Modulation of Hepatic Functions by Chicory (Cichorium intybus L.) Extract: Preclinical Study in Rats †
Abstract
:1. Introduction
2. Results
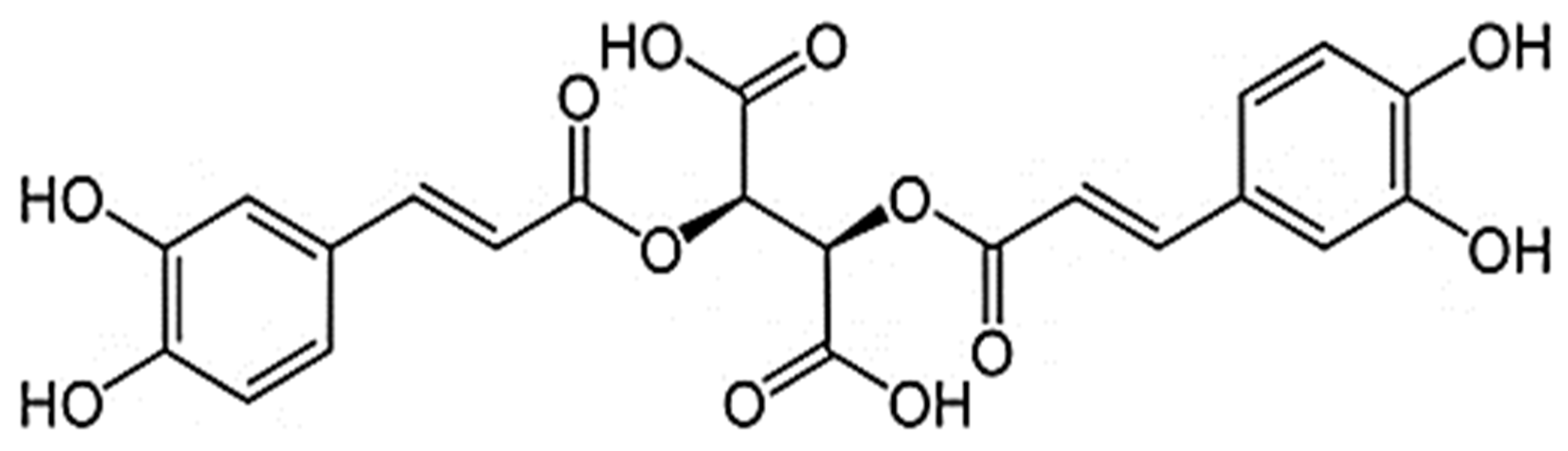
2.1. Chemical Composition of CE
2.2. Hepatoprotective Properties of CE
2.3. Hypolipidemic and Hypoglycemic Effects of CE on the Tween-80 Model of Hyperlipidemia in Rats
2.4. Hypolipidemic and Hypoglycemic Effects of CE on the Model of Alimentary Hyperlipidemia
3. Discussion
4. Materials and Methods
4.1. Phytodrug
4.2. Profiling of Polyphenolic Compounds Present in CE
4.3. Quantification of Total and Dominant Polyphenols in CE
4.4. Animals
4.5. Analysis of CE’s Hepatoprotective Effect on the Model of Acute Liver Injury in Rats
4.6. Analysis of CE’s Hypolipidemic Effect on the Model of Hyperlipidemia Caused by a Single Intraperitoneal Injection of Tween-80
4.7. Study of the Lipid-Lowering Effect on a Model of Alimentary Hyperlipidemia
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- NIH National Heart, Lung and Blood Institute. Metabolic Syndrome. Available online: https://www.nhlbi.nih.gov/health/metabolic-syndrome (accessed on 26 March 2023).
- Ren, H.; Wang, J.; Gao, Y.; Yang, F.; Huang, W. Metabolic syndrome and liver-related events: A systematic review and meta-analysis. BMC Endocr. Disord. 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K.; Zhang, Y.F.; Xie, L.; Rong, F.; Zhu, X.Y.; Xie, J.; Zhou, H.; Xu, T. Progress in the treatment of drug-induced liver injury with natural products. Pharmacol. Res. 2022, 183, 106361. [Google Scholar] [CrossRef] [PubMed]
- Enioutina, E.Y.; Job, K.M.; Sherwin, C.M.T. Why we need to pay attention to toxicity associated with herbal medicines. Br. J. Clin. Pharmacol. 2020, 86, 1793–1794. [Google Scholar] [CrossRef]
- Yuan, L.; Kaplowitz, N. Mechanisms of drug-induced liver injury. Clin. Liver Dis. 2013, 17, 507–518. [Google Scholar] [CrossRef]
- Umbaugh, D.S.; Jaeschke, H. Biomarkers of drug-induced liver injury: A mechanistic perspective through acetaminophen hepatotoxicity. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Revol, B.; Gautier-Veyret, E.; Arrivé, C.; Fouilhé Sam-Laï, N.; McLeer-Florin, A.; Pluchart, H.; Pinsolle, J.; Toffart, A.C. Pharmacokinetic herb-drug interaction between ginger and crizotinib. Br. J. Clin. Pharmacol. 2020, 86, 1892–1893. [Google Scholar] [CrossRef]
- Laube, R.; Liu, K. An unwanted complement: Rare case of potential liver injury induced by an interaction between ginseng and atorvastatin. Br. J. Clin. Pharmacol. 2019, 85, 1612–1613. [Google Scholar] [CrossRef]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Sama, S.K.; Krishnamurthy, L.; Ramachandran, K.; Lal, K. Efficacy of an indigenous compound preparation (Liv-52) in acute viral hepatitis-a double blind study. Indian. J. Med. Res. 1976, 64, 738–742. [Google Scholar]
- Saxena, A.; Garg, N.K. Effect of Liv-52 on hepatic enzymes. Indian. J. Exp. Biol. 1979, 17, 662–664. [Google Scholar]
- Thabrew, M.I.; Emerole, G.O.; Subbarao, V.V. Effect of Liv-52 on carbon tetrachloride-induced changes in hepatic microsomal drug-metabolizing enzymes of the rat. Toxicol. Lett. 1982, 14, 183–188. [Google Scholar] [CrossRef]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties—A Review. Molecules 2021, 26, 1814. [Google Scholar] [CrossRef] [PubMed]
- Jasim, R.; Antioxidant, Antimicrobial Activities and Phytochemical Constituents of Cichorium intybus L. Aerial Parts. Int. J. Bot. 2018, 14, 24–29. [Google Scholar] [CrossRef]
- Zanoni, F.; Primiterra, M.; Angeli, N.; Zoccatelli, G. Microencapsulation by spray-drying of polyphenols extracted from red chicory and red cabbage: Effects on stability and color properties. Food Chem. 2020, 307, 125535. [Google Scholar] [CrossRef]
- Cova, C.M.; Boffa, L.; Pistocchi, M.; Giorgini, S.; Luque, R.; Cravotto, G. Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers. Molecules 2019, 24, 2681. [Google Scholar] [CrossRef] [PubMed]
- Saybel, O.L.; Rendyuk, T.D.; Dargaeva, T.D.; Lupanova, I.A.; Ferubko, E.V.; Kurmanova, E.N.; Martynchik, I.A. Phenolic Compounds and Hepatoprotective Activity of Chicory Herb Extract. Drug Dev. Regist. 2021, 10, 36–45. [Google Scholar] [CrossRef]
- Kuzina, O.S.; Borovkova, M.V.; Lemyaseva, S.V.; Saybel, O.L. Toxicity of dry extract of Chicorium intybus L. following single administration. Bull. Med. Sci. 2019, 1, 29–32. [Google Scholar]
- Krepkova, L.V.; Babenko, A.N.; Saybel, O.L.; Lupanova, I.A.; Kuzina, O.S.; Job, K.M.; Sherwin, C.M.; Enioutina, E.Y. Valuable Hepatoprotective Plants—How Can We Optimize Waste Free Uses of Such Highly Versatile Resources? Front. Pharmacol. 2021, 12, 738504. [Google Scholar] [CrossRef]
- Ignat, M.V.; Coldea, T.E.; Salanță, L.C.; Mudura, E. Plants of the Spontaneous Flora with Beneficial Action in the Management of Diabetes, Hepatic Disorders, and Cardiovascular Disease. Plants 2021, 10, 216. [Google Scholar] [CrossRef]
- Mohafrash, S.M.M.; Mossa, A.H. Herbal syrup from chicory and artichoke leaves ameliorate liver damage induced by deltamethrin in weanling male rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 7672–7682. [Google Scholar] [CrossRef] [PubMed]
- Moloudi, M.R.; Hassanzadeh, K.; Abdi, M.; Zandi, F.; Rahimi, K.; Izadpanah, E. Hepatoprotective effect of the hydroalcoholic extract of Cichorium intybus in a rat model of obstructive cholestasis. Arab. J. Gastroenterol. 2021, 22, 34–39. [Google Scholar] [CrossRef]
- Keshk, W.A.; Soliman, N.A.; Ali, D.A.; Elseady, W.S. Mechanistic evaluation of AMPK/SIRT1/FXR signaling axis, inflammation, and redox status in thioacetamide-induced liver cirrhosis: The role of Cichorium intybus linn (chicory)-supplemented diet. J. Food Biochem. 2019, 43, e12938. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediators Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Mironov, A.N. Guidelines for Preclinical Studies of Drugs. Part 1; Grif and K: Moscow, Russia, 2012; pp. 80–93. [Google Scholar]
- Iqbal, Y.; Ponnampalam, E.N.; Suleria, H.A.R.; Cottrell, J.J.; Dunshea, F.R. LC-ESI/QTOF-MS Profiling of Chicory and Lucerne Polyphenols and Their Antioxidant Activities. Antioxidants 2021, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Alkandahri, M.Y.; Pamungkas, B.T.; Oktoba, Z.; Shafirany, M.Z.; Sulastri, L.; Arfania, M.; Anggraeny, E.N.; Pratiwi, A.; Astuti, F.D.; Indriyani; et al. Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1387665. [Google Scholar] [CrossRef]
- Mahdi, T.; Hossein, A. Changes in Kaempferol content of Chicory (Cichorium intybus L.) under water deficit stresses and planting densities. J. Med. Plants Res. 2014, 8, 30–35. [Google Scholar] [CrossRef]
- Inazu, A.; Brown, M.L.; Hesler, C.B.; Agellon, L.B.; Koizumi, J.; Takata, K.; Maruhama, Y.; Mabuchi, H.; Tall, A.R. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 1990, 323, 1234–1238. [Google Scholar] [CrossRef]
- Pedrelli, M.; Pramfalk, C.; Parini, P. Thyroid hormones and thyroid hormone receptors: Effects of thyromimetics on reverse cholesterol transport. World J. Gastroenterol. 2010, 16, 5958–5964. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, H.; Rudling, M.; Forrest, D.; Angelin, B.; Vennström, B. Thyroid hormone receptor beta-deficient mice show complete loss of the normal cholesterol 7alpha-hydroxylase (CYP7A) response to thyroid hormone but display enhanced resistance to dietary cholesterol. Mol. Endocrinol. 2000, 14, 1739–1749. [Google Scholar] [CrossRef]
- Keshk, W.A.; Noeman, S.A. Impact of Chicory-Supplemented Diet on HMG-CoA Reductase, Acetyl-CoA Carboxylase, Visfatin and Antioxidant Status in Triton WR-1339-Induced Hyperlipidemia. J. Food Biochem. 2015, 39, 164–172. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Ahmed, O.M.; Ahmed, R.R.; Mahmoud, A.; Abdella, E.; Ashour, M.B. Antihyperglycemic effect of crude extracts of some Egyptian plants and algae. J. Med. Food 2014, 17, 400–406. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z.; Król, B. Caffeoylquinic acid-rich extract from chicory seeds improves glycemia, atherogenic index, and antioxidant status in rats. Nutrition 2012, 28, 300–306. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Shim, D.W.; Han, J.W.; Ji, Y.E.; Shin, W.Y.; Koppula, S.; Kim, M.K.; Kim, T.K.; Park, P.J.; Kang, T.B.; Lee, K.H. Cichorium intybus Linn. Extract Prevents Type 2 Diabetes Through Inhibition of NLRP3 Inflammasome Activation. J. Med. Food 2016, 19, 310–317. [Google Scholar] [CrossRef]
- Jackson, K.M.P.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural constraints and importance of caffeic acid moiety for anti-hyperglycemic effects of caffeoylquinic acids from chicory. Mol. Nutr. Food Res. 2017, 61, 1601118. [Google Scholar] [CrossRef]
- Bokarev, I.N. The metabolic syndrome. Klin. Med. 2014, 92, 71–76. [Google Scholar]
- Pouille, C.L.; Ouaza, S.; Roels, E.; Behra, J.; Tourret, M.; Molinié, R.; Fontaine, J.-X.; Mathiron, D.; Gagneul, D.; Taminiau, B.; et al. Chicory: Understanding the Effects and Effectors of This Functional Food. Nutrients 2022, 14, 957. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, R.; Feng, B.; Cheng, Y.; Guo, Y.; Qian, H. Chicoric Acid Prevents Neuroinflammation and Neurodegeneration in a Mouse Parkinson’s Disease Model: Immune Response and Transcriptome Profile of the Spleen and Colon. Int. J. Mol. Sci. 2022, 23, 2031. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-Q.; Jian, T.-Y.; Gai, Y.-N.; Niu, G.-T.; Liu, Y.; Meng, X.-H.; Li, J.; Lyu, H.; Ren, B.-R.; Chen, J. Chicoric Acid Attenuated Renal Tubular Injury in HFD-Induced Chronic Kidney Disease Mice through the Promotion of Mitophagy via the Nrf2/PINK/Parkin Pathway. J. Agric. Food Chem. 2022, 70, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- All-Russian Research Institute of Medicinal and Aromatic Plants. Wild Medicinal Plants in Russia: Collection, Drying, Preparation of Raw Materials (Collection, Drying, Preparation of Raw Materials); FGBNU All-Russian Research Institute of Medicinal and Aromatic Plants (FGBNU VILAR): Moscow, Russia, 2015; Volume 344. [Google Scholar]
- Medicinal Plant Specialist Group Species Survival Commission IUCN—The World Conservation Union. International Standard for Sustainable Wild Collection of Medicinal and Aromatic Plants (ISSC-MAP). Available online: https://static1.squarespace.com/static/5bec424b297114f64cb908d8/t/6256dca1510d52627bf4bda3/1649859747671/ISSC-MAP_Version1_0.pdf (accessed on 12 April 2023).
- Chawech, R.; Pesnel, S.; Haddada, M.B.; Gauvin-Bialecki, A.; Morel, A.L. Polyphenol Characterization of the Aqueous Extract from Hubertia ambavilla L. (Asteraceae) by HPLC-DAD-ESI-MS(n) and Assessment of Its Antioxidant Activity. Chem. Biodivers. 2022, 19, e202200217. [Google Scholar] [CrossRef]
- European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (ETS 123). Strasbourg. 1986. Available online: https://rm.coe.int/168007a67b (accessed on 31 March 2023).
- Boivin, G.P.; Hickman, D.L.; Creamer-Hente, M.A.; Pritchett-Corning, K.R.; Bratcher, N.A. Review of CO2 as a Euthanasia Agent for Laboratory Rats and Mice. J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 491–499. [Google Scholar]
- Dobyan, D.C.; Bulger, R.E. Partial protection by chlorpromazine in mercuric chloride-induced acute renal failure in rats. Lab. Invest. 1984, 50, 578–586. [Google Scholar] [PubMed]
- Khodko, S.V.; Makarova, M.N.; Selezneva, A.I.; Saveliev, S.A.; Makarov, V.G.; Malinin, V.V. Effectiveness and mechanisms of action of Lys-Glu-Trp peptide on the model of hyperlipidemia caused by administration of Tween-80. Atheroscler. Dyslipidemia 2016, 1, 40–47. [Google Scholar]
- Polyakov, L.M.; Lushnikova, E.L.; Nepomnyashikh, L.M.; Russkikh, G.S.; Pichugin, V.I.; Yujik, E.I. Indicators of lipid metabolism and protein composition of blood plasma lipoproteins in hypothyroid rats with experimental hypercholesterolemia. Fundam. Res. 2014, 10, 342–345. [Google Scholar]


| Biomarker (Mean ± SD) | Treatment Groups a | |||
|---|---|---|---|---|
| Untreated Control | HgCl2-Treated | HgCl2-Treated + CE 100 mg/kg | HgCl2-Treated + CE 500 mg/kg | |
| Body weight on day 10 after experiment initiation (% of initial body weight) | 131.6 ± 1.7 | 112.4 ± 2.4 * | 117.1 ± 2.6 | 120.7 ± 2.9 * |
| Body weight on day 21 after experiment initiation (% of initial body weight) | 147.5 ± 2.5 | 140.1 ± 1.9 | 144.9 ± 1.1 | 145.9 ± 2.0 |
| Relative liver weight | 3.93 ± 0.13 | 5.12 ± 0.15 * | 4.53 ± 0.16 # | 4.16 ± 0.06 # |
| Total protein, g/L | 74.7 ± 1.0 | 83.7 ± 1.1 * | 75.3 ± 0.7 # | 76.3 ± 0.9 # |
| Glucose, mmol/L | 7.04 ± 0.17 | 9.01 ± 0.16 * | 7.2 ± 0.14 # | 7.5 ± 0.13 # |
| Total cholesterol, mmol/L | 1.72 ± 0.04 | 2.80 ± 0.08 * | 2.1 ± 0.08 # | 1.8 ± 0.05 # |
| Triglycerides, mmol/L | 0.98 ± 0.11 | 1.55 ± 0.12 * | 1.2 ± 0.15 # | 1.2 ± 0.15 # |
| Total bilirubin, mmol/L | 4.9 ± 0.2 | 7.8 ± 0.1 * | 4.6 ± 0.1 # | 4.8 ± 0.1 # |
| γ-glutamyl transferase (GGT), U/L | 6.5 ± 0.7 | 11.0 ± 04 * | 9.0 ± 0.5 # | 8.7 ± 0.7 # |
| Alkaline phosphatase (ALP), U/L | 784.0 ± 28.4 | 1005 ± 44.2 * | 971.0 ± 80.4 | 897.6 ± 57.0 # |
| Aspartate aminotransferase (AST), U/L | 136.8 ± 4.2 | 175.1 ± 4.4 * | 155 ± 10.5 | 151.1 ± 7.1 # |
| Alanine aminotransferase (ALT), U/L | 89.3 ± 3.7 | 126.3 ± 4.1 * | 90.0 ± 5.4 # | 80.9 ± 1.6 # |
| Biomarkers (Mean ± SD) | Treatment Groups a | |||
|---|---|---|---|---|
| Untreated Control | Tween-80-Treated | Tween-80-Treated + CE 100 mg/kg | Tween-80-Treated + CE 500 mg/kg | |
| Total cholesterol, mmol/L | 1.56 ± 0.09 | 1.83 ± 0.10 | 1.62 ± 0.08 | 1.74 ± 0.05 |
| High-density lipoproteins (HDL), mmol/L | 0.73 ± 0.03 | 0.57 ± 0.03 * | 0.65 ± 0.04 | 0.67 ± 0.03 # |
| Low-density lipoproteins (LDL), mmol/L | 0.58 ± 0.04 | 0.84 ± 0.03 * | 0.57 ± 0.02 # | 0.62 ± 0.04 # |
| Triglycerides, mmol/L | 0.68 ± 0.03 | 0.95 ± 0.04 * | 0.60 ± 0.07 # | 0.65 ± 0.06 # |
| Glucose, mmol/L | 5.23 ± 0.21 | 5.95 ± 0.13 * | 4.76 ± 0.17 # | 4.47 ± 0.15 # |
| Biomarkers (Mean ± SD) | Treatment Groups a | |||
|---|---|---|---|---|
| Untreated Control | Cholesterol–Mercazolil Treatment | Cholesterol– Mercazolil Treatment + CE 100 mg/kg | Cholesterol– Mercazolil Treatment + CE 500 mg/kg | |
| Body weight on day 29 after experiment initiation (% of initial body weight) | 119.4 ± 1.9 | 123.1 ± 2.4 | 119.2 ± 1.7 | 115.0 ± 1.5 # |
| Total cholesterol, mmol/L | 1.65 ± 0.08 | 1.82 ± 0.04 * | 1.56 ± 0.07 # | 1.53 ± 0.05 # |
| High-density lipoproteins (HDL), mmol/L | 0.80 ± 0.04 | 0.93 ± 0.03 | 0.83 ± 0.02 | 0.77 ± 0.03 |
| Low-density lipoproteins (LDL), mmol/L | 0.55 ± 0.03 | 0.53 ± 0.05 | 0.38 ± 0.04 # | 0.39 ± 0.03 # |
| Triglycerides, mmol/L | 0.76 ± 0.06 | 0.99 ± 0.08 * | 0.69 ± 0.04 # | 0.78 ± 0.02 # |
| Glucose, mmol/L | 4.80 ± 0.14 | 5.04 ± 0.13 | 5.01 ± 0.17 | 5.14 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krepkova, L.V.; Babenko, A.N.; Lemyaseva, S.V.; Saybel, O.L.; Sherwin, C.M.; Enioutina, E.Y. Modulation of Hepatic Functions by Chicory (Cichorium intybus L.) Extract: Preclinical Study in Rats. Pharmaceuticals 2023, 16, 1471. https://doi.org/10.3390/ph16101471
Krepkova LV, Babenko AN, Lemyaseva SV, Saybel OL, Sherwin CM, Enioutina EY. Modulation of Hepatic Functions by Chicory (Cichorium intybus L.) Extract: Preclinical Study in Rats. Pharmaceuticals. 2023; 16(10):1471. https://doi.org/10.3390/ph16101471
Chicago/Turabian StyleKrepkova, Lubov V., Alexandra N. Babenko, Svetlana V. Lemyaseva, Olga L. Saybel, Catherine M. Sherwin, and Elena Y. Enioutina. 2023. "Modulation of Hepatic Functions by Chicory (Cichorium intybus L.) Extract: Preclinical Study in Rats" Pharmaceuticals 16, no. 10: 1471. https://doi.org/10.3390/ph16101471
APA StyleKrepkova, L. V., Babenko, A. N., Lemyaseva, S. V., Saybel, O. L., Sherwin, C. M., & Enioutina, E. Y. (2023). Modulation of Hepatic Functions by Chicory (Cichorium intybus L.) Extract: Preclinical Study in Rats. Pharmaceuticals, 16(10), 1471. https://doi.org/10.3390/ph16101471









