Greener and Whiter Analytical Chemistry Using Cyrene as a More Sustainable and Eco-Friendlier Mobile Phase Constituent in Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Cyrene as Co-Eluent in the Mobile Phase
2.2. Greenness and Whiteness Assessments of the Methods
2.3. Potential of Heated/Superheated Cyrene Chromatography vs. Heated/Superheated Water Chromatography
3. Materials and Methods
3.1. List of Chemicals
3.2. Buffer and Sample Preparation
3.3. HPLC Analysis
3.4. Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastas, P. Green Chemistry. Frontiers 1998, 640, 850. [Google Scholar]
- Płotka-Wasylka, J.; Namieśnik, J. (Eds.) Green Analytical Chemistry: Past, Present and Perspectives; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Yabré, M.; Ferey, L.; Somé, I.T.; Gaudin, K. Greening Reversed-Phase Liquid Chromatography Methods Using Alternative Solvents for Pharmaceutical Analysis. Molecules 2018, 23, 1065. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Mechlińska, A.; Namieśnik, J. Green Analytical Chemistry—Theory and Practice. Chem. Soc. Rev. 2010, 39, 2869. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for Assessing the Greenness of Analytical Procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- National Environmental Methods Index. Available online: www.nemi.gov (accessed on 2 August 2023).
- Anastas, P.T.; Zimmerman, J.B. Peer Reviewed: Design Through the 12 Principles of Green Engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Caudet, A.; Campíns-Falcó, P.; Pérez, B.; Sancho, R.; Lorente, M.; Sastre, G.; González, C. A New Tool for Evaluating and/or Selecting Analytical Methods: Summarizing the Information in a Hexagon. TrAC Trends Anal. Chem. 2019, 118, 538–547. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Hicks, M.B.; Farrell, W.; Aurigemma, C.; Lehmann, L.; Weisel, L.; Nadeau, K.; Lee, H.; Moraff, C.; Wong, M.; Huang, Y.; et al. Making the Move towards Modernized Greener Separations: Introduction of the Analytical Method Greenness Score (AMGS) Calculator. Green Chem. 2019, 21, 1816–1826. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAC Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hussain, C.G.; Keçili, R. White Analytical Chemistry Approaches for Analytical and Bioanalytical Techniques: Applications and Challenges. TrAC Trends Anal. Chem. 2023, 159, 116905. [Google Scholar] [CrossRef]
- Fields, S.M.; Ye, C.Q.; Zhang, D.D.; Branch, B.R.; Zhang, X.J.; Okafo, N. Superheated Water as Eluent in High-Temperature High-Performance Liquid Chromatographic Separations of Steroids on a Polymer-Coated Zirconia Column. J. Chromatogr. A 2001, 913, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Droux, S.; Félix, G. Green Chiral HPLC Enantiomeric Separations Using High Temperature Liquid Chromatography and Subcritical Water on Chiralcel OD and Chiralpak AD. Chirality 2011, 23, E105–E109. [Google Scholar] [CrossRef]
- Sadeghi, F.; Navidpour, L.; Bayat, S.; Afshar, M. Validation and Uncertainty Estimation of an Ecofriendly and Stability-Indicating HPLC Method for Determination of Diltiazem in Pharmaceutical Preparations. J. Anal. Methods Chem. 2013, 2013, 353814. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.M.; Lamie, N.T. Analytical Eco-Scale for Assessing the Greenness of a Developed RP-HPLC Method Used for Simultaneous Analysis of Combined Antihypertensive Medications. J. AOAC Int. 2016, 99, 1260–1265. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Isaac, V.L.B.; Corrêa, M.A.; Salgado, H.R.N. Validation of HPLC–UV Assay of Caffeic Acid in Emulsions. J. Chromatogr. Sci. 2015, 54, bmv142. [Google Scholar] [CrossRef]
- Ribeiro, R.L.V.; Bottoli, C.B.G.; Collins, K.E.; Collins, C.H. Reevaluation of Ethanol as Organic Modifier for Use in HPLS-RP Mobile Phases. J. Braz. Chem. Soc. 2004, 15, 300–306. [Google Scholar] [CrossRef]
- Welch, C.J.; Brkovic, T.; Schafer, W.; Gong, X. Performance to Burn? Re-Evaluating the Choice of Acetonitrile as the Platform Solvent for Analytical HPLC. Green Chem. 2009, 11, 1232. [Google Scholar] [CrossRef]
- Tache, F.; Udrescu, S.; Albu, F.; Micăle, F.; Medvedovici, A. Greening Pharmaceutical Applications of Liquid Chromatography through Using Propylene Carbonate–Ethanol Mixtures Instead of Acetonitrile as Organic Modifier in the Mobile Phases. J. Pharm. Biomed. Anal. 2013, 75, 230–238. [Google Scholar] [CrossRef]
- Cheregi, M.; Albu, F.; Udrescu, Ş.; Răducanu, N.; Medvedovici, A. Greener Bioanalytical Approach for LC/MS–MS Assay of Enalapril and Enalaprilat in Human Plasma with Total Replacement of Acetonitrile throughout All Analytical Stages. J. Chromatogr. B 2013, 927, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Basci, N.E. Green Bioanalytical and Pharmaceutical Analysis of Voriconazole and Tadalafil by HPLC. Curr. Pharm. Anal. 2017, 13, 495–504. [Google Scholar] [CrossRef]
- Keppel, T.R.; Jacques, M.E.; Weis, D.D. The Use of Acetone as a Substitute for Acetonitrile in Analysis of Peptides by Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.; Ruth, W.; Kragl, U. Assessment of Acetone as an Alternative to Acetonitrile in Peptide Analysis by Liquid Chromatography/Mass Spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.T.; Fraige, K.; Leme, G.M.; da Silva Bolzani, V.; Hilder, E.F.; Cavalheiro, A.J.; Arrua, R.D.; Funari, C.S. Natural Deep Eutectic Solvents as the Major Mobile Phase Components in High-Performance Liquid Chromatography—Searching for Alternatives to Organic Solvents. Anal. Bioanal. Chem. 2018, 410, 3705–3713. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M.; El Deeb, S.; Mahmoud Swaif, M.; Ismail Ibrahim, R.; Ali Kamil, R.; Salman Abdelwahed, A.; Ehab Ibrahim, A. Micellar Organic-Solvent Free HPLC Design of Experiment for the Determination of Ertapenem and Meropenem; Assessment Using GAPI, AGREE and Analytical Eco-Scale Models. Microchem. J. 2023, 185, 108262. [Google Scholar] [CrossRef]
- Sharaf, Y.A.; El Deeb, S.; Ibrahim, A.E.; Al-Harrasi, A.; Sayed, R.A. Two Green Micellar HPLC and Mathematically Assisted UV Spectroscopic Methods for the Simultaneous Determination of Molnupiravir and Favipiravir as a Novel Combined COVID-19 Antiviral Regimen. Molecules 2022, 27, 2330. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chen, M.-L.; Rambla-Alegre, M.; Durgavanshi, A.; Bose, D.; Esteve-Romero, J. Rapid and Sensitive Determination of Nicotine in Formulations and Biological Fluid Using Micellar Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B 2010, 878, 2397–2402. [Google Scholar] [CrossRef]
- Peris-Vicente, J.; Villareal-Traver, M.; Casas-Breva, I.; Carda-Broch, S.; Esteve-Romero, J. A Micellar Liquid Chromatography Method for the Quantification of Abacavir, Lamivudine and Raltegravir in Plasma. J. Pharm. Biomed. Anal. 2014, 98, 351–355. [Google Scholar] [CrossRef]
- Ruiz-Angel, M.J.; Peris-García, E.; García-Alvarez-Coque, M.C. Reversed-Phase Liquid Chromatography with Mixed Micellar Mobile Phases of Brij-35 and Sodium Dodecyl Sulphate: A Method for the Analysis of Basic Compounds. Green Chem. 2015, 17, 3561–3570. [Google Scholar] [CrossRef]
- Citarella, A.; Amenta, A.; Passarella, D.; Micale, N. Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials. Int. J. Mol. Sci. 2022, 23, 15960. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Nemala, S.S.; De Bellis, G.; Capasso, A. Green Solvents for the Liquid Phase Exfoliation Production of Graphene: The Promising Case of Cyrene. Front. Chem. 2022, 10, 878799. [Google Scholar] [CrossRef] [PubMed]
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: A Bio-Based Novel and Sustainable Solvent for Organic Synthesis. Green Chem. 2022, 24, 6435–6449. [Google Scholar] [CrossRef]
- Wibisono, Y.; Noviani, V.; Ramadhani, A.; Baruna, A.; Devianto, L.; Adi Sulianto, A. Fabrication of Cellulose Acetate Membrane Using Cyrene as Green Solvent. In Proceedings of the First International Conference on “Green” Polymer Materials 2020, Online, 5–25 November 2020; MDPI: Basel, Switzerland, 2020; p. 7189. [Google Scholar]
- Camp, J.E. Bio-Available Solvent Cyrene: Synthesis, Derivatization, and Applications. ChemSusChem 2018, 11, 3048–3055. [Google Scholar] [CrossRef] [PubMed]
- Grune, C.; Thamm, J.; Werz, O.; Fischer, D. CyreneTM as an Alternative Sustainable Solvent for the Preparation of Poly(Lactic-Co-Glycolic Acid) Nanoparticles. J. Pharm. Sci. 2021, 110, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Milescu, R.A.; Segatto, M.L.; Stahl, A.; McElroy, C.R.; Farmer, T.J.; Clark, J.H.; Zuin, V.G. Sustainable Single-Stage Solid–Liquid Extraction of Hesperidin and Rutin from Agro-Products Using Cyrene. ACS Sustain. Chem. Eng. 2020, 8, 18245–18257. [Google Scholar] [CrossRef]
- Brouwer, T.; Schuur, B. Dihydrolevoglucosenone (Cyrene), a Biobased Solvent for Liquid–Liquid Extraction Applications. ACS Sustain. Chem. Eng. 2020, 8, 14807–14817. [Google Scholar] [CrossRef]
- Leitsch, D. A Review on Metronidazole: An Old Warhorse in Antimicrobial Chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef]
- Freeman, C.D.; Klutman, N.E.; Lamp, K.C. Metronidazole. Drugs 1997, 54, 679–708. [Google Scholar] [CrossRef]
- Ceruelos, A.H.; Romero-Quezada, L.C.; Ledezma, J.R.; Contreras, L.L. Therapeutic Uses of Metronidazole and Its Side Effects: An Update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar]
- Keating, G.M.; Scott, L.J. Moxifloxacin. Drugs 2004, 64, 2347–2377. [Google Scholar] [CrossRef] [PubMed]
- Wise, R. A Review of the Clinical Pharmacology of Moxifloxacin, a New 8-Methoxyquinolone, and Its Potential Relation to Therapeutic Efficacy. Clin. Drug Investig. 1999, 17, 365–387. [Google Scholar] [CrossRef]
- Elkhoudary, M.M.; Abdel Salam, R.A.; Hadad, G.M. Development and Optimization of HPLC Analysis of Metronidazole, Diloxanide, Spiramycin and Cliquinol in Pharmaceutical Dosage Forms Using Experimental Design. J. Chromatogr. Sci. 2016, 54, 1701–1712. [Google Scholar] [CrossRef]
- Sankar, P.R.; Deepthi, K.D.; Babu, P.S.; Rachana, G.; Geethika, A.S.; Bhargavi, J.; Kalyan, L.P.; Poojitha, K.N. Development and Validation of a Stability-Indicating Method for Assay of Moxifloxacin in Oral Pharmaceutical Dosage Forms by HPLC. IOSR J. Pharm. 2019, 9, 30–41. [Google Scholar]
- Phani Sekhar Reddy, G.; Navyasree, K.S.; Jagadish, P.C.; Bhat, K. Analytical Method Development and Validation for HPLC-ECD Determination of Moxifloxacin in Marketed Formulations. Pharm. Chem. J. 2018, 52, 674–679. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Zhao, X.; Yang, W.; Sun, H. Improved HPLC Method for the Determination of Moxifloxacin in Application to a Pharmacokinetics Study in Patients with Infectious Diseases. ISRN Pharmacol. 2013, 2013, 462918. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ansari, M.T. Quantitation of metronidazole in pharmaceutical suspension using high performance liquid chromatographic method. Pak. J. Zoology 2011, 2011, 43. [Google Scholar]
- El-Yazbi, A.F.; Aboukhalil, F.M.; Khamis, E.F.; Elkhatib, M.A.W.; El-Sayed, M.A.; Youssef, R.M. Simple Simultaneous Determination of Moxifloxacin and Metronidazole in Complex Biological Matrices. RSC Adv. 2022, 12, 15694–15704. [Google Scholar] [CrossRef]
- Shen, Y.; Lo, C.; Nagaraj, D.R.; Farinato, R.; Essenfeld, A.; Somasundaran, P. Development of Greenness Index as an Evaluation Tool to Assess Reagents: Evaluation Based on SDS (Safety Data Sheet) Information. Miner. Eng. 2016, 94, 1–9. [Google Scholar] [CrossRef]
- Islam, T.; Islam Sarker, M.Z.; Uddin, A.H.; Yunus, K.B.; Prasad, R.; Mia, M.A.R.; Ferdosh, S. Kamlet Taft Parameters: A Tool to Alternate the Usage of Hazardous Solvent in Pharmaceutical and Chemical Manufacturing/Synthesis—A Gateway towards Green Technology. Anal. Chem. Lett. 2020, 10, 550–561. [Google Scholar] [CrossRef]
- International Conference on Harmonization Geneva. ICH Guideline for Validation of Analytical Procedure: Text and Methodology, Q2 (R1); ICH: Geneva, Switzerland, 2005; Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 2 August 2023).
- Heinisch, S.; Rocca, J.-L. Sense and Nonsense of High-Temperature Liquid Chromatography. J. Chromatogr. A 2009, 1216, 642–658. [Google Scholar] [CrossRef]
- Greibrokk, T.; Andersen, T. High-Temperature Liquid Chromatography. J. Chromatogr. A 2003, 1000, 743–755. [Google Scholar] [CrossRef]
- McNeff, C.V.; Yan, B.; Stoll, D.R.; Henry, R.A. Practice and Theory of High Temperature Liquid Chromatography. J. Sep. Sci. 2007, 30, 1672–1685. [Google Scholar] [CrossRef]







| Solvent | UV Cut-Off Value (nm) | Water Solubility | Density (g/cm3) at 20 °C | Polarity Parameter Kamlet–Taft π* | Partition Coefficient N-Octanol/Water (Log Value) | Boiling Point °C | Flash Point °C at 1.013 hPa (c.c.) |
|---|---|---|---|---|---|---|---|
| Acetonitrile | 190 | Miscible in any proportion | 0.7822 | 0.75 | −0.34 | 82 | 2 |
| Methanol | 205 | 1000 g/L at 20 °C—completely miscible | 0.7913 | 0.61 | −0.77 | 64.7 | 12 |
| Ethanol | 210 | ≥1000 g/L at 20 °C | 0.81 | 0.54 | −0.31 | 78 | 9.7 |
| Water | 190 | Not applied | 0.9982 | 1.2 | Not applied | 100 | Not applied |
| Propylene carbonate | 220 | 175 g/L at 25 °C | 1.2047 | 0.9 | −0.41 | 240 | 132 |
| Acetone | 330 | Miscible in any proportion | 0.79 | 0.71 | −0.23 | 56.05 | −18 |
| Cyrene | 350 | ca.52.6 g/L at 20 °C | 1.25 | 0.93 | −1.52 | 227 | 108 |
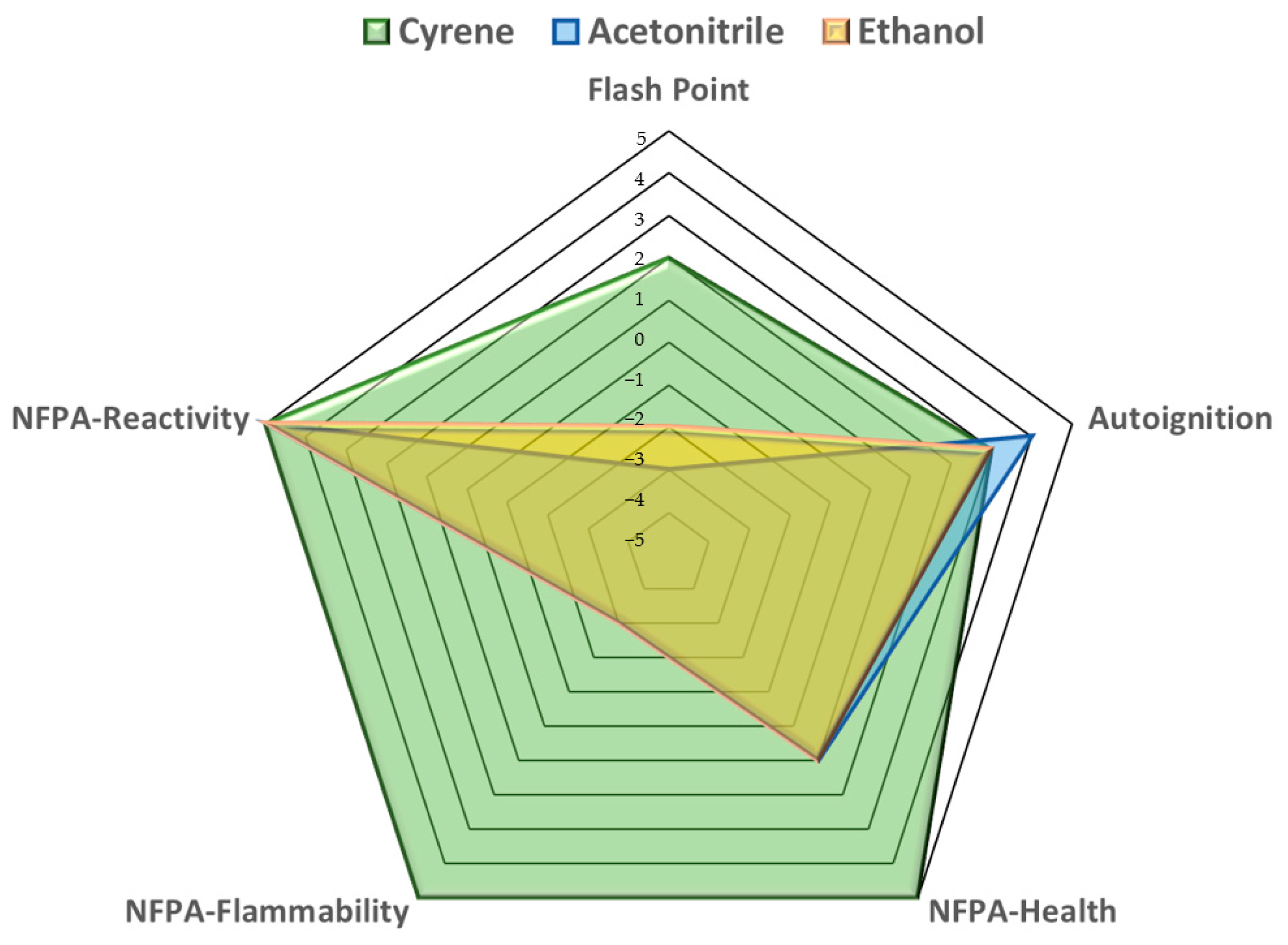
| Solvent Evaluation Criteria | Flash Point | Autoignition | NFPA Health | NFPA Flammability | NFPA Reactivity | |
|---|---|---|---|---|---|---|
| Acetonitrile | Value or Rating | 2 °C | 524 °C | 2 | 3 | 0 |
| Score | −3 | 4 | 1 | −3 | 5 | |
| Ethanol | Value or Rating | 12 °C | 455 °C | 2 | 3 | 0 |
| Score | −2 | 3 | 1 | −3 | 5 | |
| Cyrene | Value or Rating | 108 °C | 296 °C | 0 | 0 | 0 |
| Score | 2 | 3 | 5 | 5 | 5 |
| Parameter | MET | MOX |
|---|---|---|
| Linearity (R2) | 0.999 | 0.9994 |
| Linearity Range (µg/mL) | 0.3–80 | 10–80 |
| LOD (µg/mL) | 0.1 | 4 |
| LOQ (µg/mL) | 0.3 | 8 |
| Accuracy (µg/mL) | 98.4–105.6% | 96.7–104.7% |
| Precision RSD% | ||
| LQC | 1.4% | 1.9% |
| MQC | 1.2% | 1.6% |
| HQC | 0.8% | 1.6% |
| Mobile Phase Composition | Generated Backpressure in Bar |
|---|---|
| Ethanol: 0.1 M sodium acetate buffer pH 4.25 (13:87, v/v) | 71 |
| Cyrene: 0.1 M ethanol: sodium acetate buffer pH 4.25 (2:13:85, v/v/v) | 75 |
| Cyrene: 0.1 M ethanol: sodium acetate buffer pH 4.25 (4:13:83, v/v/v) | 80 |
| Cyrene: 0.1 M ethanol: sodium acetate buffer pH 4.25 (6:13:81, v/v/v) | 85 |
| Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v) | 91 |
| Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (10:13:77, v/v/v) | 98 |
| Method | Elution Conditions | Reference | |
|---|---|---|---|
| A | Reported acetonitrile-based non-green method | HPLC-DAD using RP-C18 column. Isocratic elution using ACN and phosphate buffer (30:70, v/v) as mobile phase on a Zorbax Eclipse Plus C18 column | [51] |
| B | Developed green ethanol-based reference method | HPLC-DAD using monolithic C18 column. Isocratic elution using ethanol and 0.1 M sodium acetate buffer pH 4.25 (13:87, v/v) as mobile phase | This work |
| C | Develop a greener Cyrene/ethanol-based method. | HPLC-DAD using monolithic C18 column. Isocratic elution using Cyrene: ethanol and0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v) as mobile phase | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Deeb, S.; Abdelsamad, K.; Parr, M.K. Greener and Whiter Analytical Chemistry Using Cyrene as a More Sustainable and Eco-Friendlier Mobile Phase Constituent in Chromatography. Pharmaceuticals 2023, 16, 1488. https://doi.org/10.3390/ph16101488
El Deeb S, Abdelsamad K, Parr MK. Greener and Whiter Analytical Chemistry Using Cyrene as a More Sustainable and Eco-Friendlier Mobile Phase Constituent in Chromatography. Pharmaceuticals. 2023; 16(10):1488. https://doi.org/10.3390/ph16101488
Chicago/Turabian StyleEl Deeb, Sami, Khalid Abdelsamad, and Maria Kristina Parr. 2023. "Greener and Whiter Analytical Chemistry Using Cyrene as a More Sustainable and Eco-Friendlier Mobile Phase Constituent in Chromatography" Pharmaceuticals 16, no. 10: 1488. https://doi.org/10.3390/ph16101488
APA StyleEl Deeb, S., Abdelsamad, K., & Parr, M. K. (2023). Greener and Whiter Analytical Chemistry Using Cyrene as a More Sustainable and Eco-Friendlier Mobile Phase Constituent in Chromatography. Pharmaceuticals, 16(10), 1488. https://doi.org/10.3390/ph16101488







