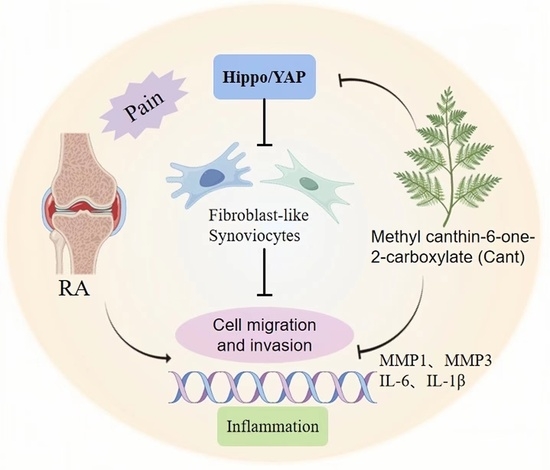
Methyl Canthin-6-one-2-carboxylate Restrains the Migration/Invasion Properties of Fibroblast-like Synoviocytes by Suppressing the Hippo/YAP Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Cant Did Not Increase Proliferation, Cell Cycle, or Apoptosis of RA-FLS Cells
2.2. Cant Suppressed Migration and Invasion of Human RA-FLS Cells
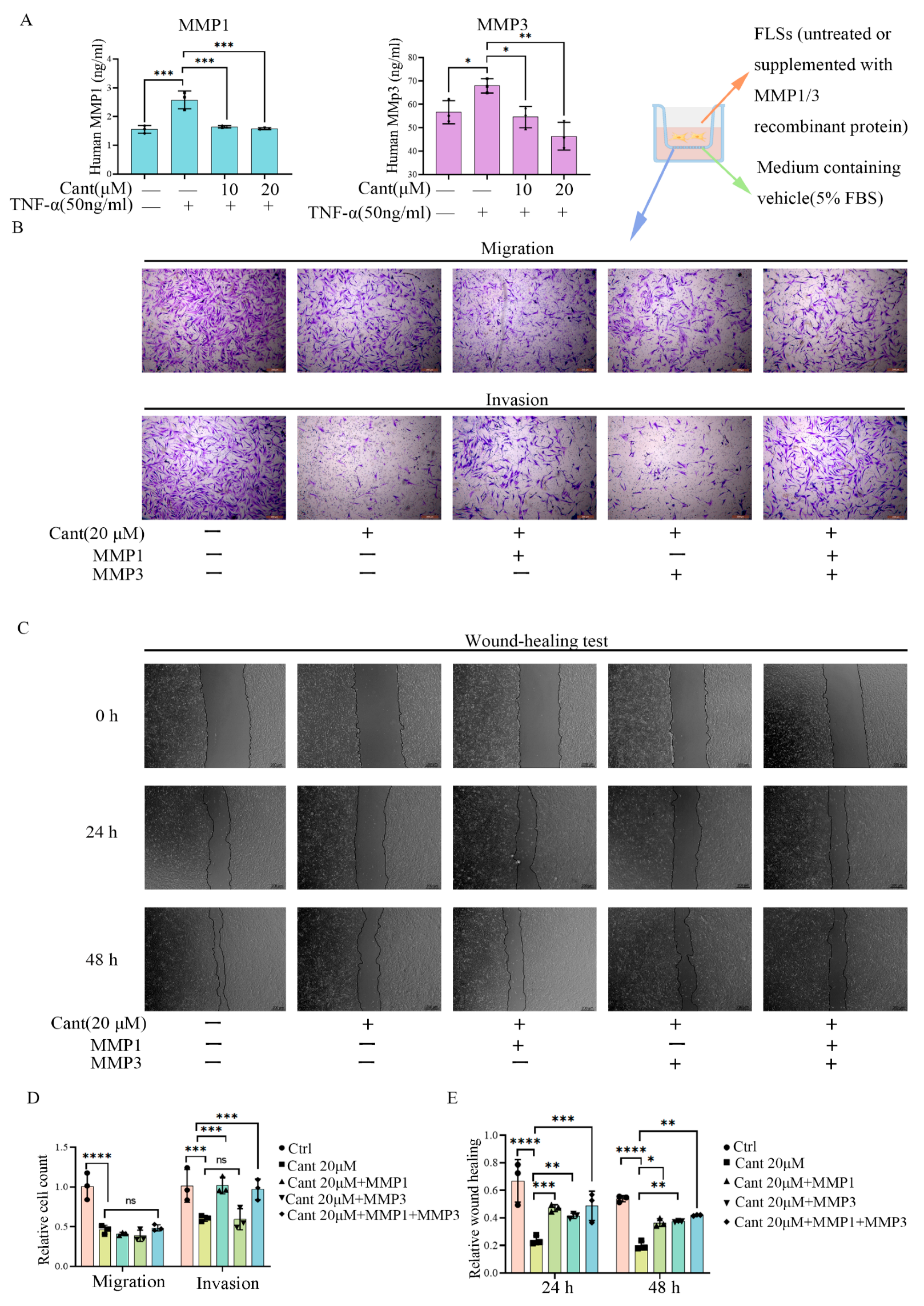
2.3. Cant Suppressed the Expression of Pro-Inflammatory Cytokines and MMPs by RA-FLS Cells
2.4. Cant Suppressed Migration and Invasion of RA-FLS Cells by Attenuating MMP1 Expression
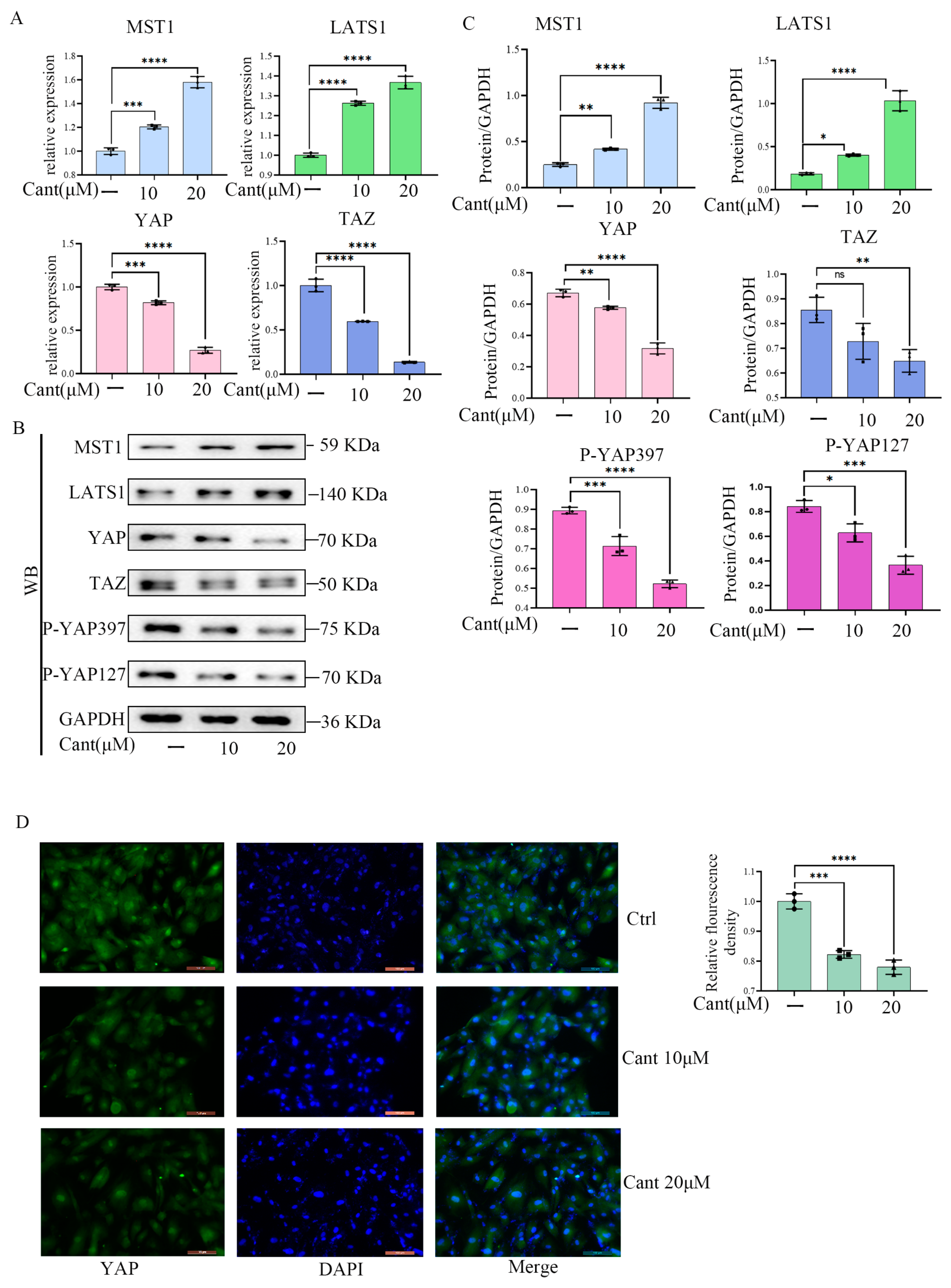
2.5. Cant Suppressed the Expression and Activation of YAP/TAZ Pathway
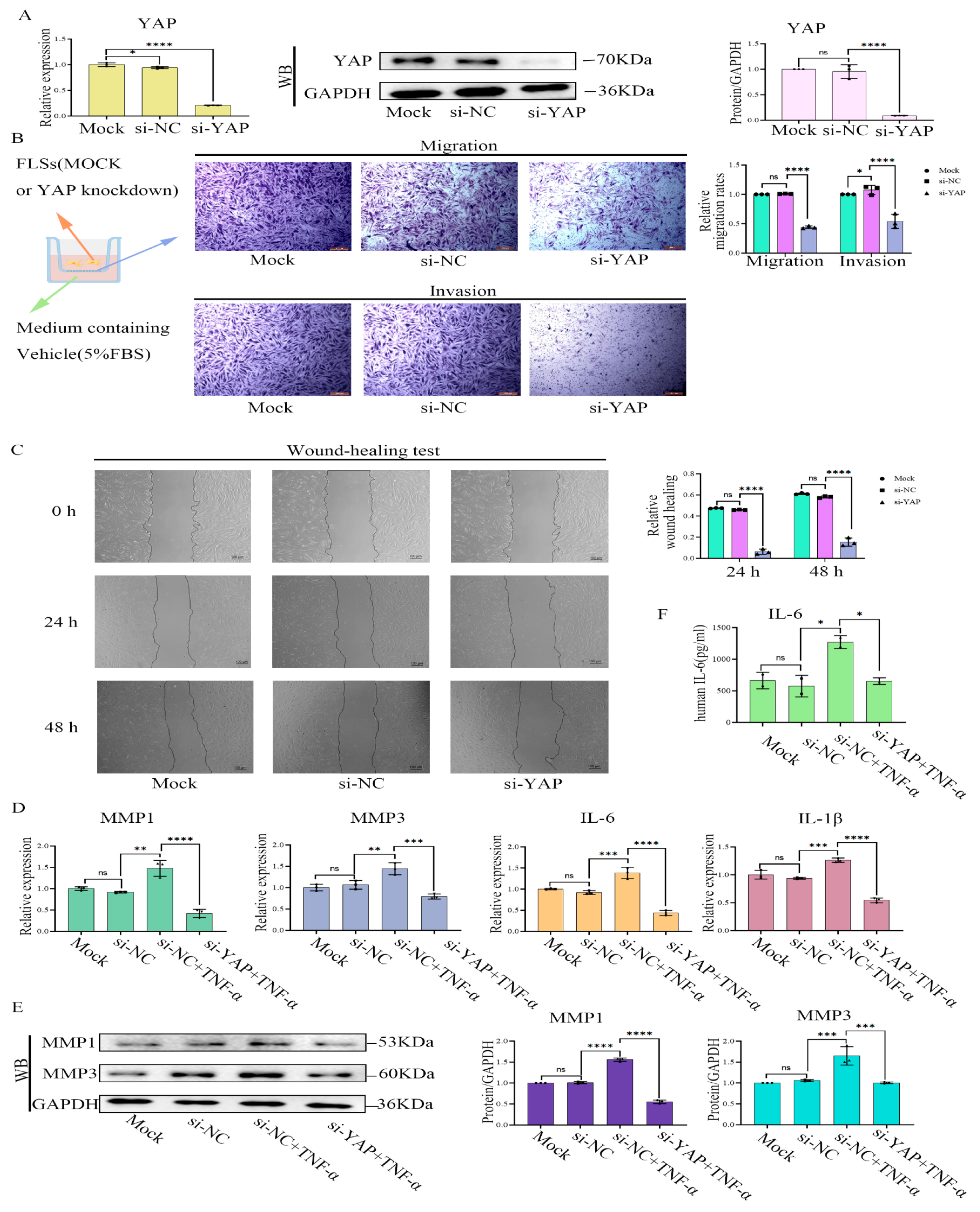
2.6. YAP Inhibition or Treatment with YAP-Specific siRNA Induced Phenotypes of RA-FLS Cells Similar to Treatment with Cant
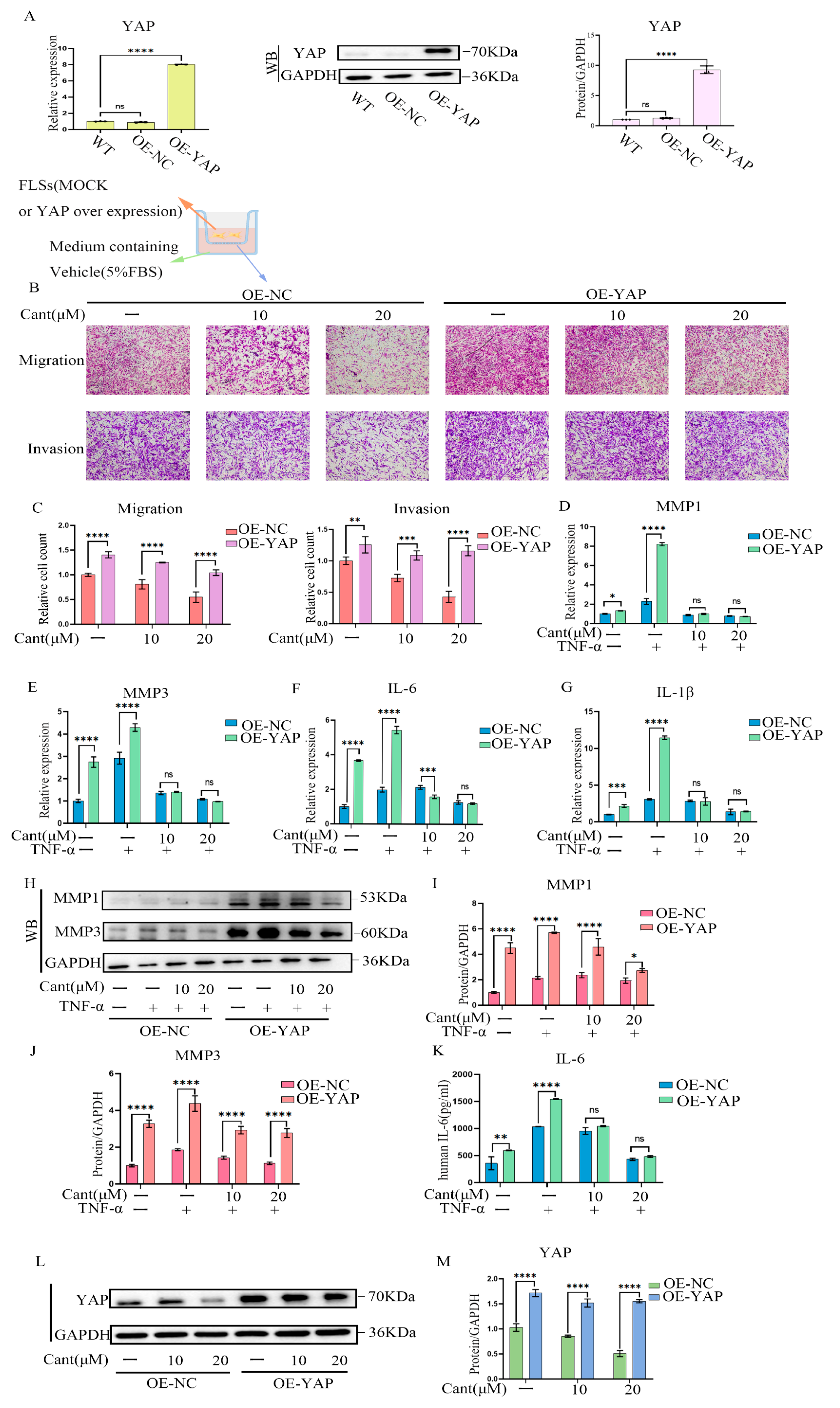
2.7. YAP Over-Expression Partially Reversed Cant-Induced Decline in RA-FLS Cell Migration and Invasion
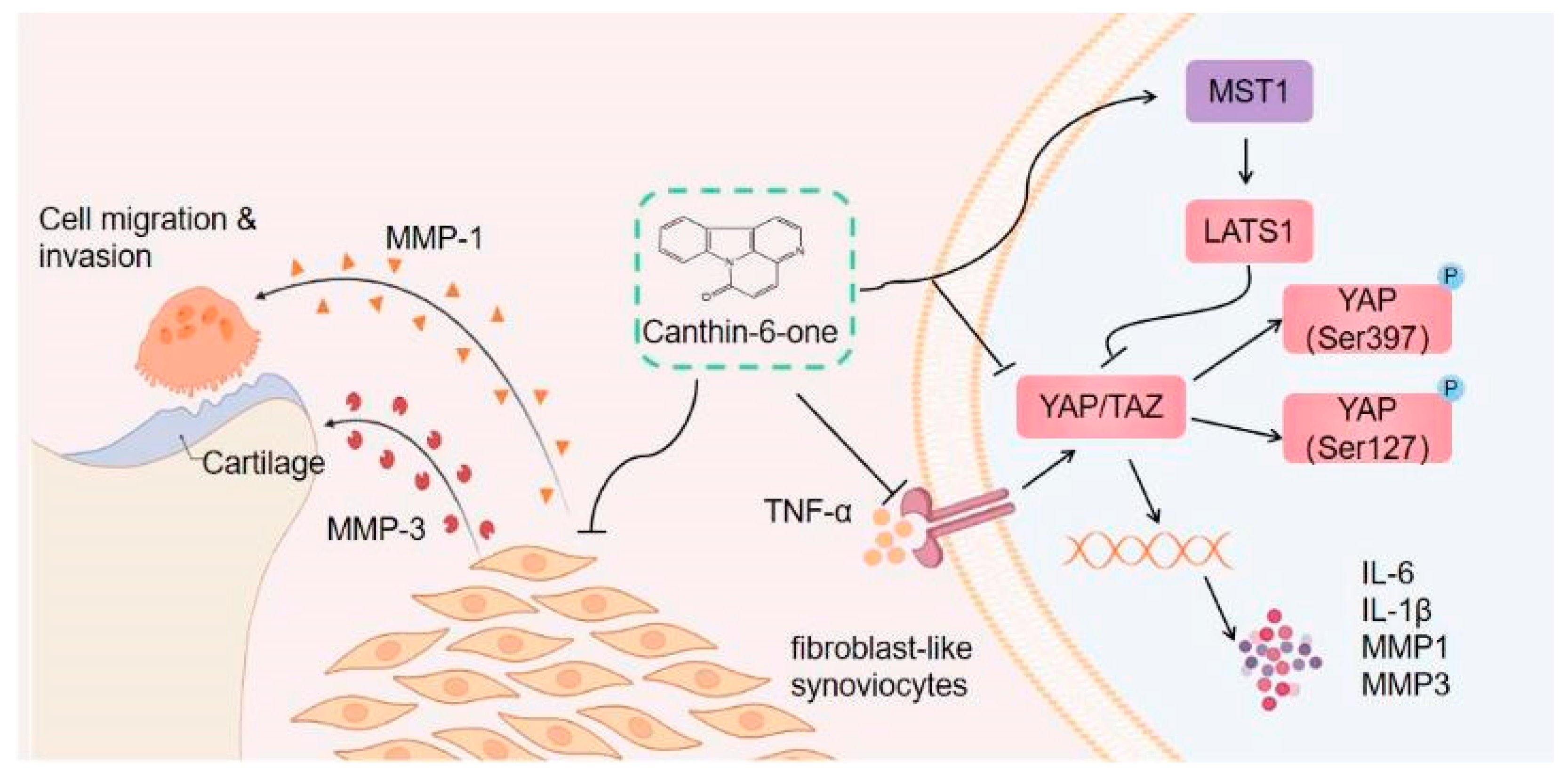
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture
4.3. CCK8 Assay
4.4. Transwell Migration Assays
4.5. Invasion Assays
4.6. Wound-Healing Assays
4.7. Western Blot
4.8. ELISA
4.9. Quantitative Polymerase Chain Reaction (qPCR)
4.10. SiRNA-Mediated Knockdown of YAP
4.11. Cell Apoptosis Assay
4.12. Immunofluorescence Analysis
4.13. EdU Proliferation Assay
4.14. Cell-Cycle Analysis
4.15. Over-Expression of YAP in RA-FLS Cells
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McInnes, I.B. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360. [Google Scholar] [CrossRef]
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward Rheumatoid Arthritis Therapy; the Old and the New. J. Cell. Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, ITC1. [Google Scholar] [CrossRef]
- Qindeel, M.; Ullah, M.H.; Fakhar-ud-Din; Ahmed, N.; Rehman, A. Recent Trends, Challenges and Future Outlook of Transdermal Drug Delivery Systems for Rheumatoid Arthritis Therapy. J. Control. Release 2020, 327, 595–615. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in Treating Patients with Arthritis. Arthritis Res. Ther. 2013, 15, S2. [Google Scholar] [CrossRef] [PubMed]
- Qindeel, M.; Ahmed, N.; Sabir, F.; Khan, S.; Ur-Rehman, A. Development of Novel pH-Sensitive Nanoparticles Loaded Hydrogel for Transdermal Drug Delivery. Drug Dev. Ind. Pharm. 2019, 45, 629–641. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; Mcshane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Rana, A.K.; Li, Y.; Dang, Q.; Yang, F. Monocytes in Rheumatoid Arthritis: Circulating Precursors of Macrophages and Osteoclasts and, Their Heterogeneity and Plasticity Role in RA Pathogenesis. Int. Immunopharmacol. 2018, 65, 348–359. [Google Scholar] [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring Synovial Homeostasis in Rheumatoid Arthritis by Targeting Fibroblast-like Synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like Synoviocytes: Key Effector Cells in Rheumatoid Arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Bottini, N.; Firestein, G.S. Duality of Fibroblast-like Synoviocytes in RA: Passive Responders and Imprinted Aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef]
- Ahn, J.K.; Huang, B.; Bae, E.-K.; Park, E.-J.; Hwang, J.-W.; Lee, J.; Koh, E.-M.; Cha, H.-S. The Role of -Defensin-1 and Related Signal Transduction Mechanisms in the Production of IL-6, IL-8 and MMPs in Rheumatoid Fibroblast-like Synoviocytes. Rheumatology 2013, 52, 1368–1376. [Google Scholar] [CrossRef]
- Sabeh, F.; Fox, D.; Weiss, S.J. Membrane-Type I Matrix Metalloproteinase-Dependent Regulation of Rheumatoid Arthritis Synoviocyte Function. J. Immunol. 2010, 184, 6396–6406. [Google Scholar] [CrossRef]
- Orr, C.; Vieira-Sousa, E.; Boyle, D.L.; Buch, M.H.; Buckley, C.D.; Cañete, J.D.; Catrina, A.I.; Choy, E.H.S.; Emery, P.; Fearon, U.; et al. Synovial Tissue Research: A State-of-the-Art Review. Nat. Rev. Rheumatol. 2017, 13, 463–475. [Google Scholar] [CrossRef]
- Dinesh, P.; Rasool, M. uPA/uPAR Signaling in Rheumatoid Arthritis: Shedding Light on Its Mechanism of Action. Pharmacol. Res. 2018, 134, 31–39. [Google Scholar] [CrossRef]
- Caire, R.; Audoux, E.; Courbon, G.; Michaud, E.; Petit, C.; Dalix, E.; Chafchafi, M.; Thomas, M.; Vanden-Bossche, A.; Navarro, L.; et al. YAP/TAZ: Key Players for Rheumatoid Arthritis Severity by Driving Fibroblast Like Synoviocytes Phenotype and Fibro-Inflammatory Response. Front. Immunol. 2021, 12, 791907. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.-L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Symons, R.A.; Colella, F.; Collins, F.L.; Rafipay, A.J.; Kania, K.; McClure, J.J.; White, N.; Cunningham, I.; Ashraf, S.; Hay, E.; et al. Targeting the IL-6–Yap–Snail Signalling Axis in Synovial Fibroblasts Ameliorates Inflammatory Arthritis. Ann. Rheum. Dis. 2022, 81, 214–224. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, Q.; Wang, H.; Yang, J.; Zhang, C.; Deng, Z.; Wu, K.; Zhou, Y.; Zeng, J.; Zhang, Y.; et al. Knockdown of YAP/TAZ Inhibits the Migration and Invasion of Fibroblast Synovial Cells in Rheumatoid Arthritis by Regulating Autophagy. J. Immunol. Res. 2020, 2020, 9510594. [Google Scholar] [CrossRef]
- Ding, J.F.; Sun, T.T.; Wu, H.M.; Zheng, H.W.; Wang, S.J.; Wang, D.Z.; Shan, W.P.; Ling, Y.; Zhang, Y. Novel Canthin-6-One Derivatives: Design, Synthesis, and Their Antiproliferative Activities via Inducing Apoptosis, DNA Damage, and Ferroptosis. ACS Omega 2023, 8, 31215–31224. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.E.; Rojas de Arias, A.; Torres de Ortiz, S.; Inchausti, A.; Nakayama, H.; Thouvenel, C.; Hocquemiller, R.; Fournet, A. Leishmanicidal Activity of Two Canthin-6-One Alkaloids, Two Major Constituents of Zanthoxylum Chiloperone Var. Angustifolium. J. Ethnopharmacol. 2002, 80, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Gazoni, V.F.; Balogun, S.O.; Arunachalam, K.; Oliveira, D.M.; Filho, V.C.; Lima, S.R.; Colodel, E.M.; Soares, I.M.; Ascêncio, S.D.; Martins, D.T. de O. Assessment of Toxicity and Differential Antimicrobial Activity of Methanol Extract of Rhizome of Simaba Ferruginea A. St.-Hil. and Its Isolate Canthin-6-One. J. Ethnopharmacol. 2018, 223, 122–134. [Google Scholar] [CrossRef]
- Yuan, N.-N.; Cai, C.-Z.; Wu, M.-Y.; Zhu, Q.; Su, H.; Li, M.; Ren, J.; Tan, J.-Q.; Lu, J.-H. Canthin-6-One Accelerates Alpha-Synuclein Degradation by Enhancing UPS Activity: Drug Target Identification by CRISPR-Cas9 Whole Genome-Wide Screening Technology. Front. Pharmacol. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, N.; Wang, J.; Schneider, U. Fruitful Decades for Canthin-6-Ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities. Molecules 2016, 21, 493. [Google Scholar] [CrossRef]
- Arunachalam, K.; Damazo, A.S.; Macho, A.; Matchado, M.S.; Pavan, E.; Figueiredo, F.d.F.; Oliveira, D.M.; Duckworth, C.A.; Thangaraj, P.; Leonti, M.; et al. Canthin-6-One Ameliorates TNBS-Induced Colitis in Rats by Modulating Inflammation and Oxidative Stress. An in Vivo and in Silico Approach. Biochem. Pharmacol. 2021, 186, 114490. [Google Scholar] [CrossRef]
- Fan, H.; Qi, D.; Yang, M.; Fang, H.; Liu, K.; Zhao, F. In Vitro and in Vivo Anti-Inflammatory Effects of 4-Methoxy-5- Hydroxycanthin-6-One, a Natural Alkaloid from Picrasma quassioides. Phytomedicine 2013, 20, 319–323. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, W.; Wei, S.; Wei, D.; Li, R.; Liu, J.; Peng, L.; Yang, S.; Gao, Y.; Wu, C.; et al. Guizhi-Shaoyao-Zhimu Decoction Possesses Anti-Arthritic Effects on Type II Collagen-Induced Arthritis in Rats via Suppression of Inflammatory Reactions, Inhibition of Invasion & Migration and Induction of Apoptosis in Synovial Fibroblasts. Biomed. Pharmacother. 2019, 118, 109367. [Google Scholar] [CrossRef]
- Ma, J.-D.; Jing, J.; Wang, J.-W.; Yan, T.; Li, Q.-H.; Mo, Y.-Q.; Zheng, D.-H.; Gao, J.-L.; Nguyen, K.-A.; Dai, L. A Novel Function of Artesunate on Inhibiting Migration and Invasion of Fibroblast-like Synoviocytes from Rheumatoid Arthritis Patients. Arthritis Res. Ther. 2019, 21, 153. [Google Scholar] [CrossRef]
- VanSaun, M.N.; Matrisian, L.M. Matrix Metalloproteinases and Cellular Motility in Development and Disease. Birth Defects Res. Part C Embryo Today Rev. 2006, 78, 69–79. [Google Scholar] [CrossRef]
- Barrette, A.M.; Ronk, H.; Joshi, T.; Mussa, Z.; Mehrotra, M.; Bouras, A.; Nudelman, G.; Jesu Raj, J.G.; Bozec, D.; Lam, W.; et al. Anti-Invasive Efficacy and Survival Benefit of the YAP-TEAD Inhibitor Verteporfin in Preclinical Glioblastoma Models. Neuro-Oncol. 2022, 24, 694–707. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.R.P.; Huizinga, T.W.J.; Toes, R.E.M. Redefining the HLA and RA Association: To Be or Not to Be Anti-CCP Positive. J. Autoimmun. 2005, 25, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Buch, M.H. Defining Refractory Rheumatoid Arthritis. Ann. Rheum. Dis. 2018, 77, 966–969. [Google Scholar] [CrossRef]
- Schett, G.; Emery, P.; Tanaka, Y.; Burmester, G.; Pisetsky, D.S.; Naredo, E.; Fautrel, B.; van Vollenhoven, R. Tapering Biologic and Conventional DMARD Therapy in Rheumatoid Arthritis: Current Evidence and Future Directions. Ann. Rheum. Dis. 2016, 75, 1428–1437. [Google Scholar] [CrossRef]
- Dudics, S.; Langan, D.; Meka, R.; Venkatesha, S.; Berman, B.; Che, C.-T.; Moudgil, K. Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. Int. J. Mol. Sci. 2018, 19, 2508. [Google Scholar] [CrossRef]
- Showalter, H.D.H. Progress in the Synthesis of Canthine Alkaloids and Ring-Truncated Congeners. J. Nat. Prod. 2013, 76, 455–467. [Google Scholar] [CrossRef]
- Hsieh, P.-W.; Chang, F.-R.; Lee, K.-H.; Hwang, T.-L.; Chang, S.-M.; Wu, Y.-C. A New Anti-HIV Alkaloid, Drymaritin, and a New C-Glycoside Flavonoid, Diandraflavone, from Drymaria d Iandra. J. Nat. Prod. 2004, 67, 1175–1177. [Google Scholar] [CrossRef]
- O’Donnell, G.; Gibbons, S. Antibacterial Activity of Two Canthin-6-One Alkaloids fromAllium Neapolitanum. Phytother. Res. 2007, 21, 653–657. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Hernández Prada, J.A.; Corsino, P.E.; Finton, K.A.; Le, N.; Rowe, T.C. Discovery of Novel DNA Gyrase Inhibitors by High-Throughput Virtual Screening. Antimicrob. Agents Chemother. 2007, 51, 3688–3698. [Google Scholar] [CrossRef]
- Thouvenel, C.; Gantier, J.-C.; Duret, P.; Fourneau, C.; Hocquemiller, R.; Ferreira, M.-E.; de Arias, A.R.; Fournet, A. Antifungal Compounds from Zanthoxylum Chiloperone Var. Angustifolium. Phytother. Res. 2003, 17, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Dejos, C.; Régnacq, M.; Bernard, M.; Voisin, P.; Bergès, T. The MFS-Type Efflux Pump Flr1 Induced by Yap1 Promotes Canthin-6-One Resistance in Yeast. FEBS Lett. 2013, 587, 3045–3051. [Google Scholar] [CrossRef] [PubMed]
- Peduto, A.; More, V.; de Caprariis, P.; Festa, M.; Capasso, A.; Piacente, S.; Martino, L.D.; Filosa, R. Synthesis and Cytotoxic Activity of New β-Carboline Derivatives. Mini Rev. Med. Chem. 2011, 11, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Ammirante, M.; Di Giacomo, R.; De Martino, L.; Rosati, A.; Festa, M.; Gentilella, A.; Pascale, M.C.; Belisario, M.A.; Leone, A.; Caterina Turco, M.; et al. 1-Methoxy-Canthin-6-One Induces c-Jun NH 2 -Terminal Kinase–Dependent Apoptosis and Synergizes with Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Activity in Human Neoplastic Cells of Hematopoietic or Endodermal Origin. Cancer Res. 2006, 66, 4385–4393. [Google Scholar] [CrossRef]
- Cho, S.-K.; Jeong, M.; Jang, D.; Choi, J.-H. Anti-Inflammatory Effects of Canthin-6-One Alkaloids from Ailanthus Altissima. Planta Med. 2018, 84, 527–535. [Google Scholar] [CrossRef]
- Klein, K.; Gay, R.E.; Gay, S. Synoviale Fibroblasten: Hauptakteure bei der rheumatoiden Arthritis. Z. Für Rheumatol. 2016, 75, 560–564. [Google Scholar] [CrossRef]
- Korb-Pap, A.; Bertrand, J.; Sherwood, J.; Pap, T. Stable Activation of Fibroblasts in Rheumatic Arthritis—Causes and Consequences. Rheumatology 2016, 55, ii64–ii67. [Google Scholar] [CrossRef]
- Green, M.J. Serum MMP-3 and MMP-1 and Progression of Joint Damage in Early Rheumatoid Arthritis. Rheumatology 2003, 42, 83–88. [Google Scholar] [CrossRef]
- Yamanaka, H.; Matsuda, Y.; Tanaka, M.; Sendo, W.; Nakajima, H.; Taniguchi, A.; Kamatani, N. Serum Matrix Metalloproteinase 3 as a Predictor of the Degree of Joint Destruction during the Six Months after Measurement, in Patients with Early Rheumatoid Arthritis. Arthritis Rheum. 2000, 43, 852. [Google Scholar] [CrossRef]
- Shinozaki, M.; Inoue, E.; Nakajima, A.; Hara, M.; Tomatsu, T.; Kamatani, N.; Yamanaka, H. Elevation of Serum Matrix Metalloproteinase-3 as a Predictive Marker for the Long-Term Disability of Rheumatoid Arthritis Patients in a Prospective Observational Cohort IORRA. Mod. Rheumatol. 2007, 17, 403–408. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De La Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, A.; Wang, Y.; Sun, W.; Zhou, X.; Xu, Q.; Mao, L.; Zhang, J. Canthin-6-Ones: Potential Drugs for Chronic Inflammatory Diseases by Targeting Multiple Inflammatory Mediators. Molecules 2023, 28, 3381. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Xu, Y.; Lin, L.; Hoi, M.P.M. Canthin-6-One (CO) from Picrasma quassioides (D.Don) Benn. Ameliorates Lipopolysaccharide (LPS)-Induced Astrocyte Activation and Associated Brain Endothelial Disruption. Phytomedicine 2022, 101, 154108. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, X.; Zeng, Y.; Huang, X.; Chen, H.; Wang, S.; Wu, J.; Li, Q.; Zhu, W.; Li, H.; et al. A Novel Phytochemical, DIM, Inhibits Proliferation, Migration, Invasion and TNF-α Induced Inflammatory Cytokine Production of Synovial Fibroblasts From Rheumatoid Arthritis Patients by Targeting MAPK and AKT/mTOR Signal Pathway. Front. Immunol. 2019, 10, 1620. [Google Scholar] [CrossRef] [PubMed]



| Genes | Sequences | |
|---|---|---|
| GAPDH | F: | CCACTCCTCCACCTTTGACGC |
| R: | CCACCCTGTTGCTGTAGCCA | |
| MMP1 | F: | TTTGTCAGGGGAGATCATCGG |
| R: | TCCAAGAGAATGGCCGAGTT | |
| MMP3 | F: | TGGACAAAGGATACAACAGGGAC |
| R: | ATCTTGAGACAGGCGGAACC | |
| IL-6 | F: | TTCTCCACAAGCGCCTTC |
| R: | AGAGGTGAGTGGCTGTCTGT | |
| IL-1β | F: | AACCTCTTCGAGGCACAAGG |
| R: | GTCCTGGAAGGAGCACTTCAT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, Y.; Xu, Q.; Zhou, X.; Ling, Y.; Zhang, J.; Mao, L. Methyl Canthin-6-one-2-carboxylate Restrains the Migration/Invasion Properties of Fibroblast-like Synoviocytes by Suppressing the Hippo/YAP Signaling Pathway. Pharmaceuticals 2023, 16, 1440. https://doi.org/10.3390/ph16101440
Zhang Z, Wang Y, Xu Q, Zhou X, Ling Y, Zhang J, Mao L. Methyl Canthin-6-one-2-carboxylate Restrains the Migration/Invasion Properties of Fibroblast-like Synoviocytes by Suppressing the Hippo/YAP Signaling Pathway. Pharmaceuticals. 2023; 16(10):1440. https://doi.org/10.3390/ph16101440
Chicago/Turabian StyleZhang, Zongying, Yunhan Wang, Qiuyun Xu, Xiaorong Zhou, Yong Ling, Jie Zhang, and Liming Mao. 2023. "Methyl Canthin-6-one-2-carboxylate Restrains the Migration/Invasion Properties of Fibroblast-like Synoviocytes by Suppressing the Hippo/YAP Signaling Pathway" Pharmaceuticals 16, no. 10: 1440. https://doi.org/10.3390/ph16101440
APA StyleZhang, Z., Wang, Y., Xu, Q., Zhou, X., Ling, Y., Zhang, J., & Mao, L. (2023). Methyl Canthin-6-one-2-carboxylate Restrains the Migration/Invasion Properties of Fibroblast-like Synoviocytes by Suppressing the Hippo/YAP Signaling Pathway. Pharmaceuticals, 16(10), 1440. https://doi.org/10.3390/ph16101440