Medicinal Plants Used for Eye Conditions in Mexico—A Review
Abstract
:1. Introduction
2. Results
2.1. Ethnobotanical Information of Medicinal Plants Used for Eye Conditions in Mexico
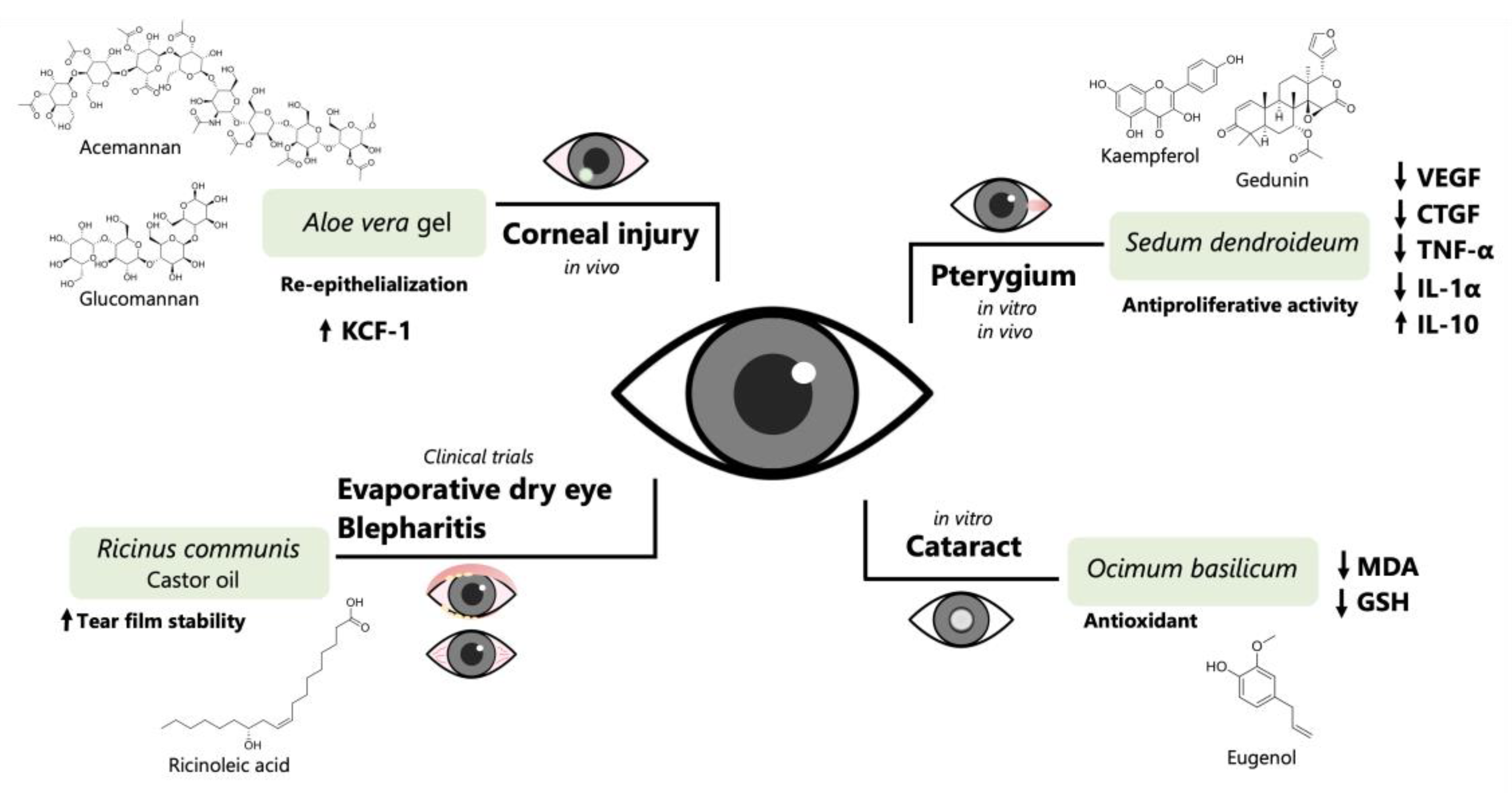
2.2. Pharmacological Efficacy and Safety Studies
3. Future Perspectives
4. Limitations of the Study
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Report on Vision; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Eze, B.I.; Chuka-Okosa, C.M.; Uche, J.N. Traditional Eye Medicine Use by Newly Presenting Ophthalmic Patients to a Teaching Hospital in South-Eastern Nigeria: Socio-Demographic and Clinical Correlates. BMC Complement. Altern. Med. 2009, 9, 40. [Google Scholar] [CrossRef]
- Gupta, N.; Vashist, P.; Tandon, R.; Gupta, S.K.; Kalaivani, M.; Dwivedi, S.N. Use of Traditional Eye Medicine and Self-Medication in Rural India: A Population-Based Study. PLoS ONE 2017, 12, e0183461. [Google Scholar] [CrossRef]
- Eticha, B.L.; Alemu, H.W.; Assaye, A.K.; Tilahun, M.M. Attitude towards Traditional Eye Medicine and Associated Factors among Adult Ophthalmic Patients Attending University of Gondar Comprehensive Specialized Hospital-Tertiary Eye Care and Training Center, Northwest Ethiopia. Clin. Optom. 2021, 13, 323–332. [Google Scholar] [CrossRef]
- Sandhu, P.S.; Singh, B.; Gupta, V.; Bansal, P.; Kumar, D. Potential Herbs Used in Ocular Diseases. J. Pharm. Sci. Res. 2011, 3, 1127. [Google Scholar]
- Calvo, M.I.; Cavero, R.Y. Medicinal Plants Used for Ophthalmological Problems in Navarra (Spain). J. Ethnopharmacol. 2016, 190, 212–218. [Google Scholar] [CrossRef]
- Shayanfar, J.; Ghasemi, H.; Esmaili, S.S.; Alijaniha, F.; Davati, A. Useful Medicinal Plants for Vision Impairment in Traditional Iranian Medicine. Galen Med. J. 2019, 8, e1285. [Google Scholar] [CrossRef]
- Graue y Díaz González, E. La Oftalmología Entre Los Indígenas del Anáhuac en la Época Prehispánica. Rev. Cult. Nuestra Am. 2018, 25, 100. [Google Scholar]
- Peralta Rodríguez, J.R. Eye Diseases and Their Treatment in the Novohispanic Population of México City. 16 Th and 17 Th Centuries. Secuencia 2008, 70, 11–44. [Google Scholar]
- Cordero-Galindo, E. La Materia Médica Mexicana En La Obra de Alonso López de Hinojosos. Cir. Cir. 1996, 64, 54–57. [Google Scholar]
- Camou-Guerrero, A.; Casas, A.; Moreno-Calles, A.I.; Aguilera-Lara, J.; Garrido-Rojas, D.; Rangel-Landa, S.; Torres, I.; Pérez-Negrón, E.; Solís, L.; Blancas, J. Ethnobotany in Mexico: History, Development, and Perspectives. In Ethnobotany of Mexico: Interactions of People and Plants in Mesoamerica; Springer: New York, NY, USA, 2016; pp. 21–39. [Google Scholar]
- Estrada-Castillón, E.; Villarreal-Quintanilla, J.Á.; Encina-Domínguez, J.A.; Jurado-Ybarra, E.; Cuéllar-Rodríguez, L.G.; Garza-Zambrano, P.; Arévalo-Sierra, J.R.; Cantú-Ayala, C.M.; Himmelsbach, W.; Salinas-Rodríguez, M.M. Ethnobotanical Biocultural Diversity by Rural Communities in the Cuatrociénegas Valley, Coahuila; Mexico. J. Ethnobiol. Ethnomed. 2021, 17, 21. [Google Scholar] [CrossRef]
- Medrano-Guerrero, A.; Carranza, E.; Juárez-Vázquez, M.C.; Solano, E.; Ruiz-Padilla, A.J.; Ruiz-Noa, Y.; Deveze-Alvarez, M.A.; Brennan-Bourdon, L.M.; Alonso-Castro, A.J. Medicinal Plants Used in Rural Communities from the Municipality of Dolores Hidalgo, Guanajuato, Mexico. Bol. Latinoam. Caribe Plantas Med. Aromáticas 2023, 22, 524–536. [Google Scholar]
- Azam, M.N.K.; Biswas, S.; Ahmed, M.N. A Cross-Sectional Study of Ethnopharmacology in the Noakhali District of Bangladesh and Exploring Potential Ocular Immunostimulatory Activity of the Medicinal Plants for the Treatment of Eye Infections. PharmacologyOnline 2015, 1, 77–89. [Google Scholar]
- Kashanipour, R.A.; McGee, R.J. Northern Lacandon Maya Medicinal Plant Use in the Communities of Lacanja Chan Sayab and Nahá Chiapas, Mexico. J. Ecol. Anthropol. 2004, 8, 47–66. [Google Scholar] [CrossRef]
- White-Olascoaga, L.; Juan-Pérez, J.I.; Chávez-Mejía, C.; Gutiérrez-Cedillo, J.G. Flora Medicinal En San Nicolás, Municipio de Malinalco, Estado de México. Polibotánica 2013, 35, 173–206. [Google Scholar]
- Hopkins, A. Use of Network Centrality Measures to Explain Individual Levels of Herbal Remedy Cultural Competence among the Yucatec Maya in Tabi, Mexico. Field methods 2011, 23, 307–328. [Google Scholar] [CrossRef]
- Lara, E.A.; Fernández, E.; del Valle, J.M.Z.; Lara, D.J.; Aguilarq, A.; Van Damme, P. Etnomedicina En Los Altos de Chiapas, México. Bol. Latinoam. Caribe Plantas Med. Aromáticas 2019, 18, 42–57. [Google Scholar] [CrossRef]
- Magaña Alejandro, M.A.; Gama Campillo, L.M.; Mariaca Méndez, R. El Uso de Las Plantas Medicinales En Las Comunidades Maya-Chontales de Nacajuca, Tabasco, México. Polibotánica 2010, 29, 213–262. [Google Scholar]
- Alfaro, M.A.M. Medicinal Plants Used in a Totonac Community of the Sierra Norte de Puebla: Tuzamapan de Gale Ana, Puebla, Mexico. J. Ethnopharmacol. 1984, 11, 203–221. [Google Scholar] [CrossRef]
- Lara Reimers, E.A.; Lara Reimers, D.J.; Chaloupkova, P.; Zepeda del Valle, J.M.; Milella, L.; Russo, D. An Ethnobotanical Survey of Medicinal Plants Used in Papantla, Veracruz, Mexico. Plants 2019, 8, 246. [Google Scholar] [CrossRef]
- Camacho-Hernández, C.; Lagunez-Rivera, L.; Aguilar-Contreras, A.; Solano, R. Ethnobotany of Medicinal Flora in Two Communities of the Mixteca Alta in Oaxaca, Mexico. Bot. Sci. 2022, 100, 912–934. [Google Scholar] [CrossRef]
- Vargas-Vizuet, A.L.; Lobato-Tapia, C.A.; Tobar-Reyes, J.R.; Solano-De la Cruz, M.T.; Marínez, A.I.; Fernández, A.R. Medicinal Plants Used in the Region of Teziutlán, Puebla, Mexico. Bol. Latinoam. Caribe Plantas Med. Aromáticas 2022, 21, 224–241. [Google Scholar] [CrossRef]
- Ortiz Palacios, L.; Cervantes Gutiérrez, V.; Chimal Hernandez, A. Plantas Medicinales de San Francisco Tlaltenco, Tláhuac, 1st ed.; Universidad Autónoma Metropolitana: Mexico City, Mexico, 2017; ISBN 978-607-28-1278-9. [Google Scholar]
- Canales, M.; Hernández, T.; Caballero, J.; De Vivar, A.R.; Avila, G.; Duran, A.; Lira, R. Informant Consensus Factor and Antibacterial Activity of the Medicinal Plants Used by the People of San Rafael Coxcatlán, Puebla, México. J. Ethnopharmacol. 2005, 97, 429–439. [Google Scholar] [CrossRef]
- Estrada-Castillón, E.; Villarreal-Quintanilla, J.Á.; Rodríguez-Salinas, M.M.; Encinas-Domínguez, J.A.; González-Rodríguez, H.; Figueroa, G.R.; Arévalo, J.R. Ethnobotanical Survey of Useful Species in Bustamante, Nuevo León, Mexico. Hum. Ecol. 2018, 46, 117–132. [Google Scholar] [CrossRef]
- del Carmen Juárez-Vázquez, M.; Carranza-Álvarez, C.; Alonso-Castro, A.J.; González-Alcaraz, V.F.; Bravo-Acevedo, E.; Chamarro-Tinajero, F.J.; Solano, E. Ethnobotany of Medicinal Plants Used in Xalpatlahuac, Guerrero, México. J. Ethnopharmacol. 2013, 148, 521–527. [Google Scholar] [CrossRef]
- Nicholson, M.S.; Arzeni, C.B. Las Plantas Medicinales de Los Mercados de Monterrey, Nuevo León, México. Econ. Bot. 1993, 47, 184–192. [Google Scholar] [CrossRef]
- Reimers, E.; Cusimamani, E.; Rodriguez, E.; Zepeda del Valle, J.; Polesny, Z.; Pawera, L. An Ethnobotanical Study of Medicinal Plants Used in Zacatecas State, Mexico. Acta Soc. Bot. Pol. 2018, 87, 1–15. [Google Scholar] [CrossRef]
- García-Hernández, K.Y.; Vibrans, H.; Rivas-Guevara, M.; Aguilar-Contreras, A. This Plant Treats That Illness? The Hot–Cold System and Therapeutic Procedures Mediate Medicinal Plant Use in San Miguel Tulancingo, Oaxaca, Mexico. J. Ethnopharmacol. 2015, 163, 12–30. [Google Scholar] [CrossRef]
- Aguilar, M.G.F.; Hernández, V.H.; Mull, J.G.C. Plantas Útiles en el Cerro del Cubilete, Silao, Guanajuato. Jóvenes Cienc. 2018, 4, 32–36. [Google Scholar]
- Alvarez-Quiroz, V.; Caso-Barrera, L.; Aliphat-Fernández, M.; Galmiche-Tejeda, Á. Plantas Medicinales Con Propiedades Frías y Calientes en la Cultura Zoque de Ayapa, Tabasco, México. Bol. Latinoam. Caribe Plantas Med. Aromáticas 2017, 16, 428–454. [Google Scholar]
- Zamora-Martínez, M.C.; de Pascual Pola, C.N. Medicinal Plants Used in Some Rural Populations of Oaxaca, Puebla and Veracruz, Mexico. J. Ethnopharmacol. 1992, 35, 229–257. [Google Scholar] [CrossRef] [PubMed]
- Orantes-García, C.; Moreno-Moreno, R.A.; Caballero-Roque, A.; Farrera-Sarmiento, O. Plantas Utilizadas en la Medicina Tradicional de Comunidades Campesinas e Indígenas de la Selva Zoque, Chiapas, México. Bol. Latinoam. Caribe Plantas Med. Aromáticas 2018, 17, 503–521. [Google Scholar]
- Estrada-Castillón, E.; Villarreal-Quintanilla, J.Á.; Cuéllar-Rodríguez, L.G.; March-Salas, M.; Encina-Domínguez, J.A.; Himmeslbach, W.; Salinas-Rodríguez, M.M.; Guerra, J.; Cotera-Correa, M.; Scott-Morales, L.M. Ethnobotany in Iturbide, Nuevo León: The Traditional Knowledge on Plants Used in the Semiarid Mountains of Northeastern Mexico. Sustainability 2022, 14, 12751. [Google Scholar] [CrossRef]
- Arias Toledo, A.A.; Valverde Valdés, M.T.; Reyes Santiago, J. Las Plantas de La Región de Zapotitlán Salinas, Puebla, 1st ed.; Instituto Nacional de Ecología: Mexico City, Mexico, 2000; Available online: http://centro.paot.org.mx/documentos/ine/plantas_zapo.pdf (accessed on 24 June 2023).
- Moreno Castillo, E.E. Herbolaria Empleada en Santa Cruz Acalpixca, Xochimilco. In Comparación Entre La Época Prehispánica Según El Códice Badiano y La Actualidad; Universidad Autónoma Metropolitana Unidad Xochimilco: Mexico City, Mexico, 2019. [Google Scholar]
- Arellano, B. Etnobotánica Medicinal de La Cultura Mephaa en La Ciénega, Municipio de Malinaltepec, Guerrero, México; Universidad Autónoma del Estado de Guerrero: Iguala, Mexico, 2017. [Google Scholar]
- Sánchez-González, A.; Granados-Sánchez, D.; Simón-Nabor, R. Uso Medicinal de Las Plantas por Los Otomíes del Municipio de Nicolás Flores, Hidalgo, México. Rev. Chapingo Ser. Hortic. 2008, 14, 271–279. [Google Scholar] [CrossRef]
- Jerezano Alberto, V.; Ríos Saúl, A.; Tepancal-Gomez, E.; Salas-Mendosa, E.; Villanueva, L. Some Traditional Medicinal Plants of North Region from Puebla, Mexico: Uses and Potential Pharmacological Activity of Rumex Spp. Nat. Prod. Chem. Res. 2016, 4, 2. [Google Scholar]
- Domínguez, X.A.; Alcorn, J.B. Screening of Medicinal Plants Used by Huastec Mayans of Northeastern Mexico. J. Ethnopharmacol. 1985, 13, 139–156. [Google Scholar] [CrossRef]
- Ankli, A.; Heinrich, M.; Bork, P.; Wolfram, L.; Bauerfeind, P.; Brun, R.; Schmid, C.; Weiss, C.; Bruggisser, R.; Gertsch, J. Yucatec Mayan Medicinal Plants: Evaluation Based on Indigenous Uses. J. Ethnopharmacol. 2002, 79, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M.; Vibrans, H.; Sticher, O.; Heinrich, M. Ethnopharmacology of the Popoluca, Mexico: An Evaluation. J. Pharm. Pharmacol. 2001, 53, 1653–1669. [Google Scholar] [CrossRef]
- García-Alvarado, J.S.; Verde-Star, M.J.; Heredia, N.L. Traditional Uses and Scientific Knowledge of Medicinal Plants from Mexico and Central America. J. Herbs. Spices Med. Plants 2001, 8, 37–89. [Google Scholar] [CrossRef]
- Escamilla Pérez, B.E.; Moreno Casasola, P.; Utrera Pérez, E.; Utrera Urea, E.; Tronco López, C.; Tronco López, B.; Tronco Morales, G. Plantas Medicinales de La Matamba y El Piñonal, Municipio de Jamapa, Veracruz, 1st ed.; Instituto de Ecología A. C. (INECOL): Veracruz, México, 2015; ISBN 978-607-7579-44-1. [Google Scholar]
- Instituto Municipal de Planeación. Paleta Vegetal Municipal de Valle de Santiago, Gto.; Instituto Municipal de Planeación: Guanajuato, México, 2020. [Google Scholar]
- Rosado-Vallado, M.; Brito-Loeza, W.; Mena-Rejon, G.J.; Quintero-Marmol, E.; Flores-Guido, J.S. Antimicrobial Activity of Fabaceae Species Used in Yucatan Traditional Medicine. Fitoterapia 2000, 71, 570–573. [Google Scholar] [CrossRef]
- Dimayuga, R.E.; Agundez, J. Traditional Medicine of Baja California Sur (Mexico) I. J. Ethnopharmacol. 1986, 17, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Arroniz, J.V.; Rosado, D.E.P.; Córdoba, P.Z.; García, E.M.G.; Muñoz, C.A.V.; Hernández, J.L.S. Conocimiento y Uso de Plantas Medicinales en Calpan, Puebla, México: Percepción de Varios Sectores Sociales. Bol. Latinoam. Caribe Plantas Med. Aromáticas 2023, 22, 676–688. [Google Scholar] [CrossRef]
- Andrade-Cetto, A. Ethnobotanical Study of the Medicinal Plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 2009, 122, 163–171. [Google Scholar] [CrossRef]
- Barrera-Catalán, E.; Herrera-Castro, N.D.; Catalán-Heverástico, C.; Ávila-Sánchez, P. Plantas Medicinales del Municipio de Tixtla de Guerrero, México. Rev. Fitotec. Mex. 2015, 38, 109–111. [Google Scholar] [CrossRef]
- Coronado-Aceves, E.W.; Sánchez-Escalante, J.J.; López-Cervantes, J.; Robles-Zepeda, R.E.; Velázquez, C.; Sánchez-Machado, D.I.; Garibay-Escobar, A. Antimycobacterial Activity of Medicinal Plants Used by the Mayo People of Sonora, Mexico. J. Ethnopharmacol. 2016, 190, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Baltisberger, M.; Sticher, O.; Heinrich, M. Medical Ethnobotany of the Zapotecs of the Isthmus-Sierra (Oaxaca, Mexico): Documentation and Assessment of Indigenous Uses. J. Ethnopharmacol. 1998, 62, 149–165. [Google Scholar] [CrossRef]
- Nava, R.F.; Zamora, D.R.; González, E.C. Notas Sobre Plantas Medicinales Del Estado de Querétaro, México. Polibotánica 2001, 12, 1–39. [Google Scholar]
- Webster, G.L. Euphorbiaceae BT—Flowering Plants. Eudicots: Malpighiales; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–216. ISBN 978-3-642-39417-1. [Google Scholar]
- Xu, Z.; Deng, M. Crassulaceae BT—Identification and Control of Common Weeds: Volume 2; Xu, Z., Deng, M., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 475–486. ISBN 978-94-024-1157-7. [Google Scholar]
- Heyduk, K. The Genetic Control of Succulent Leaf Development. Curr. Opin. Plant Biol. 2021, 59, 101978. [Google Scholar] [CrossRef]
- Benjamaa, R.; Moujanni, A.; Kaushik, N.; Choi, E.H.; Essamadi, A.K.; Kaushik, N.K. Euphorbia Species Latex: A Comprehensive Review on Phytochemistry and Biological Activities. Front. Plant Sci. 2022, 13, 1008881. [Google Scholar] [CrossRef]
- Licá, I.C.L.; dos Santos Soares, A.M.; de Mesquita, L.S.S.; Malik, S. Biological Properties and Pharmacological Potential of Plant Exudates. Food Res. Int. 2018, 105, 1039–1053. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and Limitations of Drug Delivery Systems Formulated as Eye Drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Leffler, C.T.; Schwartz, S.G.; Wainsztein, R.D.; Pflugrath, A.; Peterson, E. Ophthalmology in North America: Early Stories (1491–1801). Ophthalmol. Eye Dis. 2017, 9, 1179172117721902. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, G. A Review of Plant Species Used to Treat Conjunctivitis. Phyther. Res. 2002, 16, 1–22. [Google Scholar] [CrossRef]
- Esteva Espinosa, E. Conjuntivitis: Sintomatología, Tratamiento y Medidas Preventivas. Offarm Farm. y Soc. 2004, 23, 60–66. [Google Scholar]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M. Ethnobotany of the Genus Taraxacum—Phytochemicals and Antimicrobial Activity. Phyther. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Jayaprakash, N.; Bharadwaj, N.; Narayanappa, R. Exploring Insights for Virulent Gene Inhibition of Multidrug Resistant Salmonella Typhi, Vibrio Cholerae, and Staphylococcus Areus by Potential Phytoligands via In Silico Screening. J. Biomol. Struct. Dyn. 2014, 32, 1379–1395. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M. Matricaria Genus as a Source of Antimicrobial Agents: From Farm to Pharmacy and Food Applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Kemal, M.E.; Bakchiche, B.; Kemal, M.; Cheraif, K.; Kara, Y.; Bardaweel, S.K.; Miguel, M.G.; Yildiz, O.; Ghareeb, M.A. Six Algerian Plants: Phenolic Profile, Antioxidant, Antimicrobial Activities Associated with Different Simulated Gastrointestinal Digestion Phases and Antiproliferative Properties. J. Herb. Med. 2023, 38, 100636. [Google Scholar] [CrossRef]
- Leong, W.-H.; Lai, K.-S.; Lim, S.-H.E. Combination Therapy Involving Lavandula Angustifolia and Its Derivatives in Exhibiting Antimicrobial Properties and Combatting Antimicrobial Resistance: Current Challenges and Future Prospects. Processes 2021, 9, 609. [Google Scholar] [CrossRef]
- Sandoval-Montemayor, N.E.; García, A.; Elizondo-Treviño, E.; Garza-González, E.; Alvarez, L.; del Rayo Camacho-Corona, M. Chemical Composition of Hexane Extract of Citrus Aurantifolia and Anti-Mycobacterium Tuberculosis Activity of Some of Its Constituents. Molecules 2012, 17, 11173–11184. [Google Scholar] [CrossRef]
- Escuder, A.G.; Hunter, D.G. The Role of Botulinum Toxin in the Treatment of Strabismus. In Proceedings of the Seminars in Ophthalmology; Taylor & Francis: Abingdon, UK, 2019; Volume 34, pp. 198–204. [Google Scholar]
- Matos, F.J.A.; Machado, M.I.L.; Alencar, J.W.; Matos, M.E.O.; Craveiro, A.A. Plants Used in Traditional Medicine of China and Brazil. Mem. Inst. Oswaldo Cruz 1991, 86, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Poddar, S.; Sarkar, T.; Choudhury, S.; Chatterjee, S.; Ghosh, P. Indian Traditional Medicinal Plants: A Concise Review. Int. J. Bot. Stud. 2020, 5, 174–190. [Google Scholar]
- Bortolotti, M.; Zanello, A.; Serra, L.; Biscotti, F.; Polito, L.; Bolognesi, A. Plant Toxins as Potential Alternatives to Botulinum Toxin for Eye-Movement Disorder Therapy. Stresses 2023, 3, 270–281. [Google Scholar] [CrossRef]
- Eid, O.; Gonaid, M. Crassulaceae (Chemistry and Pharmacology)—A Review. Futur. J. Pharm. Sci. 2018, 4, 234–240. [Google Scholar] [CrossRef]
- Aragón-Parada, J.; Carrillo-Reyes, P.; Rodríguez, A.; Munguía-Lino, G. Diversidad y Distribución Geográfica del Género Sedum (Crassulaceae) en La Sierra Madre del Sur, México. Rev. Mex. Biodivers. 2019, 90, 1–17. [Google Scholar] [CrossRef]
- Villaseñor, J.L. Checklist of the Native Vascular Plants of Mexico. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Pérez, G.; Chocarro, C.; Juárez, A.; Coma, J. Evaluation of the Development of Five Sedum Species on Extensive Green Roofs in a Continental Mediterranean Climate. Urban For. Urban Green. 2020, 48, 126566. [Google Scholar] [CrossRef]
- Pieroni, A.; Giusti, M.E. Alpine Ethnobotany in Italy: Traditional Knowledge of Gastronomic and Medicinal Plants among the Occitans of the Upper Varaita Valley, Piedmont. J. Ethnobiol. Ethnomed. 2009, 5, 32. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An Ethnobotanical Survey of Traditionally Used Plants on Suva Planina Mountain (South-Eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Rigat, M.; Valles, J.; Gras, A.; Iglésias, J.; Garnatje, T. Plants with Topical Uses in the Ripollès District (Pyrenees, Catalonia, Iberian Peninsula): Ethnobotanical Survey and Pharmacological Validation in the Literature. J. Ethnopharmacol. 2015, 164, 162–179. [Google Scholar] [CrossRef]
- Mosaddegh, M.; Naghibi, F.; Moazzeni, H.; Pirani, A.; Esmaeili, S. Ethnobotanical Survey of Herbal Remedies Traditionally Used in Kohghiluyeh va Boyer Ahmad Province of Iran. J. Ethnopharmacol. 2012, 141, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Piñón, Y.; Mejía, A.; Díaz-Ruiz, G.; Aguilar, M.I.; Sánchez-Nieto, S.; Rivero-Cruz, J.F. Ethnobotanical Survey and Antibacterial Activity of Plants Used in the Altiplane Region of Mexico for the Treatment of Oral Cavity Infections. J. Ethnopharmacol. 2012, 141, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Garcıa, V.M.N.; Gonzalez, A.; Fuentes, M.; Aviles, M.; Rios, M.Y.; Zepeda, G.; Rojas, M.G. Antifungal Activities of Nine Traditional Mexican Medicinal Plants. J. Ethnopharmacol. 2003, 87, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Torrescano-De Labra, L.; Jiménez-Ferrer, E.; Camacho-Díaz, B.H.; Vargas-Villa, G.; González-Cortazar, M.; Herrera-Ruiz, M.; Ávila Reyes, S.V.; Solorza-Feria, J.; Jiménez-Aparicio, A.R. Corneal Healing and Recovery of Ocular Crystallinity with a Dichloromethane Extract of Sedum dendroideum DC in a Novel Murine Model of Ocular Pterygium. Molecules 2021, 26, 4502. [Google Scholar] [CrossRef] [PubMed]
- López-Montemayor, P.; Zavala, J.; Montalvo-Parra, M.D.; Guerrero-Ramírez, G.I.; Mayolo-Deloisa, K.; Enriquez-Ochoa, D.; Martínez-García, B.; Loya-García, D.; Guerrero-Martínez, A.M.; Valdez-García, J.E. Phytochemical Profile and Antioxidant and Antiproliferative Activity of Sedum dendroideum on Pterygium Fibroblasts. Evid.-Based Complement. Altern. Med. 2021, 2021, 5814221. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.-H.; Chen, Y.C. Kaempferol Inhibits Angiogenesis and VEGF Expression through Both HIF Dependent and Independent Pathways in Human Ovarian Cancer Cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxidative Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.K.; Srivastava, S.; Agrawal, S.S.; Saxena, R. Lowering of Intraocular Pressure by Topical Application of Daucus carota Seed Extract in Rabbits. Indian J. Exp. Biol. 2008, 46, 541–546. [Google Scholar]
- El-Mansi, A.A.; Al-Kahtani, M.A.; Rady, A.M.; El-Bealy, E.A.; Al-Asmari, A.M. Vitamin A and Daucus carota Root Extract Mitigate STZ-induced Diabetic Retinal Degeneration in Wistar Albino Rats by Modulating Neurotransmission and Downregulation of Apoptotic Pathways. J. Food Biochem. 2021, 45, e13688. [Google Scholar] [CrossRef]
- Ahmed, M.; Sahibzada, M.U.K.; Rasheed, H.M.; Khan, T.; Wahid, F.; Farooq, U.; Khusro, A.; Uddin, J.; Afzal, S.; Khan, A. Inhibition of Inflammation Associated Corneal Neovascularization by Dalbergia sissoo and Catharanthus roseus Leaf Extracts in an Animal Model. S. Afr. J. Bot. 2022, 151, 379–386. [Google Scholar] [CrossRef]
- Moghadam, M.R.; Jafarinasab, M.-R.; Yousefi, Z.; Moghaddam, A.S.; Memarzadeh, H.; Kanavi, M.R. Aloe vera Gel-Derived Eye Drops for Alkaline Corneal Injury in a Rabbit Model. J. Ophthalmic Vis. Res. 2020, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Atiba, A.; Wasfy, T.; Abdo, W.; Ghoneim, A.; Kamal, T.; Shukry, M. Aloe vera Gel Facilitates Re-Epithelialization of Corneal Alkali Burn in Normal and Diabetic Rats. Clin. Ophthalmol. 2015, 9, 2019–2026. [Google Scholar] [PubMed]
- Green, K.; Tsai, J.; Luxenberg, M.N. Effect of Aloe vera on Corneal Epithelial Wound Healing. J. Toxicol. Cutan. Ocul. Toxicol. 1996, 15, 301–304. [Google Scholar] [CrossRef]
- Vaghela, J.J.; Barvaliya, M.J.; Parmar, S.J.; Tripathi, C.R. Evaluation of Efficacy of Aloe vera (L.) Burm. f. Gel Solution in Methylcellulose-Induced Ocular Hypertension in New Zealand White Rabbits. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 20190158. [Google Scholar] [CrossRef]
- Woźniak, A.; Paduch, R. Aloe vera Extract Activity on Human Corneal Cells. Pharm. Biol. 2012, 50, 147–154. [Google Scholar] [CrossRef]
- Ceravolo, I.; Mannino, F.; Irrera, N.; Squadrito, F.; Altavilla, D.; Ceravolo, G.; Pallio, G.; Minutoli, L. Health Potential of Aloe vera against Oxidative Stress Induced Corneal Damage: An “In Vitro” Study. Antioxidants 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Rathnakumar, K.; Jaikumar, S.; Sengottuvelu, S. Effect of Euphorbia Hirta in Napthalene Induced Cataract in Rats. Res. J. Pharm. Technol. 2013, 6, 908–911. [Google Scholar]
- Sandford, E.C.; Muntz, A.; Craig, J.P. Therapeutic Potential of Castor Oil in Managing Blepharitis, Meibomian Gland Dysfunction and Dry Eye. Clin. Exp. Optom. 2021, 104, 315–322. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.K.; Srivastava, S.; Saxena, R. IOP Lowering Effects of Ocimum basilicum Seed Extract in Two Rabbit Models of Ocular Hypertension. J. Clin. Health Sci. 2019, 4, 39–46. [Google Scholar] [CrossRef]
- Anand, T.; Sundararajan, M.; Anbukkarasi, M.; Thomas, P.A.; Geraldine, P. A Methanolic Extract of Ocimum basilicum Exhibits Antioxidant Effects and Prevents Selenite-Induced Cataract Formation in Cultured Lenses of Wistar Rats. Pharmacogn. J. 2019, 11, 496–504. [Google Scholar] [CrossRef]
- Anand, T.; Anbukkarasi, M.; Teresa, P.A.; Thomas, P.A.; Geraldine, P. Evaluation of the Putative Efficacy of a Methanolic Extract of Ocimum basilicum in Preventing Disruption of Structural Proteins in an in Vitro System of Selenite-Induced Cataractogenesis. Curr. Eye Res. 2020, 45, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, M.; Asokkumar, K.; Lalitha, V.; Sivashanmugam, T.; Subhadradevi, V. Anticataract and Antioxidant Activities of Citrus aurantium L. Peel Extract against Naphthalene Induced Cataractogenesis in Rats. J. Pharm. Res. 2011, 4, 680–682. [Google Scholar]
- Tewari, D.; Samoilă, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D. Medicinal Plants and Natural Products Used in Cataract Management. Front. Pharmacol. 2019, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients 2022, 14, 534. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Ohia, S.; Bagchi, D. Naphthalene Toxicity and Antioxidant Nutrients. Toxicology 2002, 180, 97–105. [Google Scholar] [CrossRef]
- Muthaiah, R. Screening Methods for the Evaluation of Antiglaucoma and Anticataract Drugs. In Introduction to Basics of Pharmacology and Toxicology: Volume 3: Experimental Pharmacology: Research Methodology and Biostatistics; Springer: Berlin/Heidelberg, Germany, 2022; pp. 523–539. [Google Scholar]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.N.; Chaicumpa, W.; Michalak, I. A Comprehensive Review on Chemical Profile and Pharmacological Activities of Ocimum Basilicum. Food Rev. Int. 2023, 39, 119–147. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-Inflammatory Potential and Antioxidant Profile of Eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Shah, M.; Cabrera-Ghayouri, S.; Christie, L.-A.; Held, K.S.; Viswanath, V. Translational Preclinical Pharmacologic Disease Models for Ophthalmic Drug Development. Pharm. Res. 2019, 36, 58. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, B.; Singh, R. Catharanthus roseus (L.) G. Don: A Review of Its Ethnobotany, Phytochemistry, Ethnopharmacology and Toxicities. J. Ethnopharmacol. 2022, 284, 114647. [Google Scholar] [CrossRef]
- Bozzi, A.; Perrin, C.; Austin, S.; Vera, F.A. Quality and Authenticity of Commercial Aloe vera Gel Powders. Food Chem. 2007, 103, 22–30. [Google Scholar] [CrossRef]
- Massoud, D.; Alrashdi, B.M.; Fouda, M.; El-kott, A.; Soliman, S.A.; Abd-Elhafeez, H.H. Aloe vera and Wound Healing: A Brief Review. Braz. J. Pharm. Sci. 2023, 58, 1–11. [Google Scholar] [CrossRef]
- Jettanacheawchankit, S.; Sasithanasate, S.; Sangvanich, P.; Banlunara, W.; Thunyakitpisal, P. Acemannan Stimulates Gingival Fibroblast Proliferation; Expressions of Keratinocyte Growth Factor-1, Vascular Endothelial Growth Factor, and Type I Collagen; and Wound Healing. J. Pharmacol. Sci. 2009, 109, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Fraunfelder, F.W. Ocular Side Effects from Herbal Medicines and Nutritional Supplements. Am. J. Ophthalmol. 2004, 138, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Al Ghadeer, H.; Al Gethami, A.; Al Sulaiman, H.; Bukhari, T. Corneal Toxicity after Self-Application of Calotropis Procera (Ushaar) Latex: Case Report and Analysis of the Active Components. Middle East Afr. J. Ophthalmol. 2019, 26, 40. [Google Scholar] [CrossRef]
- Basak, S.K.; Bakshi, P.K.; Basu, S.; Basak, S. Keratouveitis Caused by Euphorbia Plant Sap. Indian J. Ophthalmol. 2009, 57, 311. [Google Scholar] [CrossRef]
- da Silva Conci, L.; Alves, D.L.S.; Agostini, F.S.; Frossard, J.C.; Alves, D.C.S.; Alves, D.H.S.; Pinheiro, A.G. Keratouveitis Caused by Euphorbia milii Sap: Case Report and Overview. Rev. Bras. Oftalmol. 2021, 80, 1–3. [Google Scholar]



| Plant Family | Plant Name | Common Name (Spanish or Autochthonal) | Plant Part Used | Medicinal Use | Ethnobotanical Information/Estate | Reference |
|---|---|---|---|---|---|---|
| Anacardiaceae | Metopium brownei (Jacq.) Urb. | Che’ chen (Lacandon); Chechem (spanish) | Sap/crude “Bleed sap from the base of the tree” | Eye irritation | “Wash eyelids with sap”/Chiapas | [16] |
| Schinus molle L. | Pirú (Spanish) | Not specified | Eye cleaner | Not specified/Estado de México | [17] | |
| Spondias purpurea L. | Ciruela (Spanish) | Not specified | Eye pain | Not specified/Yucatán | [18] | |
| Apiaceae | Daucus carota L. | Zanahoria (Spanish) | Fresh leaves | Eye problems | Squeezed/Chiapas | [19] |
| Apocynaceae | Catharanthus roseus (L.) G.Don | Vicaria (Spanish and Chontal) | Root | Conjunctivitis | Not specified/Tabasco | [20] |
| Araceae | Philodendron sagittifolium Liebm. | “chapiz grande”, “malaste grande” (Spanish); “tantai” (Totonaca) | Leaves | To cure strabismus | “Leaves applied to eyelids of sleeping children, to cure strabismus”/Puebla | [21] |
| Arecaceae | Acrocomia aculeata (Jacq.) Lodd. ex Mart. | Coyol redondo, palma (Spanish) | Fresh bark | Eye problems | Decoction/Veracruz | [22] |
| Asphodelaceae | Aloe vera (L.) Burm. f. | Sábila (Spanish) | Leaf gel | Eye cleaner | Crushed/Oaxaca | [23] |
| Asteraceae | Arnica montana L. | Arnica (Spanish) | Aerial parts | Improves eyesight | Decoction and topical administration/Puebla | [24] |
| Chamaemelum nobile (L.) All. | Manzanilla (Spanish) | Whole plant | Eye infection | Oral infusion of whole plant/Guanajuato | [14] | |
| Helianthus annuus L. | Girasol (Spanish) | Fresh leaves | Eye problems | Infusion/Chiapas | [19] | |
| M. chamomilla | Manzanilla (Spanish) | Not specified | Eye cleaner | Decoction, infusion, washes/Oaxaca | [23] | |
| Not specified | Eye irritation | Not specified/Mexico city | [25] | |||
| Aerial parts | Eye infection | “Tea, the infected eyes are washed with it (topical)”/Puebla | [26] | |||
| Leaves and inflorescences | Eye infection | “Applied as eye drops”/Nuevo León | [27] | |||
| Leaves, whole plant | Conjunctivitis | Infusion, oral/Guerrero | [28] | |||
| Whole plant | Not specified | The infusion is used as eye drops/Nuevo León | [29] | |||
| Leaves and flowers | Eye problems | Not specified/Zacatecas | [30] | |||
| Not specified | Eye irritation | Not specified/Oaxaca | [31] | |||
| Fresh leaves | Eyes problem | Bath/Veracruz | [22] | |||
| Leaves and flowers | Eye cleaner | “Put two warm drops of the 5apónica directly in the eye”/Coahuila | [13] | |||
| Taraxacum officinale F.H. Wigg | Diente de león (Spanish) | Aerial part, root | Conjuntivitis | Decoction, oral/Puebla | [24] | |
| Zinnia peruviana (L.) L. | Mal de ojo (Spanish) | Not specified | Eye irritation | Not specified/Guanajuato | [32] | |
| Commelinaceae | Commelina erecta L. | “matalín” (Spanish); “kasmalj” (Totonaca) | Sap | Eye cleaner | “Sap used as eyedrops to clean the eyes”/Puebla | [21] |
| Tradescantia spathacea Sw. | Maguey morado (Spanish) | Leaves | Eye irritation | Infusion of leaves and applied as eye drops/Tabasco | [33] | |
| Tradescantia zebrina var. zebrina | Matlali color morado (Spanish); hierba de 1os ojos (Spanish) | Leaves | Cataract | “The juice from its leaves and twigs is put directly over the cataract of the eye”/Oaxaca, Puebla, and Veracruz | [34] | |
| Costaceae | Costus pulverulentus C. Presl | Cañita agría (Spanish) | Stem | Eye irritation | Infusion of stem and applied as eye drops/Chiapas | [35] |
| Crassulaceae | Echeveria elegans var. simulans Poelln. | Siempre viva (Spanish) | Sap | Red eyes, irritated eyes | Eye drops/Nuevo León | [36] |
| Kalanchoe pinnata (Lam.) Pers. | Siempre viva, “hoja fresca” (Spanish); “Tkuya tuwan” (Totonaca) | Leaves | Eye cleaner | “Sap of leaves used to clean the eyes”/Puebla | [21] | |
| Sedum allantoides Rose | Cola de Borrego (Spanish) | Sap | Conjuntivitis | The sap of this plant is used as an antiseptic in mild eye infections, conjunctivitis, and children with thrush (Candida albicans infection)/Puebla | [37] | |
| Sedum dendroideum Moc. & Sessé ex DC. | Siempre viva (Spanish) | Leaves/sap | Eye pain | Detached leaves are squeezed directly into the eye. A small amount of sap is directly applied to the eye to avoid a burning sensation/Mexico City | [38] | |
| Siempre viva (Spanish) | Not specified | Eye infection | Crushed/Oaxaca | [23] | ||
| Sedum diffusum S. Watson | Chismes (Spanish) | Sap | Red eyes, irritated eyes | Eye drops/Nuevo León | [36] | |
| Sedum morganianum E.Walther | Cola de Borrego (Spanish) | Fresh leaves | Eye infection | Squeeze/Chiapas | [19] | |
| Iná meda (Me 'phaa) | Leaves | “Carnosidad” 1 Eye infection | Topical/Guerrero | [39] | ||
| Sedum oxypetalum Kunth | Siempre viva (Spanish) | Leaves, stem | “Carnosidad” 1, eye cleaner | Extract drops and poultice (Cataplasm)/Mexico City | [25] | |
| Sedum praealtum A.DC. | Siempre viva “damdo” (Spanish) | Leaves | Eye irritation | Not specified/Hidalgo | [40] | |
| Siempreviva; flor de siempreviva (Spanish); su sá (Ngiba) | Not specified | Eye irritation | Not specified/Oaxaca | [31] | ||
| Sedum × rubrotinctum R.T.Clausen | Dedo de niño (Spanish) | Leaves | Eye infection | “Cut leaves and squeeze out the liquid until it contains and apply a few drops in the ear or eye if it is the case”/Puebla | [41] | |
| Euphorbiaceae | Croton cortesianus Kunth | Not specified | Sap | Eye infection | Not specified/southeast San Luis Potosi and northern Veracruz | [42] |
| Croton reflexifolius Kunth | Not specified | Resin | Eye problems | “The resin of Croton reflexifolius is used for treating pimples in the mouth (herpes) and eye problems”/Yucatán | [43] | |
| Croton repens Schltdl. | Sangre de grado de la sabana/Soj kobak/Soj muk (Popoluca) | Sap | Retina complication | “The sap of astringent plants is applied to the eye for cleaning the retina”/Veracruz | [44] | |
| Euphorbia hirta L. | Hierba de la golondrina (Spanish) | Stem | Eye irritation | Not specified/Hidalgo | [40] | |
| Not specified | Conjuntivitis | Not specified/Estado de México | [17] | |||
| E. prostrata | Hierba de la golondrina (Spanish) | Whole plant | Cataracts | “An infusion of the whole plant is used for diarrhea and cataracts”/Nuevo León | [29] | |
| Leaves | Eye diseases | “Two or three leaves of the plant are squeezed, and the fluid is applied to the eyes”/Not specified | [45] | |||
| Golondrina (Spanish) | Leaves | Watering eyes, “nubes” 2 | Two or three leaves of the plant are squeezed into the eye/Veracruz | [46] | ||
| J. dioica | Sangre de drago, sangregado (Spanish) | Aerial parts and roots | Eye irritation | Infusion, oral/Not specified | [45] | |
| Sangregado (Spanish) | Not specified | Eye irritation, “nubes” 2, blindness | Not specified/Guanajuato | [47] | ||
| Sangre de drago, sangre de grado (Spanish) | Sap | Eye cleaner | “Apply a drop of sap to the eye”/Coahuila | [13] | ||
| Ricinus communis L. | Higuerilla (Spanish) | Not specified | Conjunctivitis | Not specified/Estado de México | [17] | |
| Fabaceae | Senna spectabilis (DC.) H.S. Irwin & Barneby | Flor de todos 1os santos (Spanish) | Flowers | To wash the eyes | “The concoction of the flowers is used to wash the eyes and avoiding ‘evil eye’ (mal de ojo) occurs”/Oaxaca, Puebla and Veracruz | [34] |
| Dalbergia glabra (Mill.) Standl. | Not specified | Leaves | Eye infections | “Infusion used to treat eye infections”/Yucatán | [48] | |
| Gliricidia sepium (Jacq.) Kunth | Cocohite (Spanish); aj chánté (Chontal) | Leaves | Conjunctivitis | Not specified/Tabasco | [20] | |
| Prosopis laevigata (Humb. & Bonpl. ex Willd.) M.C.Johnst. | Mezquite (Spanish) | Leaves | Eye problems | “Tea from leaf shoots, the eyes are washed”/Puebla | [26] | |
| Mezquite (Spanish); Mizquitl (Nahuatl) | Leaves | Eye infection | Infusion, oral/Guerrero | [28] | ||
| Senna racemosa (Mill.) H.S.Irwin & Barneby | Not specified | Bark | Eye infections | “Infusion is used to treat eye infections”/Yucatán | [48] | |
| Fouquieriaceae | Fouquieria diguetii (Tiegh.) I.M.Johnst. | Palo Adán (Spanish) | Flower’s sap | Cataract | “Applied directly to the eye”/Baja California Sur | [49] |
| Lamiaceae | Agastache mexicana (Kunth) Lint & Epling. | Toronjil (Spanish) | Not specified | Eye cleaner | Infusion, washes/Oaxaca | [23] |
| Lavandula angustifolia Mill. | Lavanda (Spanish) | Leaves | Conjunctivitis | Infusion, oral/Puebla | [50] | |
| Ocimum basilicum L. | Albahaca (Spanish) | Not specified | Eye pain | Not specified/Yucatán | [18] | |
| Albahacar (Spanish); Albajaka (Chontal) | Aerial part | Conjunctivitis | Not specified/Tabasco | [20] | ||
| Ocimum carnosum (Spreng.) Link & Otto ex Benth | Siempreviva (Spanish) | Leaves | Cataract | Maceration, topical/Hidalgo | [51] | |
| Rosmarinus officinalis L. | Romero (Spanish) | Aerial part | Blurry vision | Decoction, infusion, crushed; oral/topical/Puebla | [24] | |
| Salvia hispanica L. | Chía (Spanish) | Seeds | “Basura en el ojo” (foreign body sensation) | Seeds are mashed and put into the eye. Tear production helps to eliminate the foreign body sensation/Guerrero | [52] | |
| Lauraceae | Persea americana Mill. | Aguacate (Spanish); tchunue (Ngiba) | Not specified | Eye irritation | Not specified/Oaxaca | [31] |
| Malvaceae | Malvaviscus arboreus Dill. ex Cav. | Sibí; Sibil (Spanish); Yopo 'aj ts 'ibi (Chontal) | Leaves | “Carnosidad” 1 | Not specified/Tabasco | [20] |
| Pachira aquatica Aubl. | Zapote de agua (Spanish); Ajp 'o 'te c (Chontal) | Cortex | Conjuntivitis | Not specified/Tabasco | [20] | |
| Papaveraceae | Argemone mexicana L. | Tachina, Táchino (Mayo); Xazácös; (Seri); Cardo (Spanish); Chicalote (Spanish) | Shoots | Eye infection | Decoction/Sonora | [53] |
| Chicale (Spanish); Chicalotl (Nahuatl) | Flower, latex | Eye infection | Maceration, topical/Guerrero | [28] | ||
| Argemone ochroleuca Sweet | Chicalote (Spanish) | Not specified | Eye infection, cataracts | Infusion, washes/Oaxaca | [23] | |
| Flowers, latex | “Carnosidad” 1, eye irritation | Extract drops/Mexico City | [25] | |||
| Chicatl (náhuatl) | Latex | “Carnosidad” 1 | “1 or 2 drops of latex into the eye. To improve effectiveness, 1 drop is recommended before going to sleep/Guerrero | [52] | ||
| Argemone platyceras Link & Otto | Chicalote blanco (Spanish) | Flowers, latex | “Carnosidad” 1,eye irritation | Extract drops/Mexico city | [25] | |
| Chicalote (Spanish) | Not specified | Eye pain | Not specified/Estado de México | [17] | ||
| Plantaginaceae | Plantago major L. | Lantén (Spanish) | Not specified | Eye cleaner | Washes/Oaxaca | [23] |
| Polypodiaceae | Pleopeltis polypodioides (L.) E.G. Andrews & Windham | Siempre viva (Spanish) | Aerial part | Conjuntivitis | Infusion, oral/Puebla | [24] |
| Rhamnaceae | Sarcomphalus obtusifolius (Hook. ex Torr. & A.Gray) Hauenschild | Jutuqui, Jo’otoro (Mayo); Bachata, Ciruela del monte, Hui-chillame (Spanish) | Shoots | Eye infections | Decoction/Sonora | [53] |
| Rosaceae | Rosa sp. | Rosa de castilla (Spanish) | Flowers | Eye cleaner | “Boiling two to three roses and wash the eyes with the infusion”/Veracruz | [46] |
| Rosa × centifolia | Not specified | Petal; flower | Ophthalmological problems | Not specified/Oaxaca | [54] | |
| Rosa de Castilla (Spanish) | Flowers | “Tea (oral) and used to wash the eye (topical)”/Puebla | [26] | |||
| Not specified | Eye irritation | Not specified/Oaxaca | [31] | |||
| Not specified | Eye irritation | Not specified/Mexico city | [25] | |||
| Rosa chinensis Jacq. | Rosa concha (Spanish) | Flowers | To reduce swelling | “The concoction of the flower is put directly over the eyes to reduce swelling”/Oaxaca, Puebla, and Veracruz | [34] | |
| Rosa (Spanish) | Not specified | Eye problems | Not specified/Estado de México | [17] | ||
| Rosa gallica L. | Rosa de castilla (Spanish) | Not specified | Conjunctivitis; improves eyesight; eyes wash | Infusion, washes/Oaxaca | [23] | |
| Rosa de castilla (Spanish); Nich i castilla (Chontal) | Flowers | “Carnosidad” 1 | Not specified/Tabasco | [20] | ||
| Rosa de castilla (Spanish) | Flowers | Eye infection | Infusion, washes/Guanajuato | [14] | ||
| Rosa moschata Herrm. | Flor de concha, Rosa Concha (Spanish); U nich pat (Chontal) | Flowers | “Carnosidad” 1,eye irritation | Not specified/Tabasco | [20] | |
| Rosa multiflora Thunb. | Rosa blanca (Spanish) | Flowers | Eye irritation | Infusion, vaporization, topical/Puebla | [24] | |
| Rutaceae | Citrus × aurantiifolia (Christm.) Swingle | Limón (Spanish) | Leaves; fruit | Conjunctivitis | Infusion, essential oil/oral/Central and southern Mexico | [45] |
| Citrus × aurantium L. | Limonero (Spanish) | Not specified | Eye cleaner | Infusion, washes/Oaxaca | [23] | |
| Citrus × limon (L.) Osbeck | Lima chichi (Spanish) | Fresh fruit | Infection | “Infection in the eye” Squeezed/Veracruz | [22] | |
| Ruta chalepensis L. | Ruda (Spanish) | Whole plant | “Vista venteada” | “The powdered plant is mixed with aguardiente, an alcoholic beverage, and camphor, the resultant paste is applied close to the eyes to treat blurred sight”/Querétaro | [55] | |
| Solanaceae | Capsicum baccatum L. | Hoja de chile (Spanish) | Aerial parts; fruit; leaf | Ophthalmological problems | Not specified/Oaxaca | [54] |
| Viburnaceae | Sambucus mexicana C.Presl ex DC. | Not specified | Flower; leaf | Ophthalmological problems | Not specified/Oaxaca | [54] |
| Vitaceae | Cissus verticillata subsp. verticillata | Not specified | Aerial parts; fruit | Ophthalmological problems | Not specified/Oaxaca | [54] |
| Vitis tiliifolia Humb. & Bonpl. ex Schult. | Bejuco de uva (Spanish) | Leaves | Eye cleaner | “Boiling the leaves with water, the infusion is used to wash the eyes”/Querétaro | [55] | |
| Uva silvestre (Spanish) | Not specified | “Carnosidad” 1 | Not specified/Estado de México | [17] | ||
| Zingiberaceae | Hellenia speciosa (J. Koenig) S.R. Dutta | Päsak (Lacandon); Jengibre (Spanish) | Sap/crude “Cut stalk and drain sap” | Eye irritation | “The sap is directly applied to eyes”/Chiapas | [16] |
| Species Name | Extract | Vehicle or Formulation | Study/Model or Clinical Intervention | Way of Administration | Outcome | Reference |
|---|---|---|---|---|---|---|
| D. carota | Aqueous seed extract | Dried and powdered extract was dissolved in 0.25% hydroxy propyl methylcellulose | In vivo/water-loading model and steroid induced model IOP in rabbits | Topically (instilled) 50 µL | IOP reduction of 29.39% (water-loading model) and 30.27% (steroid-induced model) at 0.6%. | [89] |
| Chloroform root extract | - | In vivo/STZ-induced diabetic retinal damage in rats | Orally administered at 200 mg/kg/day | ↑ Serum plasma retinol (~1.70%). Protection and attenuation of retinal damage via downregulation of apoptotic pathways, enhancing oxidative capacity, and modulation of retinal neurotransmission at 200 mg/kg/day. | [90] | |
| C. roseus | 70% methanol leaf extract | Eye drops (1% w/v) were prepared in normal saline and DMSO (4%) | In vivo/alkali burn-induced corneal neovascularization in rabbits | Topically applied (instilled) three eye drops three times a day | ↓ Vessel length and thickness; their propagation towards the cornea was stopped. | [91] |
| A. vera | Leaf gel | Aloe vera gel-derived eye drops | In vivo/alkali-burned corneas in rabbits | Topically (instilled) eye drops four times a day for seven days | ↓ Corneal epithelial defect area; ↓ rate of keratocyte loss. | [92] |
| Eye drops, Gel lyophilized powder (60 mg/mL) in saline. | In vivo/alkali-burned corneas in normal and diabetic rats | Topically applied (instilled) eye drops four times daily for 3 days | Promoting corneal wound healing through facilitating re-epithelization and reducing inflammation in diabetic rats. | [93] | ||
| Eye drops, gel | In vivo/mechanically induced corneal epithelial lesion in rabbits. | Topically applied (instilled) 50 µL eye drops applied three times daily | No effect | [94] | ||
| 6 and 12% gel solutions with potassium sorbate as a preservative | In vivo/methylcellulose-induced ocular hypertension in rabbits | Topically applied (instilled) eye drops applied every 8 h for 2 days | IOP reduction of 8.6 and 10.4% with 6 and 12% gel solutions, respectively | [95] | ||
| Ethanol, ethyl acetate, and heptane extracts | Dissolved in DMSO | In vitro/human normal corneal cell line 10.014 pRSV-T (ATCC No. CRL-11515) | - | ↓ NO production ↓ IL-1β, IL-6, TNF-α and IL-10 production | [96] | |
| Methanol extract from fresh leaves | Lyophilized powder (100 μg/mL) | In vitro/epithelial adenovirus 12-SV40 hybrid-transformed HCE cells (ATCC® CRL-11135™); exposure to H2O2 | - | ↑ Cell viability ↓ ROS production and MDA levels ↑ Gene expression of Nrf2, SOD2 and Catalase ↓ COX-2, TNF-α, IL-6 and IL-1β mRNA expression | [97] | |
| S. dendroideum | Dichloromethane extract from leaf juice; lyophilized powder | 20 mg/mL dichloromethane extract dissolved in vehicle (cyclodextrin and Tween 20 solution) | In vivo/tetradecanoylphorbol acetate-induced pterygium-like eye lesion in mice | Topically applied (instilled) daily for fifteen days | ↓ Corneal opacity ↓ TNF-α and IL1β ↑ IL-10 | [85] |
| The stems and leaves were blended with distilled H2O | 100 mg of lyophilized powder in 1 mL of DMSO | In vitro/human pterygium fibroblast primary culture | - | ↓ Proliferation ↓ VEGF and CTGF expression at 250 μg/mL | [86] | |
| E. hirta | Ethanolic extract of aerial parts | - | In vivo/naphthalene-induced cataract in rats | Orally for 28 days | ↓ Opacity index at 200 and 400 mg/kg | [98] |
| R. communis | Castor oil | Castor oil emulsions | Six clinical trials 1/meibomian gland dysfunction; dry eye; contact lens discomfort; blepharitis | Topically applied (instilled) eye drops | Beneficial effects on the lipid layer, tear film integrity, eyelash health, and meibomian gland functionality | [99] |
| O. basilicum | Aqueous extract from seeds | Extract in 0.25% Hydroxypropyl methylcellulose | In vivo/water-loading and steroid-induced ocular hypertension in rabbit eyes | Topically applied (instilled) eye drops, single drop of extract | IOP reduction at 0.5% of extract | [100] |
| Methanolic leaves extract | 200 μg/mL DMEM | In vitro/selenite-induced cataractogenesis in rat lenses | - | Prevented selenite-induced cataract formation. ↑ GSH level ↓ MDA | [101] | |
| Methanolic leaves Extract | 200 μg/mL DMEM | In vitro/selenite-induced cataractogenesis in rat lenses | - | Prevented alterations of the insoluble-to-soluble-protein ratio and the protein carbonyl and sulfhydryl levels. Prevented the reduction in mRNA transcript levels of the αA- crystallin and βB1-crystallin genes | [102] | |
| C. aurantium | Hydromethanol peel extract | - | In vivo/naphthalene-induced cataractogenesis in rats | Orally for 28 days | ↓ Opacity index at 200 and 400 mg/kg ↓ Carbonyl and ↑ sulfhydryl levels ↓ MDA and LH Restored enzymatic and non-enzymatic antioxidant enzymes | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Gómez, A.; Velo-Silvestre, A.A.; Alonso-Castro, A.J.; Hernández-Zimbrón, L.F. Medicinal Plants Used for Eye Conditions in Mexico—A Review. Pharmaceuticals 2023, 16, 1432. https://doi.org/10.3390/ph16101432
Salazar-Gómez A, Velo-Silvestre AA, Alonso-Castro AJ, Hernández-Zimbrón LF. Medicinal Plants Used for Eye Conditions in Mexico—A Review. Pharmaceuticals. 2023; 16(10):1432. https://doi.org/10.3390/ph16101432
Chicago/Turabian StyleSalazar-Gómez, Anuar, Amabile A. Velo-Silvestre, Angel Josabad Alonso-Castro, and Luis Fernando Hernández-Zimbrón. 2023. "Medicinal Plants Used for Eye Conditions in Mexico—A Review" Pharmaceuticals 16, no. 10: 1432. https://doi.org/10.3390/ph16101432
APA StyleSalazar-Gómez, A., Velo-Silvestre, A. A., Alonso-Castro, A. J., & Hernández-Zimbrón, L. F. (2023). Medicinal Plants Used for Eye Conditions in Mexico—A Review. Pharmaceuticals, 16(10), 1432. https://doi.org/10.3390/ph16101432








