Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2
Abstract
:1. Introduction
2. Results
2.1. Extraction Yield of MCEOs
2.2. Chemical Profile of EOs from Myrtus communis L.
2.3. Antioxidant Activity
2.4. Anti-Inflammatory Activity
2.4.1. In Vitro Study: Inhibition of BSA Denaturation
2.4.2. In Vivo Study: Carrageenan-Induced Paw Edema in Rats
2.4.3. In Silico Results
3. Discussion
3.1. Phytochemical Analysis
3.2. Antioxidant Activity of MCEOs
3.3. Anti-Inflammatory Activity
3.4. In Silico Analysis
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection of Plant Material
4.3. Essential Oil Extraction
4.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis of EO
4.5. Antioxidant Activity
4.5.1. Scavenging Activity of the DPPH• Radical Assay
4.5.2. Hydroxyl Radical (OH•) Scavenging Assay
4.5.3. Scavenging ABTS•+ Radical Test
4.6. Anti-Inflammatory Activity
4.6.1. In Vitro: Inhibition of BSA Denaturation Test
4.6.2. In Vivo: Carrageenan-Induced Paw Edema in Rats
| Groups | Status | Optimum Doses and Route of Administration | Justification of Optimal Doses Choice |
|---|---|---|---|
| Group I | Control group | Vehicle (distilled water) (per os) + 100 µL of carrageenan (1%) (ipl) | [93] |
| Group II | Experimental group A | Diclofenac (50 mg/kg) (per os) + 100 µL carrageenan (1%) (ipl) | [94,95] |
| Group III | Experimental group B | MCEOs (25 mg/kg) (per os) + 100 µL carrageenan (1%) (ipl) | [35] |
| Group IV | Experimental group C | MCEOs (50 mg/kg) (per os) + 100 µL carrageenan (1%) (ipl) | [35] |
4.7. In Silico Study
4.7.1. Preparation of Cyclooxygenase-2 Crystallographic Structure and Generation of Receptor Grid
4.7.2. Preparation of Compounds
4.7.3. Virtual Screening Procedure
4.7.4. MM–GBSA Analysis
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Kalhoro, M.T.; Zhang, H.; Kalhoro, G.M.; Wang, F.; Chen, T.; Faqir, Y.; Nabi, F.J.S.R. Fungicidal properties of ginger (Zingiber officinale) essential oils against Phytophthora colocasiae. Sci. Rep. 2022, 12, 2191. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Al-Hamoud, K.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z. Diversity of Citrullus colocynthis (L.) Schrad Seeds Extracts: Detailed Chemical Profiling and Evaluation of Their Medicinal Properties. Plants 2023, 12, 567. [Google Scholar] [CrossRef]
- Harassi, Y.; Tilaoui, M.; Idir, A.; Frederic, J.; Baudino, S.; Ajouaoi, S.; Mouse, H.A.; Zyad, A. Phytochemical analysis, cytotoxic and antioxidant activities of Myrtus communis essential oil from Morocco. J. Complement. Integr. Med. 2019, 16, 23–43. [Google Scholar] [CrossRef]
- Saha, K.; Lajis, N.H.; Israf, D.A.; Hamzah, A.S.; Khozirah, S.; Khamis, S.; Syahida, A. Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J. Ethnopharmacol. 2004, 92, 263–267. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Bharadvaja, N. Potential Benefits of Nutraceuticals for Oxidative Stress Management. Rev. Bras. Farm. 2022, 32, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, A.; Abdalla, S.; Baghiani, A.; Charef, N.J.A. Phytochemical analysis, hypotensive effect and antioxidant properties of Myrtus communis L. growing in Algeria. Asian J. Trop. Biomed. 2015, 5, 19–28. [Google Scholar] [CrossRef]
- Gyesi, J.N.; Opoku, R.; Borquaye, L.S.J. Chemical composition, total phenolic content, and antioxidant activities of the essential oils of the leaves and fruit pulp of Annona muricata L.(Soursop) from Ghana. Biochem. Res. Int. 2019, 2019, 4164576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Ning, B.T.J.S.T.; Therapy, T. Signaling pathways and intervention therapies in sepsis. Signal Transduct. Target. Ther. 2021, 6, 407–443. [Google Scholar] [CrossRef] [PubMed]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Eid, B.G. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed. Pharmacother. 2019, 120, 109567. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef]
- García-Rayado, G.; Navarro, M.; Lanas, A. NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert. Rev. Clin. Pharmacol. 2018, 11, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Akinrinde, A.S.; Soetan, K.O.; Tijani, M.O. Exacerbation of diclofenac-induced gastroenterohepatic damage by concomitant exposure to sodium fluoride in rats: Protective role of luteolin. Drug Chem. Toxicol. 2022, 45, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- De Siqueira Patriota, L.L.; De Brito Marques Ramos, D.; Silva, M.G.; Dos Santos, A.C.L.A.; Silva, Y.A.; Paiva, P.M.G.; Pontual, E.V.; De Albuquerque, L.P.; Mendes, R.L.; Napoleão, T.H. Inhibition of Carragenan-Induced Acute Inflammation in Mice by the Microgramma vacciniifolia Frond Lectin (MvFL). Polymers 2022, 14, 1609. [Google Scholar] [CrossRef]
- Izak-Shirian, F.; Najafi-Asl, M.; Azami, B.; Heidarian, E.; Najafi, M.; Khaledi, M.; Nouri, A. Quercetin exerts an ameliorative effect in the rat model of diclofenac-induced renal injury through mitigation of inflammatory response and modulation of oxidative stress. Eur. J. Inflamm. 2022, 20, 172. [Google Scholar] [CrossRef]
- Hassan, R.A.; Hozayen, W.G.; Abo Sree, H.T.; Al-Muzafar, H.M.; Amin, K.A.; Ahmed, O.M. Naringin and Hesperidin Counteract Diclofenac-Induced Hepatotoxicity in Male Wistar Rats via their Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Oxidative Med. Cell. Longev. 2021, 2021, 9990091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Zhang, Z.B.; Zheng, Y.F.; Chen, H.M.; Yu, X.T.; Chen, X.Y.; Zhang, X.; Xie, J.H.; Su, Z.Q.; Feng, X.X.; et al. Gastro-protective effect of andrographolide sodium bisulfite against indomethacin-induced gastric ulceration in rats. Int. Immunopharmacol. 2015, 26, 384–391. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Ragno, R.; Del Bufalo, D. Essential oils and their main chemical components: The past 20 years of preclinical studies in Melanoma. Cancers 2020, 12, 2650. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Martineau, A.R. Inflammation-mediated tissue damage in pulmonary tuberculosis and host-directed therapeutic strategies. Semin. Immunol. 2023, 65, 101672. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; Tahir Ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, P.; Thangam, E.B. Vitex trifolia L. modulates inflammatory mediators via down-regulation of the NF-κB signaling pathway in carrageenan-induced acute inflammation in experimental rats. J. Ethnopharmacol. 2022, 298, 115583. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Bottoni, M.; Milani, F.; Todero, S.; Berera, P.; Maggi, F.; Santagostini, L.; Fico, G. Botanic Garden as a Factory of Molecules: Myrtus communis L. subsp. communis as a Case Study. Plants 2022, 11, 754. [Google Scholar] [CrossRef]
- Berka-Zougali, B.; Ferhat, M.A.; Hassani, A.; Chemat, F.; Allaf, K.S. Comparative Study of Essential Oils Extracted from Algerian Myrtus communis L. Leaves Using Microwaves and Hydrodistillation. Int. J. Mol. Sci. 2012, 13, 4673–4695. [Google Scholar] [CrossRef]
- Giampieri, F.; Cianciosi, D.; Forbes-Hernández, T.Y. Myrtle (Myrtus communis L.) berries, seeds, leaves, and essential oils: New undiscovered sources of natural compounds with promising health benefits. Food Front. 2020, 1, 276–295. [Google Scholar] [CrossRef]
- Aggul, A.G.; Demir, G.M.; Gulaboglu, M. Ethanol Extract of Myrtle (Myrtus communis L.) Berries as a Remedy for Streptozotocin-Induced Oxidative Stress in Rats. Appl. Biochem. Biotechnol. 2022, 194, 1645–1658. [Google Scholar] [CrossRef]
- Zanetti, S.; Cannas, S.; Molicotti, P.; Bua, A.; Cubeddu, M.; Porcedda, S.; Marongiu, B.; Sechi, L.A. Evaluation of the Antimicrobial Properties of the Essential Oil of Myrtus communis L. against Clinical Strains of Mycobacterium spp. Interdiscip. Perspect. Infect. Dis. 2010, 10, 931330. [Google Scholar]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, F.; Vivenzio, G.; Nazzaro, F.J. Chemical composition, antibiofilm, cytotoxic, and anti-acetylcholinesterase activities of Myrtus communis L. leaves essential oil. BMC Complement. Med. Ther. 2022, 22, 142. [Google Scholar] [CrossRef]
- Tichati, L. Chemical characterization with GC-MS and evaluation of antioxidant and anti-inflammatory activities of Algerian essential oil from Myrtus communis. South. Asian J. 2022, 12, 267–274. [Google Scholar]
- Okaiyeto, K.; Kerebba, N.; Rautenbach, F.; Singh, S.K.; Dua, K.; Oguntibeju, O.O. UPLC-ESI-QTOF-MS phenolic compounds identification and quantification from ethanolic extract of Myrtus communis ‘Variegatha’: In vitro antioxidant and anti-diabetic potentials. Arab. J. Chem. 2023, 16, 104447. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Ugurlu, P.; Celik, C.; Sen, N.B.; Helvacıoglu, S.; Charehsaz, M.; Erdogan, M.; Ockun, M.A.; Kırmızıbekmez, H.; Aydın, A.J. Myrtus communis L. (Myrtle) plant parts: Comparative assessment of their chemical compositions, antioxidant, anticancer, and anti-mutagenic activities. South. Afr. J. Bot. 2022, 150, 711–720. [Google Scholar] [CrossRef]
- Khosropour, P.; Sajjadi, S.E.; Talebi, A.; Minaiyan, M.J. Anti-inflammatory effect of Myrtus communis hydroalcoholic extract and essential oil on acetic acid–induced colitis in rats. J. Rep. Pharm. Sci. 2019, 8, 204. [Google Scholar]
- Touaibia, M. Composition and Anti-inflammatory Effect of the Common Myrtle’s (Myrtus communis L.) Essential Oil Growing Wild in Algeria. Phytotherapie 2020, 18, 156–161. [Google Scholar] [CrossRef]
- Acree, A.; Arn, H. Gas Chromatography-Olfactometry (GCO) of Natural Products. DATU Inc. Available online: https://www.flavornet.org/f_kovats.html (accessed on 22 July 2023).
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Foudil-Cherif, Y.; Boutarene, N.; Yassaa, N. Chemical composition of essential oils of Algerian Myrtus communis and chiral analysis of their leave volatiles. J. Essen. Oil Res. 2013, 25, 402–408. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Al-Hajj, N.; Jaber, S. Myrtus communis L.: Essential oil chemical composition, total phenols and flavonoids contents, antimicrobial, antioxidant, anticancer, and α-amylase inhibitory activity. Chem. Biol. Technol. Agric. 2023, 10, 41. [Google Scholar] [CrossRef]
- Pereira, P.C.; Cebola, M.J.; Bernardo-Gil, M.G. Evolution of the Yields and Composition of Essential Oil from Portuguese Myrtle (Myrtus comunis L.) through the Vegetative Cycle. Molecules 2009, 14, 3094–3105. [Google Scholar] [CrossRef] [PubMed]
- Dejam, M.; Farahmand, Y. Essential oil content and composition of myrtle (Myrtus communis L.) leaves from South of Iran. J. Essen. Oil Bear. Plants 2017, 20, 869–872. [Google Scholar] [CrossRef]
- Asllani, U. Chemical composition of Albanian myrtle oil (Myrtus communis L.). J. Essen. Oil Res. 2000, 12, 140–142. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Bugarin, D.; Grbović, S.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Orčić, D.; Jovin, E.; Couladis, M. Essential oil of Myrtus communis L. as a potential antioxidant and anti-mutagenic agents. Molecules 2010, 15, 2759–2770. [Google Scholar] [CrossRef]
- Bradesi, P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A.F. Chemical Composition of Myrtle Leaf Essential Oil from Corsica (France). J. Essent. Oil Res. 1997, 9, 283–288. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Garry, R.P.; Michet, A. Essential oils of myrtle (Myrtus communis L.) of the Mediterranean littoral. J. Essent. Oil Res. 1998, 10, 613–617. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Jemia, M.B.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Mirzakhani, M.; Pirbalouti, A.G. Essential oil variation among 21 wild myrtle (Myrtus communis L.) populations collected from different geographical regions in Iran. Ind. Crops Prod. 2013, 51, 328–333. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Hamdi, N.; Miladi, R.; Abdelkafi, S. Myrtus communis essential oil: Chemical composition and antimicrobial activities against food spoilage pathogens. Chem. Biodivers. 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Bekhechi, C.; Watheq-Malti, C.E.; Boussaïd, M.; Achouri, I.; Belilet, K.; Gibernau, M.; Casanova, J.; Tomi, F. Composition and chemical variability of Myrtus communis leaf oil from Northwestern Algeria. Nat. Prod. Comm. 2019, 14, 1934578X19850030. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I.; Maccioni, S.; Baldini, R. Phytochemical typologies in some populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy). Food Chem. 2004, 85, 599–604. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Mohafez, O.M.; Khalil, H.E.; Alhaider, I.A. Essential oil from myrtle leaves growing in the eastern part of Saudi Arabia: Components, anti-inflammatory and cytotoxic activities. J. Essen. Oil Bear. Plants 2019, 22, 877–892. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Mulas, M. Climate Variables of the Sites of Origin and Genotype Influence on Phenolic Compounds Accumulation in Cultivars of Myrtus communis L. Horticulture 2022, 8, 928. [Google Scholar] [CrossRef]
- Hong, M.; Kim, M.; Jang, H.; Bo, S.; Deepa, P.; Sowndhararajan, K.; Kim, S. Multivariate Analysis of Essential Oil Composition of Artemisia annua L. Collected from Different Locations in Korea. Molecules 2023, 28, 1131. [Google Scholar] [CrossRef]
- Hazrati, S.; Hosseini, S.J.; Ebadi, M.T.; Nicola, S. Evolution of Phytochemical Variation in Myrtle (Myrtus communis L.) Organs during Different Phenological Stages. Horticulture 2022, 8, 757. [Google Scholar] [CrossRef]
- Khan, M.H.; Dar, N.A.; Alie, B.A.; Dar, S.A.; Lone, A.A.; Mir, G.H.; Fayaz, U.; Ali, S.; Tyagi, A.; El-Sheikh, M.A. Unraveling the Variability of Essential Oil Composition in Different Accessions of Bunium persicum Collected from Different Temperate Micro-Climates. Molecules 2023, 28, 2404. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef]
- Snoussi, A.; Chaabouni, M.M.; Bouzouita, N.; Kachouri, F. Chemical Composition and Antioxidant Activity of Myrtus communis L. Floral Buds Essential Oil. J. Essent. Oil Res. 2011, 23, 10–14. [Google Scholar] [CrossRef]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El-Barnossi, A.; Benziane, Z.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef]
- Lim, A.C.; Tang, S.G.H.; Zin, N.M.; Maisarah, A.M.; Ariffin, I.A.; Ker, P.J.; Mahlia, T.M.I. Chemical Composition, Antioxidant, Antibacterial, and Anti-biofilm Activities of Backhousia citriodora Essential Oil. Molecules 2022, 27, 4895. [Google Scholar] [CrossRef]
- Hennia, A.; Nemmiche, S.; Guerreiro, A.; Faleiro, M.L.; Antunes, M.D.; Aazza, S.; Miguel, M.G. Antioxidant and anti-proliferative activities of Myrtus communis L. essential oils from different Algerian regions. J. Essen. Oil Bear. Plants 2019, 22, 1488–1499. [Google Scholar] [CrossRef]
- Gladikostić, N.; Ikonić, B.; Teslić, N.; Zeković, Z.; Božović, D.; Putnik, P.; Bursać Kovačević, D.; Pavlić, B. Essential Oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae Families Grown in Serbia: Comparative Chemical Profiling with In Vitro Antioxidant Activity. Plants 2023, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Loizzo, M.R.; Riccobono, L.; Bruno, M.; Tenuta, M.C.; Leporini, M.; Falco, T.; Leto, C.; Tuttolomondo, T.; Cammalleri, I.J. Comparative chemical composition and bioactivity of leaves essential oils from nine Sicilian accessions of Myrtus communis L. J. Essen. Oil Res. 2019, 31, 546–555. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical Compositions and Antioxidant Activities of Essential Oils, and Their Combinations, Obtained from Flavedo By-Product of Seven Cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Sehaki, C.; Jullian, N.; Choque, E.; Dauwe, R.; Fontaine, J.X.; Molinie, R.; Ayati, F.; Fernane, F.; Gontier, E. Profiling of Essential Oils from the Leaves of Pistacia lentiscus Collected in the Algerian Region of Tizi-Ouzou: Evidence of Chemical Variations Associated with Climatic Contrasts between Littoral and Mountain Samples. Molecules 2022, 27, 4148. [Google Scholar] [CrossRef]
- Othman, H.I.A.; Alkatib, H.H.; Zaid, A.; Sasidharan, S.; Rahiman, S.S.F.; Lee, T.P.; Dimitrovski, G.; Althakafy, J.T.; Wong, Y.F. Phytochemical Composition, Antioxidant and Antiproliferative Activities of Citrus hystrix, Citrus limon, Citrus pyriformis, and Citrus microcarpa Leaf Essential Oils against Human Cervical Cancer Cell Line. Plants 2023, 12, 134. [Google Scholar] [CrossRef]
- Simo, M.K.; Siwe, G.T.; Taboula, K.M.; Chen, Z.; Mangoua, K.M.; Dize, D.; Jazet, P.D.; Sameza, M.L.; Fekam, F.B.; Froldi, G.J. Anti-inflammatory, Antinociceptive, and Toxicological Properties of Uvaria comperei Stem Crude Extract and Fractions. Biomed. Res. Int. 2023, 23, 2754725. [Google Scholar] [CrossRef] [PubMed]
- Zouari, B.K.; Makni, S.; Tounsi, A.; Jlaiel, L.; Trigui, M.; Tounsi, S. Effects of Juniperus phoenicea Hydroalcoholic Extract on Inflammatory Mediators and Oxidative Stress Markers in Carrageenan-Induced Paw Oedema in Mice. Biomed. Res. Int. 2018, 2018, 3785487. [Google Scholar]
- Sivapalan, S.; Dharmalingam, S.; Venkatesan, V.; Angappan, M.; Ashokkumar, V. Phytochemical analysis, anti-inflammatory, antioxidant activity of Calotropis gigantea and its therapeutic applications. J. Ethnopharmacol. 2023, 303, 115963. [Google Scholar] [CrossRef]
- Dhami, A.; Palariya, D.; Singh, A.; Kumar, R.; Prakash, O.; Kumar, R.; Pant, A. Chemical composition, antioxidant, in vitro anti-inflammatory and antibacterial activity of seeds essential oil of Zanthoxylum armatum DC. Collected from two different altitudes of Kumaun region, Uttarakhand. Int. J. Chem. Sci. 2018, 6, 363–370. [Google Scholar]
- Akbar, A.; Gul, Z.; Chein, S.H.; Sadiq, M.B. Investigation of Anti-Inflammatory Properties, Phytochemical Constituents, Antioxidant, and Antimicrobial Potentials of the Whole Plant Ethanolic Extract of Achillea santolinoides subsp. wilhelmsii (K. Koch) Greuter of Balochistan. Oxidative Med. Cell. Longev. 2023, 23, 2567333. [Google Scholar] [CrossRef]
- Williams, L.; O’connar, A.; Latore, L.; Dennis, O.; Ringer, S.; Whittaker, J.; Conrad, J.; Vogler, B.; Rosner, H.; Kraus, W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West. Indian. Med. J. 2008, 57, 327–331. [Google Scholar]
- Kiliç, F.S.; Aydın, Ş.; Yıldırım, C.; Dönertaş, B.; Öner, S.; Kaygısız, B. Effects of gabapentin on carrageenan-induced inflammation, acute phase reactants and gastric mucus secretion in rats. Eur. J. Ther. 2019, 25, 23–27. [Google Scholar] [CrossRef]
- Boarescu, I.; Boarescu, P.M.; Pop, R.M.; Bocșan, I.C.; Gheban, D.; Râjnoveanu, R.M.; Râjnoveanu, A.; Bulboacă, A.E.; Buzoianu, A.D.; Bolboacă, S.D. Curcumin Nanoparticles Enhance Antioxidant Efficacy of Diclofenac Sodium in Experimental Acute Inflammation. Biomedicines 2022, 10, 61. [Google Scholar] [CrossRef]
- Al-Majed, A.; Khattab, M.; Raza, M.; Al-Shabanah, O.; Mostafa, A. Potentiation of diclofenac-induced anti-inflammatory response by aminoguanidine in carrageenan-induced acute inflammation in rats: The role of nitric oxide. Inflamm. Res. 2003, 52, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Jafarian, S.; Majnooni, M.B.; Farzaei, M.H.; Mohammadi-Noori, E.; Khan, H. Anti-nociceptive and anti-inflammatory activities of the essential oil isolated from Cupressus arizonica Greene fruits. Korean J. Pain. 2022, 35, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Oubihi, A.; Ballaoui, F.Z.; Imtara, H.; Jaber, H.; Ettouil, A.; Haida, S.; Ouhssine, M.; Noman, O.M.; Mothana, R.A.; Tarayrah, M.; et al. Phytochemical Compounds, Acute Toxicity, Anti-Inflammatory and Antioxidant Activities of Thymus leptobotrys Murb Essential Oil. Molecules 2023, 28, 1355. [Google Scholar] [CrossRef] [PubMed]
- Mbiri, J.W.; Kasili, S.; Patrick, K.; Mbinda, W.; Piero, N. Anti-inflammatory properties of methanolic bark extracts of Terminalia brownii in wistar albino rats. Int. J. Curr. Pharm. Rev. Res. 2016, 8, 100–104. [Google Scholar]
- Hasan, M.K.; Akhter, S.; Fatema, K.; Hossain, M.R.; Sultana, T.; Uzzaman, M. Selective modification of diclofenac to reduce the adverse effects; A computer-aided drug design approach. Inform. Med. Unlocked 2023, 36, 101159. [Google Scholar] [CrossRef]
- Calva, J.; Cartuche, L.; Castillo, L.N.; Morocho, V. Biological Activities and Chemical Composition of Essential Oil from Hedyosmum purpurascens (Todzia)—An Endemic Plant in Ecuador. Molecules 2023, 28, 2366. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Food 2022, 11, 464. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Pharm. Biol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Omoboyowa, D.A. Sterols from Jatropha tanjorensis leaves exhibit anti-inflammatory potential: In vitro and in silico studies. Bull. Natl. Res. Cent. 2021, 45, 194. [Google Scholar] [CrossRef]
- Omoboyowa, D.A.; Kareem, J.A.; Saibu, O.A.; Bodun, D.S.; Ajayi, T.M.; Oyeneyin, O.E. Identification of Phyto compounds from Ilex kudingcha as Inhibitors of Sterol-14α-Demethylase Protease: A Computational Approach against chagas Disease. Chem. Afr. 2022, 6, 1335–1347. [Google Scholar] [CrossRef]
- Omoboyowa, D.A. Virtual screening of phyto-compounds from Blighia sapida as protein tyrosine phosphatase 1B inhibitor: A computational approach against diabetes. Chem. Afr. 2022, 5, 871–881. [Google Scholar] [CrossRef]
- Olawale, F.; Olofinsan, K.; Iwaloye, O.; Chukwuemeka, P.O. Screening of compounds from Nigerian anti-diabetes plants as protein tyrosine phosphatase IB inhibitor. Comput. Toxicol. 2021, 21, 100200. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, T.; Takim, K.; Çeken, B.; Kizil, M. DNA damage protecting activity and in vitro antioxidant potential of the methanol extract of Cherry (Prunus avium L). J. Med. Plant Res. 2014, 8, 715–726. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kar, B.; Kumar, R.S.; Karmakar, I.; Dola, N.; Bala, A.; Mazumder, U.K.; Hadar, P. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S976–S980. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Vadivu, R.; Lakshmi, K.S. In vitro and in vivo-anti-inflammatory activity of leaves of Symplocos cochinchnensis (Lour) moore ssp laurina. Bangladesh J. Pharmacol. 2008, 3, 121–124. [Google Scholar] [CrossRef]
- Sari, D.P.; Wulandari, R.L. Anti-inflammatory effects of ethanol extract of Cymbopogon nardus herbs on rats induced by carrageenan. Proc. Mandala Waluya Int. Conf. Pharm. Sci. Pract. 2022, 1, 16–23. [Google Scholar]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L. OPLS3: A force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Omoboyowa, D.A.; Singh, G.; Fatoki, J.O.; Oyeneyin, O.E. Computational investigation of phytochemicals from Abrus precatorius seeds as modulators of peroxisome proliferator-activated receptor gamma (PPARγ). J. Biomol. Struct. Dyn. 2022, 41, 5568–5582. [Google Scholar] [CrossRef]
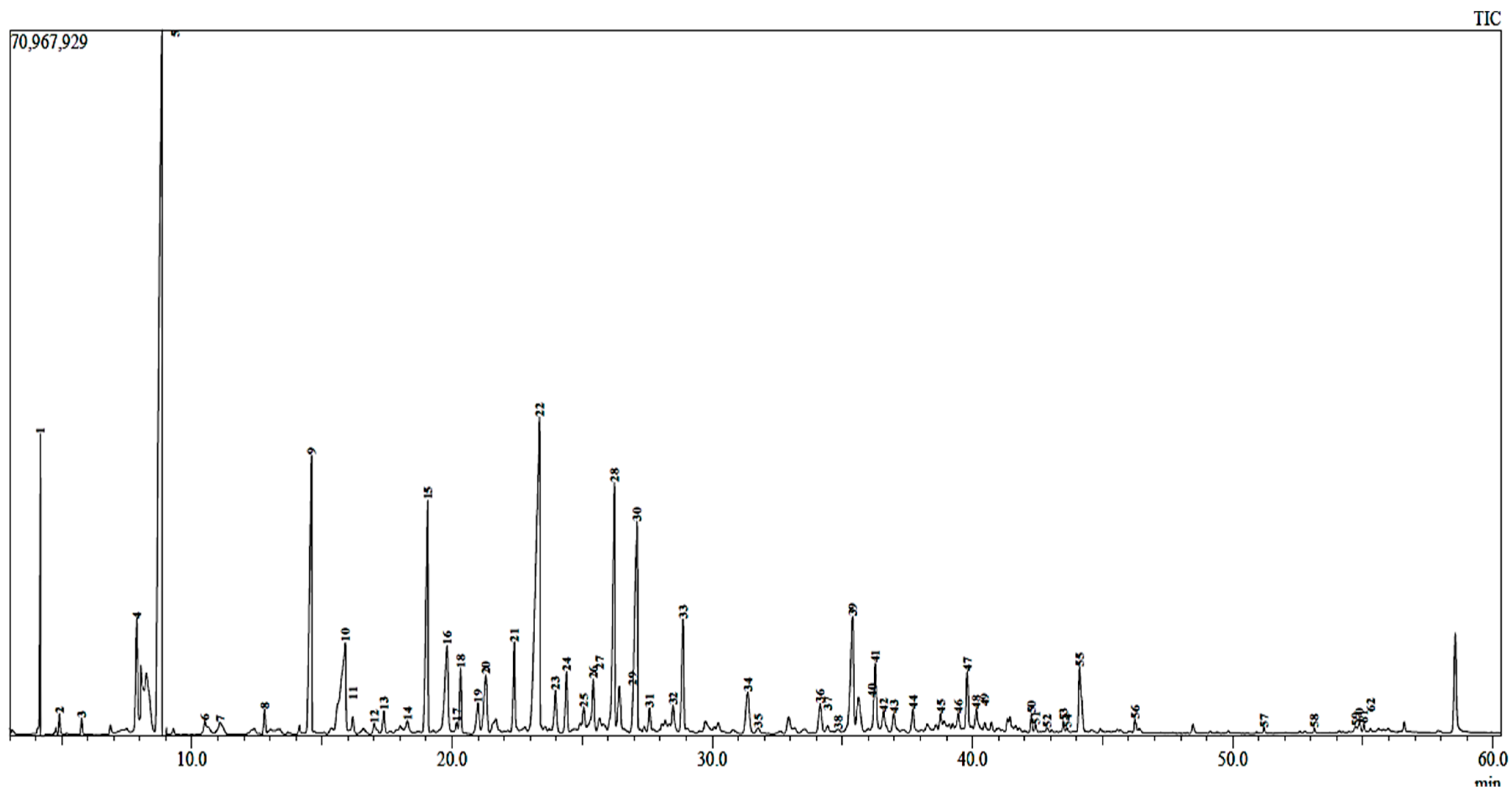







| Plant Species | Oil Weight (g) | Plant Weight (g) | Yield (%) |
|---|---|---|---|
| Myrtus communis L. | 0.9 | 300 | 0.3 |
| Compounds | Percentage (%) | Rt | RI | Base m/z |
|---|---|---|---|---|
| 1. α-pinene | 1.81 | 4.180 | 945 | 93.10 |
| 2. β-pinene | 0.22 | 5.764 | 994 | 93.10 |
| 3. β-myrcene | 4.40 | 7.888 | 1020 | 68.05 |
| 4. D-Limonene | 2.08 | 8.846 | 1056 | 43.00 |
| 5. 1,8-Cineol | 19.05 | 10.50 | 1087 | 93.10 |
| 6. Bicyclo [4.1.0] hept-2-ene, 3,7,7-trimethyl-(2-Carene) | 0.24 | 11.088 | 1095 | 93.10 |
| 7. cis-p-Mentha-2,8-dien-1-ol | 0.40 | 12.795 | 1134 | 70.10 |
| 8. Butanoic acid, 2-methyl-, 2-methylbutyl ester | 0.40 | 14.597 | 1187 | 93.10 |
| 9. β-Linalool | 5.70 | 15.893 | 1202 | 69.10 |
| 10. (-)-Cis-Sabinol | 0.20 | 16.183 | 1271 | 92.05 |
| 11. 4-Terpineol (p-Menth-1-en-4-ol) | 0.42 | 17.011 | 1273 | 91.05 |
| 12. 1,4-Benzodioxan-2-ylmethyl 2-furoate | 0.34 | 17.377 | NF | 71.05 |
| 13. p-Menth-1-en-8-ol (α-Terpineol) | 4.62 | 18.288 | 1300 | 95.10 |
| 14. p-Menth-1-en-8-ol, acetate (α-Terpinyl-Acetate) | 2.50 | 19.063 | 1347 | 59.05 |
| 15. Dimethylbenzylcarbinyl Acetate | 0.17 | 19.801 | NF | 93.05 |
| 16. Linalyl acetate | 1.04 | 20.177 | 1352 | 132.10 |
| 17. (1S-(1Alpha,2alpha,4beta))-1-isopropenyl-4-methyl-1,2-cyclohexanediol | 0.77 | 20.326 | NF | 93.10 |
| 18. Geraniol | 1.53 | 20.994 | 1367 | 43 |
| 19. Trans-Pinocarveol | 0.32 | 21.292 | 1375 | 93.10 |
| 20. Geranyl Acetate | 11.74 | 22.396 | 1473 | 69.10 |
| 21. 3-Isopropenyl-5-methyl-1-cyclohexene | 1.13 | 23.363 | 1489 | 69.10 |
| 22. Eugenol | 0.50 | 23.975 | 1500 | 93.10 |
| 23. α-Patchoulene | 1.22 | 24.404 | NF | 93.10 |
| 24. γ-Selinene | 0.33 | 25.064 | 1546 | 164.10 |
| 25. β-Caryophyllene | 1.67 | 25.426 | 1556 | 135.10 |
| 26. α-Humulene | 0.95 | 25.671 | 1558 | 105.10 |
| 27. nerol- Acetate | 5.07 | 26.242 | 1578 | 69.1 |
| 28. Ingol 12-acetate | 0.89 | 26.427 | NF | 139.10 |
| 29. Eugenol Methyl ether | 5.58 | 27.108 | NF | 178.10 |
| 30. 2-Ethyl-5-n-propylphenol | 0.48 | 27.591 | NF | 135.10 |
| 31. 2,4,4-Trimethyl-3-(3-methylbuta-1,3-dienyl)cyclohexanone | 0.69 | 28.492 | NF | 139.10 |
| 32. (E)-Methyl isoeugenol | 2.24 | 28.876 | NF | 178.10 |
| 33. Widdrol hydroxyether | 1.35 | 31.369 | NF | 139.10 |
| 34. Viridiflorol | 0.17 | 31.77 | 1845 | 58.00 |
| 35. Cohumulinic acid | 0.83 | 34.147 | NF | 252.10 |
| 36. Bicyclo [4.3.0] nonan-2-one, 8-isopropylidene- | 0.23 | 34.44 | NF | 178.10 |
| 37. 2-Hydroxy-3,5,5-trimethyl-2-cyclohexen-1-one | 0.22 | 34.846 | NF | 173.10 |
| 38. Durohydroquinone | 3.64 | 35.398 | NF | 166.10 |
| 39. Caryophyllene oxide 1664 | 1.24 | 35.627 | 1966 | 41.05 |
| 40. Cinerolone | 1.45 | 36.264 | NF | 166.10 |
| 41. Androstan-17-one, 3-ethyl-3-hydroxy-, (5. alpha.) | 0.73 | 36.591 | NF | 41.05 |
| 42. 1-Heptatriacotanol | 0.60 | 36.982 | NF | 41.05 |
| 43. 2-Dodecen-1-yl (-) succinic anhydride | 0.62 | 37.718 | NF | 41.05 |
| 44. Furan, 2,3-dihydro-2,2-dimethyl-3-(1-methylethenyl)-5-(1-methylethyl)- | 0.40 | 38.776 | NF | 43.00 |
| 45. Z-5,17-Octadecadien-1-ol acetate | 0.58 | 39.456 | NF | 43.00 |
| 46. 1,2,4-Cyclopentanetrione, 3-(2-pentenyl)– | 1.09 | 39.798 | NF | 180.10 |
| 47. 4′-Ethoxy-2′-hydroxyoctadecanophenone | 0.46 | 40.149 | NF | 180.10 |
| 48. 3-Octen-2-one, 3-butyl- | 0.28 | 42.258 | NF | 43.00 |
| 49. Bicyclo [2.2.1]heptan-2-one, 4-hydroxy-1,7,7-trimethyl-, acetate | 0.10 | 42.427 | NF | 43.00 |
| 50. 3-Isopropyl-6,7-dimethyltricyclo [4.4.0.0(2,8)] decane-9,10-diol | 0.07 | 42.868 | NF | 159.15 |
| 51. 6Z-2,5,5,10-Tetramethyl-undeca-2,6,9-trien-8-one | 0.17 | 43.492 | NF | 83.05 |
| 52. 2-Butenoic acid, 2-methyl-, 2-(acetyloxy)-1,1a,2,3,4,6,7,10,11,11a-decahydro | 0.05 | 43.632 | NF | 83.05 |
| -7,10-dihydroxy-1,1,3,6,9-pentamethyl-4a,7a-epoxy- | ||||
| 53. (+/−)-Phytone | 1.69 | 44.115 | 2314 | 43.00 |
| 54. 5-Isopropyl-2,2,7a-trimethylhexahydrobenzo [1,3] dioxo-4-ol | 0.26 | 46.246 | NF | 43.00 |
| 55. Grandlure II | 0.07 | 51.204 | NF | 93.05 |
| 56. 2,5-Cyclohexadiene-1,4-dione, 2,5-bis(1,1-dimethylpropyl)- | 0.06 | 53.141 | NF | 318.10 |
| 57. Androst-1-en-3-one, 17-(acetyloxy)-4,5-epoxy-, (4. beta.,5. beta.,17. beta.)- | 0.13 | 54.719 | NF | 329.20 |
| 58. 4-Norlanosta-17(20),24-diene-11,16-diol-21-oic acid, 3-oxo-16,21-lactone | 0.13 | 54.873 | NF | 69.05 |
| 59. (+)-Cuparene | 0.10 | 55.041 | NF | 132.10 |
| 60. 4-Hexen-1-ol, 6-(2,6,6-trimethyl-1-cyclohexenyl) -4-methyl-, (E)- | 0.06 | 55.293 | NF | 329.20 |
| Total identified | 98.78% | |||
| Monoterpene hydrocarbons | 8.75 | |||
| Sesquiterpene hydrocarbons | 4.17 | |||
| Oxygenated monoterpenes | 70.56 | |||
| Oxygenated sesquiterpenes | 3.10 | |||
| Other compounds | 12.20 |
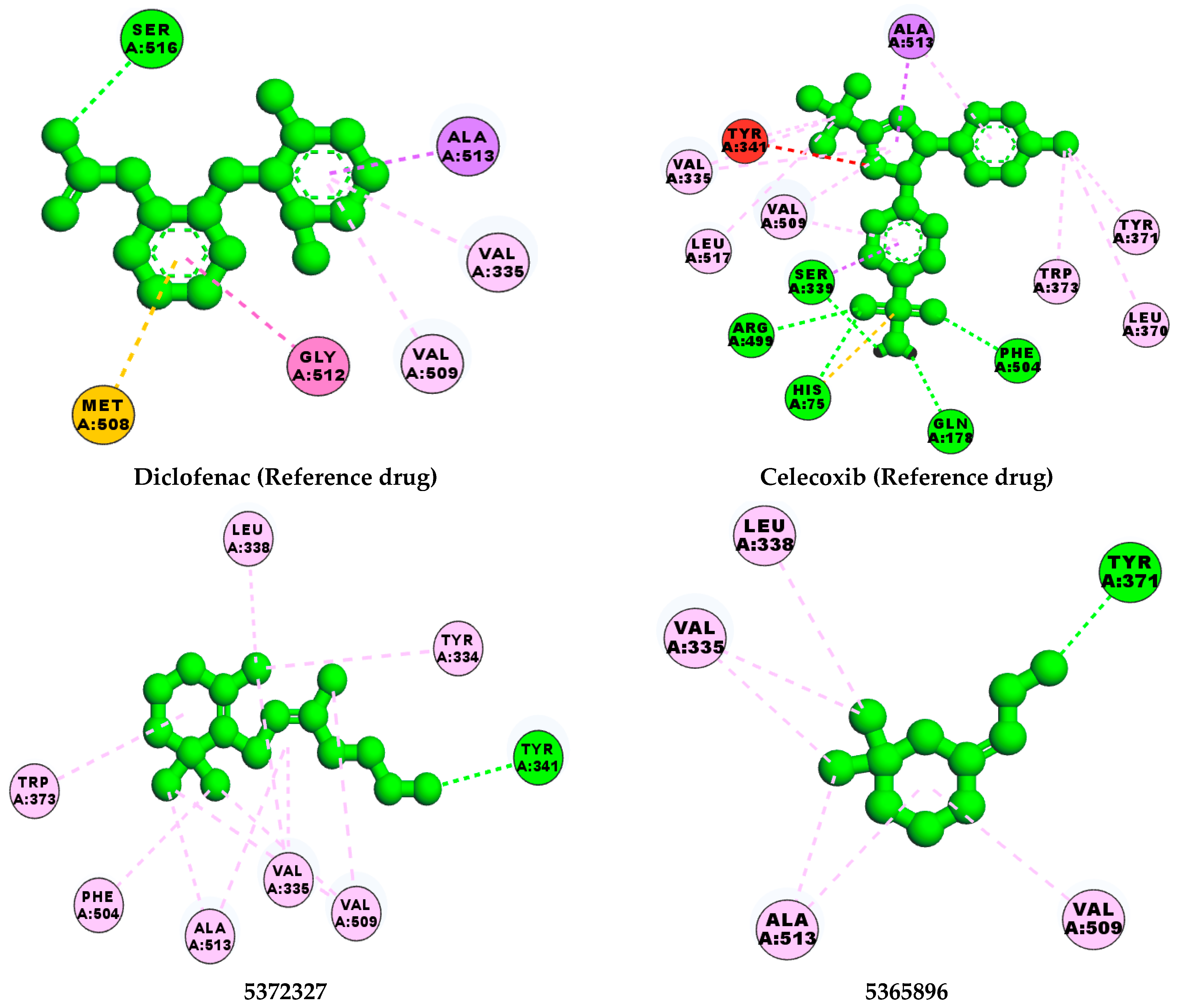
| PubChem ID | No H-Bonds | Interacting Residues | Chemical Structure of Compounds |
|---|---|---|---|
| 97456 | 1 | SER 516 |  |
| 2662 | 5 | PHE 504; GLN 178; HIS 75; ARG 499; SER 339 |  |
| 5365821 | 1 | SER 516 |  |
| 3033 | 1 | SER 516 |  |
| 540492 | 1 | SER 516 |  |
| 28237 | 0 | Nil |  |
| 5372327 | 1 | TYR 341 |  |
| 565273 | 0 | Nil |  |
| 5365896 | 1 | TYR 371 |  |
| 5281520 | 0 | Nil |  |
| 86895 | 0 | Nil |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belahcene, S.; Kebsa, W.; Omoboyowa, D.A.; Alshihri, A.A.; Alelyani, M.; Bakkour, Y.; Leghouchi, E. Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2. Pharmaceuticals 2023, 16, 1343. https://doi.org/10.3390/ph16101343
Belahcene S, Kebsa W, Omoboyowa DA, Alshihri AA, Alelyani M, Bakkour Y, Leghouchi E. Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2. Pharmaceuticals. 2023; 16(10):1343. https://doi.org/10.3390/ph16101343
Chicago/Turabian StyleBelahcene, Samia, Widad Kebsa, Damilola A. Omoboyowa, Abdulaziz A. Alshihri, Magbool Alelyani, Youssef Bakkour, and Essaid Leghouchi. 2023. "Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2" Pharmaceuticals 16, no. 10: 1343. https://doi.org/10.3390/ph16101343
APA StyleBelahcene, S., Kebsa, W., Omoboyowa, D. A., Alshihri, A. A., Alelyani, M., Bakkour, Y., & Leghouchi, E. (2023). Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2. Pharmaceuticals, 16(10), 1343. https://doi.org/10.3390/ph16101343






