Targeting CD44 Receptor Pathways in Degenerative Joint Diseases: Involvement of Proteoglycan-4 (PRG4)
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. CD44 Receptor: Overview of Structure, Function, and Signaling
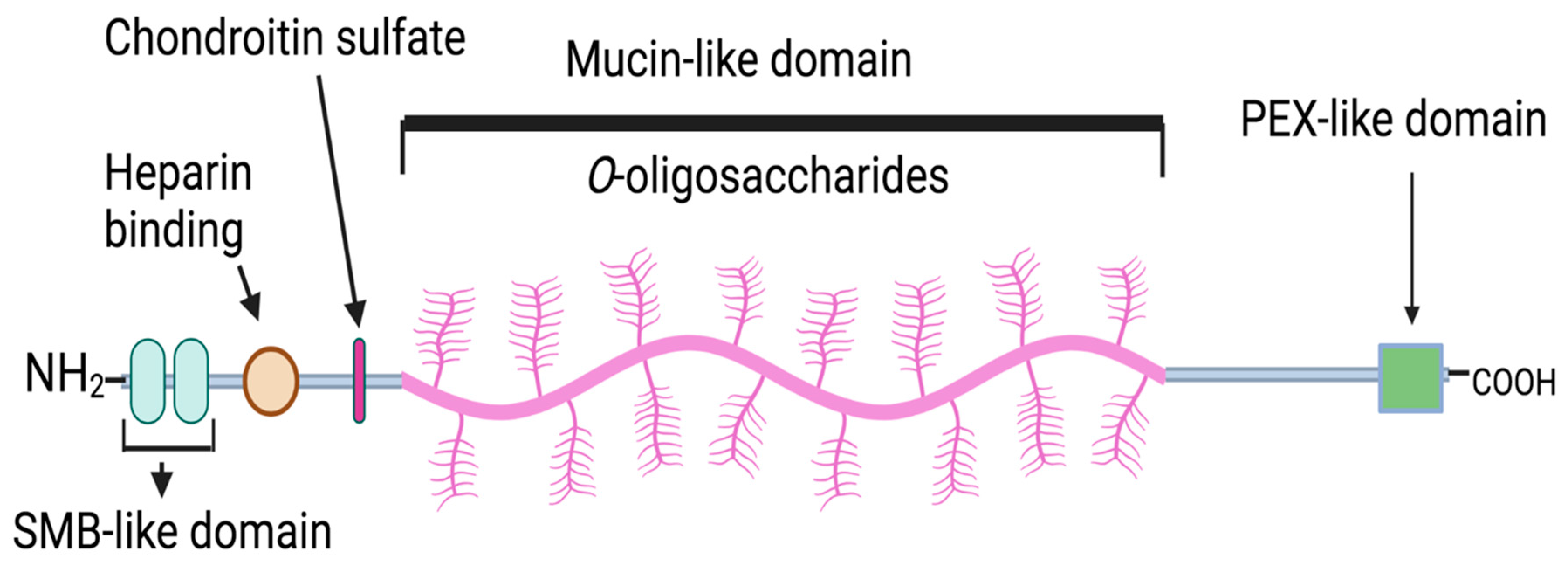
4. Proteoglycan-4 (PRG4): Localization, Structure, and Biological Function in the Joint
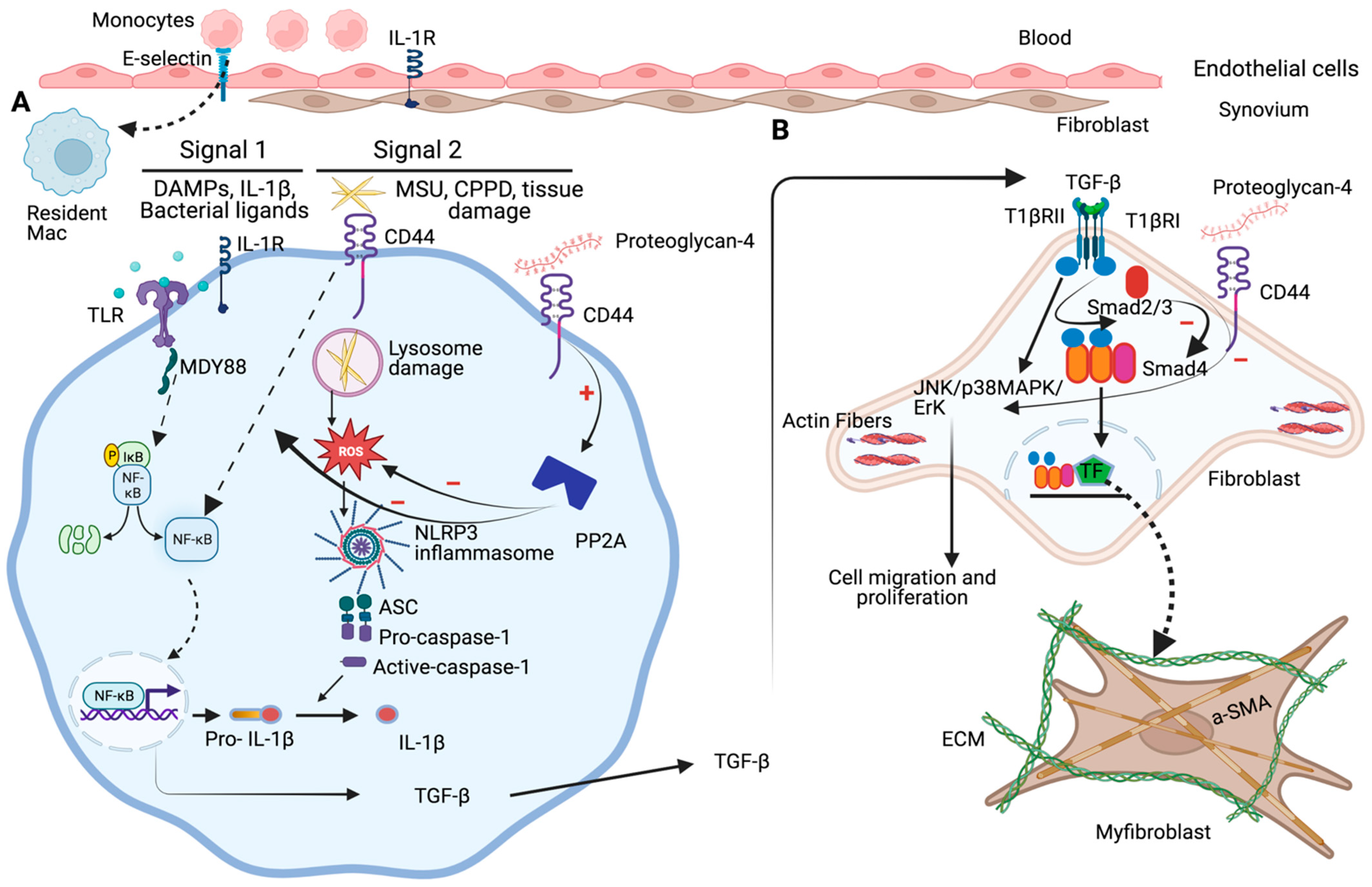
5. Role of PRG4-CD44 in Regulating Synovitis and Synovial Hyperplasia in Rheumatoid Arthritis (RA)
6. Role of PRG4-CD44 in Regulating Synovial Inflammation and Arthrofibrosis in Osteoarthritis (OA)
7. Role of PRG4-CD44 in Mitigating Inflammatory Response in Gout Arthritis
8. Summary and Future Considerations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [PubMed]
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Mohammed, A.; Alshamarri, T.; Adeyeye, T.; Lazariu, V.; McNutt, L.A.; Carpenter, D.O. A comparison of risk factors for osteo-and rheumatoid arthritis using NHANES data. Prev. Med. Rep. 2020, 20, 101242. [Google Scholar]
- Desai, J.; Steiger, S.; Anders, H.-J. Molecular pathophysiology of gout. Trends Mol. Med. 2017, 23, 756–768. [Google Scholar]
- Haskin, C.L.; Milam, S.B.; Cameron, I.L. Pathogenesis of degenerative joint disease in the human temporomandibular joint. Crit. Rev. Oral Biol. Med. 1995, 6, 248–277. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J. Pathophysiology of osteoarthritis. Osteoarthr. Cartil. 2004, 12, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [PubMed]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar]
- Remst, D.F.; Davidson, E.N.B.; van der Kraan, P.M. Unravelling osteoarthritis-related synovial fibrosis: A step closer to solving joint stiffness. Rheumatology 2015, 54, 1954–1963. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, K.; Jiang, P.; Chang, C.; Xu, L.; Xu, L.; Shi, Y.; Guo, S.; Xue, Y.; He, D. Inflammatory response to regulated cell death in gout and its functional implications. Front. Immunol. 2022, 13, 888306. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.C.; Rigon, C. Epidemiology of osteoarthritis: Prevalence, risk factors and functional impact. Aging Clin. Exp. Res. 2003, 15, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, M.; Yang, J.; Wang, J.; Qiao, Y.; Li, X. The immunological basis in the pathogenesis of gout. Iran. J. Immunol. 2017, 14, 90–98. [Google Scholar] [PubMed]
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar]
- Köller, M.; Aringer, M.; Kiener, H.; Erlacher, L.; Machold, K.; Eberl, G.; Studnicka-Benke, A.; Graninger, W.; Smolen, J. Expression of adhesion molecules on synovial fluid and peripheral blood monocytes in patients with inflammatory joint disease and osteoarthritis. Ann. Rheum. Dis. 1999, 58, 709–712. [Google Scholar] [CrossRef]
- Nassonov, E.; Samsonov, M.Y.; Chichasova, N.V.; Nikiphorova, E.L.; Tilz, G.P.; Demel, U.; Widner, B.; Fuchs, D. Soluble adhesion molecules in rheumatoid arthritis. Rheumatology 2000, 39, 808–810. [Google Scholar] [CrossRef]
- Schett, G.; Kiechl, S.; Bonora, E.; Zwerina, J.; Mayr, A.; Axmann, R.; Weger, S.; Oberhollenzer, F.; Lorenzini, R.; Willeit, J. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 2381–2389. [Google Scholar] [CrossRef]
- Veale, D.J.; Maple, C. Cell adhesion molecules in rheumatoid arthritis: Implications for therapy. Drugs Aging 1996, 9, 87–92. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [PubMed]
- Fuchs, S.; Dankbar, B.; Wildenau, G.; Goetz, W.; Lohmann, C.H.; Tibesku, C.O. Expression of the CD44 variant isoform 5 in the human osteoarthritic knee joint: Correlation with radiological, histomorphological, and biochemical parameters. J. Orthop. Res. 2004, 22, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Grisar, J.; Munk, M.; Steiner, C.W.; Amoyo-Minar, L.; Tohidast-Akrad, M.; Zenz, P.; Steiner, G.; Smolen, J.S. Expression patterns of CD44 and CD44 splice variants in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. Incl. Suppl. 2012, 30, 64. [Google Scholar]
- Knudson, W.; Loeser, R. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell. Mol. Life Sci. CMLS 2002, 59, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Naor, D.; Nedvetzki, S. CD44 in rheumatoid arthritis. Arthritis Res. Ther. 2003, 5, 105. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Luo, W.; Gao, S.G.; Su, D.Z.; Li, Y.S.; Zeng, C.; Lei, G.H. Expression of CD44 in articular cartilage is associated with disease severity in knee osteoarthritis. Mod. Rheumatol. 2013, 23, 1186–1191. [Google Scholar] [CrossRef]
- Herishanu, Y.; Gibellini, F.; Njuguna, N.; Keyvanfar, K.; Wiestner, A. CD44 signaling via PI3K/AKT and MAPK/ERK pathways protects CLL cells from spontaneous and drug induced apoptosis through MCL-1. Leuk. Lymphoma 2011, 52, 1758. [Google Scholar] [CrossRef]
- Amash, A.; Wang, L.; Wang, Y.; Bhakta, V.; Fairn, G.D.; Hou, M.; Peng, J.; Sheffield, W.P.; Lazarus, A.H. CD44 antibody inhibition of macrophage phagocytosis targets Fcγ receptor–and complement receptor 3–dependent mechanisms. J. Immunol. 2016, 196, 3331–3340. [Google Scholar] [CrossRef]
- Bousoik, E.; Qadri, M.; Elsaid, K.A. CD44 receptor mediates urate crystal phagocytosis by macrophages and regulates inflammation in a murine peritoneal model of acute gout. Sci. Rep. 2020, 10, 5748. [Google Scholar] [CrossRef] [PubMed]
- Vachon, E.; Martin, R.; Kwok, V.; Cherepanov, V.; Chow, C.W.; Doerschuk, C.M.; Plumb, J.; Grinstein, S.; Downey, G.P. CD44-mediated phagocytosis induces inside-out activation of complement receptor-3 in murine macrophages. Blood J. Am. Soc. Hematol. 2007, 110, 4492–4502. [Google Scholar] [CrossRef] [PubMed]
- Vachon, E.; Martin, R.; Plumb, J.; Kwok, V.; Vandivier, R.W.; Glogauer, M.; Kapus, A.; Wang, X.; Chow, C.W.; Grinstein, S.; et al. CD44 is a phagocytic receptor. Blood 2006, 107, 4149–4158. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Liu, Y.; He, Y.; Wang, W.; Du, Y.; Yang, C.; Gao, F. CD44 clustering is involved in monocyte differentiation. Acta Biochim. Biophys. Sin. 2014, 46, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.S.; Majumdar, S.; Jacob, M.; Hayden, J.; Mrass, P.; Weninger, W.; Assoian, R.K.; Puré, E. Fibroblast migration is mediated by CD44-dependent TGFβ activation. J. Cell Sci. 2008, 121, 1393–1402. [Google Scholar] [CrossRef]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Puré, E. CD44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrix remodeling and tensile strength during cutaneous wound healing. Matrix Biol. 2019, 75, 314–330. [Google Scholar]
- Osawa, Y.; Kawai, H.; Tsunoda, T.; Komatsu, H.; Okawara, M.; Tsutsui, Y.; Yoshida, Y.; Yoshikawa, S.; Mori, T.; Yamazoe, T.; et al. Cluster of differentiation 44 promotes liver fibrosis and serves as a biomarker in congestive hepatopathy. Hepatol. Commun. 2021, 5, 1437–1447. [Google Scholar] [CrossRef]
- Svee, K.; White, J.; Vaillant, P.; Jessurun, J.; Roongta, U.; Krumwiede, M.; Johnson, D.; Henke, C. Acute lung injury fibroblast migration and invasion of a fibrin matrix is mediated by CD44. J. Clin. Investig. 1996, 98, 1713–1727. [Google Scholar] [CrossRef]
- Jay, G.D.; Haberstroh, K.; Cha, C.J. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and Healon. J. Biomed. Mater. Res. 1998, 40, 414–418. [Google Scholar] [CrossRef]
- Jay, G.D.; Waller, K.A. The biology of lubricin: Near frictionless joint motion. Matrix Biol. 2014, 39, 17–24. [Google Scholar] [PubMed]
- Swann, D.A.; Silver, F.H.; Slayter, H.S.; Stafford, W.; Shore, E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem. J. 1985, 225, 195–201. [Google Scholar] [CrossRef]
- Al-Sharif, A.; Jamal, M.; Zhang, L.X.; Larson, K.; Schmidt, T.A.; Jay, G.D.; Elsaid, K.A. Lubricin/proteoglycan 4 binding to CD44 receptor: A mechanism of the suppression of proinflammatory cytokine–induced synoviocyte proliferation by lubricin. Arthritis Rheumatol. 2015, 67, 1503–1513. [Google Scholar] [CrossRef]
- Borland, G.; Ross, J.; Guy, K. Forms and functions of CD44. Immunology 1998, 93, 139. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Lantier, L.; Kennedy, A.; Bonner, J.S.; Mayes, W.H.; Bracy, D.P.; Bookbinder, L.H.; Hasty, A.H.; Thompson, C.B.; Wasserman, D.H. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes 2013, 62, 1888–1896. [Google Scholar] [CrossRef]
- Ouhtit, A.; Rizeq, B.; Abou Saleh, H.; Rahman, M.M.; Zayed, H. Novel CD44-downstream signaling pathways mediating breast tumor invasion. Int. J. Biol. Sci. 2018, 14, 1782. [Google Scholar] [CrossRef]
- Sherman, L.; Sleeman, J.; Herrlich, P.; Ponta, H. Hyaluronate receptors: Key players in growth, differentiation, migration and tumor progression. Curr. Opin. Cell Biol. 1994, 6, 726–733. [Google Scholar] [PubMed]
- Tammi, R.H.; Kultti, A.; Kosma, V.-M.; Pirinen, R.; Auvinen, P.; Tammi, M.I. Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Stamenkovic, I.; Amiot, M.; Pesando, J.M.; Seed, B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell 1989, 56, 1057–1062. [Google Scholar] [CrossRef]
- Mackay, C.R.; Terpe, H.J.; Stauder, R.; Marston, W.L.; Stark, H.; Günthert, U. Expression and modulation of CD44 variant isoforms in humans. J. Cell Biol. 1994, 124, 71–82. [Google Scholar] [CrossRef]
- Sen, Y.; Yip, G.W. CD44 (CD44 molecule (Indian blood group)). Atlas Genet. Cytogenet. Oncol. Haematol. 2010, 14, 178–191. [Google Scholar] [CrossRef]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar]
- Naor, D.; Nedvetzki, S.; Golan, I.; Melnik, L.; Faitelson, Y. CD44 in cancer. Crit. Rev. Clin. Lab. Sci. 2002, 39, 527–579. [Google Scholar]
- Lesley, J.; Hyman, R.; English, N.; Catterall, J.B.; Turner, G.A. CD44 in inflammation and metastasis. Glycoconj. J. 1997, 14, 611–622. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 2015, 6, 201. [Google Scholar]
- Singleton, P.A.; Bourguignon, L.Y. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp. Cell Res. 2004, 295, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Hirao, M.; Doi, Y.; Takahashi, N.; Kondo, T.; Tsukita, S.; Tsukita, S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J. Cell Biol. 1998, 140, 885–895. [Google Scholar] [PubMed]
- Basu, S.; S Cheriyamundath, A. Ben-Ze’ev, Cell–cell adhesion: Linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7, F1000 Faculty Rev-1488. [Google Scholar] [CrossRef]
- Kajita, M.; Itoh, Y.; Chiba, T.; Mori, H.; Okada, A.; Kinoh, H.; Seiki, M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 2001, 153, 893–904. [Google Scholar] [PubMed]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, inflammation, and breast cancer progression. Front. Immunol. 2015, 6, 236. [Google Scholar]
- Knudson, W.; Chow, G.; Knudson, C.B. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol. 2002, 21, 15–23. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol. Pathol. 1999, 52, 189. [Google Scholar]
- Miletti-González, K.E.; Murphy, K.; Kumaran, M.N.; Ravindranath, A.K.; Wernyj, R.P.; Kaur, S.; Miles, G.D.; Lim, E.; Chan, R.; Chekmareva, M.; et al. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): Modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J. Biol. Chem. 2012, 287, 18995–19007. [Google Scholar] [CrossRef]
- Weber, G.F.; Ashkar, S.; Glimcher, M.J.; Cantor, H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 1996, 271, 509–512. [Google Scholar] [CrossRef]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.; Lokeshwar, V.B. The role of CD44 in disease pathophysiology and targeted treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Suenaga, N.; Mori, H.; Itoh, Y.; Seiki, M. CD44 binding through the hemopexin-like domain is critical for its shedding by membrane-type 1 matrix metalloproteinase. Oncogene 2005, 24, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.P.; Haynes, B.F.; McCachren, S.S. Expression of CD44 variants in human inflammatory synovitis. J. Clin. Immunol. 1995, 15, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Chawla, K.; Ham, H.O.; Nguyen, T.; Messersmith, P.B. Molecular resurfacing of cartilage with proteoglycan 4. Acta Biomater. 2010, 6, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Damen, A.; van Donkelaar, C.C.; Cardinaels, R.M.; Brandt, J.M.; Schmidt, T.A.; Ito, K. Proteoglycan 4 reduces friction more than other synovial fluid components for both cartilage-cartilage and cartilage-metal articulation. Osteoarthr. Cartil. 2021, 29, 894–904. [Google Scholar] [CrossRef]
- Elsaid, K.; Fleming, B.C.; Oksendahl, H.L.; Machan, J.T.; Fadale, P.D.; Hulstyn, M.J.; Shalvoy, R.; Jay, G.D. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2008, 58, 1707–1715. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Ludwig, T.E.; Liebisch, G.; Zhang, R.; Siebert, H.C.; Wilhelm, J.; Kaesser, U.; Dettmeyer, R.B.; Klein, H.; Ishaque, B.; et al. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS ONE 2015, 10, e0125192. [Google Scholar]
- Elsaid, K.; Machan, J.T.; Waller, K.; Fleming, B.C.; Jay, G.D. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor α on chondroprotection in an animal model. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 2997–3006. [Google Scholar] [CrossRef]
- Young, A.A.; McLennan, S.; Smith, M.M.; Smith, S.M.; Cake, M.A.; Read, R.A.; Melrose, J.; Sonnabend, D.H.; Flannery, C.R.; Little, C.B. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res. Ther. 2006, 8, R41. [Google Scholar] [CrossRef]
- Elsaid, K.A.; Jay, G.D.; Chichester, C.O. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007, 56, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2015, 25, e222–e230. [Google Scholar] [CrossRef] [PubMed]
- Blewis, M.E.; Lao, B.J.; Schumacher, B.L.; Bugbee, W.D.; Sah, R.L.; Firestein, G.S. Interactive cytokine regulation of synoviocyte lubricant secretion. Tissue Eng. Part A 2010, 16, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, A.; Reddi, A.H. Stimulation of superficial zone protein/lubricin/PRG4 by transforming growth factor-β in superficial zone articular chondrocytes and modulation by glycosaminoglycans. Tissue Eng. Part A 2015, 21, 1973–1981. [Google Scholar] [CrossRef]
- Jones, A.; Flannery, C. Bioregulation of lubricin expression by growth factors and cytokines. Eur. Cells Mater. 2007, 13, 40–45. [Google Scholar] [CrossRef]
- Schmidt, T.; Gastelum, N.S.; Han, E.H.; Nugent-Derfus, G.E.; Schumacher, B.L.; Sah, R.L. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1α, IGF-I, and TGF-β1. Osteoarthr. Cartil. 2008, 16, 90–97. [Google Scholar] [CrossRef]
- Rhee, D.K.; Marcelino, J.; Baker, M.; Gong, Y.; Smits, P.; Lefebvre, V.; Jay, G.D.; Stewart, M.; Wang, H.; Warman, M.L.; et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Investig. 2005, 115, 622–631. [Google Scholar] [CrossRef]
- Waller, K.A.; Zhang, L.X.; Elsaid, K.A.; Fleming, B.C.; Warman, M.L.; Jay, G.D. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc. Natl. Acad. Sci. USA 2013, 110, 5852–5857. [Google Scholar] [CrossRef]
- Elsaid, K.; Zhang, L.; Shaman, Z.; Patel, C.; Schmidt, T.A.; Jay, G.D. The impact of early intra-articular administration of interleukin-1 receptor antagonist on lubricin metabolism and cartilage degeneration in an anterior cruciate ligament transection model. Osteoarthr. Cartil. 2015, 23, 114–121. [Google Scholar] [CrossRef]
- Hurtig, M.; Zaghoul, I.; Sheardown, H.; Schmidt, T.A.; Liu, L.; Zhang, L.; Elsaid, K.A.; Jay, G.D. Two compartment pharmacokinetic model describes the intra-articular delivery and retention of rhprg4 following ACL transection in the Yucatan mini pig. J. Orthop. Res. 2019, 37, 386–396. [Google Scholar] [CrossRef]
- Jay, G.D.; Elsaid, K.A.; Kelly, K.A.; Anderson, S.C.; Zhang, L.; Teeple, E.; Waller, K.; Fleming, B.C. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012, 64, 1162–1171. [Google Scholar] [CrossRef]
- Jay, G.D.; Fleming, B.C.; Watkins, B.A.; McHugh, K.A.; Anderson, S.C.; Zhang, L.X.; Teeple, E.; Waller, K.A.; Elsaid, K.A. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010, 62, 2382–2391. [Google Scholar] [CrossRef]
- Teeple, E.; Elsaid, K.A.; Jay, G.D.; Zhang, L.; Badger, G.J.; Akelman, M.; Bliss, T.F.; Fleming, B.C. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament–deficient rat knee. Am. J. Sports Med. 2011, 39, 164–172. [Google Scholar] [CrossRef]
- Ludwig, T.E.; Hunter, M.M.; Schmidt, T.A. Cartilage boundary lubrication synergism is mediated by hyaluronan concentration and PRG4 concentration and structure. BMC Musculoskelet. Disord. 2015, 16, 386. [Google Scholar] [CrossRef]
- Jay, G.D.; Harris, D.A.; Cha, C.-J. Boundary lubrication by lubricin is mediated by O-linked β(1-3) Gal-GalNAc oligosaccharides. Glycoconj. J. 2001, 18, 807–815. [Google Scholar] [CrossRef]
- Larson, K.M.; Zhang, L.; Badger, G.J.; Jay, G.D. Early genetic restoration of lubricin expression in transgenic mice mitigates chondrocyte peroxynitrite release and caspase-3 activation. Osteoarthr. Cartil. 2017, 25, 1488–1495. [Google Scholar] [CrossRef][Green Version]
- Waller, K.A.; Zhang, L.X.; Jay, G.D. Friction-induced mitochondrial dysregulation contributes to joint deterioration in Prg4 knockout mice. Int. J. Mol. Sci. 2017, 18, 1252. [Google Scholar] [CrossRef]
- Estrella, R.P.; Whitelock, J.M.; Packer, N.H.; Karlsson, N.G. The glycosylation of human synovial lubricin: Implications for its role in inflammation. Biochem. J. 2010, 429, 359–367. [Google Scholar] [CrossRef]
- Jin, C.; Ekwall, A.K.H.; Bylund, J.; Björkman, L.; Estrella, R.P.; Whitelock, J.M.; Eisler, T.; Bokarewa, M.; Karlsson, N.G. Human synovial lubricin expresses sialyl Lewis x determinant and has L-selectin ligand activity. J. Biol. Chem. 2012, 287, 35922–35933. [Google Scholar]
- Qadri, M.; Jay, G.D.; Zhang, L.X.; Schmidt, T.A.; Totonchy, J.; Elsaid, K.A. Proteoglycan-4 is an essential regulator of synovial macrophage polarization and inflammatory macrophage joint infiltration. Arthritis Res. Ther. 2021, 23, 241. [Google Scholar] [CrossRef]
- Qadri, M.; Jay, G.D.; Zhang, L.X.; Richendrfer, H.; Schmidt, T.A.; Elsaid, K.A. Proteoglycan-4 regulates fibroblast to myofibroblast transition and expression of fibrotic genes in the synovium. Arthritis Res. Ther. 2020, 22, 113. [Google Scholar] [PubMed]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51 (Suppl. S5), v3–v11. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M. Rheumatoid arthritis pathogenesis, prediction, and prevention: An emerging paradigm shift. Arthritis Rheumatol. 2021, 73, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D. Suppl 1: The normal synovium. Open Rheumatol. J. 2011, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.; Mikecz, K.; Glant, T.T.; Jobanputra, P.; Pinder, S.; Bavington, C.; Morrison, P.; Nuki, G. CD44 expression by leucocytes in rheumatoid arthritis and modulation by specific antibody: Implications for lymphocyte adhesion to endothelial cells and synoviocytes in vitro. Scand. J. Immunol. 1997, 45, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Golan, I.; Nedvetzki, S.; Golan, I.; Eshkar-Sebban, L.; Levartovsky, D.; Elkayam, O.; Caspi, D.; Aamar, S.; Amital, H.; Rubinow, A.; et al. Expression of extra trinucleotide in CD44 variant of rheumatoid arthritis patients allows generation of disease-specific monoclonal antibody. J. Autoimmun. 2007, 28, 99–113. [Google Scholar] [CrossRef]
- Qadri, M.; Almadani, S.; Jay, G.D.; Elsaid, K.A. Role of CD44 in regulating TLR2 activation of human macrophages and downstream expression of proinflammatory cytokines. J. Immunol. 2018, 200, 758–767. [Google Scholar] [CrossRef]
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar]
- Shen, J.; Chen, D. Recent progress in osteoarthritis research. J. Am. Acad. Orthop. Surg. 2014, 22, 467. [Google Scholar] [CrossRef]
- Goldring, S.; Goldring, M. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuronal Interact. 2006, 6, 376. [Google Scholar] [PubMed]
- Goldring, S.R.; Goldring, M.B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 2004, 427, S27–S36. [Google Scholar]
- Inada, M.; Wang, Y.; Byrne, M.H.; Rahman, M.U.; Miyaura, C.; López-Otín, C.; Krane, S.M. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 2004, 101, 17192–17197. [Google Scholar] [CrossRef] [PubMed]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Fuchs, S.; Rolauffs, B.; Arndt, S.; Tibesku, C.O.; Prehm, P. CD44H and the isoforms CD44v5 and CD44v6 in the synovial fluid of the osteoarthritic human knee joint. Osteoarthr. Cartil. 2003, 11, 839–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alquraini, A.; Jamal, M.; Zhang, L.; Schmidt, T.; Jay, G.D.; Elsaid, K.A. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res. Ther. 2017, 19, 89. [Google Scholar] [CrossRef]
- Cheuy, V.A.; Foran, J.R.; Paxton, R.J.; Bade, M.J.; Zeni, J.A.; Stevens-Lapsley, J.E. Arthrofibrosis associated with total knee arthroplasty. J. Arthroplast. 2017, 32, 2604–2611. [Google Scholar]
- Jeroen, C.; EGMOND, H.V.; Stephan, B.W.; Nina, M.C. Early follow-up after primary total knee and total hip arthroplasty with rapid recovery: Focus groups. Acta Orthop. Belg. 2015, 81, 447–453. [Google Scholar]
- Qadri, M.M.; Jay, G.D.; Ostrom, R.S.; Zhang, L.X.; Elsaid, K.A. cAMP attenuates TGF-β’s profibrotic responses in osteoarthritic synoviocytes: Involvement of hyaluronan and PRG4. Am. J. Physiol. Cell Physiol. 2018, 315, C432–C443. [Google Scholar] [CrossRef]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar]
- Ma, C.A.; Leung, Y.Y. Exploring the link between uric acid and osteoarthritis. Front. Med. 2017, 4, 225. [Google Scholar] [CrossRef]
- Rees, F.; Hui, M.; Doherty, M. Optimizing current treatment of gout. Nat. Rev. Rheumatol. 2014, 10, 271–283. [Google Scholar] [PubMed]
- Busso, N.; So, A. Gout. Mechanisms of inflammation in gout. Arthritis Res. Ther. 2010, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Qadri, M.; Jay, G.D.; Zhang, L.X.; Wong, W.; Reginato, A.M.; Sun, C.; Schmidt, T.A.; Elsaid, K.A. Recombinant human proteoglycan-4 reduces phagocytosis of urate crystals and downstream nuclear factor kappa B and inflammasome activation and production of cytokines and chemokines in human and murine macrophages. Arthritis Res. Ther. 2018, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. How interleukin-1β induces gouty arthritis. Arthritis Rheum. 2010, 62, 3140. [Google Scholar] [CrossRef]
- ElSayed, S.; Jay, G.D.; Cabezas, R.; Qadri, M.; Schmidt, T.A.; Elsaid, K.A. Recombinant human proteoglycan 4 regulates phagocytic activation of monocytes and reduces IL-1β secretion by urate crystal stimulated gout PBMCs. Front. Immunol. 2021, 12, 771677. [Google Scholar] [CrossRef]
- Lambrecht, C.; Haesen, D.; Sents, W.; Ivanova, E.; Janssens, V. Structure, regulation, and pharmacological modulation of PP2A phosphatases. Phosphatase Modul. 2013, 1053, 283–305. [Google Scholar]
- Baskaran, R.; Velmurugan, B.K. Protein phosphatase 2A as therapeutic targets in various disease models. Life Sci. 2018, 210, 40–46. [Google Scholar] [CrossRef]
- Qadri, M.; ElSayed, S.; Elsaid, K.A. Fingolimod Phosphate (FTY720-P) Activates Protein Phosphatase 2A in Human Monocytes and Inhibits Monosodium Urate Crystal–Induced Interleukin-1β Production. J. Pharmacol. Exp. Ther. 2021, 376, 222–230. [Google Scholar] [CrossRef]
- Elsaid, K.; Merriman, T.R.; Rossitto, L.A.; Liu-Bryan, R.; Karsh, J.; Phipps-Green, A.; Jay, G.D.; Elsayed, S.; Qadri, M.; Miner, M.; et al. Amplification of inflammation by lubricin deficiency implicated in incident, erosive gout independent of hyperuricemia. Arthritis Rheumatol. 2023, 75, 794–805. [Google Scholar] [CrossRef]
- Ruan, M.Z.; Erez, A.; Guse, K.; Dawson, B.; Bertin, T.; Chen, Y.; Jiang, M.M.; Yustein, J.; Gannon, F.; Lee, B.H. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci. Transl. Med. 2013, 5, ra34–ra176. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.; Grol, M.W.; Ruan, M.Z.; Dawson, B.; Chen, Y.; Jiang, M.M.; Song, I.W.; Jayaram, P.; Cela, R.; Gannon, F.; et al. Combinatorial Prg4 and Il-1ra gene therapy protects against hyperalgesia and cartilage degeneration in post-traumatic osteoarthritis. Hum. Gene Ther. 2019, 30, 225–235. [Google Scholar] [CrossRef] [PubMed]
| Study | Model | Main Outcome |
|---|---|---|
| Al-Sharif et al. [40] |
|
|
| Alquraini et al. [107] |
|
|
| Qadri et al. [98] |
|
|
| Qadri et al. [92] |
|
|
| Qadri et al. [115] |
|
|
| Bousoik et al. [29] |
|
|
| Qadri et al. and ElSayed et al. [117,120] |
|
|
| Elsaid et al. [121] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadri, M.M. Targeting CD44 Receptor Pathways in Degenerative Joint Diseases: Involvement of Proteoglycan-4 (PRG4). Pharmaceuticals 2023, 16, 1425. https://doi.org/10.3390/ph16101425
Qadri MM. Targeting CD44 Receptor Pathways in Degenerative Joint Diseases: Involvement of Proteoglycan-4 (PRG4). Pharmaceuticals. 2023; 16(10):1425. https://doi.org/10.3390/ph16101425
Chicago/Turabian StyleQadri, Marwa M. 2023. "Targeting CD44 Receptor Pathways in Degenerative Joint Diseases: Involvement of Proteoglycan-4 (PRG4)" Pharmaceuticals 16, no. 10: 1425. https://doi.org/10.3390/ph16101425
APA StyleQadri, M. M. (2023). Targeting CD44 Receptor Pathways in Degenerative Joint Diseases: Involvement of Proteoglycan-4 (PRG4). Pharmaceuticals, 16(10), 1425. https://doi.org/10.3390/ph16101425






