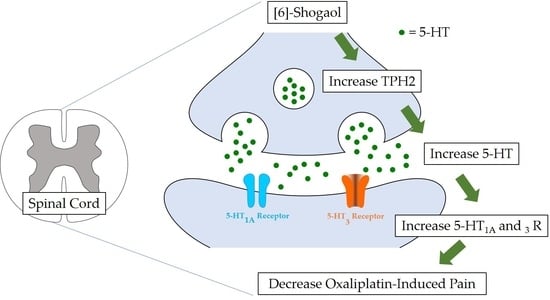
Involvement of the Spinal Serotonergic System in the Analgesic Effect of [6]-Shogaol in Oxaliplatin-Induced Neuropathic Pain in Mice
Abstract
:1. Introduction
2. Results
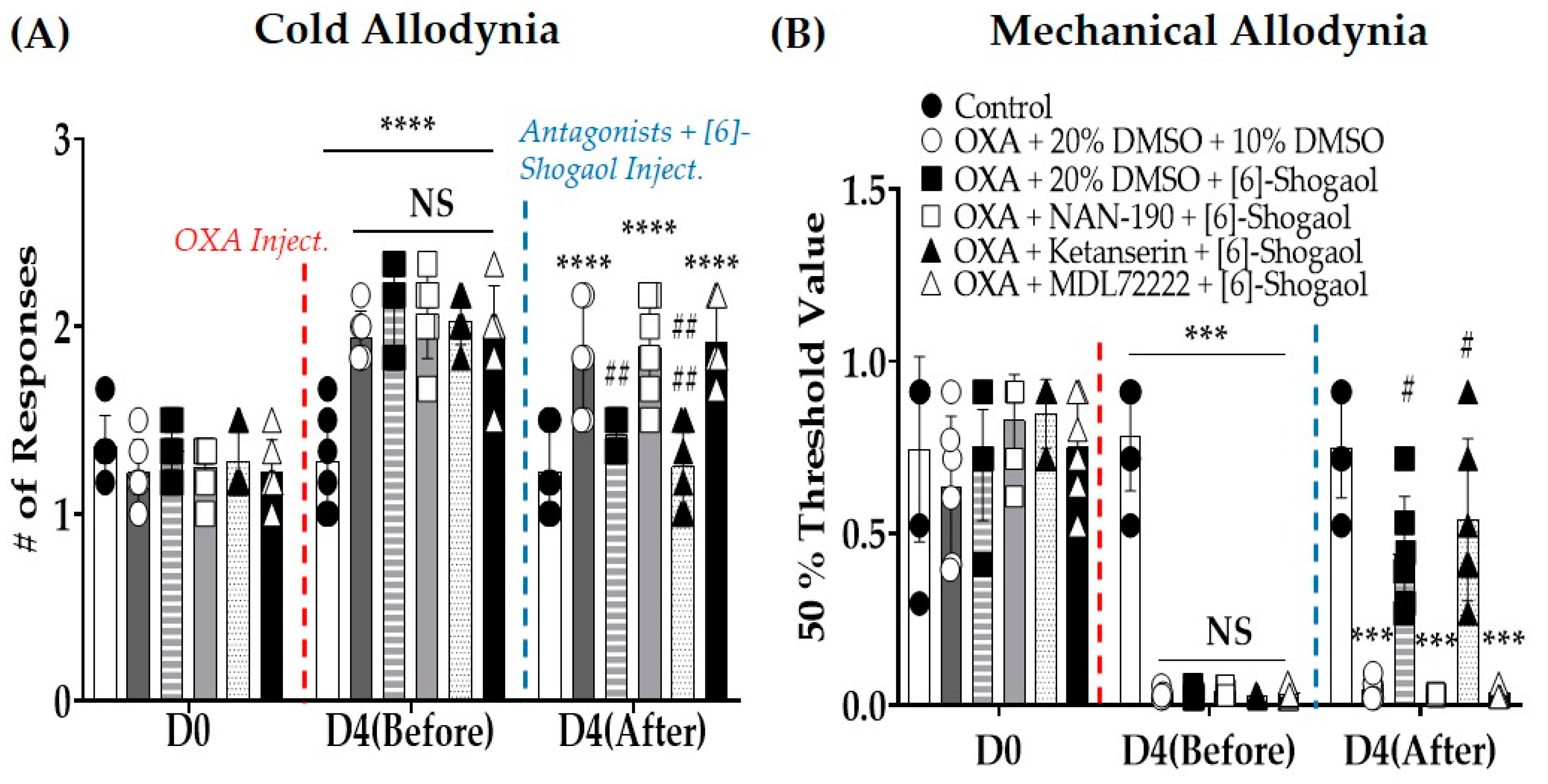
2.1. [6]-Shogaol Alleviates Oxaliplatin-Induced Cold and Mechanical Allodynia in Mice
2.2. Intrathecal Injections of Serotonin Receptor Antagonists Prevent the Analgesic Effect Induced by [6]-Shogaol Injections
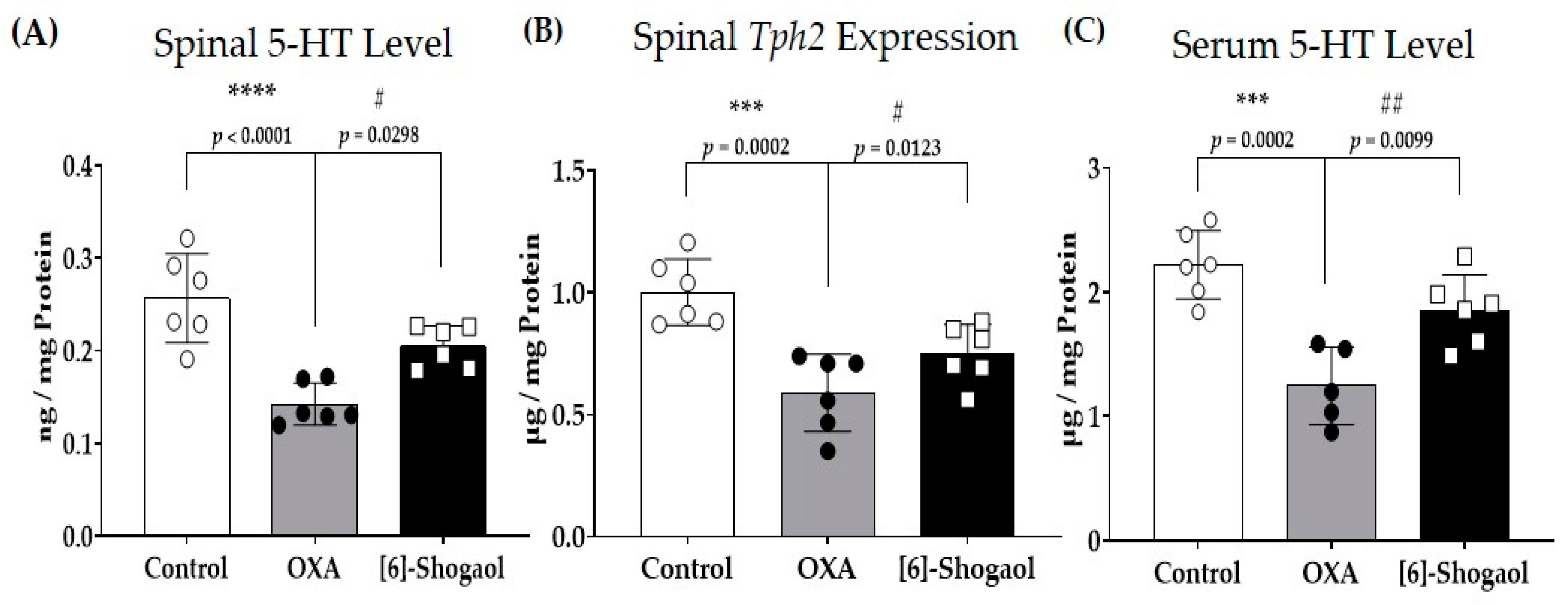
2.3. Spinal Serotonin Level Increases after [6]-Shogaol Administration
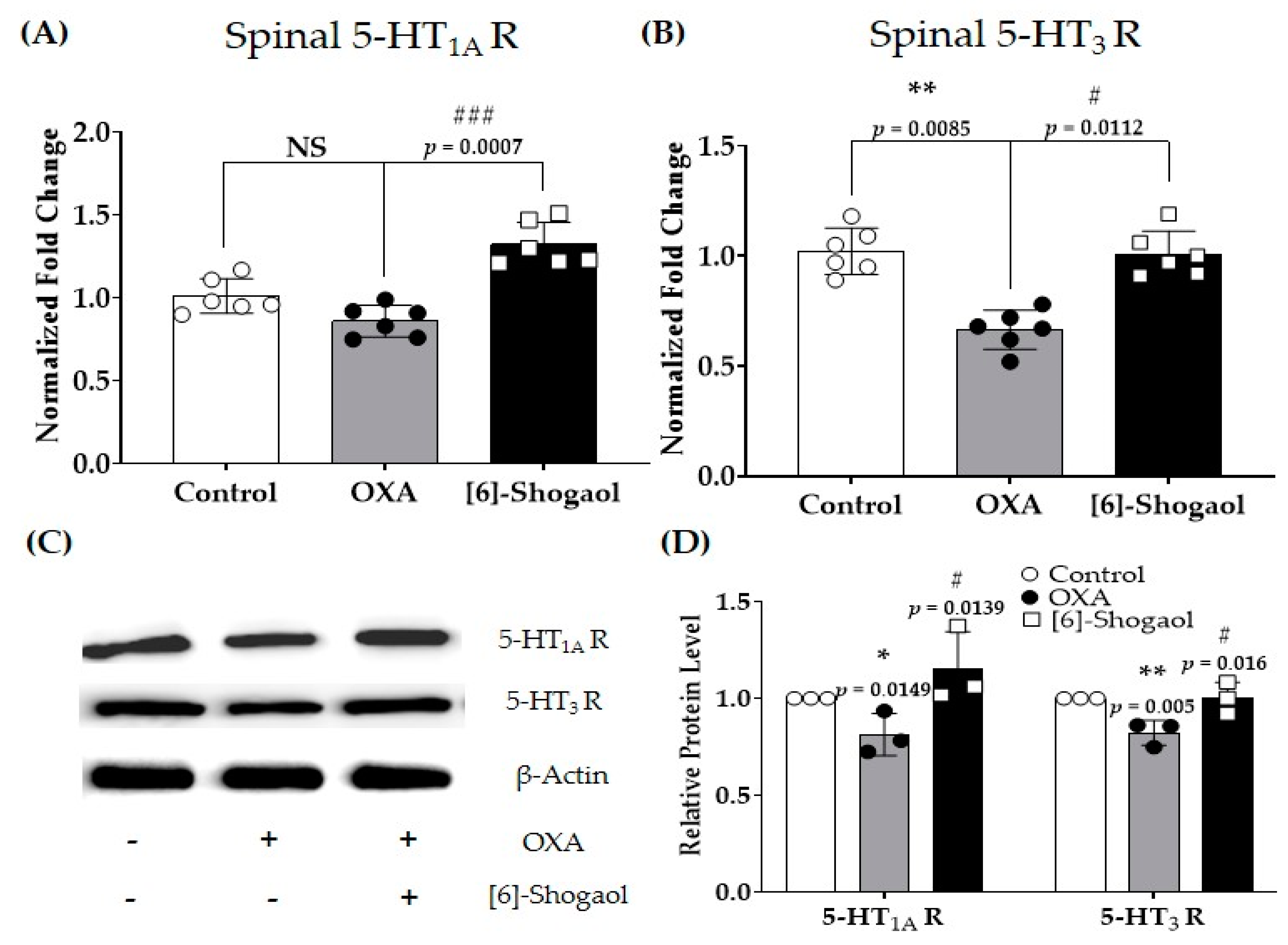
2.4. Spinal Serotonergic Receptors Are Involved in the Analgesic Effect of [6]-Shogaol
2.5. Intrathecal Injections of Serotonin Receptor Agonists Decrease Oxaliplatin-Induced Cold and Mechanical Allodynia in Mice
3. Discussion
3.1. [6]-Shogaol and Spinal Serotonin Synthesis
3.2. Serotonergic Receptors and [6]-Shogaol-Induced Analgesia
3.3. Role of [6]-Shogaol and Future Studies
4. Materials and Methods
4.1. Animals
4.2. Behavioral Tests
4.2.1. Measurement of Cold Allodynia
4.2.2. Measurement of Mechanical Allodynia
4.3. Oxaliplatin Injection
4.4. Administration of [6]-Shogaol
4.5. Intrathecal Injection
4.5.1. 5-HT Antagonist Administration
4.5.2. 5-HT Agonist Administration
4.6. Tissue Preparation
4.7. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Western Blots
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raymond, E.; Chaney, S.; Taamma, A.; Cvitkovic, E. Oxaliplatin: A review of preclinical and clinical studies. Ann. Oncol. 1998, 9, 1053–1071. [Google Scholar] [CrossRef]
- Cassidy, J.; Misset, J.-L. Oxaliplatin-related side effects: Characteristics and management. Semin. Oncol. 2002, 29, 11–20. [Google Scholar] [CrossRef]
- Kato, N.; Tateishi, K.; Tsubaki, M.; Takeda, T.; Matsumoto, M.; Tsurushima, K.; Ishizaka, T.; Nishida, S. Gabapentin and duloxetine prevent oxaliplatin-and paclitaxel-induced peripheral neuropathy by inhibiting extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation in spinal cords of mice. Pharmaceuticals 2020, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Quintero, G.C. Review about gabapentin misuse, interactions, contraindications and side effects. J. Exp. Pharmacol. 2017, 9, 13–21. [Google Scholar] [CrossRef]
- Perahia, D.; Kajdasz, D.; Walker, D.; Raskin, J.; Tylee, A. Duloxetine 60 mg once daily in the treatment of milder major depressive disorder. Int. J. Clin. Pract. 2006, 60, 613–620. [Google Scholar] [CrossRef]
- Kim, S.; Gang, J.; Lee, J.-H.; Yang, H.; Cheon, C.; Ko, S.-G.; Bae, H.; Kim, W. [6]-Shogaol Attenuates Oxaliplatin-Induced Allodynia through Serotonergic Receptors and GABA in the Spinal Cord in Mice. Pharmaceuticals 2022, 15, 726. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Min, D.; Lee, D.; Kim, W. Zingiber officinale roscoe rhizomes attenuate oxaliplatin-induced neuropathic pain in mice. Molecules 2021, 26, 548. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gang, J.; Yang, E.; Kim, W.; Jin, Y.-H. Bee venom acupuncture attenuates oxaliplatin-induced neuropathic pain by modulating action potential threshold in a-fiber dorsal root ganglia neurons. Toxins 2020, 12, 737. [Google Scholar] [CrossRef]
- Lee, J.H.; Ji, H.; Ko, S.-G.; Kim, W. Ji017 attenuates oxaliplatin-induced cold allodynia via spinal trpv1 and astrocytes inhibition in mice. Int. J. Mol. Sci. 2021, 22, 8811. [Google Scholar] [CrossRef] [PubMed]
- Fajrin, F.A.; Nurrochmad, A.; Nugroho, A.E.; Susilowati, R. The improvement of pain behavior and sciatic nerves morphology in mice model of painful diabetic neuropathy upon administration of ginger (Zingiber officinale roscoe.) extract and its pungent compound, 6-shogaol. J. Nat. Sci. Biol. Med. 2019, 10, 149. [Google Scholar]
- Fajrin, F.A.; Nugroho, A.E.; Nurrochmad, A.; Susilowati, R. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J. Ethnopharmacol. 2020, 249, 112396. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C. Serotonin in pain and pain control. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2010; Volume 21, pp. 457–471. [Google Scholar]
- Jin, Z.; Lee, G.; Kim, S.; Park, C.-S.; Park, Y.S.; Jin, Y.-H. Ginger and its pungent constituents non-competitively inhibit serotonin currents on visceral afferent neurons. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Walstab, J.; Krüger, D.; Stark, T.; Hofmann, T.; Demir, I.; Ceyhan, G.; Feistel, B.; Schemann, M.; Niesler, B. Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterol. Motil. 2013, 25, 439-e302. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, D.; Troost, F.; Masclee, A. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil. 2009, 21, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Bader, M. Inhibition of serotonin synthesis: A novel therapeutic paradigm. Pharmacol. Ther. 2020, 205, 107423. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.-P.; Araragi, N.; Waider, J.; van den Hove, D.; Gutknecht, L. Targeting brain serotonin synthesis: Insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2426–2443. [Google Scholar] [CrossRef] [PubMed]
- Marston, O.J.; Garfield, A.S.; Heisler, L.K. Role of central serotonin and melanocortin systems in the control of energy balance. Eur. J. Pharmacol. 2011, 660, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bardin, L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011, 22, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, A.; Almeida, A. Descending inhibitory systems. Handb. Clin. Neurol. 2006, 81, 179–192. [Google Scholar] [PubMed]
- Bardin, L.; Bardin, M.; Lavarenne, J.; Eschalier, A. Effect of intrathecal serotonin on nociception in rats: Influence of the pain test used. Exp. Brain Res. 1997, 113, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Megumu, Y.; Hidemasa, F. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J. Pharmacol. Sci. 2006, 101, 107–117. [Google Scholar]
- Musa, A.; Maje, I.; Chindo, B.; Ahmad, M.H. Potential involvement of opioidergic, α1-adrenergic and serotonergic pathways in the anti-nociceptive actions of Tapinanthus globiferus A. Rich (Loranthaceae) in mice. Adv. Tradit. Med. 2022, 23, 803–818. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, W. Involvement of serotonergic system in oxaliplatin-induced neuropathic pain. Biomedicines 2021, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.; Windeck, T.; Ploch, M.; Verspohl, E.J. Mode of action of gingerols and shogaols on 5-HT3 receptors: Binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur. J. Pharmacol. 2006, 530, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Iwamoto, M.; Aoki, S.; Tanaka, N.; Tajima, K.; Yamahara, J.; Takaishi, Y.; Yoshida, M.; Tomimatsu, T.; Tamai, Y. Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem. Pharm. Bull. 1991, 39, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Peter, J.-U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Gao, J.; Pan, Z.; Jiao, Z.; Li, F.; Zhao, G.; Wei, Q.; Pan, F.; Evangelou, E. TPH2 gene polymorphisms and major depression–a meta-analysis. PLoS ONE 2012, 7, e36721. [Google Scholar] [CrossRef]
- Aggarwal, M.; Puri, V.; Puri, S. Serotonin and CGRP in migraine. Ann. Neurosci. 2012, 19, 88. [Google Scholar] [CrossRef]
- Messing, R.B.; Lytle, L.D. Serotonin-containing neurons: Their possible role in pain and analgesia. Pain 1977, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kanova, M.; Kohout, P. Serotonin—Its synthesis and roles in the healthy and the critically ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef]
- Bowker, R.; Westlund, K.; Coulter, J. Origins of serotonergic projections to the spinal cord in rat: An immunocytochemical-retrograde transport study. Brain Res. 1981, 226, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Darcsi, A.; Kéry, Á.; Riethmüller, E. Blood-brain barrier permeability study of ginger constituents. J. Pharm. Biomed. Anal. 2020, 177, 112820. [Google Scholar] [CrossRef] [PubMed]
- Laporte, A.; Doyen, C.; Nevo, I.; Chauveau, J.; Hauw, J.; Hamon, M. Autoradiographic mapping of serotonin 5-HT1A, 5-HT1D, 5-HT2A and 5-HT3 receptors in the aged human spinal cord. J. Chem. Neuroanat. 1996, 11, 67–75. [Google Scholar] [CrossRef]
- Marlier, L.; Teilhac, J.-R.; Cerruti, C.; Privat, A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991, 550, 15–23. [Google Scholar] [CrossRef]
- Abe, K.; Kato, G.; Katafuchi, T.; Tamae, A.; Furue, H.; Yoshimura, M. Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience 2009, 159, 316–324. [Google Scholar] [CrossRef]
- Fukushima, T.; Ohtsubo, T.; Tsuda, M.; Yanagawa, Y.; Hori, Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. J. Neurophysiol. 2009, 102, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Whorton, M.R.; MacKinnon, R. X-ray structure of the mammalian GIRK2–βγ G-protein complex. Nature 2013, 498, 190–197. [Google Scholar] [CrossRef]
- Ok, S.; Jeong, W.-S. Optimization of extraction conditions for the 6-shogaol-rich extract from ginger (Zingiber officinale Roscoe). Prev. Nutr. Food Sci. 2012, 17, 166. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Kont, I.; Fürst, R. Benefits of ginger and its constituent 6-shogaol in inhibiting inflammatory processes. Pharmaceuticals 2021, 14, 571. [Google Scholar] [CrossRef]
- Haghighi, M.; Rohani, M.S. The effects of powdered ginger (Zingiber officinale) on the haematological and immunological parameters of rainbow trout Oncorhynchus mykiss. J. Med. Plant Herb. Ther. Res. 2013, 1, 8–12. [Google Scholar]
- Jiao, W.; Mi, S.; Sang, Y.; Jin, Q.; Chitrakar, B.; Wang, X.; Wang, S. Integrated network pharmacology and cellular assay for the investigation of an anti-obesity effect of 6-shogaol. Food Chem. 2022, 374, 131755. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Huang, Y.; Yu, M.; Dong, X.; Undem, B.J.; Yu, S. Ginger constituent 6-shogaol attenuates vincristine-induced activation of mouse gastroesophageal vagal afferent c-fibers. Molecules 2022, 27, 7465. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cheon, C.; Kim, B.; Kim, W. The effect of ginger and its sub-components on pain. Plants 2022, 11, 2296. [Google Scholar] [CrossRef]
- Park, K.-T.; Jo, H.; Kim, B.; Kim, W. Red Ginger Extract Prevents the Development of Oxaliplatin-Induced Neuropathic Pain by Inhibiting the Spinal Noradrenergic System in Mice. Biomedicines 2023, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-T.; Kim, S.; Choi, I.; Han, I.-H.; Bae, H.; Kim, W. The involvement of the noradrenergic system in the antinociceptive effect of cucurbitacin D on mice with paclitaxel-induced neuropathic pain. Front. Pharmacol. 2023, 13, 1055264. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J.; Bennett, G.J. Ethosuximide reverses paclitaxel-and vincristine-induced painful peripheral neuropathy. Pain 2004, 109, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.L.; Hansen, R.B.; Storm, M.A.; Olesen, J.; Hansen, T.F.; Ossipov, M.; Izarzugaza, J.M.; Porreca, F.; Kristensen, D.M. Von Frey testing revisited: Provision of an online algorithm for improved accuracy of 50% thresholds. Eur. J. Pain 2020, 24, 783–790. [Google Scholar] [CrossRef]
- Dixon, W. Staircase bioassay: The up-and-down method. Neurosci. Biobehav. Rev. 1991, 15, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.; Chung, J.; Yaksh, T. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]
- Clark, M.F.; Lister, R.M.; Bar-Joseph, M. ELISA techniques. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1986; Volume 118, pp. 742–766. [Google Scholar]
- Liu, Z.-Q.; Mahmood, T.; Yang, P.-C. Western blot: Technique, theory and trouble shooting. N. Am. J. Med. Sci. 2014, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Yang, P.-C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gang, J.; Park, K.-T.; Kim, S.; Kim, W. Involvement of the Spinal Serotonergic System in the Analgesic Effect of [6]-Shogaol in Oxaliplatin-Induced Neuropathic Pain in Mice. Pharmaceuticals 2023, 16, 1465. https://doi.org/10.3390/ph16101465
Gang J, Park K-T, Kim S, Kim W. Involvement of the Spinal Serotonergic System in the Analgesic Effect of [6]-Shogaol in Oxaliplatin-Induced Neuropathic Pain in Mice. Pharmaceuticals. 2023; 16(10):1465. https://doi.org/10.3390/ph16101465
Chicago/Turabian StyleGang, Juan, Keun-Tae Park, Suyong Kim, and Woojin Kim. 2023. "Involvement of the Spinal Serotonergic System in the Analgesic Effect of [6]-Shogaol in Oxaliplatin-Induced Neuropathic Pain in Mice" Pharmaceuticals 16, no. 10: 1465. https://doi.org/10.3390/ph16101465