Formulation and Characterization of Curcumin Niosomes: Antioxidant and Cytotoxicity Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation and Optimization of Curcumin Niosomes (Curcusomes) by Box–Behnken Design (BBD)
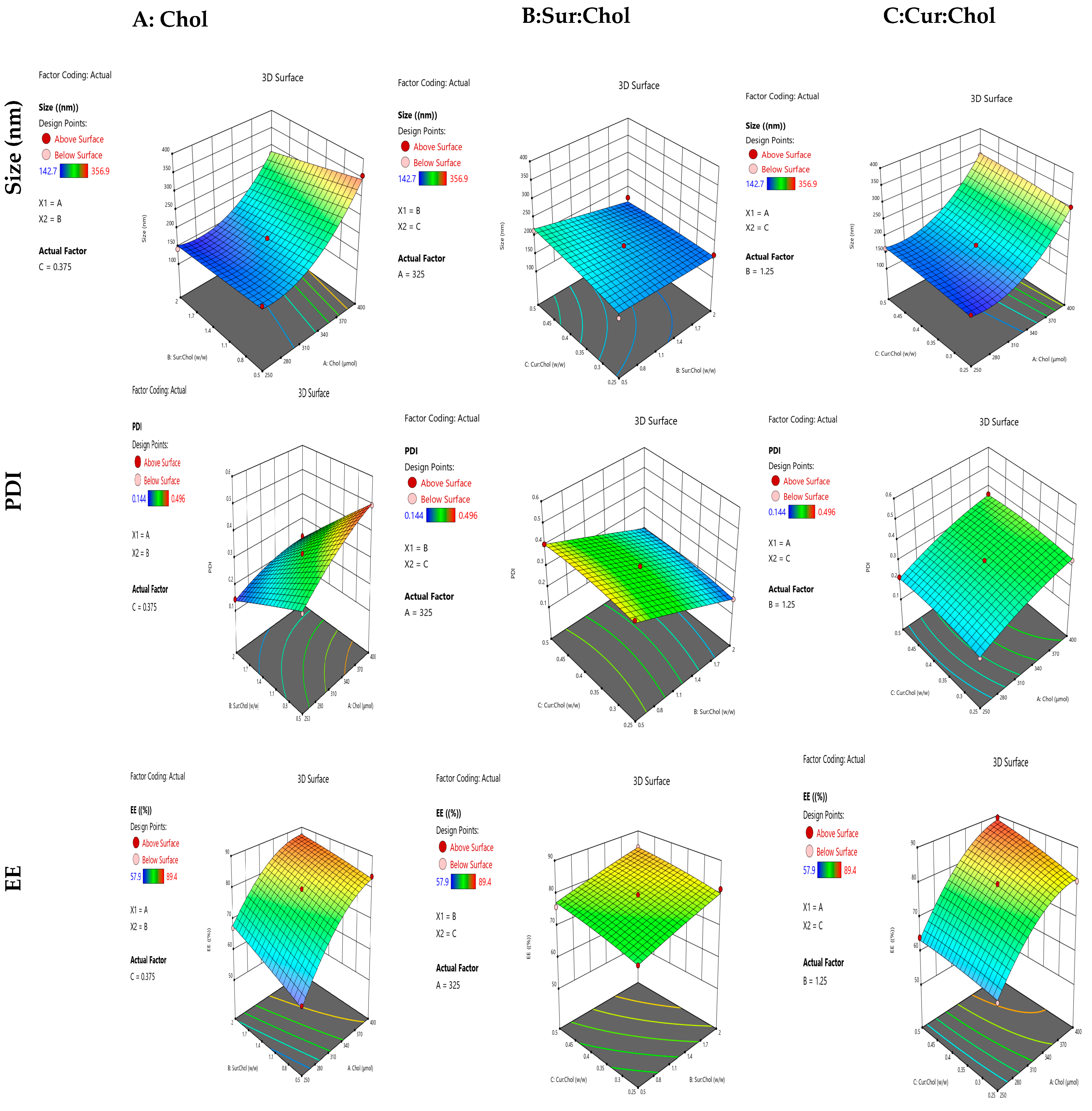
2.1.1. Influence of Independent Variables on Size
2.1.2. Influence of Independent Variables on PDI
2.1.3. Influence of Independent Variables on EE
2.2. Characterization of Optimized Formulation
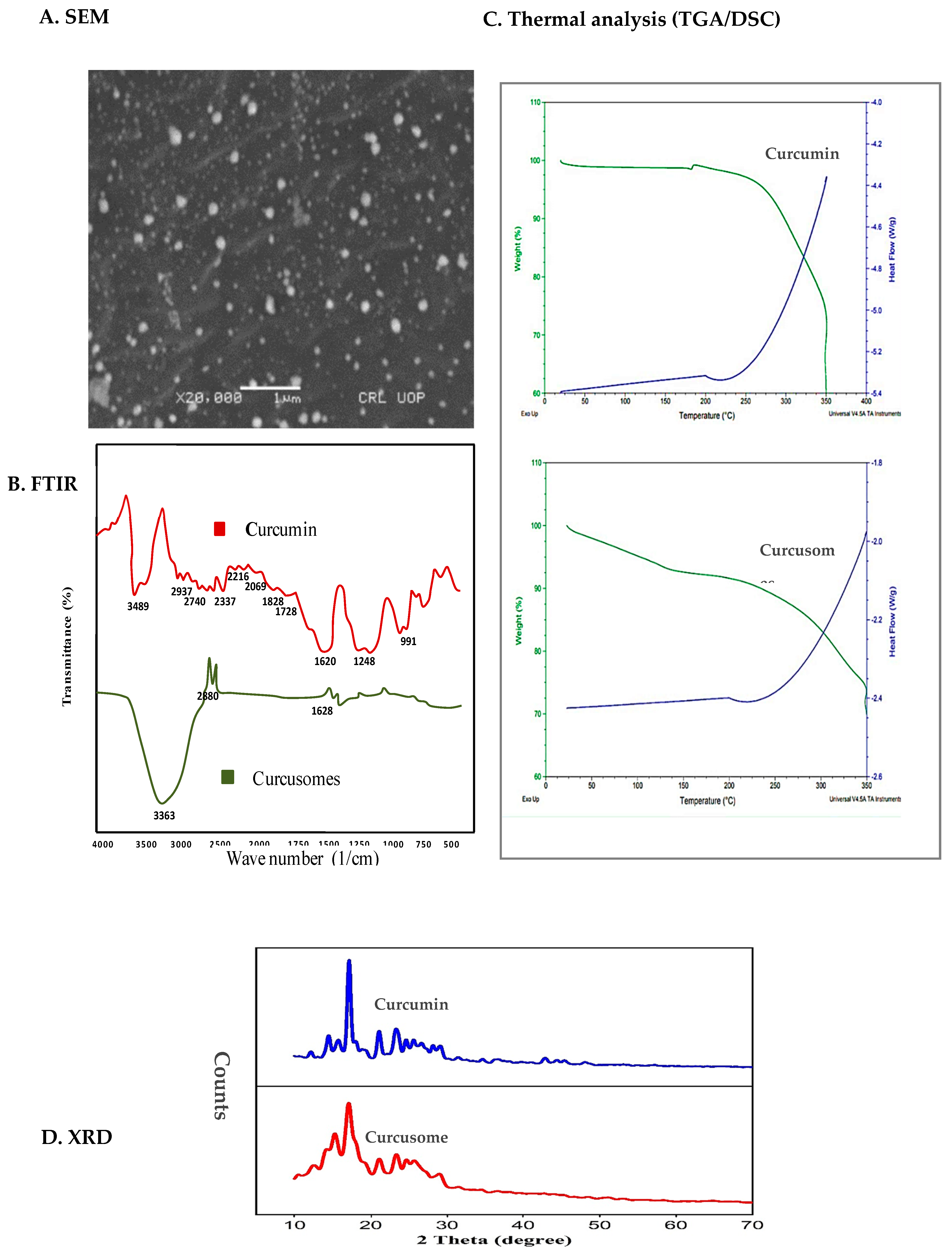
2.2.1. Size Distribution, Surface Charge, and Morphology
2.2.2. FTIR
2.2.3. DSC/TGA
2.2.4. X-ray Diffraction (XRD)
2.2.5. In Vitro Drug Release and Kinetics
2.2.6. Antioxidant Potential
2.2.7. Cytotoxic Potential
2.2.8. Stability Studies
2.3. Drug–Excipients Compatibility Study
3. Experimental
3.1. Materials
3.2. Methods
3.2.1. Preparation of Curcusomes
3.2.2. Optimization of Curcusomal Preparations with Box–Behnken Design
3.2.3. Characterization of Optimal Formulation
Size Distribution Analysis
Entrapment Efficiency
X-ray Diffraction (XRD)
Chemical Compatibility of Formulation Components (FTIR)
Thermal Analysis (DSC/TGA)
Morphology
Surface Charge Measurement
Storage Stability
In Vitro Drug Release and Kinetics
Antioxidant Potential
Cytotoxicity Studies
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afshari, A.R.; Jalili-Nik, M.; Abbasinezhad-Moud, F.; Javid, H.; Karimi, M.; Mollazadeh, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. Anti-tumor effects of curcuminoids in glioblastoma multiforme: An updated literature review. Curr. Med. Chem. 2021, 28, 8116–8138. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Cicero, A.F.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Gaire, B.P. Neuroprotective effects of curcumin in cerebral ischemia: Cellular and molecular mechanisms. ACS Chem. Neurosci. 2021, 12, 2562–2572. [Google Scholar] [CrossRef]
- Han, X.; Xu, B.; Beevers, C.S.; Odaka, Y.; Chen, L.; Liu, L.; Luo, Y.; Zhou, H.; Chen, W.; Shen, T. Curcumin inhibits protein phosphatases 2A and 5, leading to activation of mitogen-activated protein kinases and death in tumor cells. Carcinogenesis 2012, 33, 868–875. [Google Scholar] [CrossRef]
- Sundram, V.; Chauhan, S.C.; Ebeling, M.; Jaggi, M. Curcumin attenuates β-catenin signaling in prostate cancer cells through activation of protein kinase D1. PLoS ONE 2012, 7, e35368. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.K.; Mishra, J.; Mishra, A.K. Introducing Tween-curcumin niosomes: Preparation, characterization and microenvironment study. Soft Matter 2020, 16, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Ibrahim, M.M.; Elsewedy, H.S. Curcumin niosomes prepared from proniosomal gels: In vitro skin permeability, kinetic and in vivo studies. Polymers 2021, 13, 791. [Google Scholar] [CrossRef]
- Zadeh, E.S.; Ghanbari, N.; Salehi, Z.; Derakhti, S.; Amoabediny, G.; Akbari, M.; Tokmedash, M.A. Smart pH-responsive magnetic graphene quantum dots nanocarriers for anticancer drug delivery of curcumin. Mater. Chem. Phys. 2023, 297, 127336. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Varaprasad, K.; Akbari-Fakhrabadi, A.; Hameed, A.S.H.; Sadiku, R. Biomolecule chitosan, curcumin and ZnO-based antibacterial nanomaterial, via a one-pot process. Carbohydr. Polym. 2020, 249, 116825. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Chen, W.-R.; Tsosie, J.K.; Xie, X.; Li, P.; Wan, J.-B.; He, C.-W.; Chen, M.-W. Niosome encapsulation of curcumin: Characterization and cytotoxic effect on ovarian cancer cells. J. Nanomater. 2016, 2016, 6365295. [Google Scholar] [CrossRef]
- Azeem, A.; Anwer, M.K.; Talegaonkar, S. Niosomes in sustained and targeted drug delivery: Some recent advances. J. Drug Target. 2009, 17, 671–689. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Mashal, M.; Zárate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Navarrete, G.M.; Avilés-Trigueros, M. A novel cationic niosome formulation for gene delivery to the retina. J. Control. Release 2014, 174, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Hanieh, P.N.; Imbriano, A.; Passeri, D.; Del Favero, E.; Rossi, M.; Marianecci, C.; De Panfilis, S.; Carafa, M. Different instrumental approaches to understand the chitosan coated niosomes/mucin interaction. J. Drug Deliv. Sci. Technol. 2020, 55, 101339. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Walter, J.-G.; Stahl, F.; Scheper, T. Niosomes as nanoparticular drug carriers: Fundamentals and recent applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef]
- Tavano, L.; Alfano, P.; Muzzalupo, R.; de Cindio, B. Niosomes vs microemulsions: New carriers for topical delivery of Capsaicin. Colloids Surf. B Biointerfaces 2011, 87, 333–339. [Google Scholar] [CrossRef]
- Mavaddati, M.A.; Moztarzadeh, F.; Baghbani, F. Effect of formulation and processing variables on dexamethasone entrapment and release of niosomes. J. Clust. Sci. 2015, 26, 2065–2078. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Demirbolat, G.M.; Aktas, E.; Coskun, G.P.; Erdogan, O.; Cevik, O. New approach to formulate methotrexate-loaded niosomes: In vitro characterization and cellular effectiveness. J. Pharm. Innov. 2021, 17, 622–637. [Google Scholar] [CrossRef]
- Perera, K.D.; Weragoda, G.K.; Haputhanthri, R.; Rodrigo, S.K. Study of concentration dependent curcumin interaction with serum biomolecules using ATR-FTIR spectroscopy combined with Principal Component Analysis (PCA) and Partial Least Square Regression (PLS-R). Vib. Spectrosc. 2021, 116, 103288. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Bhatt, H.; Shah, A.; Komanduri, N.; Vijayasarathy, D.; Ghosh, B.; Biswas, S. Formulation optimization, characterization, and evaluation of in vitro cytotoxic potential of curcumin loaded solid lipid nanoparticles for improved anticancer activity. Chem. Phys. Lipids 2017, 208, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, Z.S.; Yuksel, N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J. Pharm. Sci. 2010, 99, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Rawtani, D.; Barot, T. Formulation and optimization of long acting dual niosomes using box-Behnken experimental design method for combinative delivery of ethionamide and D-cycloserine in tuberculosis treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 131–142. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, L.F.; Huitson, A.; Deering, R.B.; Brodie, N.H. Efficacy and Tolerability of Sustained-Release Clomipramine (Anafranil SR®) in the Treatment of Phobias: A Comparison with the Conventional Formulation of Clomipramine (Anafranil®). J. Int. Med. Res. 1985, 13, 203–208. [Google Scholar] [CrossRef]
- Agarwal, P.S.; Sahi, A.K.; Vajanthri, K.Y.; Pallawi Singh, K.N.; Mahto, S.K. Fabrication and cytocompatibility evaluation of psyllium husk (Isabgol)/gelatin composite scaffolds. Appl. Biochem. Biotechnol. 2019, 188, 750–768. [Google Scholar]
- Panigrahi, R.; Chowdary, K.A.; Mishra, G.; Bhowmik, M.; Behera, S. Formulation of fast dissolving tablets of Lisinopril using combination of synthetic superdisintegrants. Asian J. Pharm. Technol. 2012, 2, 94–98. [Google Scholar]
- Nadzir, M.M.; Fen, T.W.; Mohamed, A.R.; Hisham, S.F. Size and stability of curcumin niosomes from combinations of Tween 80 and Span 80. Sains Malays. 2017, 46, 2455–2460. [Google Scholar] [CrossRef]
- García-Manrique, P.; Machado, N.D.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Effect of drug molecular weight on niosomes size and encapsulation efficiency. Colloids Surf. B Biointerfaces 2020, 186, 110711. [Google Scholar] [CrossRef] [PubMed]
- Ruckmani, K.; Sankar, V. Formulation and optimization of zidovudine niosomes. Aaps Pharmscitech 2010, 11, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, A.; Anil Kumar, V.; Sadasivan Pillai, K. Formulation and in vivo evaluation of niosome-encapsulated daunorubicin hydrochloride. Drug Dev. Ind. Pharm. 2002, 28, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin loaded monolaurate sugar esters-based niosomes: Sustained release and mutual antioxidant—Hepatoprotective interplay. Pharmaceutics 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Murthy, P.; Sahu, J.; Sahoo, P.; Amir, F. Vesicles of non-ionic surfactants (niosomes) and drug delivery potential. Int. J. Pharm. Sci. Nanotechnol. (IJPSN) 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Chudasama, A.; Patel, V.; Nivsarkar, M.; Vasu, K.; Shishoo, C. A novel lipid-based oral drug delivery system of nevirapine. Int. J. PharmTech Res. 2011, 3, 1159–1168. [Google Scholar]
- Bragagni, M.; Mennini, N.; Ghelardini, C.; Mura, P. Development and characterization of niosomal formulations of doxorubicin aimed at brain targeting. J. Pharm. Pharm. Sci. 2012, 15, 184–196. [Google Scholar] [CrossRef]
- Obeid, M.A.; Khadra, I.; Albaloushi, A.; Mullin, M.; Alyamani, H.; Ferro, V.A. Microfluidic manufacturing of different niosomes nanoparticles for curcumin encapsulation: Physical characteristics, encapsulation efficacy, and drug release. Beilstein J. Nanotechnol. 2019, 10, 1826–1832. [Google Scholar] [CrossRef]
- Honarvari, B.; Karimifard, S.; Akhtari, N.; Mehrarya, M.; Moghaddam, Z.S.; Ansari, M.J.; Jalil, A.T.; Matencio, A.; Trotta, F.; Yeganeh, F.E. Folate-targeted curcumin-loaded niosomes for site-specific delivery in breast cancer treatment: In silico and In vitro study. Molecules 2022, 27, 4634. [Google Scholar] [CrossRef]
- Mazzotta, E.; Orlando, C.; Muzzalupo, R. New nanomaterials with intrinsic antioxidant activity by surface functionalization of niosomes with natural phenolic acids. Pharmaceutics 2021, 13, 766. [Google Scholar] [CrossRef]
- Mousazadeh, N.; Gharbavi, M.; Rashidzadeh, H.; Nosrati, H.; Danafar, H.; Johari, B. Anticancer evaluation of methotrexate and curcumin-coencapsulated niosomes against colorectal cancer cell lines. Nanomedicine 2022, 17, 201–217. [Google Scholar] [CrossRef] [PubMed]

| Std | Run | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|---|
| Chol | Sur: Chol | Cur: Chol | Average Size | PDI | EE | ||
| (μmol) | (w/w) | (w/w) | (nm) | (%) | |||
| 2 | 1 | 1 | −1 | 0 | 356.9 ± 3.19 | 0.496 ± 0.006 | 83.6 ± 4.7 |
| 12 | 2 | 0 | 1 | 1 | 168.4 ± 1.56 | 0.150 ± 0.002 | 85.4 ± 3.8 |
| 4 | 3 | 1 | 1 | 0 | 276.3 ± 1.32 | 0.228 ± 0.007 | 86.9 ± 6.5 |
| 8 | 4 | 1 | 0 | 1 | 318.5 ± 4.25 | 0.362 ± 0.003 | 89.4 ± 2.4 |
| 15 | 5 | 0 | 0 | 0 | 180.9 ± 1.36 | 0.307 ± 0.006 | 79.7 ± 3.0 |
| 14 | 6 | 0 | 0 | 0 | 181.6 ± 1.02 | 0.318 ± 0.005 | 78.9 ± 2.9 |
| 10 | 7 | 0 | 1 | −1 | 163.7 ± 0.95 | 0.174 ± 0.002 | 81.4 ± 4.1 |
| 11 | 8 | 0 | −1 | 1 | 215.6 ± 3.11 | 0.407 ± 0.009 | 75.9 ± 1.8 |
| 13 | 9 | 0 | 0 | 0 | 180.5 ± 1.32 | 0.311 ± 0.004 | 79.2 ± 4.3 |
| 5 | 10 | −1 | 0 | −1 | 153.9 ± 0.74 | 0.192 ± 0.006 | 60.7 ± 5.2 |
| 3 | 11 | −1 | 1 | 0 | 142.7 ± 0.92 | 0.144 ± 0.006 | 67.2 ± 2.6 |
| 9 | 12 | 0 | −1 | −1 | 163.8 ± 1.15 | 0.413 ± 0.008 | 72.1 ± 3.3 |
| 7 | 13 | −1 | 0 | 1 | 163.1 ± 1.47 | 0.217 ± 0.005 | 64.1 ± 2.8 |
| 6 | 14 | 1 | 0 | −1 | 302.7 ± 2.96 | 0.334 ± 0.003 | 80.5 ± 2.3 |
| 1 | 15 | −1 | −1 | 0 | 176.2 ± 1.12 | 0.285 ± 0.004 | 57.9 ± 1.5 |
| Response | Suggested Model | R2 | Adjusted R2 | Predicted R2 | SD | p-Value | |
|---|---|---|---|---|---|---|---|
| Y1 | Quadratic | 0.9968 | 0.9912 | 0.9497 | 0.006 | <0.0001 | Significant |
| Y2 | Quadratic | 0.9958 | 0.9883 | 0.9491 | 0.011 | <0.0001 | Significant |
| Y3 | Quadratic | 0.9942 | 0.9896 | 0.9719 | 0.009 | <0.0001 | Significant |
| Independent Variables | Units | Optimal Values | |
|---|---|---|---|
| A: Chol | μmol | 325 | |
| B: Sur–Chol | w/w | 2 | |
| C: Cur–Chol | w/w | 0.5 | |
| Responses | Predicted Value | Experimental Value | Residual |
| Size | 165.2 | 169.4 | 3.2 |
| PDI | 0.192 | 0.189 | −0.003 |
| EE | 84.51 | 84.50 | −0.01 |
| Independent Variables | Level | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| Chol (μmol) | 250 | 325 | 400 |
| Sur: Chol (w/w) | 0.5 | 1.25 | 2 |
| Cur: Chol (w/w) | 0.25 | 0.375 | 0.5 |
| Dependent Variables | Goal | ||
| Average size (nm) | Minimum | ||
| PDI | Minimum | ||
| EE (%) | Maximum | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghumman, S.A.; Ijaz, A.; Noreen, S.; Aslam, A.; Kausar, R.; Irfan, A.; Latif, S.; Shazly, G.A.; Shah, P.A.; Rana, M.; et al. Formulation and Characterization of Curcumin Niosomes: Antioxidant and Cytotoxicity Studies. Pharmaceuticals 2023, 16, 1406. https://doi.org/10.3390/ph16101406
Ghumman SA, Ijaz A, Noreen S, Aslam A, Kausar R, Irfan A, Latif S, Shazly GA, Shah PA, Rana M, et al. Formulation and Characterization of Curcumin Niosomes: Antioxidant and Cytotoxicity Studies. Pharmaceuticals. 2023; 16(10):1406. https://doi.org/10.3390/ph16101406
Chicago/Turabian StyleGhumman, Shazia Akram, Amna Ijaz, Sobia Noreen, Afeefa Aslam, Rizwana Kausar, Ali Irfan, Sumera Latif, Gamal A. Shazly, Pervaiz Akhtar Shah, Maria Rana, and et al. 2023. "Formulation and Characterization of Curcumin Niosomes: Antioxidant and Cytotoxicity Studies" Pharmaceuticals 16, no. 10: 1406. https://doi.org/10.3390/ph16101406
APA StyleGhumman, S. A., Ijaz, A., Noreen, S., Aslam, A., Kausar, R., Irfan, A., Latif, S., Shazly, G. A., Shah, P. A., Rana, M., Aslam, A., Altaf, M., Kotwica-Mojzych, K., & Bin Jardan, Y. A. (2023). Formulation and Characterization of Curcumin Niosomes: Antioxidant and Cytotoxicity Studies. Pharmaceuticals, 16(10), 1406. https://doi.org/10.3390/ph16101406










