Heavy Metal-Based Nanoparticles as High-Performance X-ray Computed Tomography Contrast Agents
Abstract
:1. Introduction
2. Basic Principles of CT Contrast Agents
3. Heavy Metal-Based CT Contrast Agents
3.1. Noble Metal-Based NPs
3.1.1. Pd (Z = 46)-NPs
3.1.2. Ag (Z = 47)-NPs
3.1.3. Pt (Z = 78)-NPs
3.1.4. Au (Z = 79)-NPs
3.2. Lanthanide (Ln)-Based NPs
3.2.1. Ce (Z = 58)-Based NPs
3.2.2. Gd (Z = 64)-Based NPs
3.2.3. Dy (Z = 66)-Based NPs
3.2.4. Ho (Z = 67)-Based NPs
3.2.5. Yb (Z = 70)-Based NPs
3.3. Other Heavy Metal-Based NPs (Ta, W, and Bi)
3.3.1. Ta (Z = 73)-Based NPs
3.3.2. W (Z = 74)-Based NPs
3.3.3. Bi (Z = 83)-Based NPs
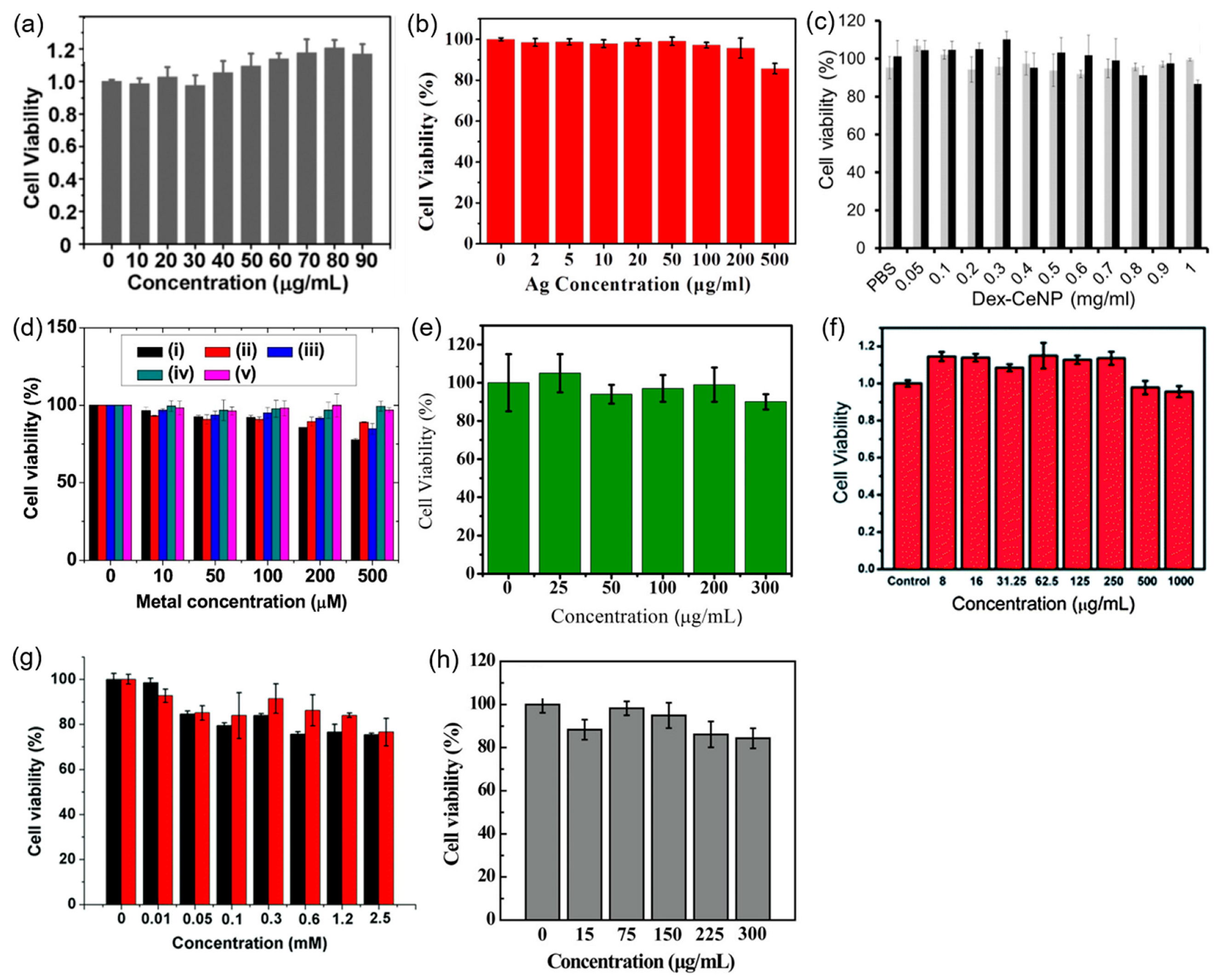
4. Cytotoxicity
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Metal-based nanoparticles, sensors, and their multifaceted application in food packing. J. Nanobiotechnol. 2021, 19, 256. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Khan, R.M.R.; Rashid, M. Gold, silver, and palladium nanoparticles: A chemical tool for biomedical applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; NIST: Gaithersburg, MD, USA, 1996. Available online: http://www.nist.gov/pml/data/xraycoef (accessed on 1 October 2023).
- Yu, S.-B.; Watson, A.D. Metal-based X-ray contrast media. Chem. Rev. 1999, 99, 2353–2377. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-sized CT contrast agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Nanoparticulate X-ray computed tomography contrast agents: From design validation to in vivo applications. Acc. Chem. Res. 2012, 45, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, J.C.; Hafeli, U.O. Utilization of nanoparticles as X-ray contrast agents for diagnostic imaging applications. Contrast. Media Mol. I 2015, 10, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Aslan, N.; Ceylan, B.; Koç, M.M.; Findik, F. Metallic Nanoparticles as X-ray Computed Tomography (CT) Contrast Agents: A Review. J. Mol. Struct. 2020, 1219, 128599. [Google Scholar] [CrossRef]
- Spampinato, M.V.; Abid, A.; Matheus, M.G. Current radiographic iodinated contrast agents. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 697–704. [Google Scholar] [CrossRef]
- Singh, J.; Daftary, A. Iodinated Contrast Media and Their Adverse Reactions. J. Nucl. Med. Technol. 2008, 36, 69–74. [Google Scholar] [CrossRef]
- Taghavi, H.; Bakhshandeh, M.; Montazerabadi, A.; Nazari Moghadam, H.; Mazloom Shahri, S.B.; Keshtkar, M. Comparison of Gold Nanoparticles and Iodinated Contrast Media in Radiation Dose Reduction and Contrast Enhancement in Computed Tomography. Iran. J. Radiol. 2020, 17, e92446. [Google Scholar] [CrossRef]
- Swain, S.; Sahu, P.K.; Beg, S.; Babu, S.M. Nanoparticles for cancer targeting: Current and future directions. Curr. Drug. Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Higashisaka, K.; Tsutsumi, Y. Biocompatibility of Nanomaterials. In Nanomaterials in Pharmacology. Methods in Pharmacology and Toxicology; Lu, Z.-R., Sakuma, S., Eds.; Humana Press: New York, NY, USA, 2016; pp. 185–199. [Google Scholar]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of nano-particles. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-W.; Chambers, E.; Mitragotri, S. Factors that control the circulation time of nanoparticles in blood: Challenges, solutions and future prospects. Curr. Pharm. Des. 2010, 16, 2298–2307. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug. Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Lyu, M.; Zhu, D.; Duo, Y.; Li, Y.; Quan, H. Bimetallic Nanodots for Tri-Modal CT/MRI/PA Imaging and Hypoxia-Resistant Thermoradiotherapy in the NIR-II Biological Windows. Biomaterials 2020, 233, 119656. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Guo, R.; Cao, X.; Zhao, J.; Luo, Y.; Shen, M.; Zhang, G.; Shi, X. Size-controlled synthesis of dendrimer-stabilized silver nanoparticles for X-ray computed tomography imaging applications. Polym. Chem. 2010, 1, 1677–1683. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, M.; Chen, M.; Li, L.; Dong, C.; Hou, Y.; Zhao, H.; Jia, B.; Wang, F. Hyaluronic Acid-Coated Silver Nanoparticles as a Nanoplatform for In Vivo Imaging Applications. ACS Appl. Mater. Interfaces 2016, 8, 25650–25653. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Cruz, E.D.; Lau, K.C.; Bouché, M.; Kim, J.; Maidment, A.D.A.; Cormode, D.P. Renally Excretable and Size-Tunable Silver Sulfide Nanoparticles for Dual-Energy Mammography or Computed Tomography. Chem. Mater. 2019, 31, 7845–7854. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, J.; Zhou, Q.; Liang, P.; Wang, Y.; Gao, X.; Wang, Y. Renal Clearable Ag Nanodots for In Vivo Computer Tomography Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 5900–5906. [Google Scholar] [CrossRef]
- Naha, P.C.; Hsu, J.C.; Kim, J.; Shah, S.; Bouché, M.; Si-Mohamed, S.; Rosario-Berrios, D.N.; Douek, P.; Hajfathalian, M.; Yasini, P.; et al. Dextran-Coated Cerium Oxide Nanoparticles: A Computed Tomography Contrast Agent for Imaging the Gastrointestinal Tract and Inflammatory Bowel Disease. ACS Nano 2020, 14, 10187–10197. [Google Scholar] [CrossRef]
- García, A.; Cámara, J.A.; Boullosa, A.M.; Gustà, M.F.; Mondragón, L.; Schwartz, S., Jr.; Casals, E.; Abasolo, I.; Bastús, N.G.; Puntes, V. Nanoceria as Safe Contrast Agents for X-ray CT Imaging. Nanomaterials 2023, 13, 2208. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Xu, W.; Kim, S.J.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Park, J.A.; Kim, T.J.; Lee, G.H. Potential dual imaging nanoparticle: Gd2O3 nanoparticle. Sci. Rep. 2015, 5, 8549. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, A.; Marasini, S.; Miao, X.; Park, J.A.; Jung, K.-H.; Ahmad, M.Y.; Yue, H.; Ho, S.L.; Liu, S.; Jang, Y.J.; et al. Synthesis, characterization, and X-ray attenuation properties of polyacrylic acid-coated ultrasmall heavy metal oxide (Bi2O3, Yb2O3, NaTaO3, Dy2O3, and Gd2O3) nanoparticles as potential CT contrast agents. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 73–81. [Google Scholar] [CrossRef]
- Lee, E.J.; Heo, W.C.; Park, J.W.; Chang, Y.; Bae, J.-E.; Chae, K.S.; Kim, T.J.; Park, J.A.; Lee, G.H. D-Glucuronic Acid Coated Gd(IO3)3·2H2O Nanomaterial as a Potential T1 MRI-CT Dual Contrast Agent. Eur. J. Inorg. Chem. 2013, 2013, 2858–2866. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, P.; Lei, P.; Song, S.; Xu, X.; Du, K.; Feng, J.; Zhang, H. PEGylated GdF3:Fe Nanoparticles as Multimodal T1/T2-Weighted MRI and X-ray CT Imaging Contrast Agents. ACS Appl. Mater. Interfaces 2017, 9, 20426–20434. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Sun, L.D.; Zheng, T.; Dong, H.; Li, Y.; Wang, Y.F.; Yan, C.H. PAA-capped GdF3 Nanoplates as Dual-mode MRI and CT Contrast Agents. Sci. Bull. 2015, 60, 1092–1100. [Google Scholar] [CrossRef]
- Olifirenko, V.; Abduraimova, A.; Kang, M.S.; Raja, I.S.; Duisenbayeva, B.; Molkenova, A.; Khamkhash, L.; Hwang, Y.-H.; Han, D.-W.; Atabaev, T.S. Potential applicability of polyethyleneimine PEI-coated Eu2O3 and Dy2O3 nanoparticles for contrast enhancement in computed tomography. Nano Express 2021, 2, 010022. [Google Scholar] [CrossRef]
- Gómez-Gónzalez, E.; Núñez, N.O.; Caro, C.; García-Martín, M.L.; Fernández-Afonso, Y.; de la Fuente, J.M.; Balcerzyk, M.; Ocaña, M. Dysprosium and holmium vanadate nanoprobes as high-performance contrast agents for high-field magnetic resonance and computed tomography imaging. Inorg. Chem. 2021, 60, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Deng, M.; Zhang, L.; Liu, Z.; Liu, Y.; Song, S.; Gong, T.; Yuan, Q. Facile Synthesis of Holmium-Based Nanoparticles as a CT and MRI Dual-Modal Imaging for Cancer Diagnosis. Front. Oncol. 2021, 11, 741383. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ni, D.L.; Bu, W.B.; Zhou, Q.; Fan, W.P.; Wu, Y.; Liu, Y.Y.; Yin, L.K.; Cui, Z.W.; Zhang, X.X.; et al. BaHoF5 nanoprobes as high-performance contrast agents for multi-modal CT imaging of ischemic stroke. Biomaterials 2015, 71, 110–118. [Google Scholar] [CrossRef]
- Ni, D.; Zhang, J.; Bu, W.; Zhang, C.; Yao, Z.; Xing, H.; Wang, J.; Duan, F.; Liu, Y.; Fan, W.; et al. PEGylated NaHoF4 nanoparticles as contrast agents for both X-ray computed tomography and ultra-high field magnetic resonance imaging. Biomaterials 2016, 76, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, A.; Marasini, S.; Tegafaw, T.; Ho, S.L.; Miao, X.; Ahmad, M.Y.; Yue, H.; Lee, G.H.; Park, J.A.; Jung, K.-H.; et al. X-ray attenuation properties of ultrasmall Yb2O3 nanoparticles as a high-performance CT contrast agent. J. Korean Phys Soc. 2019, 74, 286–291. [Google Scholar] [CrossRef]
- Silva, M.O.; Kirkwood, N.; Mulvaney, P.; Ellis, A.V.; Stok, K.S. Evaluation of a lanthanide nanoparticle-based contrast agent for microcomputed tomography of porous channels in subchondral bone. J. Orthopaed. Res. 2023, 41, 447–458. [Google Scholar] [CrossRef]
- Dong, Y.C.; Kumar, A.; Rosario-Berríos, D.N.; Si-Mohamed, S.; Hsu, J.C.; Nieves, L.M.; Douek, P.; Noël, P.B.; Cormode, D.P. Ytterbium Nanoparticle Contrast Agents for Conventional and Spectral Photon-Counting CT and Their Applications for Hydrogel Imaging. ACS Appl. Mater. Interfaces 2022, 14, 39274–39284. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xin, R.; Li, Z.; Golamaully, R.; Zhang, Y.; Zhang, J.; Yuan, Q.; Liu, X. Large-scale and facile synthesis of biocompatible Yb-based nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. Curr. Top. Med. Chem. 2013, 13, 513–518. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Yuan, Q.; He, Y.; Lu, L. A high-performance ytterbium-based nanoparticulate contrast agent for in vivo X-ray computed tomography imaging. Angew. Chem. 2012, 124, 1466–1471. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Liu, J.; Gu, S.; Yuan, Q.; Ren, J.; Qu, X. Long-circulating Er3+-doped Yb2O3 up-conversion nanoparticles as an in vivo X-ray CT imaging contrast agent. Biomaterials 2012, 33, 6748–6757. [Google Scholar] [CrossRef]
- Oh, M.H.; Lee, N.; Kim, H.; Park, S.P.; Piao, Y.; Lee, J.; Jun, S.W.; Moon, W.K.; Choi, S.H.; Hyeon, T. Large-scale synthesis of bioinert tantalum oxide nanoparticles for X-ray computed tomography imaging and bimodal image-guided sentinel lymph node mapping. J. Am. Chem. Soc. 2021, 133, 5508–5515. [Google Scholar] [CrossRef] [PubMed]
- Bonitatibus, P.J.; Torres, A.S.; Goddard, G.D.; FitzGerald, P.F.; Kulkarni, A.M. Synthesis, characterization, and computed tomography imaging of a tantalum oxide nanoparticle imaging agent. Chem. Comm. 2010, 46, 8956–8958. [Google Scholar] [CrossRef] [PubMed]
- Bonitatibus, P.J.; Torres, A.S.; Kandapallil, B.; Lee, B.D.; Goddard, G.D.; Colborn, R.E.; Marino, M.E. Preclinical assessment of a zwitterionic tantalum oxide nanoparticle X-ray contrast agent. ACS Nano 2012, 6, 6650–6658. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; Liu, J.; Tong, W.W.L.; Askhativa, D.; Shi, J. Tantalum sulfide nanosheets as a theranostic nanoplatform for computed tomography imaging-guided combinatorial chemo-photothermal therapy. Adv. Funct. Mater. 2017, 27, 1703261. [Google Scholar] [CrossRef]
- Kim, S.J.; Xu, W.; Ahmad, M.W.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Kim, T.J.; Park, J.A.; Lee, G.H. Synthesis of nanoparticle CT contrast agents: In vitro and in vivo studies. Sci. Technol. Adv. Mater. 2015, 16, 055003. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, X.; Zheng, X.; Yin, W.; Ruan, L.; Liu, X.; Zhou, L.; Yan, L.; Li, S.; Gu, Z.; et al. Multifunctional RbxWO3 nanorods for simultaneous combined chemo-photothermal therapy and photoacoustic/CT imaging. Small 2014, 10, 4160–4170. [Google Scholar]
- Zhou, Z.; Kong, B.; Yu, C.; Shi, X.; Wang, M.; Liu, W.; Sun, Y.; Zhang, Y.; Yang, H.; Yang, S. Tungsten oxide nanorods: An efficient nanoplatform for tumor CT imaging and photothermal therapy. Sci. Rep. 2014, 4, 3653. [Google Scholar] [CrossRef] [PubMed]
- Jakhmola, A.; Anton, N.; Anton, H.; Messaddeq, N.; Hallouard, F.; Klymchenko, A.; Mely, Y.; Vandamme, T.F. Poly-ε-caprolactone tungsten oxide nanoparticles as a contrast agent for X-ray computed tomography. Biomaterials 2014, 35, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Liu, Z.; Liu, J.; Huang, S.; Li, Z.; Yuan, Q.; Ren, J.; Qu, X. Biocompatible and high-performance amino acids-capped MnWO4 nanocasting as a novel non-lanthanide contrast agent for X-ray computed tomography and T1-weighted magnetic resonance imaging. Nanoscale 2014, 6, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, L.; Huang, C.; Huang, Y.; Jia, N. Albumin-mediated platinum nanocrystals for in vivo enhanced computed tomography imaging. J. Mater. Chem. B 2017, 5, 3498–3510. [Google Scholar] [CrossRef]
- Saidi, A.K.A.A.; Ghazanfari, A.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; Zhao, D.; Liu, Y.; Yang, S.H.; Hwang, D.W.; Yang, J.-U.; et al. Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers. Nanomaterials 2023, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, M.; Zhou, Q.; Dang, M.; Tang, Y.; Wang, S.; Fu, J.; Teng, Z.; Lu, G. Computed tomography and photoacoustic imaging guided photodynamic therapy against breast cancer based on mesoporous platinum with in situ oxygen generation ability. Acta Pharm. Sin. B 2020, 10, 1719–1729. [Google Scholar] [CrossRef]
- Fu, B.; Dang, M.; Tao, J.; Li, Y.; Tang, Y. Mesoporous platinum nanoparticle-based nanoplatforms for combined chemo-photothermal breast cancer therapy. J. Colloid Interface Sci. 2020, 570, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cheng, L.; Gong, F.; Dong, Z.; Liang, C.; Wang, M.; Feng, L.; Li, Y.; Liu, Z.; Li, C.; et al. Platinum nanoworms for imaging-guided combined cancer therapy in the second near-infrared window. J. Mater. Chem. B 2018, 6, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Mehrdel, B.; Khaniabadi, P.M.; Khaniabadi, B.M. Green sonochemical synthesis platinum nanoparticles as a novel contrast agent for computed tomography. Mater. Today Commun. 2021, 27, 102480. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, T.; Wang, Q.; Lv, X.; Song, X.; Ke, H.; Guo, Z.; Huang, X.; Hu, J.; Li, Z.; et al. Albumin-coordinated assembly of clearable platinum nanodots for photo-induced cancer theranostics. Biomaterials 2018, 154, 248–260. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Yang, Y.; Jin, L.; Liu, Y.; Wang, Y.; Yan, X.; Xu, J.; Gao, R.; Lei, P.; et al. Plasmonic Pt Superstructures with Boosted Near-Infrared Absorption and Photothermal Conversion Efficiency in the Second Biowindow for Cancer Therapy. Adv. Mater. 2019, 31, 1904836. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Kim, S.H.; Choi, K.S.; Kim, S.Y.; Byun, S.J.; Kim, K.W.; Park, S.H.; Juhng, S.K.; Yoon, K.-H. Colloidal gold nanoparticles as a blood-pool contrast agent for X-ray computed tomography in mice. Investig. Radiol. 2007, 42, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.D.; Cole, L.E.; Tilley, J.M.R.; Roeder, R.K. Effects of Functionalized Gold Nanoparticle Size on X-ray Attenuation and Substrate Binding Affinity. Chem. Mater. 2014, 26, 1187–1194. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Chu, Z.; Huang, C.; Huang, Y.; Jia, N. Gemcitabine-loaded gold nanospheres mediated by albumin for enhanced anti-tumor activity combining with CT imaging. Mater. Sci. Eng. C 2018, 89, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zheng, L.; Peng, C.; Li, D.; Li, J.; Wen, S.; Shen, M.; Zhang, G.; Shi, X. Synthesis and Characterization of PEGylated Polyethylenimine-Entrapped Gold Nanoparticles for Blood Pool and Tumor CT Imaging. ACS Appl. Mater. Interfaces 2014, 6, 17190–17199. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Xu, Y.; Guo, R.; Wen, S.; Huang, Y.; Liu, W.; Shen, M.; Zhao, J.; Zhang, G.; et al. Lactobionic Acid-Modified Dendrimer-Entrapped Gold Nanoparticles for Targeted Computed Tomography Imaging of Human Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 6944–6953. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, H.; Peng, C.; Shen, M.; Pan, M.; Cao, X.; Zhang, G.; Shi, X. X-ray Attenuation Property of Dendrimer-Entrapped Gold Nanoparticles. J. Phys. Chem. C 2010, 114, 50–56. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, L.; Wen, S.; Tang, Y.; Shen, M.; Zhang, G.; Shi, X. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2014, 35, 7635–7646. [Google Scholar] [CrossRef]
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; Robertson, D.J.; Chandrasekhar, M.; Kannan, R.; Katti, K.V. Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: In vivo pharmacokinetics and X-ray-contrast-imaging studies. Small 2007, 3, 333–341. [Google Scholar] [CrossRef]
- Huang, P.; Bao, L.; Zhang, C.L.; Lin, J.; Luo, T.; Yang, D.P.; He, M.; Li, Z.M.; Gao, G.; Gao, B.; et al. Folic Acid-conjugated Silica-modified Gold Nanorods for X-ray/CT Imaging-guided Dual-mode Radiation and Photo-thermal Therapy. Biomaterials 2011, 32, 9796–9809. [Google Scholar] [CrossRef]
- Sun, I.-C.; Na, J.H.; Jeong, S.Y.; Kim, D.-E.; Kwon, I.C.; Choi, K.; Ahn, C.-H.; Kim, K. Biocompatible Glycol Chitosan-Coated Gold Nanoparticles for Tumor-Targeting CT Imaging. Pharm. Res. 2014, 31, 1418–1425. [Google Scholar] [CrossRef]
- Li, C.-H.; Kuo, T.-R.; Su, H.-J.; Lai, W.-Y.; Yang, P.-C.; Chen, J.-S.; Wang, D.-Y.; Wu, Y.-C.; Chen, C.-C. Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef]
- Mzwd, E.; Ahmed, N.M.; Suradi, N.; Alsaee, S.K.; Altowyan, A.S.; Almessiere, M.A.; Omar, A.F. Green synthesis of gold nanoparticles in Gum Arabic using pulsed laser ablation for CT imaging. Sci. Rep. 2022, 12, 10549. [Google Scholar] [CrossRef]
- Liu, H.; Shen, M.; Zhao, J.; Zhu, J.; Xiao, T.; Cao, X.; Zhang, G.; Shi, X. Facile formation of folic acid-modified dendrimer-stabilized gold–silver alloy nanoparticles for potential cellular computed tomography imaging applications. Analyst 2013, 138, 1979–1987. [Google Scholar] [CrossRef]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.N.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P.; et al. Effect of Gold Nanoparticle Size on Their Properties as Contrast Agents for Computed Tomography. Sci. Rep. 2019, 9, 14912. [Google Scholar] [CrossRef]
- Tsvirkun, D.; Ben-Nun, Y.; Merquiol, E.; Zlotver, I.; Meir, K.; Weiss-Sadan, T.; Matok, I.; Popovtzer, R.; Blum, G. CT Imaging of Enzymatic Activity in Cancer Using Covalent Probes Reveal a Size-Dependent Pattern. J. Am. Chem. Soc. 2018, 140, 12010–12020. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.-C.; Eun, D.-K.; Na, J.H.; Lee, S.; Kim, I.-J.; Youn, I.-C.; Ko, C.-Y.; Kim, H.-S.; Lim, D.; Choi, K.; et al. Heparin-Coated Gold Nanoparticles for Liver-Specific CT Imaging. Chem. Eur. J. 2009, 15, 13341–13347. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Ghazanfari, A.; Jung, K.-H.; Park, J.A.; Marasini, S.; Ho, S.L.; Miao, X.; Ahmad, M.Y.; Yue, H.; Yang, S.; Chae, K.S.; et al. Size-controlled one-pot polyol synthesis and characterization of D-glucuronic acid-coated ultrasmall BiOI nanoparticles as potential x-ray contrast agent. Mater. Res. Express 2018, 6, 015039. [Google Scholar] [CrossRef]
- Kandanapitiye, M.S.; Gao, M.; Molter, J.; Flask, C.A.; Huang, S.D. Synthesis, Characterization, and X-ray Attenuation Properties of Ultrasmall BiOI Nanoparticles: Toward Renal Clearable Particulate CT Contrast Agents. Inorg. Chem. 2014, 53, 10189–10194. [Google Scholar] [CrossRef] [PubMed]
- Rabin, O.; Perez, J.M.; Grimm, J.; Wojtkiewicz, G.; Weissleder, R. An X-Ray Computed Tomography Imaging Agent Based on Long-Circulating Bismuth Sulphide Nanoparticles. Nat. Mater. 2006, 5, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Naha, P.C.; Benavides-montes, V.; Litt, H.I.; Goforth, A.M.; Cormode, D.P. Synthesis, X-ray Opacity, and Biological Compatibility of Ultra-High Payload Elemental Bismuth Nanoparticle X-ray Contrast Agents. Chem. Mater. 2014, 26, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Swy, E.R.; Schwartz-Duval, A.S.; Shuboni, D.D.; Latourette, M.T.; Mallet, C.L.; Parys, M.; Cormode, D.P.; Shapiro, E.M. Dual-Modality, Fluorescent, PLGA Encapsulated Bismuth Nanoparticles for Molecular and Cellular Fluorescence Imaging and Computed Tomography. Nanoscale 2014, 6, 13104–13112. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhang, X.; Zhang, C.; Jiang, Y.; Fu, Y.Y.; Yu, C.; Sun, S.K.; Yan, X.P. Facile Synthesis of Uniform-Sized Bismuth Nanoparticles for CT Visualization of Gastrointestinal Tract in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 12720–12726. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Wen, L.; Sun, C.; Zhang, S.; Wang, G.; Zeng, J.; Wang, Y.; Ma, J.; Gao, M.; Le, Z. Ultrasmall biocompatible Bi2Se3 nanodots for multimodal imaging-guided synergistic radiophotothermal therapy against cancer. ACS Nano. 2016, 10, 11145–11155. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.W. Principle of CT and CT technology. J. Nucl. Med. Technol. 2007, 35, 115–128. [Google Scholar] [CrossRef]
- Paeng, J.C.; Lee, D.S. Multimodal molecular imaging in vivo. Open Nucl. Med. J. 2010, 2, 145–152. [Google Scholar] [CrossRef]
- Currie, G.M. Pharmacology, part 5: CT and MRI contrast media. J. Nucl. Med. Technol. 2019, 47, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef]
- Black, J. Biological performance of tantalum. Clin. Mater. 1994, 16, 167–173. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M.; Friberg, L.T. Handbook on the Toxicology of Metals, 3rd ed.; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Zimmermann, S.; Wolff, C.; Sures, B. Toxicity of platinum, palladium and rhodium to Daphnia magna in single and binary metal exposure experiments. Environ. Pollut. 2017, 224, 368–376. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Peng, C.; Ding, Y.; He, X.; Zhang, P.; Li, N.; Lan, T.; Wang, D.; Zhang, Z.; et al. Toxicity of cerium and thorium on Daphnia magna. Ecotoxicol. Environ. Saf. 2016, 134, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Z.; Bai, W.; Zhang, L.; He, X.; Ma, Y.; Liu, Y.; Chai, Z. Effects of rare earth elements La and Yb on the morphological and functional development of zebrafish embryos. J. Environ. Sci. 2012, 24, 209–213. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Johnson, D.R.; Seiter, J.M.; Lindsay, J.H.; Boyd, R.E.; Bednar, A.; Allison, P.G. Tungsten toxicity, bioaccumulation, and compartmentalization into organisms representing two trophic levels. Environ. Sci. Technol. 2012, 46, 9646–9652. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AnNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar]
- Bradley, B.; Singleton, M.; Li Wan Po, A. Bismuth toxicity-a reassessment. J. Clin. Pharm. Ther. 1989, 14, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-W.; Fan, S.-X.; Wang, F.; Sun, L.-D.; Zhen, X.-Y.; Yan, C.-H. Porous Pd nanoparticles with high photothermal conversion efficiency for efficient ablation of cancer cells. Nanoscale 2014, 6, 4345–4351. [Google Scholar] [CrossRef] [PubMed]
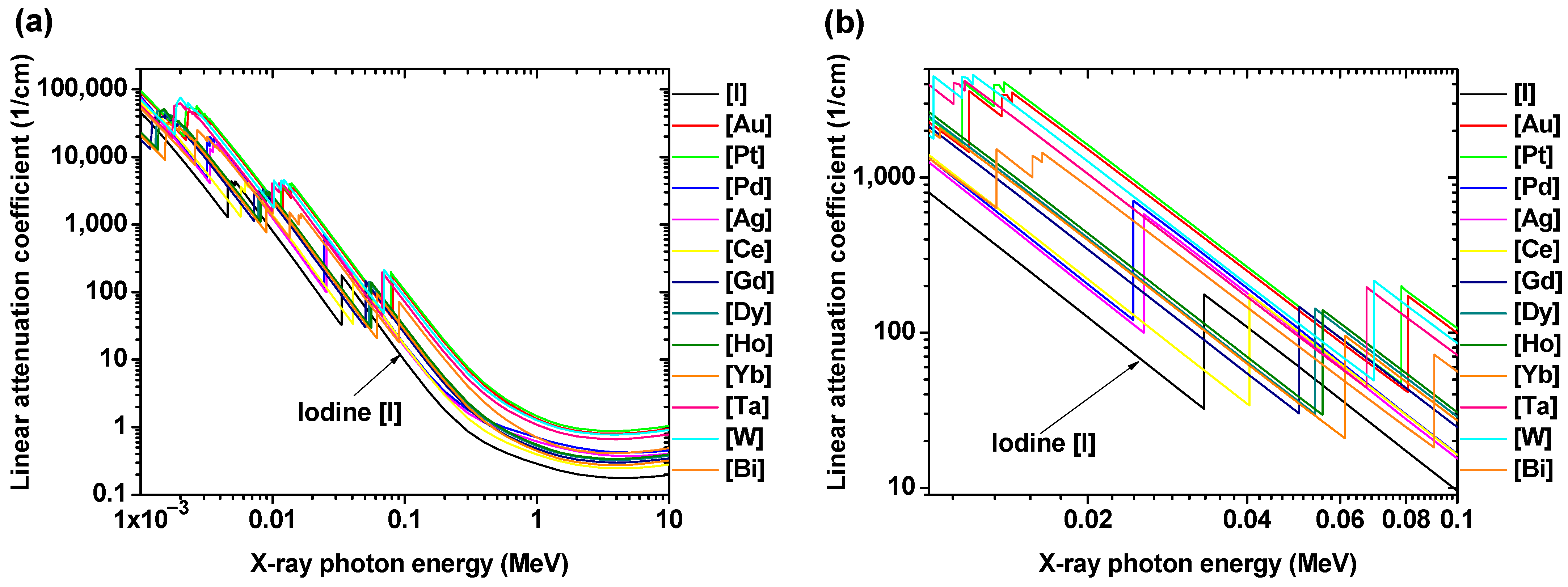
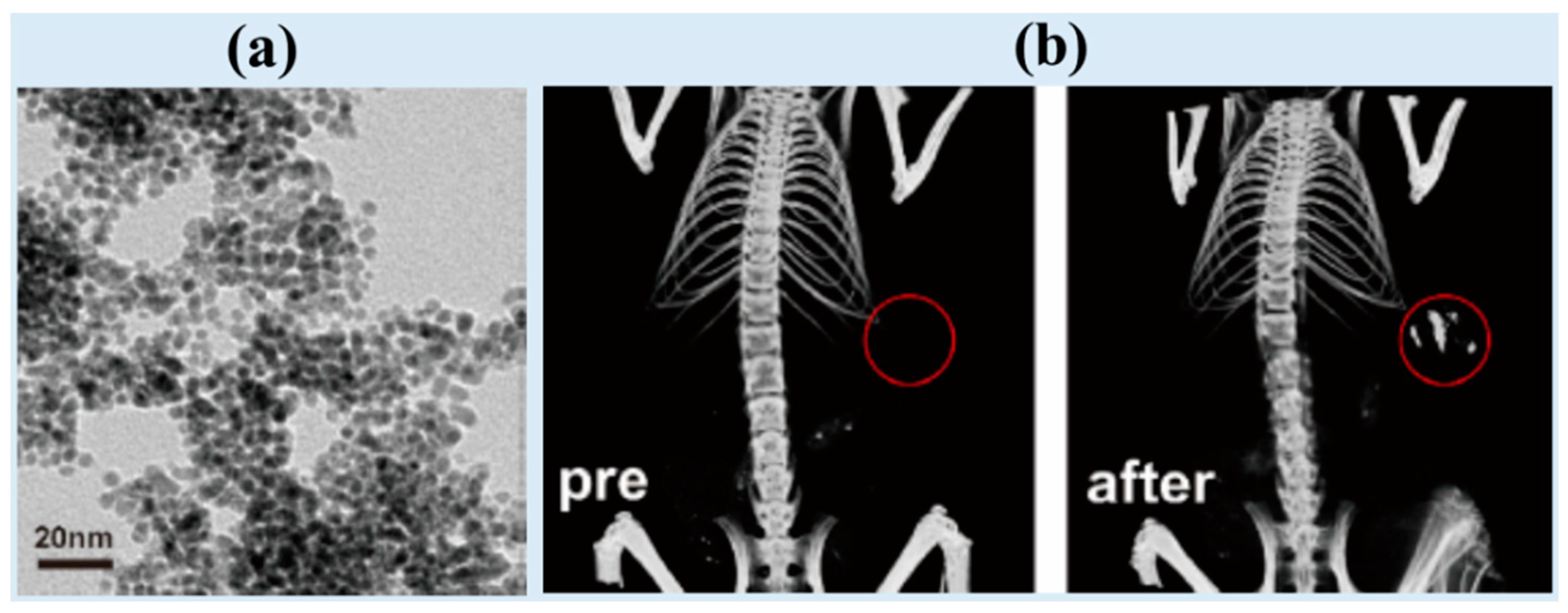


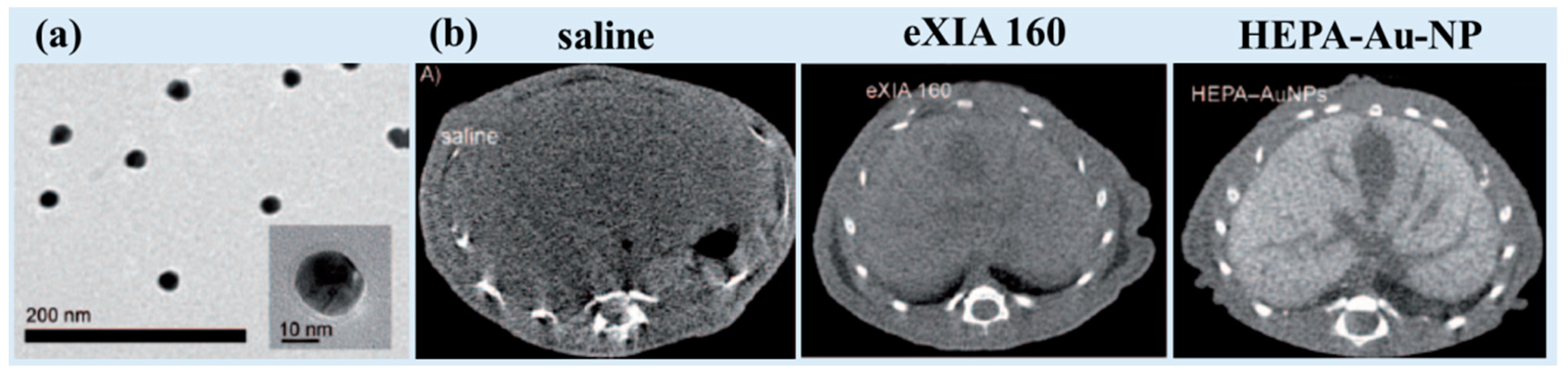
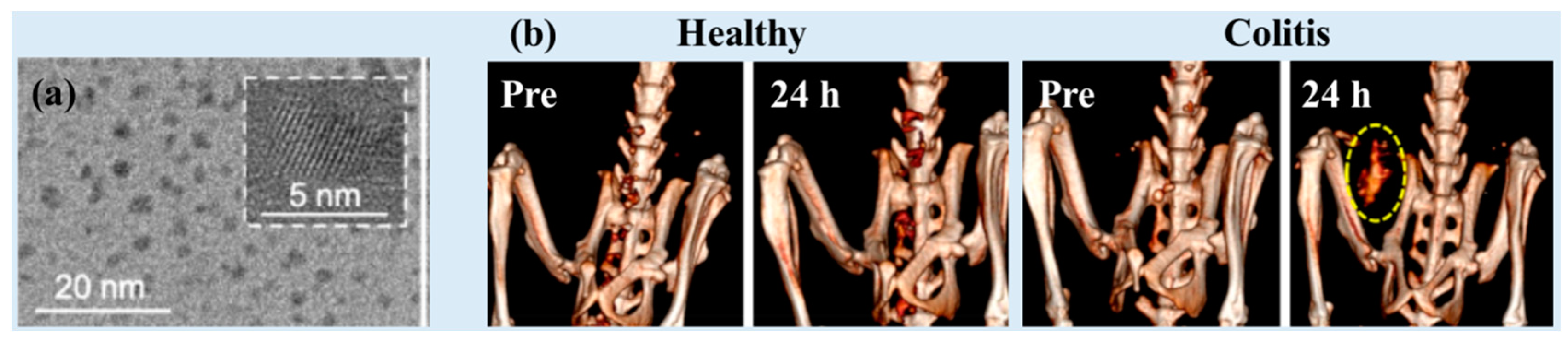


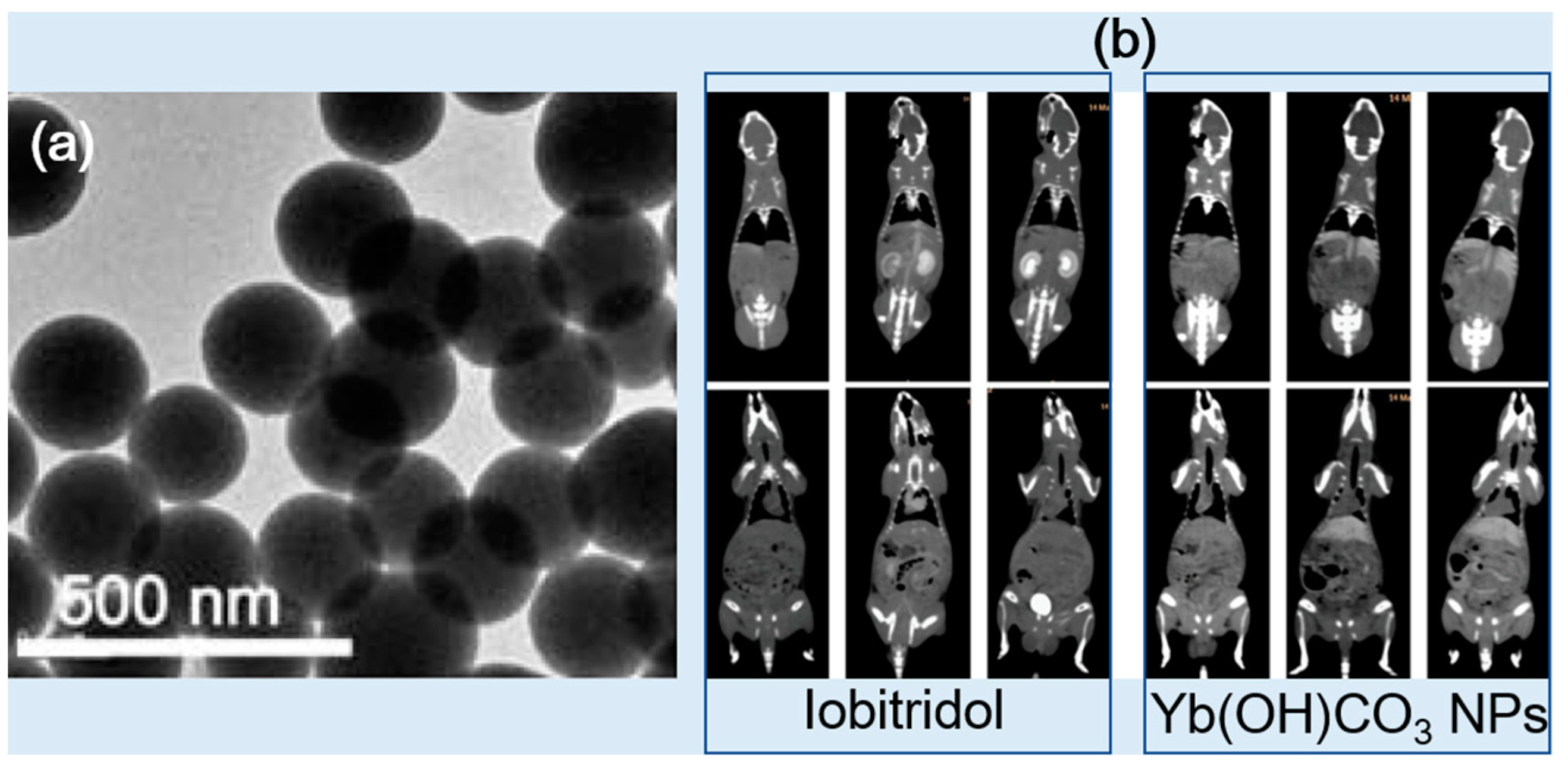
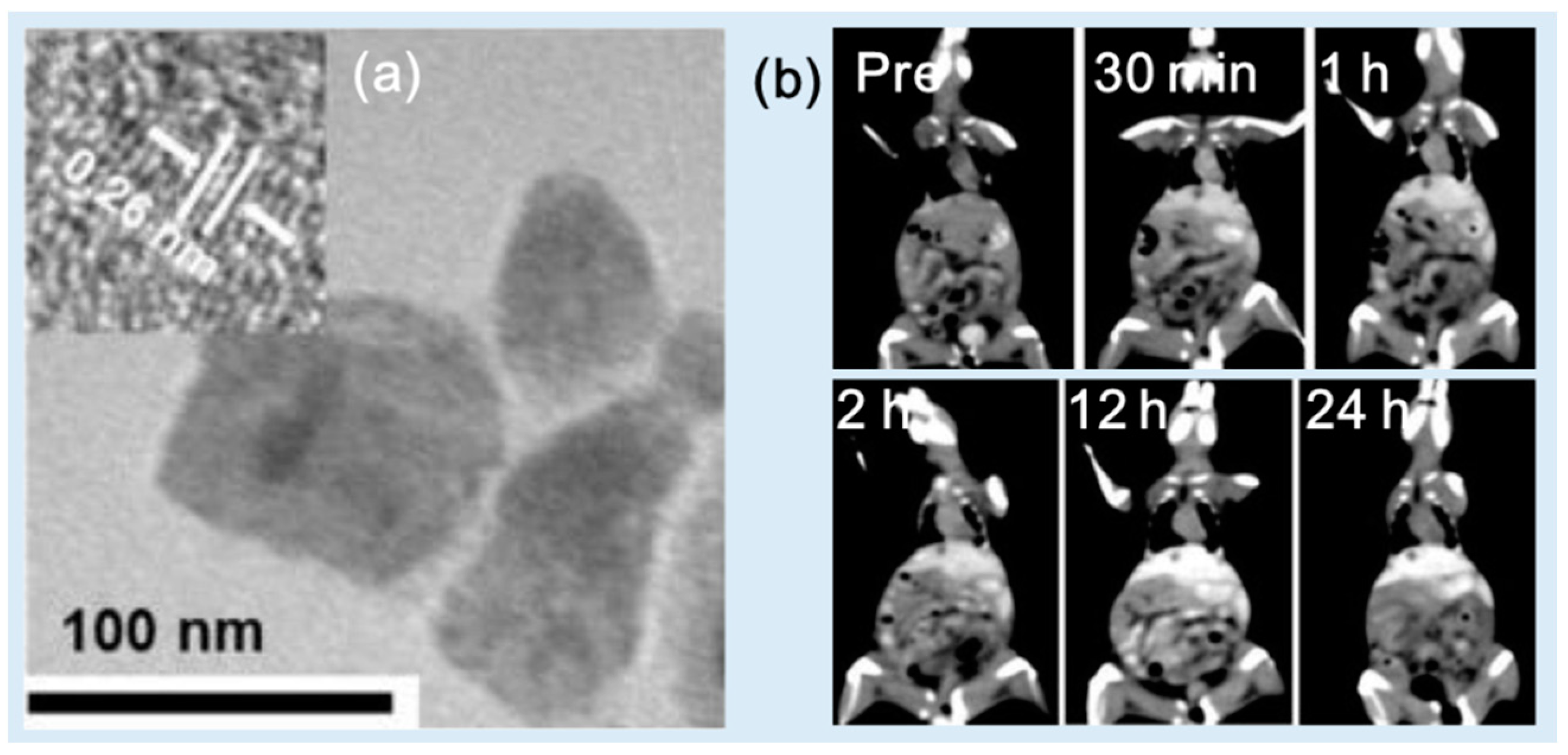


| Metal | Z 1 | Chemical Formula | Coating Ligand | Key Finding/Application | Morphology, Size (nm) 2 | η (HU/mM) | Tube Voltage (kVp) | Ref |
|---|---|---|---|---|---|---|---|---|
| Pd | 46 | FePd | Cysteamine | CT/MRI/photoacoustic tri-modal imaging probe | Spherical, 3.4 | 2.6 | - | [24] |
| Ag | 47 | Ag | Generation-5 (G5) poly(amido-amine) dendrimer | Particle diameter-dependent X-ray attenuation efficiency | Spherical, 8.8 12.4 16.1 23.2 | ~2.4 ~4.6 ~7.2 ~4.0 | 80 | [25] |
| Ag | Hyaluronic acid (HA) | SPECT 3 lung cancer imaging in vivo using 99mTc-HA-Ag NPs | Spherical, ~10 | 3.5 | - | [26] | ||
| Ag2S | Glutathione | 85% renal excretion within 24 h and nearly particle size-independent X-ray attenuation efficiency | Spherical, 2.3, 3.1, 5.1 | ~3.5 ~2.7 ~2.3 ~1.9 | 80 100 120 140 | [27] | ||
| Ag | Bovine serum albumin | Useful for CT probe and photothermal cancer therapy agent | Spherical, 5.8 | 5.7 | - | [28] | ||
| Ce | 58 | CeO2 | Dextran | Inflammatory bowel disease imaging under oxidative damage protection | Spherical, 4.8 | ~6.3 ~4.8 ~3.8 ~3.2 | 80 100 120 140 | [29] |
| CeO2 | Murine serum albumin | Long-time imaging of organs and tumor | Spherical, 5.1 | - | - | [30] | ||
| Gd | 64 | Gd2O3 | 5-amino-2,4,6-triiodoisophthalic acid | MRI/CT dual imaging in vivo application | Spherical, 2 | 11.8 | 70 | [31] |
| Gd2O3 | Polyacrylic acid | Ultrasmall NPs and high X-ray attenuation efficiency | Spherical, 1.9 | 5.9 | 70 | [32] | ||
| Gd(IO3)3H2 O | D-glucuronic acid | Properties useful as MRI/CT dual imaging agent | Mixture of nanosheet & nanorod, 110, 750 (nanosheet); 325 × 150 (nanorod) | ~5.1 | 35 | [33] | ||
| GdF3:Fe | Polyethylene glycol | In vivo MRI/CT dual imaging application | Nanorod, 51.9 × 31.3 | 6.9 | 120 | [34] | ||
| GdF3 | Polyacrylic acid | Properties useful as MRI/CT dual imaging agent | Nanoplate, 10.6 × 7.0 × 4.2 | ~7.9 | 60 | [35] | ||
| Dy | 66 | Dy2O3 | Polyacrylic acid | Ultrasmall NPs and high X-ray attenuation efficiency | Spherical, 1.8 | 6.1 | 70 | [32] |
| Dy2O3 | Polyethyleneimine | High X-ray attenuation efficiency suitable as CT contrast agent | Spherical, 79–102 | ~5 | 120 | [36] | ||
| DyVO4 | Polyacrylic acid | Properties useful as MRI/CT dual imaging agent | Spherical, 60 | 4.8 | 65 | [37] | ||
| Ho | 67 | HoF3 | Polyethylene glycol | In vivo MRI/CT dual imaging application in tumor diagnosis | Spherical, 38 | 190 | 120 | [38] |
| BaHoF5 | Polyethylene glycol | CT/CT angiography/CT perfusion and ischemic stroke imaging | Spherical, 7 | 4.8 | 80 | [39] | ||
| NaHoF4 | Polyethylene glycol | In vivo MRI/CT dual imaging and tumor imaging | Spherical, 3.2 | 6.9 | 120 | [40] | ||
| HoVO4 | Polyacrylic acid | Properties useful as MRI/CT dual imaging agent | Spherical, 65 | 4.8 | 65 | [37] | ||
| Yb | 70 | Yb2O3 | Polyacrylic acid | Ultrasmall NPs and high X-ray attenuation efficiency | Spherical, 1.7 | 6.8 | 70 | [32] |
| Yb2O3 | D-glucuronic acid | Ultrasmall NPs and high X-ray attenuation efficiency | Spherical, 2.1 | ~9.7 | 70 | [41] | ||
| BaYbF5 | - | CT contrast agent for osteochondral interface imaging | Spherical, 8, 11 | ~2.7 (8 nm), ~2.6 (11 nm) | 70 | [42] | ||
| BaYbF5@SiO2 | - | CT contrast agent for osteochondral interface imaging | Spherical, 27, 34 | ~1.8 (27 nm), ~1.2 (34 nm) | 70 | [42] | ||
| Yb | 3-mercaptopropionic acid | Applicable as CT/spectral photon-counting CT contrast agent | Spherical, 4.75 | ~10.4 | 55 | [43] | ||
| Yb(OH)CO3 | - | A large scale synthesis and in vivo CT application | Spherical, 170 | ~9.0 | 120 | [44] | ||
| NaYbF4:Er | Phospholipid-polyethylene glycol | Long circulation time and high contrasts in in vivo CT images | Spherical, 40 | ~9.9 | 120 | [45] | ||
| Yb2O3:Er | Polyethylene glycol | Long circulation time and in vivo CT/upconversion optical dual imaging | Spherical, 170 | 10.0 | 120 | [46] | ||
| Ta | 73 | NaTaO3 | Polyacrylic acid | Ultrasmall NPs and high X-ray attenuation efficiency | Spherical, 1.5 | 10.3 | 70 | [32] |
| TaOx | Polyethylene glycol-silane | Large-scale synthesis and in vivo CT/optical dual imaging through rhodamine-B-isothiocyanate conjugation | Spherical, 6, 9, 13, 15 | ~5.1 (6 nm) | 100 | [47] | ||
| Ta2O5 | (2-diethylphosphatoethyl)triethoxysilane | In vivo CT application to arterial system in high resolution | Spherical, ~6 | - | - | [48] | ||
| TaS2 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-3000 | In vivo CT-guided chemo-photothermal cancer therapy | Nanosheet, 50–100 | 6.3 | 120 | [50] | ||
| W | 74 | Na2WO4 | D-glucuronic acid | Ultrasmall NPs and in vivo CT application | Spherical, 3.2 | ~10 | 70 | [51] |
| RbxWO3 | Polyvinyl pyrrolidone | In vivo CT-guided chemo-photothermal cancer therapy | Nanorod, 5 × 20–40 | ~7.1 | 70 | [52] | ||
| WO2.9 | Polyethylene glycol | In vivo tumor CT imaging and photothermal therapy | Nanorod, 4.4 × 13.1 | 1.9 | 80 | [53] | ||
| WO3 | Poly-caprolactone and polyethylene glycol | Long circulation time up to 3 h and in vivo CT application | 2D platelet, 30–100 × 5–10 | ~10 | 49 | [54] | ||
| MnWO4 | Amino acid | In vivo CT/MRI dual imaging application | Nanorod, 20 × 50 | ~4.5 | 120 | [55] | ||
| Pt | 78 | Pt | Bovine serum albumin | Long circulation time and in vivo CT application | Spherical, 2.1 | 16.8 | 120 | [56] |
| Pt | Polyacrylic acid, poly(acrylic acid-co-maleic acid), poly(methyl vinyl ether-alt-maleic acid) | Ultrasmall NPs and high X-ray attenuation efficiency | Spherical, 2.0 | 16.4 18.4 | 50 70 | [57] | ||
| Pt | Mercaptoaminopolyglycol-chlorin e6 | In vivo CT/photoacoustic imaging-guided photothermal cancer therapy | Mesophorous, 70 | 3.1 | 120 | [58] | ||
| Pt | Polyethylene glycol | In vivo CT/chemotherapy/photothermal cancer therapy | Mesophorous, 94 | 5.5 | 120 | [59] | ||
| Pt | Poly(maleic anhydride-alt-1-octadecene)– polyethylene glycol | Long blood circulation time/in vivo CT/photoacoustic imaging/photothermal therapy/radoatherapy of cancer | Nanoworm, ~3 × ~10 | 3.9 | - | [60] | ||
| Pt | Extract from Prosopis farcta fruits | Green Pt NP synthesis | Spherical, 3.8 | 6.6 | 80 | [61] | ||
| Pt | Human serum albumin | In vivo CT/photoacoustic imaging/photothermal cancer therapy | Spherical, 6.7 | ~5.6 | - | [62] | ||
| Pt | Polyethylene glycol | Higher photothermal conversion efficiency than Pt NPs and in vivo CT-guided photothermal cancer therapy | Hollow cube, 30 | ~5.39 | - | [63] | ||
| Au | 79 | Au | Polyethylene glycol | Long blood circulation time (>4 h) and in vivo hepatoma CT imaging application | Spherical, 31 | 5.0 | 120 | [64] |
| Au | Polyethylene glycol | Application to in vivo blood pool imaging | Spherical, 10 | 4.8 | 50 | [65] | ||
| Au | Mercaptosuccinic acid | No particle size dependent X-ray attenuation efficiency | Spherical, 4.7, 13.2, 35.0, 76.4 | 10.6 13.0 | 70 45 | [66] | ||
| Au | Bovine serum albumin | In vitro CT imaging and chemotherapy of lung cancer cells | Spherical, 11.2 | ~5.6 | 120 | [67] | ||
| Au | Polyethylene glycol-polyethyleneimine | Application to in vivo blood pool CT imaging and tumor imaging | Spherical, 1.9, 2.9, 3.9, 4.6 | ~9 (2.9 nm) | - | [68] | ||
| Au | Lactobionic acid | In vivo CT imaging of cancer | Spherical, 2.7 | 8.5 | 80 | [69] | ||
| Au | G5-poly(amidoamine) dendrimer | Application in vivo CT imaging | Spherical, 1.9, 2.8, 4.0 | 9.8 (4.0 nm) | 80 | [70] | ||
| Au | NH2- fluorescein isothiocyanate-(polyethylene glycol- α-tocopheryl succinate)-(polyethylene glycol- folic acid) G5-dendrimer | In vivo targeted CT imaging and cancer therapy | Spherical, 3.3 | ~6.0 | 80 | [71] | ||
| Au | Gum Arabic | Large amounts accumulating in the liver, lung, and spleen in in vivo biodistribution | Spherical, 15–20 | ~4.9 | 80 | [72] | ||
| Au | Folic acid-conjugated silica | In vivo tumor tumor targeting CT imaging and in vitro radiation/photothermal therapy of cancer cells | Nanorod, 17.8 × 46.0 | 4.9 | - | [73] | ||
| Au | Glycol chitosan | Improved tumor accumulation and in vivo CT imaging of liver cancer | Spherical, 24 | ~2.8 | 70 | [74] | ||
| Au | Diatrizoic acid- Aptamer | In vivo tumor location via CT and fluorescence-guided resection of tumor | Spherical, 2.4 | 8.2 | - | [75] | ||
| Au | Gum Arabic | Green synthesis of colloidally stable Au NPs by laser ablation in aqueous solution | Spherical, 1.85 | ~4.3 | 80 | [76] | ||
| AuAg (3:1) | Folic acid-G5 poly(amidoamine) dendrimer | Targeted CT imaging of cancer cells in vitro | Spherical, 13.4 | ~6.3 | 100 | [77] | ||
| Au | Polyethylene glycol | Nearly particle size-independent X-ray attenuation efficiency and particle size-dependent biodistribution | Spherical, 3.9, 14.8, 50.6, 78.9, 99.2, 152.3 | 4.0–4.2 | 80 | [78] | ||
| Au | Cathepsin | Particle size-dependent in vivo accumulation/CT contrast at the tumor such that 10 nm > 30 nm > 100 nm | Spherical, 10, 30, 100 | 25.4 22.0 | 35 85 | [79] | ||
| Au | Heparin–amino acid 3,4-dihydroxyphenylalanine | Liver-specific CT imaging agent | Spherical, 24.0 | 21.9 | 70 | [80] | ||
| Bi | 83 | Bi2O3 | Polyacrylic acid | Ultrasmall NPs, high X-ray attenuation efficiency, and in vivo CT imaging | Spherical, 2.3 | 11.7 | 70 | [32] |
| BiOI | D-glucuronic acid | Ultrasmall NPs and very high X-ray attenuation efficiency | Spherical, 1.9 | ~21 | 70 | [82] | ||
| BiOI | Polyvinyl pyrrolidone | Very high X-ray attenuation efficiency | Spherical, 2.8 | ~20 | 75 | [83] | ||
| Bi2S3 | Polyvinyl pyrrolidone | High X-ray attenuation efficiency, long circulation time of >2 h, and in vivo CT imging | Nanosheet, 10–50×3–4 | ~9.7 | 50 | [84] | ||
| Bi | 1,2-propanediol and glucose | High payload element Bi NP CT contrast agent | Faceted, 74 | ~5.9 | 80 | [85] | ||
| Bi | Poly(DL-lactic-co-glycolic acid) | Potential agent for dual modality fluorescence and CT imaging | Spherical, 38 | 10.2 | 80 | [86] | ||
| Bi | Oligosaccharide | Simple synthesis of Bi NPs for in vivo gastrointestinal CT imaging | Spherical, 22 | 8.5 6.4 | 80 120 | [87] | ||
| Bi2Se3 | Bovine serum albumin | In vivo CT/photoacoustic imaging-guided synergetic radiophotothermal therapy of cancer | Spherical, 2.7 | 7.06 | 55 | [88] |
| Subject | Description |
|---|---|
| Advantages |
|
| Disadvantages |
|
| Potential side effects |
|
| Physicochemical properties affecting NP accumulation or renal excretion |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.Y.; Liu, S.; Tegafaw, T.; Saidi, A.K.A.A.; Zhao, D.; Liu, Y.; Nam, S.-W.; Chang, Y.; Lee, G.H. Heavy Metal-Based Nanoparticles as High-Performance X-ray Computed Tomography Contrast Agents. Pharmaceuticals 2023, 16, 1463. https://doi.org/10.3390/ph16101463
Ahmad MY, Liu S, Tegafaw T, Saidi AKAA, Zhao D, Liu Y, Nam S-W, Chang Y, Lee GH. Heavy Metal-Based Nanoparticles as High-Performance X-ray Computed Tomography Contrast Agents. Pharmaceuticals. 2023; 16(10):1463. https://doi.org/10.3390/ph16101463
Chicago/Turabian StyleAhmad, Mohammad Yaseen, Shuwen Liu, Tirusew Tegafaw, Abdullah Khamis Ali Al Saidi, Dejun Zhao, Ying Liu, Sung-Wook Nam, Yongmin Chang, and Gang Ho Lee. 2023. "Heavy Metal-Based Nanoparticles as High-Performance X-ray Computed Tomography Contrast Agents" Pharmaceuticals 16, no. 10: 1463. https://doi.org/10.3390/ph16101463
APA StyleAhmad, M. Y., Liu, S., Tegafaw, T., Saidi, A. K. A. A., Zhao, D., Liu, Y., Nam, S. -W., Chang, Y., & Lee, G. H. (2023). Heavy Metal-Based Nanoparticles as High-Performance X-ray Computed Tomography Contrast Agents. Pharmaceuticals, 16(10), 1463. https://doi.org/10.3390/ph16101463











