The Safety of Bacteriophages in Treatment of Diseases Caused by Multidrug-Resistant Bacteria
Abstract
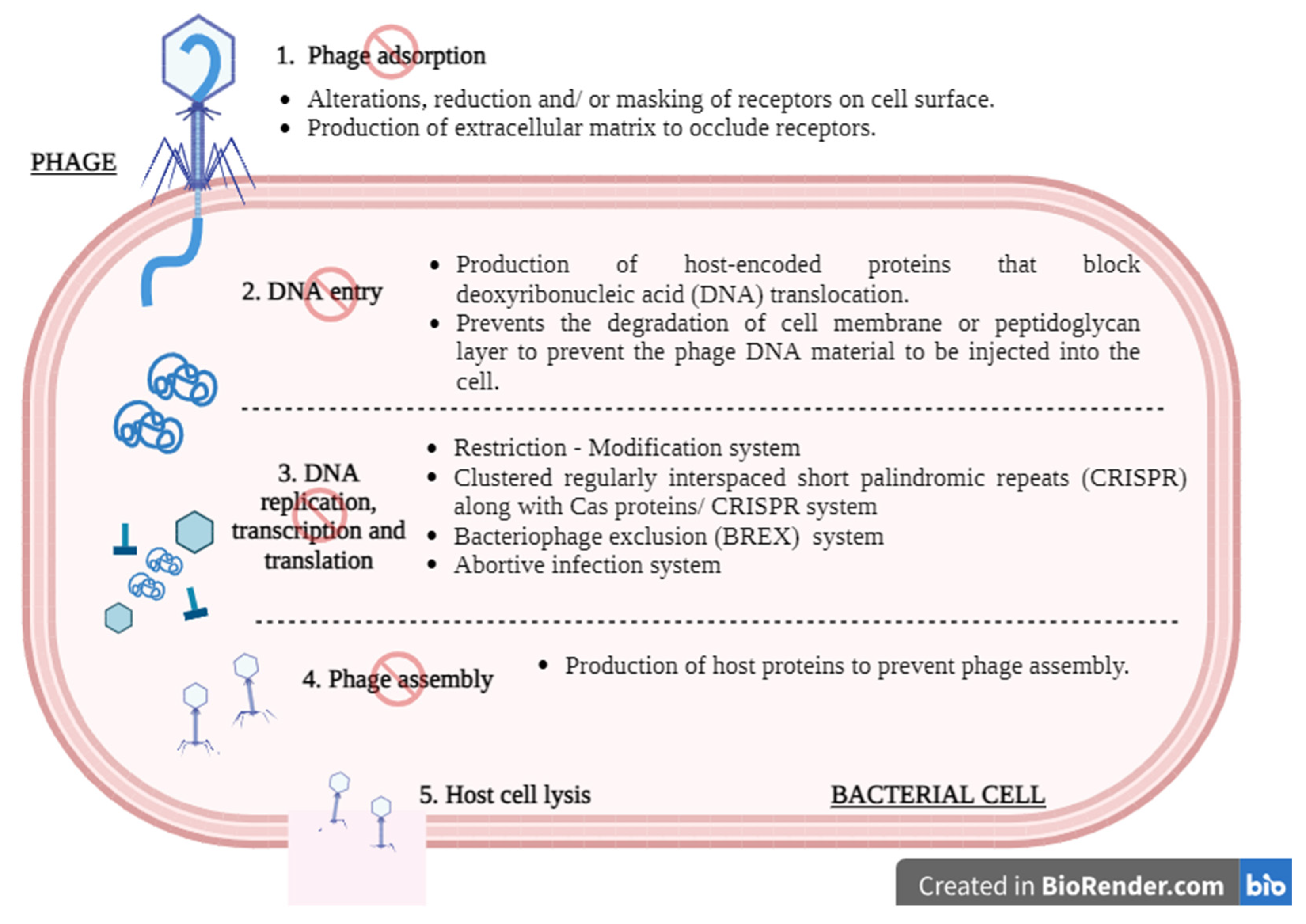
:1. Introduction
2. The Era of Phages
2.1. An Alternative to Antibiotic
2.2. Current Phage Applications
2.2.1. Phage Therapy against MDR Pathogens
2.2.2. Food Safety
2.2.3. Environment Pathogen Control
3. Safety Concerns and Challenges
3.1. Human Body
3.1.1. Disruption of Gut Microbiome and Host Genome
3.1.2. Bacterial Endotoxin Release
3.1.3. Impact of Phages on Immune Activation
3.1.4. Bacterial and Toxin Contaminants
3.2. Temperate Phages
3.3. Induction of Phage-Resistant Bacteria
3.4. Environmental Impact
4. Studies on Phage Safety
4.1. Animal Studies
4.2. Clinical Cases
4.3. Clinical Trials
5. Challenges and Future Improvements
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- De Tejada, G.M.; Heinbockel, L.; Ferrer-Espada, R.; Heine, H.; Alexander, C.; Bárcena-Varela, S.; Goldmann, T.; Correa, W.; Wiesmüller, K.; Gisch, N.; et al. Lipoproteins/peptides are sepsis-inducing toxins from bacteria that can be neutralized by synthetic anti-endotoxin peptides. Sci. Rep. 2015, 5, 14292. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Lou, X.; Luo, Q.; He, Z.; Sun, M.; Sun, J. Recent advances in bacteriophage-based therapeutics: Insight into the post-antibiotic era. Acta Pharm. Sin. B 2022, 12, 4348–4364. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gustave, C.A.; Lustig, S.; Malatray, M.; Fevre, C.; Josse, J.; Petitjean, C.; Chidiac, C.; et al. Phage Therapy as Adjuvant to Conservative Surgery and Antibiotics to Salvage Patients with Relapsing S. aureus Prosthetic Knee Infection. Front. Med. 2020, 7, 570572. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.J.; Ooi, M.L.; Paramasivan, S.; Finnie, J.W.; Morales, S.; Psaltis, A.J.; Vreugde, S.; Wormald, P. Safety and efficacy of a bacteriophage cocktail in an in vivo model of Pseudomonas aeruginosa sinusitis. Transl. Res. J. Lab. Clin. Med. 2019, 206, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Merabishvili, M.; Caudron, E.; Lannoy, D.; Van Simaey, L.; Duyvejonck, H.; Guillemain, R.; Thumerelle, C.; Podglajen, I.; Compain, F.; et al. A Case of Phage Therapy against Pandrug-Resistant Achromobacter xylosoxidans in a 12-Year-Old Lung-Transplanted Cystic Fibrosis Patient. Viruses 2021, 13, 60. [Google Scholar] [CrossRef]
- Yin, S.; Huang, G.; Zhang, Y.; Jiang, B.; Yang, Z.; Dong, Z.; You, B.; Yuan, Z.; Hu, F.; Zhao, Y.; et al. Phage Abp1 Rescues Human Cells and Mice from Infection by Pan-Drug Resistant Acinetobacter Baumannii. Cell. Physiol. Biochem. 2017, 44, 2337–2345. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Doub, J.B.; Ng, V.Y.; Johnson, A.J.; Slomka, M.; Fackler, J.; Horne, B.; Brownstein, M.J.; Henry, M.; Malagon, F.; Biswas, B. Salvage Bacteriophage Therapy for a Chronic MRSA Prosthetic Joint Infection. Antibiotics 2020, 9, 241. [Google Scholar] [CrossRef]
- LaVergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage Therapy for a Multidrug-Resistant Acinetobacter baumannii Craniectomy Site Infection. Open Forum Infect. Dis. 2018, 5, ofy064. [Google Scholar] [CrossRef] [PubMed]
- Lindford, A.; Kiuru, V.; Anttila, V.J.; Vuola, J. Successful eradication of multidrug resistant acinetobacter in the Helsinki burn centre. J. Burn. Care Res. 2015, 36, 595–601. [Google Scholar] [CrossRef]
- Karumathil, D.P.; Nair, M.S.; Gaffney, J.; Kollanoor-Johny, A.; Venkitanarayanan, K. Trans-Cinnamaldehyde and eugenol increase Acinetobacter baumannii sensitivity to beta-lactam antibiotics. Front. Microbiol. 2018, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 10–39. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Y.; Zhang, C.; Luo, X.; Chen, Y.; Peng, Y.; Gong, Y. Lytic Bacteriophage Screening Strategies for Multidrug-Resistant Bloodstream Infections in a Burn Intensive Care Unit. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8352–8362. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; World Health Organization: Geneva, Switzerland, 2017. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 8 January 2023).
- Hendrix, R.W.; Smith, M.C.M.; Burns, R.N.; Ford, M.E.; Hatfull, G.F. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. Proc. Natl. Acad. Sci. USA 1999, 96, 2192–2197. [Google Scholar] [CrossRef]
- Kim, S.H.; Adeyemi, D.E.; Park, M.K. Characterization of a new and efficient polyvalent phage infecting E. coli o157:H7, Salmonella spp., and Shigella sonnei. Microorganisms 2021, 9, 2105. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Wilson, T.; On, S.L.W. Effect of phage and host concentration on the inactivation of Escherichia coli O157:H7 on cooked and raw beef. Food Sci. Technol. Int. = Cienc. Y Tecnol. De Los Aliment. Int. 2015, 21, 104–109. [Google Scholar] [CrossRef]
- Duc, H.M.; Son, H.M.; Yi, H.P.S.; Sato, J.; Ngan, P.H.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157:H7 in different food matrices. Food Res. Int. 2020, 131, 108977. [Google Scholar] [CrossRef]
- Sui, B.; Han, L.; Ren, H.; Liu, W.; Zhang, C. A Novel Polyvalent Bacteriophage vB_EcoM_swi3 Infects Pathogenic Escherichia coli and Salmonella enteritidis. Front. Microbiol. 2021, 12, 649673. [Google Scholar] [CrossRef]
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, Á.; Almuiña-González, R.; De Miguel, T.; Sánchez, S.; Sánchez-Pérez, Á.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- d’Herelle, F. An Address on Bacteriophagy and Recovery from Infectious Diseases. Can. Med. Assoc. J. 1931, 24, 619–628. [Google Scholar]
- d’Herelle, F. Annual Graduate Fortnight. Medical and Surgical Aspects of Acute Bacterial Infections, October 20 to 31, 1930: Bacteriophage as a Treatment in Acute Medical and Surgical Infections. Bull. N. Y. Acad. Med. 1931, 7, 329–348. [Google Scholar] [PubMed]
- Pirnay, J.P.; De Vos, D.; Verbeken, G.; Merabishvili, M.; Chanishvili, N.; Vaneechoutte, M.; Zizi, M.; Laire, G.; Lavigne, R.; Huys, I.; et al. The phage therapy paradigm: Prêt-à-porter or sur-mesure? Pharm. Res. 2011, 28, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.N.; Rudraradhya, A.C.; Sadagopan, S.; Sukumaran, S.; Sambasivam, G.; Ramesh, N. Analysis of susceptibility patterns of pseudomonas aeruginosa and Isolation, Characterization of lytic bacteriophages targeting multi drug resistant Pseudomonas aeruginosa. Biomed. Pharmacol. J. 2018, 11, 1105–1117. [Google Scholar] [CrossRef]
- Nepal, R.; Houtak, G.; Karki, S.; Dhungana, G.; Vreugde, S.; Malla, R. Genomic characterization of three bacteriophages targeting multidrug resistant clinical isolates of Escherichia, Klebsiella and Salmonella. Arch. Microbiol. 2022, 204, 334. [Google Scholar] [CrossRef]
- Tao, C.; Yi, Z.; Zhang, Y.; Wang, Y.; Zhu, H.; Afayibo, D.J.A.; Li, T.; Tian, M.; Qi, J.; Ding, C.; et al. Characterization of a Broad-Host-Range Lytic Phage SHWT1 Against Multidrug-Resistant Salmonella and Evaluation of Its Therapeutic Efficacy in vitro and in vivo. Front. Vet. Sci. 2021, 8, 683853. [Google Scholar] [CrossRef]
- Deng, L.Y.; Yang, Z.C.; Gong, Y.L.; Huang, G.T.; Yin, S.P.; Jiang, B.; Peng, Y.Z. Therapeutic effect of phages on extensively drug-resistant Acinetobacter baumannii-induced sepsis in mice. Chin. J. Burn. 2016, 32, 523–528. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails to Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef]
- Aslam, S.; Courtwright, A.M.; Koval, C.; Lehman, S.M.; Morales, S.; Furr, C.L.L.; Rosas, F.; Brownstein, M.J.; Fackler, J.R.; Sisson, B.M.; et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am. J. Transplantation 2019, 19, 2631–2639. [Google Scholar] [CrossRef]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef]
- Wu, N.; Dai, J.; Guo, M.; Li, J.; Zhou, X.; Li, F.; Gao, Y.; Qu, H.; Lu, H.; Jin, J.; et al. Pre-optimized phage therapy on secondary Acinetobacter baumannii infection in four critical COVID-19 patients. Emerg. Microbes Infect. 2021, 10, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Nick, J.A.; Dedrick, R.M.; Gray, A.L.; Vladar, E.K.; Smith, B.E.; Freeman, K.G.; Malcolm, K.C.; Epperson, L.E.; Hasan, N.A.; Hendrix, J.; et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 2022, 185, 1860–1874.e12. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Thottathil, S.E.; Newman, T.B. Antibiotics overuse in animal agriculture: A call to action for health care providers. Am. J. Public Health 2015, 105, 2409–2410. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fèvre, E.M.; Woolhouse, M.E.J.; Van Bunnik, B.A.D. Are Food Animals Responsible for Transfer of Antimicrobial-Resistant Escherichia coli or Their Resistance Determinants to Human Populations? A Systematic Review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Zolfo, M.; Williams, A.; Ashubwe-Jalemba, J.; Tweya, H.; Adeapena, W.; Labi, A.; Adomako, L.A.B.; Addico, G.N.D.; Banu, R.A.; et al. Antibiotic-Resistant Bacteria in Drinking Water from the Greater Accra Region, Ghana: A Cross-Sectional Study, December 2021–March 2022. Int. J. Environ. Res. Public Health 2022, 19, 12300. [Google Scholar] [CrossRef] [PubMed]
- Bamigboye, C.O.; Amao, J.A.; Ayodele, T.A.; Adebayo, A.S.; Ogunleke, J.D.; Abass, T.B.; Oyedare, T.A.; Adetutu, T.J.; Adeeyo, A.O.; Oyedemi, A.A. An appraisal of the drinking water quality of groundwater sources in Ogbomoso, Oyo state, Nigeria. Groundw. Sustain. Dev. 2020, 11, 100453. [Google Scholar] [CrossRef]
- Odonkor, S.T.; Simpson, S.V.; Morales Medina, W.R.; Fahrenfeld, N.L. Antibiotic-Resistant Bacteria and Resistance Genes in Isolates from Ghanaian Drinking Water Sources. J. Environ. Public Health 2022, 2022, 2850165. [Google Scholar] [CrossRef]
- Adesoji, A.T.; Onuh, J.P.; Musa, A.O.; Akinrosoye, P.F. Bacteriological qualities and antibiogram studies of bacteria from “suya” and smoked fish (Clarias gariepinus) in Dutsin-Ma, Katsina State, Nigeria. Pan Afr. Med. J. 2019, 33, 219. [Google Scholar] [CrossRef]
- Lauteri, C.; Festino, A.R.; Conter, M.; Vergara, A. Prevalence and antimicrobial resistance profile in Salmonella spp. isolates from swine food chain. Ital. J. Food Saf. 2022, 11, 9980. [Google Scholar] [CrossRef]
- Rau, R.B.; Ribeiro, A.R.; Dos Santos, A.; Barth, A.L. Antimicrobial resistance of Salmonella from poultry meat in Brazil: Results of a nationwide survey. Epidemiol. Infect. 2021, 149, 26–39. [Google Scholar] [CrossRef]
- Andreoletti, O.; Lau Baggesen, D.; Bolton, D.; Butaye, P.; Cook, P.; Davies, R.; Escámez, P.S.F.; Griffin, J.; Hald, T.; Havelaar, A.; et al. Scientific Opinion on the risk posed by pathogens in food of non-animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations). EFSA J. 2013, 11, 3025. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, M.U.; Luies, S.K.; Kamal, A.; Ferdous, S.; Lin, A.; Sharior, F.; Khan, R.; Rahman, Z.; Parvez, S.M.; et al. Contamination of fresh produce with antibiotic-resistant bacteria and associated risks to human health: A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Saharan, V.V.; Nimesh, S.; Singh, A.P. Phenotypic and virulence traits of Escherichia coli and Salmonella strains isolated from vegetables and fruits from India. J. Appl. Microbiol. 2018, 125, 270–281. [Google Scholar] [CrossRef]
- Olowe, O.A.; Adefioye, O.J.; Ajayeoba, T.A.; Schiebel, J.; Weinreich, J.; Ali, A.; Burdukiewicz, M.; Rödiger, S.; Schierack, P. Phylogenetic grouping and biofilm formation of multidrug resistant Escherichia coli isolates from humans, animals and food products in South-West Nigeria. Sci. Afr. 2019, 6, e00158. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Karimi Torshizi, M.A.; Rahimi, S.; Engberg, R.M.; Sørensen Dalgaard, T. Effect of serum anti-phage activity on colibacillosis control by repeated phage therapy in broilers. Vet. Microbiol. 2019, 234, 61–71. [Google Scholar] [CrossRef]
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage Cocktails Protect Dairy Cows Against Mastitis Caused By Drug Resistant Escherichia coli Infection. Front. Cell. Infect. Microbiol. 2021, 11, 690377. [Google Scholar] [CrossRef]
- Tolen, T.N.; Xie, Y.; Hairgrove, T.B.; Gill, J.J.; Matthew Taylor, T. Evaluation of commercial prototype bacteriophage intervention designed for reducing O157 and non-O157 Shiga-toxigenic Escherichia coli (STEC) on beef cattle hide. Foods 2018, 7, 114. [Google Scholar] [CrossRef]
- Verstappen, K.M.; Tulinski, P.; Duim, B.; Fluit, A.C.; Carney, J.; Van Nes, A.; Wagenaar, J.A. The Effectiveness of Bacteriophages against Methicillin-Resistant Staphylococcus aureus ST398 Nasal Colonization in Pigs. PLoS ONE 2016, 11, e0160242. [Google Scholar] [CrossRef]
- Thanki, A.M.; Brown, N.; Millard, A.D.; Clokie, M.R.J. Genomic characterization of jumbo Salmonella phages that effectively target United Kingdom pig-associated Salmonella serotypes. Front. Microbiol. 2019, 10, 1491. [Google Scholar] [CrossRef]
- Chen, L.; Fan, J.; Yan, T.; Liu, Q.; Yuan, S.; Zhang, H.; Yang, J.; Deng, D.; Huang, S.; Ma, Y. Isolation and Characterization of Specific Phages to Prepare a Cocktail Preventing Vibrio sp. Va-F3 Infections in Shrimp (Litopenaeus vannamei). Front. Microbiol. 2019, 10, 2337. [Google Scholar] [CrossRef] [PubMed]
- Le, T.S.; Southgate, P.C.; O’connor, W.; Vu, S.V.; İpek Kurtböke, D. Application of Bacteriophages to Control Vibrio alginolyticus Contamination in Oyster (Saccostrea glomerata) Larvae. Antibiotics 2020, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Z.; Yang, K.; Wang, J.; Jousset, A.; Xu, Y.; Shen, Q.; Friman, V.P. Phage combination therapies for bacterial wilt disease in tomato. Nat. Biotechnol. 2019, 37, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Carstens, A.B.; Djurhuus, A.M.; Kot, W.; Hansen, L.H. A novel six-phage cocktail reduces Pectobacterium atrosepticum soft rot infection in potato tubers under simulated storage conditions. FEMS Microbiol. Lett. 2019, 366, fnz101. [Google Scholar] [CrossRef] [PubMed]
- Zaczek-Moczydłowska, M.A.; Young, G.K.; Trudgett, J.; Plahe, C.; Fleming, C.C.; Campbell, K.; O’Hanlon, R. Phage cocktail containing Podoviridae and Myoviridae bacteriophages inhibits the growth of Pectobacterium spp. under in vitro and in vivo conditions. PLoS ONE 2020, 15, e0230842. [Google Scholar] [CrossRef] [PubMed]
- Papaianni, M.; Paris, D.; Woo, S.L.; Fulgione, A.; Rigano, M.M.; Parrilli, E.; Tutino, M.L.; Marra, R.; Manganiello, G.; Casillo, A.; et al. Plant Dynamic Metabolic Response to Bacteriophage Treatment After Xanthomonas campestris pv. campestris Infection. Front. Microbiol. 2020, 11, 732. [Google Scholar] [CrossRef]
- Vikram, A.; Tokman, J.I.; Woolston, J.; Sulakvelidze, A. Phage Biocontrol Improves Food Safety by Significantly Reducing the Level and Prevalence of Escherichia coli O157:H7 in Various Foods. J. Food Prot. 2020, 83, 668–676. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, R.; Yu, X.; Zhang, X.; Liu, G.; Liu, X. Molecular Characteristics of Novel Phage vB_ShiP-A7 Infecting Multidrug-Resistant Shigella flexneri and Escherichia coli, and Its Bactericidal Effect in vitro and in vivo. Front. Microbiol. 2021, 12, 698962. [Google Scholar] [CrossRef]
- Rangasamy, K.; Murugan, A.; Devarajan, N.; Parray, J.A. Emergence of multi drug resistance among soil bacteria exposing to insecticides. Microb. Pathog. 2017, 105, 153–165. [Google Scholar] [CrossRef]
- Mahdiyah, D.; Farida, H.; Riwanto, I.; Mustofa, M.; Wahjono, H.; Laksana Nugroho, T.; Reki, W. Screening of Indonesian peat soil bacteria producing antimicrobial compounds. Saudi J. Biol. Sci. 2020, 27, 2604–2611. [Google Scholar] [CrossRef]
- Mafiz, A.I.; He, Y.; Zhang, W.; Zhang, Y. Soil Bacteria in Urban Community Gardens Have the Potential to Disseminate Antimicrobial Resistance Through Horizontal Gene Transfer. Front. Microbiol. 2021, 12, 771707. [Google Scholar] [CrossRef]
- Yahya, M.; Hmaied, F.; Jebri, S.; Jofre, J.; Hamdi, M. Bacteriophages as indicators of human and animal faecal contamination in raw and treated wastewaters from Tunisia. J. Appl. Microbiol. 2015, 118, 1217–1225. [Google Scholar] [CrossRef]
- Calero-Cáceres, W.; Balcázar, J.L. Antibiotic resistance genes in bacteriophages from diverse marine habitats. Sci. Total Environ. 2019, 654, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Lekunberri, I.; Subirats, J.; Borrego, C.M.; Balcázar, J.L. Exploring the contribution of bacteriophages to antibiotic resistance. Environ. Pollut. 2017, 220, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- McCallin, S.; Alam Sarker, S.; Barretto, C.; Sultana, S.; Berger, B.; Huq, S.; Krause, L.; Bibiloni, R.; Schmitt, B.; Reuteler, G.; et al. Safety analysis of a Russian phage cocktail: From MetaGenomic analysis to oral application in healthy human subjects. Virology 2013, 443, 187–196. [Google Scholar] [CrossRef]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE study: Effects of supplemental bacteriophage intake on inflammation and gut microbiota in healthy adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef]
- Galtier, M.; De Sordi, L.; Maura, D.; Arachchi, H.; Volant, S.; Dillies, M.A.; Debarbieux, L. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microbiol. 2016, 18, 2237–2245. [Google Scholar] [CrossRef]
- Grubb, D.S.; Wrigley, S.D.; Freedman, K.E.; Wei, Y.; Vazquez, A.R.; Trotter, R.E.; Wallace, T.C.; Johnson, S.A.; Weir, T.L. PHAGE-2 Study: Supplemental Bacteriophages Extend Bifidobacterium animalis subsp. lactis BL04 Benefits on Gut Health and Microbiota in Healthy Adults. Nutrients 2020, 12, 2474. [Google Scholar] [CrossRef]
- Gunathilaka, G.U.; Tahlan, V.; Mafiz, A.I.; Polur, M.; Zhang, Y. Phages in urban wastewater have the potential to disseminate antibiotic resistance. Int. J. Antimicrob. Agents 2017, 50, 678–683. [Google Scholar] [CrossRef]
- Liu, J.; Liu, P.; Feng, F.; Zhang, J.; Li, F.; Wang, M.; Sun, Y. Evaluation of Potential ARG Packaging by Two Environmental T7-Like Phage during Phage-Host Interaction. Viruses 2020, 12, 1060. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Qiu, T.; Gao, M.; Wang, X. Bacteriophages: Underestimated vehicles of antibiotic resistance genes in the soil. Front. Microbiol. 2022, 13, 936267. [Google Scholar] [CrossRef]
- Frazão, N.; Sousa, A.; Lässig, M.; Gordo, I. Horizontal gene transfer overrides mutation in Escherichia coli colonizing the mammalian gut. Proc. Natl. Acad. Sci. USA 2019, 116, 17906–17915. [Google Scholar] [CrossRef]
- Fernández-Orth, D.; Miró, E.; Brown-Jaque, M.; Rodríguez-Rubio, L.; Espinal, P.; Rodriguez-Navarro, J.; González-López, J.J.; Muniesa, M.; Navarro, F. Faecal phageome of healthy individuals: Presence of antibiotic resistance genes and variations caused by ciprofloxacin treatment. J. Antimicrob. Chemother. 2019, 74, 854–864. [Google Scholar] [CrossRef]
- Enault, F.; Briet, A.; Bouteille, L.; Roux, S.; Sullivan, M.B.; Petit, M.A. Phages rarely encode antibiotic resistance genes: A cautionary tale for virome analyses. ISME J. 2016, 11, 237–247. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Leitner, L.; Mehnert, U.; Chkhotua, A.; Kessler, T.M.; Sybesma, W. Adapted bacteriophages for treating urinary tract infections. Front. Microbiol. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Infusion Related Reactions Guidance 2013; Canterbury District Health Board (CRCHS): Canterbury, New Zealand, 2013; Available online: https://edu.cdhb.health.nz/Hospitals-Services/health-professionals/Cytotoxic-Biotherapy/Documents/Infusion%20Related%20Reactions%20Guidance%202013.pdf (accessed on 12 April 2023).
- Moghadam, M.T.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Jazi, F.M. How phages overcome the challenges of drug resistant bacteria in clinical infections. Infect. Drug Resist. 2020, 13, 45–61. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Clement, F.; Merabishvili, M.; Lavigne, R.; Vaneechoutte, M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci. Rep. 2017, 7, 8004. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, X.; Sun, L.; He, T.; Wei, R.; Pang, M.; Wang, R. Corrigendum: Staphylococcus aureus Bacteriophage Suppresses LPS-Induced Inflammation in MAC-T Bovine Mammary Epithelial Cells. Front. Microbiol. 2018, 9, 331–333. [Google Scholar] [CrossRef]
- Dufour, N.; Delattre, R.; Chevallereau, A.; Ricard, J.D.; Debarbieux, L. Phage Therapy of Pneumonia Is Not Associated with an Overstimulation of the Inflammatory Response Compared to Antibiotic Treatment in Mice. Antimicrob. Agents Chemother. 2019, 63, e00379-19. [Google Scholar] [CrossRef]
- Majewska, J.; Kaźmierczak, Z.; Lahutta, K.; Lecion, D.; Szymczak, A.; Miernikiewicz, P.; Drapała, J.; Harhala, M.; Marek-Bukowiec, K.; Jędruchniewicz, N.; et al. Induction of Phage-Specific Antibodies by Two Therapeutic Staphylococcal bacteriophages Administered per os. Front. Immunol. 2019, 10, 2607. [Google Scholar] [CrossRef]
- Jun, J.W.; Shin, T.H.; Kim, J.H.; Shin, S.P.; Han, J.E.; Heo, G.J.; De Zoysa, M.; Shin, G.W.; Chai, J.Y.; Park, S.C. Bacteriophage Therapy of a Vibrio parahaemolyticus Infection Caused by a Multiple-Antibiotic–Resistant O3:K6 Pandemic Clinical Strain. J. Infect. Dis. 2014, 210, 72–78. [Google Scholar] [CrossRef]
- Sunagar, R.; Patil, S.A.; Chandrakanth, R.K. Bacteriophage therapy for Staphylococcus aureus bacteremia in streptozotocin-induced diabetic mice. Res. Microbiol. 2010, 161, 854–860. [Google Scholar] [CrossRef]
- Gainey, A.B.; Burch, A.K.; Brownstein, M.J.; Brown, D.E.; Fackler, J.; Horne, B.; Biswas, B.; Bivens, B.N.; Malagon, F.; Daniels, R. Combining bacteriophages with cefiderocol and meropenem/vaborbactam to treat a pan-drug resistant Achromobacter species infection in a pediatric cystic fibrosis patient. Pediatr. Pulmonol. 2020, 55, 2990–2994. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Khoshbayan, A.; Chegini, Z.; Farahani, I.; Shariati, A. Bacteriophages, a New Therapeutic Solution for Inhibiting Multidrug-Resistant Bacteria Causing Wound Infection: Lesson from Animal Models and Clinical Trials. Drug Des. Dev. Ther. 2020, 14, 1867–1883. [Google Scholar] [CrossRef]
- Park, K.; Cha, K.E.; Myung, H. Observation of inflammatory responses in mice orally fed with bacteriophage T7. J. Appl. Microbiol. 2014, 117, 627–633. [Google Scholar] [CrossRef]
- Setting Endotoxin Acceptance Criteria for Biologics Intravenous (IV) and Subcutaneous (SC) Mono- and Combination Therapies| American Pharmaceutical Review—The Review of American Pharmaceutical Business & Technology. American Pharmaceutical Review. 2018. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/353671-Setting-Endotoxin-Acceptance-Criteria-for-Biologics-Intravenous-IV-and-Subcutaneous-SC-Mono-and-Combination-Therapies/ (accessed on 27 April 2023).
- Cooper, C.J.; Mirzaei, M.K.; Nilsson, A.S. Adapting drug approval pathways for bacteriophage-based therapeutics. Front. Microbiol. 2016, 7, 1209. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, H.; Zhou, Y.; Zhu, S.; Pang, M.; Shahin, K.; Shahin, K.; Olaniran, A.; Schmidt, S.; Wang, R. Transient carriage and low-level colonization of orally administrated lytic and temperate phages in the gut of mice. Food Prod. Process. Nutr. 2020, 2, 14. [Google Scholar] [CrossRef]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In Vitro Characterization of Anti-biofilm Activity. Clin. Infect. Dis. 2021, 73, e144–e151. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Wang, Y.; Han, S. Bacterial DNA involvement in carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 996778. [Google Scholar] [CrossRef]
- Gilbey, T.; Ho, J.; Cooley, L.A.; Petrovic Fabijan, A.; Iredell, J.R. Adjunctive bacteriophage therapy for prosthetic valve endocarditis due to Staphylococcus aureus. Med. J. Aust. 2019, 211, 142–143.e1. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Ceremuga, M.; Bijak, J.S.; Bijak, M. Biological Toxins as the Potential Tools for Bioterrorism. Int. J. Mol. Sci. 2019, 20, 1181. [Google Scholar] [CrossRef] [PubMed]
- Kissner, T.L.; Cisney, E.D.; Ulrich, R.G.; Fernandez, S.; Saikh, K.U. Staphylococcal enterotoxin A induction of pro-inflammatory cytokines and lethality in mice is primarily dependent on MyD88. Immunology 2010, 130, 516–526. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef]
- Ho, S.S.; Michalek, S.M.; Nahm, M.H. Lipoteichoic Acid Is Important in Innate Immune Responses to Gram-Positive Bacteria. Infect. Immun. 2008, 76, 206–213. [Google Scholar] [CrossRef]
- Berube, B.J.; Wardenburg, J.B. Staphylococcus aureus α-Toxin: Nearly a Century of Intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef]
- Szentirmai, É.; Massie, A.R.; Kapás, L. Lipoteichoic acid, a cell wall component of Gram-positive bacteria, induces sleep and fever and suppresses feeding. Brain Behav. Immun. 2021, 92, 184–192. [Google Scholar] [CrossRef]
- Guerin, K.; Choi, V.; Aranda, J.; Demirs, J.; Li, H.; Yang, J.; Nguyen, N.; Bottega, S.; Jaffee, B.; Dryja, T.; et al. Residual Cesium Chloride in AAV Vectors Purified by CsCl Gradient Centrifugation Does Not Cause Obvious Inflammation or Retinal Degeneration in C57Bl6/J Mice Following Subretinal Injection. Mol. Ther. 2015, 23, S240. [Google Scholar] [CrossRef]
- Hietala, V.; Horsma-Heikkinen, J.; Carron, A.; Skurnik, M.; Kiljunen, S. The Removal of Endo- and Enterotoxins from Bacteriophage Preparations. Front. Microbiol. 2019, 10, 1674. [Google Scholar] [CrossRef]
- Chaudhary, K. BacteRiophage EXclusion (BREX): A novel anti-phage mechanism in the arsenal of bacterial defense system. J. Cell. Physiol. 2018, 233, 771–773. [Google Scholar] [CrossRef]
- Liu, M.; Deora, R.; Doulatov, S.R.; Gingery, M.; Eiserling, F.A.; Preston, A.; Maskell, D.J.; Simons, R.W.; Cotter, P.A.; Parkhill, J.; et al. Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 2002, 295, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Fineran, P.C.; Blower, T.R.; Foulds, I.J.; Humphreys, D.P.; Lilley, K.S.; Salmond, G.P.C. The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair. Proc. Natl. Acad. Sci. USA 2009, 106, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Blower, T.R.; Fineran, P.C.; Johnson, M.J.; Toth, I.K.; Humphreys, D.P.; Salmond, G.P.C. Mutagenesis and functional characterization of the RNA and protein components of the toxIN abortive infection and toxin-antitoxin locus of Erwinia. J. Bacteriol. 2009, 191, 6029–6039. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J. Antibiotics in the environment. Upsala J. Med. Sci. 2014, 119, 108–112. [Google Scholar] [CrossRef]
- Litt, P.K.; Jaroni, D. Isolation and Physiomorphological Characterization of Escherichia coli O157:H7-Infecting Bacteriophages Recovered from Beef Cattle Operations. Int. J. Microbiol. 2017, 2017, 7013236. [Google Scholar] [CrossRef]
- Meaden, S.; Koskella, B. Exploring the risks of phage application in the environment. Front. Microbiol. 2013, 4, 358. [Google Scholar] [CrossRef]
- Drilling, A.J.; Ooi, M.L.; Miljkovic, D.; James, C.; Speck, P.; Vreugde, S.; Clark, J.; Wormald, P.J. Long-Term Safety of Topical Bacteriophage Application to the Frontal Sinus Region. Front. Cell. Infect. Microbiol. 2017, 7, 49. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The safety and toxicity of phage therapy: A review of animal and clinical studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Onsea, J.; Soentjens, P.; Djebara, S.; Merabishvili, M.; Depypere, M.; Spriet, I.; De Munter, P.; Debaveye, Y.; Njis, S.; Vanderschot, P.; et al. Bacteriophage Application for Difficult-To-Treat Musculoskeletal Infections: Development of a Standardized Multidisciplinary Treatment Protocol. Viruses 2019, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Khatami, A.; Lin, R.C.Y.; Petrovic-Fabijan, A.; Alkalay-Oren, S.; Almuzam, S.; Britton, P.N.; Brownstein, M.J.; Dao, Q.; Fackler, J.; Hazan, R.; et al. Bacterial lysis, autophagy and innate immune responses during adjunctive phage therapy in a child. EMBO Mol. Med. 2021, 13, e13936. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons Learned From the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wu, N.; Zeng, Y.; Chen, L.; Li, L.; Yang, L.; Zhang, Y.; Guo, M.; Li, L.; Li, J.; et al. Non-active antibiotic and bacteriophage synergism to successfully treat recurrent urinary tract infection caused by extensively drug-resistant Klebsiella pneumoniae. Emerg. Microbes Infect. 2020, 9, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, A.; Lood, C.; Wubbolts, J.; Hites, M.; Balarjishvili, N.; Leshkasheli, L.; Askilashvili, L.; Kvachadze, L.; van Noort, V.; Wagemans, J.; et al. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nat. Commun. 2022, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.V.; Johri, P.; Hoyle, N.; Pipia, L.; Nadareishvili, L.; Nizharadze, D. Case Report: Chronic Bacterial Prostatitis Treated With Phage Therapy After Multiple Failed Antibiotic Treatments. Front. Pharmacol. 2021, 12, 692614. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, C.; Gonzales, F.; Buckley, M.; Biswas, B.; Henry, M.; Deschenes, M.V.; Horne, B.; Fackler, J.; Brownstein, M.J.; Schooley, R.T.; et al. Successful Treatment of Staphylococcus aureus Prosthetic Joint Infection with Bacteriophage Therapy. Viruses 2021, 13, 1182. [Google Scholar] [CrossRef]
- Rostkowska, O.M.; Międzybrodzki, R.; Miszewska-Szyszkowska, D.; Górski, A.; Durlik, M. Treatment of recurrent urinary tract infections in a 60-year-old kidney transplant recipient. The use of phage therapy. Transpl. Infect. Dis. 2021, 23, e13391. [Google Scholar] [CrossRef]
- Corbellino, M.; Kieffer, N.; Kutateladze, M.; Balarjishvili, N.; Leshkasheli, L.; Askilashvili, L.; Tsertsvadze, G.; Rimoldi, S.G.; Nizharadze, D.; Hoyle, N.; et al. Eradication of a Multidrug-Resistant, Carbapenemase-Producing Klebsiella pneumoniae Isolate Following Oral and Intra-rectal Therapy With a Custom Made, Lytic Bacteriophage Preparation. Clin. Infect. Dis. 2020, 70, 1998–2001. [Google Scholar] [CrossRef]
- Nir-Paz, R.; Gelman, D.; Khouri, A.; Sisson, B.M.; Fackler, J.; Alkalay-Oren, S.; Khalifa, L.; Rimon, A.; Yerushalmy, O.; Bader, R.; et al. Successful Treatment of Antibiotic-resistant, Poly-microbial Bone Infection With Bacteriophages and Antibiotics Combination. Clin. Infect. Dis. 2019, 69, 2015–2018. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Winkler, T.; Müller, M.; Perka, C.; Trampuz, A. Bacteriophages as Adjuvant to Antibiotics for the Treatment of Periprosthetic Joint Infection Caused by Multidrug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, e00924-19. [Google Scholar] [CrossRef]
- Maddocks, S.; Fabijan, A.P.; Ho, J.; Lin, R.C.Y.; Ben Zakour, N.L.; Dugan, C.; Kliman, I.; Branston, S.; Morales, S.; Iredell, J.R. Bacteriophage Therapy of Ventilator-associated Pneumonia and Empyema Caused by Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2019, 200, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Logan, C.; Yung, G.; Furr, C.L.L.; Lehman, S.M.; Morales, S.; Rosas, F.; Gaidamaka, A.; Bilinsky, I.; Grint, P.; et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 2019, 47, 665–668. [Google Scholar] [CrossRef]
- Kuipers, S.; Ruth, M.M.; Mientjes, M.; de Sévaux, R.G.L.; van Ingen, J. A Dutch Case Report of Successful Treatment of Chronic Relapsing Urinary Tract Infection with Bacteriophages in a Renal Transplant Patient. Antimicrob. Agents Chemother. 2020, 64, e01281-19. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Pretorius, V.; Lehman, S.M.; Morales, S.; Schooley, R.T. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J. Heart Lung Transplantation 2019, 38, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Boucher, F.; Fevre, C.; Perpoint, T.; Chateau, J.; Petitjean, C.; Josse, J.; Chidiac, C.; L’hostis, G.; Leboucher, G.; et al. Innovations for the treatment of a complex bone and joint infection due to XDR Pseudomonas aeruginosa including local application of a selected cocktail of bacteriophages. J. Antimicrob. Chemother. 2018, 73, 2901–2903. [Google Scholar] [CrossRef] [PubMed]
- Duplessis, C.; Biswas, B.; Hanisch, B.; Perkins, M.; Henry, M.; Quinones, J.; Wolfe, D.; Estrella, L.; Hamilton, T. Refractory Pseudomonas Bacteremia in a 2-Year-Old Sterilized by Bacteriophage Therapy. J. Pediatr. Infect. Dis. Soc. 2018, 7, 253–256. [Google Scholar] [CrossRef]
- Ferry, T.; Leboucher, G.; Fevre, C.; Herry, Y.; Conrad, A.; Josse, J.; Batailler, C.; Chidiac, C.; Medina, M.; Lustig, S.; et al. Salvage Debridement, Antibiotics and Implant Retention (“DAIR”) With Local Injection of a Selected Cocktail of Bacteriophages: Is It an Option for an Elderly Patient With Relapsing Staphylococcus aureus Prosthetic-Joint Infection? Open Forum Infect. Dis. 2018, 5, ofy269. [Google Scholar] [CrossRef]
- Zhvania, P.; Hoyle, N.S.; Nadareishvili, L.; Nizharadze, D.; Kutateladze, M. Phage therapy in a 16-year-old boy with netherton syndrome. Front. Med. 2017, 4, 94. [Google Scholar] [CrossRef]
- Jennes, S.; Merabishvili, M.; Soentjens, P.; Pang, K.W.; Rose, T.; Keersebilck, E.; Soete, O.; François, P.; Teodorescu, S.; Verween, G.; et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—A case report. Crit. Care 2017, 21, 129. [Google Scholar] [CrossRef]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.O.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet. Infect. Dis. 2021, 21, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Fabijan, A.; Lin, R.C.Y.; Ho, J.; Maddocks, S.; Zakour, N.L.; Iredell, J.R.; Westmead Bacteriophage Therapy Team. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Hawkins, C.H.; Änggård, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-S.; Kumar Biswas, S.; Tan, W.S.; Saha, A.K.; Leo, B.-F. Efficacy and potential of phage therapy against multidrug resistant Shigella spp. PeerJ 2019, 7, e6225. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jo, Y.; Hwang, Y.J.; Hong, H.W.; Hong, S.S.; Park, K.; Myung, H. Phage-Antibiotic Synergy via Delayed Lysis. Appl. Environ. Microbiol. 2018, 84, e02085-18. [Google Scholar] [CrossRef]
- Uchiyama, J.; Shigehisa, R.; Nasukawa, T.; Mizukami, K.; Takemura-Uchiyama, I.; Ujihara, T.; Murakami, H.; Imanishi, I.; Nishifuji, K.; Sakaguchi, M.; et al. Piperacillin and ceftazidime produce the strongest synergistic phage–antibiotic effect in Pseudomonas aeruginosa. Arch. Virol. 2018, 163, 1941–1948. [Google Scholar] [CrossRef]
- Han, M.L.; Nang, S.C.; Lin, Y.W.; Zhu, Y.; Yu, H.H.; Wickremasinghe, H.; Barlow, C.K.; Creek, D.J.; Crawford, S.; Rao, G.; et al. Comparative metabolomics revealed key pathways associated with the synergistic killing of multidrug-resistant Klebsiella pneumoniae by a bacteriophage-polymyxin combination. Comput. Struct. Biotechnol. J. 2022, 20, 485–495. [Google Scholar] [CrossRef]
- Zuo, P.; Yu, P.; Alvarez, P.J.J. Aminoglycosides Antagonize Bacteriophage Proliferation, Attenuating Phage Suppression of Bacterial Growth, Biofilm Formation, and Antibiotic Resistance. Appl. Environ. Microbiol. 2021, 87, e00468-21. [Google Scholar] [CrossRef]
| Studies | Phage/s | Against | Phage Distribution | Normal Imaging/Lab Assessment | Presence of Abnormal (Increase or Decrease) of | Phage-Related Adverse Events (Cytotoxicity or Physiological Effects) | ||
|---|---|---|---|---|---|---|---|---|
| Phage/s (Administration Route) | Endotoxin within Acceptable Range | Cell Infiltration/Cytokine Production | Antibodies Production | |||||
| Dufour et al., 2019 [83] Mice | E. coli phages LM33_P1 and 536_P1 (Intranasal) | Yes, 0.072 and 0.003 EU/mL, respectively | Pathogenic E. coli | Yes | Not significant | |||
| Fong et al., 2019 [6] Sheep | P. aeruginosa phage cocktail (Local) | Chronic rhinosinusitis (CRS) P. aeruginosa strain | Detected in feces on Day 7 of treatment Detected in blood samples of certain sheep on Day 1 and Day 7 of treatment Detected in organ samples after 16–18 h of treatment | Yes | No significant adverse effects such as loss of appetite, fever, or other signs of systemic illness | |||
| Drilling et al., 2017 [112] Sheep | NOV012 cocktail (Local) | CRS S. aureus | Not detected in blood during 20 days of treatment | None | No adverse effects | |||
| Yin et al., 2017 [8] Mice | Abp1 phage (Intraperitoneal) | Only mentioned that the endotoxin is removed using a kit | MDR A. baumannii | Detected in liver and kidney 7 days after infection | No cytotoxic effect | |||
| Studies | Phage/s | Against | Phage Distribution | Normal Imaging/Lab Assessment (e.g., X-rays, Liver Function) | Presence of Abnormal (Increase or Decrease) of | Phage-Related Adverse Events (Physiological Effects) | ||
|---|---|---|---|---|---|---|---|---|
| Phage/s (Administration Route) | Endotoxin within Acceptable Range | Cell Infiltration/Cytokine Production | Antibodies Production | |||||
| Eskenazi et al., 2022 [118] | Phage vB_KpnM_M1 (M1) (Local) | Fracture-related pandrug-resistant Klebsiella pneumoniae infection | Yes | Neutralizing antibodies are triggered | None | |||
| Lebeaux et al., 2021 [7] | APC 2.1 cocktail (Inhalation) | Yes, 30 mL of APC 2.1 was diluted tenfold from stock (5 × 109 pfu/mL, 1760 EU/mL endotoxin level) | Pandrug-resistant Achromobacter xylosoxidans in lung transplanted cystic fibrosis infection | Initial persisting airway colonization with no adverse effects | ||||
| Johri et al., 2021 [119] | Pyo, Intesti, and Staphylococcal phage (Oral, intrarectal, and urethral instillations) | Methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus haemolyticus, Enterococcus faecalis, and Streptococcus mitis in chronic bacterial prostatitis | Yes | None | None | None | ||
| Wu et al., 2021 [33] | ɸAb124 (Inhalation) | Carbapenem- resistant A. baumannii (CRAB) secondary to COVID-19 infection | Yes | Present (Atypical cytokine storm, dramatic increase of IL6 and IL8 during initiation) | Transient fever | |||
| Ramirez-Sanchez et al., 2021 [120] | AB-SA01 cocktail and SaGR51ø1 phage (IV and IA) | Yes, <250 EU/mL (<5 EU/kg per dose) and <1 EU/mL, respectively | Persistent methicillin- sensitive S. aureus (MSSA) in prosthetic joint infection | Yes | None | Neutralizing antibodies are triggered | Liver function tests, renal function, and complete cell blood count remained stable | |
| Khatami et al., 2021 [115] | PASA16 (IV) | Yes, 170 EU/mL. | MDR P. aeruginosa | Detected in blood during initiation (Day 2 and 5) | Yes | Present (CRP peaked) | Present (Increased serum IgG) | Transient fever and transient increase in pain at the infected site (heel) |
| Ferry et al., 2020 [5] | PP1493, PP1815, and PP1957 phage cocktail (Local) | Orosthetic knee infection (PKI) Methicillin- susceptible S. aureus | Present (Mild synovitis) | Mild discharge of synovial fluid and mild synovial inflammation without adverse effects or superinfection | ||||
| Bao et al., 2020 [117] | Cocktail III composed of Kp152, Kp154, Kp155, Kp164, Kp6377, and HD001 (Local) | Extensively drug-resistant Klebsiella pneumoniae in UTI | None | |||||
| Rostkowska et al., 2020 [121] | Phage therapy (Intrarectal) | MDR ESBL- producing Klebsiella pneumoniae in UTI | Yes | None | Yes | |||
| Doub et al., 2020 [10] | S. aureus phage, SaGR51Φ1 (Intraarticular/Intravenous) | Yes, <1 EU/mL | Chronic methicillin-resistant S. aureus prosthetic joint infection | Yes | Present (Transaminitis) | Transient transaminitis | ||
| Rubalskii et al., 2020 [32] | Various phage cocktails (Local, Oral, Inhalation) | MDR/especially recalcitrant S. aureus, Enterococcus faecium, P. aeruginosa, K. pneumoniae, and E. coli in cardiothoracic surgery infection | Yes | Present | None | |||
| Aslam et al., 2020 [116] | Patient 8 SDSU1 cocktail, SDSU2 cocktail, and PAK_P1 single phage (IV) | Yes, 4.3 EU per dose. Diluted. | MDR and antibiotic-recalcitrant P. aeruginosa | Transient fever, wheezing, and shortness of breath | ||||
| Gainey et al., 2020 [87] | Ax2CJ45ϕ2 (Intravenous) | Pan-drug resistance Achromobacter spp. In cystic fibrosis | Yes | No infusion site reactions, anaphylaxis, elevated liver enzymes, increased serum creatinine, electrolyte abnormalities, seizures, abnormal vitals, and gastro- intestinal disturbances | ||||
| Corbellino et al., 2020 [122] | vB_KpnM_GF (Oral and Intrarectal) | MDR Carbapenemase-Producing Klebsiella pneumoniae | Yes | Normal clinical examinations, complete blood count, the serum C-reactive protein levels, and the concentrations of liver enzymes and serum electrolytes. No adverse effects | ||||
| Aslam et al., 2019 [31] | Various phage cocktails and single phage (Intravenous/Inhalation) | Yes, between 0.2 EU/mL and 7300 EU/mL | MDR infections caused by P. aeruginosa and Burkholderia dolosa in lung transplant | Yes | Present | None | ||
| Nir-Paz et al., 2019 [123] | ɸAbKT21phi3; MK278859; and ɸKpKT21phi1; MK278861 (Intravenous) | Yes, 35 EU/mL for ɸKpKT21phi1 and 5 EU/mL for ɸAbKT21phi3 | Extensively drug-resistant A. baumannii and MDR K. pneumoniae in bone infection | Not detected in blood, stool, urine, or saliva after 8 months | Yes | None | None | |
| Tkhilaishvili et al., 2019 [124] | Phage (Local) | MDR P. aeruginosa in Periprosthetic Joint Infection | None | |||||
| Onsea et al., 2019 [114] | BFC 1 and Pro phage cocktails (Local) | Yes. | MDR S. epidermidis, MDR P. aeruginosa, S. aureus, Sagalactiae, and E. faecalis in musculoskeletal infections | Yes | None | None | No severe systemic side effects or immune reactions The systemic inflammatory markers (CRP and WBC count) decreased to normal levels after one month, and no antibodies were produced against the administered phages | |
| Maddocks et al., 2019 [125] | AB-PA01 (Inhalation, IV) | MDR P. aeruginosa in ventilator-associated pneumonia and empyema | None | |||||
| Law et al., 2019 [126] | AB-PA01 (IV) | MDR P. aeruginosa in cystic fibrosis | Yes | No adverse events noted clinically or on laboratory monitoring (liver function tests, complete blood counts, electrolytes) | ||||
| Dedrick et al., 2019 [9] | Three phage cocktail—Muddy, BPs33ΔHTH-HRM10, and ZoeJΔ45 (IV) | Undetectable levels of endotoxin | MDR Mycobacterium abscessus | Detected in serum after 1 day of treatment Detected in feces 4 and 6 days post-treatment Detected in wound swabs at 3 and 5 days post-treatment Undetected in saliva, although a high phage titer was observed on Day 9 after treatment initiation | Yes | Present | Transient sweats and flushing for the first 2 days of therapy, but continued therapy without event | |
| Kuipers et al., 2019 [127] | Anti-Klebsiella pneumoniae phages (Oral/intravesical) | No exact details of endotoxin concentration | MDR-ESBL-producing K. pneumoniae in chronic UTI | None | ||||
| Aslam et al., 2019 [128] | AB-SA01 cocktail (IV) | MSSA in left ventricular assist device infection | No adverse clinical or laboratory events | |||||
| Ferry et al., 2018 [129] | Staphylococcal phage Sb-1 (Local) | Yes, <1–5 (EU)/mL for 1010 pfu/mL | XDR P. aeruginosa in complex bone and joint infection | |||||
| Duplessis et al., 2018 [130] | Phage cocktail (IV) | Yes, <5 EU/kg per hour Diluted to meet standard. | MDR P. aeruginosa in bacteremia | None | ||||
| LaVergne et al., 2018 [11] | Phage (IV) | Yes, <5 EU/kg per hour Diluted to meet standard. | MDR A. baumannii in craniectomy site infection | Detected in blood during initial administration | Yes | Transient hypotension | ||
| Ferry et al., 2018 [131] | Phage cocktail (Local) | MDR P. aeruginosa and methicillin- susceptible S. aureus in Prosthetic-Joint Infection | None | |||||
| Ujmajuridze et al., 2018 [78] | Adapted Pyo phage (Intravesical) | MDR uropathogens (S. aureus, E. coli, Streptococcus spp., P. aeruginosa, and Proteus mirabilis) in UTI | Transient fever and chills | |||||
| Schooley et al., 2017 [30] | ΦPC, ΦIV, and ΦIVB (IV and Intracavitary) | Yes, 2.4 × 103 endotoxin units (EU)/mL, 5.89 × 103 EU/mL, and 1.64 × 103 EU/mL, respectively. Diluted to meet standard. | MDR A. baumannii in infection | No detectable phage titer in plasma samples after 6 h following initial injection | Yes | None | ||
| Zhvania et al., 2017 [132] | Sb1, Pyo, and Fersis phages (Local) | Antibiotic-resistant chronic S. aureus in skin infection | Yes | None | ||||
| Jennes et al., 2017 [133] | BFC1 cocktail (IV) | MDR P. aeruginosa in acute kidney injury | Yes | None | No unexpected adverse events, clinical abnormalities, or changes in laboratory tests | |||
| Studies | Phage/s | Against | Phage Distribution | Normal Imaging/Lab Assessment (e.g., X-rays, Liver Function) | Presence of Abnormal (Increase or Decrease) of | Adverse Events | ||
|---|---|---|---|---|---|---|---|---|
| Phage/s (Administration Route) | Endotoxin within Acceptable Range | Cell Infiltration/Cytokine Production | Antibodies Production | |||||
| Leitner et al., 2020 [134] | Pyo Phage (Local) | Yes, <0.5 EU/mL | MDR uropathogens (Enterococcus spp., E. coli, Proteus mirabilis, P. aeruginosa, Staphylococcus spp., and Streptococcus spp.) in UTI | Yes | Sudden onset of fever (>38 °C) | |||
| Fabijan et al., 2020 [135] | AB-SA01 (IV) | MRSA in infection | Detected in blood up to 12 h after dosing, and valve tissues at day 14 of phage treatment. | Yes | Not present. (Decline in inflammatory markers) | No adverse reactions (fever, tachycardia, hypotension, diarrhea, or abdominal pain and the development of renal or hepatic dysfunction) | ||
| Wright et al., 2009 [136] | Biophage-PA. (Local) | Antibiotic-resistant P. aeruginosa in chronic otitis | Detected in ear during post- treatment | Present in some patients. Assessed via VAS | None | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.M.; Nang, S.C.; Tang, S.S. The Safety of Bacteriophages in Treatment of Diseases Caused by Multidrug-Resistant Bacteria. Pharmaceuticals 2023, 16, 1347. https://doi.org/10.3390/ph16101347
Chung KM, Nang SC, Tang SS. The Safety of Bacteriophages in Treatment of Diseases Caused by Multidrug-Resistant Bacteria. Pharmaceuticals. 2023; 16(10):1347. https://doi.org/10.3390/ph16101347
Chicago/Turabian StyleChung, Ka Mun, Sue C. Nang, and Swee Seong Tang. 2023. "The Safety of Bacteriophages in Treatment of Diseases Caused by Multidrug-Resistant Bacteria" Pharmaceuticals 16, no. 10: 1347. https://doi.org/10.3390/ph16101347




