. Behav. Brain Res. 2012, 228, 66–73. [Google Scholar] [CrossRef] [PubMed]

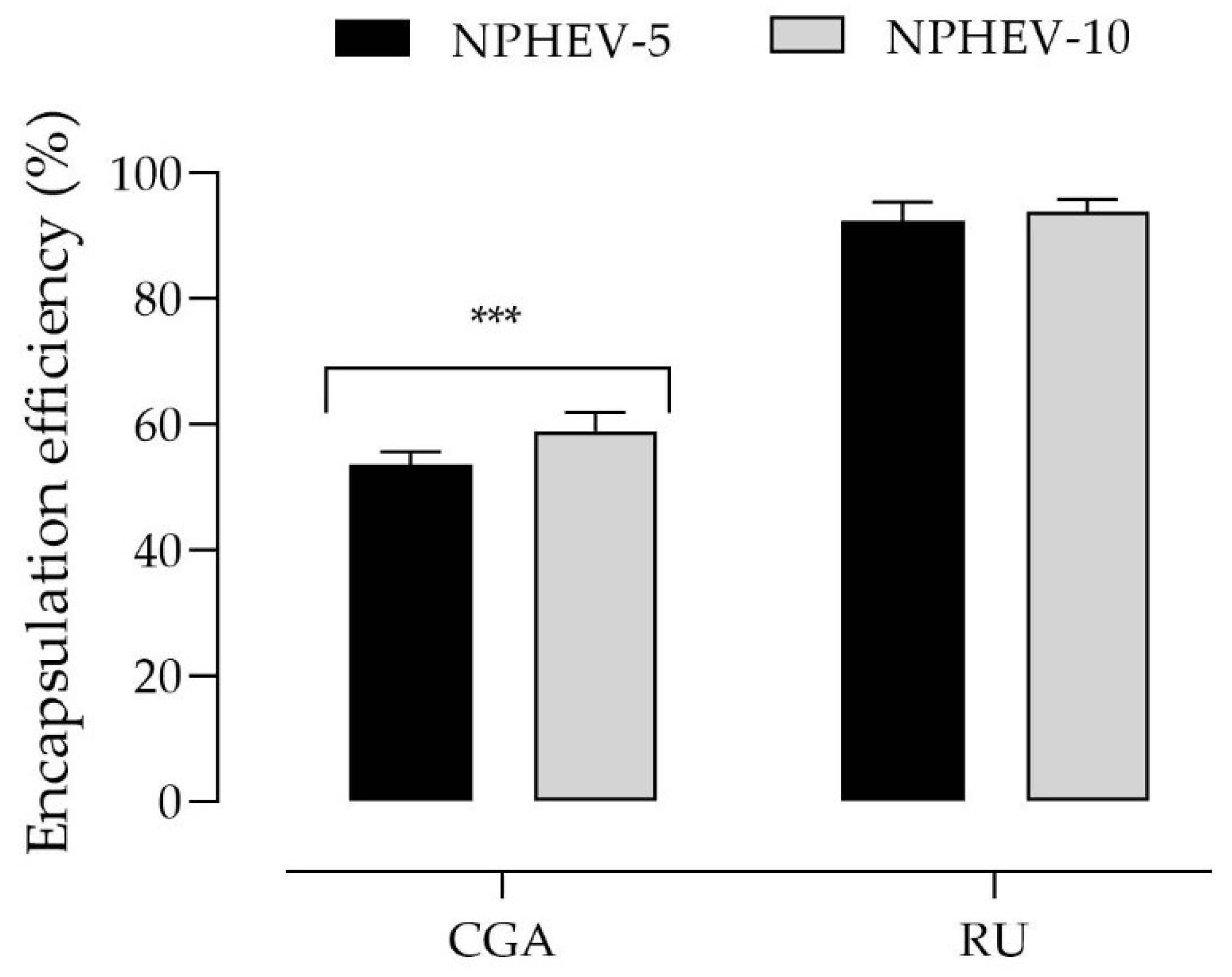

| Average Diameter (nm) | PDI | Zeta Potential (mV) | pH | CGA Content (%) | RU Content (%) | |
|---|---|---|---|---|---|---|
| NPB | 206 ± 7 | 0.163 ± 0.00 | +8.8 ± 0.12 | 4.6 ± 0.03 | - | - |
| NPHEV-5 | 143 ± 5 * | 0.164 ± 0.03 | +13.1 ± 10.3 | 3.9 ± 0.12 | 87.1 ± 5.9 | 92.3 ± 12.5 |
| NPHEV-10 | 144 ± 1 * | 0.142 ± 0.01 | +15.5 ± 8.24 | 3.7 ± 0.10 | 91.7 ± 12.56 | 99.9 ± 8.4 |
| DPPH (µg/mL) $ | ORAC Values (mmol/L) | |
|---|---|---|
| HEV | 12.39 ± 0.02 | 70 ± 4 |
| NPHEV-5 | 16.30 ± 0.50 * | 91 ± 8 * |
| NPHEV-10 | 14.40 ± 0.40 *# | 133 ± 11 *# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cezarotto, V.S.; Franceschi, E.P.; Stein, A.C.; Emanuelli, T.; Maurer, L.H.; Sari, M.H.M.; Ferreira, L.M.; Cruz, L. Nanoencapsulation of Vaccinium ashei Leaf Extract in Eudragit® RS100-Based Nanoparticles Increases Its In Vitro Antioxidant and In Vivo Antidepressant-like Actions. Pharmaceuticals 2023, 16, 84. https://doi.org/10.3390/ph16010084
Cezarotto VS, Franceschi EP, Stein AC, Emanuelli T, Maurer LH, Sari MHM, Ferreira LM, Cruz L. Nanoencapsulation of Vaccinium ashei Leaf Extract in Eudragit® RS100-Based Nanoparticles Increases Its In Vitro Antioxidant and In Vivo Antidepressant-like Actions. Pharmaceuticals. 2023; 16(1):84. https://doi.org/10.3390/ph16010084
Chicago/Turabian StyleCezarotto, Verciane Schneider, Eduarda Piovesan Franceschi, Ana Cristina Stein, Tatiana Emanuelli, Luana Haselein Maurer, Marcel Henrique Marcondes Sari, Luana Mota Ferreira, and Letícia Cruz. 2023. "Nanoencapsulation of Vaccinium ashei Leaf Extract in Eudragit® RS100-Based Nanoparticles Increases Its In Vitro Antioxidant and In Vivo Antidepressant-like Actions" Pharmaceuticals 16, no. 1: 84. https://doi.org/10.3390/ph16010084
APA StyleCezarotto, V. S., Franceschi, E. P., Stein, A. C., Emanuelli, T., Maurer, L. H., Sari, M. H. M., Ferreira, L. M., & Cruz, L. (2023). Nanoencapsulation of Vaccinium ashei Leaf Extract in Eudragit® RS100-Based Nanoparticles Increases Its In Vitro Antioxidant and In Vivo Antidepressant-like Actions. Pharmaceuticals, 16(1), 84. https://doi.org/10.3390/ph16010084








