Modulation of Neuron and Astrocyte Dopamine Receptors via Receptor–Receptor Interactions
Abstract
:1. Introduction
2. Dopamine Receptors
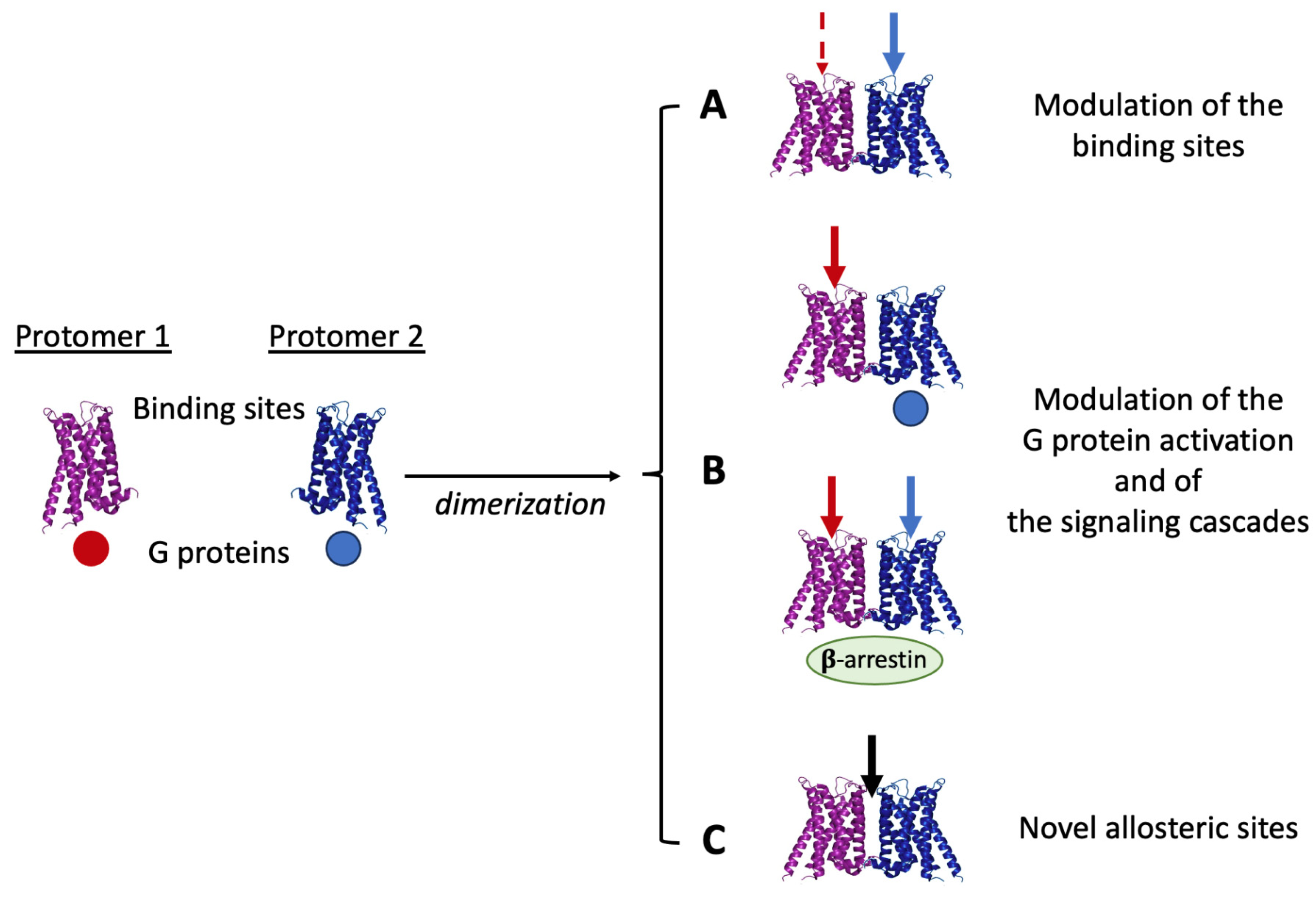
3. Structural Receptor–Receptor Interactions
4. Receptor Complexes Involving Dopamine Receptors
4.1. Receptor Complexes Involving D2-like Dopamine Receptors
4.2. Receptor Complexes Involving D1-like Dopamine Receptors
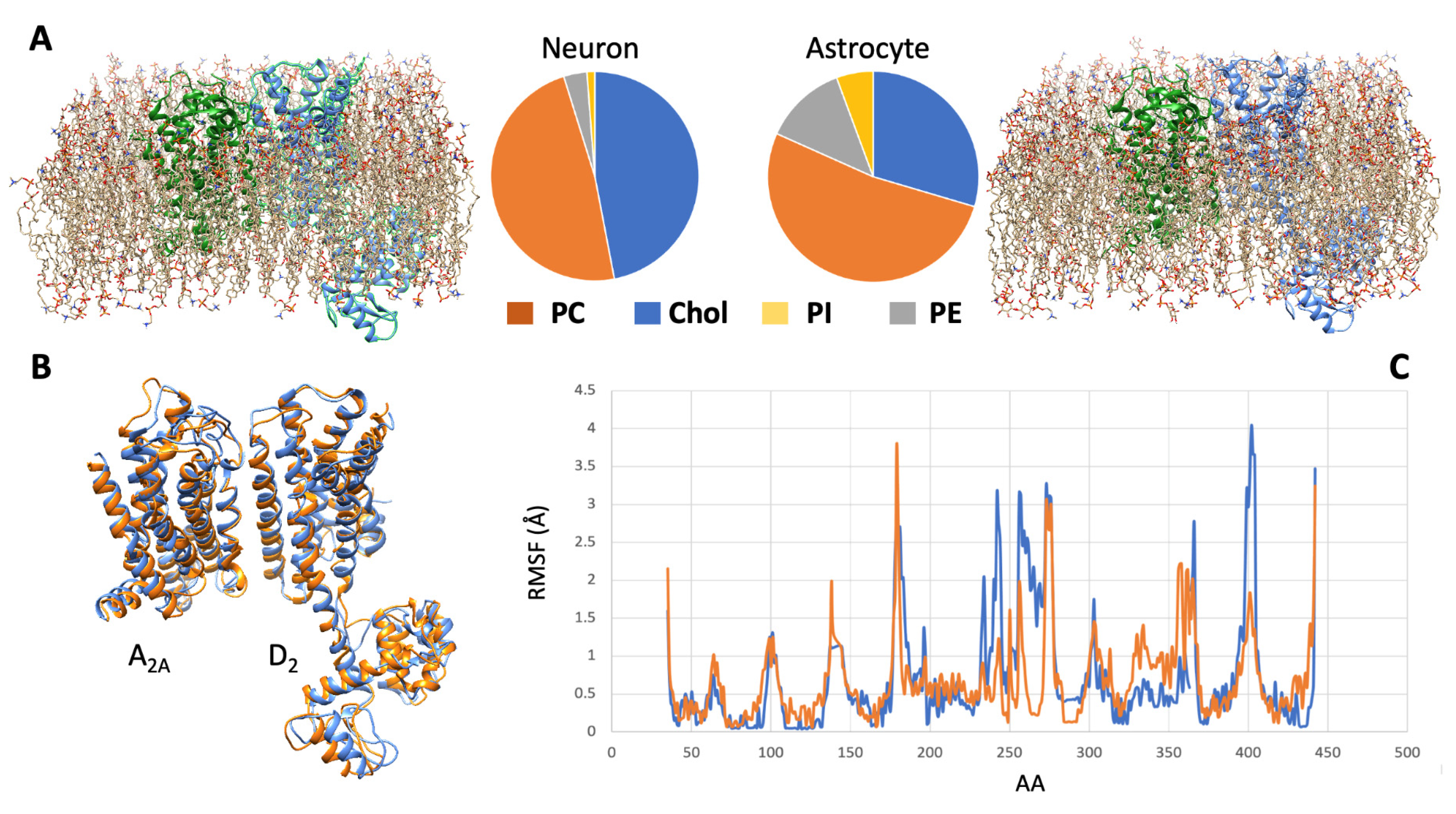
4.3. Possible Differences in Receptor Complex Dynamics in Neurons and Astrocytes
5. Complexes Involving Dopamine Receptors in the Main Dopaminergic Pathways: Impact on Neuropharmacology
5.1. Nigro-Striatal Dopamine Pathway
5.2. Mesolimbic Dopamine Pathway
5.3. Mesocortical Dopamine Pathway
5.4. Neuron–Astrocyte Crosstalk
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dahlström, A.; Fuxe, K. A method for the demonstration of monoamine-containing nerve fibres in the central nervous system. Acta Physiol. 1964, 60, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Andén, N.-E.; Carlsson, A.; Dahlström, A.; Fuxe, K.; Hillarp, N.A.; Larsson, K. Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci. 1964, 3, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, M.; Morelli, M. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In Dopamine; Dunnet, S.B., Bentivoglio, M., Björklund, A., Hökfelt, T., Eds.; Handbook of Chemical Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 21, pp. 1–107. [Google Scholar]
- Hurd, Y.L.; Hall, H. Human forebrain dopamine systems: Characterization of the normal brain and in relation to psychiatric disorders. In Dopamine; Dunnet, S.B., Bentivoglio, M., Björklund, A., Hökfelt, T., Eds.; Handbook of Chemical Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 21, pp. 525–571. [Google Scholar]
- Albanese, A.; Altavista, M.C.; Rossi, P. Organization of central nervous system dopaminergic pathways. J. Neural Transm. 1986, 22, 3–17. [Google Scholar]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Function, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar]
- Fuxe, K.; Rivera, A.; Jacobsen, K.X.; Höistad, M.; Leo, G.; Horvath, T.L.; Staines, W.; De la Calle, A.; Agnati, L.F. Dynamics of volume transmission in the brain. Focus on catecholamine and opioid peptide communication and the role of uncoupling protein 2. J. Neural Transm. 2005, 112, 65–76. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Guescini, M.; Genedani, S.; Fuxe, K. Understanding wiring and volume transmission. Brain Res. Rev. 2010, 64, 137–159. [Google Scholar]
- Guidolin, D.; Marcoli, M.; Maura, G.; Agnati, L.F. New dimensions of connectomics and network plasticity in the central nervous system. Rev. Neurosci. 2017, 28, 113–132. [Google Scholar]
- Eid, L.; Parent, M. Chemical anatomy of pallidal afferents in primates. Brain Struct. Funct. 2016, 221, 4291–4317. [Google Scholar]
- Rice, M.E.; Cragg, S.J. Dopamine spillover after quantal release: Rethinking dopamine transmission in the nigrostriatal pathway. Brain Res. Rev. 2008, 58, 303–313. [Google Scholar] [CrossRef]
- Rice, M.E.; Patel, J.C. Somatodendritic dopamine release: Recent mechanistic insights. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140185. [Google Scholar] [CrossRef]
- Goto, Y.; Otani, S.; Grace, A.A. The yin and yang of dopamine release: A new perspective. Neuropharmacology 2007, 53, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Floresco, S.B. Dopaminergic regulation of limbic-striatal interplay. J. Psychiatry Neurosci. 2007, 32, 400–411. [Google Scholar] [PubMed]
- Hirasawa, H.; Contini, M.; Raviola, E. Extrasynaptic release of GABA and dopamine by retinal dopaminergic neurons. Phylos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140186. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M.; Diaz-Corrales, F.J.; Miyoshi, K.; Ogawa, N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res. 2004, 1029, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Mladinov, M.; Mayer, D.; Brčic, L.; Wolstencroft, E.; thi Man, N.; Holt, I.; Hof, P.R.; Morris, G.E.; Šimic, G. Astrocyte expression of D2-like dopamine receptors in the prefrontal cortex. Transl. Neurosci. 2010, 1, 238–243. [Google Scholar] [CrossRef]
- Montoya, A.; Elgueta, D.; Campos, J.; Chovar, O.; Falcòn, P.; Matus, S.; Alfaro, I.; Bono, M.R.; Pacheco, R. Dopamine receptor D3 signalling in astrocytes promotes neuroinflammation. J. Neuroinflamm. 2019, 16, 258. [Google Scholar] [CrossRef]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor-receptor interactions and microvesicle exchange as mechanisms modulating signaling between neurons and astrocytes. Neuropharmacology 2023, 231, 109509. [Google Scholar]
- Kebabian, J.W.; Petzold, G.L.; Greengard, P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the “dopamine receptor”. Proc. Natl. Acad. Sci. USA 1972, 69, 2145–2149. [Google Scholar] [CrossRef]
- Burt, D.R.; Enna, S.J.; Creese, I.; Snyder, S.H. Dopamine receptor binding in the corpus striatum of mammalian brain. Proc. Natl. Acad. Sci. USA 1975, 72, 4655–4659. [Google Scholar] [CrossRef]
- Seeman, P.; Chau-Wong, M.; Tedesco, J.; Wong, K. Brain receptors for antipsychotic drugs and dopamine: Direct binding assays. Proc. Natl. Acad. Sci. USA 1975, 72, 4376–4380. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.; Benfenati, F.; Celani, M.; Zini, I.; Zoli, M.; Mutt, V. Evidence for the existence of receptor-receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. In Basic Aspects of Receptor Biochemistry; Springer: Vienna, Austria, 1983; Volume 18, pp. 165–179. [Google Scholar]
- Bockaert, J.; Pin, J.P. Molecular tinkering of G protein coupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Marshall, F.H.; White, J.; Main, M.; Green, A.; Wise, A. GABA(B) receptors function as heterodimers. Biochem. Soc. Trans. 1999, 27, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lee, S.P.; O’Dowd, B.F.; George, S.R. Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed. FEBS Lett. 1999, 456, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Xie, Z.; Varghese, G.; Nguyen, T.; O’Dowd, B.F.; George, S.R. Oligomerization of dopamine and serotonin receptors. Neuropsychopharmacology 2000, 23, S32–S40. [Google Scholar] [CrossRef]
- Overton, M.C.; Blumer, K.J. G protein-coupled receptors function as oligomers in vivo. Curr. Biol. 2000, 10, 341–344. [Google Scholar] [CrossRef]
- Zeng, F.; Wess, J. Molecular aspects of muscarinic receptor dimerization. Neuropsychopharmacology 2000, 23, S19–S31. [Google Scholar] [CrossRef]
- Angers, S.; Salahpour, A.; Bouvier, M. Biochemical and biophysical demonstration of GPCR oligomerization in mammalian cells. Life Sci. 2001, 68, 2243–2250. [Google Scholar] [CrossRef]
- Dean, M.K.; Higgs, C.; Smith, R.E.; Bywater, R.P.; Snell, C.R.; Scott, P.D.; Upton, G.J.; Howe, T.J.; Reynolds, C.A. Dimerization of G protein-coupled receptors. J. Med. Chem. 2001, 44, 4595–4614. [Google Scholar]
- Kenakin, T. Drug efficacy at G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 349–379. [Google Scholar] [CrossRef]
- Waldhoer, M.; Fong, J.; Jones, R.M.; Lunzer, M.M.; Sharma, S.K.; Kostenis, E.; Portoghese, P.S.; Whistler, J.L. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. USA 2005, 102, 9050–9055. [Google Scholar] [CrossRef]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. Receptor-receptor interactions as a widespread phenomenon: Novel targets for drug development? Front. Endocrinol. 2019, 10, 53. [Google Scholar]
- Changeux, J.P.; Christopoulos, A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Diabetes Obes. Metabol. 2017, 19, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.-H. Dopamine Signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G.; Wishart, D.S.; Anselmi, L.; Toti, L.; Bove, C.; Prakash, Y.S.; et al. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar]
- Beaulieu, J.-M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar]
- Tritsch, N.X.; Sabatini, B.L. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 2012, 76, 33–50. [Google Scholar]
- Gingrich, J.A.; Caron, M.G. Recent advances in the molecular biology of dopamine receptors. Annu. Rev. Neurosci. 1993, 16, 299–321. [Google Scholar] [CrossRef]
- Dal Toso, R.; Sommer, B.; Ewert, M.; Herb, A.; Pritchett, D.B.; Bach, A.; Shivers, B.D.; Seeburg, P.H. The dopamine D2 receptor: Two molecular forms generated by alternative splicing. EMBO J. 1989, 8, 4025–4034. [Google Scholar] [CrossRef]
- Giros, B.; Sokoloff, P.; Martres, M.P.; Riou, J.-F.; Emorine, L.J.; Schwartz, J.-C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature 1989, 342, 923–926. [Google Scholar] [CrossRef]
- Kim, K.-M. Unveiling the differences in signaling and regulatory mechanisms between dopamine D2 and D3 receptors and their impact on behavioral sensitization. Int. J. Mol. Sci. 2023, 24, 6742. [Google Scholar]
- Jose, P.A.; Yu, P.-Y.; Yamapchi, I.; Eisner, G.M.; Mouradian, M.M.; Felder, C.C.; Felder, R.A. Dopamine D1 receptor regulation of phospholipase C. Hypertens. Res. 1995, 18 (Suppl. 1), S39–S42. [Google Scholar] [CrossRef]
- Rashid, A.; O’Dowd, B.F.; Verma, V.; George, S.R. Neuromal Gq/11-coupled dopamine receptors: An uncharted role for dopamine. Trends Pharmacol. Sci. 2007, 28, 551–555. [Google Scholar] [CrossRef]
- Sahu, A.; Tyeryar, K.R.; Vongtau, H.O.; Sibley, D.R.; Undieh, A.S. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol. Pharmacol. 2009, 75, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.C.; Jose, P.A.; Axelrod, J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J. Pharmacol. Exp. Ther. 1989, 248, 171–175. [Google Scholar] [PubMed]
- Kofuji, P.; Araque, A. G-protein-coupled receptors in astrocyte-neuron communication. Neuroscience 2021, 456, 71–84. [Google Scholar] [PubMed]
- Oda, S.; Funato, H. D1- and D2-type dopamine receptors are immunolocalized in pial and layer I astrocytes in the rat cerebral cortex. Front. Neuroanat. 2023, 17, 1111008. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Tyurikova, O.; Bard, L.; Zheng, K.; Semyanov, A.; Henneberger, C.; Rusakov, D.A. Dopamine elevates and lowers astroglial Ca(2+) through distinct pathways depending on local synaptic circuitry. Glia 2017, 65, 447–459. [Google Scholar] [CrossRef]
- Xin, W.; Schuebel, K.E.; Jair, K.W.; Cimbro, R.; De Biase, L.M.; Goldman, D.; Bonci, A. Ventral midbrain astrocytes display unique physiological features and sensitivity to dopamine D2 receptor signaling. Neuropsychopharmacology 2019, 44, 344–355. [Google Scholar]
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.; Loke, K.; Quintana, R.; Rothwell, P.E.; Lujan, R.; Marsicano, G.V.; et al. Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. Neuron 2020, 105, 1036–1047. [Google Scholar]
- Li, C.; Saliba, N.B.; Martin, H.; Losurdo, N.A.; Kolahdouzan, K.; Siddiqui, R.; Medeiros, D.; Li, W. Purkinje cell dopaminergic inputs to astrocytes regulate cerebellar-dependent behavior. Nat. Commun. 2023, 14, 1613. [Google Scholar]
- Gurevich, V.V.; Gurevich, E.V. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 2006, 110, 465–502. [Google Scholar] [PubMed]
- Komolov, K.E.; Benovic, J.L. G protein-coupled receptor kinases: Past, present and future. Cell. Signal. 2018, 41, 17–24. [Google Scholar] [PubMed]
- Pitcher, J.A.; Freedman, N.J.; Lefkowitz, R.J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998, 67, 653–692. [Google Scholar]
- Lohse, M.J.; Benovic, J.L.; Codina, J.; Caron, M.G.; Lefkowitz, R.J. beta-Arrestin: A protein that regulates beta-adrenergic receptor function. Science 1990, 248, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, L.M.; Lefkowitz, R.J. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002, 115, 455–465. [Google Scholar] [CrossRef]
- Hollinger, S.; Hepler, J.R. Cellular regulation of RGS proteins: Modulators and integrators of G protein signaling. Pharmacol. Rev. 2002, 34, 527–559. [Google Scholar] [CrossRef]
- Woodard, G.E.; Jardin, I.; Berna-Erro, A.; Salido, G.M.; Rosado, J.A. Regulators of G-protein-signaling proteins: Negative modulators of G-protein-coupled receptor signaling. Int. Rev. Cell Mol. Biol. 2015, 317, 97–183. [Google Scholar]
- Kovoor, A.; Seyffarth, P.; Ebert, J.; Barghshoon, S.; Chen, C.-K.; Schwarz, S.; Axelrod, J.D.; Cheyette, B.N.R.; Simon, M.I.; Lester, H.A.; et al. D2 dopamine receptors colocalize regulator of G-protein signaling 9-2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J. Neurosci. 2005, 25, 2157–2165. [Google Scholar] [CrossRef]
- Celver, J.; Sharma, M.; Kovoor, A. RGS9-2 mediates specific inhibition of agonist-induced internalization of D2-dopamine receptors. J. Neurochem. 2010, 114, 739–749. [Google Scholar] [CrossRef]
- Fuxe, K.; Canals, M.; Torvinen, M.; Marcellino, D.; Terasmaa, A.; Genedani, S.; Leo, G.; Guidolin, D.; Diaz-Cabiale, Z.; Rivera, A.; et al. Intramembrane receptor-receptor interactions: A novel principle in molecular medicine. J. Neural Transm. 2007, 114, 49–75. [Google Scholar]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Maura, G.; Agnati, L.F. Intercellular communication in the central nervous system as deduced by chemical neuroanatomy and quantitative analysis of images: Impact on neuropharmacology. Int. J. Mol. Sci. 2022, 23, 5805. [Google Scholar]
- Prezeau, L.; Rives, M.L.; Comps-Agrar, L.; Maurel, D.; Knlazeff, J.; Pin, J.P. Functional crosstalk between GPCRs: With or without oligomerization. Curr. Opin. Pharm. 2010, 10, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Fuxe, K.; Giardino, L.; Calzà, L.; Zoli, M.; Battistini, N.; Benfenati, F.; Vanderhaeghen, J.J.; Guidolin, D.; Ruggeri, M. Evidence for cholecystokinin-dopamine receptor interactions in the central nervous system of the adult and old rat. Studies on their functional meaning. Ann. N. Y. Acad. Sci. 1985, 448, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T.; Agnati, L.F.; Caron, M.; Fredholm, B.; Guidolin, D.; Kobilka, B.; Lefkowitz, R.W.; Lohse, M.; Woods, A.; Fuxe, K. International workshop at the Nobel Forum, Karolinska Institutet, on G protein-coupled receptors: Finding the words to describe monomers, oligomers, and their molecular mechanisms and defining their meaning. Can a consensus be reached? J. Recept. Signal Transduct. Res. 2010, 30, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Trifilieff, P.; Rives, M.L.; Urizar, E.; Piskorowski, R.A.; Vishwasrao, H.D.; Castrillon, J.; Schmauss, C.; Stätmann, M.; Gullberg, M.; Javitch, J.A. Detection of antigen interactions ex vivo by proximity ligation assay: Endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 2011, 51, 111–118. [Google Scholar] [CrossRef]
- Fernández-Dueñas, V.; Gòmez-Soler, M.; Valle-Leòn, M.; Watanabe, M.; Ferrer, I.; Ciruela, F. Revealing adenosine A2A-dopamine D2 receptor heteromers in Parkinson’s disease post-mortem brain through a new AlphaScreen-based approach. Int. J. Mol. Sci. 2019, 20, 3600. [Google Scholar] [CrossRef]
- Petazzi, R.A.; Aji, A.K.; Chiantia, S. Fluorescence microscopy methods for the study of protein oligomerization. In Progress in Molecular Biology and Translational Science; Giraldo, J., Ciruela, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 169, pp. 1–42. [Google Scholar]
- De Oliveira, P.; Moreno, E.; Casajuana-Martin, N.; Casadò-Anguera, V.; Cai, N.-S.; Camacho-Hernandez, G.A.; Zhu, H.; Bonifazi, A.; Hall, M.D.; Weinshenker, D.; et al. Preferential Gs protein coupling of the galanin Gal1 receptor in the μ-opioid-Gal1 receptor heterotetramer. Pharmacol. Res. 2022, 182, 106322. [Google Scholar] [CrossRef]
- Franco, R.; Martinez-Pinilla, E.; Lanciego, J.L.; Navarro, G. Basic pharmacological and structural evidence for class A G-protein-coupled receptor heteromerization. Front. Pharmacol. 2016, 7, 76. [Google Scholar]
- Changeux, J.P. The origins of allostery: From personal memories to material for the future. J. Mol. Biol. 2013, 425, 1396–1406. [Google Scholar]
- Kenakin, T.; Miller, I.J. Seven transmembrane receptors as shape shifting proteins: The impact of allosteric modulation and functional selectivity on new drug discovery. Pharm. Rev. 2010, 62, 265–304. [Google Scholar]
- Smith, N.J.; Milligan, G. Allostery of G protein-coupled receptors homo- and heteromers: Uncharted pharmacological landscapes. Pharm. Rev. 2010, 62, 701–725. [Google Scholar] [PubMed]
- Liu, J.; Nussinov, R. Allostery: An overview of its history, concepts, methods and applications. PLoS Comput. Biol. 2016, 12, e1004966. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Ciruela, F.; Woods, A.S.; Lluis, C.; Franco, R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007, 30, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Filizola, M.; Weinstein, H. The study of G-protein coupled receptor oligomerization with computational modeling and bioinformatics. FEBS J. 2005, 272, 2926–2938. [Google Scholar] [PubMed]
- Simpson, L.M.; Taddese, B.; Wall, I.D.; Reynolds, C.A. Bioinformatics and molecular modelling approaches to GPCR oligomerization. Curr. Opin. Pharm. 2010, 10, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Ciruela, F.; Genedani, S.; Guescini, M.; Tortorella, C.; Albertin, G.; Fuxe, K.; Agnati, L.F. Bioinformatics and mathematical modeling in the study of receptor-receptor interactions and receptor oligomerization. Focus on adenosine receptors. Biochim. Biophys. Acta 2011, 1808, 1267–1283. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Tarakanov, A.O.; Brito, I.; Fuxe, K. Glutamate heteroreceptor complexes in the brain. Pharmacol. Rep. 2018, 70, 936–950. [Google Scholar]
- Pinna, A.; Bonaventura, J.; Farré, D.; Sànchez, M.; Simola, N.; Mallol, J.; Lluís, C.; Costa, G.; Baqi, Y.; Müller, C.E.; et al. L-DOPA disrupts adenosine A(2A)-cannabinoid CB(1)-dopamine D(2) receptor heteromer cross-talk in the striatum of hemiparkinsonian rats: Biochemical and behavioral studies. Exp. Neurol. 2014, 253, 180–191. [Google Scholar] [CrossRef]
- Cabello, N.; Gandia, J.; Bertarelli, D.C.; Watanabe, M.; Lluis, C.; Franco, R.; Ferré, S.; Luján, R.; Ciruela, F. Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J. Neurochem. 2009, 109, 1497–1507. [Google Scholar] [CrossRef]
- Beggiato, S.; Tomasini, M.C.; Borelli, A.C.; Borroto-Escuela, D.O.; Fuxe, K.; Antonelli, T.; Tanganelli, S.; Ferraro, L. Functional role of striatal A2A, D2, and mGlu5 receptor interactions in regulating striatopallidal GABA neuronal transmission. J. Neurochem. 2016, 138, 254–264. [Google Scholar] [CrossRef]
- Farran, B. An update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol. Res. 2017, 117, 303–327. [Google Scholar] [PubMed]
- Gainetdinov, R.R.; Premont, R.T.; Bohn, L.M.; Lefkowitz, R.J.; Caron, M.G. Desensitization of G protein-coupled receptors and neural functions. Annu. Rev. Neurosci. 2004, 27, 107–144. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Rajagopal, S. The β-arrestin: Multifunctional regulators of G protein-coupled receptors. J. Biol. Chem. 2016, 291, 8969–8977. [Google Scholar] [PubMed]
- Agnati, L.F.; Leo, G.; Genedani, S.; Andreoli, N.; Marcellino, D.; Woods, A.; Piron, L.; Guidolin, D.; Fuxe, K. Structural plasticity in G-protein coupled receptors as demonstrated by the allosteric actions of homocysteine and computer-assisted analysis of disordered domains. Brain Res. Rev. 2008, 58, 459–474. [Google Scholar] [PubMed]
- Agnati, L.F.; Guidolin, D.; Vilardaga, J.P.; Ciruela, F.; Fuxe, K. On the expanding terminology in the GPCR field: The meaning of receptor mosaics and receptor heteromers. J. Recept. Signal Transduct. Res. 2010, 30, 287–303. [Google Scholar] [PubMed]
- Alemany, R.; Perona, J.S.; Sánchez-Dominguez, J.M.; Montero, E.; Cañizares, J.; Bressani, R.; Escribà, P.V.; Ruiz-Gutierrez, V.G. protein-coupled receptor systems and their lipid environment in health disorders during aging. Biochim. Biophys. Acta 2007, 1768, 964–975. [Google Scholar]
- Foord, S.M.; Jupe, S.; Holbrook, J. Bioinformatics and type II G-protein-coupled receptors. Biochem. Soc. Trans. 2002, 30, 473–479. [Google Scholar] [CrossRef]
- Whorton, M.R.; Bokoch, M.P.; Rasmussen, S.G.F.; Huang, B.; Zare, R.N.; Kobilka, B.; Sunahara, R.K. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. USA 2007, 104, 7682–7687. [Google Scholar]
- Gurevich, V.V.; Gurevich, E.V. How and why do GPCRs dimerize? Trends Pharmacol. Sci. 2008, 29, 234–240. [Google Scholar]
- Teichmann, A.; Gibert, A.; Lampe, A.; Grzesik, P.; Rutz, C.; Furkert, J.; Schmoranzer, J.; Krause, G.; Wiesner, B.; Schülein, R. The specific monomer/dimer equilibrium of the corticotropin-releasing factor receptor type 1 is established in the endoplasmic reticulum. J Biol. Chem. 2014, 289, 24250–24262. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Guidolin, D.; Woods, A.; Agnati, L.F. Dopamine Receptor Oligomerization. In The Dopamine Receptors; Neve, K.A., Ed.; Humana Press: Totowa, NJ, USA; Springer: Berlin/Heidelberg, Germany, 2010; pp. 255–280. [Google Scholar]
- Pelassa, S.; Guidolin, D.; Venturini, A.; Averna, M.; Frumento, G.; Campanini, L.; Bernardi, R.; Cortelli, P.; Buonaura, G.C.; Maura, G.; et al. A2A-D2 heteromers on striatal astrocytes: Biochemical and biophysical evidence. Int. J. Mol. Sci. 2019, 20, 2457. [Google Scholar] [CrossRef] [PubMed]
- Torvinen, M.; Marcellino, D.; Canals, M.; Agnati, L.F.; Lluis, C.; Franco, R.; Fuxe, K. Adenosine a2a receptor and dopamine d3 receptor interactions: Evidence of functional a2a/d3 heteromeric complexes. Mol. Pharmacol. 2005, 67, 400–407. [Google Scholar] [PubMed]
- Fuxe, K.; Borroto-Escuela, D.O. Receptor-Receptor Interactions in the Central Nervous System; Humana Press: New York, NY, USA, 2018; Volume 140, p. 346. [Google Scholar]
- Liu, X.Y.; Chu, X.P.; Mao, L.M.; Wang, M.; Lan, H.X.; Li, M.H.; Zhang, G.C.; Parelkar, N.K.; Fibuch, E.E.; Haines, M.; et al. Modulation of D2R–NR2B interactions in response to cocaine. Neuron 2006, 52, 897–909. [Google Scholar] [PubMed]
- Koschatzky, S.; Tschammer, N.; Gmeiner, P. Cross-receptor interactions between dopamine D2L and neurotensin NTS1 receptors modulate binding affinities of dopaminergics. ACS Chem. Neurosci. 2011, 2, 308–316. [Google Scholar]
- Plach, M.; Schäfer, T.; Borroto-Escuela, D.O.; Weickert, D.; Gmeiner, P.; Fuxe, K.; Friedland, K. Differential allosteric modulation within dopamine D2R-neurotensin NTS1R and D2R-serotonin 5-HT2AR receptor complexes gives bias to intracellular calcium signaling. Sci. Rep. 2019, 9, 16312. [Google Scholar]
- Przybyla, J.A.; Watts, V.J. Ligand-induced regulation and localization of cannabinoid CB1 and dopamine D2L receptor heterodimers. J. Pharmacol. Exp. Ther. 2010, 332, 710–719. [Google Scholar] [CrossRef]
- Kolasa, M.; Solich, J.; Faron-Górecka, A.; Żurawek, D.; Pabian, P.; Łukasiewicz, S.; Kuśmider, M.; Szafran-Pilch, K.; Szlachta, M.; Dziedzicka-Wasylewska, M. Paroxetine and Low-dose Risperidone Induce Serotonin 5-HT1A and Dopamine D2 Receptor Heteromerization in the Mouse Prefrontal Cortex. Neuroscience 2018, 377, 184–196. [Google Scholar]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Marcellino, D.; Ciruela, F.; Agnati, L.F.; Fuxe, K. Dopamine D2 and 5-hydroxytryptamine 5-HT2A receptors assemble into functionally interacting heteromers. Biochem. Biophys. Res. Commun. 2010, 401, 605–610. [Google Scholar] [CrossRef]
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor–receptor interactions. Mol. Psychiatry 2013, 18, 849–850. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Ceccoli, C.; Gatta, E.; Candiani, S.; Pedrazzi, M.; Capraro, M.; Maura, G.; Agnati, L.F.; et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2023, 24, 4677. [Google Scholar]
- Kern, A.; Albarran-Zeckler, R.; Walsh, H.E.; Smith, R.G. Apo-grelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron 2012, 73, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Becher, A.M.; Bonaventura, J.; Quiroz, C.; Sanchez-Soto, M.; Casadò-Anguera, V.; Cai, N.-S.; Moreno, E.; Boateng, C.A.; Keck, T.M.; et al. Functional and pharmacological role of the dopamine D4 receptor and its polymorphic variants. Front. Endocrinol. 2022, 13, 1014678. [Google Scholar]
- Rebois, R.V.; Maki, K.; Meeks, J.A.; Fishman, P.H.; Hébert, T.E.; Northup, J.K. D2-like dopamine and β-adrenergic receptors form a signaling complex that integrates Gs- and Gi-mediated regulation of adenylyl cyclase. Cell. Signal. 2012, 24, 2051–2060. [Google Scholar]
- Gago, B.; Fuxe, K.; Agnati, L.; Penafiel, A.; De La Calle, A.; Rivera, A. Dopamine D(4) receptor activation decreases the expression of mu-opioid receptors in the rat striatum. J. Comp. Neurol. 2007, 502, 358–366. [Google Scholar] [PubMed]
- Petkova-Kirova, P.; Giovannini, M.G.; Kalfin, R.; Rakovska, A. Modulation of acetylcholine release by cholecystokinin in striatum: Receptor specificity; role of dopaminergic neuronal activity. Brain Res. Bull. 2012, 89, 177–184. [Google Scholar]
- Pittolo, S.; Yokoyama, S.; Willoughby, D.D.; Taylor, C.R.; Reitman, M.E.; Tse, V.; Wu, Z.; Etchenique, R.; Li, Y.; Poskanzer, K.E. Dopamine activates astrocytes in prefrontal cortex via α1-adrenergic receptors. Cell Rep. 2022, 40, 111426. [Google Scholar]
- Pinton, L.; Borroto-Escuela, D.O.; Narváez, M.; Oflijan, J.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2R and Sigma 1 allosteric receptor–receptor interaction in the rat brain: Role in brain plasticity and cocaine action. Springerplus 2015, 4, P37. [Google Scholar] [CrossRef]
- Rashid, A.J.; So, C.H.; Kong, M.M.C.; Furtak, T.; El-Ghundi, M.; Cheng, R.; O’Dowd, B.F.; George, S.R. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. USA 2007, 104, 654–659. [Google Scholar] [CrossRef]
- Marcellino, D.; Ferré, S.; Casado, V.; Cortés, A.; Le Foll, B.; Mazzola, C.; Drago, F.; Saur, O.; Stark, H.; Soriano, A.; et al. Identification of dopamine D1-D3 receptor heteromers: Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem. 2008, 283, 26016–26025. [Google Scholar]
- Ginés, S.; Hillion, J.; Torvinen, M.; Le Crom, S.; Casadó, V.; Canela, E.I.; Rondin, S.; Lew, J.Y.; Watson, S.; Zoli, M.; et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 8606–8611. [Google Scholar] [CrossRef]
- Franco, R.; Lluis, C.; Canela, E.I.; Mallol, J.; Agnati, L.; Casadó, V.; Ciruela, F.; Ferré, S.; Fuxe, K. Receptor-receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. J. Neural Transm. 2007, 114, 93–104. [Google Scholar] [PubMed]
- Lee, F.J.; Xue, S.; Pei, L.; Vukusic, B.; Chéry, N.; Wang, Y.; Wang, Y.T.; Niznik, H.B.; Yu, X.-M.; Liu, F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell 2002, 111, 219–230. [Google Scholar] [PubMed]
- Mitrano, D.A.; Pare, J.F.; Smith, Y.; Weinshenker, D. D1-dopamine and a1-adrenergic receptors co-localize in dendrites of the rat prefrontal cortex. Neuroscience 2014, 258, 90–100. [Google Scholar]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. Adenosine A2A-Dopamine D2 Receptor-Receptor Interaction in Neurons and Astrocytes: Evidence and Perspectives. Prog. Mol. Biol. Transl. Sci. 2020, 169, 247–277. [Google Scholar] [PubMed]
- Ciruela, F.; Burgueno, J.; Casado, V.; Canals, M.; Marcellino, D.; Goldberg, S.R.; Bader, M.; Fuxe, K.; Agnati, L.F.; Lluis, C.; et al. Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electro- static interactions between adenosine A2A and dopamine D2 receptors. Anal. Chem. 2004, 76, 5354–5363. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.S.; Ciruela, F.; Fuxe, K.; Agnati, L.F.; Lluis, C.; Franco, R.; Ferré, S. Role of electrostatic interaction in receptor–receptor heteromerization. J. Mol. Neurosci. 2005, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Rodriguez, D.; Romero-Fernandez, W.; Kapla, J.; Jaiteh, M.; Ranganathan, A.; Lazarova, T.; Fuxe, K.; Carlsson, J. Mapping the interface of a GPCR dimer: A structural model of the A2A adenosine and D2 dopamine receptor heteromer. Front. Pharmacol. 2018, 9, 829. [Google Scholar]
- Ferré, S.; von Euler, G.; Johansson, B.; Fredholm, B.B.; Fuxe, K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc. Natl. Acad. Sci. USA 1991, 88, 7238–7241. [Google Scholar] [CrossRef]
- Fuxe, K.; Ferré, S.; Zoli, M.; Agnati, L.F. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res. Rev. 1998, 26, 258–273. [Google Scholar] [CrossRef]
- Diaz-Cabiale, Z.; Hurd, Y.; Guidolin, D.; Finnman, U.B.; Zoli, M.; Agnati, L.F.; Vanderhaeghen, J.J.; Fuxe, K.; Ferré, S. Adenosine A2A agonist CGS 21680 decreases the affinity of dopamine D2 receptors for dopamine in human striatum. Neuroreport 2001, 12, 1831–1834. [Google Scholar] [CrossRef]
- Cervetto, C.; Venturini, A.; Passalacqua, M.; Guidolin, D.; Genedani, S.; Fuxe, K.; Borroto- Escuela, D.O.; Cortelli, P.; Woods, A.; Maura, G.; et al. A2A-D2 receptor- receptor interaction modulates gliotransmitter release from striatal astrocyte processes. J. Neurochem. 2016, 140, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.; Romero-Fernandez, W.; Tarakanov, A.; Ciruela, F.; Agnati, L.; Fuxe, K. On the existence of a possible A2A-D2-beta- arrestin2 complex: A2A agonist modulation of D2 agonist-induced beta-arrestin2 recruitment. J. Mol. Biol. 2011, 406, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Ferré, S.; Genedani, S.; Leo, G.; Guidolin, D.; Filaferro, M.; Carriba, P.; Casado, V.; Lluis, C.; Franco, R.; et al. Allosteric modulation of dopamine D2 receptors by homocysteine. J. Proteome Res. 2006, 5, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 receptor-receptor interaction at striatal astrocyte processes. J. Mol. Neurosci. 2018, 65, 456–466. [Google Scholar] [PubMed]
- Borgkvist, A.; Marcellino, D.; Fuxe, K.; Greengard, P.; Fisone, G. Regulation of DARPP-32 phosphorylation by Delta(9)-tetrahydrocannabinol. Neuropharmacology 2008, 54, 31–35. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Błasiak, E.; Szafran-Pilch, K.; Dziedzicka-Wasylewska, M. Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics—In Vitro studies. J Neurochem. 2016, 137, 549–560. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Rivera, A.; Diaz-cabiale, Z.; Filip, M.; Gago, B.; Roberts, D.C.S.; Lange, U.; Genedani, S.; Ferraro, L.; et al. Receptor-receptor interactions within receptor mosaics. Impact on neuropharmacology. Brain Res. Rev. 2008, 58, 415–452. [Google Scholar]
- Ferré, S.; Sarasola, L.I.; Quiroz, C.; Ciruela, F. Presynaptic adenosine receptor heteromers as key modulators of glutamatergic and dopaminergic neurotransmission in the striatum. Neuropharmacology 2023, 223, 109329. [Google Scholar]
- Liu, F.; Wan, Q.; Pristupa, Z.B.; Yu, X.M.; Wang, Y.T.; Niznik, H.B. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature 2000, 403, 274–280. [Google Scholar] [CrossRef]
- McNeill, J.; Rudyk, C.; Hildebrand, M.E.; Salmaso, N. Ion channels and electrophysiological properties of astrocytes: Implications for emergent stimulation technologies. Front. Cell. Neurosci. 2021, 15, 644126. [Google Scholar]
- Fitzner, D.; Bader, J.M.; Penkert, H.; Bergner, C.G.; Su, M.; Weil, M.-T.; Surma, M.A.; Mann, M.; Klose, C.; Simons, M. Cell-type- and brain-region-resolved mouse brain lipidome. Cell Rep. 2020, 32, 108132. [Google Scholar] [PubMed]
- Lee, J.; Patel, D.S.; Ståhle, J.; Park, S.-J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI Membrane Builder for Complex Biological Membrane Simulations with glycolipids and lipoglycans. Chem. Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Kuriata, A.; Gierut, A.M.; Oleniecki, T.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. CABS-Flex 2.0: A Web Server for fast simulations of flexibility of protein structures. Nucleic Acids Res. 2018, 46, W338–W343. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Carlsson, J.; Ambrogini, P.; Narvàez, M.; Wydra, K.; Tarakanov, A.D.; Li, X.; Millòn, C.; Ferraro, L.; Cuppini, R.; et al. Understanding the role of GPCR heteroreceptor complexes in modulating the brain networks in health and disease. Front. Cell. Neurosci. 2017, 11, 37. [Google Scholar] [PubMed]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor–Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors. Int. J. Mol. Sci. 2021, 22, 8656. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.; Hasbi, A.; O’Dowd, B.F.; George, S.R. heteromeric dopamine receptor signaling complexes: Emerging neurobiology and disease relevance. Neuropsychopharmacology 2014, 39, 156–168. [Google Scholar]
- Lindvall, O.; Björklund, A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol. Scand. 1974, 412, 1–48. [Google Scholar]
- Gerfen, C.R. The neostriatal mosaic: Multiple levels of compartmental organization. Trends Neurosci. 1992, 15, 133–139. [Google Scholar]
- Liu, Y.-J.; Chen, J.; Li, X.; Zhou, X.; Hu, Y.-M.; Chu, S.-F.; Peng, Y.; Chen, N.-H. Research progress on adenosine in central nervous system diseases. CNS Neurosci. Ther. 2019, 25, 899–910. [Google Scholar]
- Ferré, S.; Popoli, P.; Gimenez-Llort, L.; Finnman, U.B.; Martinez, E.; Scotti de Carolis, A.; Fuxe, K. Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport 1994, 6, 73–76. [Google Scholar] [CrossRef]
- Emmi, A.; Antonini, A.; Sandre, M.; Baldo, A.; Contran, M.; Macchi, V.; Guidolin, D.; Porzionato, A.; De Caro, R. Topography and distribution of adenosine A2A and dopamine D2 receptors in the human subthalamic nucleus. Front. Neurosci. 2022, 16, 945574. [Google Scholar] [CrossRef] [PubMed]
- Cotzias, G.C. Levodopa in the treatment of Parkinsonism. JAMA 1971, 218, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.D. Problems with long-term levodopa therapy for Parkinson’s disease. Clin. Neuropharmacol. 1994, 17, S32–S544. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Guidolin, D.; Agnati, L.F.; Borroto-Escuela, D.O. Dopamine heteroreceptor complexes as therapeutic targets in Parkinson’s disease. Exp. Opin. Ther. Targets 2015, 19, 377–398. [Google Scholar]
- Chen, J.F.; Cunha, R.A. The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal. 2020, 16, 167–174. [Google Scholar]
- Marcellino, D.; Carriba, P.; Filip, M.; Borgkvist, A.; Frankowska, M.; Bellido, I.; Tanganelli, S.; Muller, C.E.; Roberts, D.C.; Fisone, G.; et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioural analysis. Neuropharmacology 2008, 54, 815–828. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Mechoulam, R.; Offen, D. The CB1 cannabinoid receptor agonist, HU-210, reduces levodopa-induced rotations in 6-hydroxydopamine-lesioned rats. Pharmacol. Toxicol. 2003, 93, 66–70. [Google Scholar] [CrossRef]
- Haber, S.N. Neuroanatomy of reward: A view from the ventral striatum. In Neurobiology of Sensation and Reward; Gottfried, J.A., Ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Fuxe, K.; Dahlstrom, A.; Jonsson, G.; Marcellino, D.; Guescini, M.; Dam, M.; Manger, P.; Agnati, L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 2010, 90, 82–100. [Google Scholar]
- Borroto-Escuela, D.O.; Ferraro, L.; Narvaez, M.; Tanganelli, S.; Beggiato, S.; Liu, F.; Rvera, A.; Fuxe, K. Multiple adenosine-dopamine (A2A-D2 like) heteroreceptor complexes in the brain and their role in schizophrenia. Cells 2020, 9, 1077. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Pintsuk, J.; Schäfer, T.; Friedland, K.; Ferraro, L.; Tanganelli, S.; Liu, F.; Fuxe, K. Multiple D2 heteroreceptor complexes: New targets for treatment of schizophrenia. Ther. Adv. Psychopharmacol. 2016, 6, 77–94. [Google Scholar] [CrossRef]
- Edwards, S.; Whisler, K.N.; Fuller, D.C.; Orsulak, P.J.; Self, D.W. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology 2007, 32, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Briand, L.A.; Flagel, S.B.; Garcia-Fuster, M.J.; Watson, S.J.; Akil, H.; Sarter, M.; Robinson, T.E. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 2008, 33, 2969–2980. [Google Scholar] [PubMed]
- Wydra, K.; Suder, A.; Borroto-Escuela, D.O.; Filip, M.; Fuxe, K. On the role of A(2)A and D(2) receptors in control of cocaine and food-seeking behaviors in rats. Psychopharmacology 2015, 232, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Romieu, P.; Phan, V.L.; Martin-Fardon, R.; Maurice, T. Involvement of the sigma1 receptor in cocaine-induced conditioned place preference: Possible dependence on dopamine uptake blockade. Neuropsychopharmacology 2002, 26, 444–455. [Google Scholar] [CrossRef]
- Canales, J.J. Stimulant-induced adaptations in neostriatal matrix and striosome systems: Transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol. Learn. Mem. 2005, 83, 93–103. [Google Scholar] [CrossRef]
- Seeman, P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin. Ther. Targets 2006, 10, 515–531. [Google Scholar] [CrossRef]
- Carlsson, A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1988, 1, 179–186. [Google Scholar] [CrossRef]
- Andersen, M.B.; Fuxe, K.; Werge, T.; Gerlach, J. The adenosine A2A receptor agonist CGS 21680 exhibits antipsychotic-like activity in Cebus apella monkeys. Behav. Pharmacol. 2022, 13, 639–644. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Stephens, S.L.; Young, W.S. III. Oxytocin as a natural antipsychotic: A study using oxytocin knockout mice. Mol. Psychiatry 2009, 14, 190–196. [Google Scholar] [CrossRef]
- Feifel, D. Oxytocin as a potential therapeutic target for schizophrenia and other neuropsychiatric conditions. Neuropsychopharmacology 2012, 37, 304–305. [Google Scholar] [CrossRef]
- Hökfelt, T.; Fuxe, K.; Johansson, O.; Ljungdahl, Å. Pharmaco-histochemical evidence of the existence of dopamine nerve terminals in the limbic cortex. Eur. J. Pharmacol. 1974, 25, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.; Gaspar, P.; Verney, C. Dopaminergic innervation of the cerebral cortex: Unexpected differences between rodents and primates. Trends Neurosci. 1991, 14, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Malenka, E.J.; Nestler, S.E.; Hyman, R.C. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, 2nd ed.; McGraw-Hill Medical: New York, NY, USA, 2009; Chapter 13; p. 318. [Google Scholar]
- Casadó-Anguera, V.; Moreno, E.; Sánchez-Soto, M.; Cai, N.S.; Bonaventura, J.; Homar-Ruano, P.; Rubinstein, M.; Cortés, A.; Canela, E.I.; Ferré, S.; et al. Heteromerization between a2A adrenoceptors and different polymorphic variants of the dopamine D4 receptor determines pharmacological and functional differences. implications for impulsive-control disorders. Pharmacol. Res. 2021, 170, 105745. [Google Scholar] [CrossRef] [PubMed]
- LaHoste, G.J.; Swanson, J.M.; Wigal, S.B.; Glabe, C.; Wigal, T.; King, N.; Kennedy, J. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol. Psychiatry 1996, 1, 121–124. [Google Scholar]
- Sancho, L.; Contreras, M.; Allen, N.J. Glia as sculptors of synaptic plasticity. Neurosci. Res. 2021, 167, 17–29. [Google Scholar]
- Allen, N.J.; Barres, B.A. Signaling between glia and neurons: Focus on synaptic plasticity. Curr. Opin. Neurobiol. 2005, 15, 542–548. [Google Scholar] [CrossRef]
- Fellin, T.; Carmignoto, G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J. Physiol. 2004, 559, 3–15. [Google Scholar]
- Cervetto, C.; Maura, G.; Guidolin, D.; Amato, S.; Ceccoli, C.; Agnati, L.F.; Marcoli, M. Striatal astrocytic A2A-D2 receptor-receptor interactions and their role in neuropsychiatric disorders. Neuropharmacology 2023, 237, 109636. [Google Scholar]
- Ren, C.; He, K.J.; Hu, H.; Zhang, J.B.; Dong, L.G.; Li, D.; Chen, J.; Mao, C.J.; Wang, F.; Liu, C.F. Induction of parkinsonian-like changes via targeted downregulation of astrocytic glutamate transporter GLT-1 in the striatum. J. Park. Dis. 2022, 12, 295–314. [Google Scholar]
- Martín, R.; Bajo-Grañeras, R.; Moratalla, R.; Perea, G.; Araque, A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 2015, 349, 730–734. [Google Scholar] [CrossRef]
- Huang, G.; Dragan, M.; Freeman, D.; Wilson, J.X. Activation of catechol-O-methyltransferase in astrocytes stimulates homocysteine synthesis and export to neurons. Glia 2005, 51, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Shen, H.Y.; Augusto, E.; Wang, Y.; Wei, C.J.; Wang, Y.T.; Agostinho, P.; Boison, D.; Cunha, R.A.; Chen, J.F. Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: Relevance to schizophrenia. Biol. Psychiatr. 2015, 78, 763–774. [Google Scholar]
- Kruyer, A.; Kalivas, P.W.; Scofield, M.D. Astrocyte regulation of synaptic signaling in psychiatric disorders. Neuropsychopharmacology 2023, 48, 21–36. [Google Scholar] [PubMed]
- Berretta, S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology 2012, 62, 1584–1597. [Google Scholar] [PubMed]
- Wardas, J. Potential role of adenosine A2A receptors in the treatment of schizophrenia. Front. Biosci. 2008, 13, 4071–4096. [Google Scholar] [CrossRef]
- Valle-Léon, M.; Casajuana-Martin, N.; del Torrent, C.L.; Argerich, J.; Gòmez-Acero, L.; Sahlholm, K.; Ferré, S.; Pardo, L.; Ciruela, F. Unique effect of clozapine on adenosine A2A-dopamine D2 receptor heteromerization. Biomed. Pharmacother. 2023, 160, 114327. [Google Scholar]
- Kruyer, A.; Kalivas, P.W. Astrocytes as cellular mediators of cue reactivity in addiction. Curr. Opin. Pharmacol. 2021, 56, 1–6. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990, 13, 266–271. [Google Scholar]
- Daniels, D.J.; Lenard, N.R.; Etienne, C.L.; Law, P.-Y.; Roerig, S.C.; Portoghese, P.S. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. USA 2005, 102, 19208–19213. [Google Scholar] [CrossRef]

| Pathway | Description | Functional Features |
|---|---|---|
| Nigro-striatal | From the substantia nigra pars compacta to the dorsal striatum | Motor control |
| Mesolimbic | From the ventral tegmental area to the ventral striatum | Reward-/aversion-related cognition |
| Mesocortical | From the ventral tegmental area to the prefrontal cortex | Executive functions |
| Tubero-infundibular | From the hypothalamus to the pituitary gland | Regulation of prolactin secretion |
| Incerto-hypothalamic | From the zona incerta to the hypothalamus | Visceral and sensorimotor activities |
| Hypothalamo-spinal | From the hypothalamus to the spinal cord | Modulation of locomotor networks |
| Receptor Complex | Cell Location | Reference |
|---|---|---|
| A2A-D2 | Neurons Astrocytes | [68] [96] |
| A2A-D3 | Neurons | [97] |
| A2A-D4 | Neurons | [98] |
| NMDA-D2 | Neurons | [99] |
| NTS1-D2 | Neurons | [100,101] |
| CB1-D2 | Neurons | [102] |
| D2-5HT1 | Astrocytes | [103] |
| D2-5HT2A | Neurons | [101,104] |
| D2-OTR | Neurons Astrocytes | [105] [106] |
| D2-GHS1A | Neurons | [107] |
| D2-D4 | Neurons | [108] |
| α2A-D4 | Neurons | [108] |
| β2-D4 | Neurons | [109] |
| D4-MOR | Neurons | [110] |
| CCK2-D2 (putative) | Neurons | [66,111] |
| α1-D2 (putative) | Astrocytes | [112] |
| A2A-D2-sigma1 | Neurons | [113] |
| A2A-D2-mGluR5 | Neurons | [84] |
| A2A-D2-CB1 | Neurons | [82] |
| D1-D2 | Neurons | [114] |
| D1-D3 | Neurons | [115] |
| A1-D1 | Neurons | [116,117] |
| NMDA-D1 | Neurons | [118] |
| GABAA-D5 | Neurons | [119] |
| α1-D1 (putative) | Astrocytes | [112] |
| DA Pathway | Receptor Complexes | Type of Interaction | Location | Major Pathologies |
|---|---|---|---|---|
| Nigro-striatal | A2A-D2 | Antagonistic | Dorsal striatum | PD |
| CB1-D2 | Antagonistic | |||
| NMDA-D2 | Antagonistic | |||
| A2A-D2-CB1 | Antagonistic | |||
| A2A-D2-mGluR5 | Antagonistic | |||
| A1-D1 | Antagonistic | |||
| D1-D3 | Synergistic | |||
| Mesolimbic | A2A-D2 | Antagonistic | Ventral striatum | Addiction Schizophrenia |
| A2A-D3 | Antagonistic | |||
| NMDA-D2 | Antagonistic | |||
| NMDA-D1 | Antagonistic | |||
| NTS1-D2 | Antagonistic | |||
| D2-5HT2A | Synergistic | |||
| D2-OTR | Synergistic | |||
| D1-D2 | Signaling cascade change | |||
| D1-D3 | Synergistic | |||
| A2A-D2-sigma1 | Antagonistic | |||
| A2A-D2-mGluR5 | Antagonistic | |||
| GABAA-D5 | Antagonistic | |||
| D4-MOR | Synergistic | |||
| Mesocortical | α2A-D4 | Dependent on D4 polymorphism | Prefrontal cortex | ADHD |
| β2-D4 | Signaling cascade change | |||
| D2-D4 | Dependent on D4 polymorphism | |||
| A2A-D4 | Antagonistic | |||
| Neuron–Astrocyte crosstalk | A2A-D2 | Antagonistic | Astrocytes | PD Addiction Schizophrenia |
| D2-OTR | Synergistic | |||
| D2-5HT1 | Not detailed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; De Caro, R.; Maura, G.; Agnati, L.F. Modulation of Neuron and Astrocyte Dopamine Receptors via Receptor–Receptor Interactions. Pharmaceuticals 2023, 16, 1427. https://doi.org/10.3390/ph16101427
Guidolin D, Tortorella C, Marcoli M, Cervetto C, De Caro R, Maura G, Agnati LF. Modulation of Neuron and Astrocyte Dopamine Receptors via Receptor–Receptor Interactions. Pharmaceuticals. 2023; 16(10):1427. https://doi.org/10.3390/ph16101427
Chicago/Turabian StyleGuidolin, Diego, Cinzia Tortorella, Manuela Marcoli, Chiara Cervetto, Raffaele De Caro, Guido Maura, and Luigi F. Agnati. 2023. "Modulation of Neuron and Astrocyte Dopamine Receptors via Receptor–Receptor Interactions" Pharmaceuticals 16, no. 10: 1427. https://doi.org/10.3390/ph16101427
APA StyleGuidolin, D., Tortorella, C., Marcoli, M., Cervetto, C., De Caro, R., Maura, G., & Agnati, L. F. (2023). Modulation of Neuron and Astrocyte Dopamine Receptors via Receptor–Receptor Interactions. Pharmaceuticals, 16(10), 1427. https://doi.org/10.3390/ph16101427








