Role of Resolvins in Inflammatory and Neuropathic Pain
Abstract
:1. Introduction
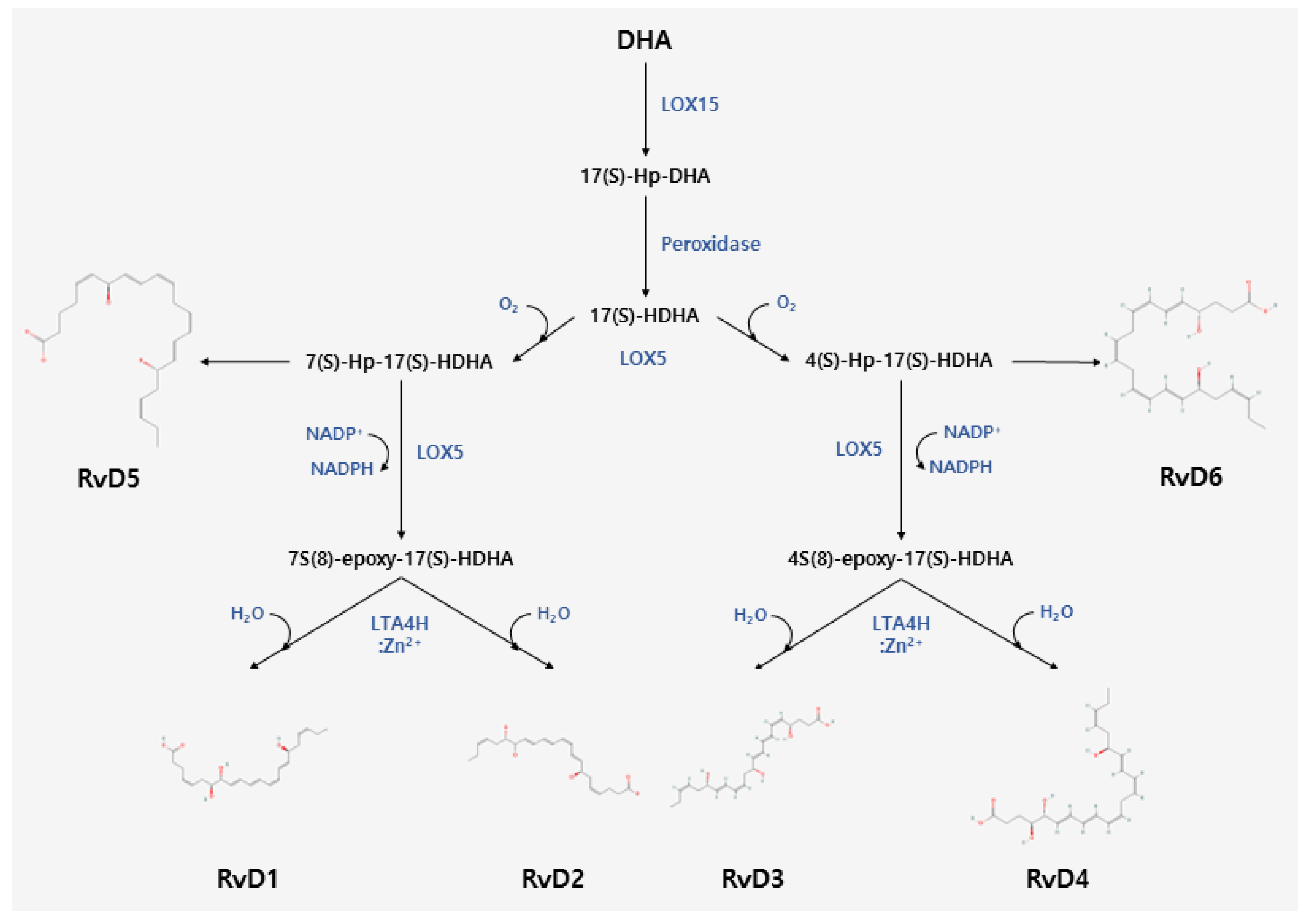
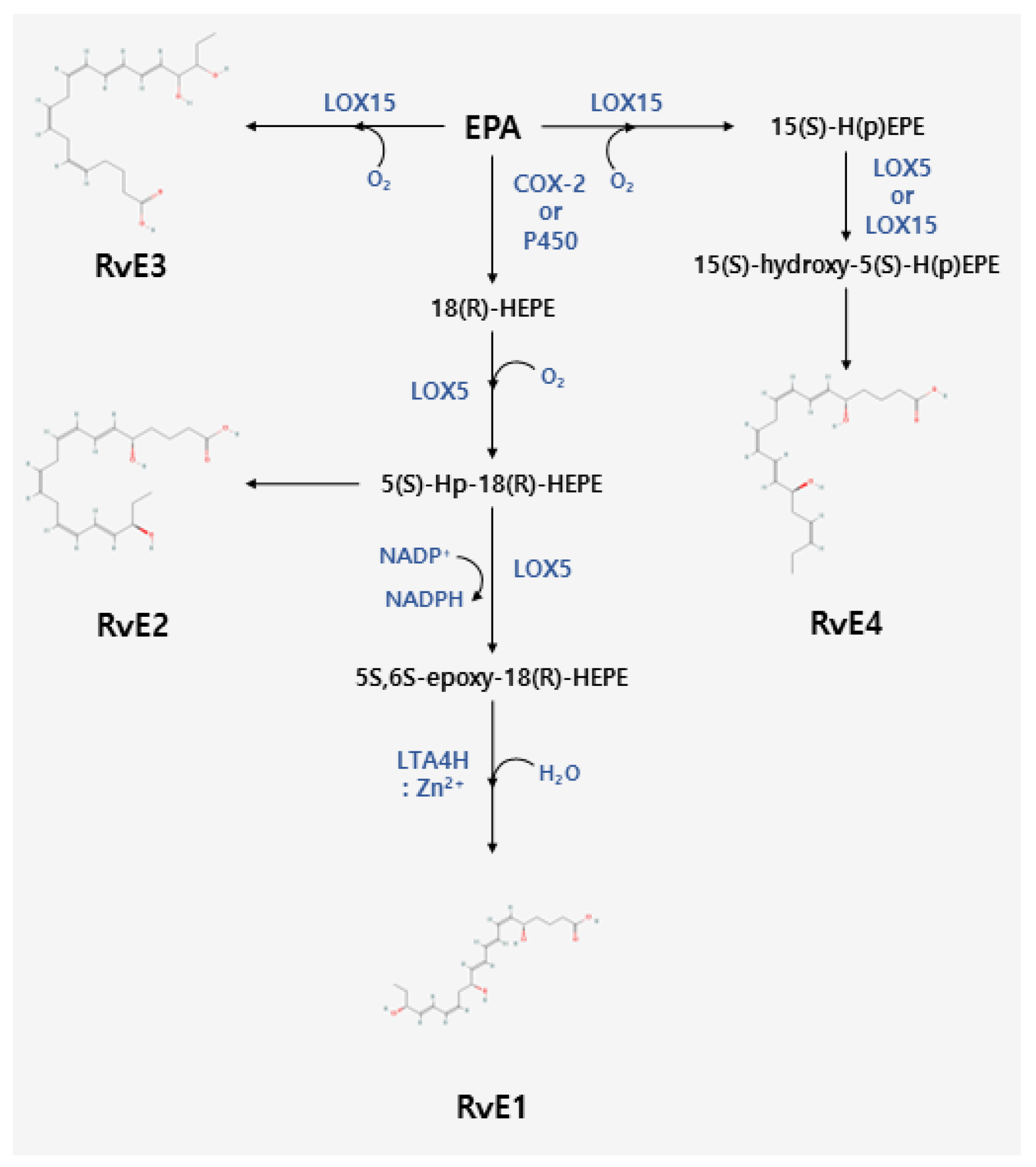
2. Biosynthesis of Resolvins
2.1. Biosynthesis of D-Series Resolvins
2.2. Biosynthesis of E-Series Resolvins
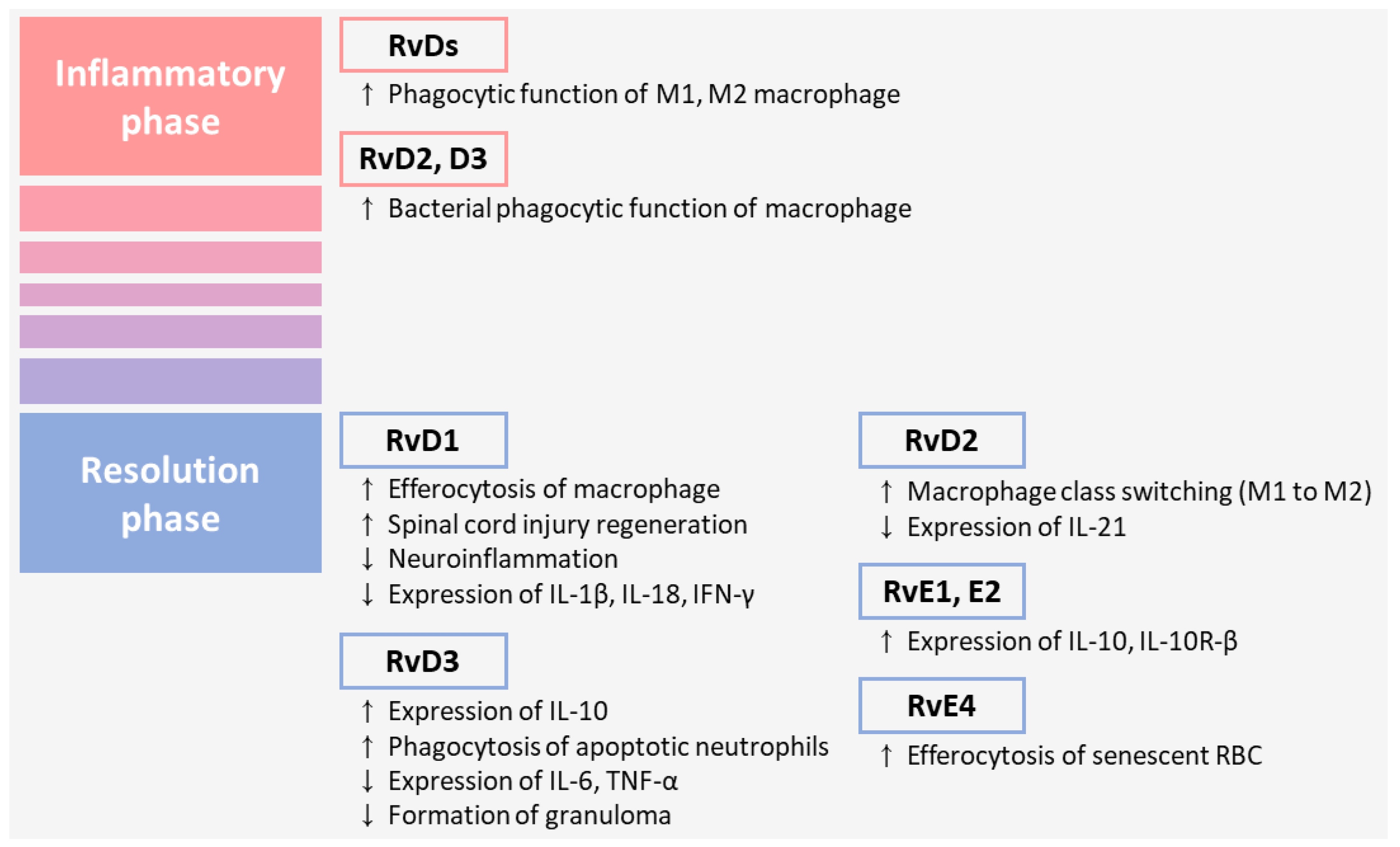
3. Resolvins as Potential Therapeutic Targets in Inflammation
4. Functions of Resolvins in Inflammatory Pain
4.1. Formalin-Induced Pain Model
4.2. Complete Freund’s Adjuvant (CFA)-Induced Pain Model
4.3. Capsaicin-Induced Pain Model
4.4. Carrageenan-Induced Pain Model
4.5. Allyl Isothiocyanate (AITC)-Induced Pain Model
4.6. Cytokine-Induced Pain Model
5. Functions of Resolvins in Neuropathic Pain
5.1. Chemotherapy-Induced Peripheral Neuropathy (CIPN) Pain Model
5.2. Chronic Constrictive Injury (CCI) Mouse Model
5.3. Spinal Nerve Ligation (SNL) Rat Model
6. Perspective
6.1. The Preparation of NSAIDs and Resolvins
6.2. Challenges in Resolvins Production
6.3. Administration Routes for Resolvins
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Herr, M.J. Physiology, Nociception. In StatPearls; National Institutes of Health: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hwang, S.M.; Chung, G.; Kim, Y.H.; Park, C.K. The Role of Maresins in Inflammatory Pain: Function of Macrophages in Wound Regeneration. Int. J. Mol. Sci. 2019, 20, 5849. [Google Scholar] [CrossRef]
- Van der Vlist, M.; Raoof, R.; Willemen, H.; Prado, J.; Versteeg, S.; Martin Gil, C.; Vos, M.; Lokhorst, R.E.; Pasterkamp, R.J.; Kojima, T.; et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron 2022, 110, 613–626.e9. [Google Scholar] [CrossRef]
- Parisien, M.; Lima, L.V.; Dagostino, C.; El-Hachem, N.; Drury, G.L.; Grant, A.V.; Huising, J.; Verma, V.; Meloto, C.B.; Silva, J.R.; et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci. Transl. Med. 2022, 14, eabj9954. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef]
- Limerick, G.; Christo, D.K.; Tram, J.; Moheimani, R.; Manor, J.; Chakravarthy, K.; Karri, J.; Christo, P.J. Complex Regional Pain Syndrome: Evidence-Based Advances in Concepts and Treatments. Curr. Pain Headache Rep. 2023, 27, 269–298. [Google Scholar] [CrossRef]
- Haanpaa, M.; Attal, N.; Backonja, M.; Baron, R.; Bennett, M.; Bouhassira, D.; Cruccu, G.; Hansson, P.; Haythornthwaite, J.A.; Iannetti, G.D.; et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011, 152, 14–27. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Fiore, N.T.; Debs, S.R.; Hayes, J.P.; Duffy, S.S.; Moalem-Taylor, G. Pain-resolving immune mechanisms in neuropathic pain. Nat. Rev. Neurol. 2023, 19, 199–220. [Google Scholar] [CrossRef]
- Hanc, P.; Messou, M.A.; Wang, Y.; von Andrian, U.H. Control of myeloid cell functions by nociceptors. Front. Immunol. 2023, 14, 1127571. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, S.; Cheng, J.; Zhang, X.; Jing, D.; Lin, Z.; Zhou, J. Integrated analysis reveals Atf3 promotes neuropathic pain via orchestrating JunB mediated release of inflammatory cytokines in DRG macrophage. Life Sci. 2023, 329, 121939. [Google Scholar] [CrossRef]
- Tansley, S.; Uttam, S.; Urena Guzman, A.; Yaqubi, M.; Pacis, A.; Parisien, M.; Deamond, H.; Wong, C.; Rabau, O.; Brown, N.; et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat. Commun. 2022, 13, 843. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, M.W.; Wang, X.W.; Cui, X.; Liu, J.; Huang, Q.; Cao, X.; Zhou, F.Q.; Qian, J.; He, S.Q.; et al. scRNA-sequencing reveals subtype-specific transcriptomic perturbations in DRG neurons of Pirt(EGFPf) mice in neuropathic pain condition. eLife 2022, 11, e76063. [Google Scholar] [CrossRef]
- Ji, H.; Kim, K.R.; Park, J.J.; Lee, J.Y.; Sim, Y.; Choi, H.; Kim, S. Combination gene delivery reduces spinal cord pathology in rats with peripheral neuropathic pain. J. Pain. 2023. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, W.; Ou, W.; Zhang, M.; Cui, S.; Zhang, S. Daphnetin alleviates neuropathic pain in chronic constrictive injury rats via regulating the NF-kappaB dependent CXCL1/CXCR2 signaling pathway. Pharm. Biol. 2023, 61, 746–754. [Google Scholar] [CrossRef]
- Welberg, L. Inflammation helps resolve pain. Nat. Neurosci. 2022, 25, 677. [Google Scholar] [CrossRef]
- Xu, M.; Bennett, D.L.H.; Querol, L.A.; Wu, L.J.; Irani, S.R.; Watson, J.C.; Pittock, S.J.; Klein, C.J. Pain and the immune system: Emerging concepts of IgG-mediated autoimmune pain and immunotherapies. J. Neurol. Neurosurg. Psychiatry 2020, 91, 177–188. [Google Scholar] [CrossRef]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Ju, Z.; Shang, Z.; Mahmud, T.; Fang, J.; Liu, Y.; Pan, Q.; Lin, X.; Chen, F. Synthesis and Anti-Inflammatory Activity of the Natural Cyclooxygenase-2 Inhibitor Axinelline A and Its Analogues. J. Nat. Prod. 2023, 86, 958–965. [Google Scholar] [CrossRef]
- Sung, C.S.; Cheng, H.J.; Chen, N.F.; Tang, S.H.; Kuo, H.M.; Sung, P.J.; Chen, W.F.; Wen, Z.H. Antinociceptive Effects of Aaptamine, a Sponge Component, on Peripheral Neuropathy in Rats. Mar. Drugs 2023, 21, 113. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Robson, P.J. Therapeutic potential of cannabinoid medicines. Drug Test. Anal. 2014, 6, 24–30. [Google Scholar] [CrossRef]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef]
- Sofia, R.D.; Vassar, H.B.; Knobloch, L.C. Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. Psychopharmacologia 1975, 40, 285–295. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Karuppagounder, V.; Nowak, I.; Sepulveda, D.E.; Lewis, G.S.; Christopher, N.C.; Raup-Konsavage, W.M.; Vrana, K.E.; Kamal, F.; Elbarbary, R.A. Cannabidiol and cannabigerol, non-psychotropic cannabinoids, as analgesics that effectively manage bone fracture pain and promote healing in mice. J. Bone Miner Res. 2023. [Google Scholar] [CrossRef]
- Frane, N.; Stapleton, E.; Iturriaga, C.; Ganz, M.; Rasquinha, V.; Duarte, R. Cannabidiol as a treatment for arthritis and joint pain: An exploratory cross-sectional study. J. Cannabis Res. 2022, 4, 47. [Google Scholar] [CrossRef]
- Uberall, M.A. A Review of Scientific Evidence for THC:CBD Oromucosal Spray (Nabiximols) in the Management of Chronic Pain. J. Pain Res. 2020, 13, 399–410. [Google Scholar] [CrossRef]
- Roman-Vargas, Y.; Porras-Arguello, J.D.; Blandon-Naranjo, L.; Perez-Perez, L.D.; Benjumea, D.M. Evaluation of the Analgesic Effect of High-Cannabidiol-Content Cannabis Extracts in Different Pain Models by Using Polymeric Micelles as Vehicles. Molecules 2023, 28, 4299. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Bylund, D.B.; Enna, S.J.; Elsevier, S. xPharm: The comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef]
- Nettleton, J.A. Omega-3 fatty acids: Comparison of plant and seafood sources in human nutrition. J. Am. Diet Assoc. 1991, 91, 331–337. [Google Scholar] [CrossRef]
- Surette, M.E. The science behind dietary omega-3 fatty acids. CMAJ 2008, 178, 177–180. [Google Scholar] [CrossRef]
- Oh, S.F.; Dona, M.; Fredman, G.; Krishnamoorthy, S.; Irimia, D.; Serhan, C.N. Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 2012, 188, 4527–4534. [Google Scholar] [CrossRef]
- Isobe, Y.; Arita, M.; Iwamoto, R.; Urabe, D.; Todoroki, H.; Masuda, K.; Inoue, M.; Arai, H. Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3. J. Biochem. 2013, 153, 355–360. [Google Scholar] [CrossRef]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.L.; Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar] [CrossRef]
- Biringer, R.G. The enzymology of human eicosanoid pathways: The lipoxygenase branches. Mol. Biol. Rep. 2020, 47, 7189–7207. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- McGee, J.; Fitzpatrick, F. Enzymatic hydration of leukotriene A4. Purification and characterization of a novel epoxide hydrolase from human erythrocytes. J. Biol. Chem. 1985, 260, 12832–12837. [Google Scholar] [CrossRef]
- Gerlach, B.D.; Ampomah, P.B.; Yurdagul, A., Jr.; Liu, C.; Lauring, M.C.; Wang, X.; Kasikara, C.; Kong, N.; Shi, J.; Tao, W.; et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 2021, 33, 2445–2463.e2448. [Google Scholar] [CrossRef]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef]
- Sun, Y.P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng, C.Y.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef]
- Winkler, J.W.; Orr, S.K.; Dalli, J.; Cheng, C.Y.; Sanger, J.M.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci. Rep. 2016, 6, 18972. [Google Scholar] [CrossRef]
- Primdahl, K.G.; Tungen, J.E.; De Souza, P.R.S.; Colas, R.A.; Dalli, J.; Hansen, T.V.; Vik, A. Stereocontrolled synthesis and investigation of the biosynthetic transformations of 16(S),17(S)-epoxy-PD(n-3 DPA). Org. Biomol. Chem. 2017, 15, 8606–8613. [Google Scholar] [CrossRef]
- Kok Kendirlioglu, B.; Unalan Ozpercin, P.; Yuksel Oksuz, O.; Sozen, S.; Cihnioglu, R.; Kalelioglu, T.; Ilnem, M.C.; Karamustafalioglu, N. Resolvin D1 as a novel anti-inflammatory marker in manic, depressive and euthymic states of bipolar disorder. Nord. J. Psychiatry 2020, 74, 83–88. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Gilligan, M.M.; Gartung, A.; Sulciner, M.L.; Norris, P.C.; Sukhatme, V.P.; Bielenberg, D.R.; Huang, S.; Kieran, M.W.; Serhan, C.N.; Panigrahy, D. Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 6292–6297. [Google Scholar] [CrossRef]
- Dos Santos, H.T.; Nam, K.; Gil, D.; Yellepeddi, V.; Baker, O.J. Current experimental methods to investigate the impact of specialized pro-resolving lipid mediators on Sjogren’s syndrome. Front. Immunol. 2022, 13, 1094278. [Google Scholar] [CrossRef]
- Luciano, N.; Valentini, V.; Calabro, A.; Elefante, E.; Vitale, A.; Baldini, C.; Bartoloni, E. One year in review 2015: Sjogren’s syndrome. Clin. Exp. Rheumatol. 2015, 33, 259–271. [Google Scholar]
- Eickmeier, O.; Seki, H.; Haworth, O.; Hilberath, J.N.; Gao, F.; Uddin, M.; Croze, R.H.; Carlo, T.; Pfeffer, M.A.; Levy, B.D. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal. Immunol. 2013, 6, 256–266. [Google Scholar] [CrossRef]
- Benabdoune, H.; Rondon, E.P.; Shi, Q.; Fernandes, J.; Ranger, P.; Fahmi, H.; Benderdour, M. The role of resolvin D1 in the regulation of inflammatory and catabolic mediators in osteoarthritis. Inflamm. Res. 2016, 65, 635–645. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Colby, J.K.; Abdulnour, R.E.; Sham, H.P.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Hellmann, J.; Wong, B.; Cui, Y.; El-Chemaly, S.; et al. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Protective for Injured Epithelia. Am. J. Pathol. 2016, 186, 1801–1813. [Google Scholar] [CrossRef]
- Libreros, S.; Shay, A.E.; Nshimiyimana, R.; Fichtner, D.; Martin, M.J.; Wourms, N.; Serhan, C.N. A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution. Front. Immunol. 2020, 11, 631319. [Google Scholar] [CrossRef]
- Serhan, C.N.; Libreros, S.; Nshimiyimana, R. E-series resolvin metabolome, biosynthesis and critical role of stereochemistry of specialized pro-resolving mediators (SPMs) in inflammation-resolution: Preparing SPMs for long COVID-19, human clinical trials, and targeted precision nutrition. Semin. Immunol. 2022, 59, 101597. [Google Scholar] [CrossRef]
- Mas, E.; Croft, K.D.; Zahra, P.; Barden, A.; Mori, T.A. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012, 58, 1476–1484. [Google Scholar] [CrossRef]
- Yu, D.; Jiang, F.; Xu, W.; He, P.; Chen, F.; Liu, X.; Bao, X. Declined Serum Resolvin D1 Levels to Predict Severity and Prognosis of Human Aneurysmal Subarachnoid Hemorrhage: A Prospective Cohort Study. Neuropsychiatr. Dis. Treat. 2023, 19, 1463–1476. [Google Scholar] [CrossRef]
- Karayigit, O.; Nurkoc, S.G.; Basyigit, F.; Kiziltunc, E. The Role of Serum Resolvin D1 Levels in Determining the Presence and Prognosis of ST-Segment Elevation Myocardial Infarction. Med. Princ. Pract. 2022, 31, 548–554. [Google Scholar] [CrossRef]
- Karatay, E.; Utku, O.G. Serum resolvin D1 levels as a marker of inflammation in constipation dominant irritable bowel syndrome. Turk. J. Gastroenterol. 2020, 31, 113–119. [Google Scholar] [CrossRef]
- Costa, D.; Pellicano, C.; Mercuri, V.; Arnone, J.M.; Rizzo, F.; Leodori, G.; Gargiulo, P.; Rosato, E. In acromegalic patients the serum levels of interleukin-33 and Resolvin D1 influence skin perfusion of hands: A pilot study. Microvasc. Res. 2023, 149, 104571. [Google Scholar] [CrossRef]
- Song, J.; Sun, R.; Zhang, Y.; Ke, J.; Zhao, D. Serum resolvin E1 levels and its relationship with thyroid autoimmunity in Hashimoto’s thyroiditis: A preliminary study. BMC Endocr. Disord. 2021, 21, 66. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Lee, H.L.; Wee, S.; Lee, H.; Hwang, G.; Hwang, S.; Yoon, S.; Yang, Y.I.; Han, I.; Kim, K.N. Co-Administration of Resolvin D1 and Peripheral Nerve-Derived Stem Cell Spheroids as a Therapeutic Strategy in a Rat Model of Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 10971. [Google Scholar] [CrossRef]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Author Correction: Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 4725. [Google Scholar] [CrossRef]
- Hardesty, J.E.; Warner, J.B.; Song, Y.L.; Rouchka, E.C.; McClain, C.J.; Warner, D.R.; Kirpich, I.A. Resolvin D1 attenuated liver injury caused by chronic ethanol and acute LPS challenge in mice. FASEB J. 2023, 37, e22705. [Google Scholar] [CrossRef]
- Sundarasivarao, P.Y.K.; Walker, J.M.; Rodriguez, A.; Spur, B.W.; Yin, K. Resolvin D2 induces anti-microbial mechanisms in a model of infectious peritonitis and secondary lung infection. Front. Immunol. 2022, 13, 1011944. [Google Scholar] [CrossRef]
- Kim, J.; Joshi, H.P.; Sheen, S.H.; Kim, K.T.; Kyung, J.W.; Choi, H.; Kim, Y.W.; Kwon, S.Y.; Roh, E.J.; Choi, U.Y.; et al. Resolvin D3 Promotes Inflammatory Resolution, Neuroprotection, and Functional Recovery after Spinal Cord Injury. Mol. Neurobiol. 2021, 58, 424–438. [Google Scholar] [CrossRef]
- Hamaguchi, A.; Fukuda, H.; Fujiwara, K.; Harada, T.; Fukushima, K.; Shuto, S.; Fujino, H. Individual resolvin E family members work distinctly and in a coordinated manner in the resolution of inflammation. Prostaglandins Lipid Mediat. 2023, 168, 106759. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Backhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Ganesan, R.; Henkels, K.M.; Shah, K.; De La Rosa, X.; Libreros, S.; Cheemarla, N.R.; Serhan, C.N.; Gomez-Cambronero, J. D-series Resolvins activate Phospholipase D in phagocytes during inflammation and resolution. FASEB J. 2020, 34, 15888–15906. [Google Scholar] [CrossRef]
- Norris, P.C.; Arnardottir, H.; Sanger, J.M.; Fichtner, D.; Keyes, G.S.; Serhan, C.N. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot. Essent. Fatty Acids 2018, 138, 81–89. [Google Scholar] [CrossRef]
- Shay, A.E.; Nshimiyimana, R.; Samuelsson, B.; Petasis, N.A.; Haeggstrom, J.Z.; Serhan, C.N. Human leukocytes selectively convert 4S,5S-epoxy-resolvin to resolvin D3, resolvin D4, and a cys-resolvin isomer. Proc. Natl. Acad. Sci. USA 2021, 118, e2116559118. [Google Scholar] [CrossRef]
- Fukuda, H.; Ikeda, H.; Muromoto, R.; Hirashima, K.; Ishimura, K.; Fujiwara, K.; Aoki-Saito, H.; Hisada, T.; Watanabe, M.; Ishihara, J.; et al. Synthesis of Resolvin E3, a Proresolving Lipid Mediator, and Its Deoxy Derivatives: Identification of 18-Deoxy-resolvin E3 as a Potent Anti-Inflammatory Agent. J. Org. Chem. 2020, 85, 14190–14200. [Google Scholar] [CrossRef]
- Sadhu, S.; Decker, C.; Sansbury, B.E.; Marinello, M.; Seyfried, A.; Howard, J.; Mori, M.; Hosseini, Z.; Arunachalam, T.; Finn, A.V.; et al. Radiation-Induced Macrophage Senescence Impairs Resolution Programs and Drives Cardiovascular Inflammation. J. Immunol. 2021, 207, 1812–1823. [Google Scholar] [CrossRef]
- Rymut, N.; Heinz, J.; Sadhu, S.; Hosseini, Z.; Riley, C.O.; Marinello, M.; Maloney, J.; MacNamara, K.C.; Spite, M.; Fredman, G. Resolvin D1 promotes efferocytosis in aging by limiting senescent cell-induced MerTK cleavage. FASEB J. 2020, 34, 597–609. [Google Scholar] [CrossRef]
- Dort, J.; Orfi, Z.; Fabre, P.; Molina, T.; Conte, T.C.; Greffard, K.; Pellerito, O.; Bilodeau, J.F.; Dumont, N.A. Resolvin-D2 targets myogenic cells and improves muscle regeneration in Duchenne muscular dystrophy. Nat. Commun. 2021, 12, 6264. [Google Scholar] [CrossRef]
- Ji, R.-R. Specialized Pro-Resolving Mediators as Resolution Pharmacology for the Control of Pain and Itch. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 273–293. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Valente, M.; Dentoni, M.; Bellizzi, F.; Kuris, F.; Gigli, G.L. Specialized Pro-Resolving Mediators in Neuroinflammation: Overview of Studies and Perspectives of Clinical Applications. Molecules 2022, 27, 4836. [Google Scholar] [CrossRef]
- Kelley, D.S.; Taylor, P.C.; Nelson, G.J.; Mackey, B.E. Dietary docosahexaenoic acid and immunocompetence in young healthy men. Lipids 1998, 33, 559–566. [Google Scholar] [CrossRef]
- Bright, J.M.; Sullivan, P.S.; Melton, S.L.; Schneider, J.F.; McDonald, T.P. The effects of n-3 fatty acid supplementation on bleeding time, plasma fatty acid composition, and in vitro platelet aggregation in cats. J. Vet. Intern. Med. 1994, 8, 247–252. [Google Scholar] [CrossRef]
- Cooper, A.L.; Gibbons, L.; Horan, M.A.; Little, R.A.; Rothwell, N.J. Effect of dietary fish oil supplementation on fever and cytokine production in human volunteers. Clin. Nutr. 1993, 12, 321–328. [Google Scholar] [CrossRef]
- Lee, T.H.; Hoover, R.L.; Williams, J.D.; Sperling, R.I.; Ravalese, J., 3rd; Spur, B.W.; Robinson, D.R.; Corey, E.J.; Lewis, R.A.; Austen, K.F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N. Engl. J. Med. 1985, 312, 1217–1224. [Google Scholar] [CrossRef]
- Hodge, W.; Barnes, D.; Schachter, H.M.; Pan, Y.; Lowcock, E.C.; Zhang, L.; Sampson, M.; Morrison, A.; Tran, K.; Miguelez, M.; et al. Effects of omega-3 fatty acids on eye health. Evid Rep. Technol. Assess (Summ.) 2005, 1–6. [Google Scholar]
- Arora, V.; Campbell, J.N.; Chung, M.K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef]
- Luo, X.; Gu, Y.; Tao, X.; Serhan, C.N.; Ji, R.R. Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Front. Pharmacol. 2019, 10, 745. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef]
- Park, C.K.; Xu, Z.Z.; Liu, T.; Lu, N.; Serhan, C.N.; Ji, R.R. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: Distinct roles of resolvin D1, D2, and E1. J. Neurosci. 2011, 31, 18433–18438. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Kim, Y.G.; Hwang, S.W. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br. J. Pharmacol. 2010, 161, 707–720. [Google Scholar] [CrossRef]
- Lima-Garcia, J.F.; Dutra, R.C.; da Silva, K.; Motta, E.M.; Campos, M.M.; Calixto, J.B. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 2011, 164, 278–293. [Google Scholar] [CrossRef]
- Pang, J.; Xin, P.; Kong, Y.; Wang, Z.; Wang, X. Resolvin D2 Reduces Chronic Neuropathic Pain and Bone Cancer Pain via Spinal Inhibition of IL-17 Secretion, CXCL1 Release and Astrocyte Activation in Mice. Brain. Sci. 2023, 13, 152. [Google Scholar] [CrossRef]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Lopez-Cano, M.; Fernandez-Duenas, V.; Llebaria, A.; Ciruela, F. Formalin Murine Model of Pain. Bio Protocol 2017, 7, e2628. [Google Scholar] [CrossRef]
- Hanlon, K.E.; Vanderah, T.W. Constitutive activity at the cannabinoid CB(1) receptor and behavioral responses. Methods Enzymol. 2010, 484, 3–30. [Google Scholar] [CrossRef]
- Recognition and Alleviation of Pain in Laboratory Animals; National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2009. [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Andersen, H.H.; Lo Vecchio, S.; Gazerani, P.; Arendt-Nielsen, L. Dose-response study of topical allyl isothiocyanate (mustard oil) as a human surrogate model of pain, hyperalgesia, and neurogenic inflammation. Pain 2017, 158, 1723–1732. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Kaur, J.; Ghosh, S.; Sahani, A.K.; Sinha, J.K. Mental imagery training for treatment of central neuropathic pain: A narrative review. Acta Neurol. Belg. 2019, 119, 175–186. [Google Scholar] [CrossRef]
- Su, C.J.; Zhang, J.T.; Zhao, F.L.; Xu, D.L.; Pan, J.; Liu, T. Resolvin D1/N-formyl peptide receptor 2 ameliorates paclitaxel-induced neuropathic pain through the activation of IL-10/Nrf2/HO-1 pathway in mice. Front. Immunol. 2023, 14, 1091753. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Berta, T.; Ji, R.R. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J. Neuroimmune Pharmacol. 2013, 8, 37–41. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tang, Y.R.; Gao, X.; Zhang, N.N.; Lv, Q.Q.; Liu, J.; Li, Y. Aspirin-triggered Resolvin D1 ameliorates activation of the NLRP3 inflammasome via induction of autophagy in a rat model of neuropathic pain. Front. Pharmacol. 2023, 14, 971136. [Google Scholar] [CrossRef]
- Hama, A.; Takamatsu, H. Chemotherapy-Induced Peripheral Neuropathic Pain and Rodent Models. CNS Neurol. Disord. Drug Targets 2016, 15, 7–19. [Google Scholar] [CrossRef]
- Brandolini, L.; d’Angelo, M.; Antonosante, A.; Allegretti, M.; Cimini, A. Chemokine Signaling in Chemotherapy-Induced Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 2904. [Google Scholar] [CrossRef]
- Pennypacker, S.D.; Fonseca, M.M.; Morgan, J.W.; Dougherty, P.M.; Cubillos-Ruiz, J.R.; Strowd, R.E.; Romero-Sandoval, E.A. Methods and protocols for chemotherapy-induced peripheral neuropathy (CIPN) mouse models using paclitaxel. Methods Cell Biol. 2022, 168, 277–298. [Google Scholar] [CrossRef]
- Klein, I.; Lehmann, H.C. Pathomechanisms of Paclitaxel-Induced Peripheral Neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef]
- Medeiros, P.; Dos Santos, I.R.; Junior, I.M.; Palazzo, E.; da Silva, J.A.; Machado, H.R.; Ferreira, S.H.; Maione, S.; Coimbra, N.C.; de Freitas, R.L. An Adapted Chronic Constriction Injury of the Sciatic Nerve Produces Sensory, Affective, and Cognitive Impairments: A Peripheral Mononeuropathy Model for the Study of Comorbid Neuropsychiatric Disorders Associated with Neuropathic Pain in Rats. Pain Med. 2021, 22, 338–351. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Chung, J.M.; Kim, H.K.; Chung, K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol. Med. 2004, 99, 35–45. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). In StatPearls; National Institutes of Health: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef]
- Ha, M.W.; Paek, S.M. Recent Advances in the Synthesis of Ibuprofen and Naproxen. Molecules 2021, 26, 4792. [Google Scholar] [CrossRef]
- Nicolaou, K.C. ORGN 243-Molecules that Changed the World: Evolution of the Art of Total Synthesis over the Last Century. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2008; Volume 236. [Google Scholar]
- Wang, L.; Liu, M.; Jiang, M.; Wan, L.; Li, W.; Cheng, D.; Chen, F. Six-Step Continuous Flow Synthesis of Diclofenac Sodium via Cascade Etherification/Smiles Rearrangement Strategy: Tackling the Issues of Batch Processing. Chemistry 2022, 28, e202202097. [Google Scholar] [CrossRef]
- Liu, C.; Fan, D.; Lei, Q.; Lu, A.; He, X. Roles of Resolvins in Chronic Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 14883. [Google Scholar] [CrossRef]
- Meesawatsom, P.; Burston, J.; Hathway, G.; Bennett, A.; Chapman, V. Inhibitory effects of aspirin-triggered resolvin D1 on spinal nociceptive processing in rat pain models. J. Neuroinflamm. 2016, 13, 233. [Google Scholar] [CrossRef]
- Recchiuti, A.; Codagnone, M.; Pierdomenico, A.M.; Rossi, C.; Mari, V.C.; Cianci, E.; Simiele, F.; Gatta, V.; Romano, M. Immunoresolving actions of oral resolvin D1 include selective regulation of the transcription machinery in resolution-phase mouse macrophages. FASEB J. 2014, 28, 3090–3102. [Google Scholar] [CrossRef]
- Ramar, M.; Yano, N.; Fedulov, A.V. Intra-Airway Treatment with Synthetic Lipoxin A4 and Resolvin E2 Mitigates Neonatal Asthma Triggered by Maternal Exposure to Environmental Particles. Int. J. Mol. Sci. 2023, 24, 6145. [Google Scholar] [CrossRef]
- Dos Santos, H.T.; Maslow, F.; Nam, K.; Trump, B.; Weisman, G.A.; Baker, O.J. A combination treatment of low-dose dexamethasone and aspirin-triggered resolvin D1 reduces Sjogren syndrome-like features in a mouse model. JADA Found Sci. 2023, 2, 100016. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Luo, L.; Lin, J.; Li, D.; Zheng, S.; Huang, H.; Yan, S.; Yang, J.; Hao, Y.; et al. Resolvin D1 Improves the Resolution of Inflammation via Activating NF-kappaB p50/p50-Mediated Cyclooxygenase-2 Expression in Acute Respiratory Distress Syndrome. J. Immunol. 2017, 199, 2043–2054. [Google Scholar] [CrossRef]
- Hsiao, H.M.; Thatcher, T.H.; Colas, R.A.; Serhan, C.N.; Phipps, R.P.; Sime, P.J. Resolvin D1 Reduces Emphysema and Chronic Inflammation. Am. J. Pathol. 2015, 185, 3189–3201. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. 3), S5. [Google Scholar] [CrossRef]
- Deyama, S. Elucidation of the Mechanisms Underlying the Rapid Antidepressant Actions of Ketamine and Search for Possible Candidates for Novel Rapid-acting Antidepressants. Yakugaku Zasshi 2023, 143, 713–720. [Google Scholar] [CrossRef]
- Pirault, J.; Back, M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Roh, J.; Go, E.J.; Park, J.W.; Kim, Y.H.; Park, C.K. Resolvins: Potent Pain Inhibiting Lipid Mediators via Transient Receptor Potential Regulation. Front. Cell Dev. Biol. 2020, 8, 584206. [Google Scholar] [CrossRef]
- Herova, M.; Schmid, M.; Gemperle, C.; Hersberger, M. ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J. Immunol. 2015, 194, 2330–2337. [Google Scholar] [CrossRef]
- Andoh, T.; Kuraishi, Y. Expression of BLT1 leukotriene B4 receptor on the dorsal root ganglion neurons in mice. Brain Res. Mol. Brain Res. 2005, 137, 263–266. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Lee, J.Y.; Park, C.K. Resolvin E1 Inhibits Substance P-Induced Potentiation of TRPV1 in Primary Sensory Neurons. Mediat. Inflamm. 2016, 2016, 5259321. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Fredman, G.; Serhan, C.N. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 2012, 180, 2018–2027. [Google Scholar] [CrossRef]
| Pain Model | Treatment | Pain Behavior | Inflammatory Factor | Tissue | Ref |
|---|---|---|---|---|---|
| Formalin- induced pain | RvD5 | Spontaneous | - | - | [91] |
| RvE1 | Spontaneous | - | - | [92] | |
| RvD2 | Spontaneous | - | - | [93] | |
| RvD1 | Spontaneous | - | - | [94] | |
| Capsaicin- induced pain | RvE1 | Spontaneous | - | - | [92] |
| RvD2, RvE1 | Spontaneous | - | - | [93] | |
| CFA- induced pain | RvE1 RvD1 | Heat | - | - | [92] |
| RvD2 | Heat | - | - | [93] | |
| RVD1 | Mechanical, Heat | - | - | [94] | |
| AT-RvD1 | Mechanical | TNF-α, IL-1β↓ | Hind paw | [95] | |
| Carrageenan-induced pain | RvE1 RvD1 | Heat | TNF-α, IL-1β, IL-6↓ | Hind paw | [92] |
| RvD2 | Mechanical, Heat | - | - | [93] | |
| AITC- induced pain | RvD1, RvD2 | Spontaneous | - | - | [93] |
| IL-17- induced pain | RvD2 | Mechanical, Heat | CXCL1↓ | Spinal cord | [96] |
| TNF-α- induced pain | RvE1 | Mechanical, Heat | - | - | [92] |
| Pain Model | Treatment | Pain Behavior | Inflammatory Factor | Tissue | Ref |
|---|---|---|---|---|---|
| CIPN mouse | RvD1 | Mechanical, Heat, Cold | CD68↓/IL-10↑ | DRG, Sciatic nerve | [105] |
| RvD5 | Mechanical | - | - | [91] | |
| CCI mouse | RvD2 | Mechanical, Heat | IL-17, CXCL1, GFAP↓ | Spinal cord | [96] |
| RvE1 | Mechanical | Iba-1, GFAP, TNF-α↓ | Spinal cord | [106] | |
| SNL rat | RvE1 | Mechanical, Heat | - | - | [106] |
| AT-RvD1 | Mechanical, Heat | IL-1β, IL-18↓ TNF-α, Iba-1↓ | Spinal cord | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Roh, J.; Pan, J.; Kim, Y.H.; Park, C.-K.; Jo, Y.Y. Role of Resolvins in Inflammatory and Neuropathic Pain. Pharmaceuticals 2023, 16, 1366. https://doi.org/10.3390/ph16101366
Park J, Roh J, Pan J, Kim YH, Park C-K, Jo YY. Role of Resolvins in Inflammatory and Neuropathic Pain. Pharmaceuticals. 2023; 16(10):1366. https://doi.org/10.3390/ph16101366
Chicago/Turabian StylePark, Jaeik, Jueun Roh, Jingying Pan, Yong Ho Kim, Chul-Kyu Park, and Youn Yi Jo. 2023. "Role of Resolvins in Inflammatory and Neuropathic Pain" Pharmaceuticals 16, no. 10: 1366. https://doi.org/10.3390/ph16101366
APA StylePark, J., Roh, J., Pan, J., Kim, Y. H., Park, C.-K., & Jo, Y. Y. (2023). Role of Resolvins in Inflammatory and Neuropathic Pain. Pharmaceuticals, 16(10), 1366. https://doi.org/10.3390/ph16101366






