Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Profiling of Onion Peel Extracts Using UPLC-QTOF-MS/MS
2.2. Antimicrobial Activity of Onion Peel Extracts (Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC))
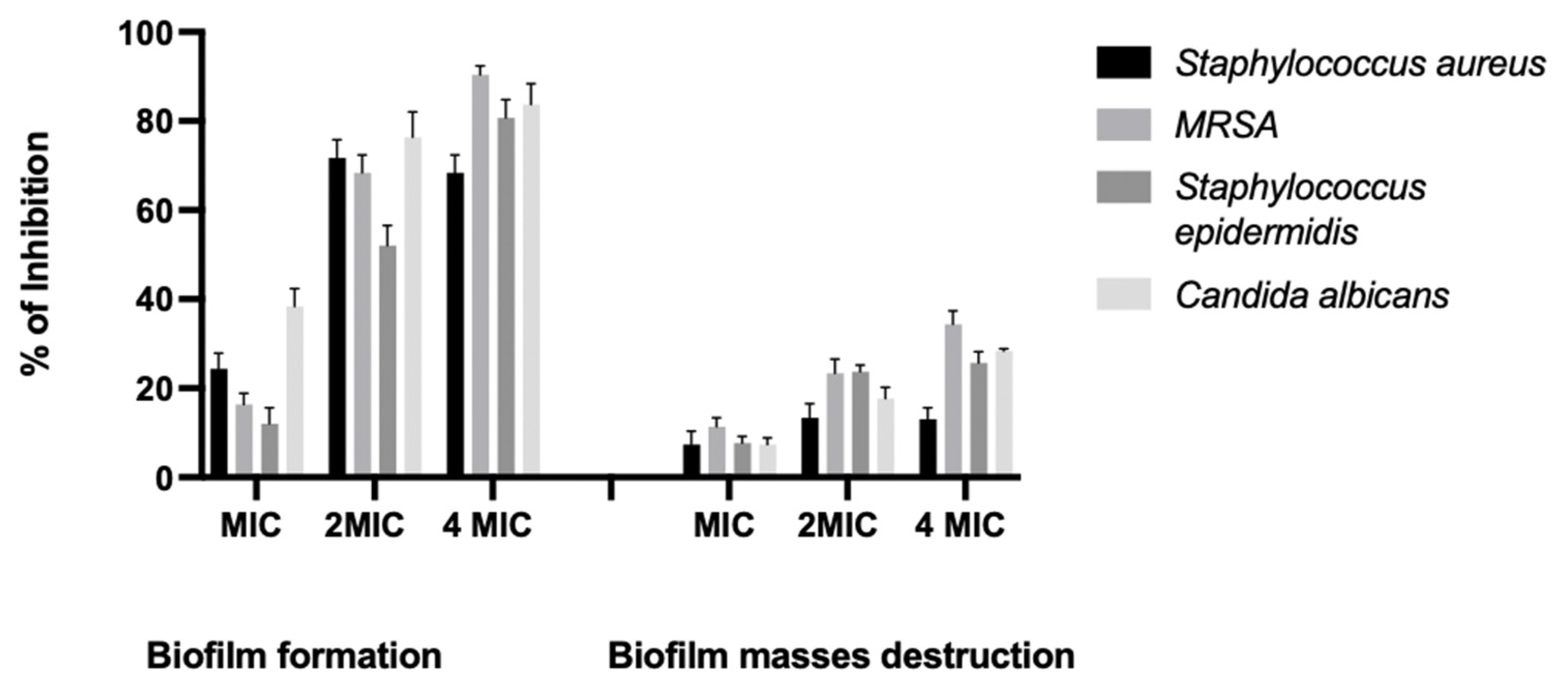
2.3. Inhibition of Biofilm Formation and Destruction of Biofilm Masses
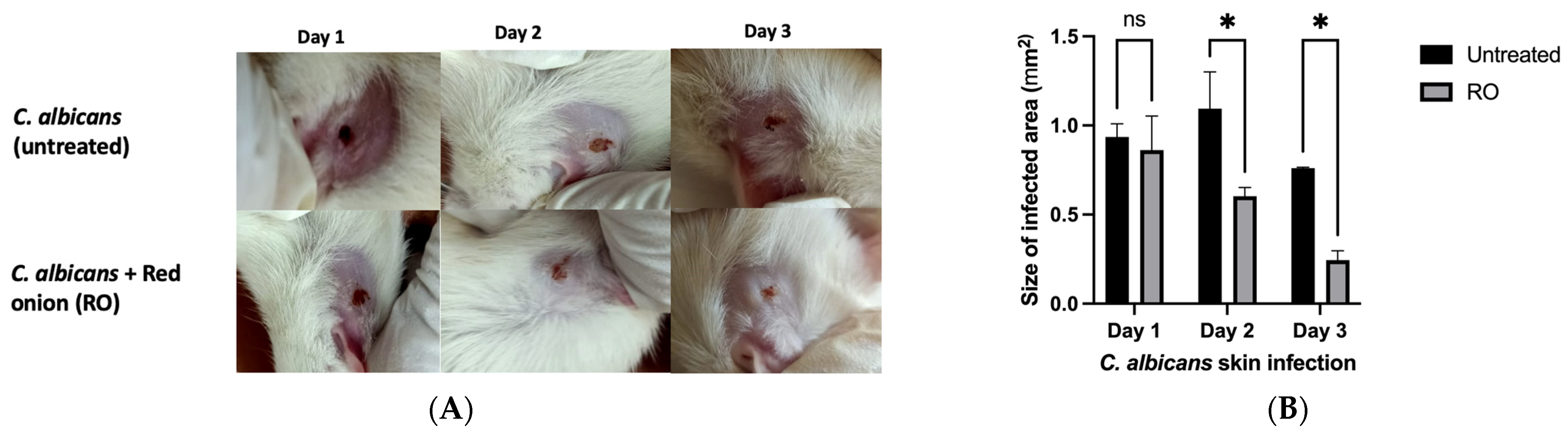
2.4. RO Peel Extract Limits C. albicans Skin Infection and Improves Skin Appearance
2.5. In Vitro Screening of the Cytotoxicity and Anti-Inflammatory Effects of Onion Peel Extracts
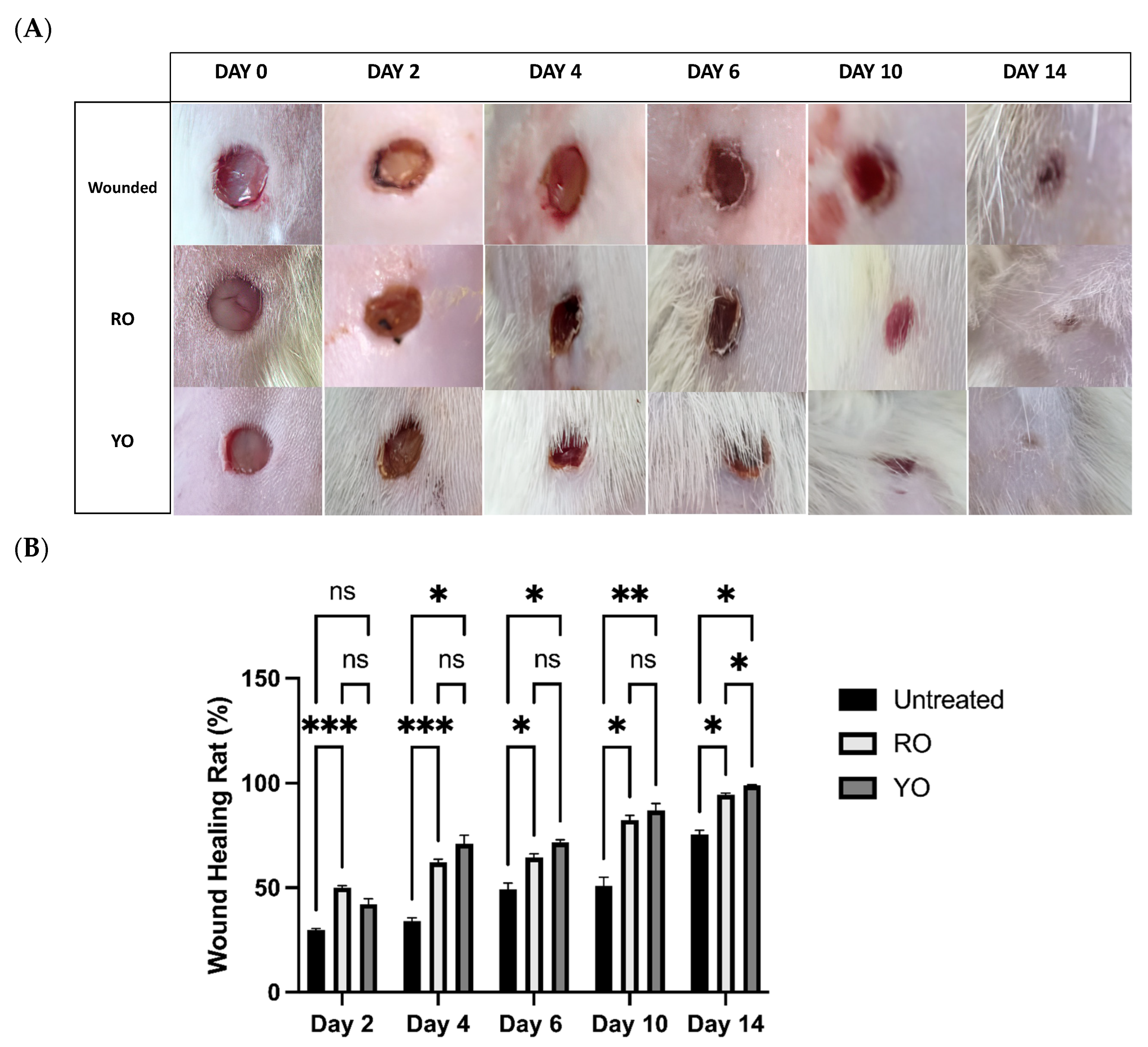
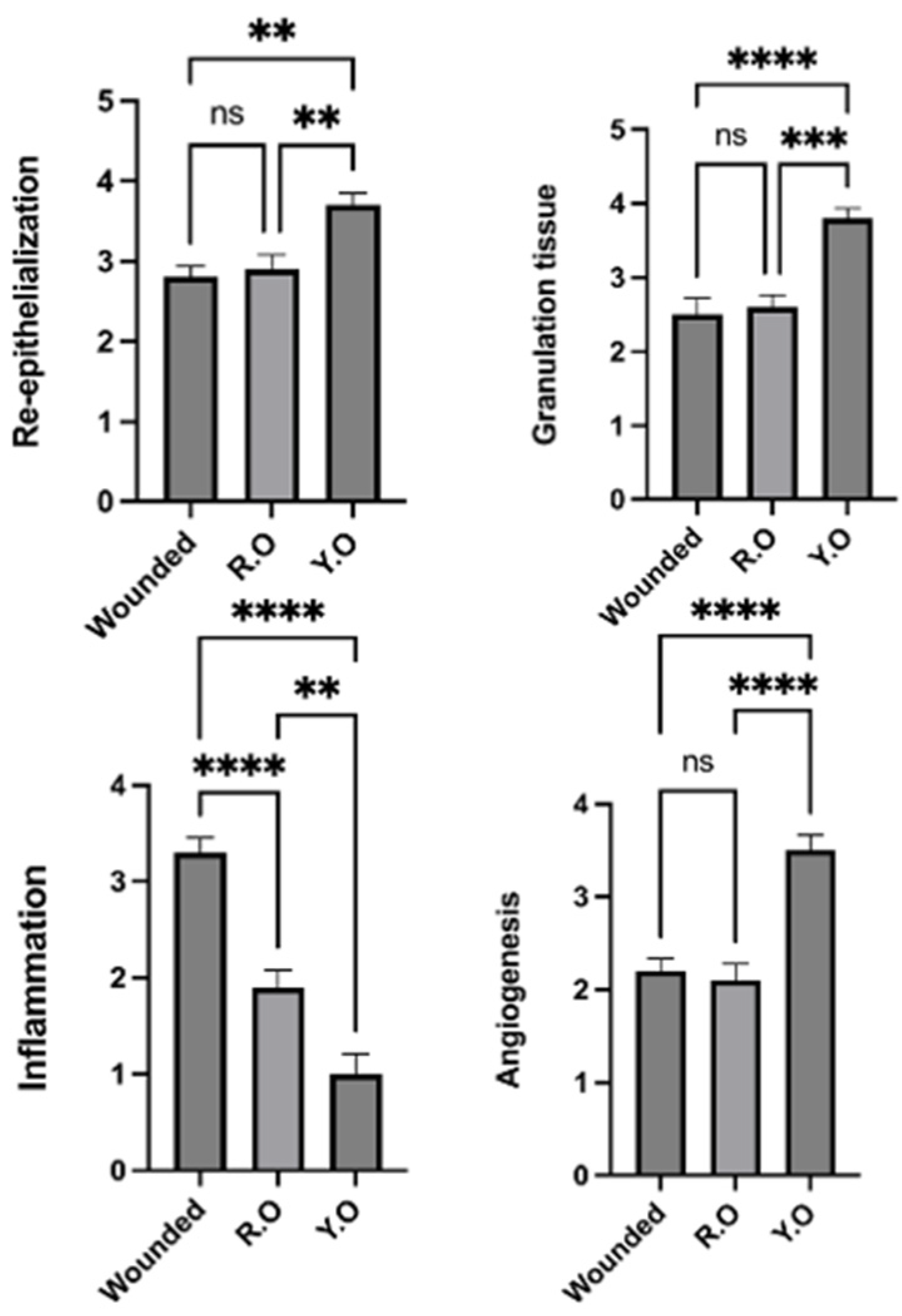
2.6. RO and YO Peel Extracts Improve Wound Healing and Tissue Repair
2.7. Onion Peel Extract Promotes Wound Healing and Ameliorates Histopathological Alterations in Wounded Tissues
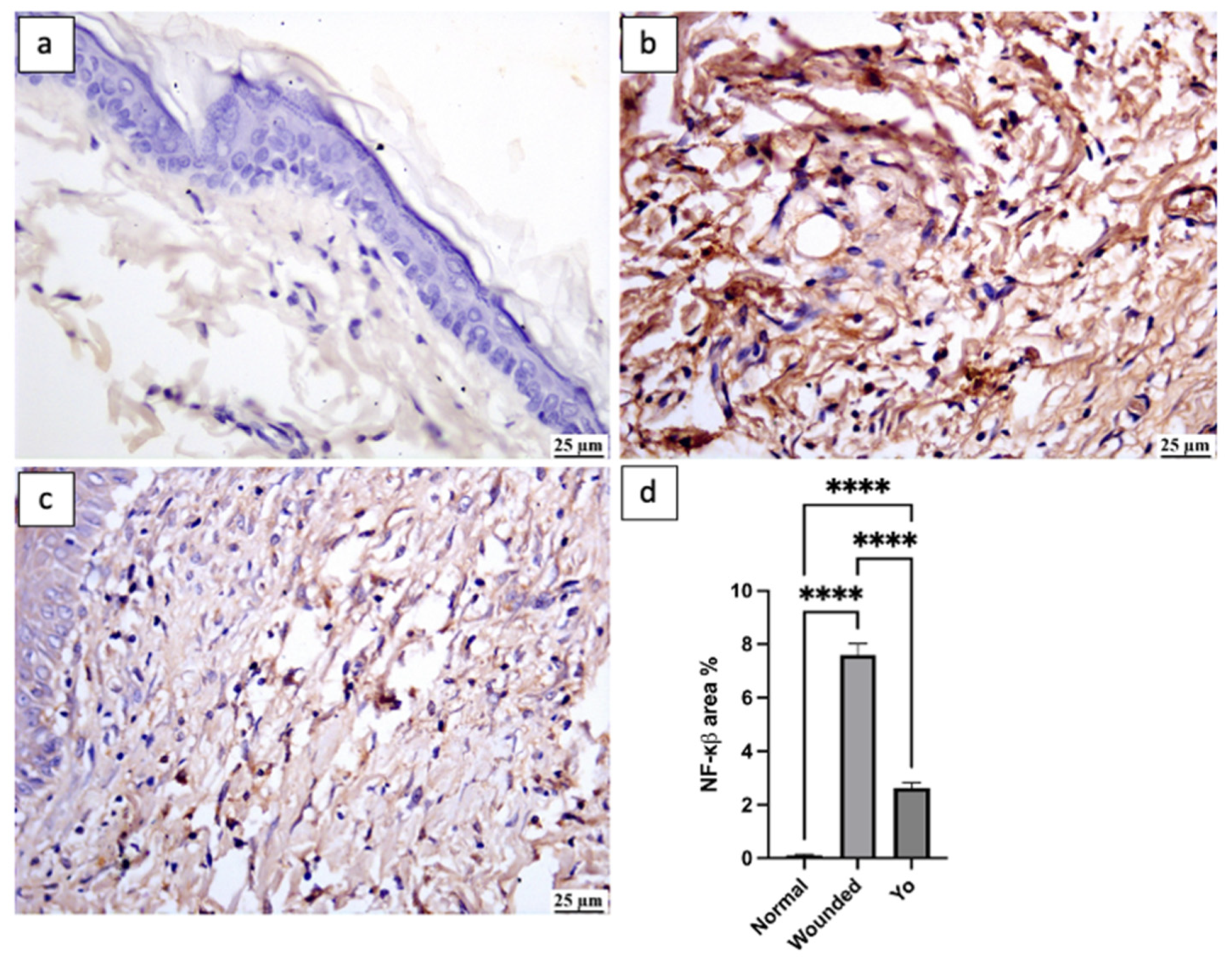
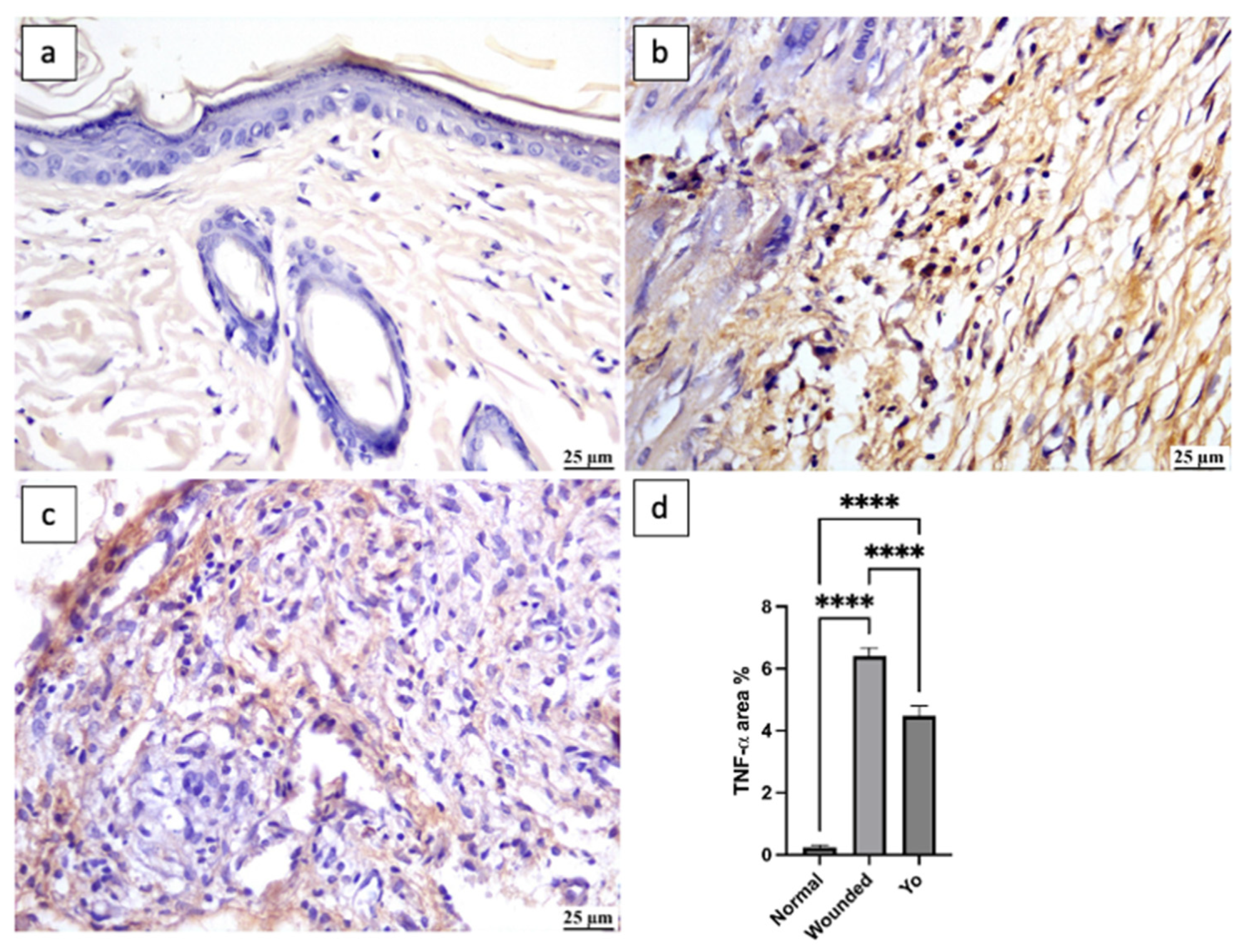
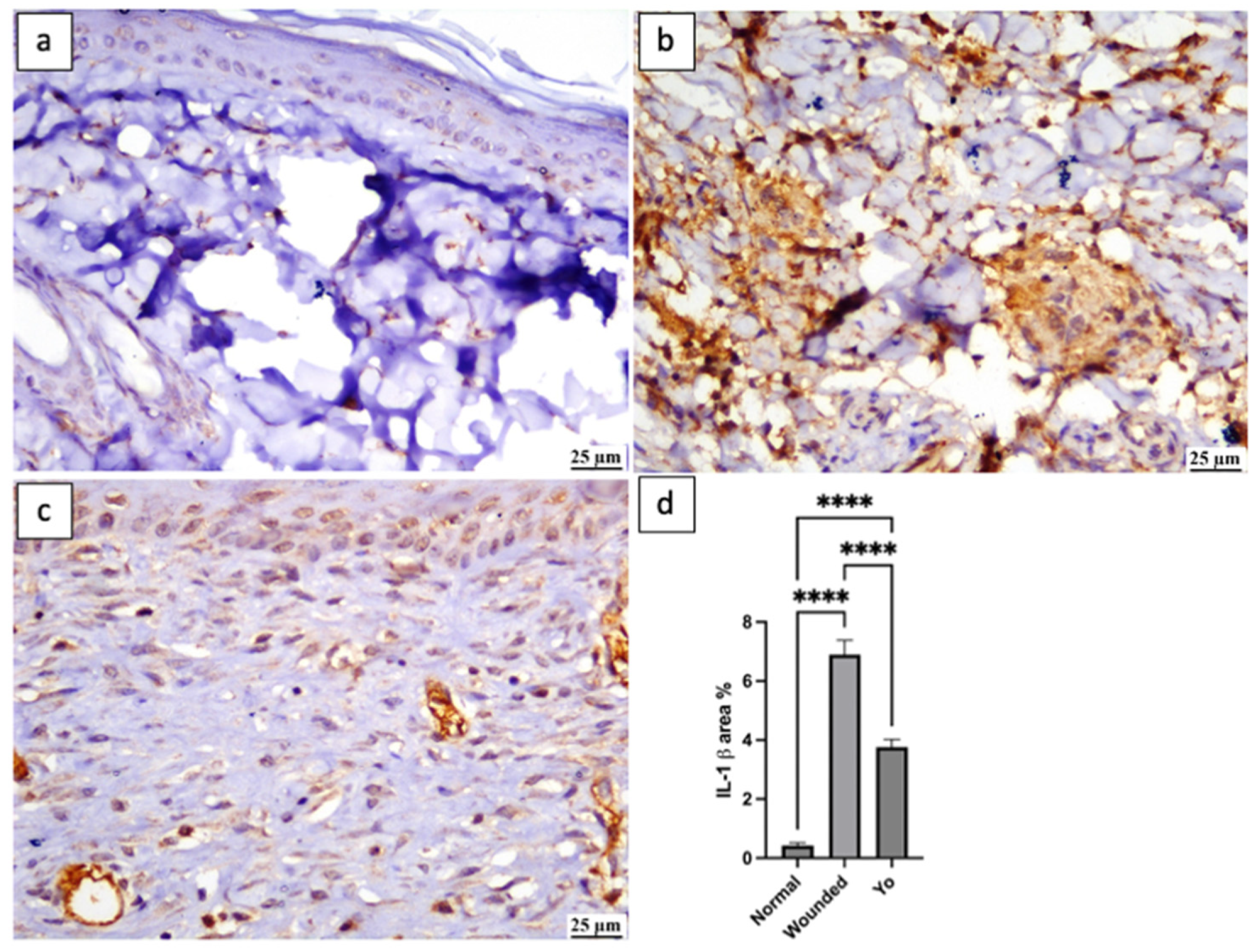
2.8. YO Peel Extract Suppresses Wound-Associated Inflammation
2.9. YO Peel Extract Accelerates Angiogenesis and Curtails Notch-1 Expression
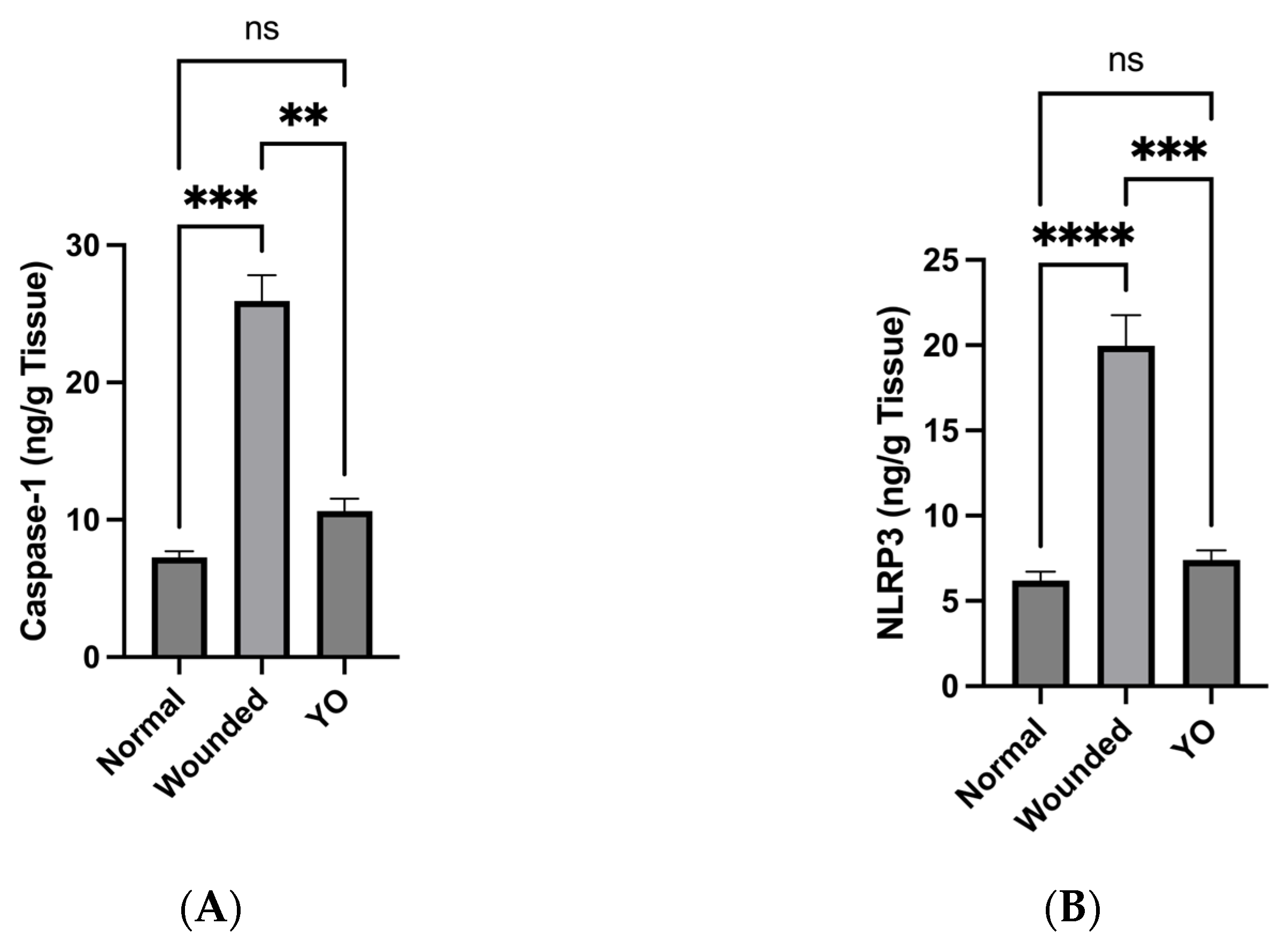
2.10. YO Peel Extract Inhibits NLRP3 Inflammasome and Caspase-1 Signaling
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extraction
3.3. UPLC-QTOF–MS/MS Analysis
3.4. Antimicrobial Activity Evaluation
3.4.1. Microbial Strain and Inoculum Preparation
3.4.2. Sensitivity Test
3.4.3. MIC and MBC Determination by Microtiter Broth Dilution Method
3.4.4. Anti-Biofilm Assay of RO Peel Extract
Effect of RO Peel Extract on Initial Adherence (Biofilm Formation)
Effect of RO Peel Extract on Preformed Biofilm
3.4.5. SP-SDS Method for Pure Yeast Cultures and Composite Samples
3.5. Pharmacological Activity Evaluation
3.5.1. In Vitro Sulforhodamine B (SRB) Cytotoxicity Assay
3.5.2. In Vitro Anti-Inflammatory Screening
3.5.3. Evaluating Excisional Wound Healing Activity In Vivo
Preparation and Characterization of Onion Peel Extract Gel for Topical Application
Animals and an Excisional Wound Model
Histopathological Evaluation
Immunohistochemistry
Enzyme-Linked Immunosorbent Assay (ELISA)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suleria, H.A.R.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions—A global benefit to health. Phyther. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. FAO Statistics Division. 2018. Available online: https://www.fao.org/faostat/en/#data (accessed on 1 May 2022).
- Omar, A.E.; Al-Khalaifah, H.S.; Mohamed, W.A.M.; Gharib, H.S.A.; Osman, A.; Al-Gabri, N.A.; Amer, S.A. Effects of Phenolic-Rich Onion (Allium cepa L.) Extract on the Growth Performance, Behavior, Intestinal Histology, Amino Acid Digestibility, Antioxidant Activity, and the Immune Status of Broiler Chickens. Front. Vet. Sci. 2020, 7, 582612. [Google Scholar] [CrossRef] [PubMed]
- Colina-Coca, C.; González-Peña, D.; de Ancos, B.; Sánchez-Moreno, C. Dietary onion ameliorates antioxidant defence, inflammatory response, and cardiovascular risk biomarkers in hypercholesterolemic Wistar rats. J. Funct. Foods 2017, 36, 300–309. [Google Scholar] [CrossRef]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Gazzerro, P.; Tucci, M.; Di Sanzo, R.; Carabetta, S.; Campone, L.; Russo, M.; Rastrelli, L. Onion peel: Turning a food waste into a resource. Antioxidants 2021, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, A.E.; Gendy, A.M.; Amin, M.M.; Alshareef, W.A.; El Gizawy, H.A. Comparative characterization of carob pulp and seeds extracts: HPLC, antimicrobial, anti-inflammatory, and cytotoxic studies. Egypt. J. Chem. 2022, 65, 279–284. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Maheswary, T.; Nurul, A.A.; Fauzi, M.B. The Insights of Microbes’ Roles in Wound Healing: A Comprehensive Review. Pharmaceutics 2021, 13, 981. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; He, Y.; Lv, Z.; Liang, Z.; Chen, J.; Li, P.; Liu, J.; Yang, H.; Tao, A.; et al. Exploring the Role of Staphylococcus aureus in Inflammatory Diseases. Toxins 2022, 14, 464. [Google Scholar] [CrossRef]
- Short, B.; Bakri, A.; Baz, A.; Williams, C.; Brown, J.; Ramage, G. There Is More to Wounds than Bacteria: Fungal Biofilms in Chronic Wounds. Curr. Clin. Microbiol. Rep. 2023, 10, 9–16. [Google Scholar] [CrossRef]
- Ali, N.B.; El-Shiekh, R.A.; Ashour, R.M.; El-Gayed, S.H.; Abdel-Sattar, E.; Hassan, M. In Vitro and In Vivo Antibiofilm Activity of Red Onion Scales: An Agro-Food Waste. Molecules 2023, 28, 355. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Zhou, F.; Zhang, G.; Feng, X.; Xiong, Y.; Wu, Y. Sustained activation of NLRP3 inflammasome contributes to delayed wound healing in aged mice. Int. Immunopharmacol. 2023, 116, 109828. [Google Scholar] [CrossRef]
- Shao, H.; Li, Y.; Pastar, I.; Xiao, M.; Prokupets, R.; Liu, S.; Yu, K.; Vazquez-Padron, R.I.; Tomic-Canic, M.; Velazquez, O.C.; et al. Notch1 signaling determines the plasticity and function of fibroblasts in diabetic wounds. Life Sci. Alliance 2020, 3, e202000769. [Google Scholar] [CrossRef]
- Puca, V.; Marulli, R.Z.; Grande, R.; Vitale, I.; Niro, A.; Molinaro, G.; Prezioso, S.; Muraro, R.; Di Giovanni, P. Microbial Species Isolated from Infected Wounds and Antimicrobial Resistance Analysis: Data Emerging from a Three-Years Retrospective Study. Antibiotics 2021, 10, 1162. [Google Scholar] [CrossRef]
- Jarić, S.; Kostić, O.; Mataruga, Z.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef]
- Budovsky, A.; Yarmolinsky, L.; Ben-Shabat, S. Effect of medicinal plants on wound healing. Wound Repair Regen. 2015, 23, 171–183. [Google Scholar] [CrossRef]
- Salem, M.A.; Mohamed, O.G.; Mosalam, E.M.; Elberri, A.I.; Abdel-Bar, H.M.; Hassan, M.; Al-Karmalawy, A.A.; Tripathi, A.; Ezzat, S.M.; Abo Mansour, H.E. Investigation of the phytochemical composition, antioxidant, antibacterial, anti-osteoarthritis, and wound healing activities of selected vegetable waste. Sci. Rep. 2023, 13, 13034. [Google Scholar] [CrossRef]
- El-Haddad, A.E.; Saadeldeen, A.M.; El-Emam, S.Z. Anti-angiogenic Activity of Major Phenolics in Tamarind Assessed with Molecular Docking Study on VEGF Kinase Proteins. Pak. J. Biol. Sci. 2019, 22, 502–509. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucoside profile of southern Italian red onion (Allium cepa L.). J. Agric. Food Chem. 2005, 53, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Metrani, R.; Singh, J.; Acharya, P.K.; Jayaprakasha, G.S.; Patil, B. Comparative metabolomics profiling of polyphenols, nutrients and antioxidant activities of two red onion (Allium cepa L.) cultivars. Plants 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Donner, H.; Gao, L.; Mazza, G. Separation and characterization of simple and malonylated anthocyanins in red onions, Allium cepa L. Food Res. Int. 1997, 30, 637–643. [Google Scholar] [CrossRef]
- Thomas, B.T.; Adeleke, A.J.; Raheem-Ademola, R.R.; Kolawole, R.; Musa, O.S. Efficiency of some disinfectants on bacterial wound pathogens. Life Sci. J. 2012, 9, 752–755. [Google Scholar]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef]
- Olawuwo, O.S.; Famuyide, I.M.; McGaw, L.J. Antibacterial and antibiofilm activity of selected medicinal plant leaf extracts against pathogens implicated in poultry diseases. Front. Vet. Sci. 2022, 9, 820304. [Google Scholar] [CrossRef]
- Mohsenipour, Z.; Hassanshahian, M. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur J. Microbiol. 2015, 8, e18971. [Google Scholar] [CrossRef]
- König, A.; Hube, B.; Kasper, L. The Dual Function of the Fungal Toxin Candidalysin during Candida albicans—Macrophage Interaction and Virulence. Toxins 2020, 12, 469. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kwon, Y.-J.; Seo, E.-B.; Kim, S.-K.; Lee, H.; Lee, J.-T.; Chang, P.-S.; Choi, Y.J.; Lee, S.-H.; Ye, S.-K. Anti-inflammatory effects of Allium cepa L. peel extracts via inhibition of JAK-STAT pathway in LPS-stimulated RAW264.7 cells. J. Ethnopharmacol. 2023, 317, 116851. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M.H. Pharmacological control of inflammation in wound healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Holzer-Geissler, J.C.J.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The Impact of Prolonged Inflammation on Wound Healing. Biomedicines 2022, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, J.-S.; Kim, S.-H.; Jeong, S.-H.; Jeong, U.-Y.; Jung, J.-E.; Lee, S.-K.; Lee, S.-H. Antioxidant and Anti-Inflammatory Effects of Ethanol Extract from Whole Onion (Allium cepa L.) with Leaves. Agriculture 2022, 12, 963. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Chen, Y.; Chen, C.; Wang, J.; Lee, Y.M.; Zheng, W.; Shang, R.; Tang, Y.; Zhang, X.; et al. P311 Facilitates the Angiogenesis and Wound Healing Function of MSCs by Increasing VEGF Production. Front. Immunol. 2022, 13, 821932. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Hamilton Outtz, H.; Wu, J.K.; Wang, X.; Kitajewski, J. Notch1 Deficiency Results in Decreased Inflammation during Wound Healing and Regulates Vascular Endothelial Growth Factor Receptor-1 and Inflammatory Cytokine Expression in Macrophages. J. Immunol. 2010, 185, 4363–4373. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, X.; Zhang, H.; Li, S.; Yang, P.; Tan, Q. Relevance of NLRP3 Inflammasome-Related Pathways in the Pathology of Diabetic Wound Healing and Possible Therapeutic Targets. Oxid. Med. Cell. Longev. 2022, 2022, 9687925. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Zhu, Q.-Q.; Yang, X.-Y.; Xu, H.-H.; Sun, B.; Wang, X.-J.; Sheng, J. Wound healing can be improved by (—)-epigallocatechin gallate through targeting Notch in streptozotocin-induced diabetic mice. FASEB J. 2019, 33, 953–964. [Google Scholar] [CrossRef]
- Hussein, M.E.; Mohamed, O.G.; El-Fishawy, A.M.; El-Askary, H.I.; El-Senousy, A.S.; El-Beih, A.A.; Nossier, E.S.; Naglah, A.M.; Almehizia, A.A.; Tripathi, A.; et al. Identification of antibacterial metabolites from endophytic fungus Aspergillus fumigatus, isolated from Albizia lucidior leaves (Fabaceae), utilizing metabolomic and molecular docking techniques. Molecules 2022, 27, 1117. [Google Scholar] [CrossRef]
- El-Mancy, S.S.; El-Haddad, A.E.; Alshareef, W.A.; Saadeldeen, A.M.; El-Emam, S.Z.; Elnahas, O.S. Enhancement of Antimicrobial and Antiproliferative Activities of Standardized Frankincense Extract Using Optimized Self-Nanoemulsifying Delivery System. Sci. Pharm. 2021, 89, 36. [Google Scholar] [CrossRef]
- Ozturk, S.; Ercisli, S. Chemical composition and in vitro antibacterial activity of Seseli libanotis. World J. Microbiol. Biotechnol. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Vollaro, A.; Esposito, A.; Esposito, E.P.; Zarrilli, R.; Guaragna, A.; De Gregorio, E. PYED-1 inhibits biofilm formation and disrupts the preformed biofilm of Staphylococcus aureus. Antibiotics 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Nazeam, J.A.; Ragab, G.M.; El-Gazar, A.A.; El-Mancy, S.S.; Jamil, L.; Fayez, S.M. Topical Nano Clove/Thyme Gel against Genetically Identified Clinical Skin Isolates: In Vivo Targeting Behavioral Alteration and IGF-1/pFOXO-1/PPAR γ Cues. Molecules 2021, 26, 5608. [Google Scholar] [CrossRef]
- Thomas, P.; Sekhar, A.C.; Upreti, R.; Mujawar, M.M.; Pasha, S.S. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnol. Rep. 2015, 8, 45–55. [Google Scholar] [CrossRef]
- Alruwad, M.I.; Sabry, M.M.; Gendy, A.M.; El-Dine, R.S.; El Hefnawy, H.M. In Vitro Cytotoxic Potential of Selected Jordanian Flora and Their Associated Phytochemical Analysis. Plants 2023, 12, 1626. [Google Scholar] [CrossRef]
- Yoo, M.-S.; Shin, J.-S.; Choi, H.-E.; Cho, Y.-W.; Bang, M.-H.; Baek, N.-I.; Lee, K.-T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-$κ$B and p38 mitogen-activated protein kinase in RAW264. 7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef]
- Oliveira, T.; Figueiredo, C.A.; Brito, C.; Stavroullakis, A.; Prakki, A.; Da Silva Velozo, E.; Nogueira-Filho, G. Effect of Allium cepa L. on lipopolysaccharide-stimulated osteoclast precursor cell viability, count, and morphology using 4′, 6-diamidino-2-phenylindole-staining. Int. J. Cell Biol. 2014, 2014, 535789. [Google Scholar] [CrossRef]
- Teaima, M.H.; Eltabeeb, M.A.; El-Nabarawi, M.A.; Abdellatif, M.M. Utilization of propranolol hydrochloride mucoadhesive invasomes as a locally acting contraceptive: In-vitro, ex-vivo, and in-vivo evaluation. Drug Deliv. 2022, 29, 2549–2560. [Google Scholar] [CrossRef]
- Refai, H.; El-Gazar, A.A.; Ragab, G.M.; Hassan, D.H.; Ahmed, O.S.; Hussein, R.A.; Shabana, S.; Waffo-Téguo, P.; Valls, J.; Al-Mokaddem, A.K.; et al. Enhanced Wound Healing Potential of Spirulina platensis Nanophytosomes: Metabolomic Profiling, Molecular Networking, and Modulation of HMGB-1 in an Excisional Wound Rat Model. Mar. Drugs 2023, 21, 149. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Bakr, R.O.; Amer, R.I.; Attia, D.; Abdelhafez, M.M.; Al-Mokaddem, A.K.; El Gendy, A.N.; El-Fishawy, A.M.; Fayed, M.A.A.; Gad, S.S. In-vivo wound healing activity of a novel composite sponge loaded with mucilage and lipoidal matter of Hibiscus species. Biomed. Pharmacother. 2021, 135, 111225. [Google Scholar] [CrossRef] [PubMed]
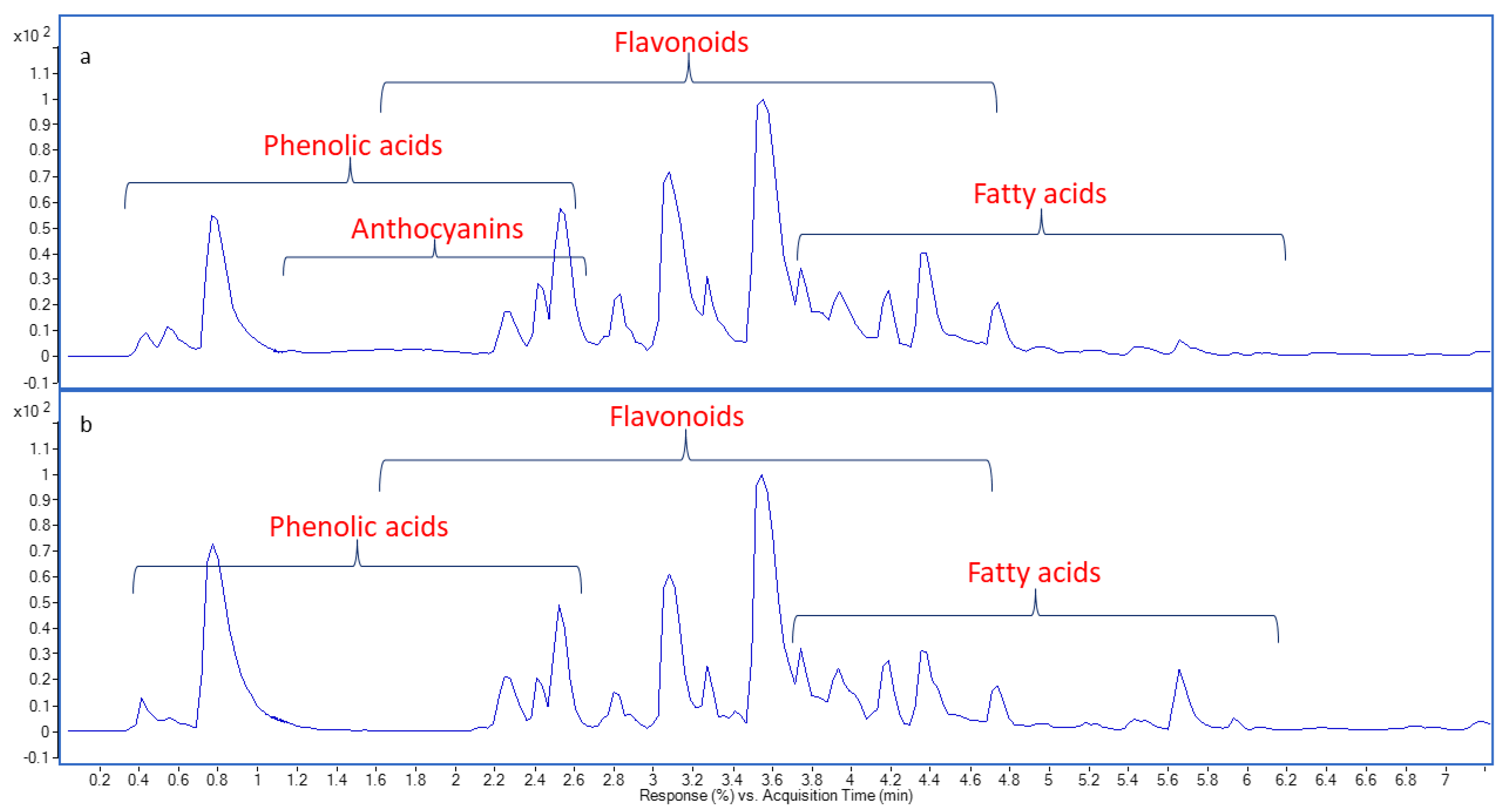



| No. | Rt | Proposed Compounds | Formula | [M−H] m/z | Diff. (ppm) | MS2 (Characteristic Fragments) | Plant |
|---|---|---|---|---|---|---|---|
| Organic acids | |||||||
| 1 | 0.25 | Dihydroxybutanoic acid | C4H8O4 | 119.0361 | −9.03 | 101, 91 | RO |
| 2 | 0.53 | Citric acid/Isocitric acid | C6H8O7 | 191.0198 | −0.55 | 155, 129, 111, 67 | YO |
| 3 | 1.35 | Quinic acid | C7H12O6 | 191.0558 | 1.89 | 173, 127, 93 | YO |
| Sugar derivatives | |||||||
| 4 | 0.4 | Hexitol | C6H14O6 | 181.0719 | −0.94 | 81, 71, 59 | YO |
| 5 | 0.4 | Tetrahexoside | C24H42O21 | 665.2126 | 2.97 | 383, 179, 101, 89 | RO |
| 6 | 0.43 | Hexuronic acid | C6H10O7 | 193.0355 | −0.41 | 113, 85, 71, 59 | RO, YO |
| 7 | 0.47 | Dihexoside | C12H22O11 | 341.1089 | −0.51 | 101, 89, 71, 59 | RO |
| Phenolic acid derivatives | |||||||
| 8 | 0.6 | Protocatechuic acid-O-hexoside | C13H16O9 | 315.0723 | −0.54 | 225, 195, 153, 109 | RO, YO |
| 9 | 0.9 | Protocatechuic acid | C7H6O4 | 153.0194 | −0.47 | 109, 81, 53 | RO, YO |
| 10 | 1.12 | Hydroxybenzoic acid | C7H6O3 | 137.0242 | 4.64 | 119, 93 | YO |
| 11 | 1.19 | Gallic acid | C7H6O5 | 169.0134 | 4.99 | 151, 125, 81 | RO |
| 12 | 1.28 | Catechol | C6H6O2 | 109.0297 | −1.84 | 91, 81, 53 | RO, YO |
| 13 | 1.28 | Methoxybenzoic acid | C8H8O3 | 151.0396 | 5.16 | 136, 108, 91 | YO |
| 14 | 1.31 | Chlorogenic acid | C16H18O9 | 353.0873 | 1.02 | 191, 173 | YO |
| 15 | 1.51 | Vanillic acid | C8H8O4 | 167.0347 | 2.03 | 152, 137, 108, 91 | RO, YO |
| 16 | 1.52 | Methyl methoxybenzoate | C9H10O3 | 165.0549 | 4.76 | 150, 121 | YO |
| 17 | 1.57 | Dimethoxybenzoic acid | C9H10O4 | 181.0504 | 1.62 | 166, 153, 122 | YO |
| 18 | 1.6 | Protocatechuic acid, methyl ether, O-hexoside | C14H18O9 | 329.088 | −0.75 | 167, 149, 137 | RO, YO |
| 19 | 1.97 | Protocatechuic acid, O-[protocatechuyl-O-hexoside] | C20H20O12 | 451.0875 | 1.54 | 153, 109 | YO |
| 20 | 2.02 | Methyl benzoate | C8H8O2 | 135.0444 | 5.49 | 122, 107, 92 | YO |
| 21 | 2.44 | Galloyl hexoside | C13H16O10 | 331.0674 | 0.16 | 169, 151, 125 | YO |
| 22 | 2.47 | Methyl gallate | C8H8O5 | 183.03 | −0.24 | 165, 152, 139 | RO |
| Flavonoids | |||||||
| 23 | 1.5 | Kaempferol-O-dihexoside | C27H30O16 | 609.1452 | 1.47 | 285, 259 | RO, YO |
| 24 | 1.86 | Taxifolin-O-hexoside | C21H22O12 | 465.1048 | 0.18 | 303, 285, 177, 166, 151 | RO, YO |
| 25 | 1.9 | Kaempferol-O-hexoside | C21H20O11 | 447.0937 | 0.07 | 285, 257 | RO, YO |
| 26 | 2.11 | O-Galloylmyricitrin | C28H24O16 | 615.0994 | −0.16 | 299, 287, 163 | RO, YO |
| 27 | 2.44 | Dihydroxyquercetin | C15H10O9 | 333.0248 | 1.54 | 315, 287, 259, 163, 151 | RO |
| 28 | 2.51 | Quercetin-O-dihexoside | C27H30O17 | 625.1421 | −1.39 | 463, 301, 179, 151 | RO, YO |
| 29 | 2.63 | Isorhamnetin-O-dihexoside | C28H32O17 | 639.1568 | 0.14 | 477, 315, 285, 151 | RO, YO |
| 30 | 2.71 | Quercetin-O-hexoside-O-acetylhexoside | C29H32O18 | 667.15 | 0.85 | 463, 301, 245 | RO |
| 31 | 2.74 | Quercetin, O-(O-malonyl-hexoside), O-hexoside | C30H32O20 | 711.1403 | −0.73 | 463, 301 | RO |
| 32 | 2.74 | Myricetin methyl ether | C16H12O8 | 331.0463 | −0.76 | 207, 151, 123 | YO |
| 33 | 2.83 | Isorhamnetin-O-galloylhexoside | C29H26O16 | 629.1145 | 1.18 | 467, 313, 285, 163 | RO, YO |
| 34 | 2.89 | Taxifolin/Dihydroquercetin | C15H12O7 | 303.0515 | −0.85 | 285, 177, 151, 125 | RO, YO |
| 35 | 2.94 | Protocatecoyl quercetin dimer | C22H14O11 | 453.0469 | −3.39 | 409, 301, 283, 229, 163 | RO, YO |
| 36 | 3.0 | Taxifolin methyl ether | C16H14O8 | 333.0617 | 0.07 | 285, 243, 165, 151, 137 | RO |
| 37 | 3.07 | Quercetin-O-hexoside | C21H20O12 | 463.0896 | −2.49 | 301, 179, 151 | RO, YO |
| 38 | 3.21 | Myricetin | C15H10O8 | 317.0292 | 3.34 | 271, 163, 152, 125 | RO, YO |
| 39 | 3.22 | Isorhamnetin-O-hexoside | C22H22O12 | 477.1035 | 0.97 | 314, 299, 271, 179, 151 | RO |
| 40 | 3.23 | Quercetin | C15H10O7 | 301.0350 | 0.19 | 273, 245, 179, 151, 121, 107 | RO, YO |
| 41 | 3.32 | Quercetin-O-acetylhexoside | C23H22O13 | 505.0986 | 0.3 | 343, 301, 179, 151, 121 | RO |
| 42 | 3.32 | Tetrahydroxyflavan, methyl ether, O-hexoside | C22H26O10 | 449.1448 | 1.22 | 287, 243, 228 | RO |
| 43 | 3.33 | Trihydroxyflavan-O-hexoside | C21H24O9 | 419.1349 | −0.37 | 257, 213, 195 | RO |
| 44 | 3.33 | Quercetin-O-hexoside (isomer 2) | C21H20O12 | 463.0896 | −2.49 | 301, 179, 151 | RO |
| 45 | 3.49 | O-Galloylquercitrin | C21H20O12 | 463.0883 | 0.34 | 447, 301, 273, 179, 151 | RO |
| 46 | 3.54 | Morin | C15H10O7 | 301.0345 | 3.01 | 273, 245, 179, 151, 121, 107 | RO, YO |
| 47 | 3.61 | Sinodiflavonoid B | C30H20O14 | 603.0778 | 0.32 | 301, 273, 179, 151 | RO, YO |
| 48 | 3.91 | Kaempferol | C15H10O6 | 285.0402 | 1.07 | 257, 229, 211, 151 | RO, YO |
| 49 | 3.94 | Isorhamnetin | C16H12O7 | 315.0508 | 1.22 | 300, 271, 151, 107 | RO, YO |
| 50 | 4.06 | Trihydroxyflavan | C15H14O4 | 257.0819 | 0.14 | 229, 213, 195, 151, 107 | RO, YO |
| 51 | 4.32 | Allium quercetin dimer (isomer 1) | C30H18O14 | 601.0625 | 1.08 | 299, 271, 243, 151 | RO, YO |
| 52 | 4.58 | Allium quercetin dimer (isomer 2) | C30H18O14 | 601.0625 | 1.39 | 299, 271, 243, 151 | RO, YO |
| 53 | 4.74 | Allium quercetin dimer (isomer 3) | C30H18O14 | 601.0625 | 4.94 | 299, 271, 179, 121 | RO, YO |
| Anthocyanins | |||||||
| 54 | 1.22 | Cyanidin-O-malonylhexoside | C24H22O14 | 533.0903 | 5.69 | 465, 285, 241, 107 | RO |
| 55 | 1.4 | Peonidin-O-hexoside | C20H30O12 | 461.1658 | 2.08 | 299, 269, 251 | RO, YO |
| 56 | 2.42 | Delphinidin-O-hexoside acetate | C23H24O13 | 507.1137 | 1.45 | 489, 285, 241 | RO |
| 57 | 2.65 | Cyanidin-O-hexoside acetate | C23H22O12 | 489.1038 | 0.21 | 284, 257, 241 | RO |
| 58 | 2.74 | Petunidin-O-hexoside | C22H24O12 | 479.1176 | 4.38 | 317, 299, 284 | RO |
| Fatty acids | |||||||
| 59 | 3.77 | Trihydroxy-Octadecenoic acid | C18H34O5 | 329.2331 | 0.34 | 293, 257, 229, 211, 171 | RO, YO |
| 60 | 4.82 | Dihydroxy-Octadecanoic acid | C18H36O4 | 315.2525 | 5.23 | 297, 267, 235, 171 | YO |
| 61 | 5.48 | Hydroxy-Pentadecanoic acid | C15H30O3 | 257.213 | −2.49 | 259, 217, 179 | YO |
| 62 | 5.56 | Dihydroxy-Octadecatrienoic acid | C18H30O4 | 309.2078 | −1.9 | 269, 241, 169, 155 | YO |
| 63 | 5.73 | Hydroxy-Palmitic acid | C16H32O3 | 271.2275 | 1.48 | 253, 225 | YO |
| 64 | 6.11 | Linoleic acid | C18H32O2 | 279.2324 | 2.47 | 259, 239, 219 | RO, YO |
| 65 | 6.24 | Palmitic acid | C16H32O2 | 255.2327 | 0.84 | 235, 215, 171 | RO, YO |
| 66 | 6.34 | Oleic acid | C18H34O2 | 281.2494 | −3.36 | 261, 241, 129, 66 | RO, YO |
| 67 | 7.86 | Behenic acid | C22H44O2 | 339.3263 | 1.62 | 319, 279 | RO, YO |
| Microbial Isolates | RO Peel Extract (mg/mL) | YO Peel Extract (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Inhibition (mm) | MIC | MBC | MBC/MIC Ratio | Inhibition (mm) | MIC | MBC | MBC/MIC Ratio | |
| P. auruginosa | 12 | 31.25 | 62.5 | 2 | -- | - | - | - |
| S. aureus | 20 | 1.9 | 1.9 | 1 | 15 | 15.6 | 15.6 | 1 |
| S. epidermidis | 13 | 7.8 | 31.25 | 4 | 8 | - | - | - |
| MRSA | 26 | 1.9 | 1.9 | 1 | 16 | 31.25 | 31.25 | 1 |
| C. albicans | 12 | 7.8 | 15.6 | 2 | 9 | 15.6 | 15.6 | 1 |
| Microbial Strains | Inhibition % of Biofilm Formation | Destruction % of Biofilm Masses | ||||
|---|---|---|---|---|---|---|
| MIC | 2MIC | 4MIC | MIC | 2MIC | 4MIC | |
| S. aureus | 25% | 70% | 85% | 6% | 12.5% | 15% |
| MRSA | 16% | 66.7% | 91.6% | 10% | 25% | 33.3% |
| S. epidermidis | 10% | 50% | 80% | 4% | 20% | 24% |
| C. albicans | 37.5% | 75% | 85% | 5% | 17.5% | 27.5 |
| Extract | Concentration (μg/mL) | Cell Viability (%) | NO Inhibition (%) | |
|---|---|---|---|---|
| MCF-7 | HT-29 | |||
| RO | 10 | 79.89 ± 1.6 | 102.13 ± 0.5 | 27.4 ± 0.6 |
| 100 | 74.57 ± 1.5 | 90.35 ± 1.5 | 75.3 ± 3 | |
| YO | 10 | 100.45 ± 1.3 | 102.03 ±0.8 | 25.7 ± 1.5 |
| 100 | 79.3 ± 1.7 | 91.59 ± 1.0 | 62.7 ± 1.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mounir, R.; Alshareef, W.A.; El Gebaly, E.A.; El-Haddad, A.E.; Ahmed, A.M.S.; Mohamed, O.G.; Enan, E.T.; Mosallam, S.; Tripathi, A.; Selim, H.M.R.M.; et al. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals 2023, 16, 1379. https://doi.org/10.3390/ph16101379
Mounir R, Alshareef WA, El Gebaly EA, El-Haddad AE, Ahmed AMS, Mohamed OG, Enan ET, Mosallam S, Tripathi A, Selim HMRM, et al. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals. 2023; 16(10):1379. https://doi.org/10.3390/ph16101379
Chicago/Turabian StyleMounir, Rafik, Walaa A. Alshareef, Eman A. El Gebaly, Alaadin E. El-Haddad, Abdallah M. Said Ahmed, Osama G. Mohamed, Eman T. Enan, Shaimaa Mosallam, Ashootosh Tripathi, Heba Mohammed Refat M. Selim, and et al. 2023. "Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling" Pharmaceuticals 16, no. 10: 1379. https://doi.org/10.3390/ph16101379
APA StyleMounir, R., Alshareef, W. A., El Gebaly, E. A., El-Haddad, A. E., Ahmed, A. M. S., Mohamed, O. G., Enan, E. T., Mosallam, S., Tripathi, A., Selim, H. M. R. M., Bukhari, S. I., Alfaraj, R., Ragab, G. M., El-Gazar, A. A., & El-Emam, S. Z. (2023). Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals, 16(10), 1379. https://doi.org/10.3390/ph16101379










